Application of Gold Nanoparticles for Improvement of Electroporation-Assisted Drug Delivery and Bleomycin Electrochemotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Electroporation Setup and Parameters
2.2. Cell Culture
2.3. Cell Permeabilization
2.4. Viability Assay
2.5. The Synthesis of 13 nm Gold Nanoparticles
2.6. Statistical Analysis
3. Results
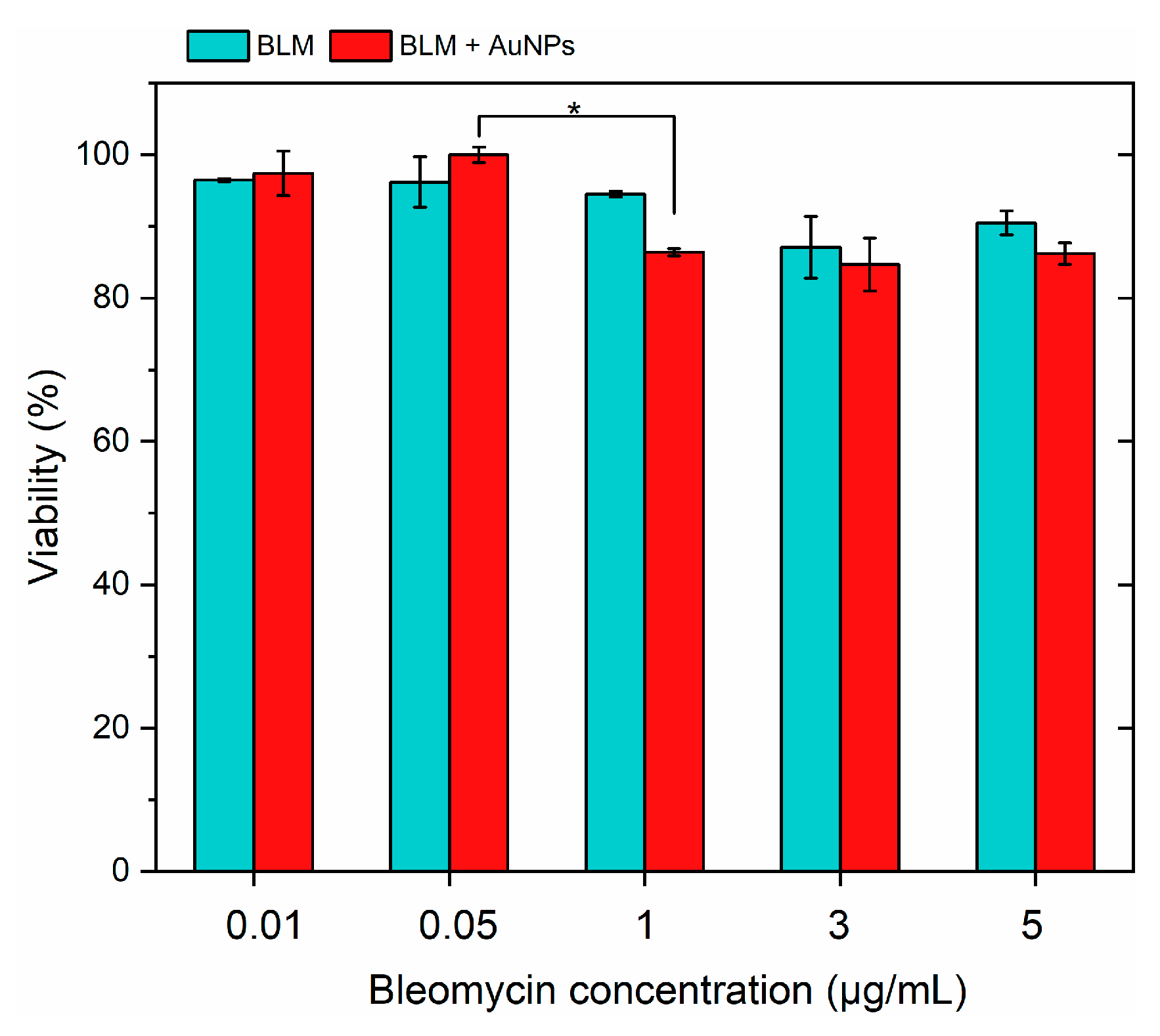
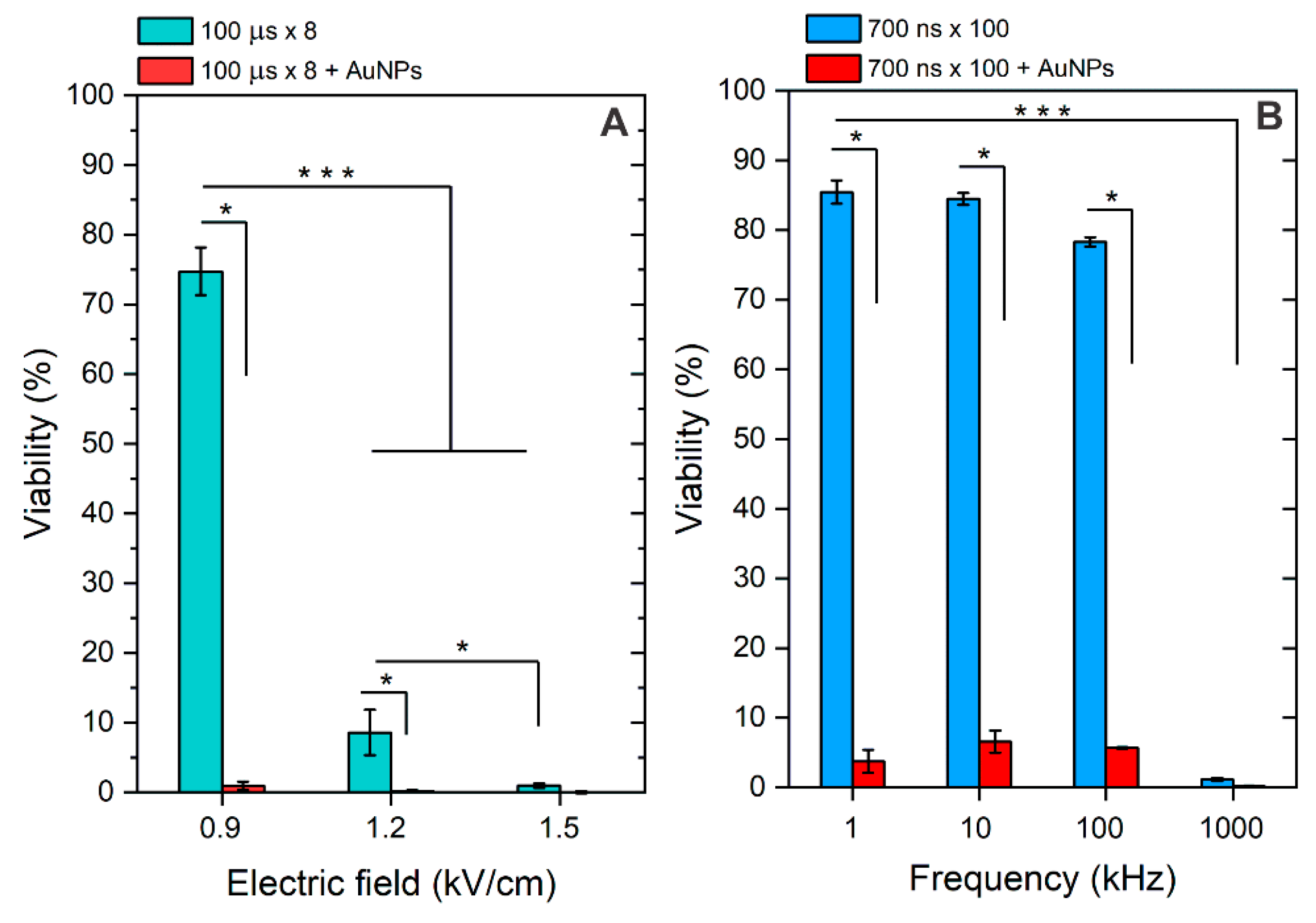
3.1. Effects of BLM and AuNPs on Cell Viability
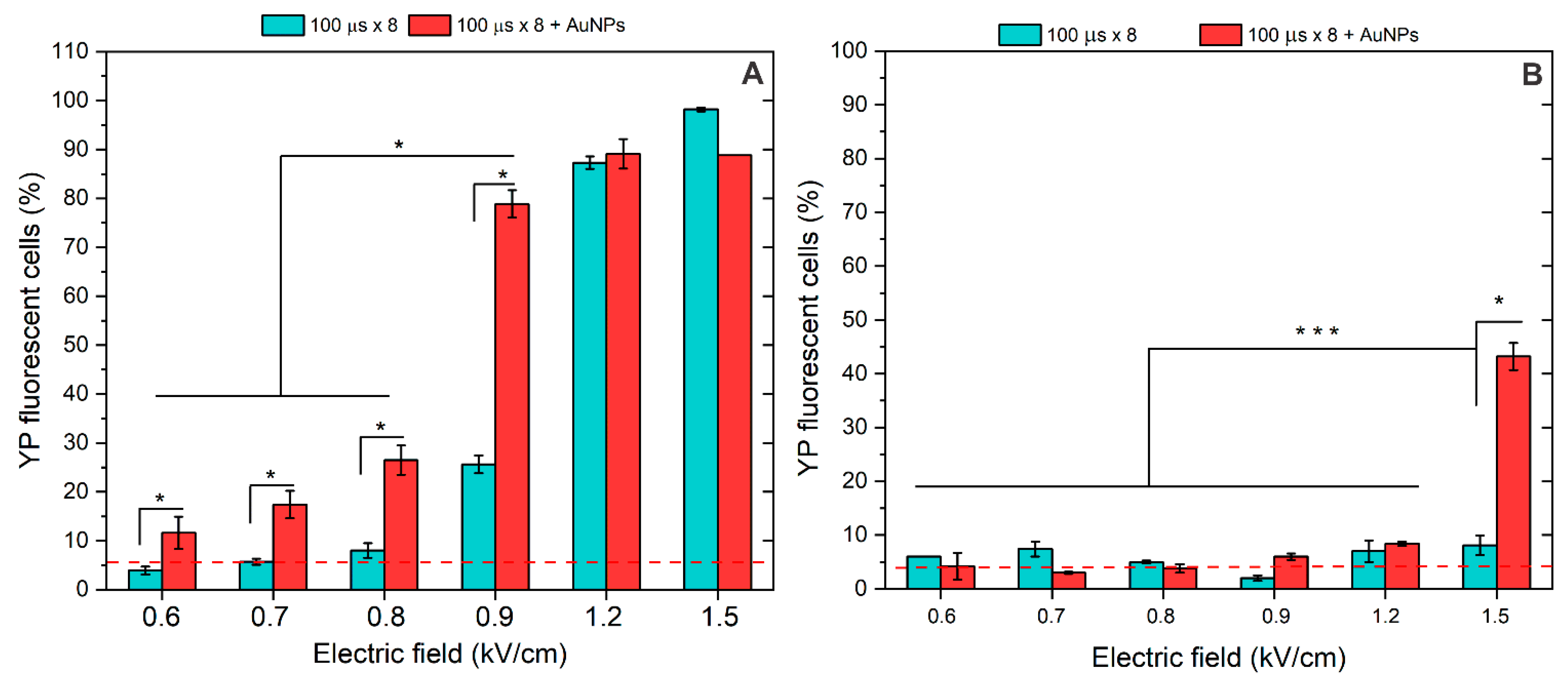
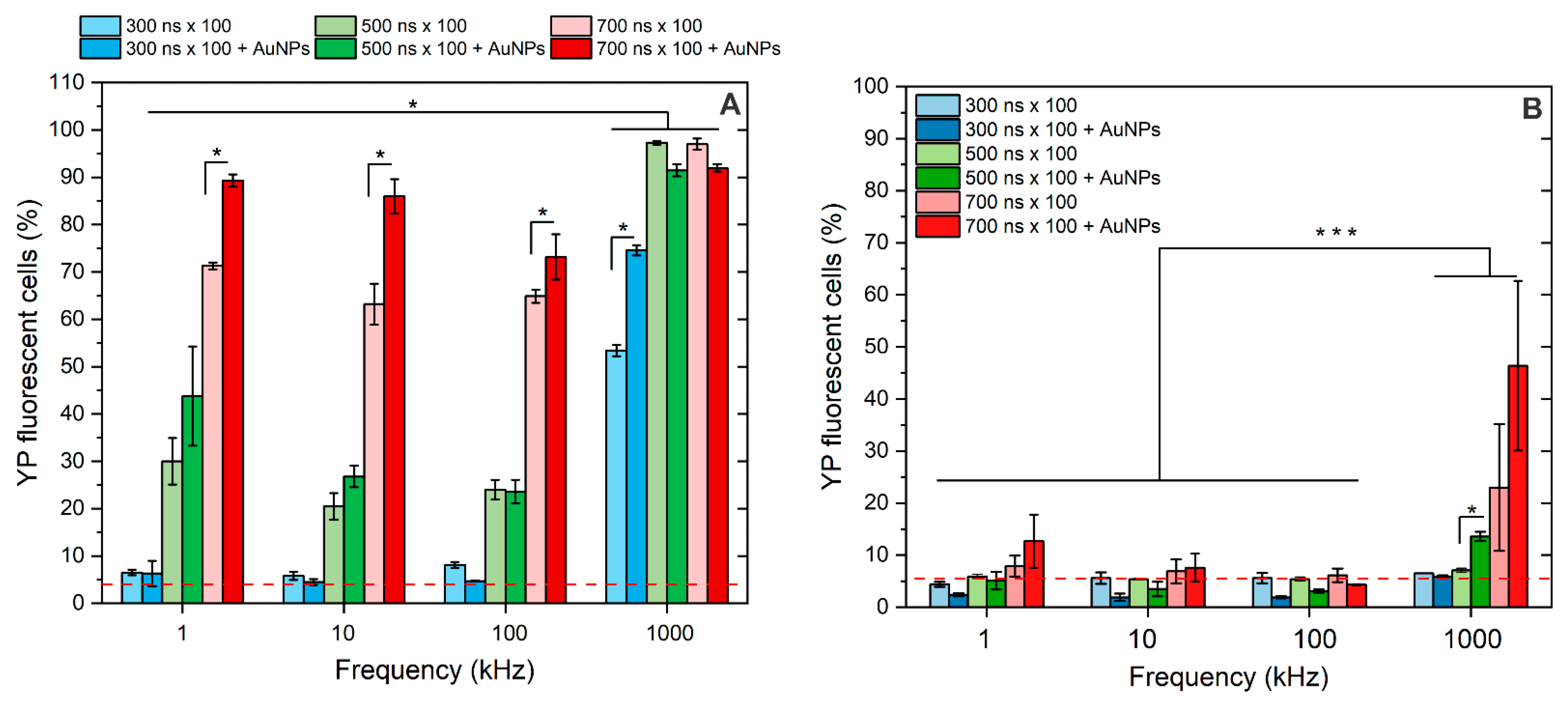
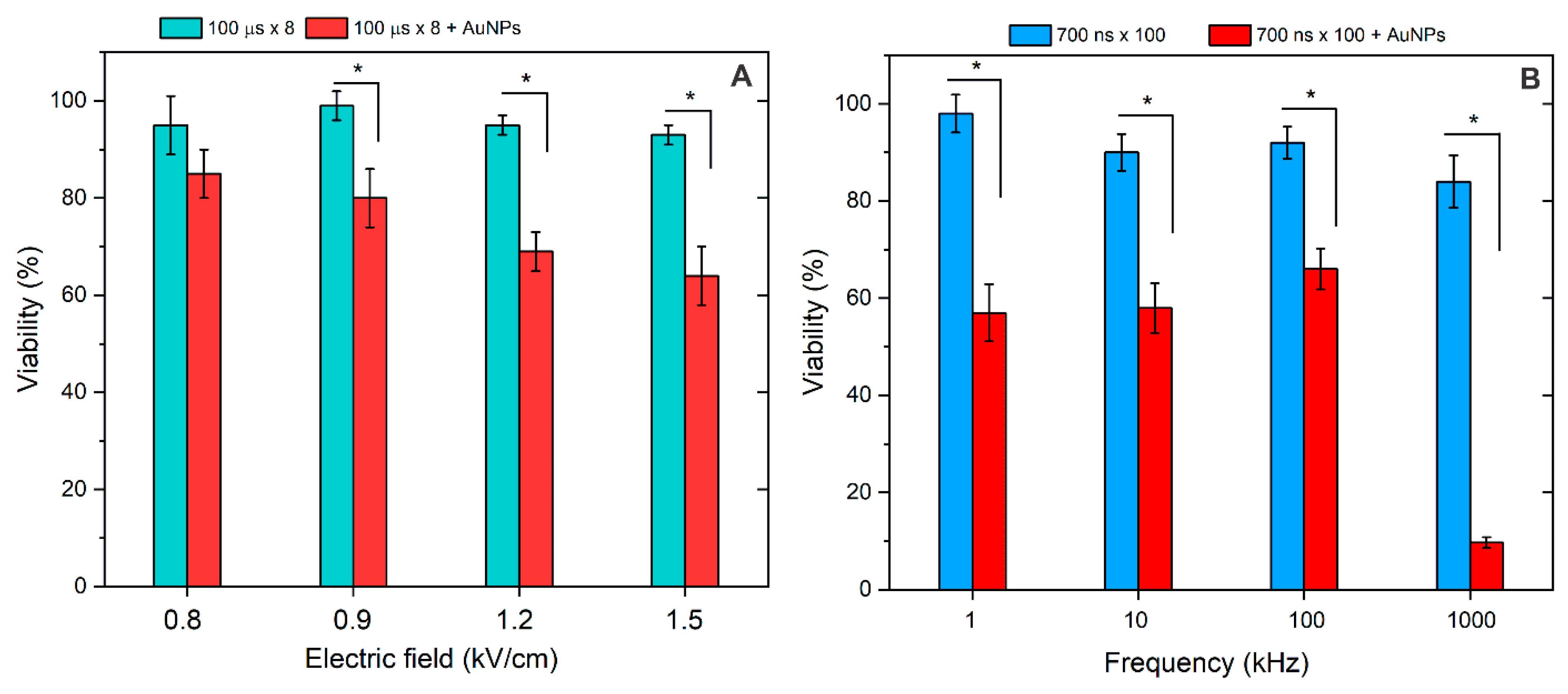
3.2. Membrane Permeabilization Using Nano- and Microsecond Electric Fields
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gehl, J. Drug and Gene Electrotransfer in Cancer Therapy. In Somatic Genome Manipulation: Advances, Methods, and Applications; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–15. ISBN 9781493923892. [Google Scholar]
- Miklavčič, D.; Mali, B.; Kos, B.; Heller, R.; Serša, G. Electrochemotherapy: From the Drawing Board into Medical Practice. Biomed. Eng. Online 2014, 13, 29. [Google Scholar] [CrossRef]
- Pucihar, G.; Krmelj, J.; Reberšek, M.; Napotnik, T.B.; Miklavčič, D. Equivalent Pulse Parameters for Electroporation. IEEE Trans. Biomed. Eng. 2011, 58, 3279–3288. [Google Scholar] [CrossRef]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D.; et al. Electrochemotherapy—An Easy, Highly Effective and Safe Treatment of Cutaneous and Subcutaneous Metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) Study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- Ivorra, A.; Al-Sakere, B.; Rubinsky, B.; Mir, L.M. Use of Conductive Gels for Electric Field Homogenization Increases the Antitumor Efficacy of Electroporation Therapies. Phys. Med. Biol. 2008, 53, 6605–6618. [Google Scholar] [CrossRef]
- Edd, J.F.; Davalos, R.V. Mathematical Modeling of Irreversible Electroporation for Treatment Planning. Technol. Cancer Res. Treat. 2007, 6, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Schoenbach, K.H.; Hargrave, B.; Joshi, R.P.; Kolb, J.F.; Nuccitelli, R.; Osgood, C.; Pakhomov, A.; Stacey, M.; Swanson, R.J.; White, J.A.; et al. Bioelectric Effects of Intense Nanosecond Pulses. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 1088–1107. [Google Scholar] [CrossRef]
- Bhonsle, S.P.; Arena, C.B.; Sweeney, D.C.; Davalos, R.V. Mitigation of Impedance Changes Due to Electroporation Therapy Using Bursts of High-Frequency Bipolar Pulses. Biomed. Eng. Online 2015, 14 (Suppl. 3), S3. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Dong, S.; Zhao, Y.; Lv, Y.; Liu, H.; Gong, L.; Ma, J.; Wang, H.; Sun, Y. Bipolar Microsecond Pulses and Insulated Needle Electrodes for Reducing Muscle Contractions during Irreversible Electroporation. IEEE Trans. Biomed. Eng. 2017, 64, 2924–2937. [Google Scholar] [CrossRef]
- Novickij, V.; Balevičiute, A.; Malysko, V.; Želvys, A.; Radzevičiute, E.; Kos, B.; Zinkeviciene, A.; Miklavčič, D.; Novickij, J.; Girkontaite, I. Effects of Time Delay Between Unipolar Pulses in High Frequency Nano-Electrochemotherapy. IEEE Trans. Biomed. Eng. 2022, 69, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Miklavčič, D.; Pucihar, G.; Pavlovec, M.; Ribarič, S.; Mali, M.; MačEk-Lebar, A.; Petkovšek, M.; Nastran, J.; Kranjc, S.; Čemažar, M.; et al. The Effect of High Frequency Electric Pulses on Muscle Contractions and Antitumor Efficiency in Vivo for a Potential Use in Clinical Electrochemotherapy. Bioelectrochemistry 2005, 65, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Mahnič-Kalamiza, S.; Miklavčič, D. Scratching the Electrode Surface: Insights into a High-Voltage Pulsed-Field Application from in Vitro & in Silico Studies in Indifferent Fluid. Electrochim. Acta 2020, 363, 137187. [Google Scholar] [CrossRef]
- Sano, M.B.; Fan, R.E.; Cheng, K.; Saenz, Y.; Sonn, G.A.; Hwang, G.L.; Xing, L. Reduction of Muscle Contractions during Irreversible Electroporation Therapy Using High-Frequency Bursts of Alternating Polarity Pulses: A Laboratory Investigation in an Ex Vivo Swine Model. J. Vasc. Interv. Radiol. 2018, 29, 893–898.e4. [Google Scholar] [CrossRef] [PubMed]
- Novickij, V.; Ruzgys, P.; Grainys, A.; Šatkauskas, S. High Frequency Electroporation Efficiency Is under Control of Membrane Capacitive Charging and Voltage Potential Relaxation. Bioelectrochemistry 2018, 119, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Semenov, I.; Casciola, M.; Ibey, B.L.; Xiao, S.; Pakhomov, A.G. Electropermeabilization of Cells by Closely Spaced Paired Nanosecond-Range Pulses. Bioelectrochemistry 2018, 121, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Radzevičiūtė, E.; Malyško-Ptašinskė, V.; Kulbacka, J.; Rembiałkowska, N.; Novickij, J.; Girkontaitė, I.; Novickij, V. Nanosecond Electrochemotherapy Using Bleomycin or Doxorubicin: Influence of Pulse Amplitude, Duration and Burst Frequency. Bioelectrochemistry 2022, 148, 108251. [Google Scholar] [CrossRef]
- Vižintin, A.; Marković, S.; Ščančar, J.; Miklavčič, D. Electroporation with Nanosecond Pulses and Bleomycin or Cisplatin Results in Efficient Cell Kill and Low Metal Release from Electrodes. Bioelectrochemistry 2021, 140, 107798. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Pliquett, U.; Chen, X.; Ford, W.; James Swanson, R.; Beebe, S.J.; Kolb, J.F.; Schoenbach, K.H. Nanosecond Pulsed Electric Fields Cause Melanomas to Self-Destruct. Biochem. Biophys. Res. Commun. 2006, 343, 351–360. [Google Scholar] [CrossRef]
- Radzeviciute-Valciuke, E.; Zelvys, A.; Mickeviciute, E.; Gecaite, J.; Zinkeviciene, A.; Malysko-Ptasinske, V.; Kaseta, V.; Novickij, J.; Ivaskiene, T.; Novickij, V. Calcium Electrochemotherapy for Tumor Eradication and the Potential of High-Frequency Nanosecond Protocols. Pharmaceuticals 2023, 16, 1083. [Google Scholar] [CrossRef]
- Balevičiūtė, A.; Radzevičiūtė, E.; Želvys, A.; Malyško-Ptašinskė, V.; Novickij, J.; Zinkevičienė, A.; Kašėta, V.; Novickij, V.; Girkontaitė, I. High-Frequency Nanosecond Bleomycin Electrochemotherapy and Its Effects on Changes in the Immune System and Survival. Cancers 2022, 14, 6254. [Google Scholar] [CrossRef]
- Lekner, J. Electroporation in Cancer Therapy without Insertion of Electrodes. Phys. Med. Biol. 2014, 59, 6031–6042. [Google Scholar] [CrossRef]
- Qiu, H.; Joshi, R.P.; Pradhan, A. Simulation of Nanoparticle Based Enhancement of Cellular Electroporation for Biomedical Applications. J. Appl. Phys. 2014, 116, 184701. [Google Scholar] [CrossRef]
- Rezaee, Z.; Yadollahpour, A.; Bayati, V.; Dehbashi, F.N. Gold Nanoparticles and Electroporation Impose Both Separate and Synergistic Radiosensitizing Effects in HT-29 Tumor Cells: An in Vitro Study. Int. J. Nanomed. 2017, 12, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Miklavcic, D.; Novickij, V.; Kranjc, M.; Polajzer, T.; Haberl Meglic, S.; Batista Napotnik, T.; Romih, R.; Lisjak, D. Contactless Electroporation Induced by High Intensity Pulsed Electromagnetic Fields via Distributed Nanoelectrodes. Bioelectrochemistry 2020, 132, 107440. [Google Scholar] [CrossRef] [PubMed]
- Radzeviciute-Valciuke, E.; Gecaite, J.; Zelvys, A.; Zinkeviciene, A.; Zalneravicius, R.; Malysko-Ptasinske, V.; Nemeikaite-Ceniene, A.; Kaseta, V.; German, N.; Novickij, J.; et al. Improving NonViral Gene Delivery Using MHz Bursts of Nanosecond Pulses and Gold Nanoparticles for Electric Field Amplification. Pharmaceutics 2023, 15, 1178. [Google Scholar] [CrossRef]
- Ghorbel, A.; Andre, F.M.; Mir, L.M.; Garcia-Sanchez, T. Electrophoresis-Assisted Accumulation of Conductive Nanoparticles for the Enhancement of Cell Electropermeabilization. Bioelectrochemistry 2021, 137, 107642. [Google Scholar] [CrossRef] [PubMed]
- Novickij, V.; Staigvila, G.; Murauskas, A.; Rembialkowska, N.; Kulbacka, J.; Novickij, J. High Frequency Bipolar Electroporator with Double-Crowbar Circuit for Load-Independent Forming of Nanosecond Pulses. Appl. Sci. 2022, 12, 1370. [Google Scholar] [CrossRef]
- Mühlpfordt, H. The Preparation of Colloidal Gold Particles Using Tannic Acid as an Additional Reducing Agent. Experientia 1982, 38, 1127–1128. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Nastajute, G.; Snitka, V.; Kausaite, A.; German, N.; Barauskas-Memenas, D.; Ramanavicius, A. Spectrophotometric Evaluation of Gold Nanoparticles as Red-Ox Mediator for Glucose Oxidase. Sens. Actuators B Chem. 2009, 137, 483–489. [Google Scholar] [CrossRef]
- German, N.; Ramanavicius, A.; Voronovic, J.; Ramanaviciene, A. Glucose Biosensor Based on Glucose Oxidase and Gold Nanoparticles of Different Sizes Covered by Polypyrrole Layer. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 413, 224–230. [Google Scholar] [CrossRef]
- Romaskevic, T.; Sedlevicius, M.; Budriene, S.; Ramanavicius, A.; Ryskevic, N.; Miasojedovas, S.; Ramanaviciene, A. Assembly and Characterization of Polyurethane-Gold Nanoparticle Conjugates. Macromol. Chem. Phys. 2011, 212, 2291–2299. [Google Scholar] [CrossRef]
- Niżnik, Ł.; Noga, M.; Kobylarz, D.; Frydrych, A.; Krośniak, A.; Kapka-Skrzypczak, L.; Jurowski, K. Gold Nanoparticles (AuNPs)-Toxicity, Safety and Green Synthesis: A Critical Review. Int. J. Mol. Sci. 2024, 25, 4057. [Google Scholar] [CrossRef] [PubMed]
- Condello, M.; D’Avack, G.; Spugnini, E.P.; Meschini, S. Electrochemotherapy: An Alternative Strategy for Improving Therapy in Drug-Resistant SOLID Tumors. Cancers 2022, 14, 4341. [Google Scholar] [CrossRef]
- Romeo, S.; Sannino, A.; Scarfì, M.R.; Vernier, P.T.; Cadossi, R.; Gehl, J.; Zeni, O. ESOPE-Equivalent Pulsing Protocols for Calcium Electroporation: An In Vitro Optimization Study on 2 Cancer Cell Models. Technol. Cancer Res. Treat. 2018, 17, 1533033818788072. [Google Scholar] [CrossRef]
- Maier, A.S.; Informatika, T.; Teknik, F.; Indonesia, U.K. Plasmonics: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 9780387378251. [Google Scholar]
- Prodan, E.; Nordlander, P. Plasmon Hybridization in Spherical Nanoparticles. J. Chem. Phys. 2004, 120, 5444–5454. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Huang, S.; Liao, W.-C.; Lu, Y.; Wang, S. Gold Nanoparticles Enhanced Electroporation for Mammalian Cell Transfection. J. Biomed. Nanotechnol. 2014, 10, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Yarmush, M.L.; Golberg, A.; Serša, G.; Kotnik, T.; Miklavčič, D. Electroporation-Based Technologies for Medicine: Principles, Applications, and Challenges. Annu. Rev. Biomed. Eng. 2014, 16, 295–320. [Google Scholar] [CrossRef] [PubMed]
- Tietze, R.; Zaloga, J.; Unterweger, H.; Lyer, S.; Friedrich, R.P.; Janko, C.; Pöttler, M.; Dürr, S.; Alexiou, C. Magnetic Nanoparticle-Based Drug Delivery for Cancer Therapy. Biochem. Biophys. Res. Commun. 2015, 468, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Wahajuddin; Arora, S. Superparamagnetic Iron Oxide Nanoparticles: Magnetic Nanoplatforms as Drug Carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lekešytė, B.; Mickevičiūtė, E.; Malakauskaitė, P.; Szewczyk, A.; Radzevičiūtė-Valčiukė, E.; Malyško-Ptašinskė, V.; Želvys, A.; German, N.; Ramanavičienė, A.; Kulbacka, J.; et al. Application of Gold Nanoparticles for Improvement of Electroporation-Assisted Drug Delivery and Bleomycin Electrochemotherapy. Pharmaceutics 2024, 16, 1278. https://doi.org/10.3390/pharmaceutics16101278
Lekešytė B, Mickevičiūtė E, Malakauskaitė P, Szewczyk A, Radzevičiūtė-Valčiukė E, Malyško-Ptašinskė V, Želvys A, German N, Ramanavičienė A, Kulbacka J, et al. Application of Gold Nanoparticles for Improvement of Electroporation-Assisted Drug Delivery and Bleomycin Electrochemotherapy. Pharmaceutics. 2024; 16(10):1278. https://doi.org/10.3390/pharmaceutics16101278
Chicago/Turabian StyleLekešytė, Barbora, Eglė Mickevičiūtė, Paulina Malakauskaitė, Anna Szewczyk, Eivina Radzevičiūtė-Valčiukė, Veronika Malyško-Ptašinskė, Augustinas Želvys, Natalija German, Almira Ramanavičienė, Julita Kulbacka, and et al. 2024. "Application of Gold Nanoparticles for Improvement of Electroporation-Assisted Drug Delivery and Bleomycin Electrochemotherapy" Pharmaceutics 16, no. 10: 1278. https://doi.org/10.3390/pharmaceutics16101278
APA StyleLekešytė, B., Mickevičiūtė, E., Malakauskaitė, P., Szewczyk, A., Radzevičiūtė-Valčiukė, E., Malyško-Ptašinskė, V., Želvys, A., German, N., Ramanavičienė, A., Kulbacka, J., Novickij, J., & Novickij, V. (2024). Application of Gold Nanoparticles for Improvement of Electroporation-Assisted Drug Delivery and Bleomycin Electrochemotherapy. Pharmaceutics, 16(10), 1278. https://doi.org/10.3390/pharmaceutics16101278












