Functionalization of Osteoplastic Material with Human Placental Growth Factor and Assessment of Biocompatibility of the Resulting Material In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Placental Growth Factor
2.2. Osteoplastic Matrix
2.3. ASC Cultures
2.4. Obtaining Human ASC Cultures
2.5. Obtaining Rabbit ASC Cultures
2.6. Microscopy
2.7. Assessment of the Impact of Placental Growth Factor on ASC Cultures
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Functionalization of the Xenogeneic Human PLGF Matrix and Assessment of Protein Release into the Medium In Vitro
2.10. Assessment of Cytotoxicity of the PLGF-Functionalized Osteoplastic Matrix
2.11. Assessment of Adhesion, Proliferation, and Migration of Human ASCs in Osteoplastic Matrix Samples
2.12. Statistical Analysis
3. Results
3.1. Interaction of PLGF with ASCs
3.1.1. Interaction of PLGF with Human ASCs
3.1.2. Interaction of Human PLGF with Rabbit ASCs
3.2. Dynamics of PLGF Release from Functionalized Osteoplastic Matrix Samples
3.3. Assessment of the Cytotoxicity of Matrix Samples Functionalized with PLGF
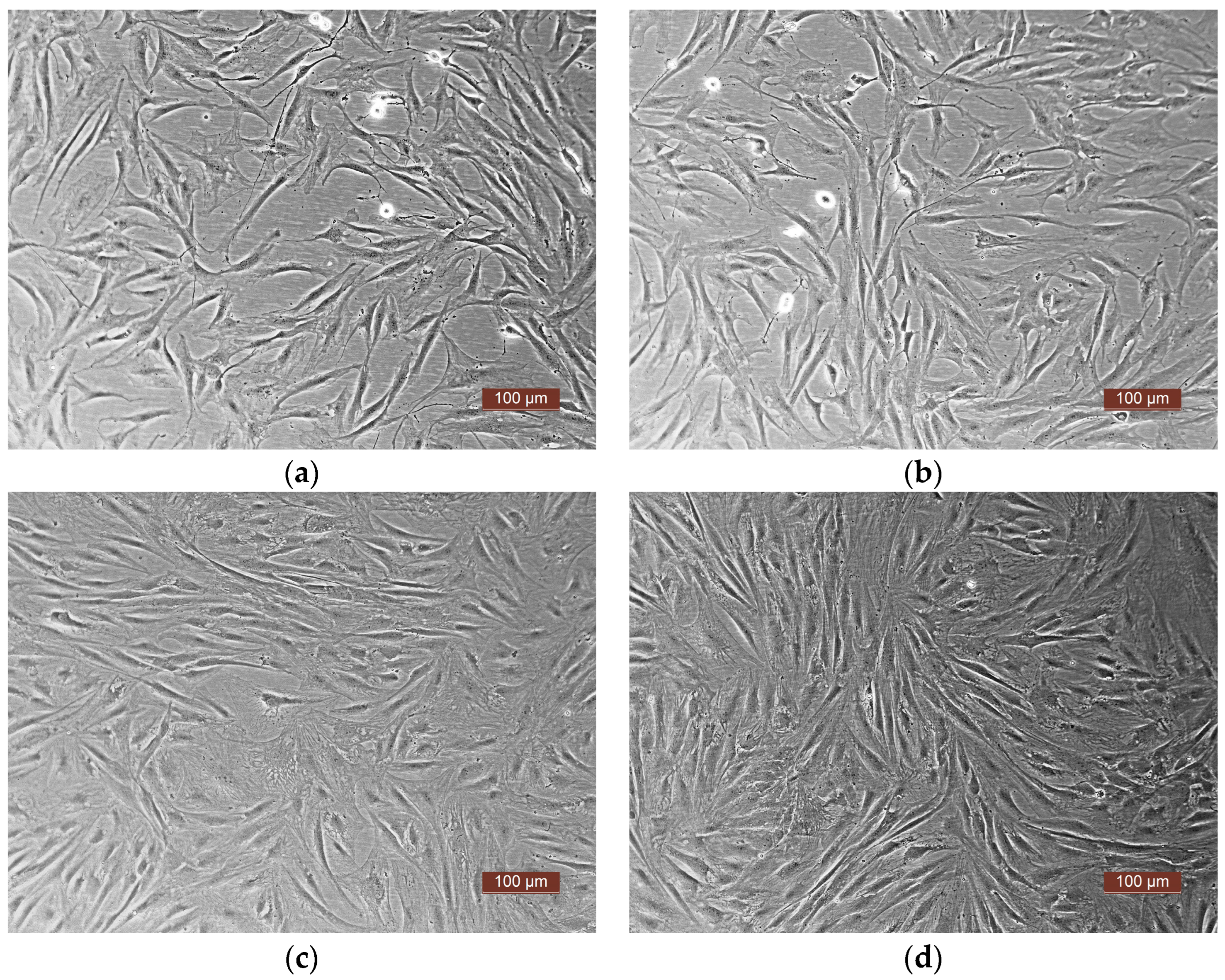
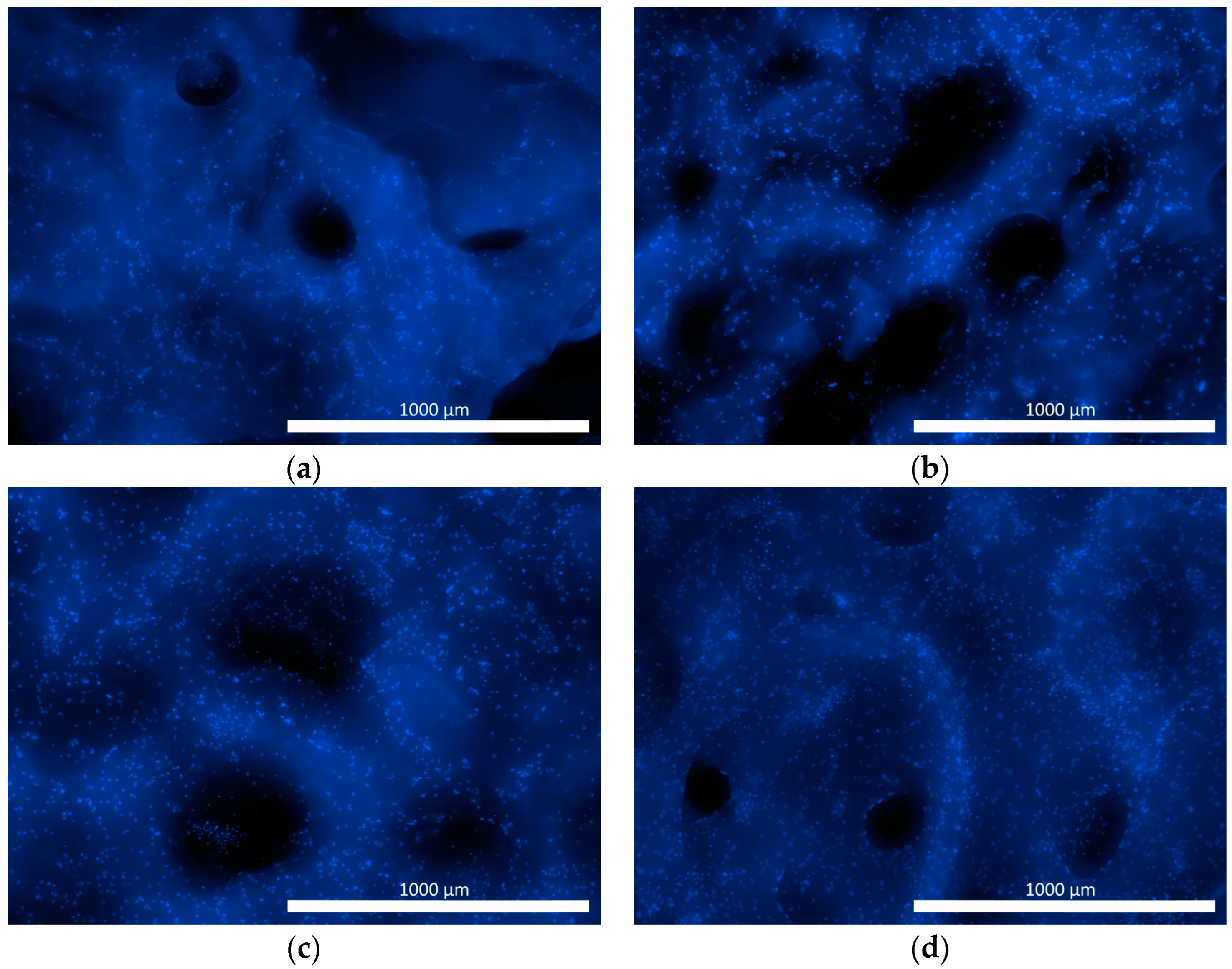
3.4. Adhesion, Proliferation, and Migration of Human ASCs in Functionalized Matrix Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, R.K.; Jensen, T.S.; Koes, B.; Hartvigsen, J. Prevalence of lumbar spinal stenosis in general and clinical populations: A systematic review and meta-analysis. Eur. Spine J. 2020, 29, 2143–2163. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Zimmerman, Z.E.; Mass, H.; Makhni, M.C. Diagnosis and Management of Lumbar Spinal Stenosis: A Review. JAMA 2022, 327, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Resnick, D.K.; Watters, W.C.; Sharan, A.; Mummaneni, P.V.; Dailey, A.T.; Wang, J.C.; Choudhri, T.F.; Eck, J.; Ghogawala, Z.; Groff, M.W.; et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: Lumbar fusion for stenosis with spondylolisthesis. J. Neurosurg. Spine 2014, 21, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Dailey, A.T.; Mummaneni, P.V.; Ghogawala, Z.; Resnick, D.K.; Watters, W.C.; Groff, M.W.; Choudhri, T.F.; Eck, J.C.; Sharan, A.; et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 8: Lumbar fusion for disc herniation and radiculopathy. J. Neurosurg. Spine 2014, 21, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Omidi-Kashani, F.; Hasankhani, E.G.; Ashjazadeh, A. Lumbar spinal stenosis: Who should be fused? An updated review. Asian Spine J. 2014, 8, 521–530. [Google Scholar] [CrossRef]
- Samuel, A.M.; Moore, H.G.; Cunningham, M.E. Treatment for Degenerative Lumbar Spondylolisthesis: Current Concepts and New Evidence. Curr. Rev. Musculoskelet. Med. 2017, 10, 521–529. [Google Scholar] [CrossRef]
- Li, B.; Sun, C.; Zhao, C.; Yao, X.; Zhang, Y.; Duan, H.; Hao, J.; Guo, X.; Fan, B.; Ning, G.; et al. Epidemiological profile of thoracolumbar fracture (TLF) over a period of 10 years in Tianjin, China. J. Spinal Cord Med. 2019, 42, 178–183. [Google Scholar] [CrossRef]
- Doud, A.N.; Weaver, A.A.; Talton, J.W.; Barnard, R.T.; Meredith, J.W.; Stitzel, J.D.; Miller, P.; Miller, A.N. Has the incidence of thoracolumbar spine injuries increased in the United States from 1998 to 2011? Clin. Orthop. Relat. Res. 2015, 473, 297–304. [Google Scholar] [CrossRef]
- Muratore, M.; Ferrera, A.; Masse, A.; Bistolfi, A. Osteoporotic vertebral fractures: Predictive factors for conservative treatment failure. A systematic review. Eur. Spine J. 2018, 27, 2565–2576. [Google Scholar] [CrossRef]
- Vaccaro, A.R.; Schroeder, G.D.; Kepler, C.K.; Cumhur Oner, F.; Vialle, L.R.; Kandziora, F.; Koerner, J.D.; Kurd, M.F.; Reinhold, M.; Schnake, K.J.; et al. The surgical algorithm for the AOSpine thoracolumbar spine injury classification system. Eur. Spine J. 2016, 25, 1087–1094. [Google Scholar] [CrossRef]
- Viswanathan, V.; Kanna, R. Management of Thoracolumbar Fractures in Adults: Current Algorithm. Int. J. Spine 2019, 4, 10–19. [Google Scholar]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Hyder, M.N.; Quadir, M.A.; Courchesne, N.M.D.; Seeherman, H.J.; Nevins, M.; Spector, M.; Hammond, P.T. Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction. Proc. Natl. Acad. Sci. USA 2014, 111, 12847–12852. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef]
- Gothard, D.; Smith, E.L.; Kanczler, J.M.; Rashidi, H.; Qutachi, O.; Henstock, J.; Rotherham, M.; El Haj, A.; Shakesheff, K.M.; Oreffo, R.O.C. Tissue engineered bone using select growth factors: A comprehensive review of animal studies and clinical translation studies in man. Eur. Cells Mater. 2014, 28, 166–208. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Hwang, J.W.; Han, Y.H. Novel bone Morphogenetic Protein (BMP)-2/4 Consensus Peptide (BCP) for the Osteogenic Differentiation of C2C12 Cells. Curr. Protein Pept. Sci. 2023, 24, 610–619. [Google Scholar] [CrossRef]
- Lee, M.; Li, W.; Siu, R.K.; Whang, J.; Zhang, X.; Soo, C.; Ting, K.; Wu, B.M. Biomimetic apatite-coated alginate/chitosan microparticles as osteogenic protein carriers. Biomaterials 2009, 30, 6094–6101. [Google Scholar] [CrossRef]
- Cao, L.; Werkmeister, J.A.; Wang, J.; Glattauer, V.; McLean, K.M.; Liu, C. Bone regeneration using photocrosslinked hydrogel incorporating rhBMP-2 loaded 2-N, 6-O-sulfated chitosan nanoparticles. Biomaterials 2014, 35, 2730–2742. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef]
- McCoy, R.J.; Widaa, A.; Watters, K.M.; Wuerstle, M.; Stallings, R.L.; Duffy, G.P.; O’Brien, F.J. Orchestrating osteogenic differentiation of mesenchymal stem cells--identification of placental growth factor as a mechanosensitive gene with a pro-osteogenic role. Stem Cells 2013, 31, 2420–2431. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, D.; Srouji, S.; Shapira-Schweitzer, K.; Kossover, O.; Ivanir, E.; Kuhn, G.; Müller, R.; Seliktar, D.; Livne, E. Low dose BMP-2 treatment for bone repair using a PEGylated fibrinogen hydrogel matrix. Biomaterials 2013, 34, 2902–2910. [Google Scholar] [CrossRef] [PubMed]
- Rahman, C.V.; Ben-David, D.; Dhillon, A.; Kuhn, G.; Gould, T.W.A.; Müller, R.; Rose, F.R.A.J.; Shakesheff, K.M.; Livne, E. Controlled release of BMP-2 from a sintered polymer scaffold enhances bone repair in a mouse calvarial defect model. J. Tissue Eng. Regen. Med. 2014, 8, 59–66. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Life-Threatening Complications Associated with Recombinant Human Bone Morphogenetic Protein in Cervical Spine Fusion, FDA Public Health Notification, July 1, 2008. Available online: www.fda.gov/cdrh/safety/070108-rhbmp.html (accessed on 5 August 2008).
- Huang, P.; Chen, A.; He, W.; Li, Z.; Zhang, G.; Liu, Z.; Liu, G.; Liu, X.; He, S.; Xiao, G.; et al. BMP-2 induces EMT and breast cancer stemness through Rb and CD44. Cell Death Discov. 2017, 3, 17039. [Google Scholar] [CrossRef]
- Horvath, L.G.; Henshall, S.M.; Kench, J.G.; Turner, J.J.; Golovsky, D.; Brenner, P.C.; O’Neill, G.F.; Kooner, R.; Stricker, P.D.; Grygiel, J.J.; et al. Loss of BMP2, Smad8, and Smad4 expression in prostate cancer progression. Prostate 2004, 59, 234–242. [Google Scholar] [CrossRef]
- Mesfin, A.; Buchowski, J.M.; Zebala, L.P.; Bakhsh, W.R.; Aronson, A.B.; Fogelson, J.L.; Hershman, S.; Kim, H.J.; Ahmad, A.; Bridwell, K.H. High-dose rhBMP-2 for adults: Major and minor complications. J. Bone Jt. Surg. 2013, 95, 1546–1553. [Google Scholar] [CrossRef]
- Moshel, Y.A.; Hernandez, E.I.; Kong, L.; Liu, C.; Samadani, U. Acute renal insufficiency, supraventricular tachycardia, and confusion after recombinant human bone morphogenetic protein-2 implantation for lumbosacral spine fusion. J. Neurosurg. Spine 2008, 8, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Selph, S.; McDonagh, M.; Peterson, K.; Tiwari, A.; Chou, R.; Helfand, M. Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: A systematic review and meta-analysis. Ann. Intern. Med. 2013, 158, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Lewandrowski, K.U.; Nanson, C.; Calderon, R. Vertebral osteolysis after posterior interbody lumbar fusion with recombinant human bone morphogenetic protein 2: A report of five cases. Spine J. 2007, 7, 609–614. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; Zara, J.N.; Zhang, X.; Askarinam, A.; Goyal, R.; Chiang, M.; Yuan, W.; Chang, L.; Corselli, M.; Shen, J.; et al. Perivascular stem cells: A prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl. Med. 2012, 1, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.; Jiang, X.; Soo, C.; Zhang, Z.; Zhang, X.; Hu, J.; Pan, H.; Hsu, T.; Wu, B.; Ting, K. A Study of the Role of Nell-1 Gene Modified Goat Bone Marrow Stromal Cells in Promoting New Bone Formation. Mol. Ther. 2007, 15, 1872–1880. [Google Scholar] [CrossRef]
- Wiszniak, S.; Schwarz, Q. Exploring the Intracrine Functions of VEGF-A. Biomolecules 2021, 11, 128. [Google Scholar] [CrossRef]
- Burger, M.G.; Grosso, A.; Briquez, P.S.; Born, G.M.E.; Lunger, A.; Schrenk, F.; Todorov, A.; Sacchi, V.; Hubbell, J.A.; Schaefer, D.J.; et al. Robust coupling of angiogenesis and osteogenesis by VEGF-decorated matrices for bone regeneration. Acta Biomater. 2022, 149, 111–125. [Google Scholar] [CrossRef]
- Geiger, F.; Lorenz, H.; Xu, W.; Szalay, K.; Kasten, P.; Claes, L.; Augat, P.; Richter, W. VEGF producing bone marrow stromal cells (BMSC) enhance vascularization and resorption of a natural coral bone substitute. Bone 2007, 41, 516–522. [Google Scholar] [CrossRef]
- Nurrachman, A.S.; Azhari, A.; Epsilawati, L.; Pramanik, F. Temporal Pattern of micro-CT Angiography Vascular Parameters and VEGF mRNA Expression in Fracture Healing: A Radiograph and Molecular Comparison. Eur. J. Dent. 2023, 17, 283–295. [Google Scholar] [CrossRef]
- Salehi, S.; Tavakoli, M.; Mirhaj, M.; Varshosaz, J.; Labbaf, S.; Karbasi, S.; Jafarpour, F.; Kazemi, N.; Salehi, S.; Mehrjoo, M.; et al. A 3D printed polylactic acid-Baghdadite nanocomposite scaffold coated with microporous chitosan-VEGF for bone regeneration applications. Carbohydr. Polym. 2023, 312, 120787. [Google Scholar] [CrossRef]
- Orth, M.; Shenar, A.K.; Scheuer, C.; Braun, B.J.; Herath, S.C.; Holstein, J.H.; Histing, T.; Yu, X.; Murphy, W.L.; Pohlemann, T.; et al. VEGF-loaded mineral-coated microparticles improve bone repair and are associated with increased expression of epo and RUNX-2 in murine non-unions. J. Orthop. Res. 2019, 37, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Wernike, E.; Montjovent, M.O.; Liu, Y.; Wismeijer, D.; Hunziker, E.B.; Siebenrock, K.A.; Hofstetter, W.; Klenke, F.M. VEGF incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. Eur. Cells Mater. 2010, 19, 30–40. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Jonckx, B.; Mazzone, M.; Zacchigna, S.; Loges, S.; Pattarini, L.; Chorianopoulos, E.; Liesenborghs, L.; Koch, M.; De Mol, M.; et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 2007, 131, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Xu, L. alphaPlGF: A new kid on the antiangiogenesis block. Cell 2007, 131, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The discovery of the placental growth factor and its role in angiogenesis: A historical review. Angiogenesis 2008, 11, 215–221. [Google Scholar] [CrossRef]
- Maes, C.; Coenegrachts, L.; Stockmans, I.; Daci, E.; Luttun, A.; Petryk, A.; Gopalakrishnan, R.; Moermans, K.; Smets, N.; Verfaillie, C.M.; et al. Placental growth factor mediates mesenchymal cell development, cartilage turnover, and bone remodeling during fracture repair. J. Clin. Investig. 2006, 116, 1230–1242. [Google Scholar] [CrossRef]
- Fischer, C.; Mazzone, M.; Jonckx, B.; Carmeliet, P. FLT1 and its ligands VEGFB and PlGF: Drug targets for anti-angiogenic therapy? Nat. Rev. Cancer 2008, 8, 942–956. [Google Scholar] [CrossRef]
- Dewerchin, M.; Carmeliet, P. PlGF: A multitasking cytokine with disease-restricted activity. Cold Spring Harb. Perspect. Med. 2012, 2, a011056. [Google Scholar] [CrossRef]
- Marrony, S.; Bassilana, F.; Seuwen, K.; Keller, H. Bone morphogenetic protein 2 induces placental growth factor in mesenchymal stem cells. Bone 2003, 33, 426–433. [Google Scholar] [CrossRef]
- Sun, Y.; Wan, B.; Wang, R.; Zhang, B.; Luo, P.; Wang, D.; Nie, J.J.; Chen, D.; Wu, X. Mechanical Stimulation on Mesenchymal Stem Cells and Surrounding Microenvironments in Bone Regeneration: Regulations and Applications. Front. Cell Dev. Biol. 2022, 10, 808303. [Google Scholar] [CrossRef] [PubMed]
- Frade, B.B.; Dias, R.B.; Gemini Piperni, S.; Bonfim, D.C. The role of macrophages in fracture healing: A narrative review of the recent updates and therapeutic perspectives. Stem Cell Investig. 2023, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, H.; Hogan, D.E.; Morgan, E.F.; Mortlock, D.P.; Einhorn, T.A.; Gerstenfeld, L.C. Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone 2012, 51, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.; Leucht, F.; Waltenberger, J.; Dehio, C.; Brenner, R.E. VEGF-A and PlGF-1 stimulate chemotactic migration of human mesenchymal progenitor cells. Biochem. Biophys. Res. Commun. 2005, 334, 561–568. [Google Scholar] [CrossRef]
- Jacobsen, K.A.; Al-Aql, Z.S.; Wan, C.; Fitch, J.L.; Stapleton, S.N.; Mason, Z.D.; Cole, R.M.; Gilbert, S.R.; Clemens, T.L.; Morgan, E.F.; et al. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J. Bone Miner. Res. 2008, 23, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Otomo, H.; Sakai, A.; Uchida, S.; Tanaka, S.; Watanuki, M.; Moriwaki, S.; Niida, S.; Nakamura, T. Flt-1 tyrosine kinase-deficient homozygous mice result in decreased trabecular bone volume with reduced osteogenic potential. Bone 2007, 40, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, L.Z.; Sun, H.P.; Xu, J.Y.; Li, Y.M.; Xie, X.; Zhang, L.M.; Deng, F.L. Sustained dual release of placental growth factor-2 and bone morphogenic protein-2 from heparin-based nanocomplexes for direct osteogenesis. Int. J. Nanomed. 2016, 11, 1147–1158. [Google Scholar] [CrossRef]
- Bertrand, A.A.; Malapati, S.H.; Yamaguchi, D.T.; Lee, J.C. The Intersection of Mechanotransduction and Regenerative Osteogenic Materials. Adv. Healthc. Mater. 2020, 9, e2000709. [Google Scholar] [CrossRef]
- Bow, A.; Anderson, D.E.; Dhar, M. Commercially available bone graft substitutes: The impact of origin and processing on graft functionality. Drug Metab. Rev. 2019, 51, 533–544. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Knöfler, W.; Barth, T.; Graul, R.; Krampe, D. Retrospective analysis of 10,000 implants from insertion up to 20 years-analysis of implantations using augmentative procedures. Int. J. Implant Dent. 2016, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 92–102. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Gopal, B. Antimicrobial and cytotoxicity evaluation of aliovalent substituted hydroxyapatite. Appl. Surf. Sci. 2014, 303, 277–281. [Google Scholar] [CrossRef]
- Egorikhina, M.N.; Aleynik, D.Y.; Rubtsova, Y.P.; Charykova, I.N. Quantitative analysis of cells encapsulated in a scaffold. MethodsX 2020, 7, 101146. [Google Scholar] [CrossRef] [PubMed]
- Egorikhina, M.N.; Rubtsova, Y.P.; Alejnik, D.Y.; Charykova, I.N.; Kovylin, R.S.; Yudin, V.V.; Chesnokov, S.A. Method of Estimating Cell Migration into Structure of Material or Scaffold. Patent No. 2740566 RU, 15 January 2021. Available online: https://rosstip.ru/patents/174011-sposob-otsenki-migratsii-kletok-v-strukturu-materiala-ili-skaffolda (accessed on 29 September 2023).
- Luo, S.; Wang, P.; Ma, M.; Pan, Z.; Lu, L.; Yin, F.; Cai, J. Genistein loaded into microporous surface of nano tantalum/PEEK composite with antibacterial effect regulating cellular response in vitro, and promoting osseointegration in vivo. J. Mech. Behav. Biomed. Mater. 2022, 125, 104972. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Lin, T.H.; Hsu, C.C.; Yeh, M.L. Restoring Osteochondral Defects through the Differentiation Potential of Cartilage Stem/Progenitor Cells Cultivated on Porous Scaffolds. Cells 2021, 10, 3536. [Google Scholar] [CrossRef]
- Hayashi, K.; Shimabukuro, M.; Ishikawa, K. Antibacterial Honeycomb Scaffolds for Achieving Infection Prevention and Bone Regeneration. ACS Appl. Mater. Interfaces 2022, 14, 3762–3772. [Google Scholar] [CrossRef]
- Klemmer, V.A.; Khera, N.; Siegenthaler, B.M.; Bhattacharya, I.; Weber, F.E.; Ghayor, C. Effect of N-Vinyl-2-Pyrrolidone (NVP), a Bromodomain-Binding Small Chemical, on Osteoblast and Osteoclast Differentiation and Its Potential Application for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 11052. [Google Scholar] [CrossRef]
- Thomas, B.; Bhat, K.; Mapara, M. Rabbit as an animal model for experimental research. Dent. Res. J. 2012, 9, 111. [Google Scholar] [CrossRef]
- Luttun, A.; Tjwa, M.; Moons, L.; Wu, Y.; Angelillo-Scherrer, A.; Liao, F.; Nagy, J.A.; Hooper, A.; Priller, J.; De Klerck, B.; et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat. Med. 2002, 8, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Heissig, B.; Wu, Y.; Dias, S.; Tejada, R.; Ferris, B.; Hicklin, D.J.; Zhu, Z.; Bohlen, P.; Witte, L.; et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat. Med. 2002, 8, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Pipp, F.; Heil, M.; Issbrücker, K.; Ziegelhoeffer, T.; Martin, S.; Van Den Heuvel, J.; Weich, H.; Fernandez, B.; Golomb, G.; Carmeliet, P.; et al. VEGFR-1–Selective VEGF Homologue PlGF Is Arteriogenic: Evidence for a Monocyte-Mediated Mechanism. Circ. Res. J. Am. Heary Assoc. 2003, 92, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Shi, Q.; Zhang, B.; Xu, J. Genetically modified mesenchymal stem cells promote spinal fusion through polarized macrophages. Lab. Investig. 2022, 102, 312–319. [Google Scholar] [CrossRef]
- Quinlan, E.; López-Noriega, A.; Thompson, E.M.; Hibbitts, A.; Cryan, S.A.; O’Brien, F.J. Controlled release of vascular endothelial growth factor from spray-dried alginate microparticles in collagen-hydroxyapatite scaffolds for promoting vascularization and bone repair. J. Tissue Eng. Regen. Med. 2017, 11, 1097–1109. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, C.C.; Wang, C.Y.; Lee, A.K.X.; Yeh, C.L.; Lin, C.P. Assessment of the Release of Vascular Endothelial Growth Factor from 3D-Printed Poly-ε-Caprolactone/Hydroxyapatite/Calcium Sulfate Scaffold with Enhanced Osteogenic Capacity. Polymers 2020, 12, 1455. [Google Scholar] [CrossRef]
- Sheehy, E.J.; Miller, G.J.; Amado, I.; Raftery, R.M.; Chen, G.; Cortright, K.; Vazquez, A.G.; O’Brien, F.J. Mechanobiology-informed regenerative medicine: Dose-controlled release of placental growth factor from a functionalized collagen-based scaffold promotes angiogenesis and accelerates bone defect healing. J. Control. Release 2021, 334, 96–105. [Google Scholar] [CrossRef]
- Karalashvili, L.; Kakabadze, A.; Uhryn, M.; Vyshnevska, H.; Ediberidze, K.; Kakabadze, Z. Bone grafts for reconstruction of bone defects (review). Georgian Med. News 2018, 282, 44–49. [Google Scholar]
- Athanasiou, V.T.; Papachristou, D.J.; Panagopoulos, A.; Saridis, A.; Scopa, C.D.; Megas, P. Histological comparison of autograft, allograft-DBM, xenograft, and synthetic grafts in a trabecular bone defect: An experimental study in rabbits. Med. Sci. Monit. 2010, 16, 31. [Google Scholar]
- Işık, G.; Özden Yüce, M.; Koçak-Topbaş, N.; Günbay, T. Guided bone regeneration simultaneous with implant placement using bovine-derived xenograft with and without liquid platelet-rich fibrin: A randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5563–5575. [Google Scholar] [CrossRef]



 —control series,
—control series,  —experimental series; ●—p < 0.05 compared with Day 1; ■—p < 0.05 comparison, control v experiment (Wilcoxon rank sum test).
—experimental series; ●—p < 0.05 compared with Day 1; ■—p < 0.05 comparison, control v experiment (Wilcoxon rank sum test).
 —control series,
—control series,  —experimental series; ●—p < 0.05 compared with Day 1; ■—p < 0.05 comparison, control v experiment (Wilcoxon rank sum test).
—experimental series; ●—p < 0.05 compared with Day 1; ■—p < 0.05 comparison, control v experiment (Wilcoxon rank sum test).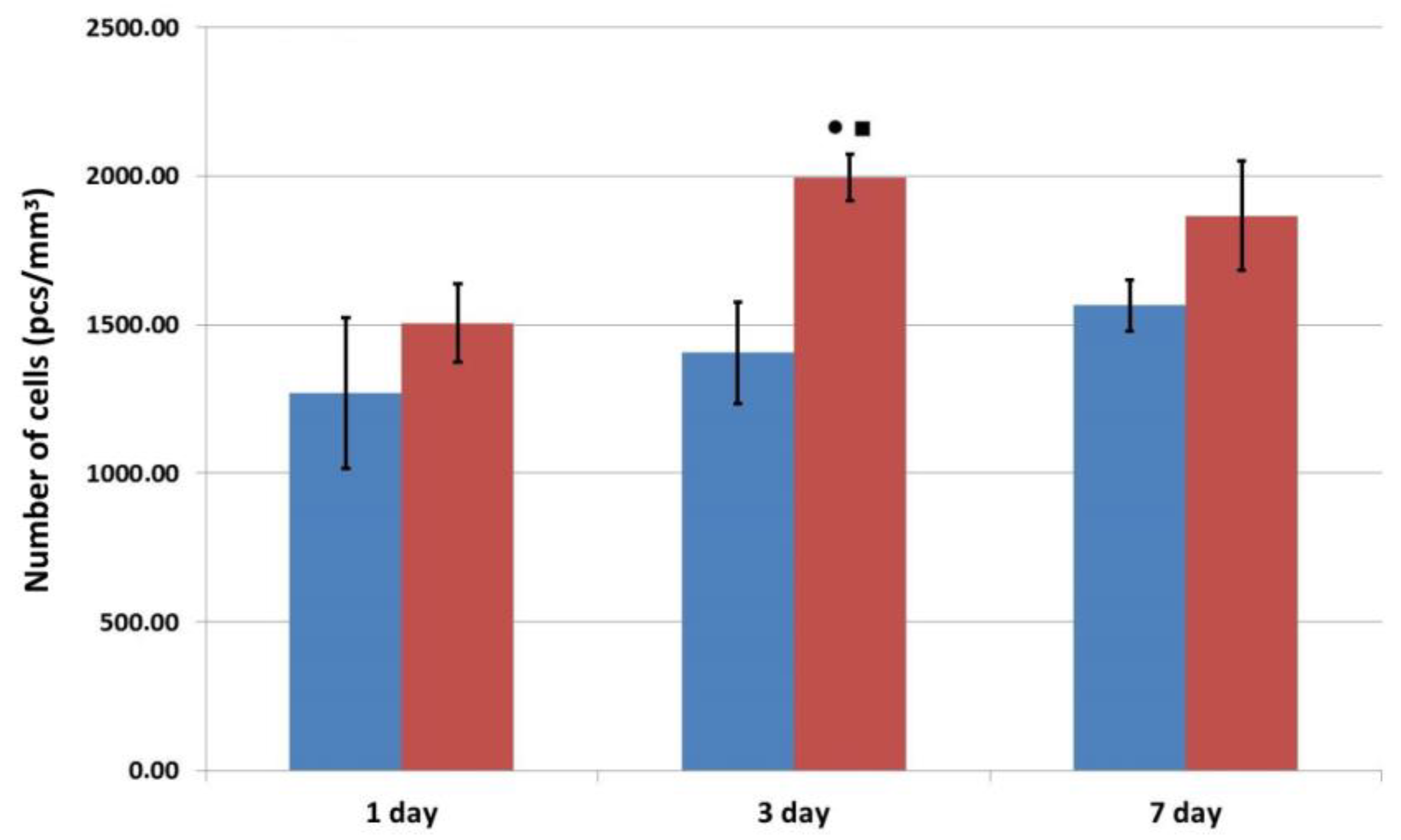
 —control series,
—control series,  —experimental series; ⋆—p < 0.05 compared with Day 1; ▲—p < 0.05 comparison, control v experiment (Wilcoxon rank sum test).
—experimental series; ⋆—p < 0.05 compared with Day 1; ▲—p < 0.05 comparison, control v experiment (Wilcoxon rank sum test).
 —control series,
—control series,  —experimental series; ⋆—p < 0.05 compared with Day 1; ▲—p < 0.05 comparison, control v experiment (Wilcoxon rank sum test).
—experimental series; ⋆—p < 0.05 compared with Day 1; ▲—p < 0.05 comparison, control v experiment (Wilcoxon rank sum test).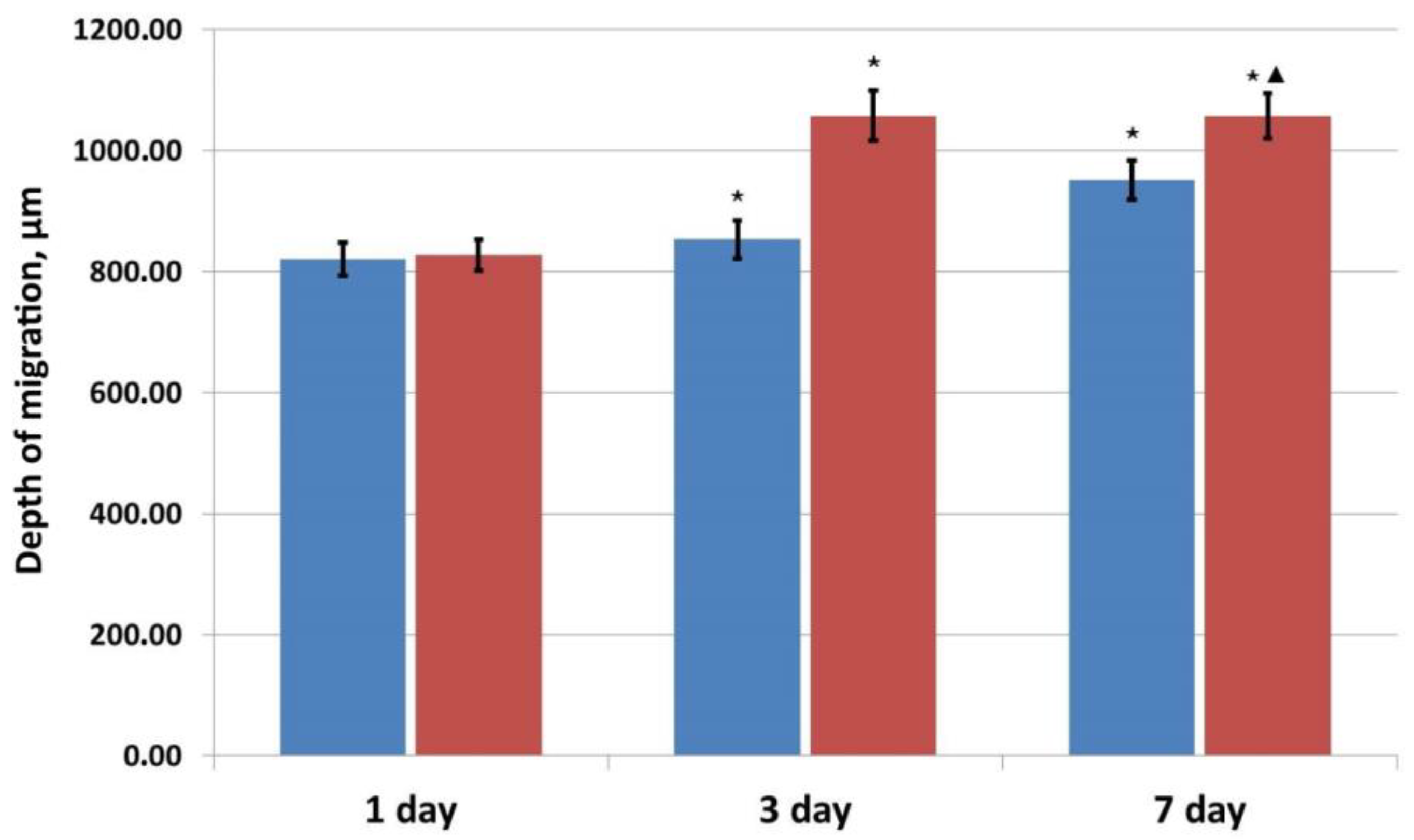
| Experiment No. | Interaction Time | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Experiment 1 n = 6 | C | 16,368.7 ± 832.7 | 22,997.7 ± 1104.7 ⋆ | 21,812.63 ± 1616.0 ⋆ |
| E | 18,072.2 ± 228.3 | 26,034.43 ± 764.2 ⋆□ | 26,293.67 ± 250.82 ⋆▲ | |
| Experiment 2 n = 6 | C | 7043.7 ± 308.6 | 10,487.8 ± 340.9 ⋆ | 18,353.7 ± 903.1 ⋆● |
| E | 8887.9 ± 252.8 ■ | 11,354.4 ± 184.4 ⋆□ | 21,886.7 ± 106.2 ⋆●▲ | |
| Experiment 3 n = 6 | C | 13,776.4 ± 593.76 | 19,998.9 ± 128.29 ⋆ | 25,701.13 ± 1321.84 ⋆ |
| E | 15,665.03 ± 886.48 | 22,931.04 ± 633.04 ⋆□ | 25,664.1 ± 449.01 ⋆● |
| Experiment No. | Interaction Time | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Experiment 1 n = 6 | C | 400.93 ± 15.18 | 668.18 ± 36.65 ⋆ | 1490.97 ± 103.87 ** |
| E | 471.72 ± 25.1 | 749.14± 42.91 ⋆ | 1506.23 ± 285.14 ** | |
| Experiment 2 n = 6 | C | 296.04 ± 15.58 | 465.97 ± 28.54 ⋆ | 592.17 ± 30.51 ** |
| E | 269.39 ± 13.89 | 497.83 ± 19.16 ⋆ | 609.21 ± 26.79 ** | |
| Experiment 3 n = 6 | C | 208.90 ± 16.61 | 363.2 ± 37.43 ⋆ | 539.96 ± 28.22 ** |
| E | 204.98 ± 20.09 | 377.88 ± 22.85 ⋆ | 658.71 ± 47.24 ** |
| Experiment No. | Interaction Time | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Experiment 1 n = 6 | C | 360 ± 20 | 710 ± 20 ⋆ | 1240 ± 90 ** |
| E | 400 ± 10 | 890 ± 70 ⋆ | 1360 ± 80 ** | |
| Experiment 2 n = 6 | C | 1640 ± 20 | 1900 ± 80 ⋆ | 1940 ± 20 |
| E | 1720 ± 40 | 1920 ± 10 ⋆ | 1900 ± 30 | |
| Experiment 3 n = 6 | C | 1430 ± 60 | 1690 ± 10 ⋆ | 1830 ± 50 ** |
| E | 1490 ± 40 | 1730 ± 30 ⋆ | 1860 ± 30 ** |
| Experiment No. | Interaction Time | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Experiment 1r n = 6 | C | 19,398.05 ± 601.02 | 32,374.54 ± 1798.17 ⋆ | 73,325.5 ± 2240.14 ⋆● |
| E | 20,153.54 ± 626.67 | 45,239.92 ± 2115.74 ⋆□ | 85,398.87 ± 1182.29 ⋆●▲ | |
| Experiment 2r n = 6 | C | 10,369.33 ± 404.33 | 26,923.23 ± 2430.30 ⋆ | 68,919.03 ± 2422.16 ⋆● |
| E | 12,072.87 ± 400.23 ■ | 44,143.73 ± 1802.12 ⋆□ | 86,880.2 ± 3493.09 ⋆●▲ | |
| Experiment 3r n = 6 | C | 10,125.98 ± 788.97 | 50,741.3 ± 2746.46 ⋆ | 50,430.87 ± 2720.10 ⋆ |
| E | 14,442.03 ± 689.93 ■ | 54,838.35 ± 2548.68 ⋆□ | 54,861.22 ± 2737.40 ⋆▲ |
| Test Points | 3 h | 24 h | 3 Days | 7 Days |
|---|---|---|---|---|
| PGLF in pg/mL (n = 9) | 2045.30 ± 103.80 | 176.43 ± 14.93 ⋆ | 119.96 ± 6.27 ⋆● | 96.31 ± 2.68 ⋆● |
| Release Period | |||
|---|---|---|---|
| 1 Day | 7 Days | ||
| Control (n = 8) | Relative density | 0.490 ± 0.020 | 0.711 ± 0.026 |
| Relative intensity of cell growth, % | 100 | 100 | |
| Cytotoxicity level | 0 | 0 | |
| Extract (n = 8) | Relative density | 0.424 ± 0.007 | 0.561 ± 0.009 |
| Relative intensity of cell growth, % | 86.53 | 78.90 | |
| Cytotoxicity level | 1 | 1 | |
| Extract 1:1 (n = 8) | Relative density | 0.417 ± 0.009 | 0.521 ± 0.210 |
| Relative intensity of cell growth, % | 85.10 | 73.28 | |
| Cytotoxicity level | 1 | 2 | |
| Extract 1:2 (n = 8) | Relative density | 0.418 ± 0.005 | 0.605 ± 0.057 |
| Relative intensity of cell growth, % | 85.30 | 85.09 | |
| Cytotoxicity level | 1 | 1 | |
| Extract 1:4 (n = 8) | Relative density | 0.459 ± 0.01 | 0.643 ± 0.048 |
| Relative intensity of cell growth, % | 93.67 | 90.43 | |
| Cytotoxicity level | 1 | 1 | |
| Extract 1:8 (n = 8) | Relative density | 0.476 ± 0.008 | 0.622 ± 0.030 |
| Relative intensity of cell growth, % | 97.14 | 87.48 | |
| Cytotoxicity level | 1 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleynik, D.Y.; Bokov, A.E.; Charykova, I.N.; Rubtsova, Y.P.; Linkova, D.D.; Farafontova, E.A.; Egorikhina, M.N. Functionalization of Osteoplastic Material with Human Placental Growth Factor and Assessment of Biocompatibility of the Resulting Material In Vitro. Pharmaceutics 2024, 16, 85. https://doi.org/10.3390/pharmaceutics16010085
Aleynik DY, Bokov AE, Charykova IN, Rubtsova YP, Linkova DD, Farafontova EA, Egorikhina MN. Functionalization of Osteoplastic Material with Human Placental Growth Factor and Assessment of Biocompatibility of the Resulting Material In Vitro. Pharmaceutics. 2024; 16(1):85. https://doi.org/10.3390/pharmaceutics16010085
Chicago/Turabian StyleAleynik, Diana Ya, Andrey E. Bokov, Irina N. Charykova, Yulia P. Rubtsova, Daria D. Linkova, Ekaterina A. Farafontova, and Marfa N. Egorikhina. 2024. "Functionalization of Osteoplastic Material with Human Placental Growth Factor and Assessment of Biocompatibility of the Resulting Material In Vitro" Pharmaceutics 16, no. 1: 85. https://doi.org/10.3390/pharmaceutics16010085
APA StyleAleynik, D. Y., Bokov, A. E., Charykova, I. N., Rubtsova, Y. P., Linkova, D. D., Farafontova, E. A., & Egorikhina, M. N. (2024). Functionalization of Osteoplastic Material with Human Placental Growth Factor and Assessment of Biocompatibility of the Resulting Material In Vitro. Pharmaceutics, 16(1), 85. https://doi.org/10.3390/pharmaceutics16010085