An Overview of the Structure–Activity Relationship in Novel Antimicrobial Thiazoles Clubbed with Various Heterocycles (2017–2023)
Abstract
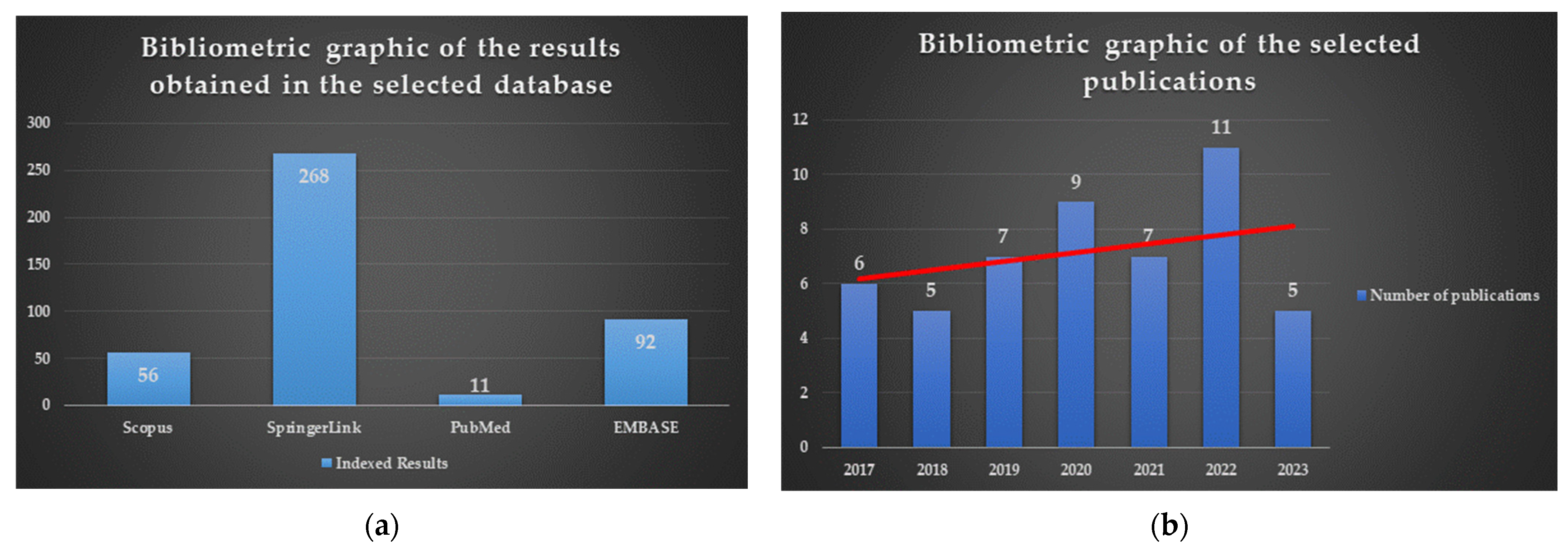
:1. Introduction
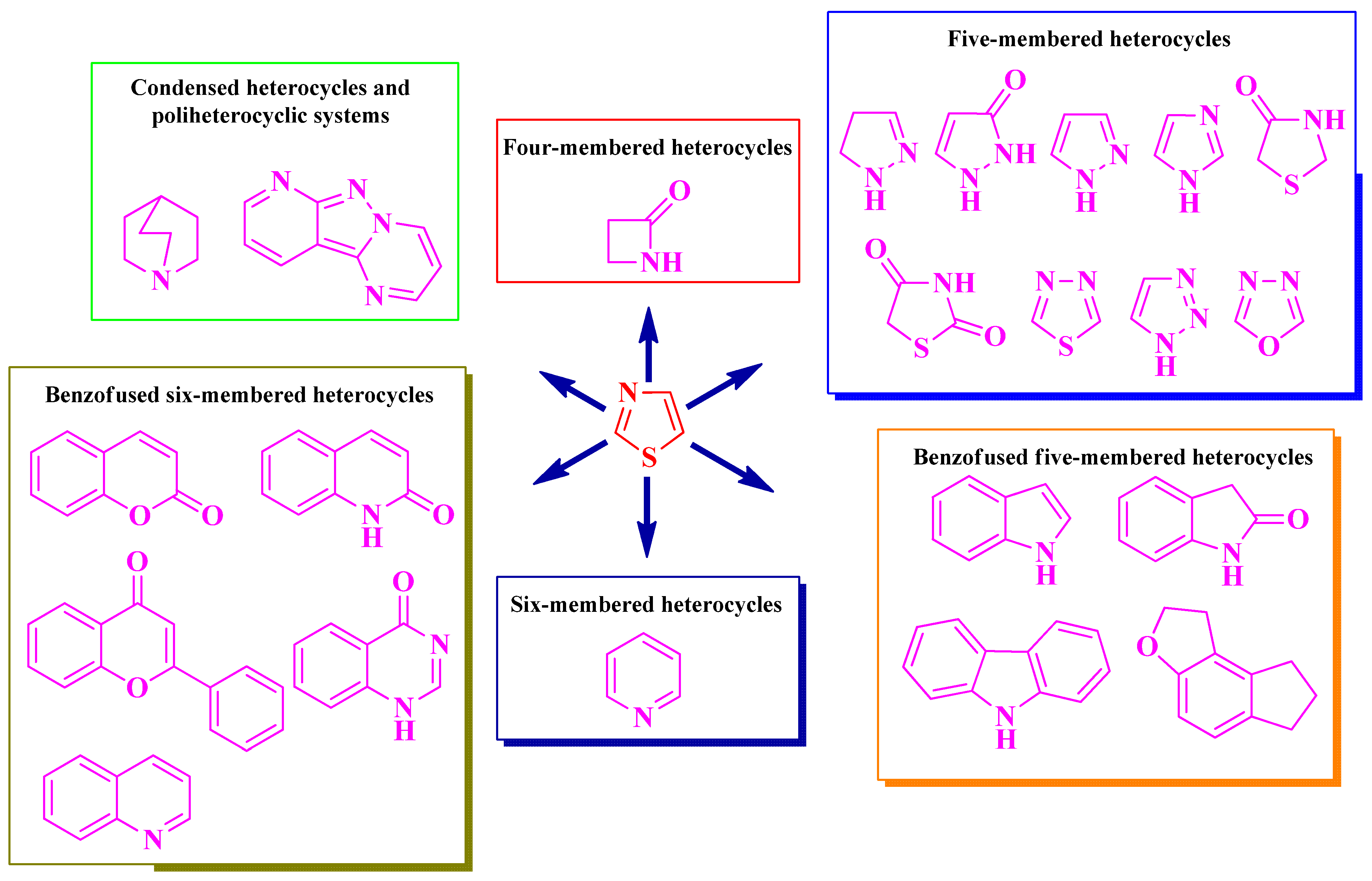
2. Structure–Activity Relationships in Antimicrobial Thiazole-Based Hybrid Compounds
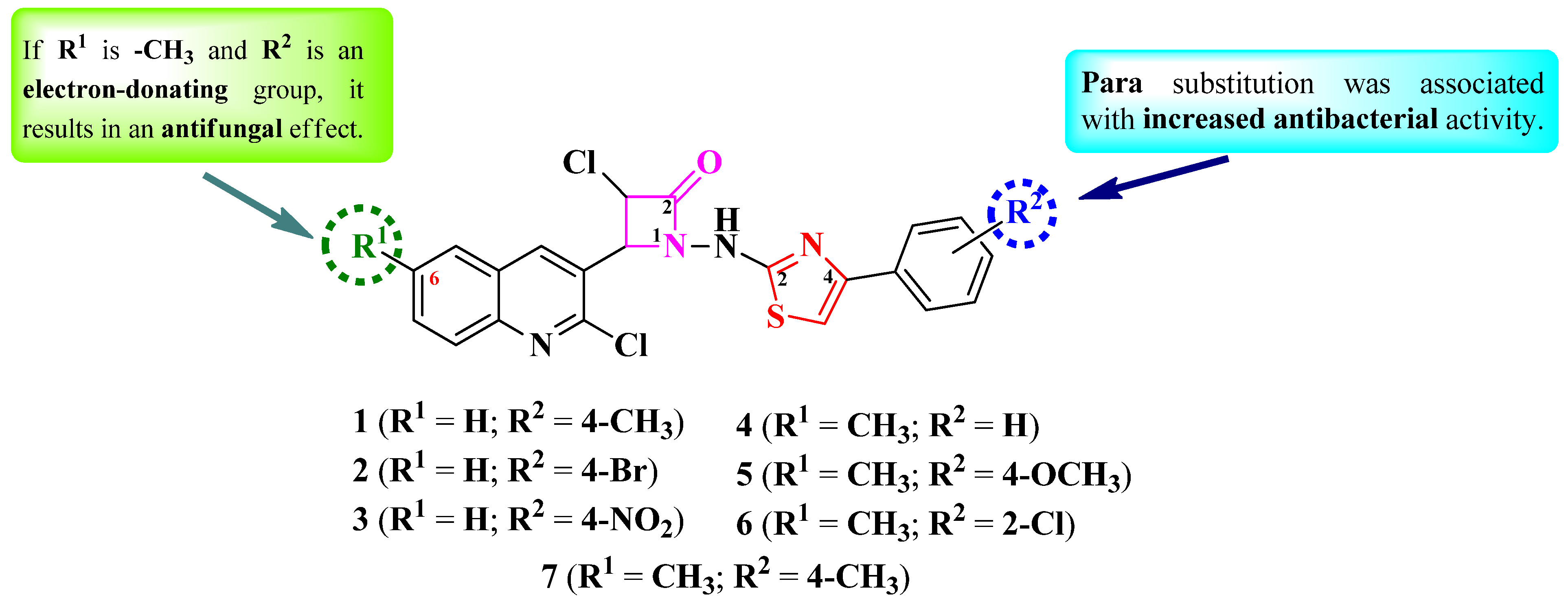
2.1. Thiazole Clubbed with Four-Membered Heterocycles
Thiazolyl–Azetidin-2-One Hybrid Compounds
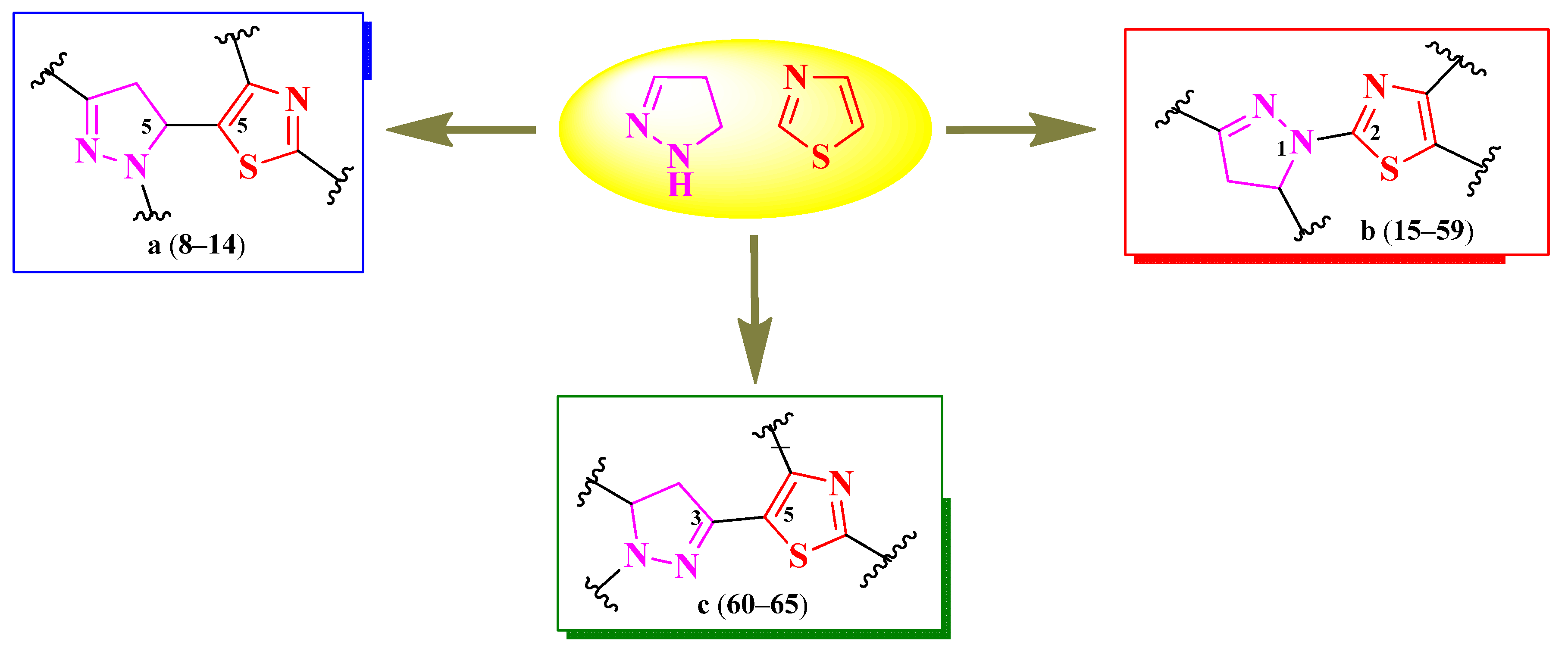
2.2. Thiazole Clubbed with Five-Membered Heterocycles
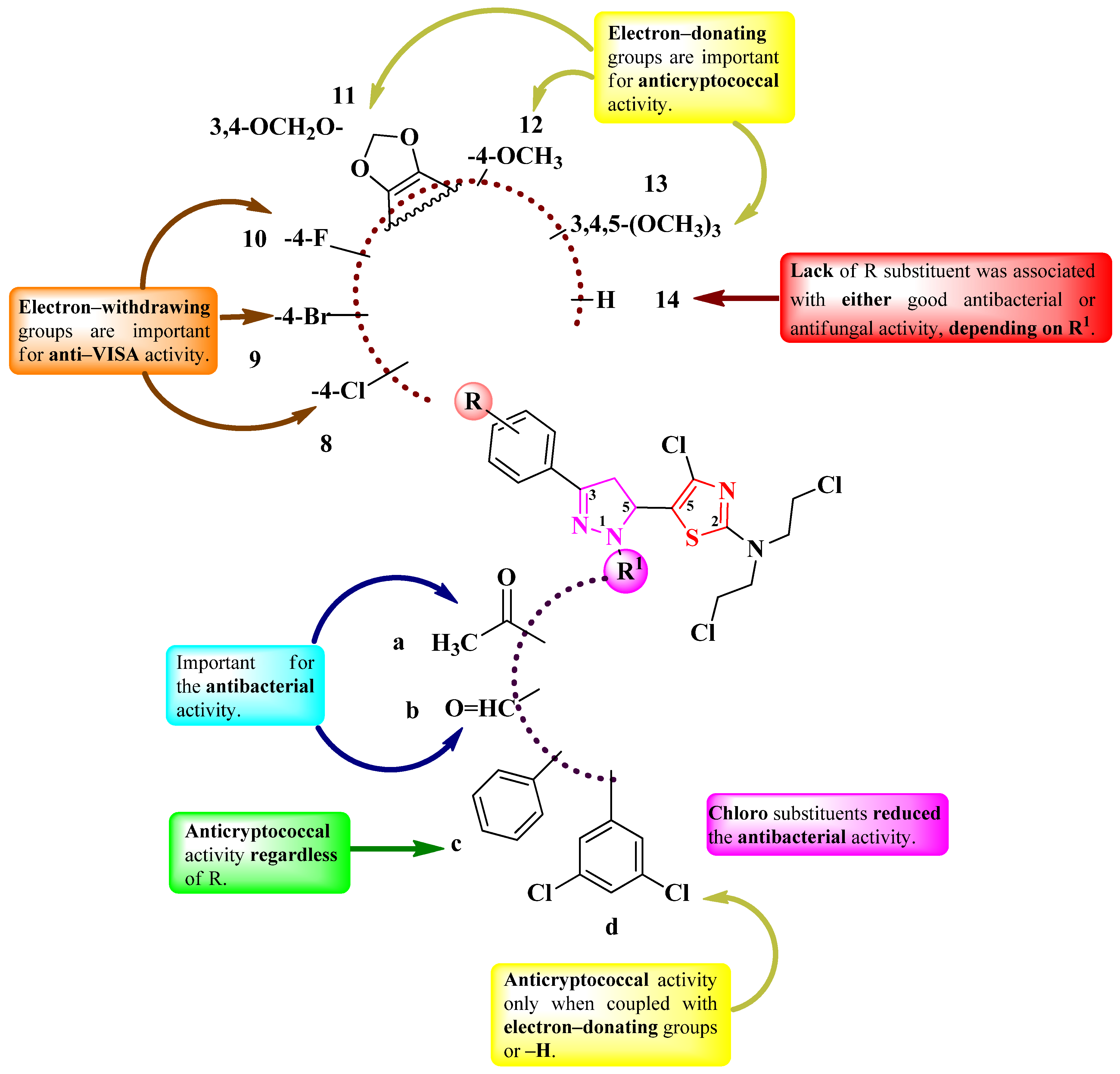
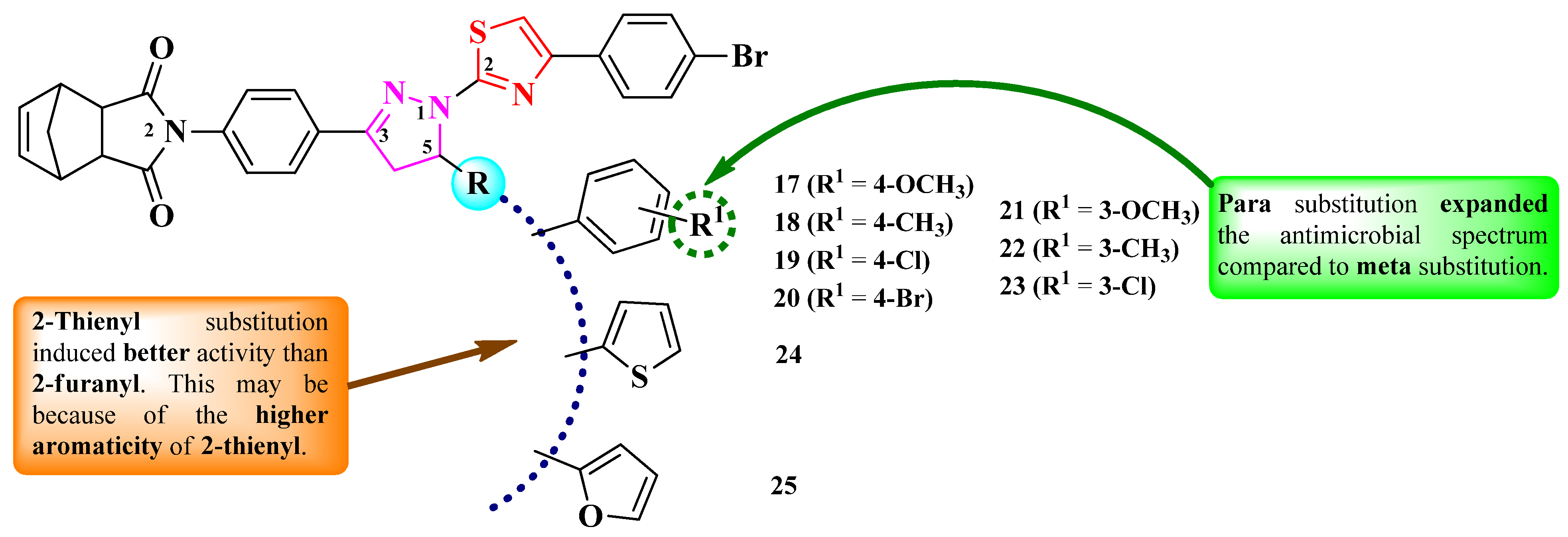
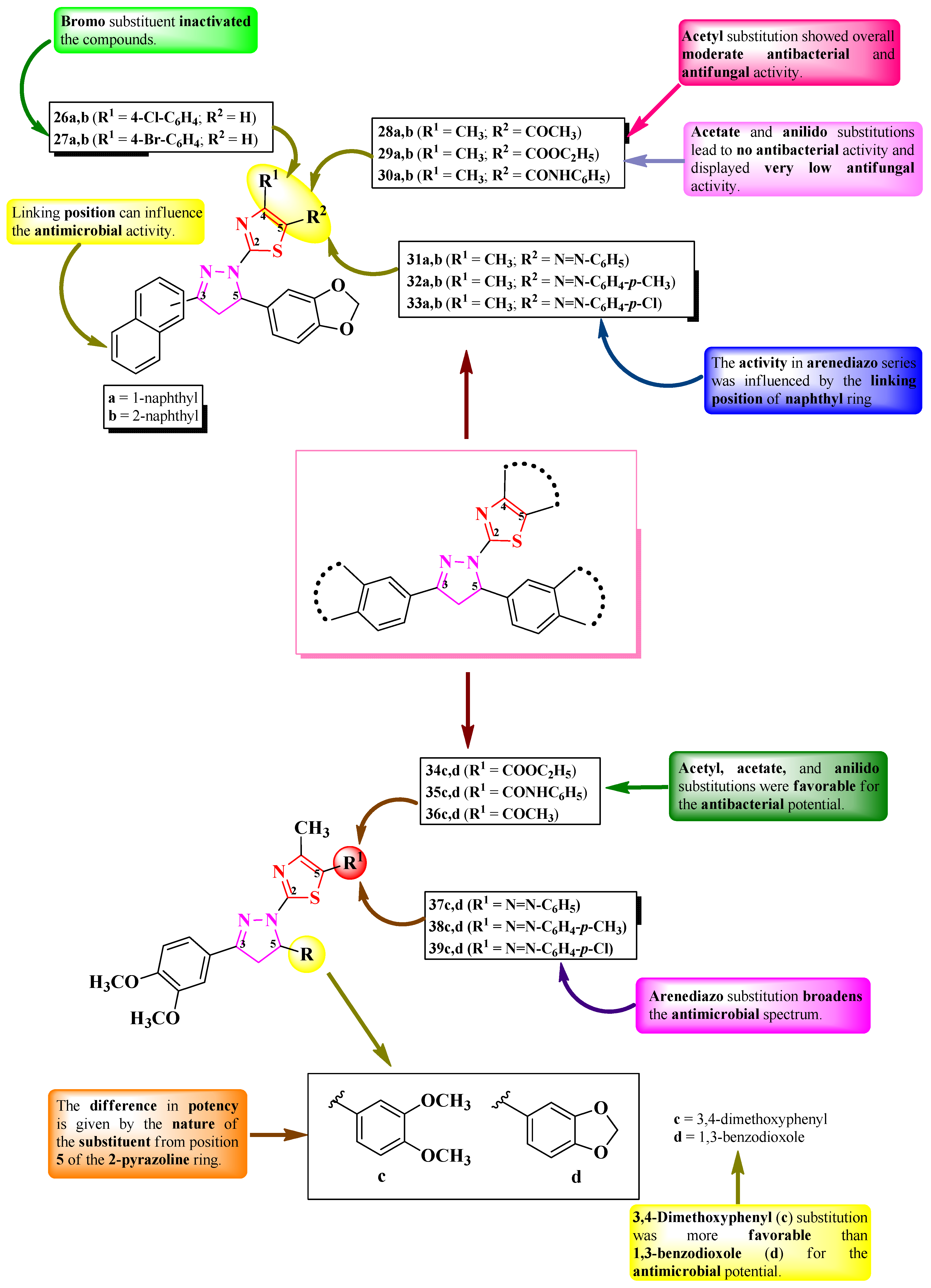
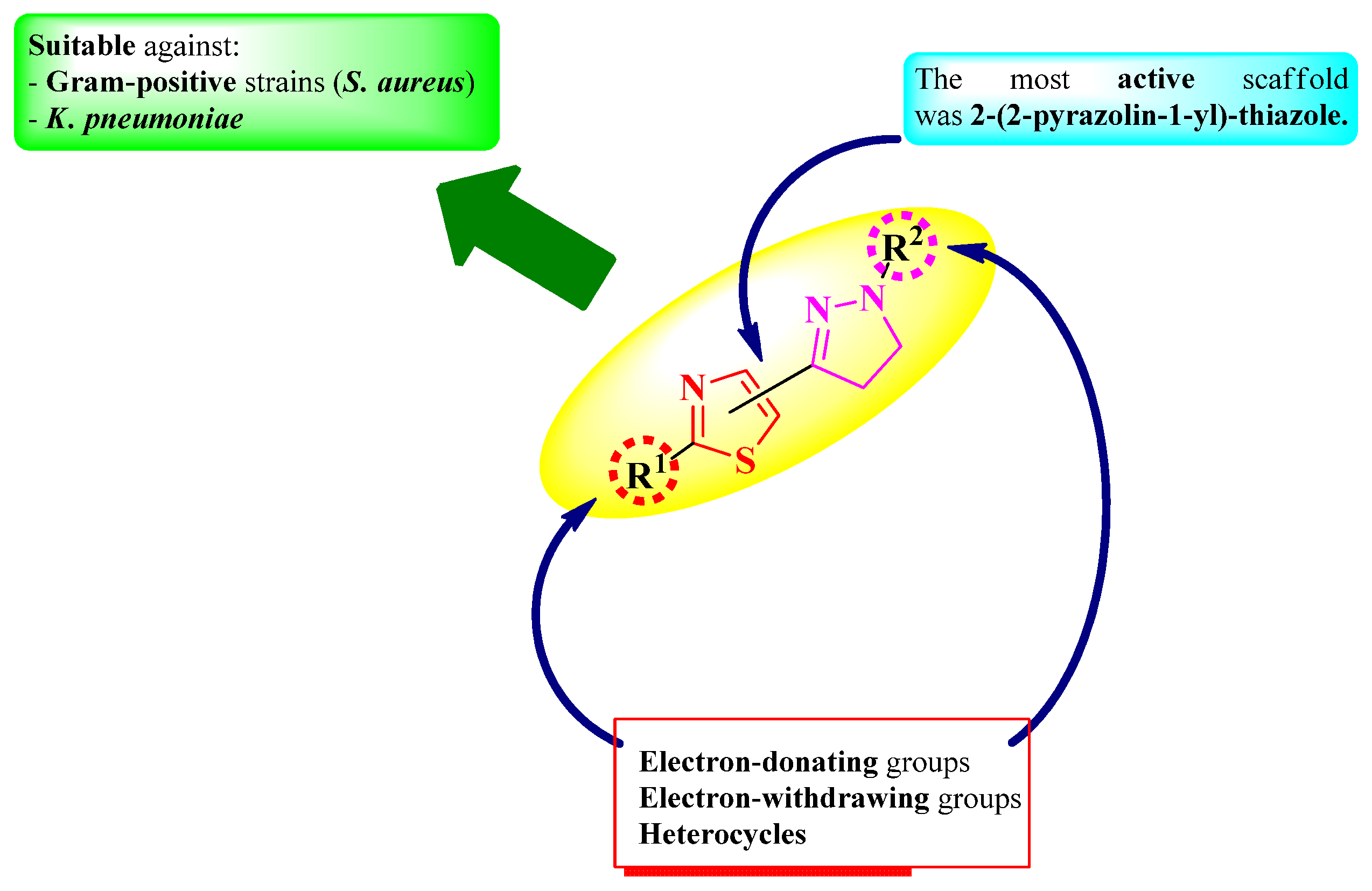
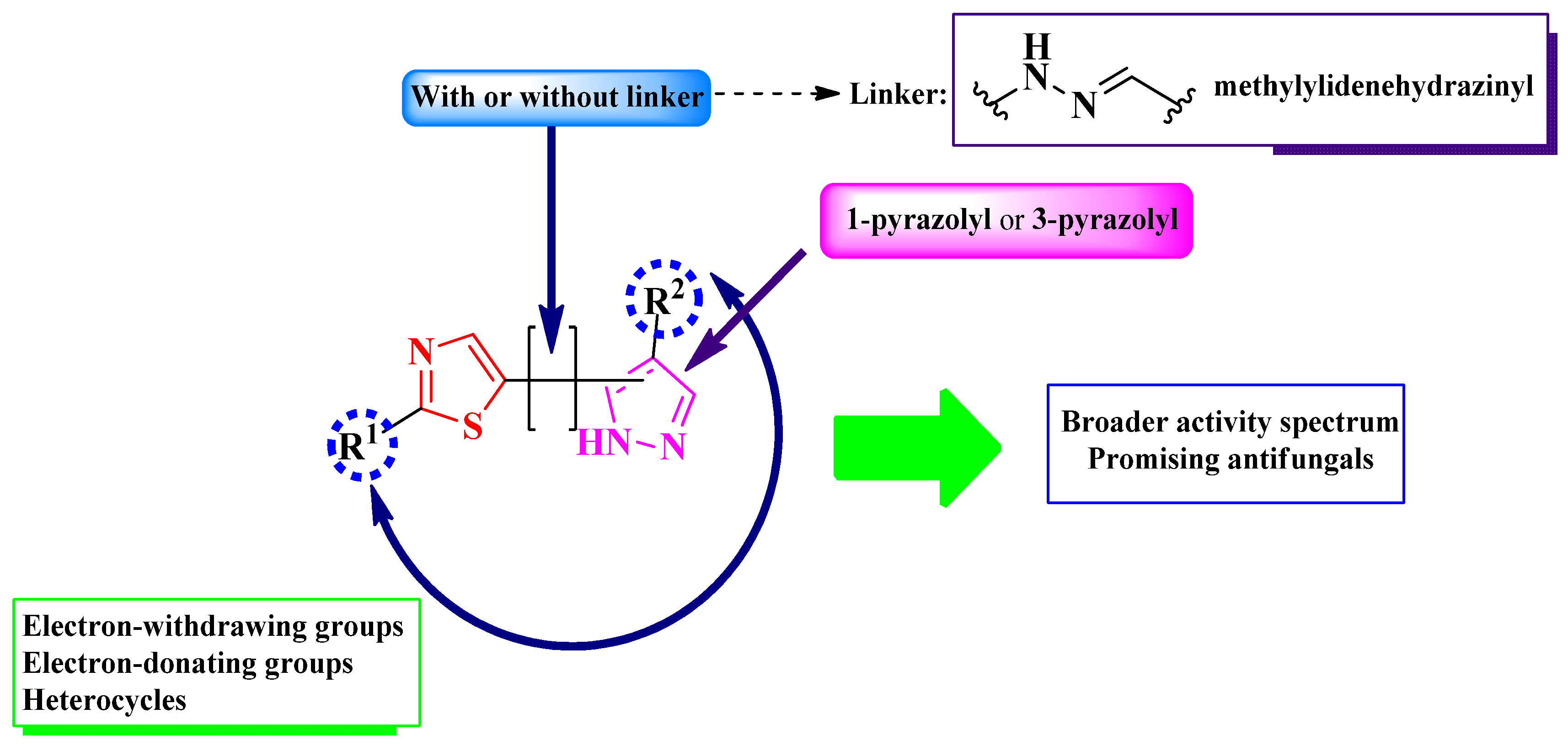
2.2.1. Thiazolyl–2-Pyrazoline Hybrid Compounds
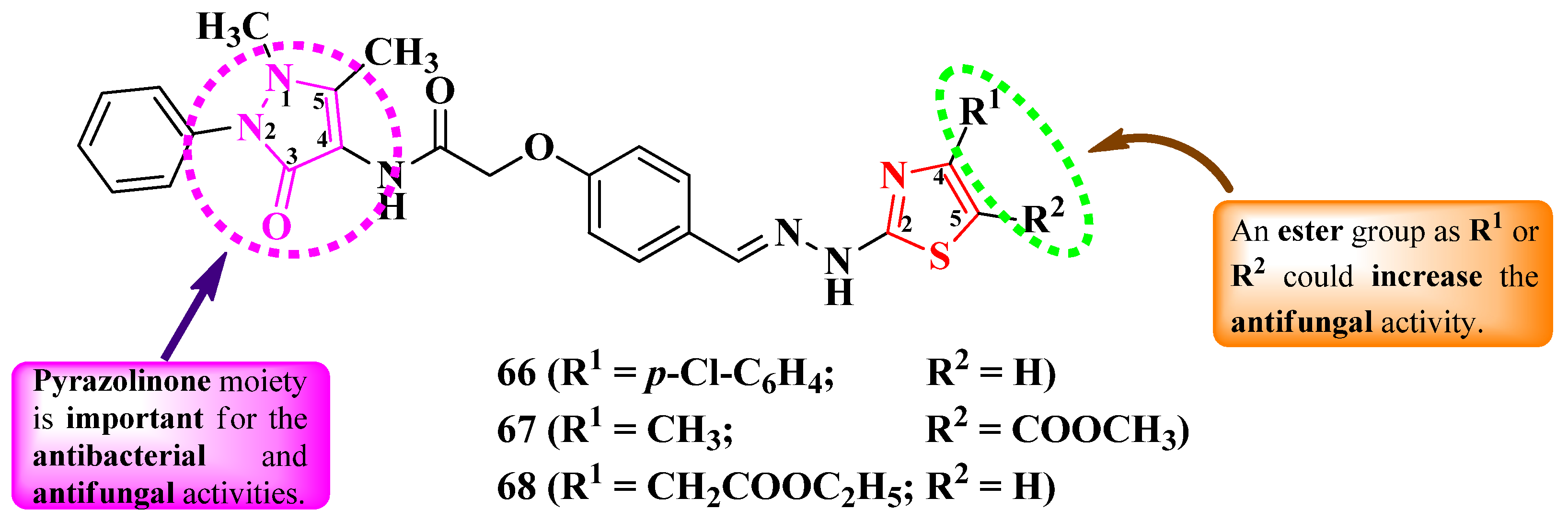
2.2.2. Thiazolyl–Pyrazolin-3-One Hybrid Compounds
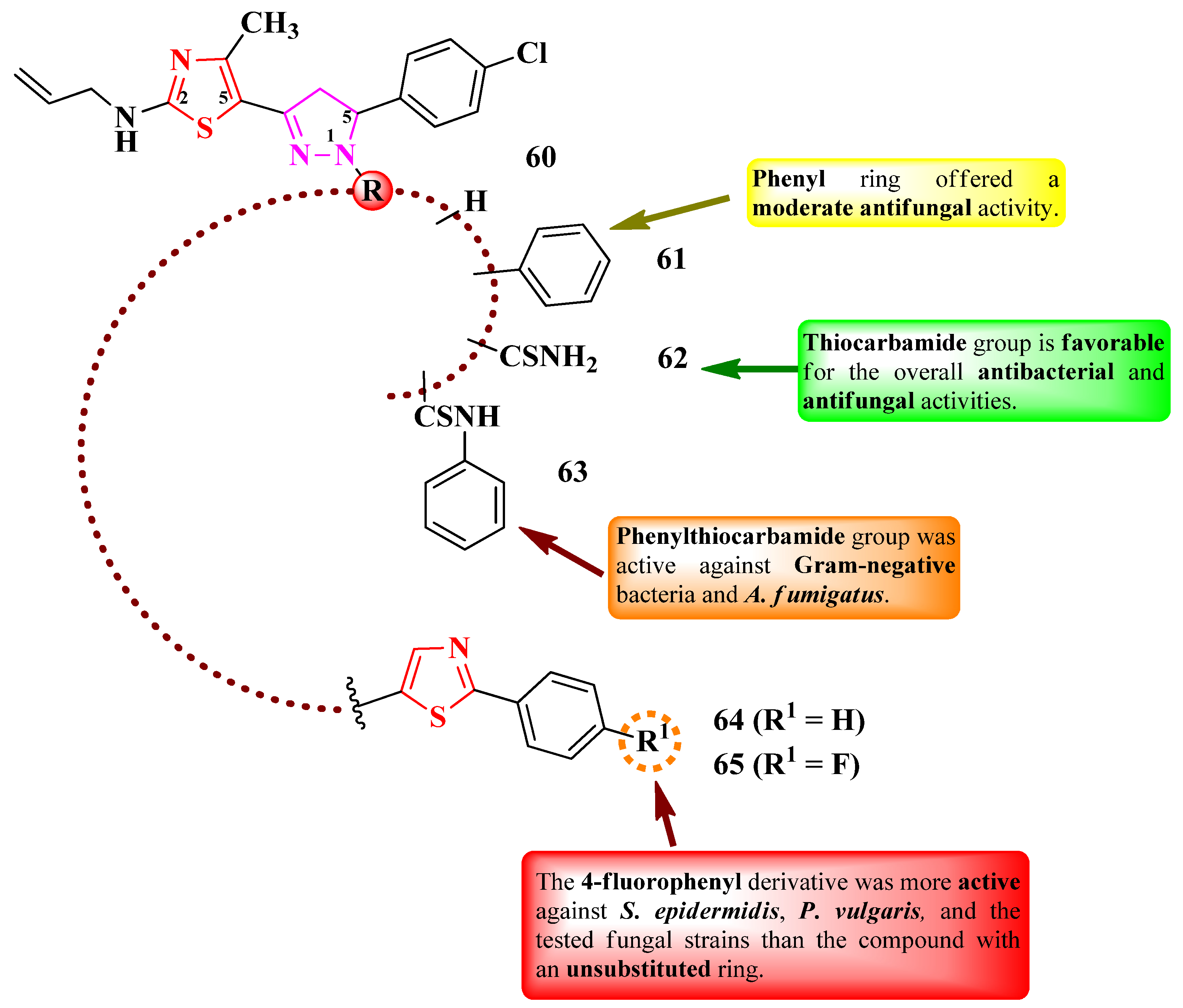
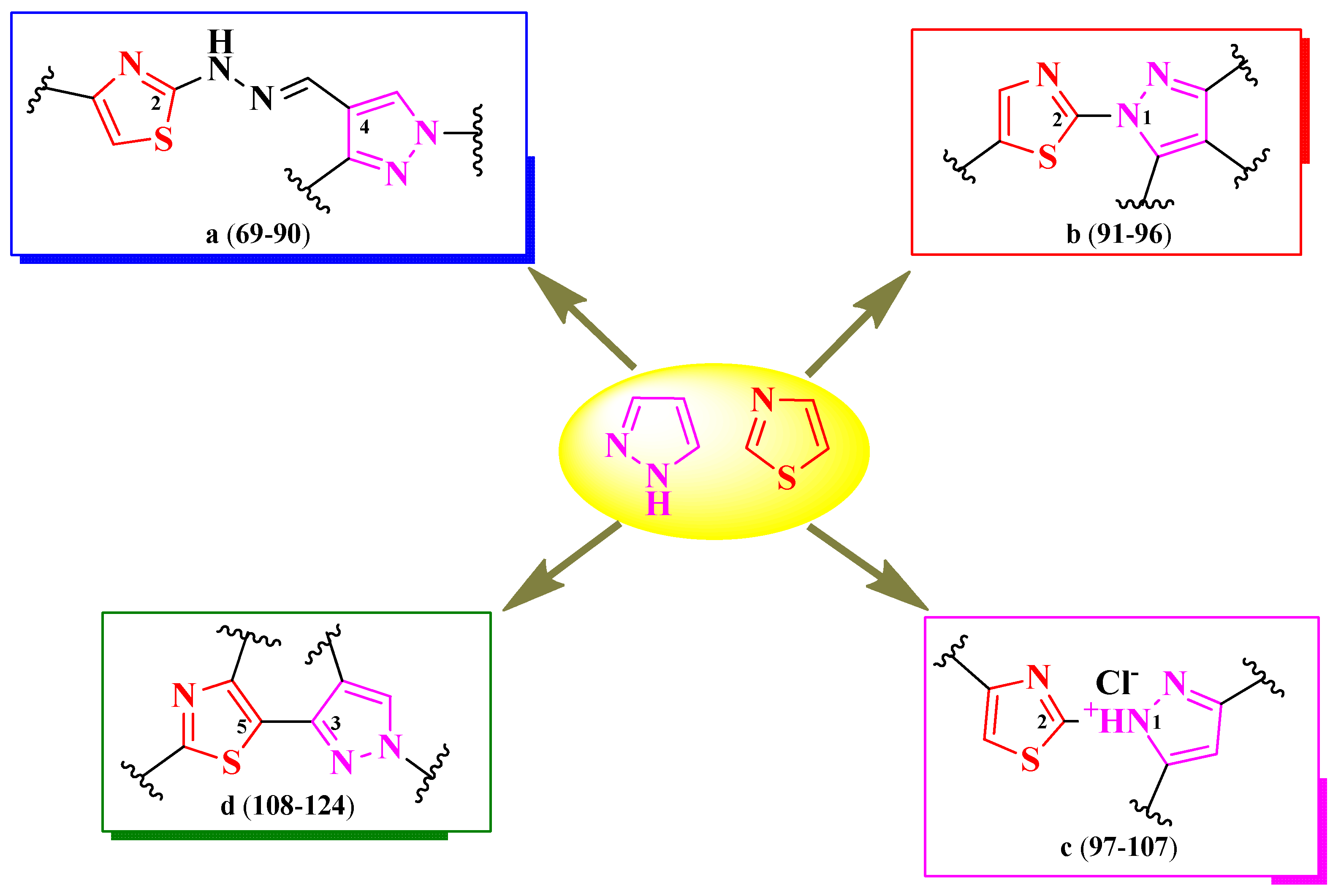
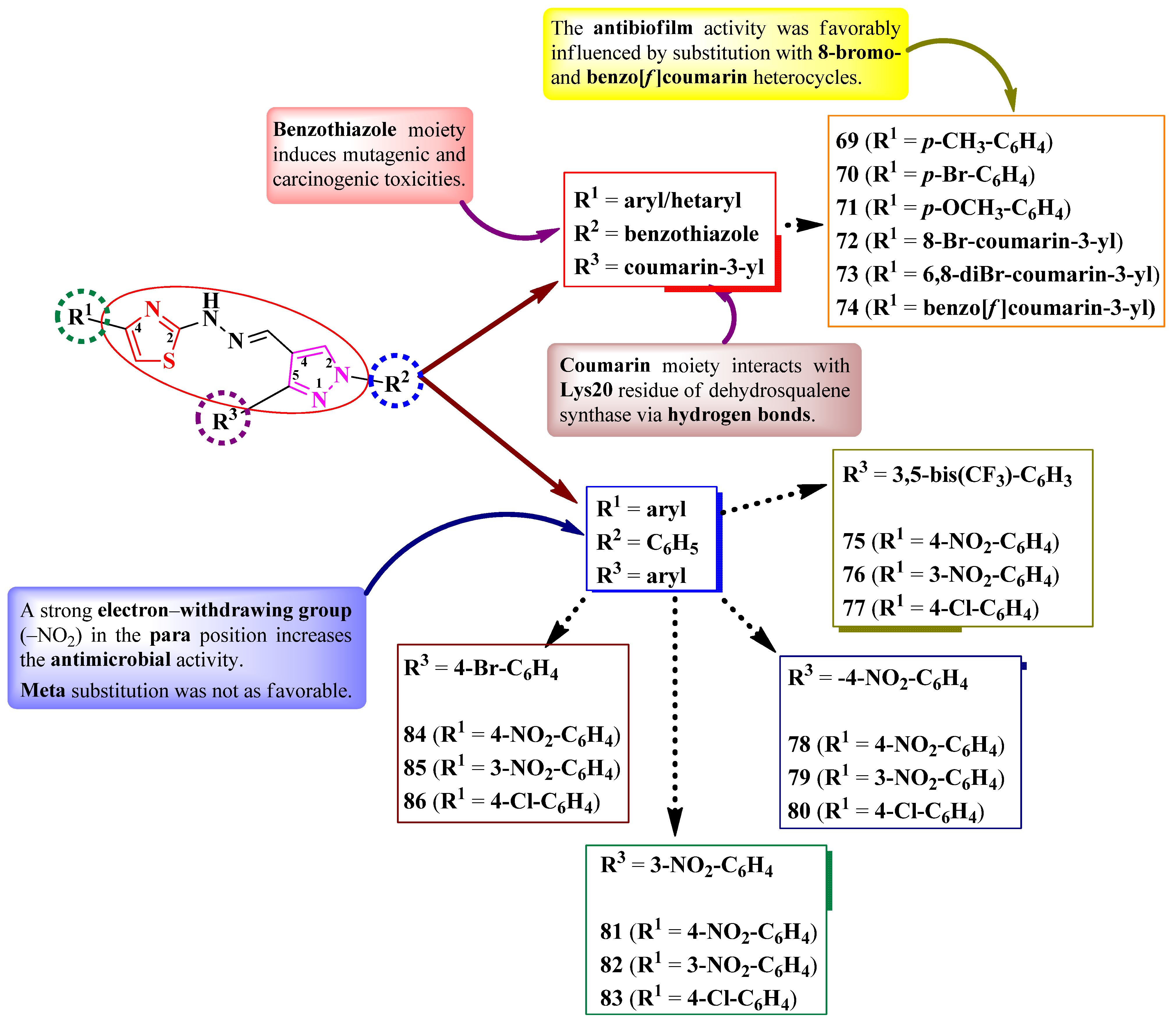
2.2.3. Thiazolyl–Pyrazole Hybrid Compounds
2.2.4. Thiazolyl–Imidazole Hybrid Compounds
2.2.5. Thiazolyl–Thiazolidin-4-One Hybrid Compounds
2.2.6. Thiazolyl–Thiazolidindione Hybrid Compounds
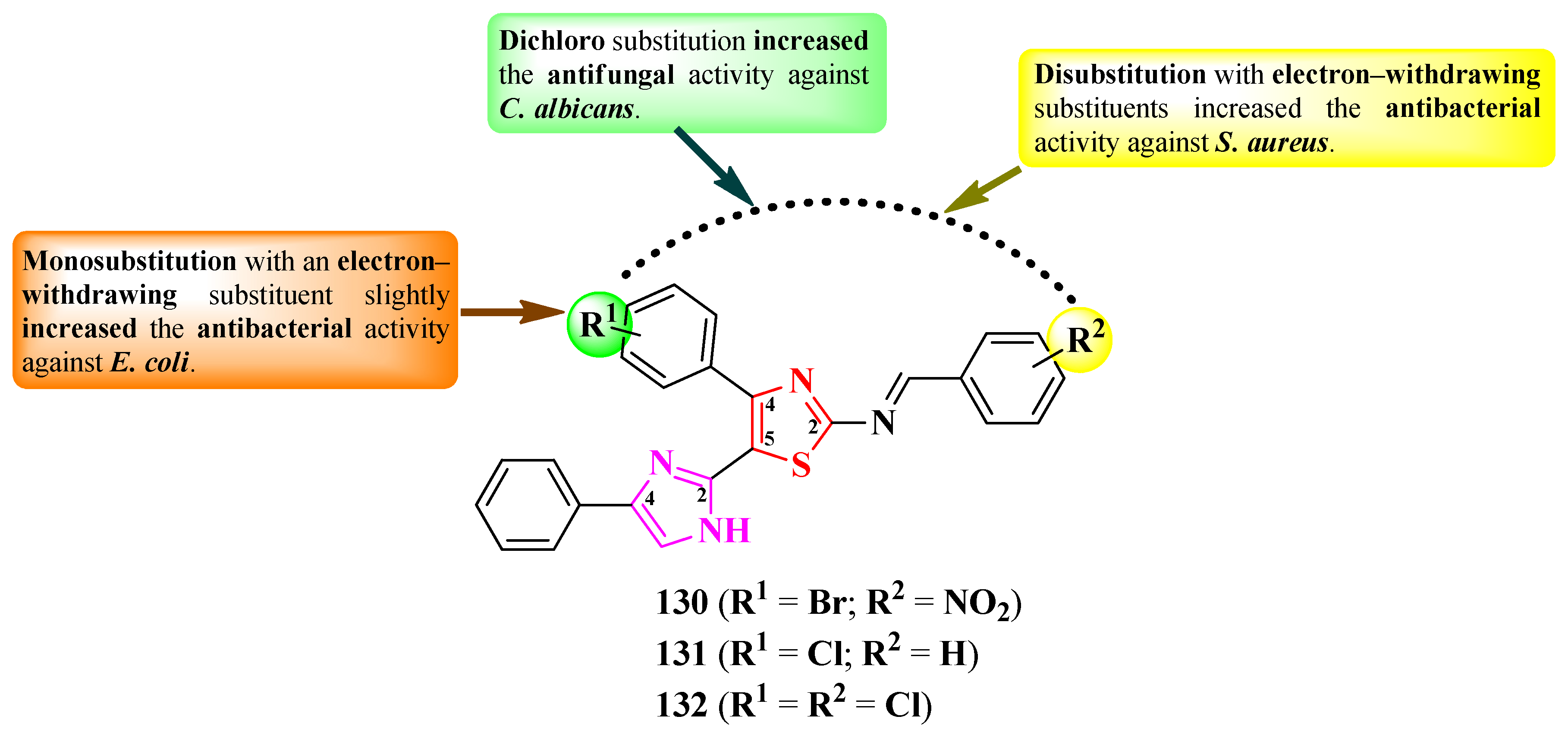
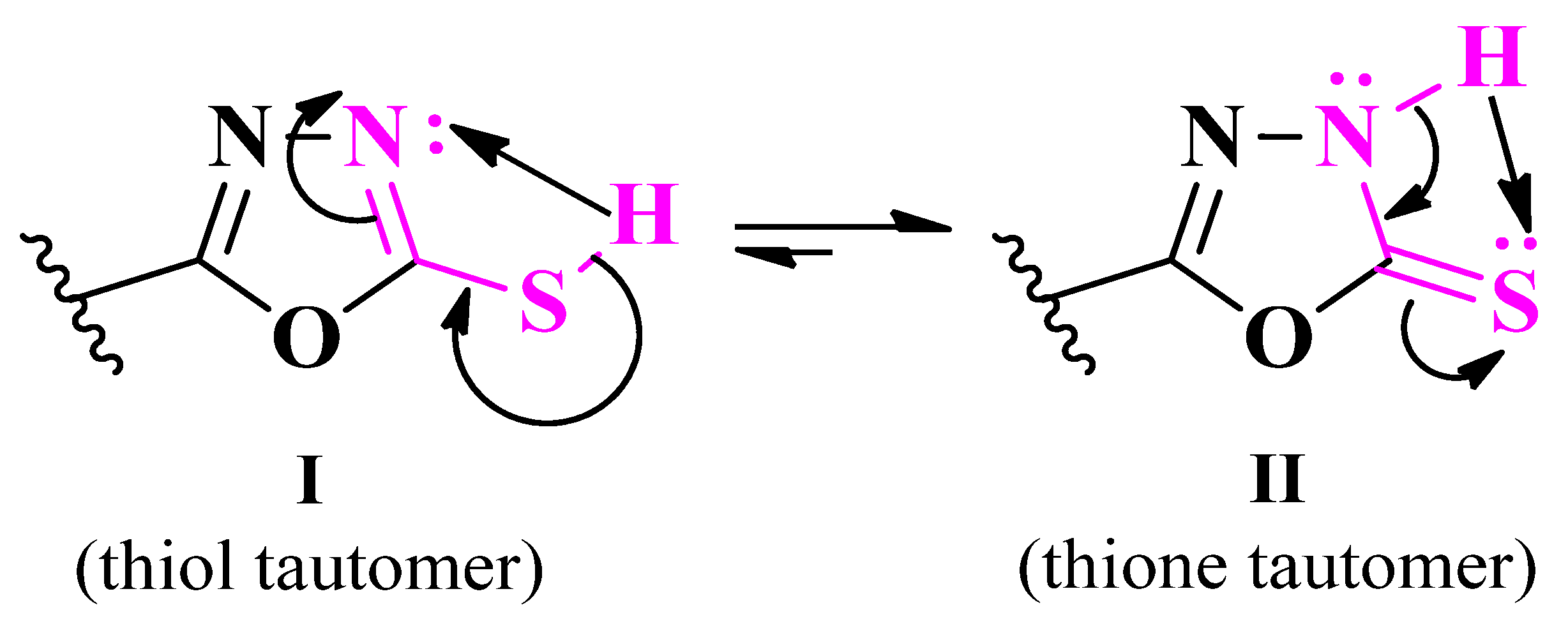
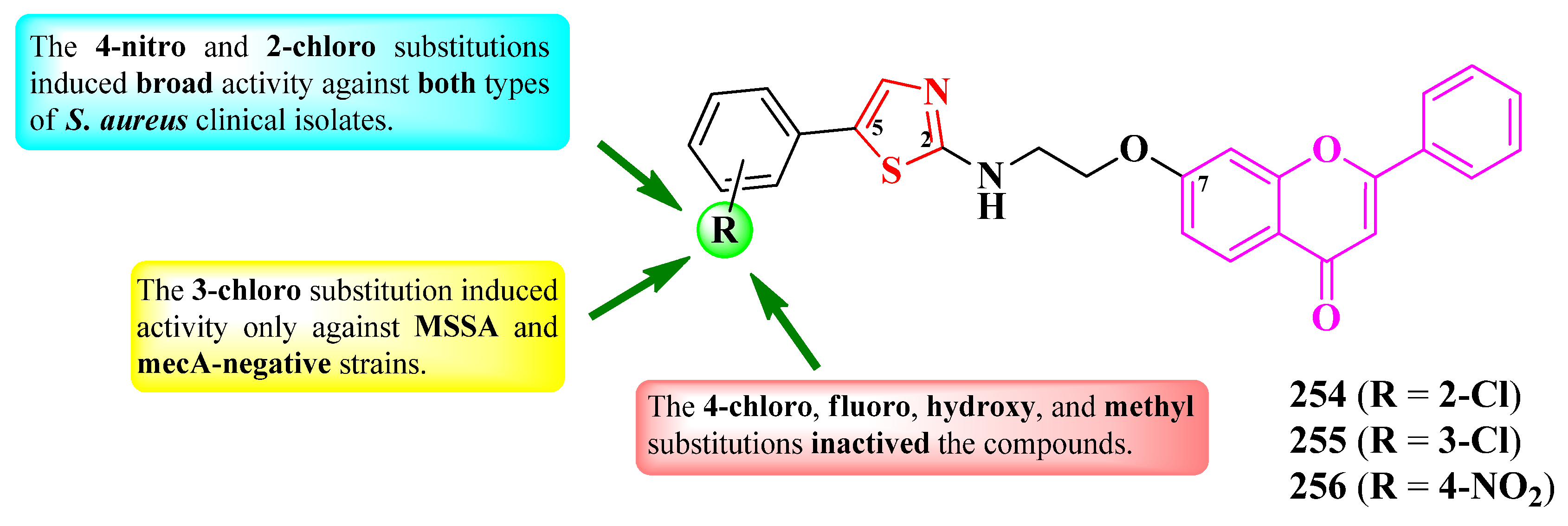
2.2.7. Thiazolyl–1,3,4-Thiadiazole Hybrid Compounds
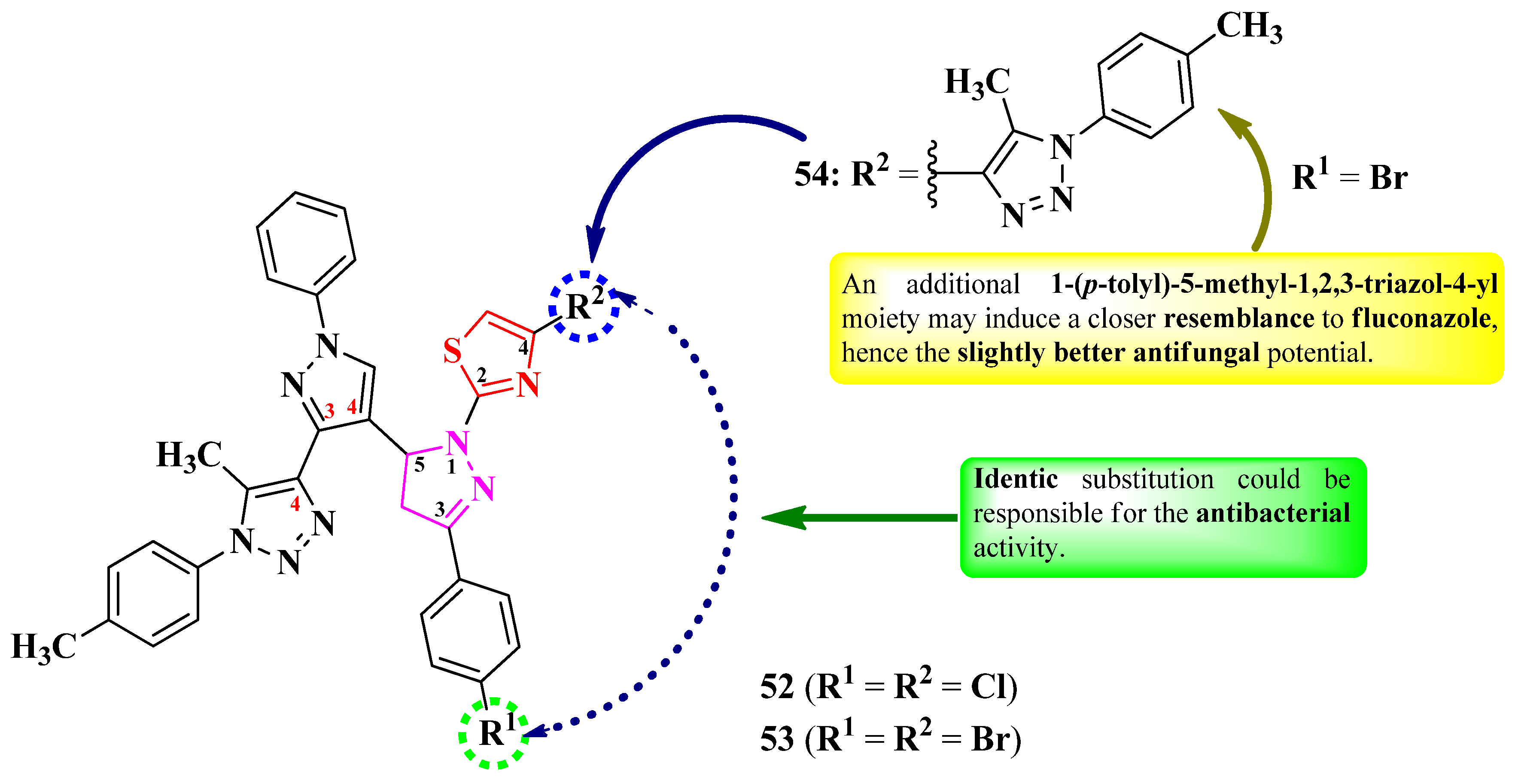
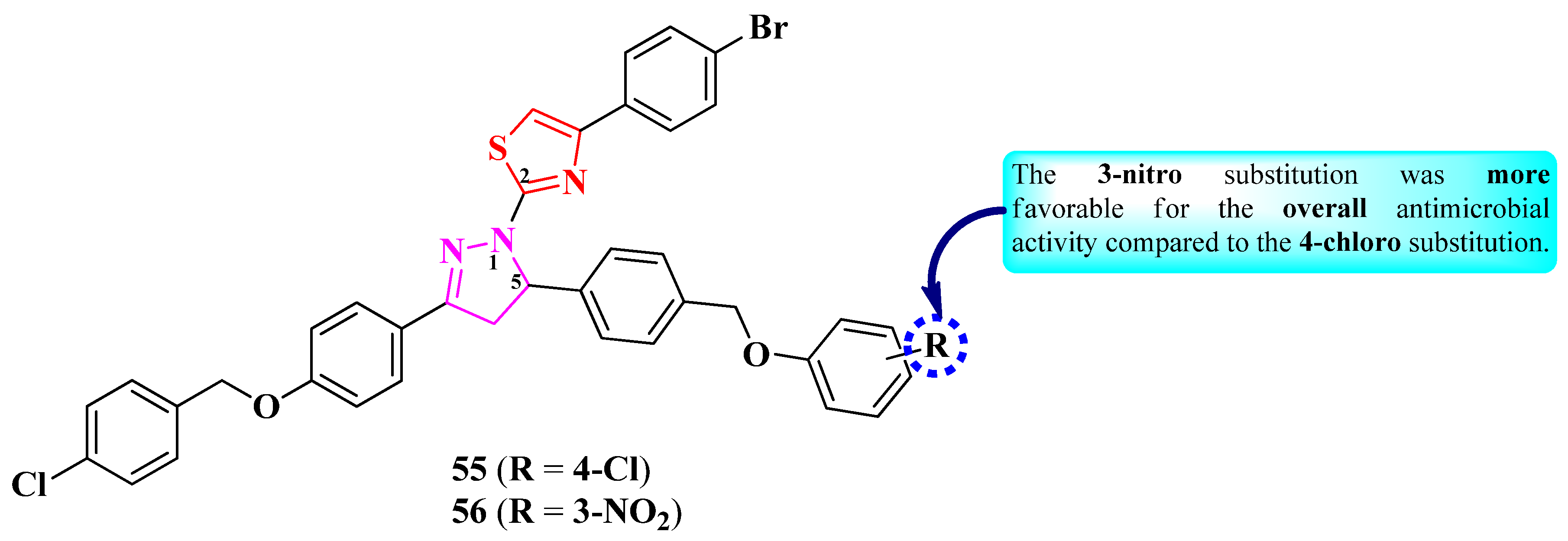
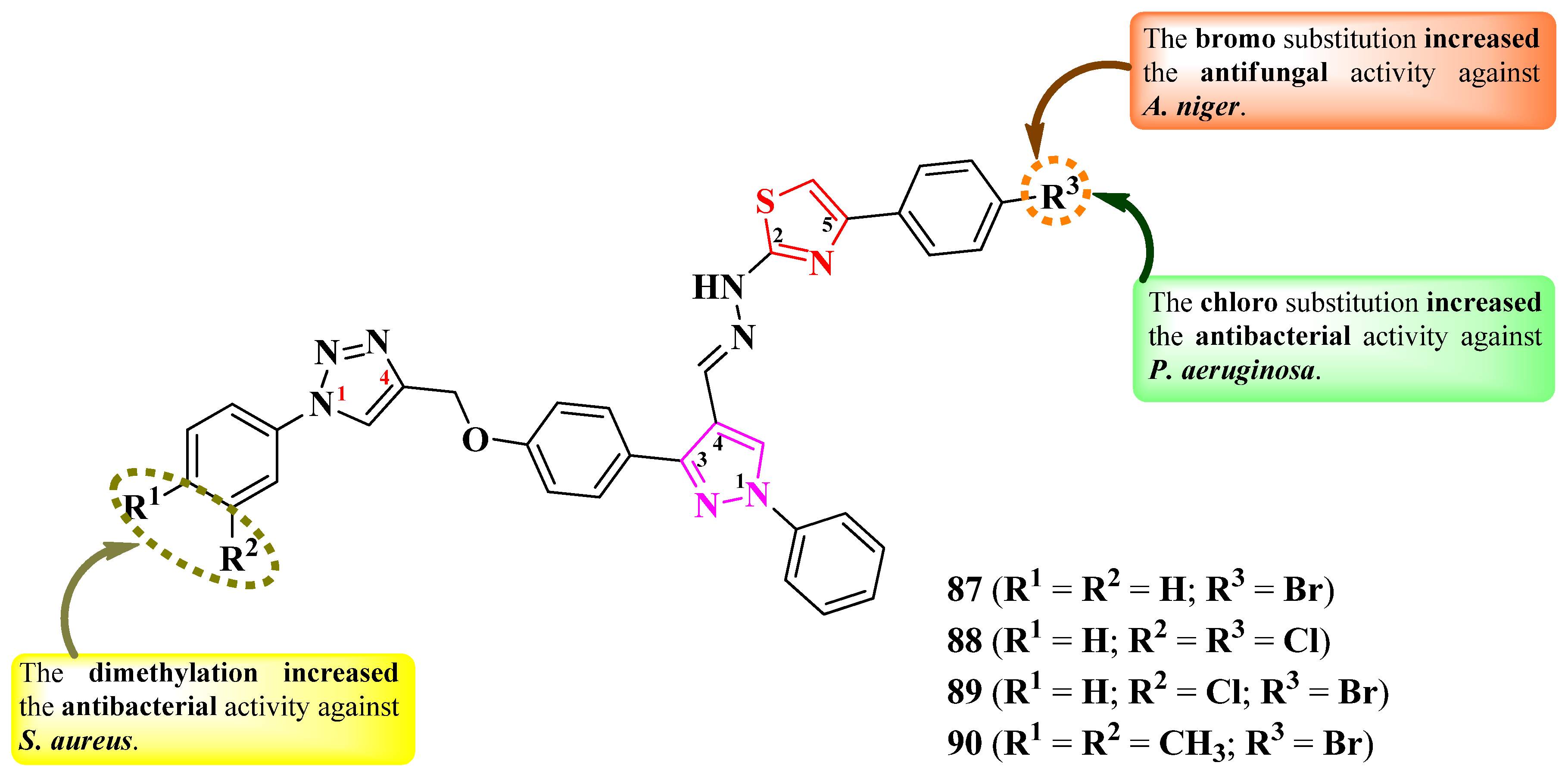
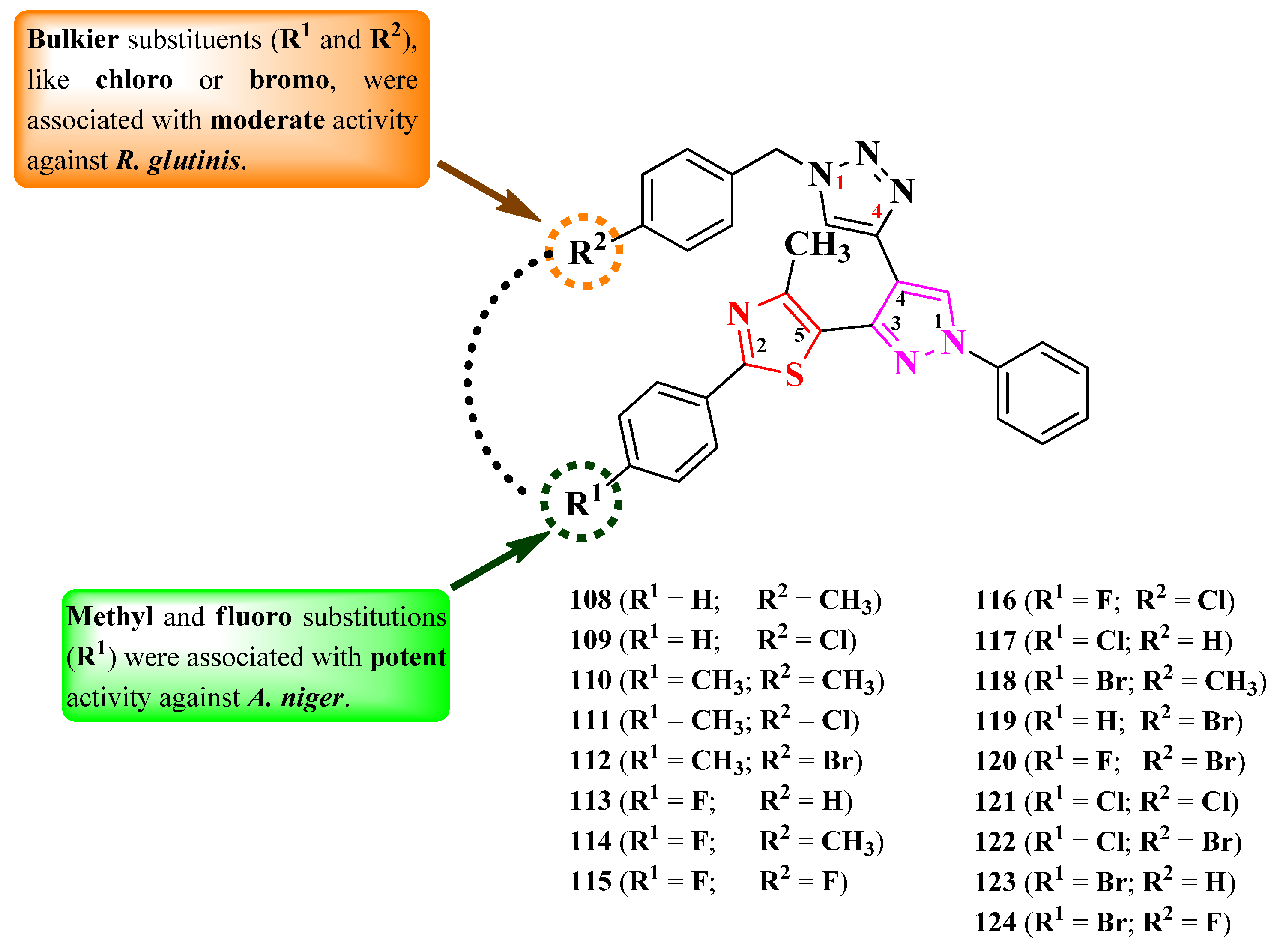
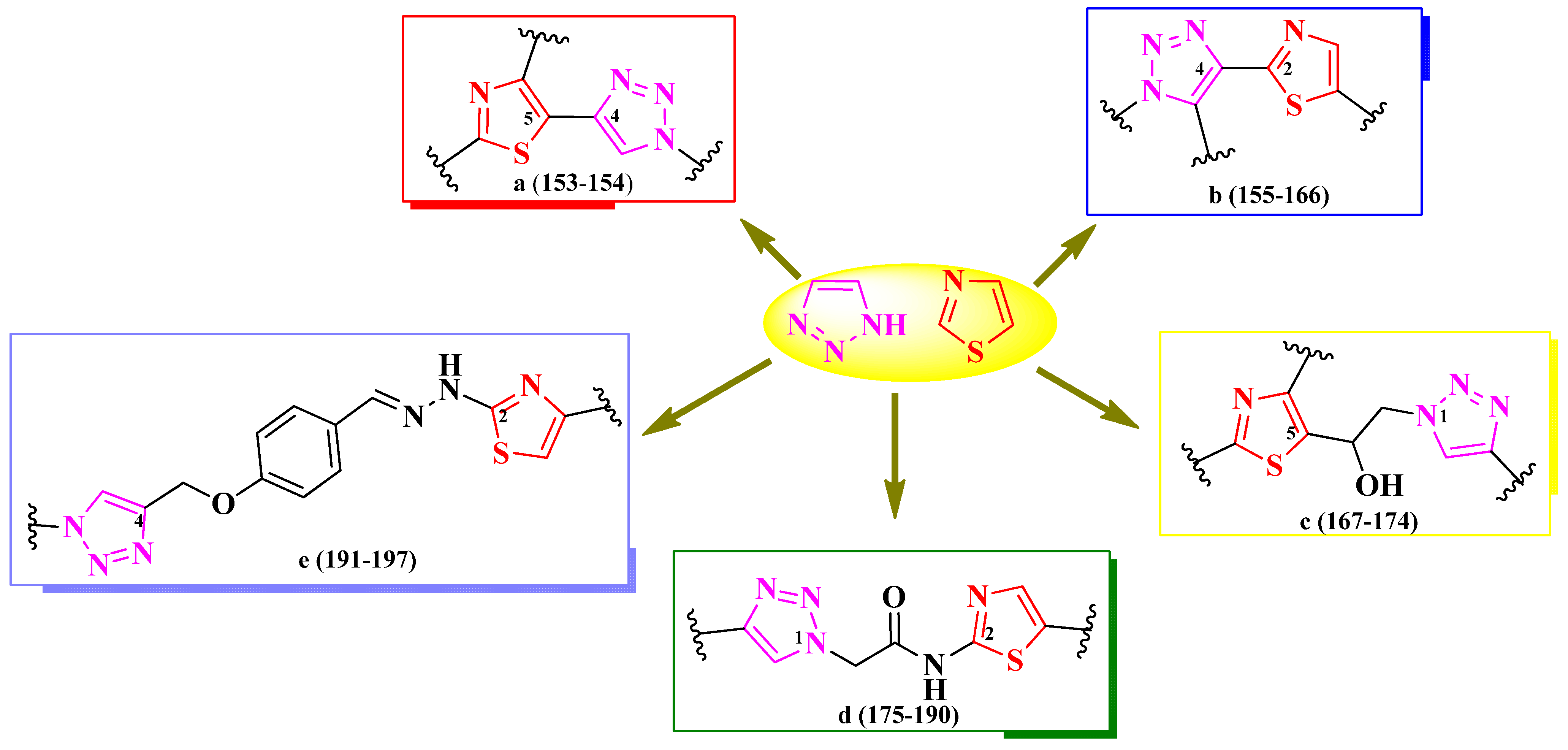
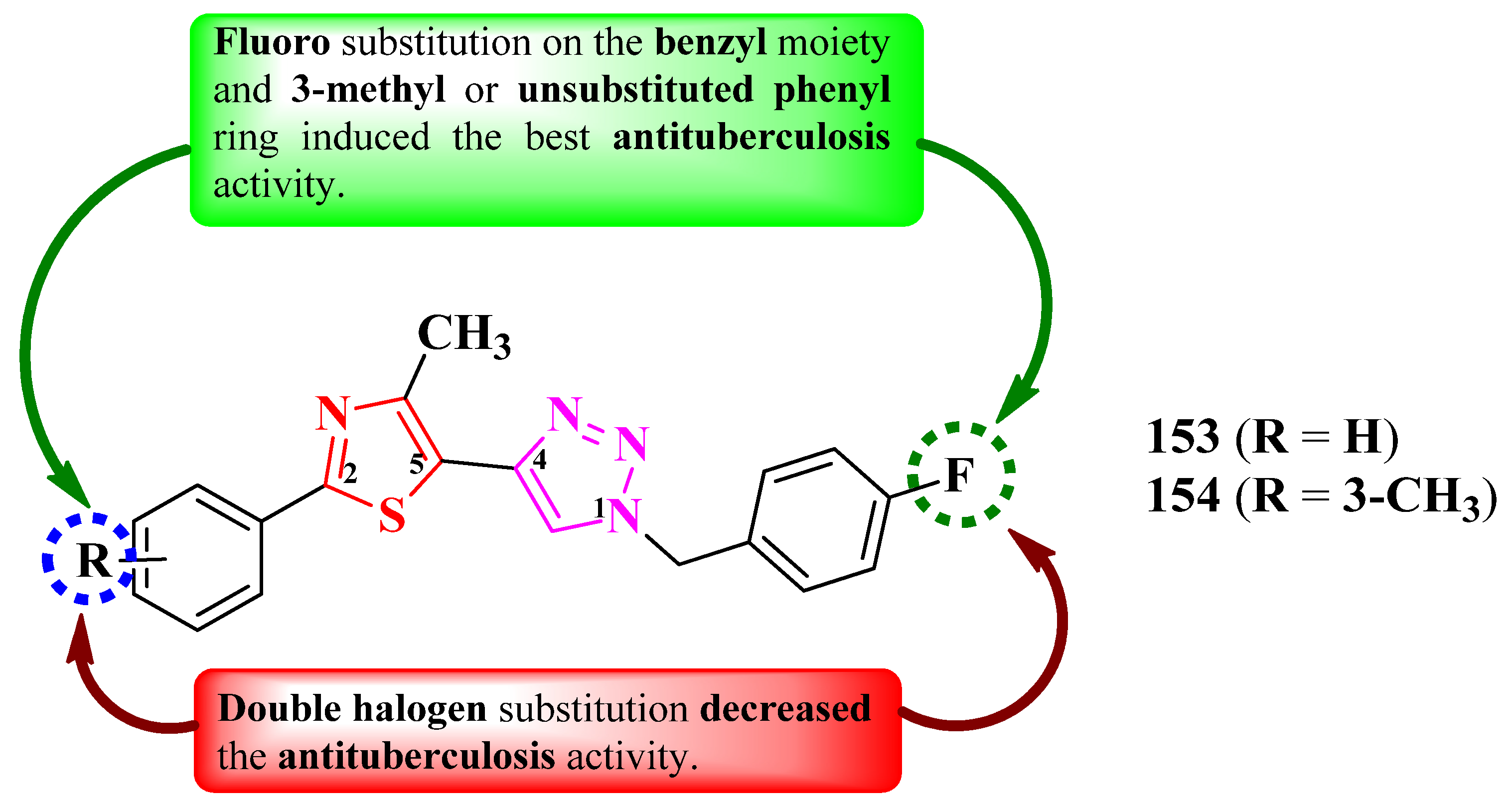
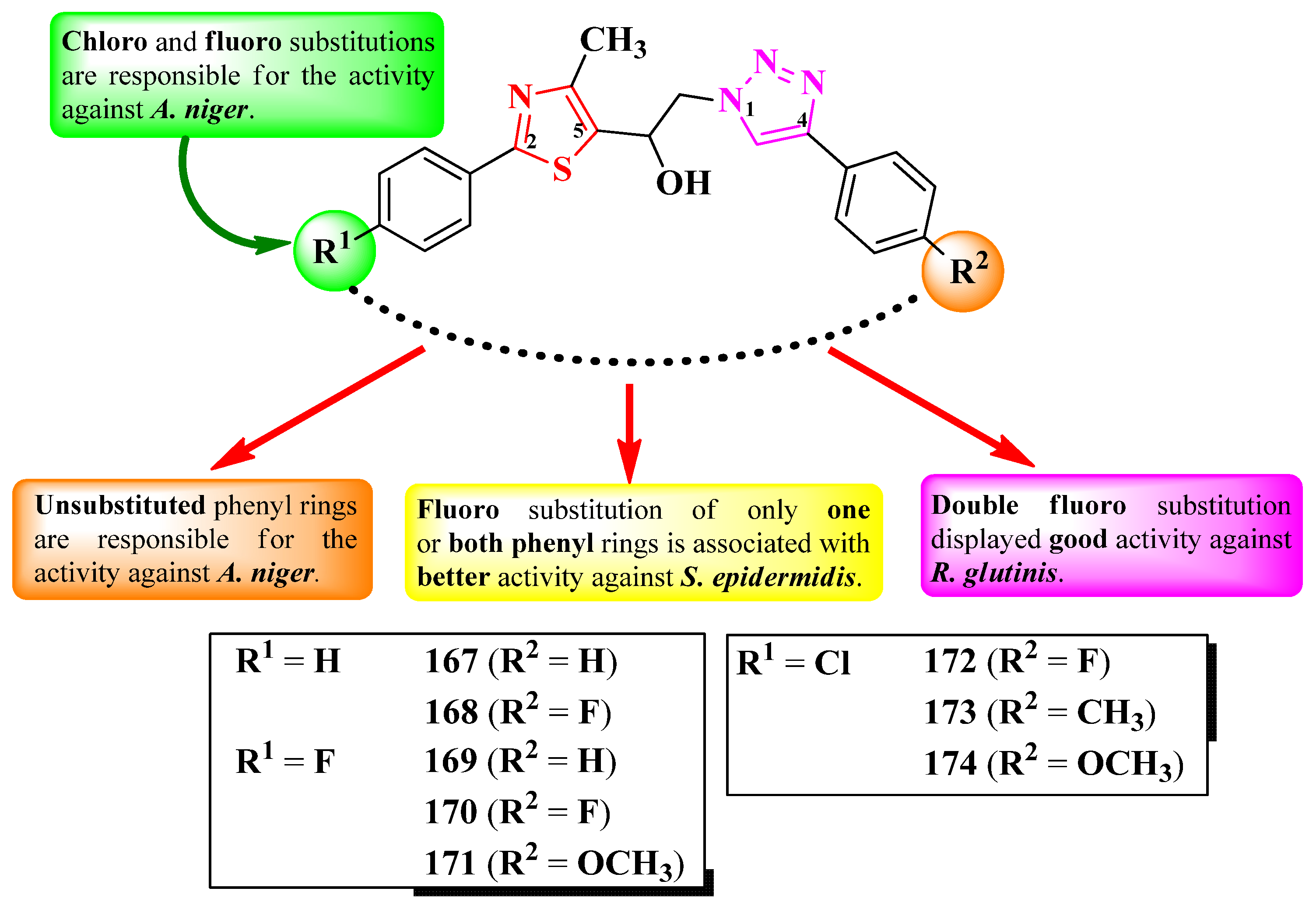
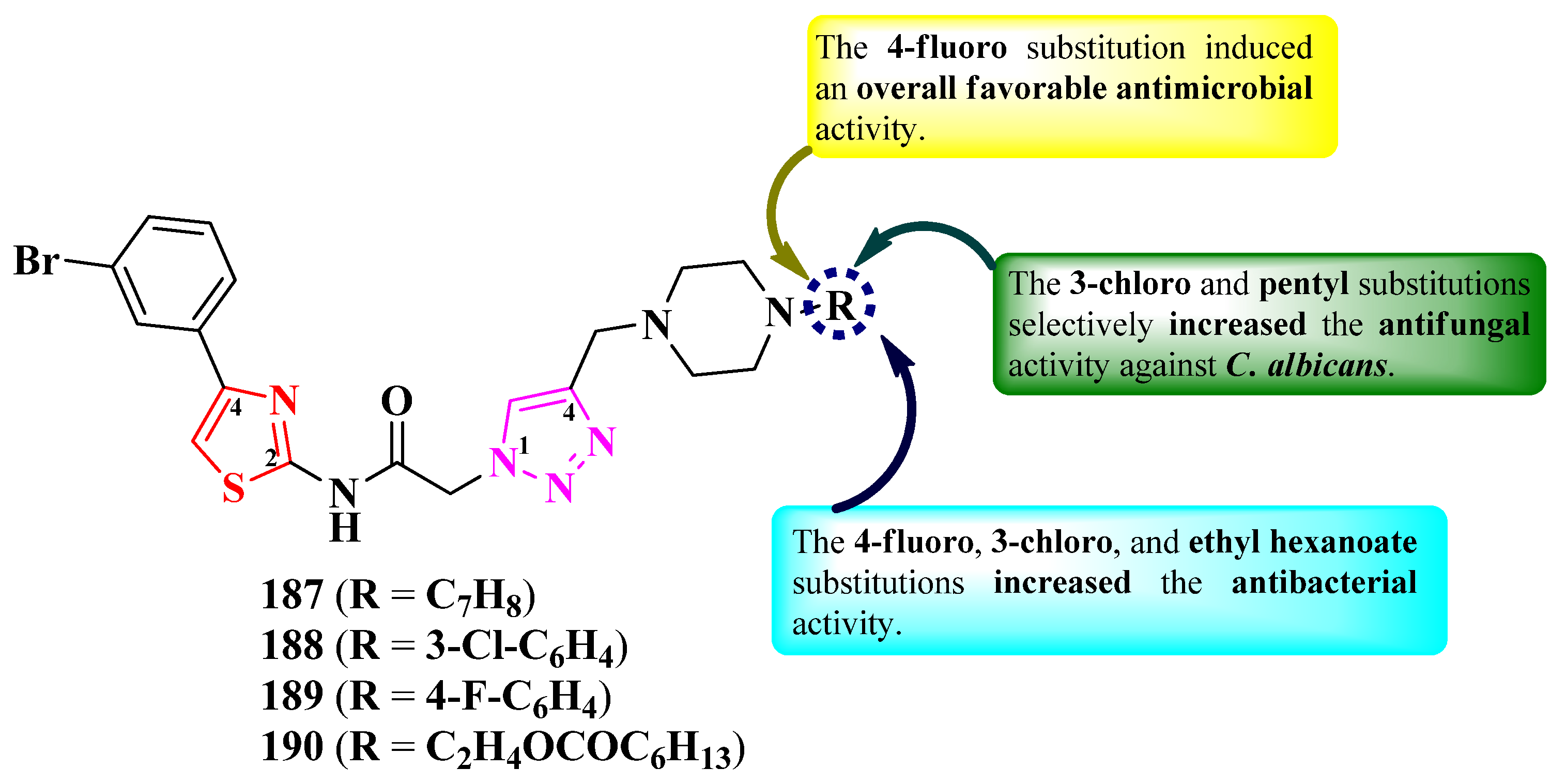
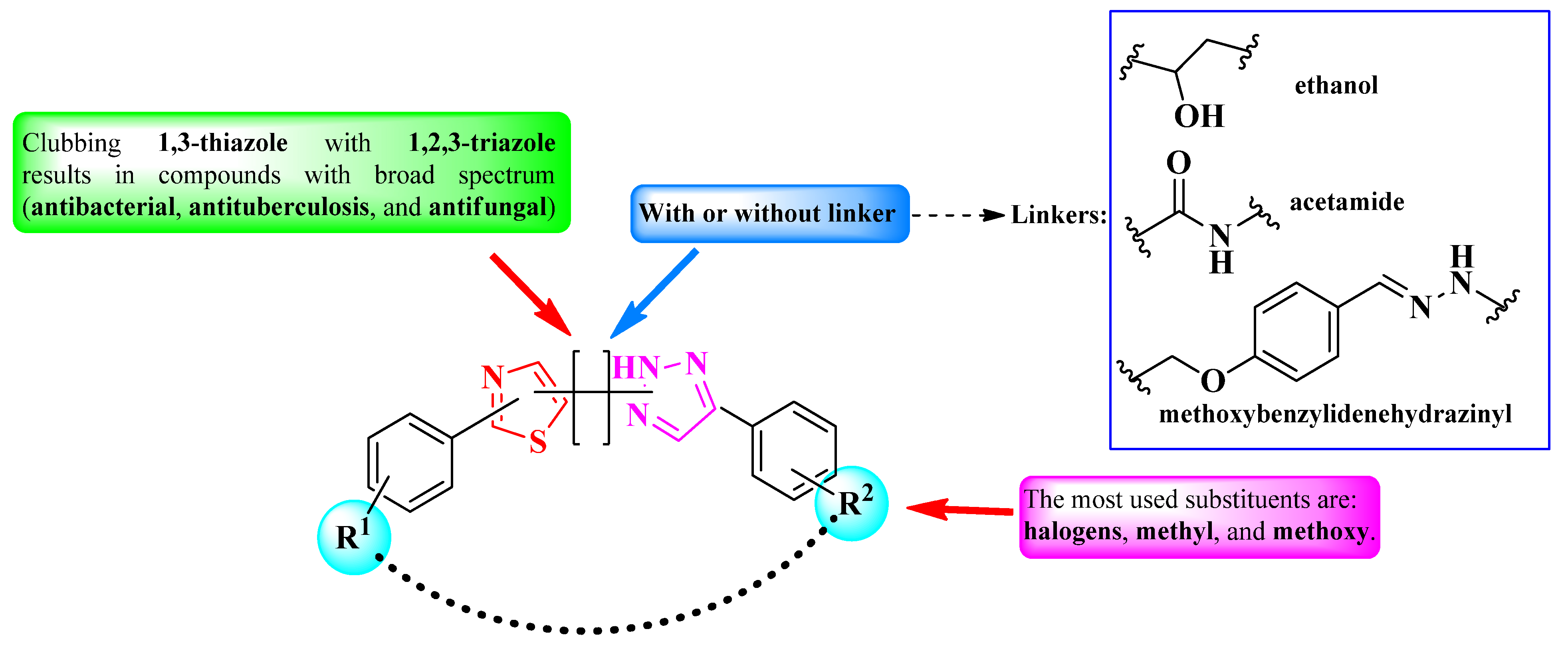
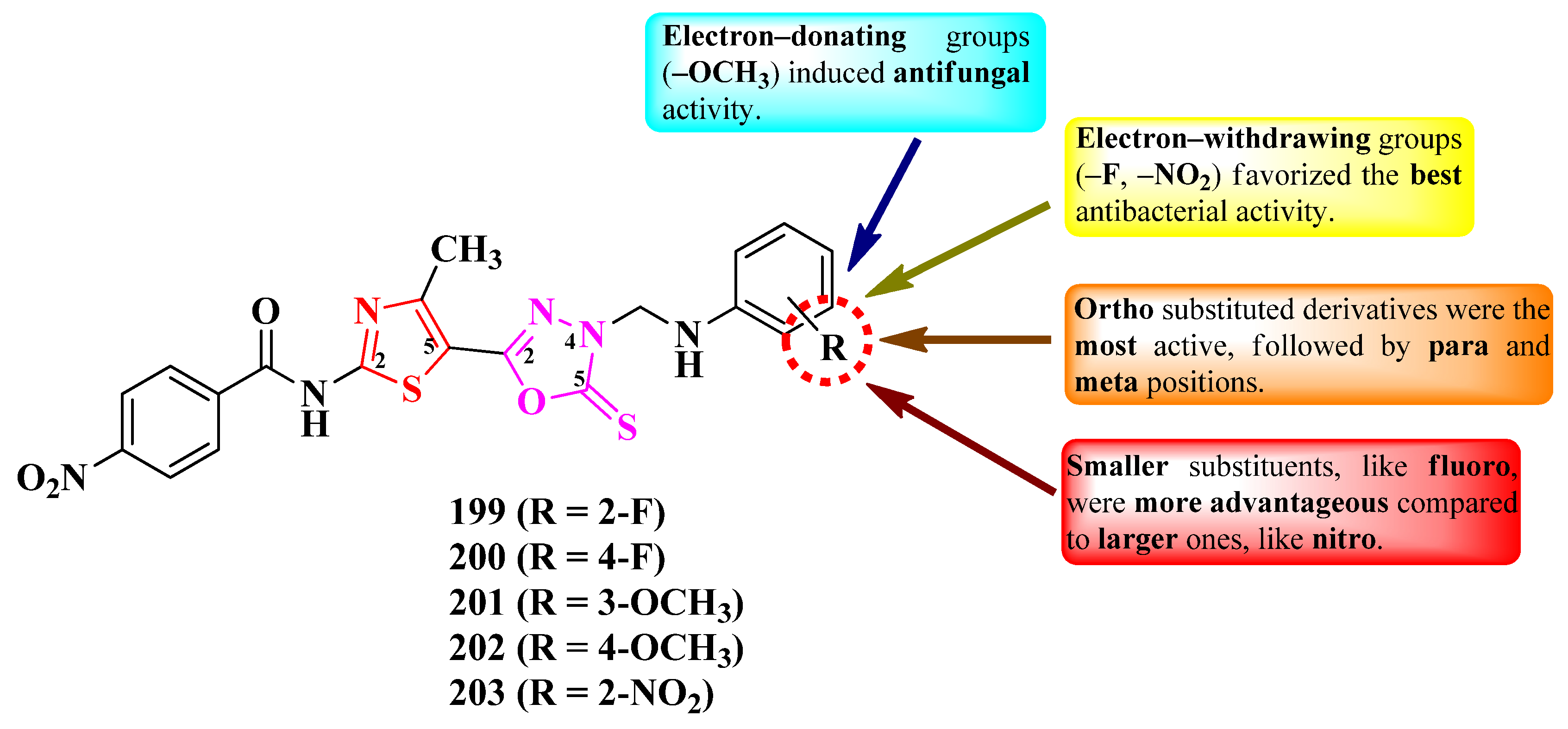
2.2.8. Thiazolyl–1,2,3-Triazole Hybrid Compounds
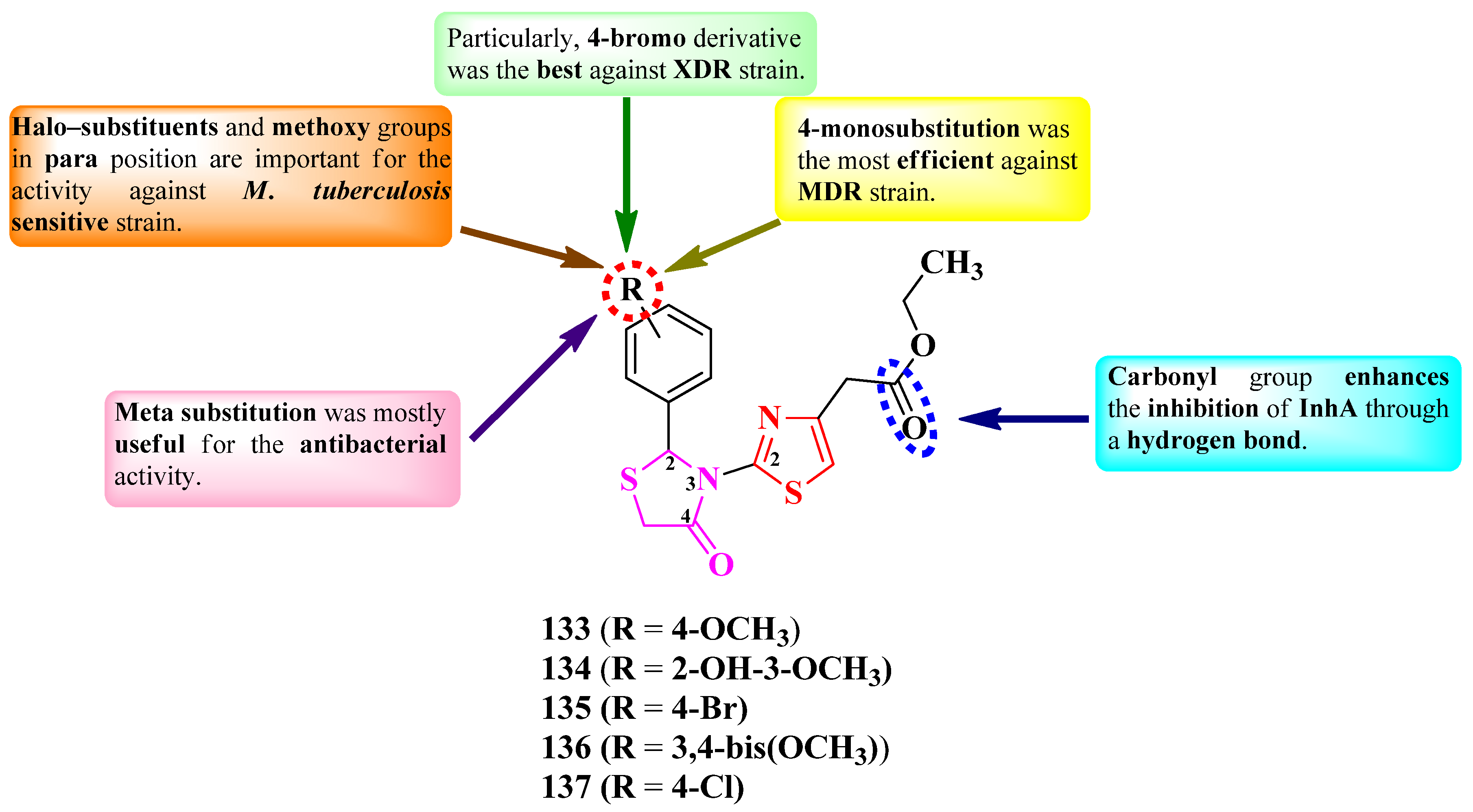
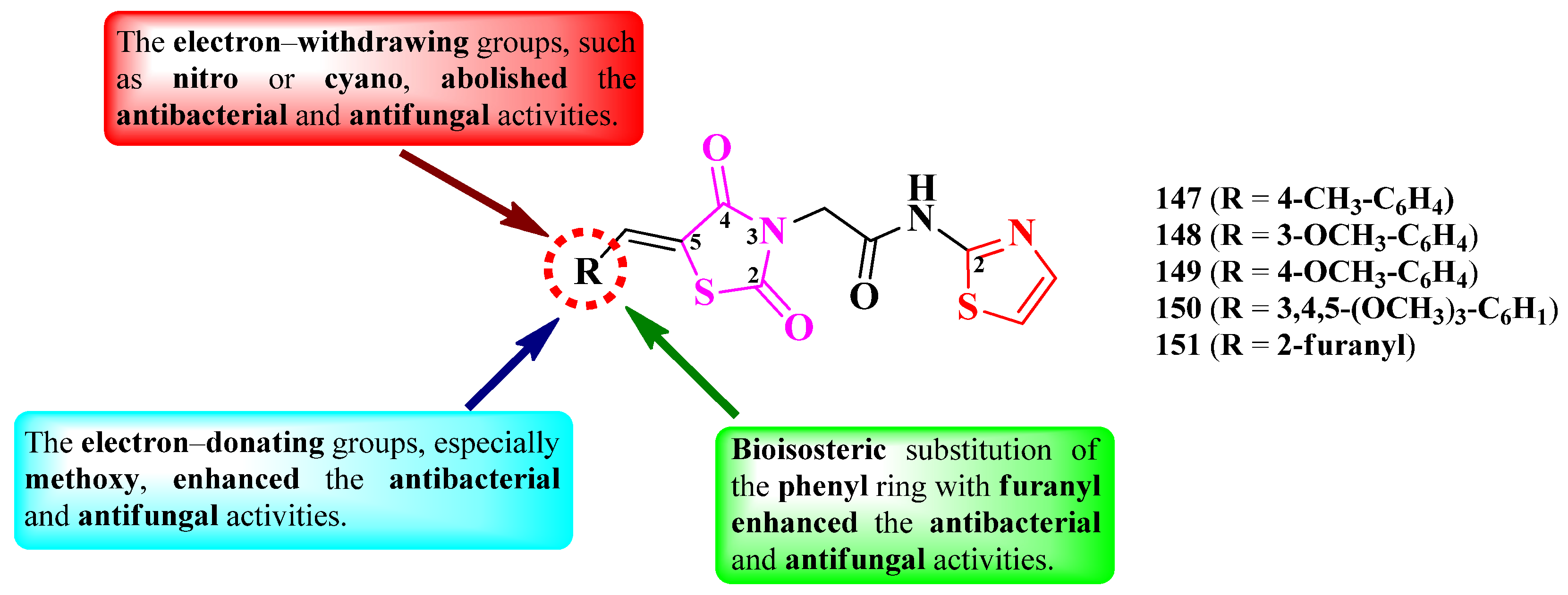
2.2.9. Thiazolyl–1,3,4-Oxadiazole Hybrid Compounds
2.3. Thiazole Clubbed with Five-Membered Benzofused Heterocycles
2.3.1. Thiazolyl–Indole and Thiazolyl–Carbazole Hybrid Compounds
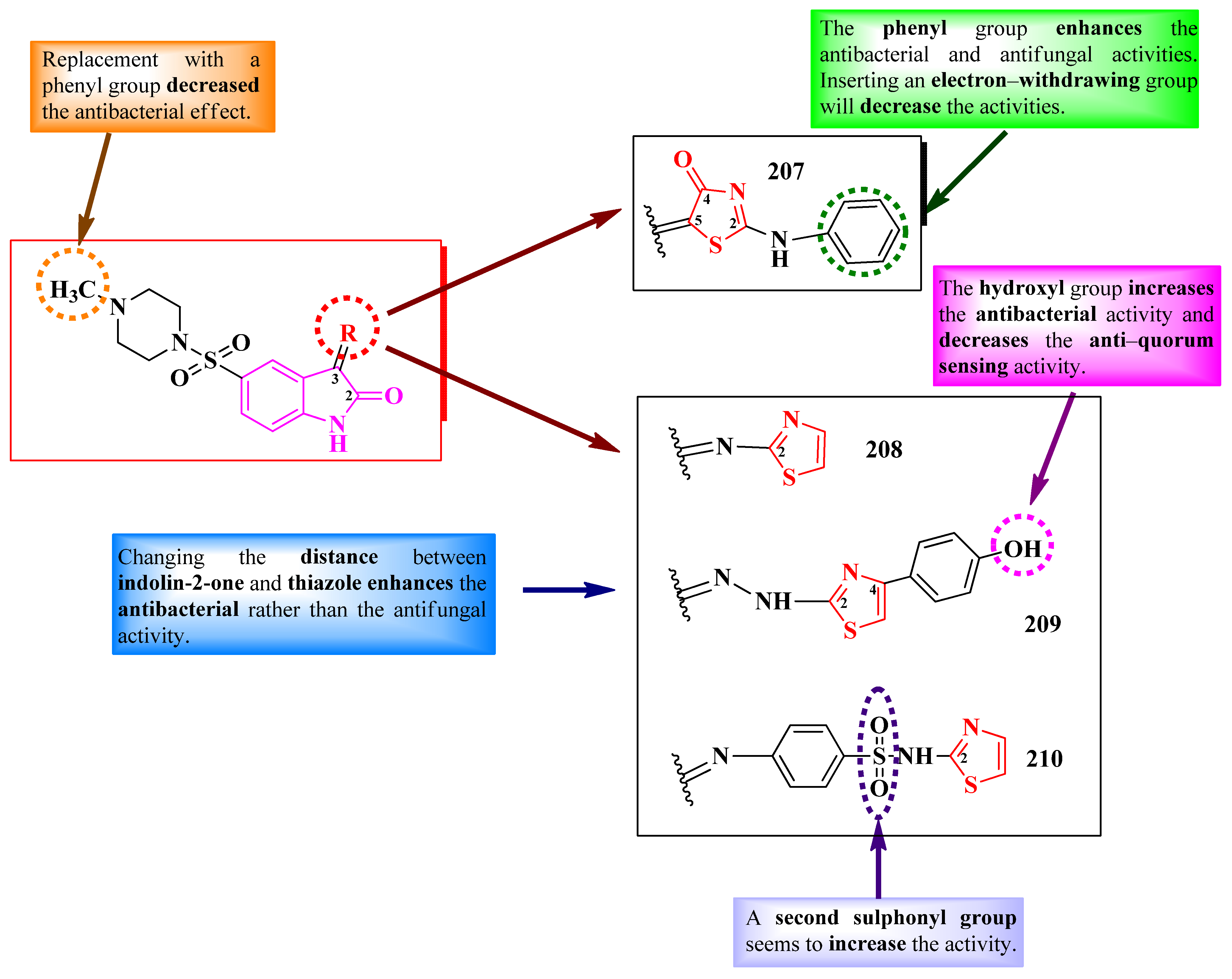
2.3.2. Thiazolyl–Indolin-2-One Hybrid Compounds
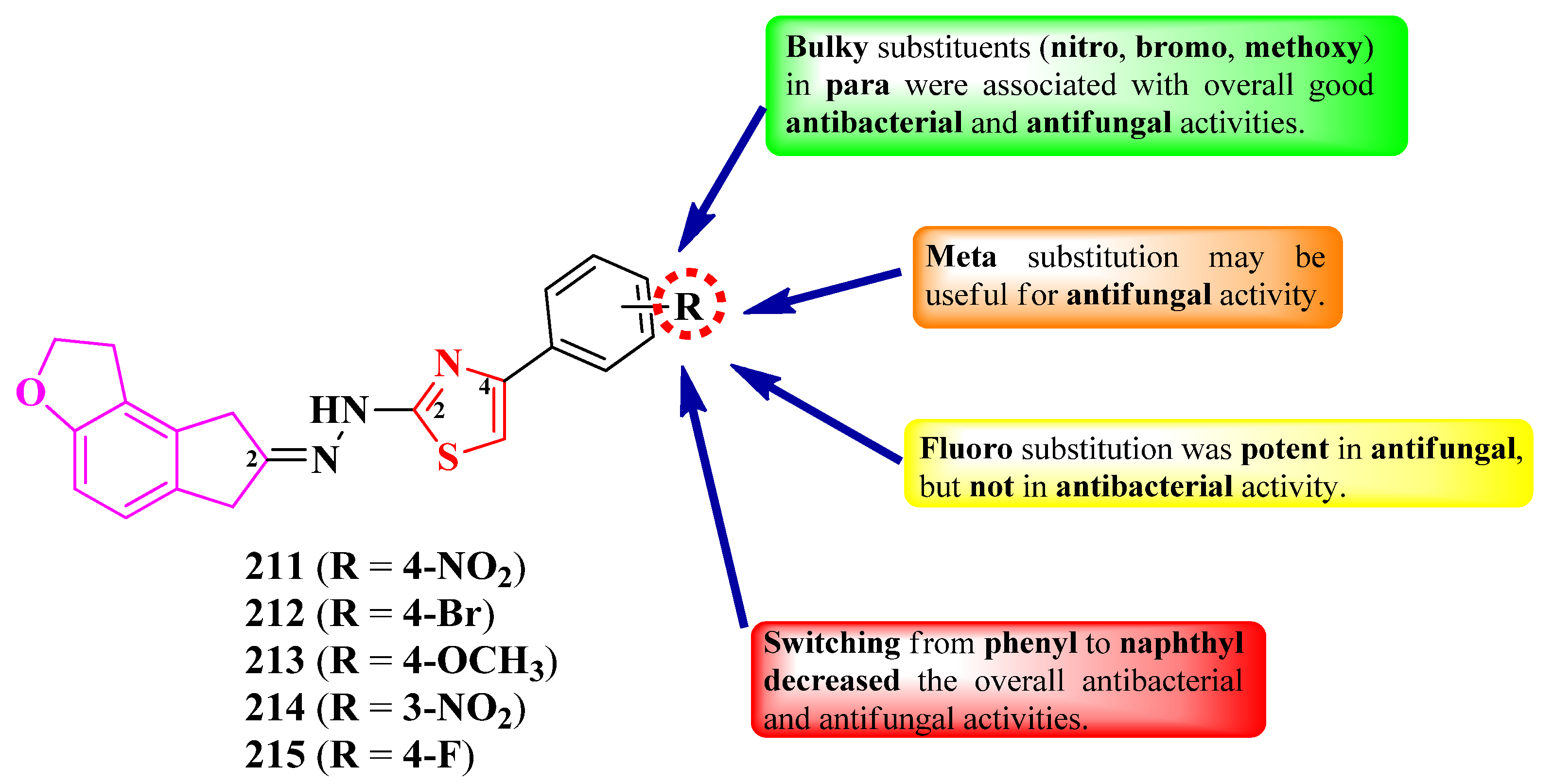
2.3.3. Thiazolyl–Tetrahydroindenofuran Hybrid Compounds
2.4. Thiazole Clubbed with Six-Membered Heterocycles
Thiazolyl–Pyridine Hybrid Compounds
2.5. Thiazole Clubbed with Six-Membered Benzofused Heterocycles
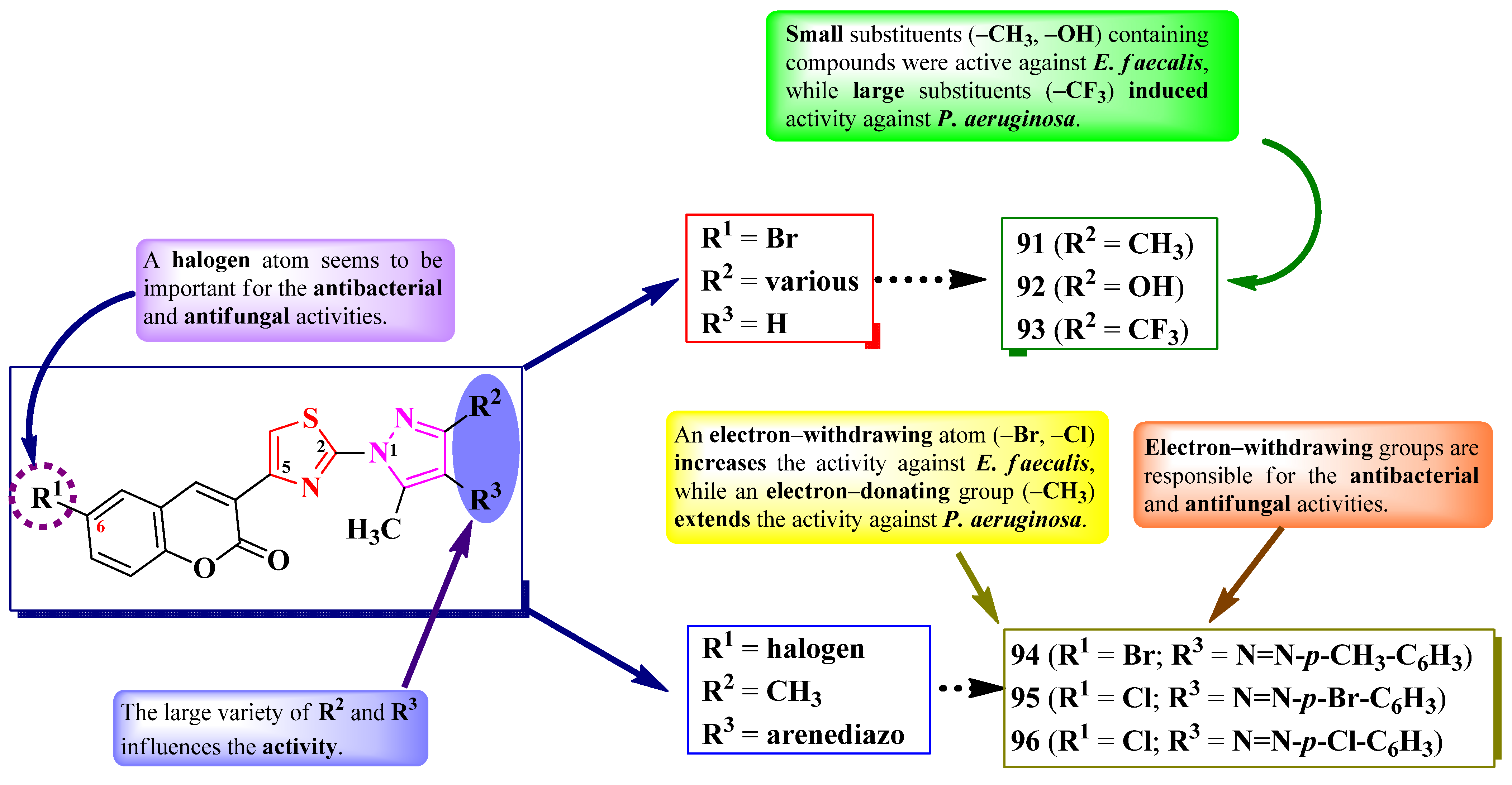
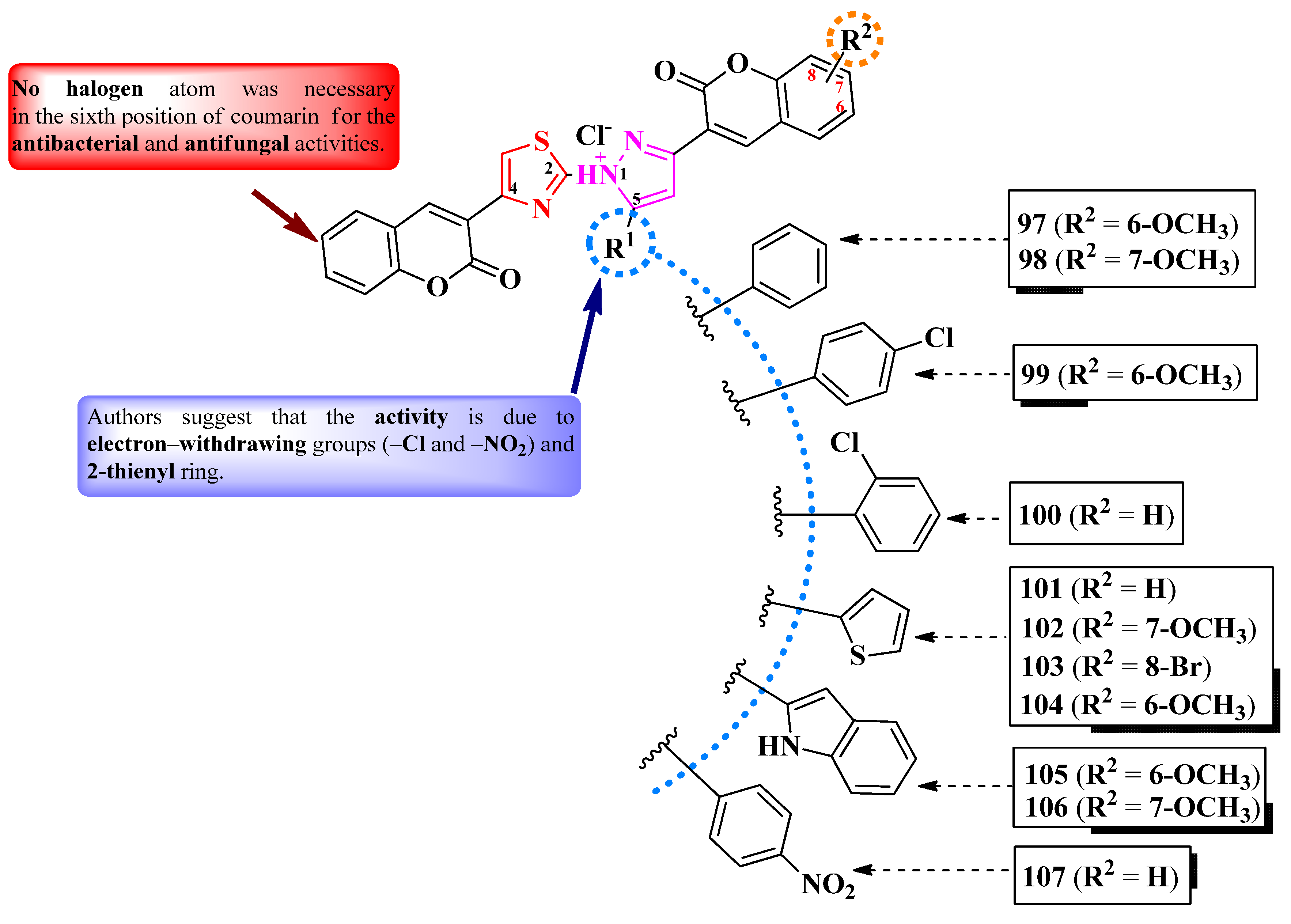
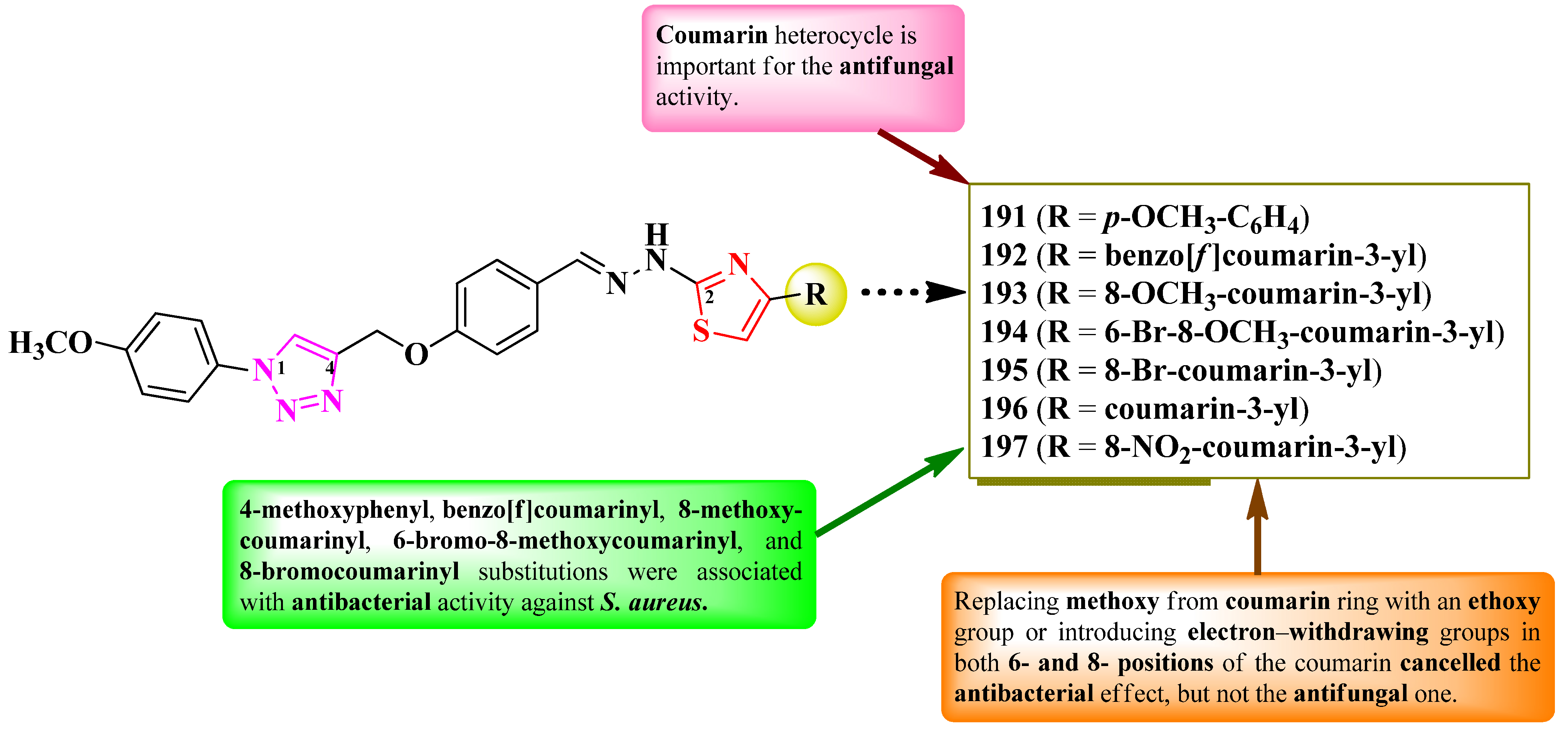
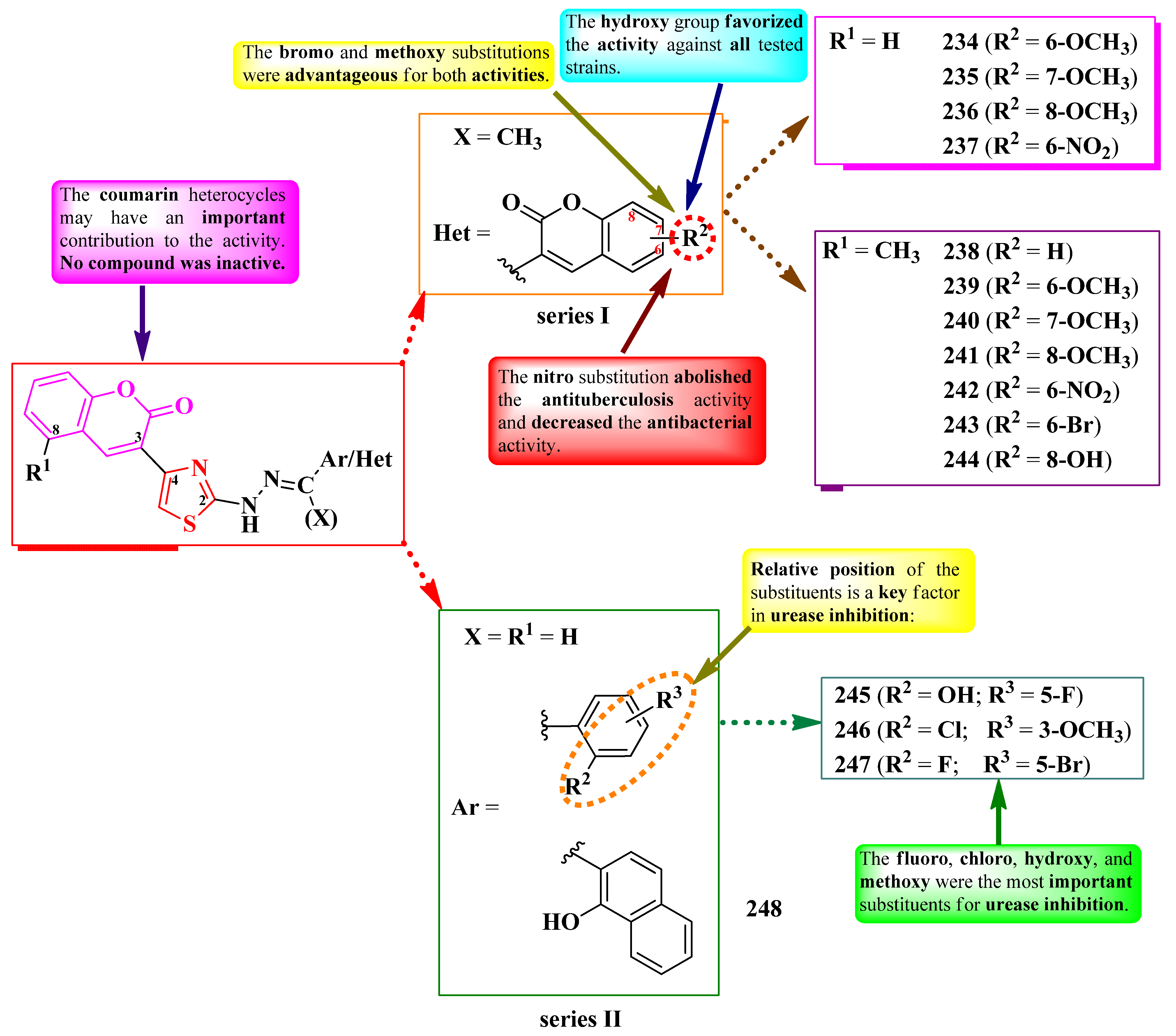
2.5.1. Thiazolyl–Coumarin Hybrid Compounds
2.5.2. Thiazolyl–Flavone Hybrid Compounds
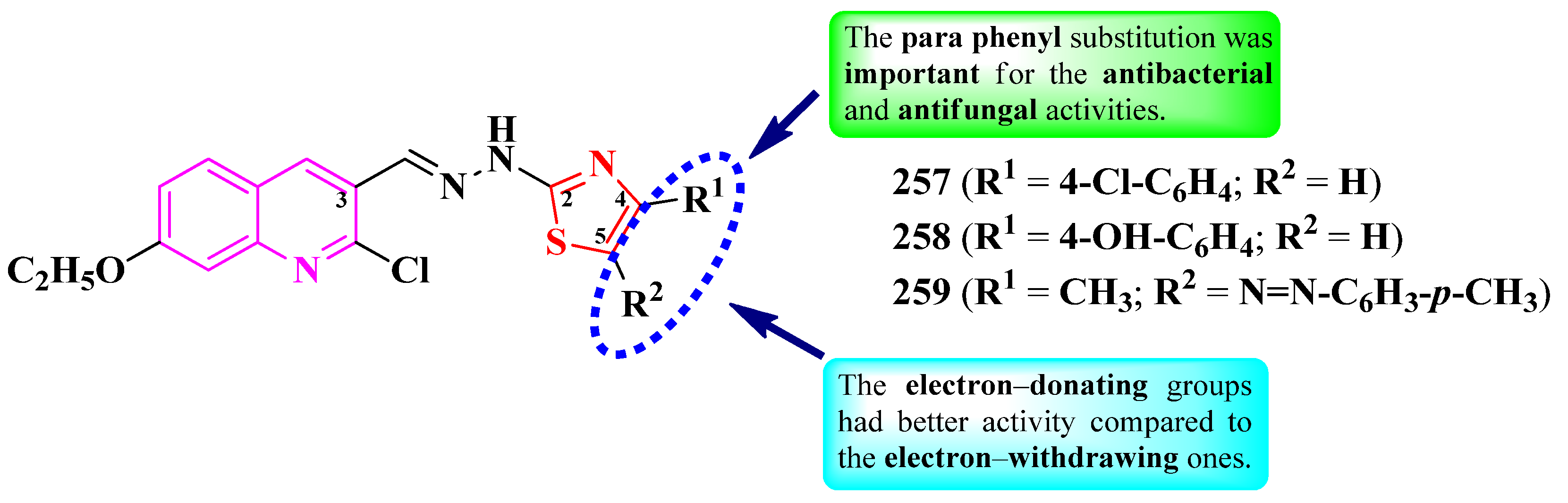
2.5.3. Thiazolyl–Quinoline and Thiazolyl–Quinolone Hybrid Compounds
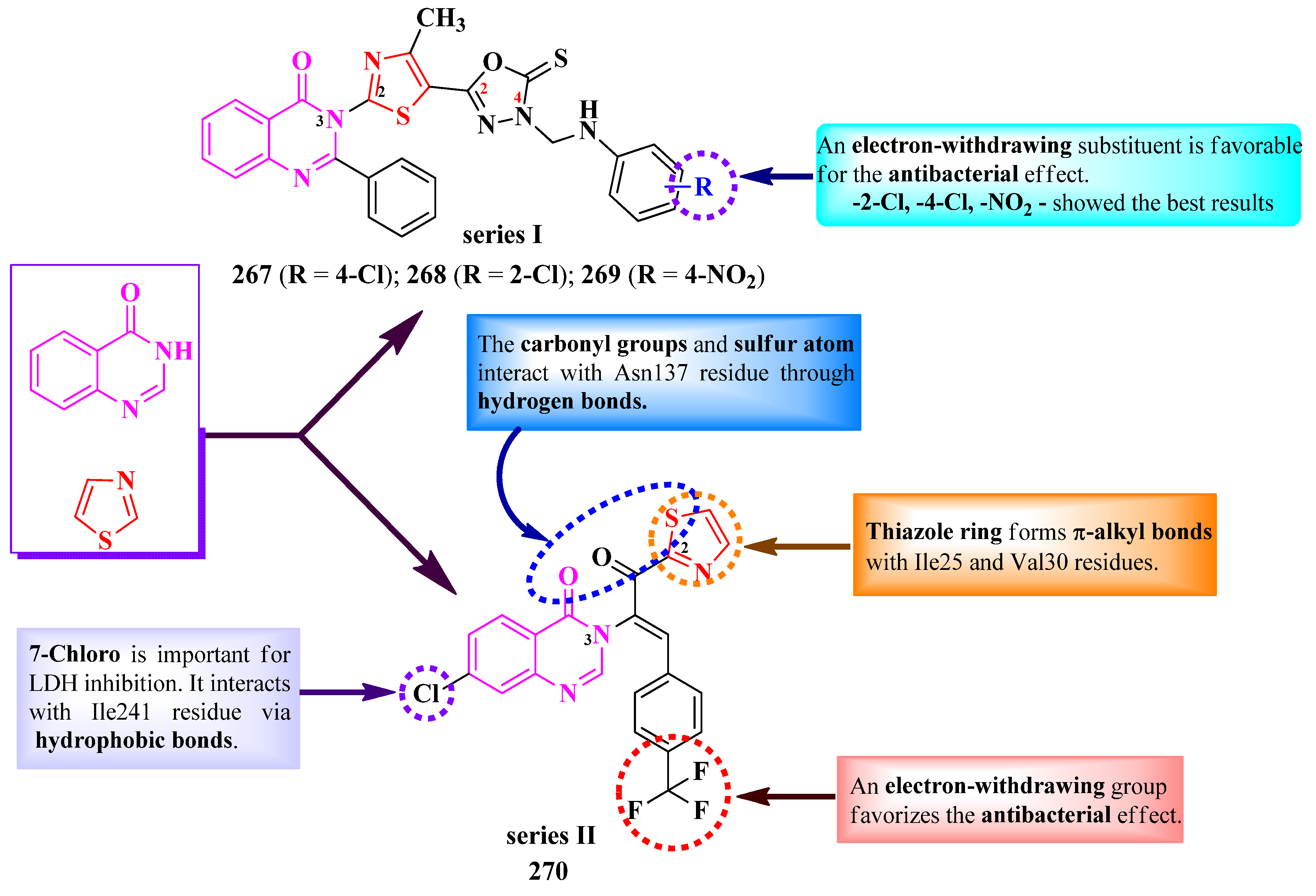
2.5.4. Thiazolyl–Quinazolone Hybrid Compounds
2.6. Thiazole Clubbed with Condensed Heterocycles
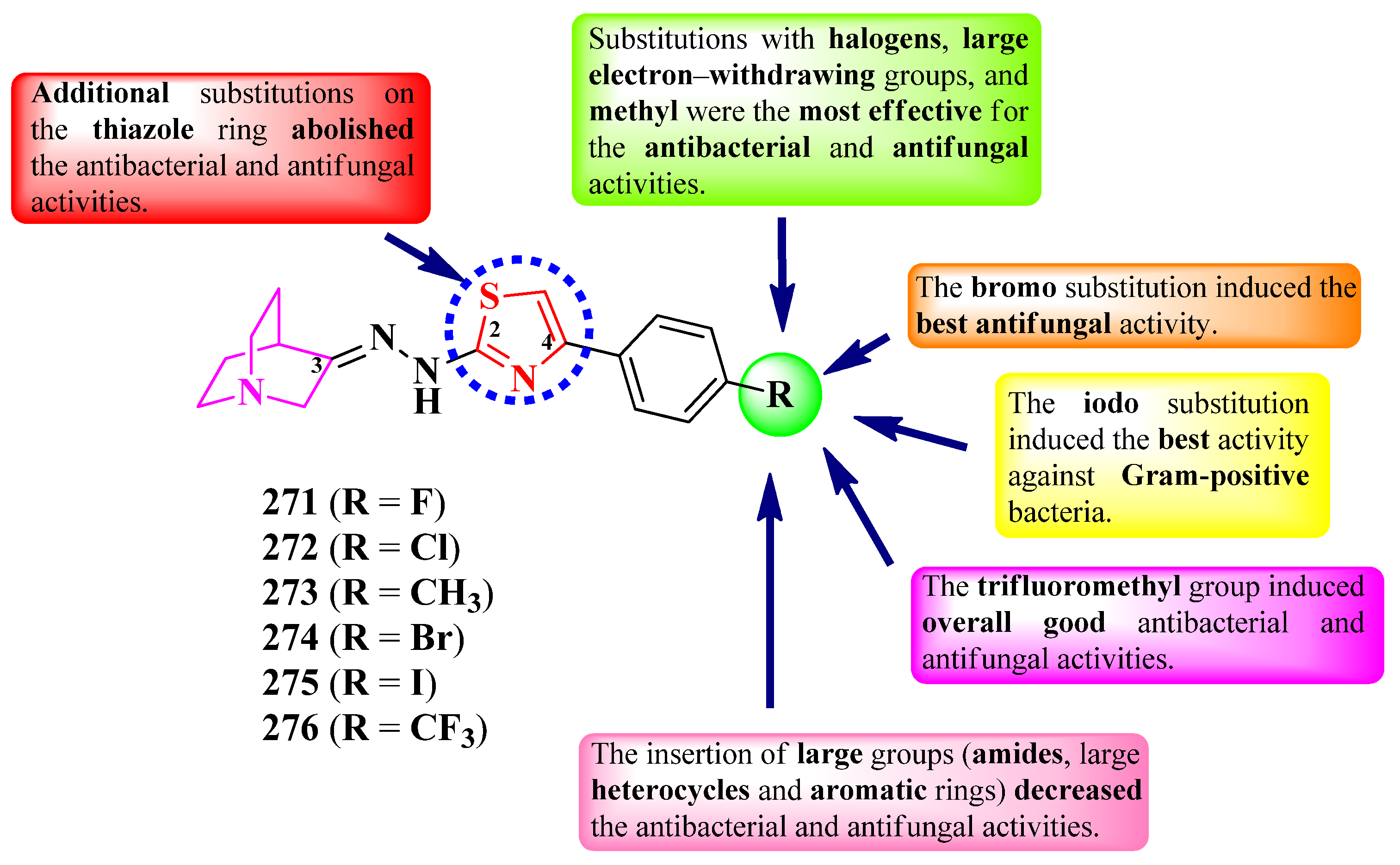
2.6.1. Thiazolyl–Quinuclidine Hybrid Compounds
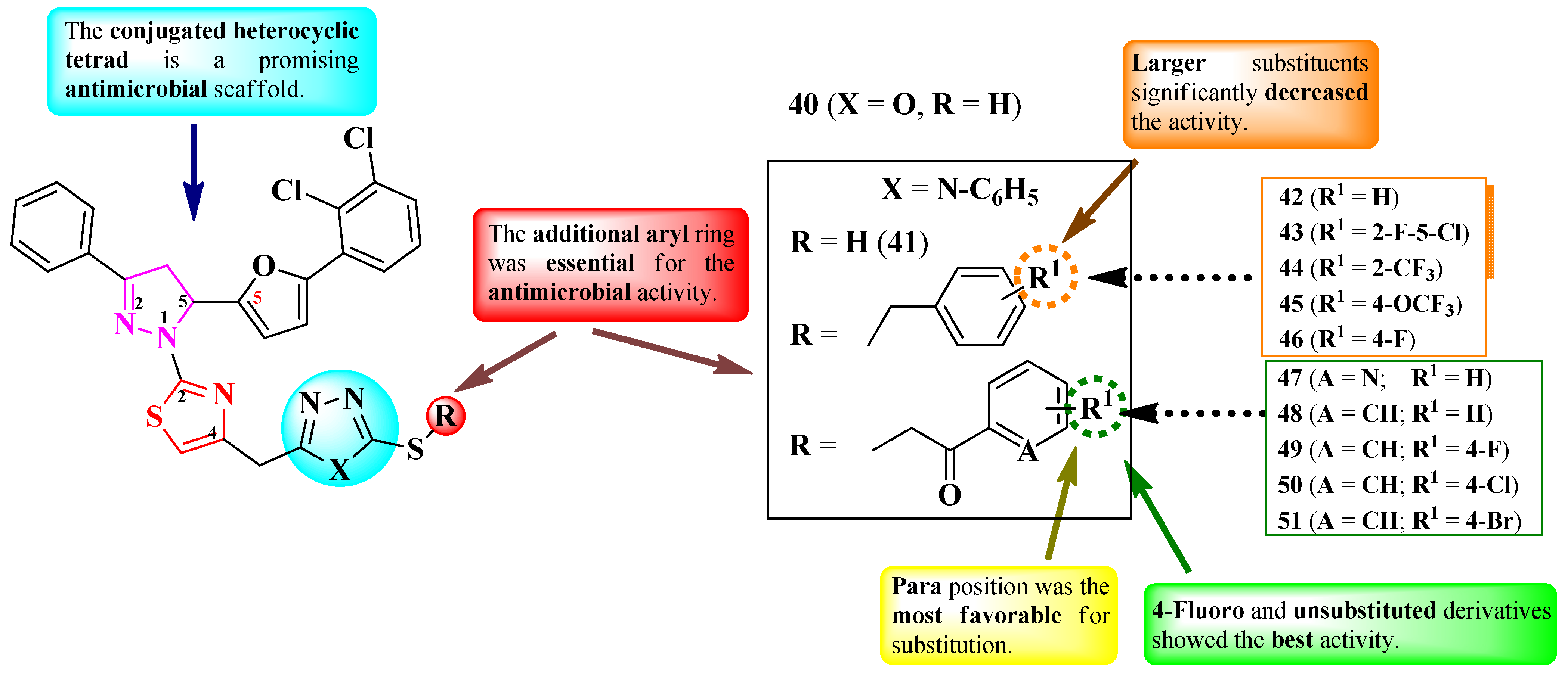
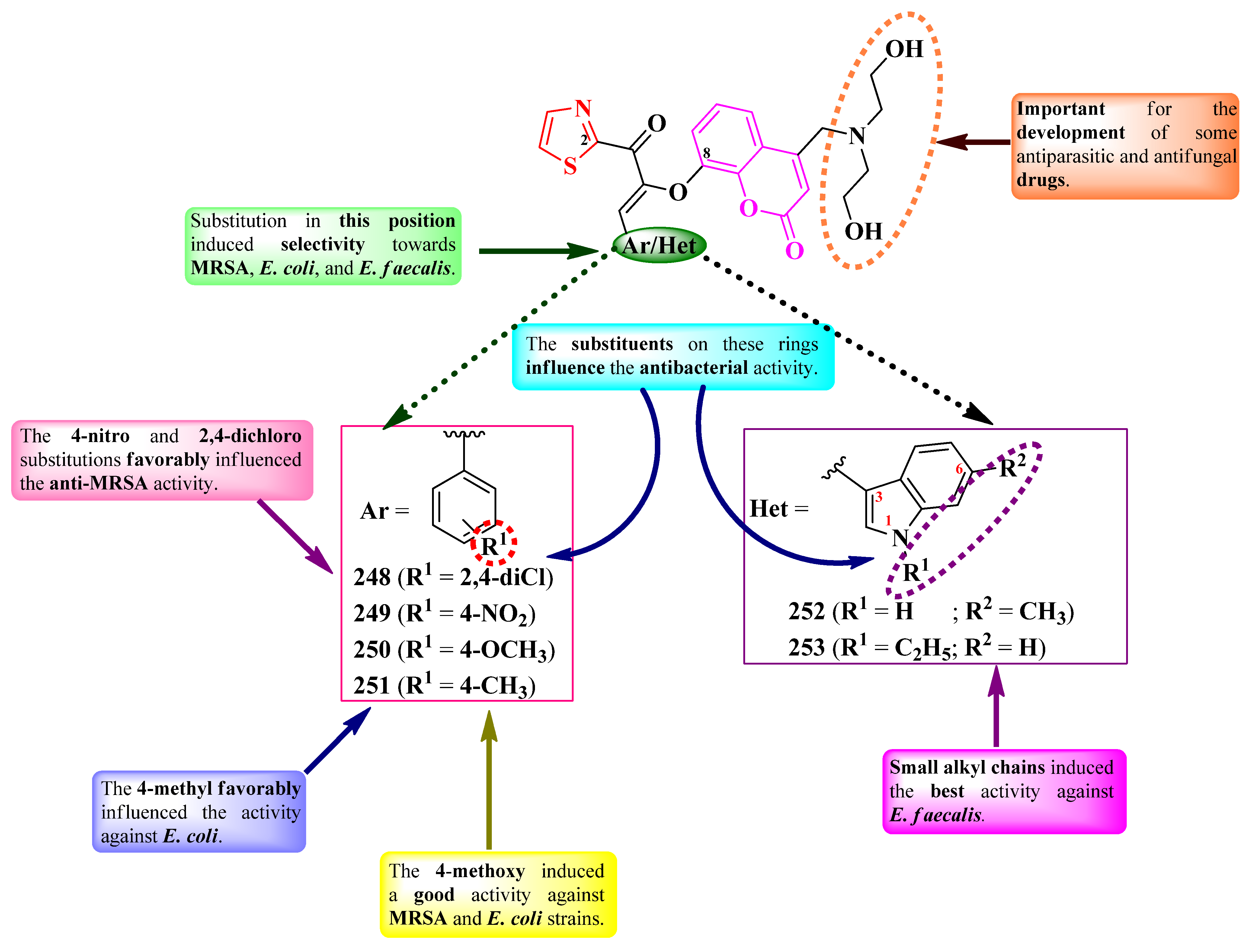
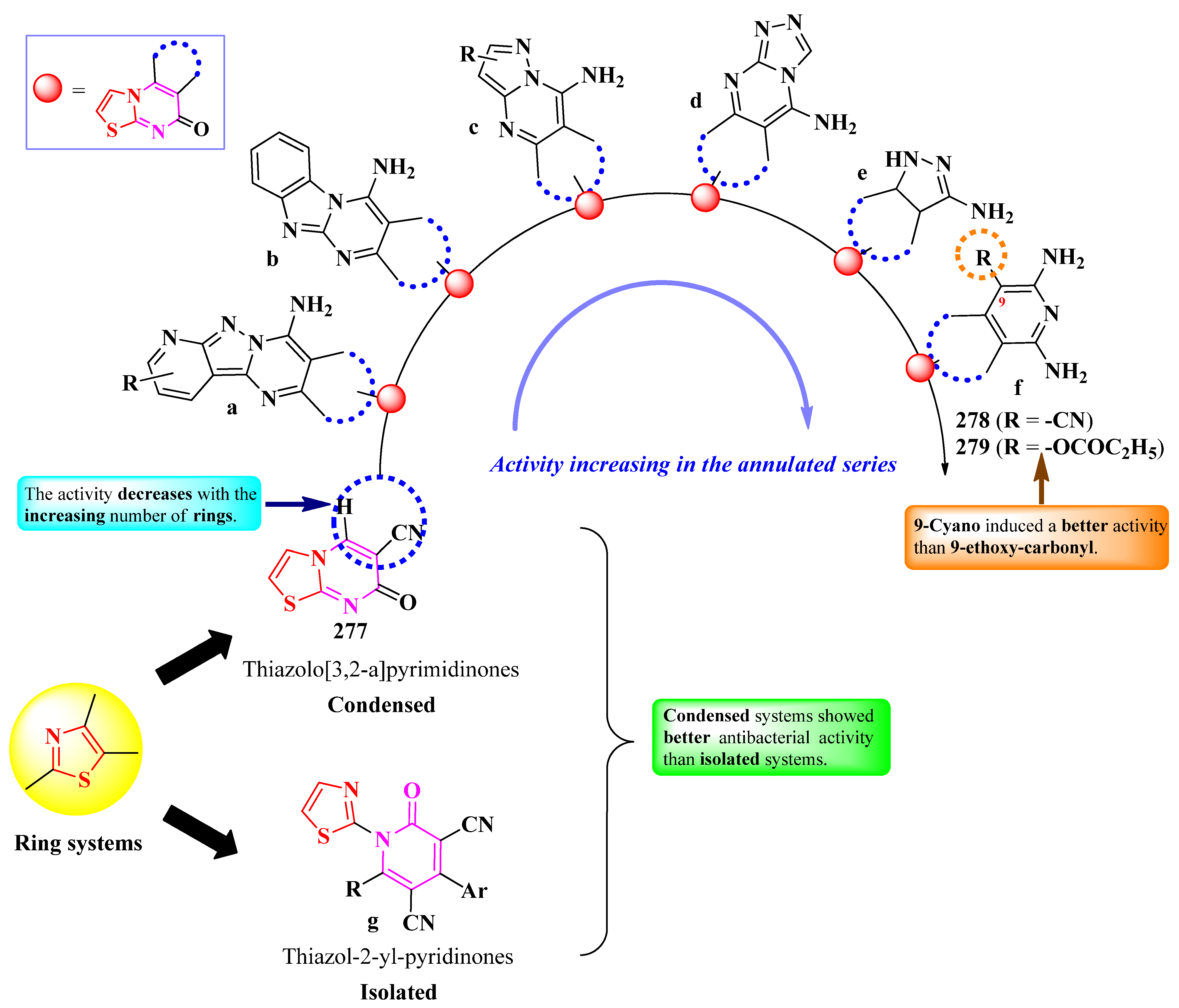
2.6.2. Thiazole Clubbed with Polyheterocyclic Systems
3. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allel, K.; Day, L.; Hamilton, A.; Lin, L.; Furuya-Kanamori, L.; Moore, C.E.; Van Boeckel, T.; Laxminarayan, R.; Yakob, L. Global antimicrobial-resistance drivers: An ecological country-level study at the human–animal interface. Lancet Planet. Health 2023, 7, e291–e303. [Google Scholar] [CrossRef] [PubMed]
- Walesch, S.; Birkelbach, J.; Jézéquel, G.; Haeckl, F.P.J.; Hegemann, J.D.; Hesterkamp, T.; Hirsch, A.K.H.; Hammann, P.; Müller, R. Fighting antibiotic resistance—Strategies and (pre)clinical developments to find new antibacterials. EMBO Rep. 2023, 24, e56033. [Google Scholar] [CrossRef] [PubMed]
- Borcea, A.-M.; Ionuț, I.; Crișan, O.; Oniga, O. An Overview of the Synthesis and Antimicrobial, Antiprotozoal, and Antitumor Activity of Thiazole and Bisthiazole Derivatives. Molecules 2021, 26, 624. [Google Scholar] [CrossRef] [PubMed]
- Mishra, I.; Mishra, R.; Mujwar, S.; Chandra, P.; Sachan, N. A retrospect on antimicrobial potential of thiazole scaffold. J. Heterocycl. Chem. 2020, 57, 2304–2329. [Google Scholar] [CrossRef]
- Petrou, A.; Fesatidou, M.; Geronikaki, A. Thiazole ring—A biologically active scaffold. Molecules 2021, 26, 3166. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, H.C. Coumarin-1,2,3-triazole Hybrid Molecules: An Emerging Scaffold for Combating Drug Resistance. Curr. Top. Med. Chem. 2021, 21, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Rittenbury, M.S. How and why aztreonam works. Surg. Gynecol. Obstet. 1990, 171, 19–23. [Google Scholar]
- Ito, A.; Sato, T.; Ota, M.; Takemura, M.; Nishikawa, T.; Toba, S.; Kohira, N.; Miyagawa, S.; Ishibashi, N.; Matsumoto, S.; et al. In Vitro Antibacterial Properties of Cefiderocol, a Novel Siderophore Cephalosporin, against Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2018, 62, e01454-17. [Google Scholar] [CrossRef]
- Borelli, C.; Schaller, M.; Niewerth, M.; Nocker, K.; Baasner, B.; Berg, D.; Tiemann, R.; Tietjen, K.; Fugmann, B.; Lang-Fugmann, S.; et al. Modes of action of the new arylguanidine abafungin beyond interference with ergosterol biosynthesis and in vitro activity against medically important fungi. Chemotherapy 2008, 54, 245–259. [Google Scholar] [CrossRef]
- Falci, D.R.; Pasqualotto, A.C. Profile of isavuconazole and its potential in the treatment of severe invasive fungal infections. Infect. Drug Resist. 2013, 6, 163–174. [Google Scholar] [CrossRef]
- Cuenca-Estrella, M.; Gomez-Lopez, A.; Mellado, E.; Garcia-Effron, G.; Rodriguez-Tudela, J.L. In vitro activities of ravuconazole and four other antifungal agents against fluconazole-resistant or -susceptible clinical yeast isolates. Antimicrob. Agents Chemother. 2004, 48, 3107–3111. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, B.; Almendros, P.; Aragoncillo, C. Highly reactive 4-membered ring nitrogen-containing heterocycles: Synthesis and properties. Curr. Opin. Drug Discov. Dev. 2010, 13, 685–697. [Google Scholar]
- Desai, N.C.; Harsora, J.P.; Monapara, J.D.; Khedkar, V.M. Synthesis, Antimicrobial Capability and Molecular Docking of Heterocyclic Scaffolds Clubbed by 2-Azetidinone, Thiazole and Quinoline Derivatives. Polycycl. Aromat. Compd. 2021, 42, 3924–3938. [Google Scholar] [CrossRef]
- Kumar, S.; Bawa, S.; Drabu, S.; Kumar, R.; Gupta, H. Biological Activities of Pyrazoline Derivatives—A Recent Development. Recent Pat. Antiinfect. Drug Discov. 2009, 4, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Cuartas, V.; Robledo, S.M.; Vélez, I.D.; Crespo, M.d.P.; Sortino, M.; Zacchino, S.; Nogueras, M.; Cobo, J.; Upegui, Y.; Pineda, T.; et al. New thiazolyl-pyrazoline derivatives bearing nitrogen mustard as potential antimicrobial and antiprotozoal agents. Arch. Pharm. 2020, 353, e1900351. [Google Scholar] [CrossRef]
- Rashdan, H.R.M.; Abdelmonsef, A.H. Towards COVID-19 TMPRSS2 enzyme inhibitors and antimicrobial agents: Synthesis, antimicrobial potency, molecular docking, and drug-likeness prediction of thiadiazole-triazole hybrids. J. Mol. Struct. 2022, 1268, 133659. [Google Scholar] [CrossRef]
- Budak, Y.; Kocyigit, U.M.; Gürdere, M.B.; Özcan, K.; Taslimi, P.; Gülçin, İ.; Ceylan, M. Synthesis and investigation of antibacterial activities and carbonic anhydrase and acetyl cholinesterase inhibition profiles of novel 4,5-dihydropyrazol and pyrazolyl-thiazole derivatives containing methanoisoindol-1,3-dion unit. Synth. Commun. 2017, 47, 2313–2323. [Google Scholar] [CrossRef]
- Mansour, E.; Aboelnaga, A.; Nassar, E.M.; Elewa, S.I. A new series of thiazolyl pyrazoline derivatives linked to benzo [1,3]dioxole moiety: Synthesis and evaluation of antimicrobial and anti-proliferative activities. Synth. Commun. 2020, 50, 368–379. [Google Scholar] [CrossRef]
- Masoud, D.M.; Azzam, R.A.; Hamdy, F.; Mekawey, A.A.I.; Abdel-Aziz, H.A. Synthesis of Some Novel Pyrazoline-Thiazole Hybrids and Their Antimicrobial Activities. J. Heterocycl. Chem. 2019, 56, 3030–3041. [Google Scholar] [CrossRef]
- Bhandare, R.R.; S.Munikrishnappa, C.; Suresh Kumar, G.V.; Konidala, S.K.; Sigalapalli, D.K.; Vaishnav, Y.; Chinnam, S.; Yasin, H.; Al-karmalawy, A.A.; Shaik, A.B. Multistep synthesis and screening of heterocyclic tetrads containing furan, pyrazoline, thiazole and triazole (or oxadiazole) as antimicrobial and anticancer agents. J. Saudi Chem. Soc. 2022, 26, 101447. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Khidre, R.E.; Mohamed, H.A.; El-Hiti, G.A. A Simple Process for the Synthesis of Novel Pyrazolyltriazole and Dihydropyrazolylthiazole Derivatives as Antimicrobial Agents. Arab. J. Sci. Eng. 2017, 42, 2441–2448. [Google Scholar] [CrossRef]
- Salih, R.H.H.; Hasan, A.H.; Hussen, N.H.; Hawaiz, F.E.; Ben Hadda, T.; Jamalis, J.; Almalki, F.A.; Adeyinka, A.S.; Coetzee, L.-C.C.; Oyebamiji, A.K. Thiazole-pyrazoline hybrids as potential antimicrobial agent: Synthesis, biological evaluation, molecular docking, DFT studies and POM analysis. J. Mol. Struct. 2023, 1282, 135191. [Google Scholar] [CrossRef]
- Dawood, D.H.; Sayed, M.M.; Tohamy, S.T.K.; Nossier, E.S. New Thiophenyl-pyrazolyl-thiazole Hybrids as DHFR Inhibitors: Design, Synthesis, Antimicrobial Evaluation, Molecular Modeling, and Biodistribution Studies. ACS Omega 2023, 8, 39250–39268. [Google Scholar] [CrossRef]
- Bondock, S.; Fouda, A.M. Synthesis and evaluation of some new 5-(hetaryl)thiazoles as potential antimicrobial agents. Synth. Commun. 2018, 48, 561–573. [Google Scholar] [CrossRef]
- Vijesh, A.M.; Isloor, A.M.; Isloor, S.; Shivananda, K.N.; Shyma, P.C.; Arulmoli, T. Synthesis of some new pyrazolone derivatives as potent antimicrobial agents. Pharma Chem. 2011, 3, 454–463. [Google Scholar]
- Abu-Melha, S. Molecular modeling and docking studies of new antimicrobial antipyrine-thiazole hybrids. Arab. J. Chem. 2022, 15, 103898. [Google Scholar] [CrossRef]
- Jamwal, A.; Javed, A.; Bhardwaj, V. A review on Pyrazole derivatives of pharmacological potential. J. Pharm. BioSci 2013, 3, 114–123. [Google Scholar]
- Gondru, R.; Sirisha, K.; Raj, S.; Gunda, S.K.; Kumar, C.G.; Pasupuleti, M.; Bavantula, R. Design, Synthesis, In Vitro Evaluation and Docking Studies of Pyrazole-Thiazole Hybrids as Antimicrobial and Antibiofilm Agents. ChemistrySelect 2018, 3, 8270–8276. [Google Scholar] [CrossRef]
- Patil, S.V.; Suryavanshi, M.B.; Nagargoje, D.R.; Kokate, S.V. Synthesis and Antimicrobial Evaluation of Some New Pyrazole Derivatives Containing Thiazole Scaffolds. Chem. Proc. 2021, 8, 46. [Google Scholar]
- Matta, R.; Pochampally, J.; Dhoddi, B.N.; Bhookya, S.; Bitla, S.; Akkiraju, A.G. Synthesis, antimicrobial and antioxidant activity of triazole, pyrazole containing thiazole derivatives and molecular docking studies on COVID-19. BMC Chem. 2023, 17, 61. [Google Scholar] [CrossRef]
- Abdel-Aziem, A.; Baaiu, B.S.; Elbazzar, A.W.; Elabbar, F. A facile synthesis of some novel thiazoles, arylazothiazoles, and pyrazole linked to thiazolyl coumarin as antibacterial agents. Synth. Commun. 2020, 50, 2522–2530. [Google Scholar] [CrossRef]
- Kumar, S.; Saini, V.; Maurya, I.K.; Sindhu, J.; Kumari, M.; Kataria, R.; Kumar, V. Design, synthesis, DFT, docking studies and ADME prediction of some new coumarinyl linked pyrazolylthiazoles: Potential standalone or adjuvant antimicrobial agents. PLoS ONE 2018, 13, e0196016. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.O.; Ghodsi, S. Thiazolyl-pyrazole-biscoumarin synthesis and evaluation of their antibacterial and antioxidant activities. Res. Chem. Intermed. 2017, 43, 661–678. [Google Scholar] [CrossRef]
- Nalawade, J.; Shinde, A.; Chavan, A.; Patil, S.; Suryavanshi, M.; Modak, M.; Choudhari, P.; Bobade, V.D.; Mhaske, P.C. Synthesis of new thiazolyl-pyrazolyl-1,2,3-triazole derivatives as potential antimicrobial agents. Eur. J. Med. Chem. 2019, 179, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Ouf, S.A.; Gomha, S.M.; Ewies, M.M.; Sharawy, I.A.A. Synthesis, Characterization, and Antifungal Activity Evaluation of Some Novel Arylazothiazoles. J. Heterocycl. Chem. 2018, 55, 258–264. [Google Scholar] [CrossRef]
- Ouf, S.A.; Gomha, S.M.; Eweis, M.; Ouf, A.S.; Sharawy, I.A.A.; Alharbi, S.A. Antidermatophytic activity of some newly synthesized arylhydrazonothiazoles conjugated with monoclonal antibody. Sci. Rep. 2020, 10, 20863. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Kant, V. A Review on Biological Activity of Imidazole and Thiazole Moieties and their Derivatives. Sci. Int. 2013, 1, 253–260. [Google Scholar] [CrossRef]
- Nikalje, A.P.G.; Tiwari, S.V.; Sarkate, A.P.; Karnik, K.S. Imidazole-thiazole coupled derivatives as novel lanosterol 14-α demethylase inhibitors: Ionic liquid mediated synthesis, biological evaluation and molecular docking study. Med. Chem. Res. 2018, 27, 592–606. [Google Scholar] [CrossRef]
- Dekate, S.M.; Hatzade, K.M.; Ghatole, A.M. Imidazole–Thiazole Hybrid: Synthesis, Brain Penetration, Cytochrome P450 Enzyme and Gastrointestinal Absorption Study. Iran. J. Sci. 2023, 47, 1081–1096. [Google Scholar] [CrossRef]
- Jain, A.K.; Vaidya, A.; Ravichandran, V.; Kashaw, S.K.; Agrawal, R.K. Recent developments and biological activities of thiazolidinone derivatives: A review. Bioorg. Med. Chem. 2012, 20, 3378–3395. [Google Scholar] [CrossRef]
- Othman, D.I.A.; Hamdi, A.; Abdel-Aziz, M.M.; Elfeky, S.M. Novel 2-arylthiazolidin-4-one-thiazole hybrids with potent activity against Mycobacterium tuberculosis. Bioorg. Chem. 2022, 124, 105809. [Google Scholar] [CrossRef]
- Abo-Ashour, M.F.; Eldehna, W.M.; George, R.F.; Abdel-Aziz, M.M.; Elaasser, M.M.; Abou-Seri, S.M.; Abdel Gawad, N.M. Synthesis and Biological Evaluation of 2-Aminothiazole-Thiazolidinone Conjugates as Potential Antitubercular Agents. Future Med. Chem. 2018, 10, 1405–1419. [Google Scholar] [CrossRef] [PubMed]
- Sucheta; Tahlan, S.; Verma, P.K. Biological potential of thiazolidinedione derivatives of synthetic origin. Chem. Cent. J. 2017, 11, 130. [Google Scholar] [CrossRef]
- Alegaon, S.G.; U, V.; Alagawadi, K.R.; Kumar, D.; Kavalapure, R.S.; Ranade, S.D.; A, S.P.; Jalalpure, S.S. Synthesis, Molecular Docking and ADME Studies of Thiazole-Thiazolidinedione Hybrids as Antimicrobial Agents. J. Biomol. Struct. Dyn. 2022, 40, 6211–6227. [Google Scholar] [CrossRef] [PubMed]
- Bhuva, H.; Sahu, D.; Shah, B.; Modi, C.; Patel, M.B. Biological Profile of Thiadiazole. Pharmacologyonline 2011, 1, 528–543. [Google Scholar]
- Stokes, J.M.; Yang, K.; Swanson, K.; Jin, W.; Cubillos-Ruiz, A.; Donghia, N.M.; MacNair, C.R.; French, S.; Carfrae, L.A.; Bloom-Ackerman, Z.; et al. A Deep Learning Approach to Antibiotic Discovery. Cell 2020, 180, 688–702.e13. [Google Scholar] [CrossRef]
- Booq, R.Y.; Tawfik, E.A.; Alfassam, H.A.; Alfahad, A.J.; Alyamani, E.J. Assessment of the Antibacterial Efficacy of Halicin against Pathogenic Bacteria. Antibiotics 2021, 10, 1480. [Google Scholar] [CrossRef]
- Hussain, Z.; Pengfei, S.; Yimin, L.; Shasha, L.; Zehao, L.; Yifan, Y.; Linhui, L.; Linying, Z.; Yong, W. Study on antibacterial effect of halicin (SU3327) against Enterococcus faecalis and Enterococcus faecium. Pathog. Dis. 2022, 80, ftac037. [Google Scholar] [CrossRef]
- Higashihira, S.; Simpson, S.J.; Collier, C.D.; Natoli, R.M.; Kittaka, M.; Greenfield, E.M. Halicin Is Effective Against Staphylococcus aureus Biofilms In Vitro. Clin. Orthop. Relat. Res. 2022, 480, 1476–1487. [Google Scholar] [CrossRef]
- van Gent, M.E.; van der Reijden, T.J.K.; Lennard, P.R.; de Visser, A.W.; Schonkeren-Ravensbergen, B.; Dolezal, N.; Cordfunke, R.A.; Drijfhout, J.W.; Nibbering, P.H. Synergism between the Synthetic Antibacterial and Antibiofilm Peptide (SAAP)-148 and Halicin. Antibiotics 2022, 11, 673. [Google Scholar] [CrossRef]
- Asif, M. Pharmacological activities of Triazole analogues as antibacterial, antifungal, antiviral agents. Pharm. Sci. Asia 2017, 44, 59–74. [Google Scholar] [CrossRef]
- Shinde, V.; Mahulikar, P.; Mhaske, P.C.; Chakraborty, S.; Choudhari, A.; Phalle, S.; Choudhari, P.; Sarkar, D. Synthesis and antimycobacterial evaluation of new 5-(1-benzyl-1H-1,2,3-triazol-4-yl)-4-methyl-2-arylthiazole derivatives. Med. Chem. Res. 2019, 28, 805–819. [Google Scholar] [CrossRef]
- Mahale, K.A.; Gosavi, K.S.; Gaikwad, N.D.; Bholay, A.D.; Patil, S. V Thiazole Substituted [1,2,3] Triazole: Synthesis and Antimicrobial Evaluation. Indian J. Chem. 2022, 61, 640–649. [Google Scholar] [CrossRef]
- Jagadale, S.; Chavan, A.; Shinde, A.; Sisode, V.; Bobade, V.D.; Mhaske, P.C. Synthesis and antimicrobial evaluation of new thiazolyl-1,2,3-triazolyl-alcohol derivatives. Med. Chem. Res. 2020, 29, 989–999. [Google Scholar] [CrossRef]
- Poonia, N.; Lal, K.; Kumar, A.; Kumar, A.; Sahu, S.; Baidya, A.T.K.; Kumar, R. Urea-Thiazole/Benzothiazole Hybrids with a Triazole Linker: Synthesis, Antimicrobial Potential, Pharmacokinetic Profile and in Silico Mechanistic Studies. Mol. Divers. 2022, 26, 2375–2391. [Google Scholar] [CrossRef]
- Veeranki, K.C.; Pochampally, J.; Maroju, R.C.; Thumma, V.; Boddu, L.S. 1,2,3-Triazoles containing Thiazole-Piperazine Moieties: Synthesis, Biological Assessment and Molecular Docking. Asian J. Chem. 2023, 35, 125–134. [Google Scholar] [CrossRef]
- Gondru, R.; Kanugala, S.; Raj, S.; Ganesh Kumar, C.; Pasupuleti, M.; Banothu, J.; Bavantula, R. 1,2,3-triazole-thiazole hybrids: Synthesis, in vitro antimicrobial activity and antibiofilm studies. Bioorganic Med. Chem. Lett. 2021, 33, 127746. [Google Scholar] [CrossRef]
- Glomb, T.; Świątek, P. Antimicrobial Activity of 1,3,4-Oxadiazole Derivatives. Int. J. Mol. Sci. 2021, 22, 6979. [Google Scholar] [CrossRef]
- Glomb, T.; Szymankiewicz, K.; Świątek, P. Anti-cancer activity of derivatives of 1,3,4-oxadiazole. Molecules 2018, 23, 3361. [Google Scholar] [CrossRef]
- Da Silva, M.M.; Comin, M.; Duarte, T.S.; Foglio, M.A.; De Carvalho, J.E.; Do Carmo Vieira, M.; Formagio, A.S.N. Synthesis, antiproliferative activity and molecular properties predictions of galloyl derivatives. Molecules 2015, 20, 5360–5373. [Google Scholar] [CrossRef]
- Savariz, F.C.; Formagio, A.S.N.; Barbosa, V.A.; Foglio, M.A.; de Carvalho, J.E.; Duarte, M.C.T.; Filho, B.P.D.; Sarragiotto, M.H. Synthesis, antitumor and antimicrobial activity of novel 1-substituted phenyl-3-[3-alkylamino(methyl)-2-thioxo-1,3,4-oxadiazol-5-yl] β-carboline derivatives. J. Braz. Chem. Soc. 2010, 21, 288–298. [Google Scholar] [CrossRef]
- Athar Abbasi, M.; Raza, H.; Aziz-ur-Rehman; Zahra Siddiqui, S.; Adnan Ali Shah, S.; Hassan, M.; Seo, S.Y. Synthesis of novel N-(1,3-thiazol-2-yl)benzamide clubbed oxadiazole scaffolds: Urease inhibition, Lipinski rule and molecular docking analyses. Bioorg. Chem. 2019, 83, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Bhatt, N.B.; Joshi, S.B. Synthetic modifications in ethyl 2-amino-4-methylthiazole-5-carboxylate: 3D QSAR analysis and antimicrobial study. Synth. Commun. 2019, 49, 1055–1066. [Google Scholar] [CrossRef]
- Tiperciuc, B.G. Design and Development of New Azoles Heterocycles with Biological Potential. Habilitation Thesis, “Iuliu Hațieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania, 2021. Chapter 1.6.1. pp. 45–47. [Google Scholar]
- Meanwell, N.A. Chapter Five—A Synopsis of the Properties and Applications of Heteroaromatic Rings in Medicinal Chemistry. In Advances in Heterocyclic Chemistry; Scriven, E.F.V., Ramsden, C.A., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 123, pp. 245–361. ISBN 0065-2725. [Google Scholar]
- Patil, S.A.; Ble-González, E.A.; Isbel, S.R.; Hampton, S.M.; Bugarin, A. Carbazole Derivatives as Potential Antimicrobial Agents. Molecules 2022, 27, 6575. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, M.S.; Matter, M.A.; Asker, M.S.; Rady, M.R. Production of indole alkaloids in hairy root cultures of Catharanthus roseus L. and their antimicrobial activity. South Afr. J. Bot. 2016, 105, 9–18. [Google Scholar] [CrossRef]
- Yu, H.F.; Qin, X.J.; Ding, C.F.; Wei, X.; Yang, J.; Luo, J.R.; Liu, L.; Khan, A.; Zhang, L.C.; Xia, C.F.; et al. Nepenthe-Like Indole Alkaloids with Antimicrobial Activity from Ervatamia chinensis. Org. Lett. 2018, 20, 4116–4120. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Ding, C.F.; Deng, S.Y.; Gao, W.; Tan, B.Y.; Wu, H.; Guo, Y.; Song, J.F.; Zhang, L.C.; Zhang, R.P.; et al. Monoterpene indole N-oxide alkaloids from Tabernaemontana corymbosa and their antimicrobial activity. Fitoterapia 2022, 158, 105178. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, Y.; Li, Y.; Chen, Y. A green synthesis and antibacterial activity of ferrocene-based thiazole derivatives in choline chloride/glycerol eutectic solvent. RSC Adv. 2022, 12, 22054–22059. [Google Scholar] [CrossRef]
- Ashok, D.; Gundu, S.; Aamate, V.K.; Devulapally, M.G. Microwave-assisted synthesis, antioxidant and antimicrobial evaluation of 2-indolinone-based bis-1,2,3-triazole derivatives. Mol. Divers. 2018, 22, 57–70. [Google Scholar] [CrossRef]
- Alzahrani, A.Y.; Ammar, Y.A.; Abu-Elghait, M.; Salem, M.A.; Assiri, M.A.; Ali, T.E.; Ragab, A. Development of novel indolin-2-one derivative incorporating thiazole moiety as DHFR and quorum sensing inhibitors: Synthesis, antimicrobial, and antibiofilm activities with molecular modelling study. Bioorg. Chem. 2022, 119, 105571. [Google Scholar] [CrossRef]
- Meng, C.W.; Zhao, H.Y.; Zhu, H.; Peng, C.; Zhou, Q.M.; Xiong, L. Novel Indane Derivatives with Antioxidant Activity from the Roots of Anisodus tanguticus. Molecules 2023, 28, 1493. [Google Scholar] [CrossRef] [PubMed]
- Obafemi, C.A.; Adelani, P.O.; Fadare, O.A.; Akinpelu, D.A.; Famuyiwa, S.O. Synthesis, crystal structure and in vitro antibacterial activity of 2,3a,8b-trihydroxy-3-(thiophen-2-ylcarbonyl)-2-(trifluoromethyl)-2,3,3a, 8b-tetrahydro-4H-indeno [1,2-b]furan-4-one. J. Mol. Struct. 2013, 1049, 429–435. [Google Scholar] [CrossRef]
- Adole, V.A.; More, R.A.; Jagdale, B.S.; Pawar, T.B.; Chobe, S.S. Efficient Synthesis, Antibacterial, Antifungal, Antioxidant and Cytotoxicity Study of 2-(2-Hydrazineyl)thiazole Derivatives. ChemistrySelect 2020, 5, 2778–2786. [Google Scholar] [CrossRef]
- Muluk, M.B.; Phatak, P.S.; Pawar, S.B.; Dhumal, S.T.; Rehman, N.N.M.A.; Dixit, P.P.; Choudhari, P.B.; Haval, K.P. Synthesis, antimicrobial, and antioxidant activities of new pyridyl- and thiazolyl-bearing carbohydrazides. J. Chin. Chem. Soc. 2019, 66, 1507–1517. [Google Scholar] [CrossRef]
- Muluk, M.B.; Ubale, A.S.; Dhumal, S.T.; Rehman, N.N.M.A.; Dixit, P.P.; Kharat, K.K.; Choudhari, P.B.; Haval, K.P. Synthesis, anticancer and antimicrobial evaluation of new pyridyl and thiazolyl clubbed hydrazone scaffolds. Synth. Commun. 2020, 50, 243–255. [Google Scholar] [CrossRef]
- Patil, P.S.; Kasare, S.L.; Badar, A.D.; Kulkarni, R.S.; Dixit, P.P.; Kulkarni, J.A.; Choudhari, P.B.; Haval, K.P. Synthesis, Antimicrobial Evaluation, and Molecular Docking Study of New Thiazole-5-phenylpropenone Derivatives. Russ. J. Gen. Chem. 2020, 90, 1523–1528. [Google Scholar] [CrossRef]
- Eryılmaz, S.; Türk Çelikoğlu, E.; İdil, Ö.; İnkaya, E.; Kozak, Z.; Mısır, E.; Gül, M. Derivatives of Pyridine and Thiazole Hybrid: Synthesis, DFT, Biological Evaluation via Antimicrobial and DNA Cleavage Activity. Bioorg. Chem. 2019, 95, 103476. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M.; Popa, C.V. Pyridine Compounds with Antimicrobial and Antiviral Activities. Int. J. Mol. Sci. 2022, 23, 5659. [Google Scholar] [CrossRef]
- Al-Majedy, Y.K.; Kadhum, A.A.H.; Al-Amiery, A.A.; Mohamad, A.B. Coumarins: The antimicrobial agents. Syst. Rev. Pharm. 2016, 8, 62–70. [Google Scholar] [CrossRef]
- Yusufzai, S.K.; Osman, H.; Khan, M.S.; Mohamad, S.; Sulaiman, O.; Parumasivam, T.; Gansau, J.A.; Johansah, N. Noviany Design, characterization, in vitro antibacterial, antitubercular evaluation and structure–activity relationships of new hydrazinyl thiazolyl coumarin derivatives. Med. Chem. Res. 2017, 26, 1139–1148. [Google Scholar] [CrossRef]
- Salar, U.; Qureshi, B.; Khan, K.M.; Lodhi, M.A.; Ul-Haq, Z.; Khan, F.A.; Naz, F.; Taha, M.; Perveen, S.; Hussain, S. Aryl hydrazones linked thiazolyl coumarin hybrids as potential urease inhibitors. J. Iran. Chem. Soc. 2022, 19, 1221–1238. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, C.; Pan, G.; Yu, C.; Ansari, M.F.; Yadav Bheemanaboina, R.R.; Cheng, Y.; Zhou, C.; Zhang, J. Novel chalcone-conjugated, multi-flexible end-group coumarin thiazole hybrids as potential antibacterial repressors against methicillin-resistant Staphylococcus aureus. Eur. J. Med. Chem. 2021, 222, 113628. [Google Scholar] [CrossRef] [PubMed]
- Leonte, D.; Ungureanu, D.; Zaharia, V. Flavones and Related Compounds: Synthesis and Biological Activity. Molecules 2023, 28, 6528. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Lan, D.; Qi, G. Design and development of some thiazole-based flavanoids as novel antibacterial against pathogens causing surgical site infection for possible benefit in bone trauma via inhibition of DNA gyrase. Chem. Biol. Drug Des. 2017, 90, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bawa, S.; Gupta, H. Biological Activities of Quinoline Derivatives. Mini-Rev. Med. Chem. 2010, 9, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Ammar, Y.A.; El-Hafez, S.M.A.A.; Hessein, S.A.; Ali, A.M.; Askar, A.A.; Ragab, A. One-Pot Strategy for Thiazole Tethered 7-Ethoxy Quinoline Hybrids: Synthesis and Potential Antimicrobial Agents as Dihydrofolate Reductase (DHFR) Inhibitors with Molecular Docking Study. J. Mol. Struct. 2021, 1242, 130748. [Google Scholar] [CrossRef]
- Litim, B.; Djahoudi, A.; Meliani, S.; Boukhari, A. Synthesis and potential antimicrobial activity of novel α-aminophosphonates derivatives bearing substituted quinoline or quinolone and thiazole moieties. Med. Chem. Res. 2022, 31, 60–74. [Google Scholar] [CrossRef]
- Alsibaee, A.M.; Al-Yousef, H.M.; Al-Salem, H.S. Quinazolinones, the Winning Horse in Drug Discovery. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Desai, N.; Shihory, N.; Khasiya, A.; Pandit, U.; Khedkar, V. Quinazoline clubbed thiazole and 1,3,4-oxadiazole heterocycles: Synthesis, characterization, antibacterial evaluation, and molecular docking studies. Phosphorus Sulfur Silicon Relat. Elem. 2021, 196, 569–577. [Google Scholar] [CrossRef]
- Wang, J.; Ansari, M.F.; Zhou, C.H. Identification of Unique Quinazolone Thiazoles as Novel Structural Scaffolds for Potential Gram-Negative Bacterial Conquerors. J. Med. Chem. 2021, 64, 7630–7645. [Google Scholar] [CrossRef]
- Łączkowski, K.Z.; Landowska, K.; Biernasiuk, A.; Sałat, K.; Furgała, A.; Plech, T.; Malm, A. Synthesis, biological evaluation and molecular docking studies of novel quinuclidinone derivatives as potential antimicrobial and anticonvulsant agents. Med. Chem. Res. 2017, 26, 2088–2104. [Google Scholar] [CrossRef]
- Abdel-Latif, E.; Almatari, A.S.; Abd-ElGhani, G.E. Synthesis and Antibacterial Evaluation of Some New Thiazole-Based Polyheterocyclic Ring Systems. J. Heterocycl. Chem. 2019, 56, 1978–1985. [Google Scholar] [CrossRef]
- Radman Kastelic, A.; Odžak, R.; Pezdirc, I.; Sović, K.; Hrenar, T.; Čipak Gašparović, A.; Skočibušić, M.; Primožič, I. New and Potent Quinuclidine-Based Antimicrobial Agents. Molecules 2019, 24, 2675. [Google Scholar] [CrossRef] [PubMed]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, D.; Tiperciuc, B.; Nastasă, C.; Ionuț, I.; Marc, G.; Oniga, I.; Oniga, O. An Overview of the Structure–Activity Relationship in Novel Antimicrobial Thiazoles Clubbed with Various Heterocycles (2017–2023). Pharmaceutics 2024, 16, 89. https://doi.org/10.3390/pharmaceutics16010089
Ungureanu D, Tiperciuc B, Nastasă C, Ionuț I, Marc G, Oniga I, Oniga O. An Overview of the Structure–Activity Relationship in Novel Antimicrobial Thiazoles Clubbed with Various Heterocycles (2017–2023). Pharmaceutics. 2024; 16(1):89. https://doi.org/10.3390/pharmaceutics16010089
Chicago/Turabian StyleUngureanu, Daniel, Brîndușa Tiperciuc, Cristina Nastasă, Ioana Ionuț, Gabriel Marc, Ilioara Oniga, and Ovidiu Oniga. 2024. "An Overview of the Structure–Activity Relationship in Novel Antimicrobial Thiazoles Clubbed with Various Heterocycles (2017–2023)" Pharmaceutics 16, no. 1: 89. https://doi.org/10.3390/pharmaceutics16010089






