Capped Plasmonic Gold and Silver Nanoparticles with Porphyrins for Potential Use as Anticancer Agents—A Review
Abstract
:1. Introduction
2. Plasmonic Metal Nanoparticles with Photothermal Effects
2.1. Plasmonic Gold Nanoparticles
2.1.1. Gold Nanorods
2.1.2. Gold Nanospheres
2.1.3. Gold Nanocages
2.1.4. Gold Nanostars
2.1.5. Gold Nanoshells
2.2. Plasmonic Silver Nanoparticles
2.2.1. Silver Nanospheres
2.2.2. Silver Nanotriangles
2.2.3. Silver Nanocages
3. Functionalization of Inorganic Nanoparticles
3.1. Functionalization of Silver Nanoparticles
- The enhancement of the electrons in the electric field of the incident radiation, which is one of the mechanisms that causes these oscillations;
- The existence of restoring forces brought on by the induction in the polarization of the particle and the medium around it;
- The confinement of the electrons to specific dimensions.
- The biomolecule’s attachment to the inorganic particle’s surface via ligand-mediated binding, frequently by chemisorption, for instance, thiol groups, to the core;
- Positive charges interact electrostatically with negatively charged nanoparticles to biomolecules or the other way around versa;
- Covalent bonding by conjugation chemistry, utilizing groups focused on both biomolecules and particles;
- Receptor–ligand systems are affinity-based but non-covalent.
3.2. Functionalization of Gold Nanoparticles
3.3. The Use of Polymers for Biofunctionality
The Effect of Surface Modification of the Plasmonic Nanoparticles
4. Application of Plasmonic Nanoparticles
Toxicity and Biodegradability of Metal Nanoparticles
5. Progress in Photodynamic Therapy
5.1. Type I and Type II Photodynamic Therapy
5.2. Hypoxia Targeting for Cancer Treatment
5.3. Combination of Methods, PDT, PTT, and Magnetic Hyperthermia (MH)
6. The Use of Plasmonic–Magnetic Nanohybrids
6.1. The Decoration/Capping of Gold and Silver Nanoparticles with Porphyrins
| Porphyrin Derivative | Metal | Surface Modification of the NP | Potential Application | Effects of the Dual Theranostic Tool |
|---|---|---|---|---|
| 5,10,15-p(ῳ-methoxypolyethyleneoxyphenyl)-20-p(hydroxyphenyl) porphyrin [326] | Ag | Poly(ethyleneglycol)methyl ether | Breast cancer |
|
| 5,10,15,20-tetrakis(3-hydroxyphenyl)porphyrin [327] | Zn-Cu-In-S/ZnS QDs | No surface modification | Skin cancer |
|
| meso-tetra-(4-sulfonatophenyl) porphyrin [328] | Ternary copper indium sulfide/zinc sulfide (CuInS2/Zn) quantum dots | Polyethylene glycol | Prostate cancer |
|
| meso-tetrakis(4-hydroxyphenyl)porphyrin [329] | Superparamagnetic iron oxide NPs and Au | Polyethene glycol | Breast cancer |
|
| Superparamagnetic iron oxide NPs and Au | Polyethene glycol | Breast cancer |
| |
| Glutathione | Kidney cancer |
| ||
| 5,10,15,20-tetrakis(3-hydroxyphenyl)porphyrin [327] | Zn-Cu-In-S/ZnS QDs |
6.2. Theranostic Applications of Modified Nanoparticles
6.3. The Influence of Nanoparticles and Lipoproteins on Porphyrin Properties
7. The Effect of the Structural Properties of the Metal Nanoparticles (Size, Shape, and Surface) on Their Biological Activity
7.1. The Morphology of the Nanoparticles
7.2. The Size of Nanoparticles
7.3. The Effect of the Surface Charge of the Nanoparticles on Biological Activity
7.4. Coating of Metal Nanoparticles for Application in Biological Use
8. The Cancer Types That Are Responsive to Plasmonic Metal Nanoparticles
9. Combination Therapy
9.1. Chemotherapy and Photodynamic Therapy
9.2. Photodynamic with Sonodynamic Therapy
9.3. Photodynamic with Immunotherapy and Radiotherapy
9.4. Photodynamic and Photothermal Therapy
10. Photothermal and Photodynamic Therapy Resistance
11. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Tee, S.Y.; Ye, E. Recent advancements in coinage metal nanostructures and bio-applications. Mater. Adv. 2021, 2, 1507–1529. [Google Scholar] [CrossRef]
- Yan, J.; Teo, B.K.; Zheng, N. Surface chemistry of atomically precise coinage metal nanoclusters: From structural control to surface reactivity and catalysis. Accounts Chem. Res. 2018, 51, 3084–3093. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.K. Introduction: Gold Chemistry. ACS Publ. 2021, 121, 8309–8310. [Google Scholar] [CrossRef]
- Mauro, N.; Utzeri, M.A.; Varvar, P.; Cavallaro, G. Functionalization of metal and carbon nanoparticles with potential in cancer theranostics. Molecules 2021, 26, 3085. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Guan, J.; Hu, J.; Bourgeois, M.R.; Odom, T.W. Manipulating light matter interactions in plasmonic nanoparticle lattices. Accounts Chem. Res. 2019, 52, 2997–3007. [Google Scholar] [CrossRef]
- Davis, T.J.; Gmez, D.E.; Roberts, A. Plasmonic circuits for manipulating optical information. Nanophotonics 2016, 6, 543–559. [Google Scholar] [CrossRef]
- Rnavri, A.; Igaz, N.R.; Adamecz, D.I.; Szerencss, B.; Molnar, C.; Knya, Z.; Pfeiffer, I.; Kiricsi, M. Green silver and gold nanoparticles: Biological synthesis approaches and potentials for biomedical applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef]
- Vu, X.H.; Dien, N.D.; Pham, T.T.H.; Van Truong, N.; Ca, N.X.; Van Thu, V. Tunable LSPR of silver/gold bimetallic nanoframes and their SERS activity for methyl red detection. RSC Adv. 2021, 11, 14596–14606. [Google Scholar] [CrossRef]
- Reguera, J.; Langer, J.; de Aberasturi, D.J.N.; Liz-Marzn, L.M. Anisotropic metal nanoparticles for surface-enhanced Raman Scattering. Chem. Soc. Rev. 2017, 46, 3866–3885. [Google Scholar] [CrossRef]
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.L.; Boujday, S. Silver-based plasmonic nanoparticles for and their use in biosensing. Biosensors 2019, 9, 78. [Google Scholar] [CrossRef]
- Pormohammad, A.; Turner, R.J. Silver antibacterial synergism activities with eight other metal (loid)-based antimicrobials against Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Antibiotics 2020, 9, 853. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver nanoparticles and silver ions as potential antibacterial agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Arkas, M.; Kithreoti, G.; Boukos, N.; Kitsou, I.; Petrakli, F.; Panagiotaki, K. Two completely different biomimetic reactions mediated by the same matrix producing inorganic/organic/inorganic hybrid nanoparticles. Nano-Struct. Nano-Obj. 2018, 14, 138–148. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Metal oxide nanoparticles as biomedical materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef]
- Gao, D.; Guo, X.; Zhang, X.; Chen, S.; Wang, Y.; Chen, T.; Huang, G.; Gao, Y.; Tian, Z.; Yang, Z. Multifunctional phototheranostic nanomedicine for cancer imaging and treatment. Mater. Today Bio 2020, 5, 100035. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Y.; Yuan, H.; Fang, Q.; Cai, N.; Suo, C.; Jin, L.; Zhang, T.; Chen, X. The trends in incidence of primary liver cancer caused by specific etiologies: Results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J. Hepatol. 2019, 70, 674–683. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cao, W.; Qin, K.; Li, F.; Chen, W. Comparative study of cancer profiles between 2020 and 2022 using global cancer statistics (GLOBOCAN). J. Natl. Cancer Inst. 2024, 4, 128–134. [Google Scholar] [CrossRef]
- Monticciolo, D.L.; Newell, M.S.; Moy, L.; Niell, B.; Monsees, B.; Sickles, E.A. Breast Cancer Screening in Women at Higher-Than-Average Risk: Recommendations From the ACR. J. Am. Coll. Radiol. 2018, 15, 408–414. [Google Scholar] [CrossRef]
- Ben-Aharon, I.; van Laarhoven, H.W.M.; Fontana, E.; Obermannova, R.; Nilsson, M.; Lordick, F. Early-Onset Cancer in the Gastrointestinal Tract Is on the Rise—Evidence and Implications. Cancer Discov. 2023, 13, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Lee, S.-E.; Kim, D.-H.; Pyo, Y.-C.; Park, J.-S. Recent advances of nanotechnology for the delivery of anticancer drugs for breast cancer treatment. J. Pharm. Investig. 2020, 50, 261–270. [Google Scholar] [CrossRef]
- Mahmoudi, M. The need for robust characterization of nanomaterials for nanomedicine applications. Nat. Commun. 2021, 12, 5246. [Google Scholar] [CrossRef] [PubMed]
- Padil, V.V.; Waclawek, S.; Cernik, M.; Varma, R.S. Tree gum-based renewable materials: Sustainable applications in nanotechnology, biomedical and environmental fields. Biotechnol. Adv. 2018, 36, 1984–2016. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Xie, A.; Zhou, J.; Liu, R.; Wang, L.; Liu, H.; Kong, N.; Tao, W. Minimally invasive nanomedicine: Nanotechnology in photo-/ultrasound-/radiation-/magnetism-mediated therapy and imaging. Chem. Soc. Rev. 2022, 51, 4996–5041. [Google Scholar] [CrossRef]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug. Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Kumar, R.; Aadil, K.R.; Ranjan, S.; Kumar, V.B. Advances in nanotechnology and nanomaterials based strategies for neural tissue engineering. J. Drug Deliv. Sci. Technol. 2020, 57, 101617. [Google Scholar] [CrossRef]
- Kumar, H.; Kuca, K.; Bhatia, S.K.; Saini, K.; Kaushal, A.; Verma, R.; Bhalla, T.C.; Kumar, D. Applications of nanotechnology in sensor-based detection of foodborne pathogens. Sensors 2020, 20, 1966. [Google Scholar] [CrossRef]
- Yang, Z.; Shen, B.; Yue, L.; Miao, Y.; Hu, Y.; Ouyang, R. Application of Nanomaterials to Enhance Polymerase Chain Reaction. Molecules 2022, 27, 8854. [Google Scholar] [CrossRef]
- Pillai, G. Nanotechnology toward treating cancer: A comprehensive review. Appl. Target. Nano Drugs Deliv. Sys. 2019, 9, 221–256. [Google Scholar]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef] [PubMed]
- Baez, D.F.; Gallardo-Toledo, E.; Oyarzn, M.P.; Araya, E.; Kogan, M.J. The influence of size and chemical composition of silver and gold nanoparticles on in vivo toxicity with potential applications to central nervous system diseases. Int. J. Nanomed. 2021, 16, 2187–2201. [Google Scholar] [CrossRef]
- Gerasimov, E. Synthesis of Nanocomposites and Catalysis Applications. Nanomaterials 2022, 12, 731. [Google Scholar] [CrossRef]
- Dykman, L.A.; Khlebtsov, N.G. Methods for chemical synthesis of colloidal gold. Russ. Chem. Rev. 2019, 88, 229. [Google Scholar] [CrossRef]
- Bhattarai, B.; Zaker, Y.; Bigioni, T.P. Green synthesis of gold and silver nanoparticles: Challenges and opportunities. Curr. Opin. Green Sust. Chem. 2018, 12, 91–100. [Google Scholar] [CrossRef]
- Sajid, M.; Pإéotka-Wasylka, J. Nanoparticles: Synthesis, characteristics, and applications in analytical and other sciences. Microchem. J. 2020, 154, 104623. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Univ.-Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Rao, X.; Tatoulian, M.L.; Guyon, C.; Ognier, S.; Chu, C.; Abou Hassan, A. A comparison study of functional groups (amine vs. Thiol) for immobilizing aunps on zeolite surface. Nanomaterials 2019, 9, 1034. [Google Scholar] [CrossRef]
- Wang, G.; Qian, K.; Mei, X. A theranostic nanoplatform: Magneto-gold@ fluorescence polymer nanoparticles for tumor targeting T 1 & T 2-MRI/CT/NIR fluorescence imaging and induction of genuine autophagy mediated chemotherapy. Nanoscale 2018, 10, 10467–10478. [Google Scholar]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold nanoparticles in cancer treatment. Mol. Pharm. 2018, 16, 1–23. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.-W. State of the art biocompatible gold nanoparticles for cancer theragnosis. Pharmaceutics 2020, 12, 701. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y. Advances in nanomaterial-mediated photothermal cancer therapies: Toward clinical applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hong, J.; Ding, Y. Biological behavior regulation of gold nanoparticles via the protein corona. Adv. Healthc. Mater. 2020, 9, 1901448. [Google Scholar] [CrossRef]
- Quach, Q.H.; Kong, R.L.X.; Kah, J.C.Y. Complement activation by PEGylated gold nanoparticles. Bioconjugate Chem. 2018, 29, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, X.; Li, J.; Nie, Y.; Liao, G.; Yu, Y.; Li, C. Overcoming the reticuloendothelial system barrier to drug delivery with a don-eat-us strategy. ACS Nano 2019, 13, 13015–13026. [Google Scholar] [CrossRef] [PubMed]
- Gmez-Vallejo, V.; Puigivila, M.A.; Plaza-García, S.; Szczupak, B.; Piol, R.; Murillo, J.L.; Sorribas, V.; Lou, G.; Veintemillas, S.; Ramos-Cabrer, P. PEG-copolymer-coated iron oxide nanoparticles that avoid the reticuloendothelial system and act as kidney MRI contrast agents. Nanoscale 2018, 10, 14153–14164. [Google Scholar] [CrossRef] [PubMed]
- Jan, H.; Zaman, G.; Usman, H.; Ansir, R.; Drouet, S.; Gigliolo-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Biogenically proficient synthesis and characterization of silver nanoparticles (Ag-NPs) employing aqueous extract of Aquilegia pubiflora along with their in vitro antimicrobial, anti-cancer and other biological applications. J. Mater. Res. Technol. 2021, 15, 950–968. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.-H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Gounden, S.; Daniels, A.; Singh, M. Chitosan-modified silver nanoparticles enhance cisplatin activity in breast cancer cells. Biointerf. Res. Appl. Chem. 2021, 11, 10572–10584. [Google Scholar]
- Ebrahimzadeh, M.A.; Tafazoli, A.; Akhtari, J.; Biparva, P.; Eslami, S. Engineered silver nanoparticles, a new nanoweapon against cancer. Anti-Cancer Agents Med. Chem. 2018, 18, 1962–1969. [Google Scholar] [CrossRef]
- Babu, B.; Soy, R.C.; Mack, J.; Nyokong, T. Non-aggregated lipophilic water-soluble tin porphyrins as photosensitizers for photodynamic therapy and photodynamic antimicrobial chemotherapy. New J. Chem. 2020, 44, 11006–11012. [Google Scholar] [CrossRef]
- Shabangu, S.M.; Babu, B.; Soy, R.C.; Managa, M.; Sekhosana, K.E.; Nyokong, T. Photodynamic antimicrobial chemotherapy of asymmetric porphyrin-silver conjugates towards photoinactivation of Staphylococcus aureus. J. Coord. Chem. 2020, 73, 593–608. [Google Scholar] [CrossRef]
- Caruso, E.; Cerbara, M.; Malacarne, M.C.; Marras, E.; Monti, E.; Gariboldi, M.B. Synthesis and photodynamic activity of novel non-symmetrical diaryl porphyrins against cancer cell lines. Photochem. Photobiol. B Biol. 2019, 195, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Makola, L.C.; Managa, M.; Nyokong, T. Enhancement of photodynamic antimicrobialtherapy through the use of cationic indium porphyrin conjugated to Ag/CuFe2O4 nanoparticles. Photodiag. Photodyn. Ther. 2020, 30, 101736. [Google Scholar] [CrossRef]
- Kirar, S.; Thakur, N.S.; Laha, J.K.; Banerjee, U.C. Porphyrin functionalized gelatin nanoparticle-based biodegradable phototheranostics: Potential tools for antimicrobial photodynamic therapy. ACS Appl. Biol. Mat. 2019, 2, 4202–4212. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kdzierska, E.; Knap-Czop, K.; Kotliska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Hlapisi, N.; Motaung, T.E.; Linganiso, L.Z.; Oluwafemi, O.S.; Songca, S.P. Encapsulation of gold nanorods with porphyrins for the potential treatment of cancer and bacterial diseases: A critical review. Bioinorg. Chem. Appl. 2019, 2019, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Grumezescu, A.M. Photodynamic therapy an up-to-date review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic therapy current limitations and novel approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Youf, R.L.; Miller, M.; Balasini, A.; Thiot, F.; Miller, M.; Hascot, A.; Jonas, U.; Schnherr, H.; Lemercier, G.; Montier, T. Antimicrobial photodynamic therapy: Latest developments with a focus on combinatory strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Huang, T.; He, X.; Zhang, K.; Kang, E.-T.; Xu, L. Cationic porphyrin-based nanoparticles for photodynamic inactivation and identification of bacteria strains. Biomater. Sci. 2022, 10, 3006–3016. [Google Scholar] [CrossRef] [PubMed]
- Shitomi, K.; Miyaji, H.; Kawsaki, H. Antibacterial/Photosensitizing Action of Thiolate-protected Metal Nanoclusters and Their Application to Antimicrobial Photodynamic Therapy. Accounts Mater. Surf. Res. 2020, 5, 68–79. [Google Scholar]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic therapy for the treatment and diagnosis of cancer a review of the current clinical status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Photodynamic therapy: A brief history. J. Clin. Med. 2019, 8, 1581. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.M.F.; de Annunzio, S.R.; Victorelli, F.D.; Frade, M.L.; Ferreira, P.S.; Chorilli, M.; Fontana, C.R. Chitosan-based drug delivery systems for optimization of photodynamic therapy: A review. Aaps Pharmscitech 2019, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-C.; Lee, H.-L.; Chiou, J.-F.; Lo, L.-W. Recent advances in gold nanomaterials for photothermal therapy. J. Nanotheranostics 2022, 3, 117–131. [Google Scholar] [CrossRef]
- Ren, Y.; Yan, Y.; Qi, H. Photothermal conversion and transfer in photothermal therapy: From macroscale to nanoscale. Adv. Colloid Interface Sci. 2022, 308, 102753. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Bukhamsin, R.; AlSaihati, H.; Alshamrani, S.A.; AlSihati, J.; Al-Afghani, H.M.; Alsubki, R.A.; Abuzaid, A.A.; Al-Abdulhadi, S.; Aldawood, Y. Recent Trends and Developments in Multifunctional Nanoparticles for Cancer Theranostics. Molecules 2022, 27, 8659. [Google Scholar] [CrossRef]
- Jung, U.; Ryu, J.; Choi, H. Optical Light Sources and Wavelengths within the Visible and Near-Infrared Range Using Photoacoustic Effects for Biomedical Applications. Biosensors 2022, 12, 1154. [Google Scholar] [CrossRef]
- Frakowiak, D.; Planner, A.; Wiktorowicz, K. Near-Infrared Applications in Biotechnology; CRC Press: Boca Raton, FL, USA, 2020; pp. 151–183. [Google Scholar]
- Marek, M.R.; Pham, T.-N.; Wang, J.; Cai, Q.; Yap, G.P.; Day, E.S.; Rosenthal, J. Isocorrole-Loaded Polymer Nanoparticles for Photothermal Therapy under 980 nm Light Excitation. ACS Omega 2022, 7, 36653–36662. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.H.; Nam, J.M. Plasmonic photothermal nanoparticles for biomedical applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef]
- Gadeval, A.; Chaudhari, S.; Bollampally, S.P.; Polaka, S.; Kalyane, D.; Sengupta, P.; Kalia, K.; Tekade, R.K. Integrated nanomaterials for non-invasive photothermal therapy of rheumatoid arthritis. Drug Discov. Today 2021, 26, 2315–2328. [Google Scholar] [CrossRef]
- Zhu, J.; Meng, L.-N.; Weng, G.-J.; Li, J.-J.; Zhao, J.-W. Tuning quadruple surface plasmon resonance in gold nanoellipsoid with platinum coating: From ultraviolet to near infrared. Appl. Phys. A 2021, 127, 1–10. [Google Scholar] [CrossRef]
- De Marchi, S.; Nez-Snchez, S.; Bodeln, G.; Prez-Juste, J.; Pastoriza-Santos, I. Pd nanoparticles as a plasmonic material: Synthesis, optical properties and applications. Nanoscale 2020, 12, 23424–23443. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, H.; Shan, X.; Di, Y.; Zhao, A.; Hu, Y.; Gan, Z. Solar steam generation based on the photothermal effect: From designs to applications, and beyond. J. Mater. Chem. 2019, 7, 19203–19227. [Google Scholar] [CrossRef]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold nanoparticle based photothermal therapy: Development and application for effective cancer treatment. Sust. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Guo, Z.; Xiao, Y.; Zhang, Y.; Sun, X.; Zhe, T.; Cao, Y.; Wang, L.; Lu, Q. Silver nanoparticle-embedded hydrogel as a photothermal platform for combating bacterial infections. Chem. Eng. J. 2020, 382, 122990. [Google Scholar] [CrossRef]
- Gherasim, O.; Puiu, R.A.; Bîrcă, A.C.; Burdușel, A.C.; Grumezescu, A.M. An updated review on silver nanoparticles in biomedicine. Nanomaterials 2020, 10, 2318. [Google Scholar] [CrossRef]
- Delille, F.; Pu, Y.; Lequeux, N.; Pons, T. Designing the surface chemistry of inorganic nanocrystals for cancer imaging and therapy. Cancers 2022, 14, 2456. [Google Scholar] [CrossRef] [PubMed]
- Londhe, S.; Haque, S.; Patra, C.R. Silver and gold nanoparticles: Potential cancer theranostic applications, recent development, challenges, and future perspectives. In Gold and Silver Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2023; pp. 247–290. [Google Scholar]
- Reznickova, A.; Slavikova, N.; Kolska, Z.; Kolarova, K.; Belinova, T.; Kalbacova, M.H.; Cieslar, M.; Svorcik, V. PEGylated gold nanoparticles: Stability, cytotoxicity and antibacterial activity. Coll. Surf. A Physicochem. Eng. Asp. 2019, 560, 26–34. [Google Scholar] [CrossRef]
- Aioub, M.; Austin, L.A.; El-Sayed, M.A. Gold nanoparticles for cancer diagnostics, spectroscopic imaging, drug delivery, and plasmonic photothermal therapy. In Inorganic Frameworks as Smart Nanomedicines; Elsevier: Amsterdam, The Netherlands, 2018; pp. 41–91. [Google Scholar]
- Su, S.; Kang, P.M. Systemic review of biodegradable nanomaterials in nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Bhagyaraj, S.M.; Oluwafemi, O.S. Nanotechnology: The science of the invisible. In Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–18. [Google Scholar]
- Pasparakis, G. Recent developments in the use of gold and silver nanoparticles in biomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotech. 2022, 14, e1817. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Amatya, R.; Hwang, S.; Lee, S.; Min, K.A.; Shin, M.C. BSA-silver nanoparticles: A potential multimodal therapeutics for conventional and photothermal treatment of skin cancer. Pharmaceutics 2021, 13, 575. [Google Scholar] [CrossRef]
- Norouzi, H.; Khoshgard, K.; Akbarzadeh, F. In vitro outlook of gold nanoparticles in photo-thermal therapy: A literature review. Lasers Med. Sci. 2018, 33, 917–926. [Google Scholar] [CrossRef]
- Mbaz, G.I.M.; Lebepe, T.C.; Maluleke, R.; Oluwafemi, O.S. The influence of the reducing agent used during the synthesis of gelatin-coated gold nanorods on its thermal properties for photothermal application. Inorg. Chem. Commun. 2024, 160, 111940. [Google Scholar] [CrossRef]
- Amina, S.J.; Guo, B. A review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle. Intern. J. Nanomed. 2020, 15, 9823–9857. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J.C. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Wang, X.; Geng, Z.; Cong, H.; Shen, Y.; Yu, B. Organic semiconductors for photothermal therapy and photoacoustic imaging. Chem. Biol. Chem. 2019, 20, 1628–1636. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Zhang, Z.; Ji, L.; Zhang, J.; Wang, Q.; Guo, T.; Ni, S.; Cai, R.; Mu, X. Recent progress on NIR-II photothermal therapy. Front. Chem. 2021, 9, 728066. [Google Scholar] [CrossRef]
- Wang, H.; Mu, Q.; Wang, K.; Revia, R.A.; Yen, C.; Gu, X.; Tian, B.; Liu, J.; Zhang, M. Nitrogen and boron dual-doped graphene quantum dots for near-infrared second window imaging and photothermal therapy. Appl. Mater. Today 2019, 14, 108–117. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Q.; Liu, Y.; Ma, T.; Su, L.; Liu, S.; Shi, X.; Han, D.; Liang, F. Decorating gold nanostars with multiwalled carbon nanotubes for photothermal therapy. R. Soc. Open Sci. 2018, 5, 180159. [Google Scholar] [CrossRef]
- Lee, H.-E.; Ahn, H.-Y.; Mun, J.; Lee, Y.Y.; Kim, M.; Cho, N.H.; Chang, K.; Kim, W.S.; Rho, J.; Nam, K.T. Amino-acid-and peptide-directed synthesis of chiral plasmonic gold nanoparticles. Nature 2018, 556, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Li, F.; Guo, X.; Chen, W.; Dong, C.; Zhang, J.; Zhang, J.; Wang, L. Gold nanostars for cancer cell-targeted SERS-imaging and NIR light-triggered plasmonic photothermal therapy (PPTT) in the first and second biological windows. J. Mater. Chem. B 2019, 7, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Zhou, Z.; Chen, Z.; Tan, H. Optical diagnostic based on functionalized gold nanoparticles. Int. J. Mol. Sci. 2019, 20, 4346. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Li, J.; Pu, K. Second near-infrared absorbing agents for photoacoustic imaging and photothermal therapy. Small Meth. 2019, 3, 1900553. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Song, Z.; Feng, Y.; Chen, Y.; Zhang, D.; Feng, L. The basic properties of gold nanoparticles and their applications in tumor diagnosis and treatment. Int. J. Mol. Sci. 2020, 21, 2480. [Google Scholar] [CrossRef]
- Kharlamov, A.N.; Zubarev, I.V.; Shishkina, E.V.; Shur, V.Y. Nanoparticles for treatment of atherosclerosis: Challenges of plasmonic photothermal therapy in translational studies. Future Cardiol. 2018, 14, 109–114. [Google Scholar] [CrossRef]
- Bridonneau, N.; Noel, V.; Zrig, S.; Carn, F. Self-Assembly of Gold Nanoparticles with Oppositely Charged, Long, Linear Chains of Periodic Copolymers. J. Phys. Chem. 2020, 124, 900–908. [Google Scholar] [CrossRef]
- Bila, G.; Rabets, A.; Bilyy, R. Nano- and Microparticles and Their Role in Inflammation and Immune Response: Focus on Neutrophil Extracellular Traps. In Biomedical Nanomaterials; Stoika, R.S., Ed.; Springer: Cham, Switzerland, 2023; Volume 24, pp. 149–170. [Google Scholar]
- Ali, M.R.; Wu, Y.; El-Sayed, M.A. Gold-nanoparticle-assisted plasmonic photothermal therapy advances toward clinical application. J. Phys. Chem. C 2019, 123, 15375–15393. [Google Scholar] [CrossRef]
- De Souza, C.D.; Nogueira, B.R.; Rostelato, M.E.C. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Comp. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A review on surface-enhanced Raman scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wang, Y.; Lin, J.; Tian, Q.; Xie, Y.; Hu, J.; Yang, S. Macrophages-mediated delivery of small gold nanorods for tumor hypoxia photoacoustic imaging and enhanced photothermal therapy. ACS Appl. Mater. Interf. 2019, 11, 15251–15261. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, X.; Chu, C.; Dong, Y.; Zhang, T.; Li, X.; Liu, G.; Cai, W.; Han, S. Preparation, toxicity reduction and radiation therapy application of gold nanorods. J. Nanobiotechnol. 2021, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xiang, Y.; Pan, W.; Wang, H.; Li, N.; Tang, B. Dual-targeted photothermal agents for enhanced cancer therapy. Chem. Sci. 2020, 11, 8055–8072. [Google Scholar] [CrossRef] [PubMed]
- McKernan, P.; Virani, N.A.; Faria, G.N.; Karch, C.G.; Prada Silvy, R.; Resasco, D.E.; Thompson, L.F.; Harrison, R.G. Targeted single-walled carbon nanotubes for photothermal therapy combined with immune checkpoint inhibition for the treatment of metastatic breast cancer. Nanoscale Res. Lett. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Scarano, S.; Pascale, E.; Palladino, P.; Fratini, E.; Minunni, M. Determination of fermentable sugars in beer wort by gold nanoparticles@ polydopamine: A layer-by-layer approach for localized surface plasmon resonance measurements at fixed wavelength. Talanta 2018, 183, 24–32. [Google Scholar] [CrossRef]
- Olesiak-Banska, J.; Waszkielewicz, M.; Obstarczyk, P.; Samoc, M. Two-photon absorption and photoluminescence of colloidal gold nanoparticles and nanoclusters. Chem. Soc. Rev. 2019, 48, 4087–4117. [Google Scholar] [CrossRef]
- Upputuri, P.K.; Pramanik, M. Photoacoustic imaging in the second near-infrared window: A review. J. Biomed. Opt. 2019, 24, 040901. [Google Scholar] [CrossRef]
- Xie, Z.; Fan, T.; An, J.; Choi, W.; Duo, Y.; Ge, Y.; Zhang, B.; Nie, G.; Xie, N.; Zheng, T. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020, 49, 8065–8087. [Google Scholar] [CrossRef]
- Lindley, S.A.; Cooper, J.K.; Rojas-Andrade, M.D.; Fung, V.; Leahy, C.J.; Chen, S.; Zhang, J.Z. Highly tunable hollow gold nanospheres: Gaining size control and uniform galvanic exchange of sacrificial cobalt boride scaffolds. ACS Appl. Mater. Interf. 2018, 10, 12992–13001. [Google Scholar] [CrossRef]
- Silva, F.; Cabral Campello, M.P.; Paulo, A. Radiolabeled gold nanoparticles for imaging and therapy of cancer. Materials 2020, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Son, Y.J.; Yoo, H.S. Gold nanospheres and nanorods for anti-cancer therapy: Comparative studies of fabrication, surface-decoration, and anti-cancer treatments. Nanoscale 2020, 12, 14996–15020. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.F.; Tan, S.F. Real-Time Imaging of Chemical Reactions Between Silver and Gold Nanoparticles; Springer Theses; Springer: Singapore, 2018; Volume 2024, pp. 83–95. [Google Scholar]
- Repenko, T.; Rix, A.; Nedilko, A.; Rose, J.; Hermann, A.; Vinokur, R.; Moli, S.; Caon, R.; Mayer, M.; von Plessen, G. Strong photoacoustic signal enhancement by coating gold nanoparticles with melanin for biomedical imaging. Adv. Funct. Mater. 2018, 28, 1705607. [Google Scholar] [CrossRef]
- Alimardani, V.; Farahavar, G.; Salehi, S.; Taghizadeh, S.; Ghiasi, M.R.; Abolmaali, S.S. Gold nanocages in cancer diagnosis, therapy, and theranostics: A brief review. Front. Mater. Sci. 2021, 15, 494–511. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Chatterjee, H.; Rahman, D.S.; Sengupta, M.; Ghosh, S.K. Gold nanostars in plasmonic photothermal therapy: The role of tip heads in the thermoplasmonic landscape. J. Phys. Chem. 2018, 122, 13082–13094. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.W.; Gholami, A. Gold nanostars-diagnosis, bioimaging and biomedical applications. Drug Metab. Rev. 2020, 52, 299–318. [Google Scholar] [CrossRef]
- Lee, J.W.; Jung, H.; Cho, H.H.; Lee, J.H.; Nam, Y. Gold nanostar-mediated neural activity control using plasmonic photothermal effects. Biomaterials 2018, 153, 59–69. [Google Scholar] [CrossRef]
- Arami, H.; Kananian, S.; Khalifehzadeh, L.; Patel, C.B.; Chang, E.; Tanabe, Y.; Zeng, Y.; Madsen, S.J.; Mandella, M.J.; Natarajan, A. Remotely controlled near-infrared-triggered photothermal treatment of brain tumours in freely behaving mice using gold nanostars. Nat. Nanotech. 2022, 17, 1015–1022. [Google Scholar] [CrossRef]
- Pu, Y.; Zhao, Y.; Zheng, P.; Li, M. Elucidating the growth mechanism of plasmonic gold nanostars with tunable optical and photothermal properties. Inorg. Chem. 2018, 57, 8599–8607. [Google Scholar] [CrossRef]
- Sui, J.; Liu, G.; Song, Y.; Li, D.; Dong, X.; Wang, J.; Yu, W. Integrating photoluminescence, magnetism and thermal conversion for potential photothermal therapy and dual-modal bioimaging. J. Colloid Interface Sci. 2018, 510, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Silva, A.K.; Snchez Iglesias, A.; Grzelczak, M.; Pechoux, C.; Desboeufs, K.; Li Marzn, L.M.; Wilhelm, C. Cancer cell internalization of gold nanostars impacts their photothermal efficiency in vitro and in vivo: Toward a plasmonic thermal fingerprint in tumoral environment. Adv. Heal. Mater. 2016, 5, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M. Green synthesis of gold nanoparticles using plant products and plants extracts aiming for cancer therapy: Helping the beauty to beat ‘cure’ the beast. Artif. Cells Nanomed. Biotechnol. 2022, 50, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Malviya, R. Understanding and advancement in gold nanoparticle targeted photothermal therapy of cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1875, 188532. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Jocelyn Dang, H.; Simmonds, S.P.; Bahrami, K.; Wyse, J.M.; Dahlhauser, S.D.; Reuther, J.F.; VandeWalle, A.N.; Anslyn, E.V.; Peppas, N.A. Electrostatic and covalent assemblies of Anionic Hydrogel-Coated Gold Nanoshells for detection of Dry Eye biomarkers in human tears. Nano Lett. 2021, 21, 8734–8740. [Google Scholar] [CrossRef]
- Fereshteh, Z.; Dang, M.; Wenck, C.; Day, E.; Slater, J. E-Selectin Targeted Gold Nanoshells to Inhibit Breast Cancer Cell Binding to Lung Endothelial Cells. ACS Appl. Nano Mater. 2023, 6, 1315–1324. [Google Scholar] [CrossRef]
- Wang, Y.C.; Théberge-Julien, G.; Tardif, J.C.; Rhéaume, É.; Lesage, F.; Kakkar, A. Multifaceted ligand design facilitates chemical-or peptide-mediated linking of hollow gold nanoshells with tuned interparticle distance, interference and cytotoxicities. Mater. Adv. 2022, 3, 7272–7284. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Shieh, M.-J. Platinum (II) drug-loaded gold nanoshells for chemo-photothermal therapy in colorectal cancer. ACS Appl. Mater. Interf. 2020, 12, 4254–4264. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, X.; Chen, L.; Gong, X.; Yang, H.; Duan, X.; Zhu, Y. Multifunctional gold nanoparticles in cancer diagnosis and treatment. Int. J. Nanomed. 2022, 17, 2041–2067. [Google Scholar] [CrossRef]
- Fajstavr, D.; Karasová, A.; Michalcová, A.; Ulbrich, P.; Slepičková Kasálková, N.; Siegel, J.; Švorčík, V.; Slepička, P. PEGylated Gold Nanoparticles Grafted with N-Acetyl-L-Cysteine for Polymer Modification. Nanomaterials 2021, 11, 1434. [Google Scholar] [CrossRef]
- Wang, J.; Potocny, A.M.; Rosenthal, J.; Day, E.S. Gold nanoshell-linear tetrapyrrole conjugates for near infrared-activated dual photodynamic and photothermal therapies. ACS Omega 2019, 5, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.R.; Sampaio, I.; Zucolotto, V. Exploring silver nanoparticles for cancer therapy and diagnosis. Coll. Surf. B Biointerf. 2022, 210, 112254. [Google Scholar] [CrossRef]
- Kumar, S.S.D.; Rajendran, N.K.; Houreld, N.N.; Abrahamse, H. Recent advances on silver nanoparticle and biopolymer-based biomaterials for wound healing applications. Int. J. Biol. Macromol. 2018, 115, 165–175. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Jin, M.; Yuan, Z.; Bond, P.; Guo, J. Both silver ions and silver nanoparticles facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes. Water Res. 2020, 169, 115229. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.S.; Shim, Y.Y.; Reaney, M.J.; Cho, J.Y. Green chemistry synthesis of silver nanoparticles and their potential anticancer effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef]
- Hao, N.V.; Tung, D.H.; Thao, T.T.; Hoa, V.X.; Thoan, N.H.; Minh, P.N.; Fal, J.; Żyła, G.; Trinh, P.V. High thermal conductivity of green nanofluid containing Ag nanoparticles prepared by using solution plasma process with Paramignya trimera extract. J. Therm. Anal. Calorim. 2023, 148, 1–12. [Google Scholar] [CrossRef]
- Vishwanath, R.; Negi, B. Conventional and green methods of synthesis of silver nanoparticles and their antimicrobial properties. CRGSC 2021, 4, 100205. [Google Scholar] [CrossRef]
- Jin, J.-C.; Wang, B.-B.; Xu, Z.-Q.; He, X.-H.; Zou, H.-F.; Yang, Q.-Q.; Jiang, F.-L.; Liu, Y. A novel method for the detection of silver ions with carbon dots: Excellent selectivity, fast response, low detection limit and good applicability. Sens. Actuators B Chem. 2018, 267, 627–635. [Google Scholar] [CrossRef]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef]
- Mohsen, E.; El-Borady, O.M.; Mohamed, M.B.; Fahim, I.S. Synthesis and characterization of ciprofloxacin loaded silver nanoparticles and investigation of their antibacterial effect. J. Radiat. Res. 2020, 13, 416–425. [Google Scholar] [CrossRef]
- Marghani, B.H.; Fehaid, A.; Ateya, A.I.; Ezz, M.A.; Saleh, R.M. Photothermal therapeutic potency of plasmonic silver nanoparticles for apoptosis and anti-angiogenesis in testosterone induced benign prostate hyperplasia in rats. Life Sci. 2022, 291, 120240. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Courrol, D.; Lopes, C.R.B.; da Silva Cordeiro, T.; Franzolin, M.R.; Junior, N.D.V.; Samad, R.E.; Courrol, L.C. Optical properties and antimicrobial effects of silver nanoparticles synthesized by femtosecond laser photoreduction. Opt. Lasers Technol. 2018, 103, 233–238. [Google Scholar] [CrossRef]
- Amirjani, A.; Koochak, N.N.; Haghshenas, D.F. Synthesis of silver nanotriangles with tunable edge length: A promising candidate for light harvesting purposes within visible and near infrared ranges. Mater. Res. Express 2018, 6, 036204. [Google Scholar] [CrossRef]
- Debnath, B.; Das, R. Controlled synthesis of saponin-capped silver nanotriangles and their optical properties. Plasmonics 2019, 14, 1365–1375. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, W.; Zhong, Y.; Liang, G.; Chen, Q.; Zhang, W. The morphology control on the preparation of silver nanotriangles. Curr. Appl. Phys. 2019, 19, 1187–1194. [Google Scholar] [CrossRef]
- Bian, K.; Zhang, X.; Liu, K.; Yin, T.; Liu, H.; Niu, K.; Cao, W.; Gao, D. Peptide-directed hierarchical mineralized silver nanocages for anti-tumor photothermal therapy. ACS Sustain. Chem. Eng. 2018, 6, 7574–7588. [Google Scholar] [CrossRef]
- Qin, Z.; Du, T.; Zheng, Y.; Luo, P.; Zhang, J.; Xie, M.; Zhang, Y.; Du, Y.; Yin, L.; Cui, D. Glutathione induced transformation of partially hollow gold–silver nanocages for cancer diagnosis and photothermal therapy. Small 2019, 15, 1902755. [Google Scholar] [CrossRef]
- Wen, H.; Tamarov, K.; Happonen, E.; Lehto, V.P.; Xu, W. Inorganic Nanomaterials for Photothermal Based Cancer Theranostics. Adv. Ther. 2021, 4, 2000207. [Google Scholar] [CrossRef]
- Nguyen, N.T.-P.; Nguyen, L.V.-H.; Thanh, N.T.; Van Toi, V.; Quyen, T.N.; Tran, P.A.; Wang, H.-M.D.; Nguyen, T.-H. Stabilization of silver nanoparticles in chitosan and gelatin hydrogel and its applications. Mater. Lett. 2019, 248, 241–245. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Lin, Y.-S.; Wu, S.-J.; Mi, F.-L. Mutlifunctional nanoparticles prepared from arginine-modified chitosan and thiolated fucoidan for oral delivery of hydrophobic and hydrophilic drugs. Carbohydr. Polym. 2018, 193, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Chelushkin, P.S.; Shakirova, J.R.; Kritchenkov, I.S.; Baigildin, V.A.; Tunik, S.P. Phosphorescent NIR emitters for biomedicine: Applications, advances and challenges. Dalton Trans. 2022, 51, 1257–1280. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E. The role of ligands in the chemical synthesis and applications of inorganic nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef]
- Wang, D.; Wu, H.; Zhou, J.; Xu, P.; Wang, C.; Shi, R.; Wang, H.; Wang, H.; Guo, Z.; Chen, Q. In situ one pot synthesis of MOF polydopamine hybrid nanogels with enhanced photothermal effect for targeted cancer therapy. Adv. Sci. 2018, 5, 1800287. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.W. Metal organic frameworks for biomedical applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, F.; Liu, S.; Wu, X.; Xu, L.; Zhang, D. In situ reduction of silver nanoparticles by sodium alginate to obtain silver-loaded composite wound dressing with enhanced mechanical and antimicrobial property. Int. J. Biol. Macromolec. 2020, 148, 501–509. [Google Scholar] [CrossRef]
- Colino, C.I.; Lanao, J.M.; Gutierrez-Millan, C. Recent advances in functionalized nanomaterials for the diagnosis and treatment of bacterial infections. Mater. Sci. Eng. C 2021, 121, 111843. [Google Scholar] [CrossRef]
- Aur Soro, C.; Nesma, T.; Juanes-Velasco, P.; Landeira-Viuela, A.; Fidalgo-Gomez, H.; Acebes-Fernandez, V.; Gongora, R.; Almendral Parra, M.J.; Manzano-Roman, R.; Fuentes, M. Interactions of nanoparticles and biosystems: Microenvironment of nanoparticles and biomolecules in nanomedicine. Nanomaterials 2019, 9, 1365. [Google Scholar] [CrossRef]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle processing: Understanding and controlling aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Niu, K.; Luo, L.; Li, L.; Cong, C.; Gao, D. Reduction and protection: One-step synthesis of polydopamine-coated silver nanowires with superior biosafety for cancer treatment. ACS Sustain. Chem. Engin. 2019, 7, 20102–20106. [Google Scholar] [CrossRef]
- Zhu, M.; Tang, J.; Wei, W.; Li, S. Recent progress in the syntheses and applications of multishelled hollow nanostructures. Mater. Chem. Front. 2020, 4, 1105–1149. [Google Scholar] [CrossRef]
- Zhang, Q. Nano-Toxicity; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Atapour, A.; Amani, A.M.; Savar Dashtaki, A.; Babapoor, A.; Arjmand, O. Green synthesis of silver nanoparticles toward bio and medical applications: Review study. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), 855–872. [Google Scholar] [CrossRef]
- Cunningham, B.; Engstrom, A.M.; Harper, B.J.; Harper, S.L.; Mackiewicz, M.R. Silver nanoparticles stable to oxidation and silver ion release show size-dependent toxicity in vivo. Nanomaterials 2021, 11, 1516. [Google Scholar] [CrossRef]
- Johnson, L.; Gray, D.M.; Niezabitowska, E.; McDonald, T.O. Multi-stimuli-responsive aggregation of nanoparticles driven by the manipulation of colloidal stability. Nanoscale 2021, 13, 7879–7896. [Google Scholar] [CrossRef] [PubMed]
- Rahimi-Moghaddam, F.; Azarpira, N.; Sattarahmady, N. Evaluation of a nanocomposite of PEG-curcumin-gold nanoparticles as a near-infrared photothermal agent: An in vitro and animal model investigation. Lasers Med. Sci. 2018, 33, 1769–1779. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, D.Y. Near-infrared-responsive cancer photothermal and photodynamic therapy using gold nanoparticles. Polymers 2018, 10, 961. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The importance of poly (ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef]
- Ciepluch, K.; Skrzyniarz, K.; Zdańska, J.; Barrios-Gumiel, A.; Sánchez-Nieves, J.; de la Mata, F.J.; Maciejewska, B.; Drulis-Kawa, Z.; Bryszewska, M.; Arabski, M. PEGylation of dendronized silver nanoparticles increases the binding affinity of antimicrobial proteins. J. Mol. Liq. 2020, 319, 114339. [Google Scholar] [CrossRef]
- Xi, W. Implications of Intermolecular Interactions on Normal Raman and Surface-Enhanced Raman Spectra Using Gold Nanostars and Polymers. Ph.D. Thesis, The University of Iowa, Iowa, IA, USA, 2019. [Google Scholar]
- Ha, M.; Kim, J.-H.; You, M.; Li, Q.; Fan, C.; Nam, J.-M. Multicomponent Plasmonic Nanoparticles: From Heterostructured Nanoparticles to Colloidal Composite Nanostructures. Chem. Rev. 2019, 119, 12208–12278. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Tsai, D.-H. Self-Assembly of Noble Metal-Based Hybrid Nanostructures Using a Combination of Colloidal and Aerosol-Based Approaches. In Targeted Nanosystems for Therapeutic Applications: New Concepts, Dynamic Properties, Efficiency, and Toxicity, ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2019; pp. 139–156. [Google Scholar]
- Jun, B.-H. Silver nano/microparticles: Modification and applications. Int. J. Mol. Sci. 2019, 20, 2609. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Yang, T.; O’hagan, J.; Zhang, S.; Donnelly, R.F. Photothermal therapy. J. Control. Release 2020, 325, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.G.; Shin, S.W.; Kim, S.-Y.; Kim, S.; Lim, Y.T.; Oh, B.-K.; Um, S.H. Protein nanoparticle fabrication for optimized reticuloendothelial system evasion and tumor accumulation. Langmuir 2019, 35, 3992–3998. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Khateri, M.; Khosravi, Z.; Kamrava, S.K.; Kooranifar, S.; Ghaznavi, H.; Shakeri-Zadeh, A. Gold nanoparticles in combinatorial cancer therapy strategies. Coord. Chem. Rev. 2019, 387, 299–324. [Google Scholar] [CrossRef]
- Sibuyi, N.R.S.; Moabelo, K.L.; Fadaka, A.O.; Meyer, S.; Onani, M.O.; Madiehe, A.M.; Meyer, M. Multifunctional gold nanoparticles for improved diagnostic and therapeutic applications: A review. Nanoscale Res. Lett. 2021, 16, 1–27. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold nanoparticles (GNPs) in biomedical and clinical applications: A review. Nano Select 2022, 3, 792–828. [Google Scholar] [CrossRef]
- Charbgoo, F.; Nejabat, M.; Abnous, K.; Soltani, F.; Taghdisi, S.M.; Alibolandi, M.; Shier, W.T.; Steele, T.W.; Ramezani, M. Gold nanoparticle should understand protein corona for being a clinical nanomaterial. J. Control. Release 2018, 272, 39–53. [Google Scholar] [CrossRef]
- Abarca-Cabrera, L.; Fraga-García, P.; Berensmeier, S. Bio-nano interactions: Binding proteins, polysaccharides, lipids and nucleic acids onto magnetic nanoparticles. Biomater. Res. 2021, 25, 12. [Google Scholar] [CrossRef]
- Mandhata, C.P.; Sahoo, C.R.; Padhy, R.N. Biomedical applications of biosynthesized gold nanoparticles from cyanobacteria: An overview. Biol. Trace Elem. Res. 2022, 200, 5307–5327. [Google Scholar] [CrossRef]
- Kumari, V.; Vishwas, S.; Kumar, R.; Kakoty, V.; Khursheed, R.; Babu, M.R.; Harish, V.; Mittal, N.; Singh, P.K.; Alharthi, N.S. An overview of biomedical applications for gold nanoparticles against lung cancer. J. Drug Deliv. Sci. Technol. 2023, 86, 1–11. [Google Scholar] [CrossRef]
- Kohout, C.; Santi, C.; Polito, L. Anisotropic gold nanoparticles in biomedical applications. Int. J. Mol. Sci. 2018, 19, 3385. [Google Scholar] [CrossRef] [PubMed]
- Piotto, V.; Litti, L.; Meneghetti, M. Synthesis and shape manipulation of anisotropic gold nanoparticles by laser ablation in solution. J. Phys. Chem. C 2020, 124, 4820–4826. [Google Scholar] [CrossRef]
- Swearer, D.F.; Bourgeois, B.B.; Angell, D.K.; Dionne, J.A. Advancing plasmon-induced selectivity in chemical transformations with optically coupled transmission electron microscopy. Accounts Chem. Res. 2021, 54, 3632–3642. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Koduru, J.R.; Desai, M.L.; Park, T.J.; Singhal, R.K.; Basu, H. Recent progress on surface chemistry of plasmonic metal nanoparticles for colorimetric assay of drugs in pharmaceutical and biological samples. TrAC Tren. Analy. Chem. 2018, 105, 106–120. [Google Scholar] [CrossRef]
- Sharifi, M.; Attar, F.; Saboury, A.A.; Akhtari, K.; Hooshmand, N.; Hasan, A.; El-Sayed, M.A.; Falahati, M. Plasmonic gold nanoparticles: Optical manipulation, imaging, drug delivery and therapy. J. Control. Release 2019, 311, 170–189. [Google Scholar] [CrossRef]
- Mazloomi Rezvani, M.; Salami Kalajahi, M.; Roghani Mamaqani, H.; Pirayesh, A. Effect of surface modification with various thiol compounds on colloidal stability of gold nanoparticles. Appl. Organomet. Chem. 2018, 32, e4079. [Google Scholar] [CrossRef]
- Hariharan, K.; Patel, P.; Mehta, T. Surface modifications of gold nanoparticles: Stabilization and recent applications in cancer therapy. Pharm. Dev. Technol. 2022, 27, 665–683. [Google Scholar] [CrossRef]
- Gifford, B.J.; He, X.; Kim, M.; Kwon, H.; Saha, A.; Sifain, A.E.; Wang, Y.; Htoon, H.; Kilina, S.; Doorn, S.K. Optical effects of divalent functionalization of carbon nanotubes. Chem. Mater. 2019, 31, 6950–6961. [Google Scholar] [CrossRef]
- Kim, D.; Shin, K.; Kwon, S.G.; Hyeon, T. Synthesis and biomedical applications of multifunctional nanoparticles. Adv. Mater. 2018, 30, 1802309. [Google Scholar] [CrossRef]
- MacFarlane, L.R.; Shaikh, H.; Garcia-Hernandez, J.D.; Vespa, M.; Fukui, T.; Manners, I. Functional nanoparticles through conjugated polymer self-assembly. Nat. Rev. Mater. 2021, 6, 7–26. [Google Scholar] [CrossRef]
- Szczech, M.; Szczepanowicz, K. Polymeric core-shell nanoparticles prepared by spontaneous emulsification solvent evaporation and functionalized by the layer-by-layer method. Nanomaterials 2020, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Teijido, R.N.; Ruiz-Rubio, L.; Echaide, A.G.; Vilas-Vilela, J.L.; Lanceros-Mendez, S.; Zhang, Q. State of the art and current trends on layered inorganic-polymer nanocomposite coatings for anticorrosion and multi-functional applications. Prog. Org. Coatings 2022, 163, 106684. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional gold nanoparticles: A novel nanomaterial for various medical applications and biological activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Avelino, K.Y.; Oliveira, L.S.; Lucena-Silva, N.; Andrade, C.A.; Oliveira, M.D. Flexible sensor based on conducting polymer and gold nanoparticles for electrochemical screening of HPV families in cervical specimens. Talanta 2021, 226, 122118. [Google Scholar] [CrossRef]
- Mishra, R.; Mishra, S.; Barot, Y.B. Greener synthesis and stabilization of metallic nanoparticles in ionic liquids. In Handbook of Greener Synthesis of Nanomaterials and Compounds; Elsevier: Amsterdam, The Netherlands, 2021; pp. 245–276. [Google Scholar]
- Irfan, M.; Moniruzzaman, M.; Ahmad, T.; Osman, O.Y.; Mandal, P.C.; Bhattacharjee, S.; Hussain, M. Stability, interparticle interactions and catalytic performance of gold nanoparticles synthesized through ionic liquid mediated oil palm leaves extract. J. Environ. Chem. Eng. 2018, 6, 5024–5031. [Google Scholar] [CrossRef]
- Nejati, K.; Dadashpour, M.; Gharibi, T.; Mellatyar, H.; Akbarzadeh, A. Biomedical applications of functionalized gold nanoparticles: A review. J. Clust. Sci. 2021, 33, 1–16. [Google Scholar] [CrossRef]
- Pan, D.; Zhong, X.; Zhao, W.; Yu, Z.; Yang, Z.; Wang, D.; Cao, H.; He, W. Meso-substituted porphyrin photosensitizers with enhanced near-infrared absorption: Synthesis, characterization and biological evaluation for photodynamic therapy. Tetrahedron 2018, 74, 2677–2683. [Google Scholar] [CrossRef]
- Hong, E.J.; Kim, Y.-S.; Choi, D.G.; Shim, M.S. Cancer-targeted photothermal therapy using aptamer-conjugated gold nanoparticles. J. Ind. Eng. Chem. 2018, 67, 429–436. [Google Scholar] [CrossRef]
- Kopac, T. Protein corona, understanding the nanoparticle–protein interactions and future perspectives: A critical review. Int. J. Biol. Macromol. 2021, 169, 290–301. [Google Scholar] [CrossRef]
- Nienhaus, K.; Wang, H.; Nienhaus, G. Nanoparticles for biomedical applications: Exploring and exploiting molecular interactions at the nano-bio interface. Mater. Adv. 2020, 5, 100036. [Google Scholar] [CrossRef]
- Suvarna, M.; Dyawanapelly, S.; Kansara, B.; Dandekar, P.; Jain, R. Understanding the stability of nanoparticle–protein interactions: Effect of particle size on adsorption, conformation and thermodynamic properties of serum albumin proteins. ACS Appl. Nano Mater. 2018, 1, 5524–5535. [Google Scholar] [CrossRef]
- Fam, S.Y.; Chee, C.F.; Yong, C.Y.; Ho, K.L.; Mariatulqabtiah, A.R.; Tan, W.S. Stealth coating of nanoparticles in drug-delivery systems. Nanomaterials 2020, 10, 787. [Google Scholar] [CrossRef]
- Hyun, H.; Park, J.; Willis, K.; Park, J.E.; Lyle, L.T.; Lee, W.; Yeo, Y. Surface modification of polymer nanoparticles with native albumin for enhancing drug delivery to solid tumors. Biomaterials 2018, 180, 206–224. [Google Scholar] [CrossRef]
- Sultana, S.; Alzahrani, N.; Alzahrani, R.; Alshamrani, W.; Aloufi, W.; Ali, A.; Najib, S.; Siddiqui, N.A. Stability issues and approaches to stabilised nanoparticles based drug delivery system. J. Drug Target. 2020, 28, 468–486. [Google Scholar] [CrossRef]
- Weiss, M.; Fan, J.; Claudel, M.; Sonntag, T.; Didier, P.; Ronzani, C.; Lebeau, L.; Pons, F. Density of surface charge is a more predictive factor of the toxicity of cationic carbon nanoparticles than zeta potential. J. Nanobiotech. 2021, 19, 1–19. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, M.S.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.S.; Pfitzner, E.; Okamura, Y.; Gordeev, G.; Kusch, P.; Lange, H.; Heberle, J.; Schulz, F.; Reich, S. Surface-enhanced Raman scattering and surface-enhanced infrared absorption by plasmon polaritons in three-dimensional nanoparticle supercrystals. ACS Nano 2021, 15, 5523–5533. [Google Scholar] [CrossRef]
- Badshah, M.A.; Koh, N.Y.; Zia, A.W.; Abbas, N.; Zahra, Z.; Saleem, M.W. Recent developments in plasmonic nanostructures for metal enhanced fluorescence-based biosensing. Nanomaterials 2020, 10, 1749. [Google Scholar] [CrossRef]
- Stafford, S.; Serrano Garcia, R.; Gunko, Y.K. Multimodal magnetic-plasmonic nanoparticles for biomedical applications. Appl. Sci. 2018, 8, 97. [Google Scholar] [CrossRef]
- Xi, D.; Xiao, M.; Cao, J.; Zhao, L.; Xu, N.; Long, S.; Fan, J.; Shao, K.; Sun, W.; Yan, X. NIR light driving barrier free group rotation in nanoparticles with an 88.3% photothermal conversion efficiency for photothermal therapy. Adv. Mater. 2020, 32, 1907855. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Cho, H.-Y.; Choi, H.K.; Lee, J.-Y.; Choi, J.-W. Application of gold nanoparticle to plasmonic biosensors. Int. J. Mol. Sci. 2018, 19, 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guan, M.; Wang, J.; Sheng, H.; Chen, Y.; Liang, Y.; Peng, Q.; Lu, G. Plasmon-mediated photochemical transformation of inorganic nanocrystals. Appl. Mater. Today 2021, 24, 101125. [Google Scholar] [CrossRef]
- Cardoso-Avila, P.E.; Pichardo Molina, J.L. Demonstrating the Photochemical Transformation of Silver Nanoparticles. J. Chem. Educ. 2018, 95, 2034–2040. [Google Scholar] [CrossRef]
- Sharma, S.; Shrivastava, N.; Rossi, F.; Thanh, N.T.K. Nanoparticles-based magnetic and photo induced hyperthermia for cancer treatment. Nano Today 2019, 29, 100795. [Google Scholar] [CrossRef]
- Hussein, E.A.; Zagho, M.M.; Nasrallah, G.K.; Elzatahry, A.A. Recent advances in functional nanostructures as cancer photothermal therapy. Int. J. Nanomedicine. 2018, 13, 2897–2906. [Google Scholar] [CrossRef]
- Bucharskaya, A.B.; Maslyakova, G.N.; Chekhonatskaya, M.L.; Terentyuk, G.S.; Navolokin, N.A.; Khlebtsov, B.N.; Khlebtsov, N.G.; Bashkatov, A.N.; Genina, E.A.; Tuchin, V.V. Plasmonic photothermal therapy: Approaches to advanced strategy. Lasers Surg. Med. 2018, 50, 1025–1033. [Google Scholar] [CrossRef]
- Bailly, A.-L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.-A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef]
- Peng, C.; Xu, J.; Yu, M.; Ning, X.; Huang, Y.; Du, B.; Hernandez, E.; Kapur, P.; Hsieh, J.T.; Zheng, J. Tuning the in vivo transport of anticancer drugs using renal clearable gold nanoparticles. Angew. Chem. Int. Ed. 2019, 58, 8479–8483. [Google Scholar] [CrossRef]
- Bi, C.; Chen, J.; Chen, Y.; Song, Y.; Li, A.; Li, S.; Mao, Z.; Gao, C.; Wang, D.; Möhwald, H. Realizing a record photothermal conversion efficiency of spiky gold nanoparticles in the second near-infrared window by structure-based rational design. Chem. Mater. 2018, 30, 2709–2718. [Google Scholar] [CrossRef]
- Meola, A.; Rao, J.; Chaudhary, N.; Sharma, M.; Chang, S.D. Gold nanoparticles for brain tumor imaging: A systematic review. Front. Neurol. 2018, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Hawash, M.; Jaradat, N.; Eid, A.M.; Abubaker, A.; Mufleh, O.; Al-Hroub, Q.; Sobuh, S. Synthesis of novel isoxazole–carboxamide derivatives as promising agents for melanoma and targeted nano-emulgel conjugate for improved cellular permeability. BMC Chem. 2022, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Lin, R.; Li, H.J.; He, W.L.; Du, J.Z.; Wang, J. Strategies to improve tumor penetration of nanomedicines through nanoparticle design. Nanomed. Nanobiotech. 2019, 11, 1519. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Kang, T.; Luo, X.; Zhu, J.; Wu, P.; Cai, C. Coral-shaped Au nanostructures for selective apoptosis induction during photothermal therapy. J. Mater. Chem. B 2019, 7, 6224–6231. [Google Scholar] [CrossRef]
- Takakura, Y.; Takahashi, Y. Strategies for persistent retention of macromolecules and nanoparticles in the blood circulation. J. Control. Release 2022, 350, 486–493. [Google Scholar] [CrossRef]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef]
- Geng, H.; Vilms Pedersen, S.; Ma, Y.; Haghighi, T.; Dai, H.; Howes, P.D.; Stevens, M.M. Noble metal nanoparticle biosensors: From fundamental studies toward point-of-care diagnostics. Accounts Chem. Res. 2022, 55, 593–604. [Google Scholar] [CrossRef]
- Lv, Z.; He, S.; Wang, Y.; Zhu, X. Noble metal nanomaterials for NIR triggered photothermal therapy in cancer. Adv. Healthc. Mat. 2021, 10, 2001806. [Google Scholar] [CrossRef]
- Cheng, T.-M.; Chu, H.-Y.; Huang, H.-M.; Li, Z.-L.; Chen, C.-Y.; Shih, Y.-J.; Whang-Peng, J.; Cheng, R.H.; Mo, J.-K.; Lin, H.-Y. Toxicologic concerns with current medical nanoparticles. Int. J. Mol. Sci. 2022, 23, 7597. [Google Scholar] [CrossRef]
- Ibrahim, K.E.; Al-Mutary, M.G.; Bakhiet, A.O.; Khan, H.A. Histopathology of the liver, kidney, and spleen of mice exposed to gold nanoparticles. Molecules 2018, 23, 1848. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.D.; Delbem, A.C.B.; Monteiro, D.R. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Khizar, S.; Ahmad, N.M.; Zine, N.; Jaffrezic-Renault, N.; Errachid-el-salhi, A.; Elaissari, A. Magnetic nanoparticles: From synthesis to theranostic applications. ACS Appl. Nano Mater. 2021, 4, 4284–4306. [Google Scholar] [CrossRef]
- Sabino, C.P.; Wainwright, M.; Ribeiro, M.S.E.; Sellera, F.P.; Dos Anjos, C.; da Silva Baptista, M.; Lincopan, N. Global priority multidrug-resistant pathogens do not resist photodynamic therapy. Photochem. Photobiol. B Biol. 2020, 208, 111893. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Ahmad, T.; Moniruzzaman, M.; Bhattacharjee, S.; Abdullah, B. Size and stability modulation of ionic liquid functionalized gold nanoparticles synthesized using Elaeis guineensis (oil palm) kernel extract. Arab. J. Chem. 2020, 13, 75–85. [Google Scholar] [CrossRef]
- Slepika, P.; Slepikov Kaslkov, N.; Siegel, J.; Kolsk Zvork, V.C. Methods of gold and silver nanoparticles preparation. Materials 2019, 13, 1. [Google Scholar] [CrossRef]
- Brilkina, A.A.; Dubasova, L.V.; Sergeeva, E.A.; Pospelov, A.J.; Shilyagina, N.Y.; Shakhova, N.M.; Balalaeva, I.V. Photobiological properties of phthalocyanine photosensitizers Photosens, Holosens and Phthalosens: A comparative in vitro analysis. Photochem. Photobiol. B Biol. 2019, 191, 128–134. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Najda, A.; Kaur, P.; Shah, M.; Singh, H.; Kaur, P.; Cavalu, S.; Jaroszuk-Sierocinska, M.; Rahman, M.H. Potentiality of nanoenzymes for cancer treatment and other diseases: Current status and future challenges. Materials 2021, 14, 5965. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, B.; Zheng, K.; He, S.; Meng, L.; Song, J.; Yang, H. Recent progress in NIR-II contrast agent for biological imaging. Front. Bioeng. Biotechnol. 2020, 7, 487. [Google Scholar] [CrossRef]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef]
- Calavia, P.G.A.; Bruce, G.; Prez-Garcأa, L.; Russell, D.A. Photosensitiser-gold nanoparticle conjugates for photodynamic therapy of cancer. Photochem. Photobiol. Sci. 2018, 17, 1534–1552. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Niu, L.; Qiao, Z.-Y.; Cheng, D.-B.; Wang, J.; Zhong, Y.; Bai, F.; Wang, H.; Fan, H. Synthesis of self-assembled porphyrin nanoparticle photosensitizers. ACS Nano 2018, 12, 3796–3803. [Google Scholar] [CrossRef] [PubMed]
- Domagala, A.; Stachura, J.; Gabrysiak, M.; Muchowicz, A.; Zagozdzon, R.; Golab, J.; Firczuk, M. Inhibition of autophagy sensitizes cancer cells to Photofrin-based photodynamic therapy. BMC Cancer 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, R.; Lee, J.; Yang, S.-G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef]
- Lyons, N.J.; Giri, R.; Begun, J.; Clark, D.; Proud, D.; He, Y.; Hooper, J.D.; Kryza, T. Reactive oxygen species as mediators of Disease Progression and Therapeutic Response in Colorectal Cancer. Antioxid. Redox Signal. 2023, 39, 186–205. [Google Scholar] [CrossRef]
- Gomes, A.T.; Neves, M.G.P.; Fernandes, R.; Ribeiro, C.F.; Cavaleiro, J.A.; Moura, N.M. Unraveling the photodynamic activity of cationic benzoporphyrin-based photosensitizers against bladder cancer cells. Molecules 2021, 26, 5312. [Google Scholar] [CrossRef]
- Thakur, N.S.; Patel, G.; Kushwah, V.; Jain, S.; Banerjee, U.C. Facile development of biodegradable polymer-based nanotheranostics: Hydrophobic photosensitizers delivery, fluorescence imaging and photodynamic therapy. Photochem. Photobiol. B Biol. 2019, 193, 39–50. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, Z.; Ma, Z.; Liu, J.; Han, G.; Ma, F.; Jia, N.; Miao, Z.; Zhang, W.; Sheng, C. Design and synthesis of novel water-soluble amino acid derivatives of chlorin p6 ethers as photosensitizer. Chin. Chem. Lett. 2019, 30, 247–249. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of third generation photosensitizers used in anticancer photodynamic therapy. Photodiag. Photodynam. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Priefer, R. Non-porphyrin dyes used as photosensitizers in photodynamic therapy. J. Drug Deliv. Sci. Technol. 2020, 60, 101979. [Google Scholar] [CrossRef]
- Gao, W.-X.; Zhang, H.-N.; Jin, G.-X. Supramolecular catalysis based on discrete heterometallic coordination-driven metallacycles and metallacages. Coord. Chem. Rev. 2019, 386, 69–84. [Google Scholar] [CrossRef]
- Ni, J.; Wang, Y.; Zhang, H.; Sun, J.Z.; Tang, B.Z. Aggregation-induced generation of reactive oxygen species: Mechanism and photosensitizer construction. Molecules 2021, 26, 268. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Liu, Y.-C.; Sun, H.; Guo, D.-S. Type I photodynamic therapy by organic–inorganic hybrid materials: From strategies to applications. Coord. Chem. Rev. 2019, 395, 46–62. [Google Scholar] [CrossRef]
- Sun, J.; Cai, X.; Wang, C.; Du, K.; Chen, W.; Feng, F.; Wang, S. Cascade reactions by nitric oxide and hydrogen radical for anti-hypoxia photodynamic therapy using an activatable photosensitizer. J. Am. Chem. Soc. 2021, 143, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.; Du, J.; Gu, Z.; Zhao, Y. Reactive oxygen species-regulating strategies based on nanomaterials for disease treatment. Adv. Sci. 2021, 8, 2002797. [Google Scholar] [CrossRef]
- Hompland, T.; Fjeldbo, C.S.; Lyng, H. Tumor Hypoxia as a Barrier in Cancer Therapy: Why Levels Matter. Cancers 2021, 13, 499. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Yuan, X.; Ruan, W.; Bobrow, B.; Carmeliet, P.; Eltzschig, H.K. Targeting hypoxia-inducible factors: Therapeutic opportunities and challenges. Nat. Rev. Drug Discov. 2024, 23, 175–200. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, W.-J.; Bai, X.-F.; Zhang, X.-Z. Nanomaterials to relieve tumor hypoxia for enhanced photodynamic therapy. Nano Today 2020, 35, 100960. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, H.; Liu, B.; Liu, Z. Tumor microenvironment-responsive dynamic inorganic nanoassemblies for cancer imaging and treatment. Adv. Drug Deliv. Rev. 2021, 179, 114004. [Google Scholar] [CrossRef]
- Wu, L.; Wang, C.; Li, Y. Iron Oxide Nanoparticle Targeting Mechanism and its Application in Tumor Magnetic Resonance Imaging and Therapy. Nanomedicine 2022, 17, 1567–1583. [Google Scholar] [CrossRef] [PubMed]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Li, J.; Shen, P.; Zhao, Z.; Tang, B.Z. Exploring and leveraging aggregation effects on reactive oxygen species generation in photodynamic therapy. Aggregate 2024, 5, 1–20. [Google Scholar] [CrossRef]
- Vonlanthen, M.; Cuétara-Guadarrama, F.; Sorroza-Martínez, K.; González-Méndez, I.; Estrada-Montaño, A.S.; Rivera, E. A review of porphyrin dendrimers as light-harvesting versatile platforms. Dye. Pigment. 2023, 111873. [Google Scholar] [CrossRef]
- Ji, C.; Lai, L.; Li, P.; Wu, Z.; Cheng, W.; Yin, M. Organic dye assemblies with aggregation-induced photophysical changes and their bio-applications. Aggregate 2021, 2, e39. [Google Scholar] [CrossRef]
- Stabile, J.; Najafali, D.; Cheema, Y.; Inglut, C.T.; Liang, B.J.; Vaja, S.; Sorrin, A.J.; Huang, H.-C. Engineering gold nanoparticles for photothermal therapy, surgery, and imaging. In Nanoparticles for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 175–193. [Google Scholar]
- Ghaznavi, H.; Hosseini-Nami, S.; Kamrava, S.K.; Irajirad, R.; Maleki, S.; Shakeri-Zadeh, A.; Montazerabadi, A. Folic acid conjugated PEG coated gold iron oxide core shell nanocomplex as a potential agent for targeted photothermal therapy of cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1594–1604. [Google Scholar] [CrossRef]
- Bucharskaya, A.B.; Khlebtsov, N.G.; Khlebtsov, B.N.; Maslyakova, G.N.; Navolokin, N.A.; Genin, V.D.; Genina, E.A.; Tuchin, V.V. Photothermal and photodynamic therapy of tumors with plasmonic nanoparticles: Challenges and prospects. Materials 2022, 15, 1606. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Khaniabadi, P.M.; Jameel, M.S.; Oladzadabbasabadi, N.; Rahman, A.A.; Braim, F.S.; Mehrdel, B. Gold nanoparticles-based photothermal therapy for breast cancer. Photodiagnosis Photodyn. Ther. 2023, 42, 103312. [Google Scholar] [CrossRef]
- Elashnikov, R.; Radocha, M.; Panov, I.; Rimpelova, S.; Ulbrich, P.; Michalcova, A.; Svorcik, V.; Lyutakov, O. Porphyrin silver nanoparticles hybrids: Synthesis, characterization and antibacterial activity. Mater. Sci. Eng. 2019, 102, 192–199. [Google Scholar] [CrossRef]
- Hlapisi, N.; Maliehe, T.; Oluwafemi, O.; Songca, S.; Linganiso, L.; Motaung, T. Antibacterial activities of cationic porphyrins and porphyrin encapsulated gold nanorods on bacterial cell lines. Pharm. J. 2021, 13, 5. [Google Scholar] [CrossRef]
- Lee, H.; Han, J.; Shin, H.; Han, H.; Na, K.; Kim, H. Combination of chemotherapy and photodynamic therapy for cancer treatment with sonoporation effects. J. Control. Release 2018, 283, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M.C.; Moura, N.M.; Gomes, A.T.; Joaquinito, A.S.; Faustino, M.A.F.; Almeida, A.; Gonçalves, I.; Serra, V.V.; Neves, M.G.P.M.S. The role of porphyrinoid photosensitizers for skin wound healing. Int. J. Mol. Sci. 2021, 22, 4121. [Google Scholar] [CrossRef] [PubMed]
- Aroso, R.T.; Schaberle, F.A.; Arnaut, L.G.; Pereira, M.M. Photodynamic disinfection and its role in controlling infectious diseases. Photochem. Photobiol. Sci. 2021, 20, 1497–1545. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.; Nkambule, T.T.; Mamba, B.B. Spinel ferrite nanoparticles and nanocomposites for biomedical applications and their toxicity. Mater. Sci. Eng. 2020, 107, 110314. [Google Scholar] [CrossRef]
- Qiu, E.; Chen, X.; Yang, D.-P.; Regulacio, M.D.; Ramos, R.M.C.R.; Luo, Z.; Wu, Y.-L.; Lin, M.; Li, Z.; Loh, X.J. Fabricating Dual-Functional Plasmonic Magnetic Au@ MgFe2O4 Nanohybrids for Photothermal Therapy and Magnetic Resonance Imaging. ACS Omega 2022, 7, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Navarro, F.; Dapper, H.; Asadpour, R.; Knebel, C.; Spraker, M.B.; Schwarze, V.; Schaub, S.K.; Mayr, N.A.; Specht, K.; Woodruff, H.C. Development and external validation of deep-learning-based tumor grading models in soft-tissue sarcoma patients using MR imaging. Cancers 2021, 13, 2866. [Google Scholar] [CrossRef]
- Ielo, I.; Rando, G.; Giacobello, F.; Sfameni, S.; Castellano, A.; Galletta, M.; Drommi, D.; Rosace, G.; Plutino, M.R. Synthesis, chemical–physical characterization, and biomedical applications of functional gold nanoparticles: A review. Molecules 2021, 26, 5823. [Google Scholar] [CrossRef]
- Lima, D.S.; Ribicki, A.; Gonalves, L.; Hacke, A.C.M.; Lopes, L.C.; Pereira, R.P.; Wohnrath, K.; Fujiwara, S.R.T.; Pessa, C.A. Nanoconjugates based on a novel organic-inorganic hybrid silsesquioxane and gold nanoparticles as hemocompatible nanomaterials for promising biosensing applications. Colloids Surf. B Biointerfaces 2022, 213, 112355. [Google Scholar] [CrossRef]
- Limon, D.; Vil, S.L.; Herrera-Olivas, A.; Vera, R.; Badia, J.; Baldom, L.; Planas, M.; Feliu, L.; Prez-Garca, L.S. Enhanced cytotoxicity of highly water-soluble gold nanoparticle-cyclopeptide conjugates in cancer cells. Colloids Surf. B Biointerfaces 2021, 197, 111384. [Google Scholar] [CrossRef]
- Zou, J.; Tang, Y.; Baryshnikov, G.; Yang, Z.; Mao, R.; Feng, W.; Guan, J.; Li, C.; Xie, Y. Porphyrins containing a tetraphenylethylene-substituted phenothiazine donor for fabricating efficient dye sensitized solar cells with high photovoltages. J. Mater. Chem. A 2022, 10, 1320–1328. [Google Scholar] [CrossRef]
- Sun, X.; He, G.; Xiong, C.; Wang, C.; Lian, X.; Hu, L.; Li, Z.; Dalgarno, S.J.; Yang, Y.-W.; Tian, J. One-pot fabrication of hollow porphyrinic MOF nanoparticles with ultrahigh drug loading toward controlled delivery and synergistic cancer therapy. ACS Appl. Mater. Interfaces 2021, 13, 3679–3693. [Google Scholar] [CrossRef] [PubMed]
- Krzemien, W.; Rohlickova, M.; Machacek, M.; Novakova, V.; Piskorz, J.; Zimcik, P. Tuning Photodynamic Properties of BODIPY Dyes, Porphyrins Little Sisters. Molecules 2021, 26, 4194. [Google Scholar] [CrossRef]
- Malacarne, M.C.; Gariboldi, M.B.; Caruso, E. BODIPYs in PDT: A Journey through the Most Interesting Molecules Produced in the Last 10 Years. Int. J. Mol. Sci. 2022, 23, 10198. [Google Scholar] [CrossRef] [PubMed]
- Peiravi, M.; Eslami, H.; Ansari, M.; Zare-Zardini, H. Magnetic hyperthermia: Potentials and limitations. J. Ind. Chem. Soc. 2022, 99, 100269. [Google Scholar] [CrossRef]
- Yang, L.; Kim, T.H.; Cho, H.Y.; Luo, J.; Lee, J.M.; Chueng, S.T.D.; Hou, Y.; Yin, P.T.T.; Han, J.; Kim, J.H. Hybrid graphene gold nanoparticle based nucleic acid conjugates for cancer specific multimodal imaging and combined therapeutics. Adv. Funct. Mater. 2021, 31, 2006918. [Google Scholar] [CrossRef]
- Stolarczyk, E.U.; Le, A.M.; Kubiszewski, M.; Strzempek, W.; Menaszek, E.; Fusaro, M.; Sidoryk, K.; Stolarczyk, K. The ligand exchange of citrates to thioabiraterone on gold nanoparticles for prostate cancer therapy. Int. J. Pharm. 2020, 583, 119319. [Google Scholar] [CrossRef] [PubMed]
- Chubinidze, K.; Partsvania, B.; Devadze, L.; Zurabishvili, T.; Sepashvili, N.; Petriashvili, G.; Chubinidze, M. Gold nanoparticle conjugated organic dye nanocomposite based photostimulated luminescent enhancement and its application in nanomedicine. Am. J. Nano Res. Appl. 2017, 5, 42–47. [Google Scholar]
- Perera, Y.R.; Xu, J.X.; Amarasekara, D.L.; Hughes, A.C.; Abbood, I.; Fitzkee, N.C. Understanding the adsorption of peptides and proteins onto PEGylated gold nanoparticles. Molecules 2021, 26, 5788. [Google Scholar] [CrossRef] [PubMed]
- Mineo, P.; Abbadessa, A.; Mazzaglia, A.; Gulino, A.; Villari, V.; Micali, N.; Millesi, S.; Satriano, C.; Scamporrino, E. Gold nanoparticles functionalized with PEGylate uncharged porphyrins. Dye. Pigment. 2017, 141, 225–234. [Google Scholar] [CrossRef]
- Yang, M.; Moroz, P.; Jin, Z.; Budkina, D.S.; Sundrani, N.; Porotnikov, D.; Cassidy, J.; Sugiyama, Y.; Tarnovsky, A.N.; Mattoussi, H. Delayed photoluminescence in metal-conjugated fluorophores. J. Am. Chem. Soc. 2019, 141, 11286–11297. [Google Scholar] [CrossRef]
- Zhang, L.; Mazouzi, Y.; Salmain, M.; Liedberg, B.; Boujday, S. Antibody-gold nanoparticle bioconjugates for biosensors: Synthesis, characterization and selected applications. Biosens. Bioelectron. 2020, 165, 112370. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.-Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef]
- Shinohara, A.; Shao, G.; Nakanishi, T.; Shinmori, H. Porphyrin photoabsorption and fluorescence variation with adsorptive loading on gold nanoparticles. Front. Chem. 2021, 9, 777041. [Google Scholar] [CrossRef]
- Shahsavari, S.; Hadian-Ghazvini, S.; Saboor, F.H.; Oskouie, I.M.; Hasany, M.; Simchi, A.; Rogach, A.L. Ligand functionalized copper nanoclusters for versatile applications in catalysis, sensing, bioimaging, and optoelectronics. Mater. Chem. Front. 2019, 3, 2326–2356. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, J.; Zhang, F.; Xu, Y.; Liang, W.; Yu, Y. Nanotechnological approaches for diagnosis and treatment of ovarian cancer: A review of recent trends. Drug Deliv. 2022, 29, 3218–3232. [Google Scholar] [CrossRef]
- Vior, M.C.G.; Awruch, J.; Dicelio, L.E.; Diz, V.E. 2, 9, 16, 23-tetrakis [(3-mercapto) propoxy] phthalocyaninate zinc (II)/gold nanoparticle conjugates: Synthesis and photophysical properties. J. Photochem. Photobiol. A 2019, 368, 242–247. [Google Scholar] [CrossRef]
- Zeng, J.; Yang, W.; Shi, D.; Li, X.; Zhang, H.; Chen, M. Porphyrin derivative conjugated with gold nanoparticles for dual-modality photodynamic and photothermal therapies in vitro. ACS Biomater. Sci. Eng. 2018, 4, 963–972. [Google Scholar] [CrossRef]
- Sahu, A.; Russ, B.; Su, N.C.; Forster, J.D.; Zhou, P.; Cho, E.S.; Ercius, P.; Coates, N.E.; Segalman, R.A.; Urban, J.J. Bottom-up design of de novo thermoelectric hybrid materials using chalcogenide resurfacing. J. Mater. A 2017, 5, 3346–3357. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, Y.; Hu, Q.; Wang, L.; Wu, J.; Yu, L. Organic–Inorganic Hybrid Nanofibers Formed by Bottom-Up Hierarchical Self-Assembly. J. Phys. Chem. C 2021, 125, 1441–1446. [Google Scholar] [CrossRef]
- Karimi, S.; Moshaii, A.; Abbasian, S.; Nikkhah, M. Surface plasmon resonance in small gold nanoparticles: Introducing a size-dependent plasma frequency for nanoparticles in quantum regime. Plasmonics 2019, 14, 851–860. [Google Scholar] [CrossRef]
- Yoon, J.H.; Selbach, F.; Schumacher, L.; Jose, J.; Schlucker, S. Surface plasmon coupling in dimers of gold nanoparticles: Experiment and theory for ideal (spherical) and nonideal (faceted) building blocks. ACS Photonics 2019, 6, 642–648. [Google Scholar] [CrossRef]
- Contino, A.; Maccarrone, G.; Fragalà, M.E.; Spitaleri, L.; Gulino, A. Conjugated gold–porphyrin monolayers assembled on inorganic surfaces. Chem. Eur. J. 2017, 23, 14937–14943. [Google Scholar] [CrossRef] [PubMed]
- Bera, K.; Maiti, S.; Maity, M.; Mandal, C.; Maiti, N.C. Porphyrin–gold nanomaterial for efficient drug delivery to cancerous cells. ACS Omega 2018, 3, 4602–4619. [Google Scholar] [CrossRef]
- Vechia, I.C.D.; Steiner, B.T.; Freitas, M.L.; dos Santos Pedroso Fidelis, G.; Galvani, N.C.; Ronchi, J.M.; Possato, J.C.; Fagundes, M.Í.; Rigo, F.K.; Feuser, P.E. Comparative cytotoxic effect of citrate-capped gold nanoparticles with different sizes on noncancerous and cancerous cell lines. J. Nanopart. Res. 2020, 22, 1–11. [Google Scholar] [CrossRef]
- Trotsiuk, L.; Antanovich, A.; Lizunova, A.; Kulakovich, O. Direct synthesis of amphiphilic polyvinylpyrrolidone-capped gold nanoparticles in chloroform. Colloid Interface Sci. Commun. 2020, 37, 100289. [Google Scholar] [CrossRef]
- Siddiq, A.M.; Thangam, R.; Madhan, B.; Alam, M.S. Green (gemini) surfactant mediated gold nanoparticles green synthesis: Effect on triple negative breast cancer cells. Nano-Struct. Nano-Objects 2019, 19, 100373. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Jin, J.; Liang, H.; Jiang, W. Structural and physicochemical properties and biocompatibility of linear and looped polymer-capped gold nanoparticles. Langmuir 2019, 35, 8316–8324. [Google Scholar] [CrossRef]
- Zhou, X.; Joshi, S.; Patil, S.; Khare, T.; Kumar, V. Reactive oxygen, nitrogen, carbonyl and sulfur species and their roles in plant abiotic stress responses and tolerance. J. Plant Growth Regul. 2021, 41, 119–142. [Google Scholar] [CrossRef]
- Misran, H.; Salim, M.A.; Ramesh, S. Effect of Ag nanoparticles seeding on the properties of silica spheres. Ceram. Int. 2018, 44, 5901–5908. [Google Scholar] [CrossRef]
- Mir, S.H.; Ochiai, B. One-Pot Fabrication of Hollow Polymer@ Ag Nanospheres for Printable Translucent Conductive Coatings. Adv. Mater. Interfaces 2017, 4, 1601198. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Henriksen-Lacey, M.; Jimenez de Aberasturi, D.; Sánchez-Iglesias, A.; Liz-Marzán, L.M. Shielded silver nanorods for bioapplications. Chem. Mater. 2020, 32, 5879–5889. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, A.; Abbadessa, A.; Vento, F.; Mazzaglia, A.; Mineo, P.G. Silver nanoparticles decorated with PEGylated porphyrins as potential theranostic and sensing agents. Materials 2021, 14, 2764. [Google Scholar] [CrossRef]
- Oliveira, D.D.; Jacques Dit Lapierre, T.J.W.; Silva, F.; Cunha, I.; Souza, R.; Matos, P.; Almeida, G.; Oliveira, C.; Araújo, T.; Tsubone, T.; et al. Advances in Breast Cancer Drug Discovery: A Review of Therapeutic Strategies and Studies Involving Photosensitizers, Caged Xanthones and Thiosemicarbazones Derivatives. J. Braz. Chem. Soc. 2023, 35. [Google Scholar] [CrossRef]
- Chiga, Y.; Suzuki, W.; Takahata, R.; Kobiyama, E.; Tahara, H.; Kanemitsu, Y.; Shibuta, M.; Sakamoto, M.; Teranishi, T. Tuning the Direction of Photoinduced Electron Transfer in Porphyrin-Protected Gold Clusters. J. Phys. Chem. C 2024, 128, 3824–3831. [Google Scholar] [CrossRef]
- Chambers, P.C.; Garno, J.C. Heterostuctures of 4-(chloromethyl)phenyltrichlorosilane and 5,10,15,20-tetra(4-pyridyl)-21H,23H-porphine prepared on Si(111) using particle lithography: Nanoscale characterization of the main steps of nanopatterning. Beilstein J. Nanotechnol. 2018, 9, 1211–1219. [Google Scholar] [CrossRef]
- Yafout, M.; Ousaid, A.; Khayati, Y.; El Otmani, I.S. Gold nanoparticles as a drug delivery system for standard chemotherapeutics: A new lead for targeted pharmacological cancer treatments. Sci. Afr. 2021, 11, e00685. [Google Scholar] [CrossRef]
- Badıllı, U.; Mollarasouli, F.; Bakirhan, N.K.; Ozkan, Y.; Ozkan, S.A. Role of quantum dots in pharmaceutical and biomedical analysis, and its application in drug delivery. TrAC 2020, 131, 116013. [Google Scholar] [CrossRef]
- Muhammad, N.; Zhao, H.; Song, W.; Gu, M.; Li, Q.; Liu, Y.; Li, C.; Wang, J.; Zhan, H. Silver nanoparticles functionalized Paclitaxel nanocrystals enhance overall anti-cancer effect on human cancer cells. Nanotechnology 2020, 32, 085105. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gao, Y.; Liu, N.; Suo, Y. Nanoparticles loading porphyrin sensitizers in improvement of photodynamic therapy for ovarian cancer. Photodiag. Photodyn. Ther. 2021, 33, 102156. [Google Scholar] [CrossRef] [PubMed]
- Borzęcka, W.; Pereira, P.M.; Fernandes, R.; Trindade, T.; Torres, T.; Tomé, J.P. Encapsulation of glycosylated porphyrins in silica nanoparticles to enhance the efficacy of cancer photodynamic therapy. Mater. Adv. 2021, 2, 1613–1620. [Google Scholar] [CrossRef]
- Mwad Mbaz, G.I.; Calvin Lebepe, T.; Maluleke, R.; Obonai, A.; Mgedle, N.; Aladesuyi, O.A.; Kalimutu, R.; Kodama, T.; Komiya, A.; Oluwafemi, O.S. Photothermal Depth Profiling of Gelatin-Stabilised Gold Nanorods-Trastuzumab Conjugate as a Potential Breast Cancer Photothermal Agent. J. Inorg. Organo. Polym. Mater. 2024, 30, 2709–2718. [Google Scholar] [CrossRef]
- Rabiee, N.; Yaraki, M.T.; Garakani, S.M.; Garakani, S.M.; Ahmadi, S.; Lajevardi, A.; Bagherzadeh, M.; Rabiee, M.; Tayebi, L.; Tahriri, M.; et al. Recent advances in porphyrin-based nanocomposites for effective targeted imaging and therapy. Biomaterials 2020, 232, 119707. [Google Scholar] [CrossRef]
- Liang, X.; Chen, M.; Bhattarai, P.; Hameed, S.; Tang, Y.; Dai, Z. Complementing Cancer Photodynamic Therapy with Ferroptosis through Iron Oxide Loaded Porphyrin-Grafted Lipid Nanoparticles. ACS Nano 2021, 15, 20164–20180. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, H.; Ding, L.; Fan, R.; Kato, T.; Lee, D.; Fujino, K.; Kinoshita, T.; Lee, C.Y.; Waddell, T.K.; Keshavjee, S.; et al. Porphyrin–High-Density Lipoprotein: A Novel Photosensitizing Nanoparticle for Lung Cancer Therapy. Ann. Thorac. Surg. 2019, 107, 369–377. [Google Scholar] [CrossRef]
- Jutkova, A.; Chorvat, D.; Miskovsky, P.; Jancura, D.; Datta, S. Encapsulation of anticancer drug curcumin and co-loading with photosensitizer hypericin into lipoproteins investigated by fluorescence resonance energy transfer. Int. J. Pharm. 2019, 564, 369–378. [Google Scholar] [CrossRef]
- Lai, M. Photodynamic Therapy of Pancreatic Cancer with Porphyrin-Lipoprotein Photosensitizers. Master’s Thesis, University of Toronto (Canada), Toronto, ON, Canada, 2019. [Google Scholar]
- Zhang, W.; Taheri-Ledari, R.; Ganjali, F.; Mirmohammadi, S.S.; Qazi, F.S.; Saeidirad, M.; KashtiAray, A.; Zarei-Shokat, S.; Tian, Y.; Maleki, A. Effects of morphology and size of nanoscale drug carriers on cellular uptake and internalization process: A review. RSC Adv. 2023, 13, 80–114. [Google Scholar] [CrossRef]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of physico-chemical properties of nanoparticles on their intracellular uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef]
- Zhu, X.; Vo, C.; Taylor, M.; Smith, B.R. Non-spherical micro-and nanoparticles in nanomedicine. Mater. Horiz. 2019, 6, 1094–1121. [Google Scholar] [CrossRef]
- Zare, E.N.; Zheng, X.; Makvandi, P.; Gheybi, H.; Sartorius, R.; Yiu, C.K.; Adeli, M.; Wu, A.; Zarrabi, A.; Varma, R.S. Nonspherical metal-based nanoarchitectures: Synthesis and impact of size, shape, and composition on their biological activity. Small 2021, 17, 2007073. [Google Scholar] [CrossRef]
- Hlapisi, N.; Songca, S.P.; Ajibade, P.A. Morphological and structural properties of silver/chlorargyrite nanoparticles prepared using Senecio madagascariensis leaf extract and interaction studies with bovine serum albumin. MRS Adv. 2024, 9, 830–836. [Google Scholar] [CrossRef]
- Irvine, D.J.; Dane, E.L. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 2020, 20, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Khurana, S.; Choudhari, R.; Kesari, K.K.; Kamal, M.A.; Garg, N.; Ruokolainen, J.; Das, B.C.; Kumar, D. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2021; Volume 69, pp. 166–177. [Google Scholar]
- Wang, J.; Zhang, L.; Peng, F.; Shi, X.; Leong, D.T. Targeting endothelial cell junctions with negatively charged gold nanoparticles. Chem. Mater. 2018, 30, 3759–3767. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Tan, Z.; Bohinc, K.; Zhang, S. Interaction between nanoparticles and charged phospholipid membranes. Phys. Chem. Chem. Phys. 2018, 20, 29249–29263. [Google Scholar] [CrossRef]
- Liu, N.; Tang, M. Toxic effects and involved molecular pathways of nanoparticles on cells and subcellular organelles. J. Appl. Toxicol. 2020, 40, 16–36. [Google Scholar] [CrossRef]
- Sun, H.; Jia, J.; Jiang, C.; Zhai, S. Gold nanoparticle-induced cell death and potential applications in nanomedicine. Int. J. Mol. Sci. 2018, 19, 754. [Google Scholar] [CrossRef]
- Sharifi, M.; Hosseinali, S.H.; Saboury, A.A.; Szegezdi, E.; Falahati, M. Involvement of planned cell death of necroptosis in cancer treatment by nanomaterials: Recent advances and future perspectives. J. Control. Release 2019, 299, 121–137. [Google Scholar] [CrossRef]
- Abdel-Hakeem, M.A.; Abdel-Haseb, O.M.; Abdel-Ghany, S.E.; Cevik, E.; Sabit, H. Doxorubicin loaded on chitosan-protamine nanoparticles triggers apoptosis via downregulating Bcl-2 in breast cancer cells. J. Drug Deliv. Sci. Technol. 2020, 55, 101423. [Google Scholar] [CrossRef]
- Takáč, P.; Michalková, R.; Čižmáriková, M.; Bedlovičová, Z.; Balážová, Ľ.; Takáčová, G. The role of silver nanoparticles in the diagnosis and treatment of cancer: Are there any perspectives for the future? Life 2023, 13, 466. [Google Scholar] [CrossRef]
- Chen, L.; Wu, L.-Y.; Yang, W.-X. Nanoparticles induce apoptosis via mediating diverse cellular pathways. Nanomedicine 2018, 13, 2939–2955. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.Ö.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, S.; Imran, M.; Sohail, M.; Shah, S.W.A.; de Matas, M. PEGylation: A promising strategy to overcome challenges to cancer-targeted nanomedicines: A review of challenges to clinical transition and promising resolution. Drug Deliv. Transl. Res. 2019, 9, 721–734. [Google Scholar] [CrossRef]
- Zhao, Y.; Sultan, D.; Liu, Y. Biodistribution, excretion, and toxicity of nanoparticles. In Theranostic Bionanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 27–53. [Google Scholar]
- Abakumov, M.A.; Semkina, A.S.; Skorikov, A.S.; Vishnevskiy, D.A.; Ivanova, A.V.; Mironova, E.; Davydova, G.A.; Majouga, A.G.; Chekhonin, V.P. Toxicity of iron oxide nanoparticles: Size and coating effects. J. Biochem. Mol. Toxicol. 2018, 32, e22225. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, C.; Manzano, M.; Vallet-Regí, M. Nanoparticles coated with cell membranes for biomedical applications. Biology 2020, 9, 406. [Google Scholar] [CrossRef]
- Fang, R.H.; Gao, W.; Zhang, L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat. Rev. Clin. Oncol. 2023, 20, 33–48. [Google Scholar] [CrossRef]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan nanoparticles at the biological interface: Implications for drug delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef]
- Anwar, M.; Muhammad, F.; Akhtar, B. Biodegradable nanoparticles as drug delivery devices. J. Drug Deliv. Sci. Technol. 2021, 64, 102638. [Google Scholar] [CrossRef]
- Andleeb, A.; Andleeb, A.; Asghar, S.; Zaman, G.; Tariq, M.; Mehmood, A.; Nadeem, M.; Hano, C.; Lorenzo, J.M.; Abbasi, B.H. A systematic review of biosynthesized metallic nanoparticles as a promising anti-cancer-strategy. Cancers 2021, 13, 2818. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, S.K.; Banala, R.R.; Mukherjee, S.; Uppula, P.; Subbaiah, G.; AV, G.R.; Malarvilli, T. Novel biosynthesized gold nanoparticles as anti-cancer agents against breast cancer: Synthesis, biological evaluation, molecular modelling studies. Mater. Sci. Eng. 2019, 99, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yu, J.; Liu, P.; Chang, J.; Ali, D.; Tian, X. Gold nanoparticles synthesized from Curcuma wenyujin inhibits HER-2/neu transcription in breast cancer cells (MDA-MB-231/HER2). Arabian J. Chem. 2020, 13, 7264–7273. [Google Scholar] [CrossRef]
- Schmidt, R.M.; Hara, D.; Vega, J.D.; Abuhaija, M.B.; Tao, W.; Dogan, N.; Pollack, A.; Ford, J.C.; Shi, J. Quantifying radiosensitization of PSMA-targeted gold nanoparticles on prostate cancer cells at megavoltage radiation energies by Monte Carlo simulation and local effect model. Pharmaceutics 2022, 14, 2205. [Google Scholar] [CrossRef] [PubMed]
- Tremi, I.; Havaki, S.; Georgitsopoulou, S.; Terzoudi, G.; Lykakis, I.N.; Iliakis, G.; Georgakilas, V.; Gorgoulis, V.G.; Georgakilas, A.G. Biological response of human cancer cells to ionizing radiation in combination with gold nanoparticles. Cancers 2022, 14, 5086. [Google Scholar] [CrossRef] [PubMed]
- Niloy, M.S.; Shakil, M.S.; Hossen, M.S.; Alam, M.; Rosengren, R.J. Promise of gold nanomaterials as a lung cancer theranostic agent: A systematic review. Int. Nano Lett. 2021, 11, 93–111. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, W.; Tian, Y.; Yang, Z.; Wang, X.; Zhang, Y.; Tang, Y.; Zhao, S.; Wang, C.; Liu, Y. Anti-EGFR peptide-conjugated triangular gold nanoplates for computed tomography/photoacoustic imaging-guided photothermal therapy of non-small cell lung cancer. ACS Appl. Mater. Interfaces 2018, 10, 16992–17003. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical applications of reactive oxygen species generation by metal nanoparticles. Materials 2020, 14, 53. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, Y.; Alnaggar, M. Silver nanoparticles induce cell death of colon cancer cells through impairing cytoskeleton and membrane nanostructure. Micron 2019, 126, 102750. [Google Scholar] [CrossRef]
- Raja, G.; Jang, Y.-K.; Suh, J.-S.; Kim, H.-S.; Ahn, S.H.; Kim, T.-J. Microcellular environmental regulation of silver nanoparticles in cancer therapy: A critical review. Cancers 2020, 12, 664. [Google Scholar] [CrossRef]
- El-Hussein, A.; Manoto, S.L.; Ombinda-Lemboumba, S.; Alrowaili, Z.A.; Mthunzi-Kufa, P. A review of chemotherapy and photodynamic therapy for lung cancer treatment. Anti-Cancer Agents Med. Chem. 2021, 21, 149–161. [Google Scholar] [CrossRef]
- Menilli, L.; Milani, C.; Reddi, E.; Moret, F. Overview of nanoparticle-based approaches for the combination of Photodynamic Therapy (PDT) and chemotherapy at the preclinical stage. Cancers 2022, 14, 4462. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Cai, M.; Zhu, R.; Fu, T.; Du, Y.; Kong, J.; Zhang, Y.; Qu, C.; Dong, X.; Ni, J. Antitumor effect of photodynamic therapy/sonodynamic therapy/sono-photodynamic therapy of chlorin e6 and other applications. Mol. Pharm. 2023, 20, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Ayala, E.T.P.; de Sousa, F.A.D.; Vollet-Filho, J.D.; Garcia, M.R.; De Boni, L.; Bagnato, V.S.; Pratavieira, S. Photodynamic and sonodynamic therapy with protoporphyrin IX: In vitro and in vivo studies. Ultrasound Med. Biol. 2021, 47, 1032–1044. [Google Scholar] [CrossRef]
- Doix, B.; Trempolec, N.; Riant, O.; Feron, O. Low photosensitizer dose and early radiotherapy enhance antitumor immune response of photodynamic therapy-based dendritic cell vaccination. Front. Oncol. 2019, 9, 811. [Google Scholar] [CrossRef]
- Ji, B.; Wei, M.; Yang, B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics 2022, 12, 434. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Li, L.; Yue, T.; Feng, J.; Zhang, Y.; Hou, J.; Wang, Y. Recent progress in lactate oxidase-based drug delivery systems for enhanced cancer therapy. Nanoscale 2024, 16, 8739–8758. [Google Scholar] [CrossRef]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and photothermal therapies: Synergy opportunities for nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef]
- Liu, P.; Yang, W.; Shi, L.; Zhang, H.; Xu, Y.; Wang, P.; Zhang, G.; Chen, W.R.; Zhang, B.; Wang, X. Concurrent photothermal therapy and photodynamic therapy for cutaneous squamous cell carcinoma by gold nanoclusters under a single NIR laser irradiation. J. Mater. Chem. B 2019, 7, 6924–6933. [Google Scholar] [CrossRef]
- Yang, K.; Zhao, S.; Li, B.; Wang, B.; Lan, M.; Song, X. Low temperature photothermal therapy: Advances and perspectives. Coord. Chem. Rev. 2022, 454, 214330. [Google Scholar] [CrossRef]
- Day, R.A.; Sletten, E.M. Perfluorocarbon nanomaterials for photodynamic therapy. Curr. Opin. Colloid Interface Sci. 2021, 54, 101454. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Cheng, F.; Pu, Y.; Cao, J.; Lin, S.; Zhu, G.; He, B. Polymeric micelles amplify tumor oxidative stresses through combining PDT and glutathione depletion for synergistic cancer chemotherapy. Chem. Eng. J. 2021, 411, 128561. [Google Scholar] [CrossRef] [PubMed]

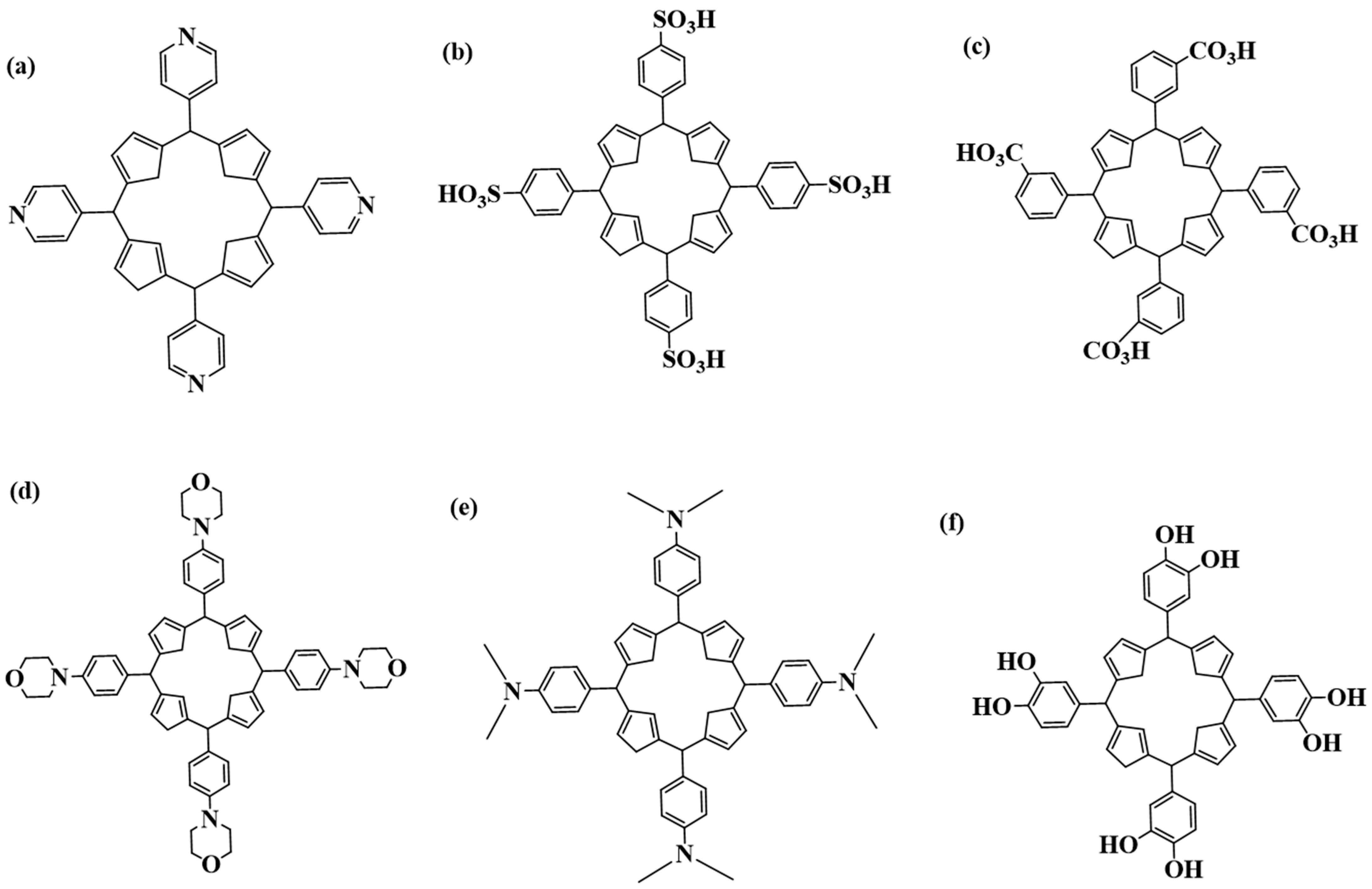
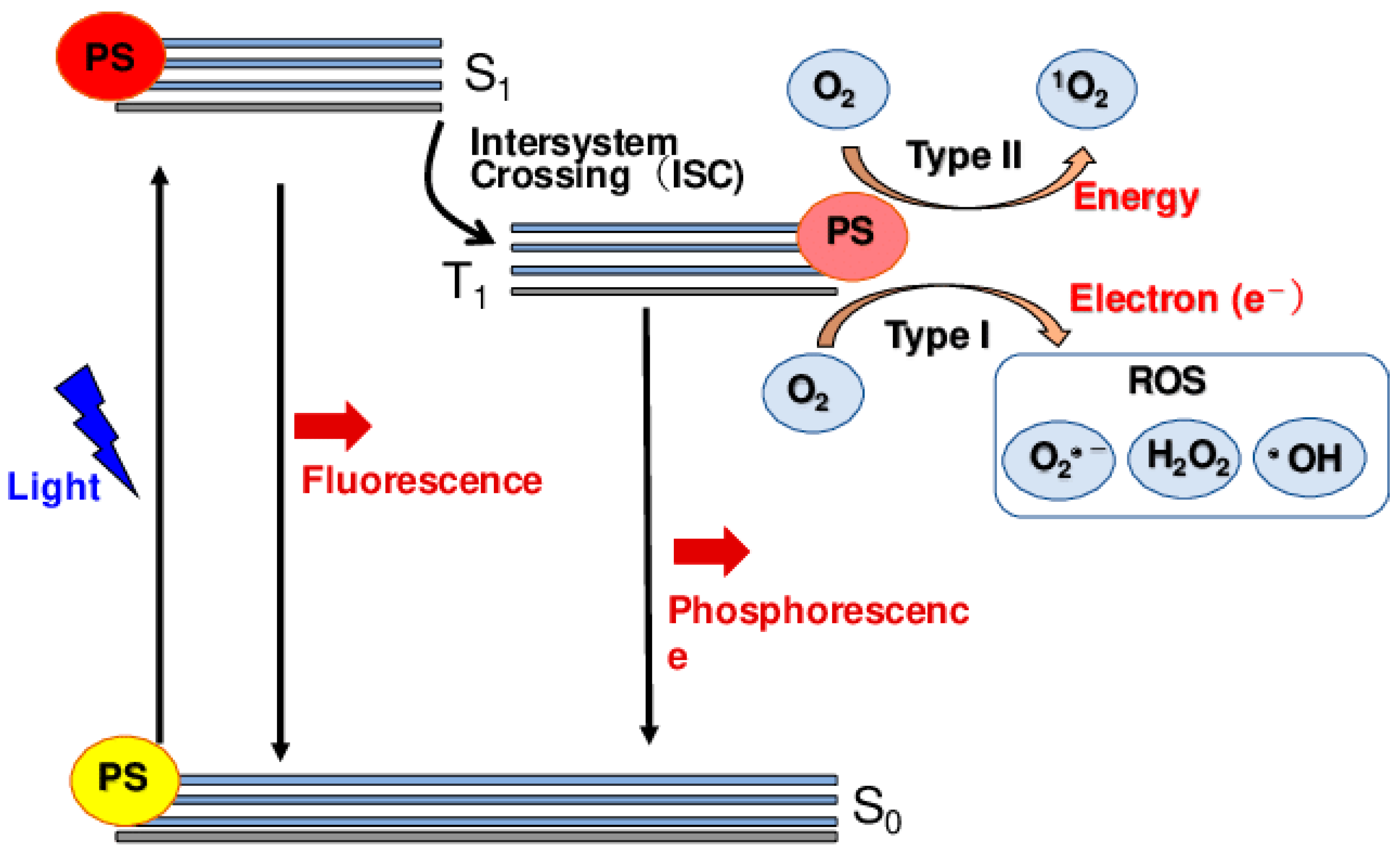
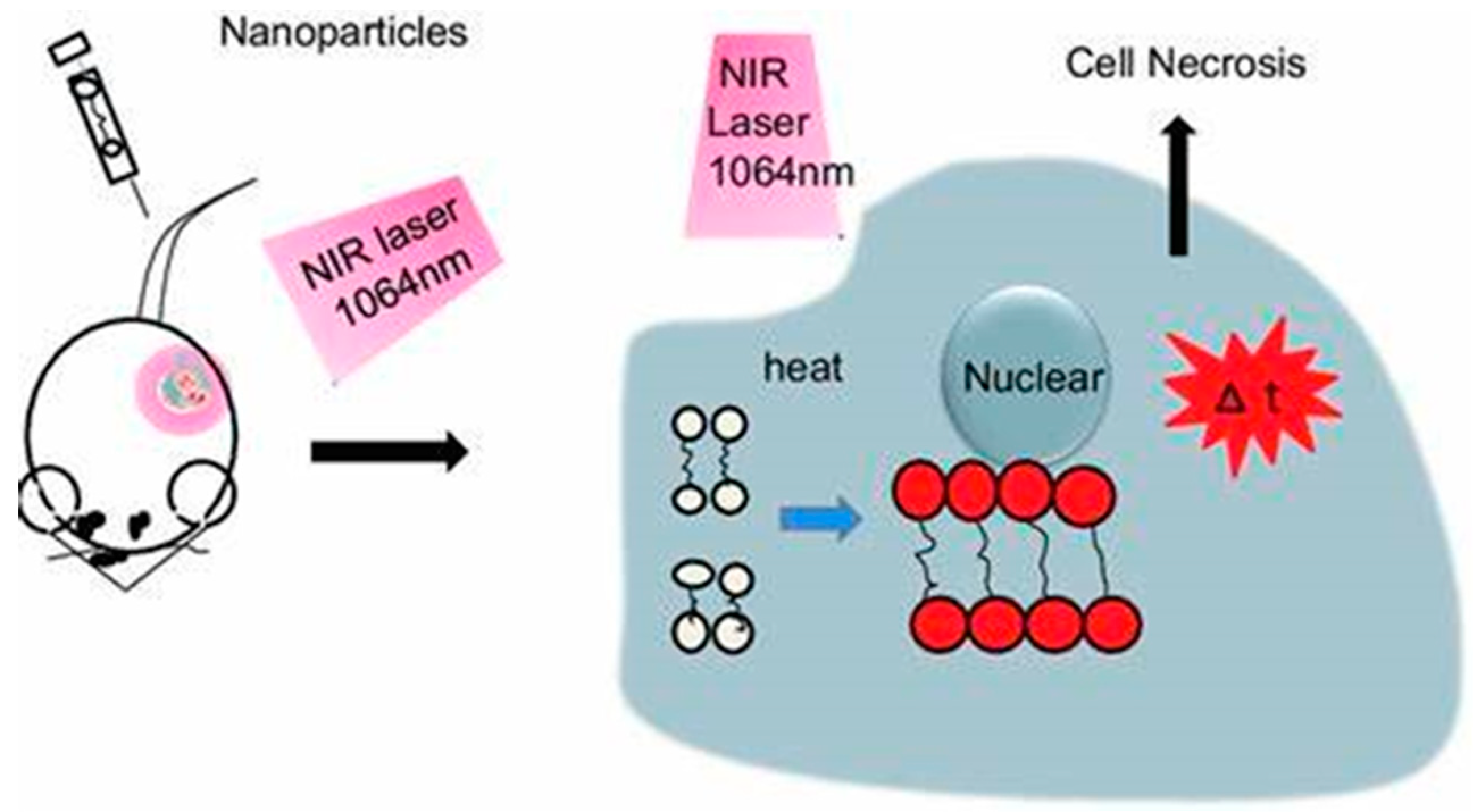
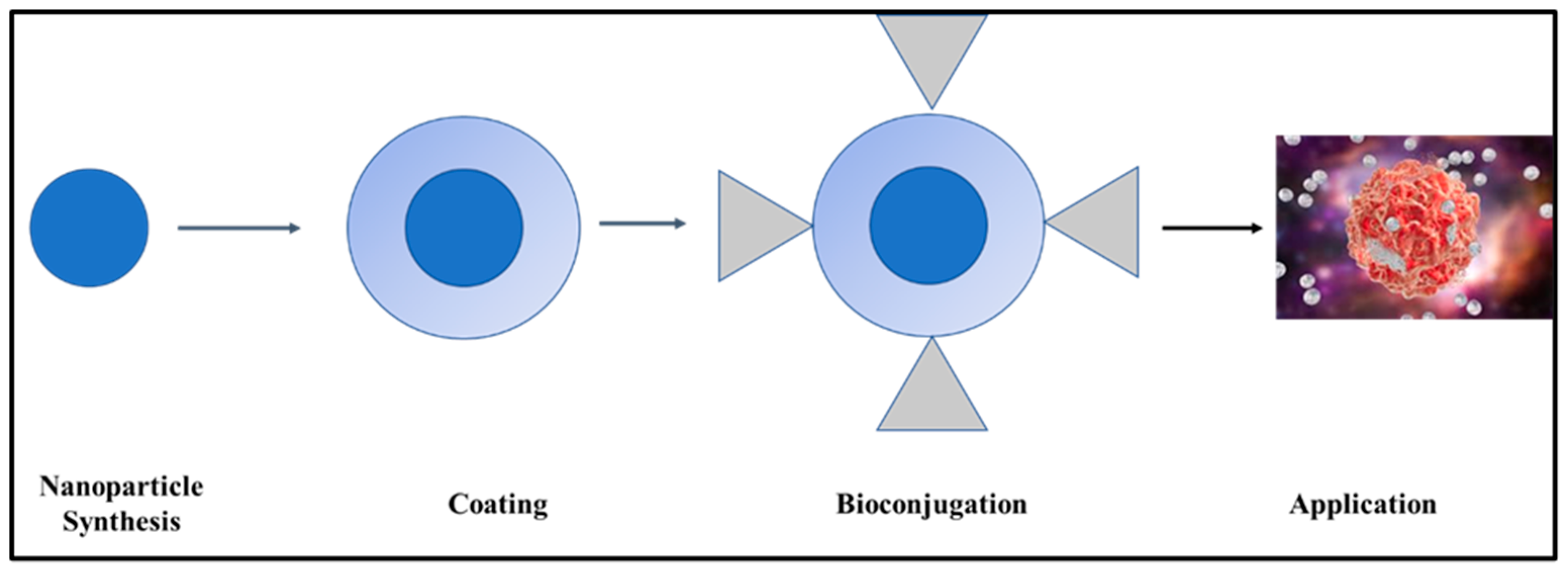
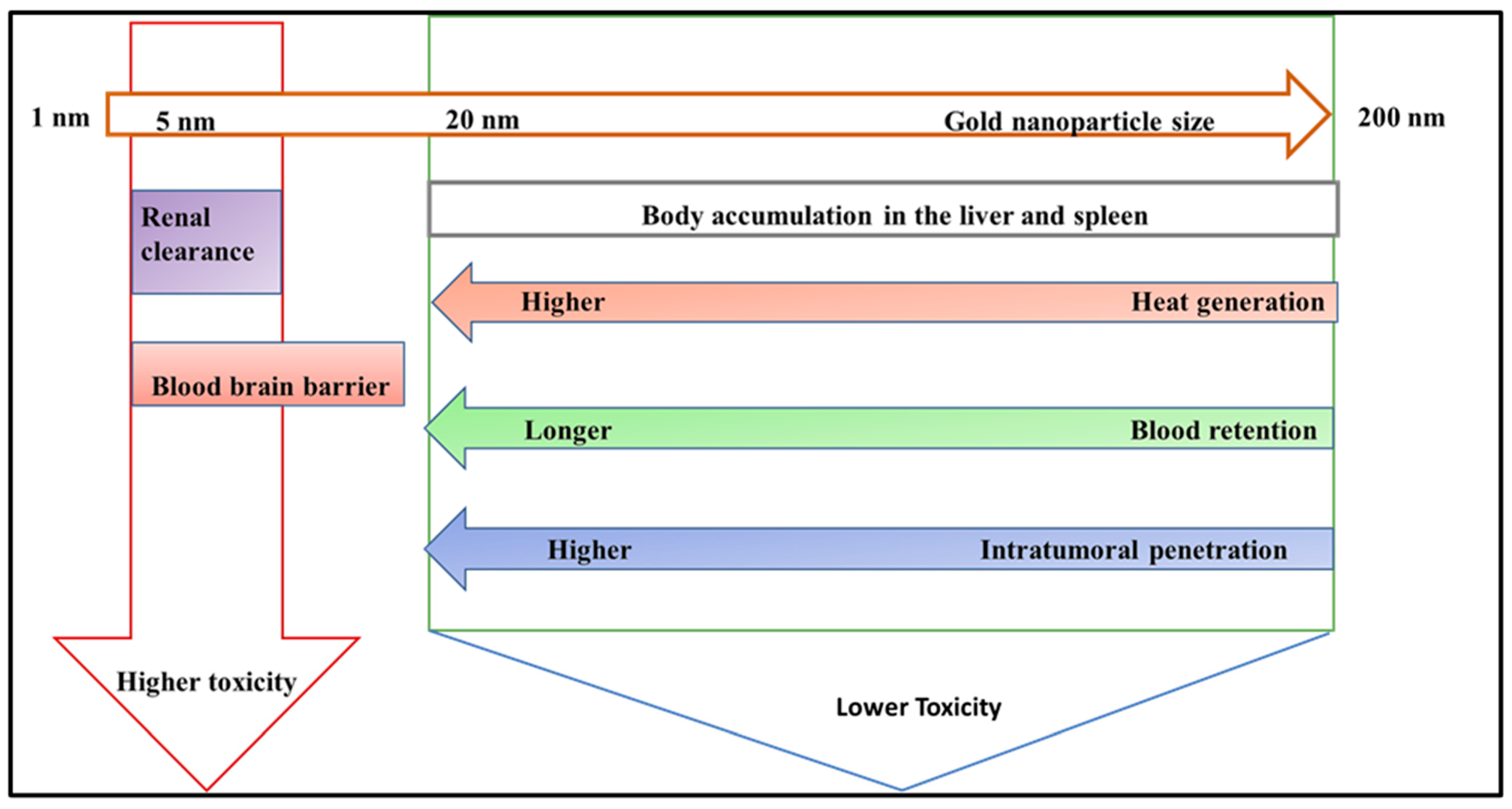
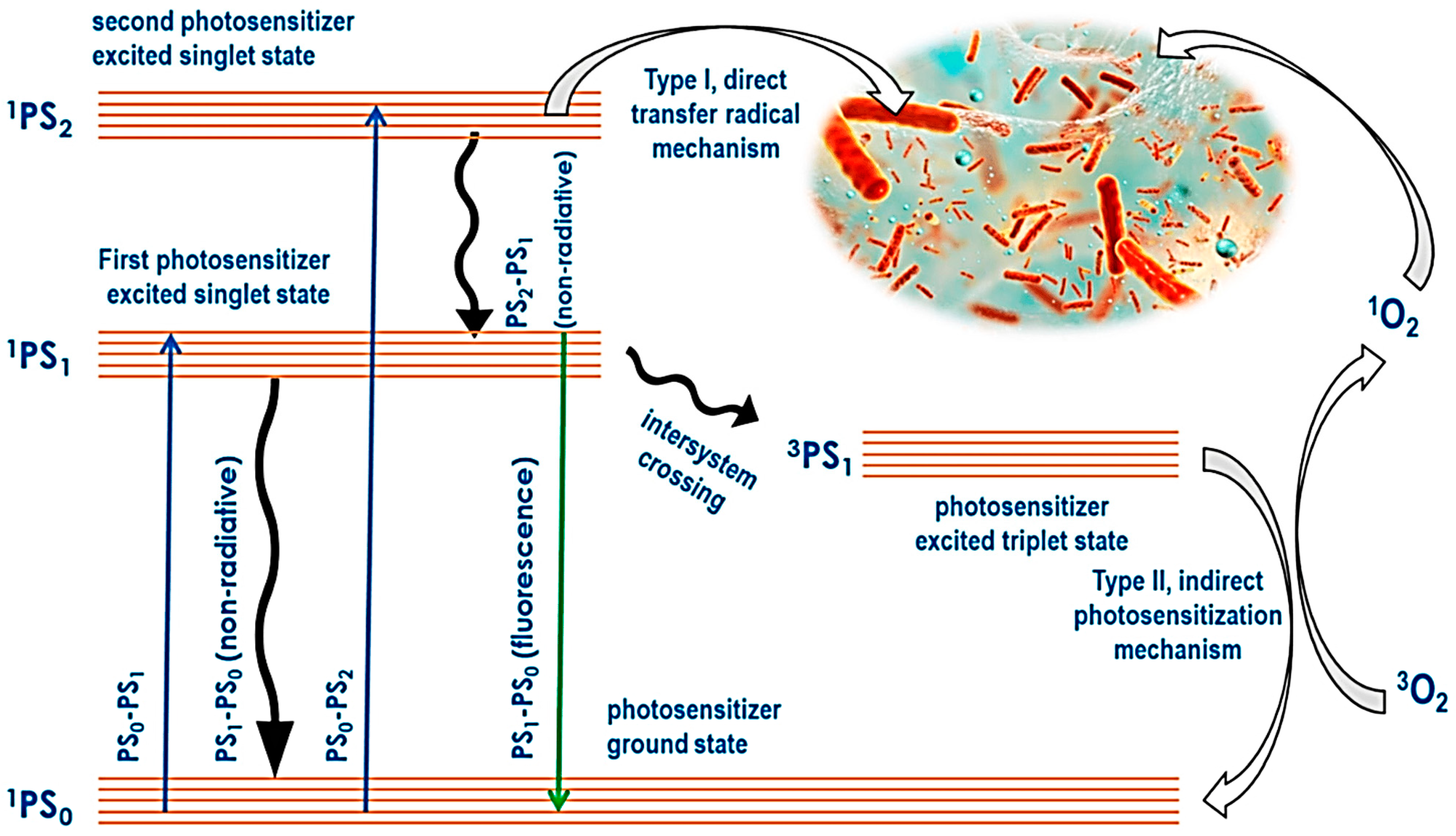


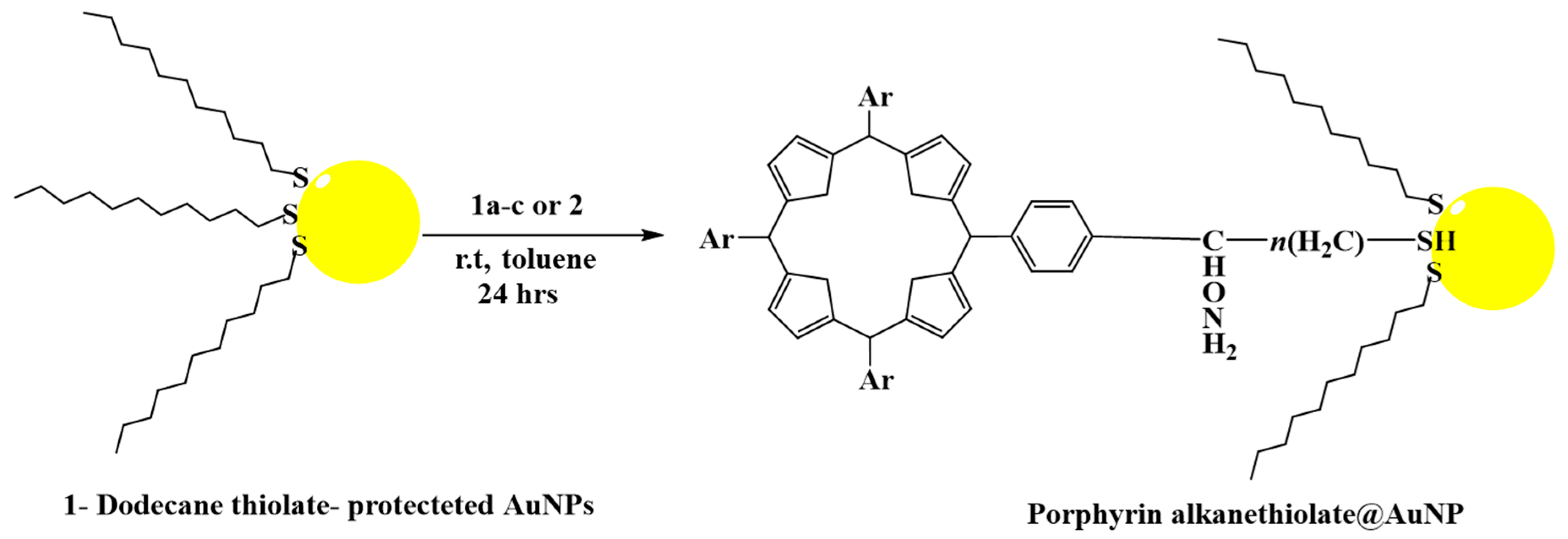
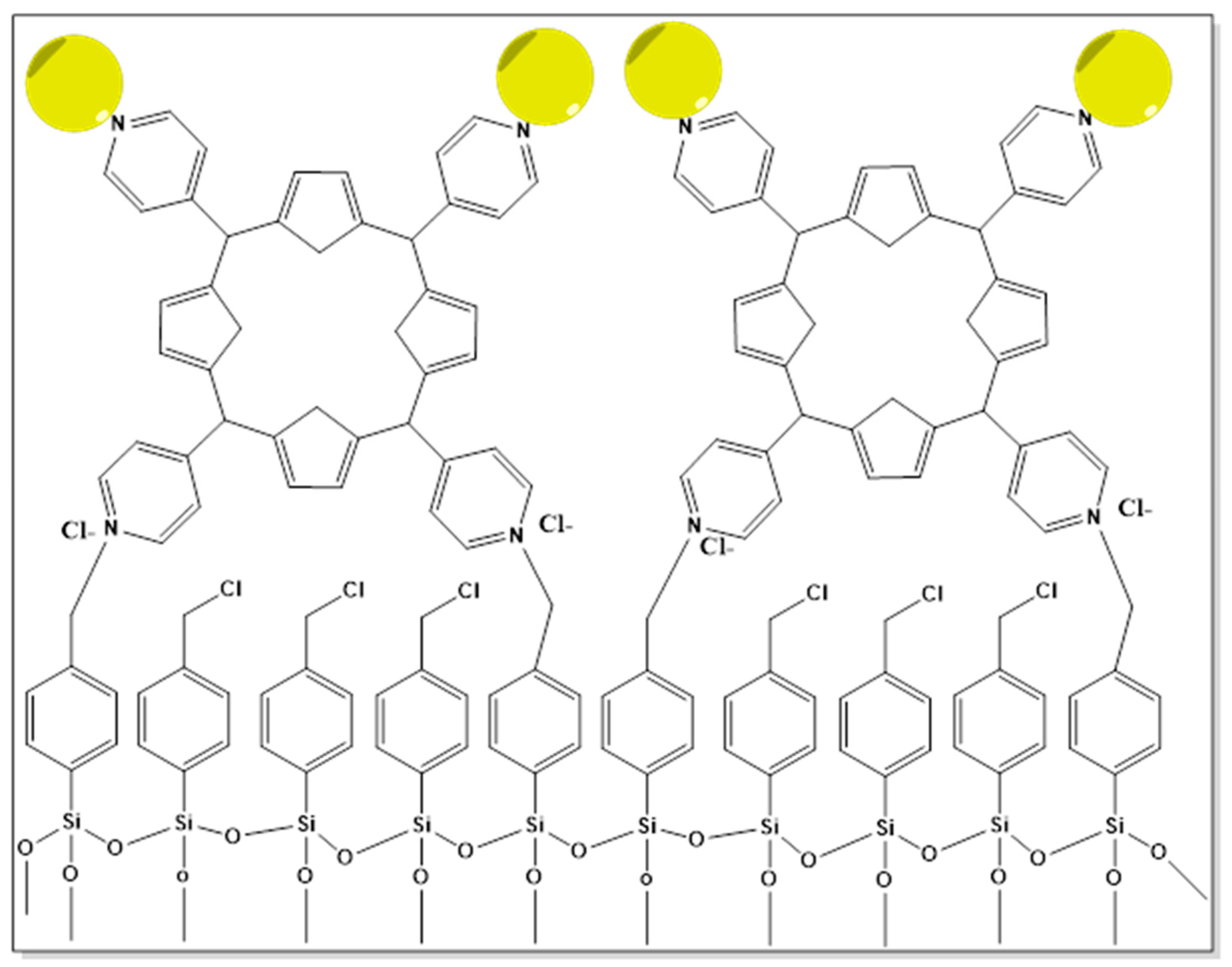

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlapisi, N.; Songca, S.P.; Ajibade, P.A. Capped Plasmonic Gold and Silver Nanoparticles with Porphyrins for Potential Use as Anticancer Agents—A Review. Pharmaceutics 2024, 16, 1268. https://doi.org/10.3390/pharmaceutics16101268
Hlapisi N, Songca SP, Ajibade PA. Capped Plasmonic Gold and Silver Nanoparticles with Porphyrins for Potential Use as Anticancer Agents—A Review. Pharmaceutics. 2024; 16(10):1268. https://doi.org/10.3390/pharmaceutics16101268
Chicago/Turabian StyleHlapisi, Nthabeleng, Sandile P. Songca, and Peter A. Ajibade. 2024. "Capped Plasmonic Gold and Silver Nanoparticles with Porphyrins for Potential Use as Anticancer Agents—A Review" Pharmaceutics 16, no. 10: 1268. https://doi.org/10.3390/pharmaceutics16101268






