Application of the Thermal Analysis of Frozen Aqueous Solutions to Assess the Miscibility of Hyaluronic Acid and Polymers Used for Dissolving Microneedles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermal Analysis of Frozen Solutions and Solids
2.3. Preparation of Polymer Films and Dissolving Microneedles
3. Results
3.1. Properties of Single-Solute Frozen Polymer Solutions
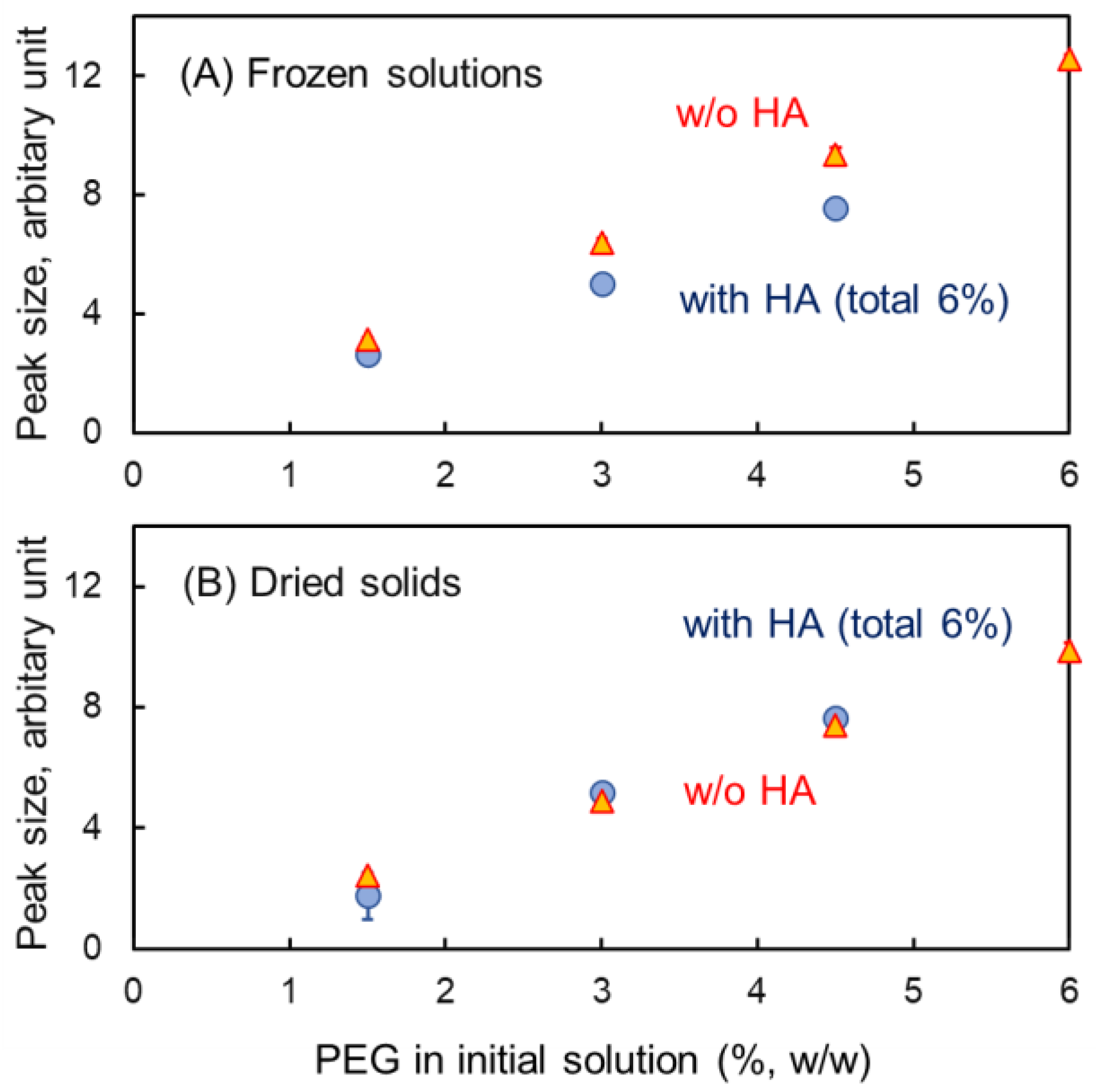
3.2. Mixing States of Freeze-Concentrated HA and Polymer Combinations
3.3. Mixing States of HA and Crystallizing Solutes in Frozen Solutions
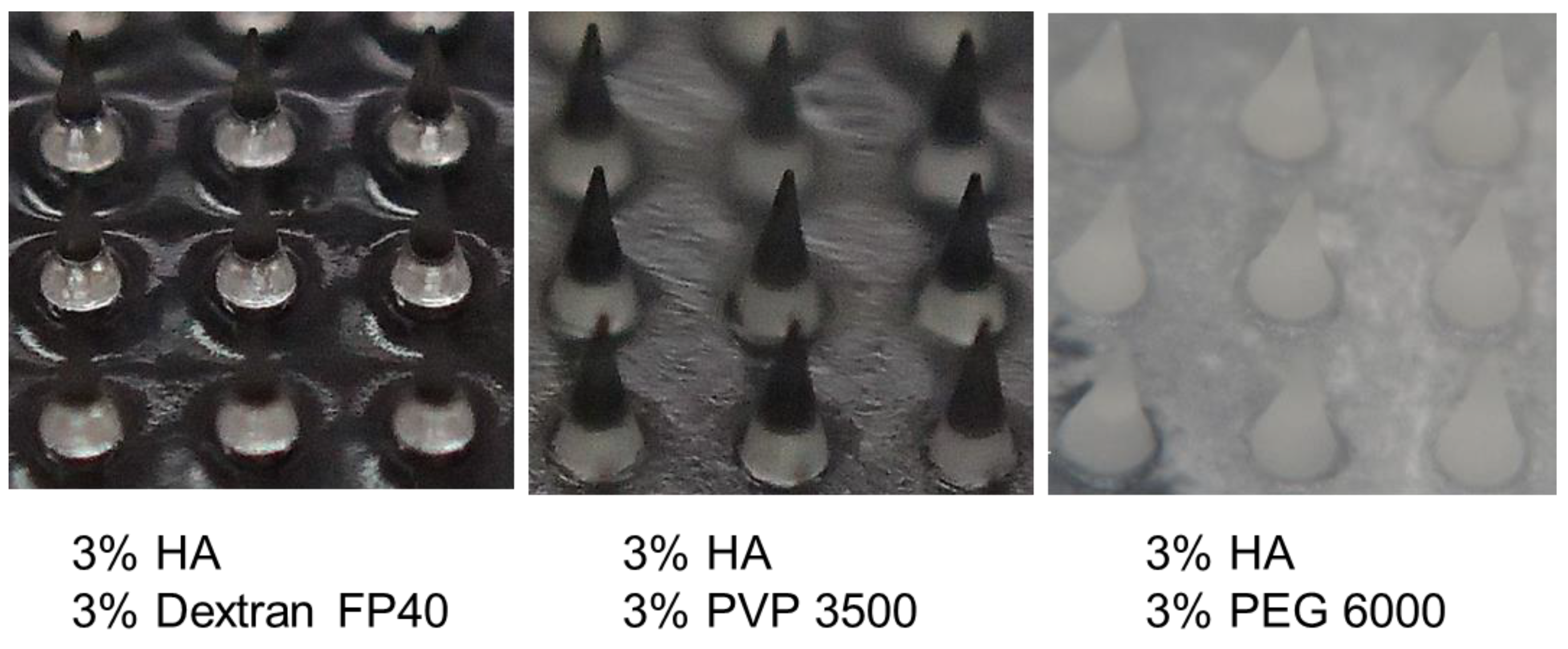
3.4. Characterization of HA-Based Polymer Films and Microneedles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harrer, D.; Armengol, E.S.; Friedl, J.D.; Jalil, A.; Jelkmann, M.; Leichner, C.; Laffleur, F. Is hyaluronic acid the perfect excipient for the pharmaceutical need? Int. J. Pharm. 2021, 601, 120589. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Lei, C.; Liu, X.R.; Chen, Q.B.; Li, Y.; Zhou, J.L.; Zhou, L.Y.; Zou, T. Hyaluronic acid and albumin based nanoparticles for drug delivery. J. Control. Release 2021, 331, 416–433. [Google Scholar] [CrossRef] [PubMed]
- Saha, I.; Rai, V.K. Hyaluronic acid based microneedle array: Recent applications in drug delivery and cosmetology Carbohydr. Polym. 2021, 267, 118168. [Google Scholar] [CrossRef]
- Ramadon, D.; Karn, P.R.; Anjani, Q.K.; Kim, M.-H.; Cho, D.Y.; Hwang, H.; Kim, D.H.; Kim, D.H.; Kim, G.; Lee, K.; et al. Development of ropivacaine hydrochloride-loaded dissolving microneedles as a local anesthetic agent: A proof-of-concept. Int. J. Pharm. 2024, 660, 124347. [Google Scholar] [CrossRef] [PubMed]
- Hirobe, S.; Azukizawa, H.; Hanafusa, T.; Matsuo, K.; Quan, Y.S.; Kamiyama, F.; Katayama, I.; Okada, N.; Nakagawa, S. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials 2015, 57, 50–58. [Google Scholar] [CrossRef]
- Aldawood, F.K.; Andar, A.; Desai, S. A comprehensive review of microneedles: Types, materials, processes, characterizations and applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef] [PubMed]
- Ando, D.; Miyatsuji, M.; Sakoda, H.; Yamamoto, E.; Miyazaki, T.; Koide, T.; Sato, Y.; Izutsu, K. Mechanical characterization of dissolving microneedles: Factors affecting physical strength of needles. Pharmaceutics 2024, 16, 200. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.J.; Liu, Y.; Lim, S.H.; Loh, X.J.; Kang, L.; Lim, C.Y.; Phua, K.K.L. Formulation, characterization and evaluation of mRNA-loaded dissolvable polymeric microneedles (RNApatch). Sci. Rep. 2018, 8, 11842. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kashiwara, S.; Fukushima, K.; Takada, K. Two-layered dissolving microneedles for percutaneous delivery of sumatriptan in rats. Drug Dev. Ind. Pharm. 2011, 37, 1387–1393. [Google Scholar] [CrossRef]
- Hiraishi, Y.; Nakagawa, T.; Quan, Y.S.; Kamiyama, F.; Hirobe, S.; Okada, N.; Nakagawa, S. Performance and characteristics evaluation of a sodium hyaluronate-based microneedle patch for a transcutaneous drug delivery system. Int. J. Pharm. 2013, 441, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Fatnassi, M.; Jacquart, S.; Brouillet, F.; Rey, C.; Combes, C.; Fullana, S.G. Optimization of spray-dried hyaluronic acid microspheres to formulate drug-loaded bone substitute materials. Powder Technol. 2014, 255, 44–51. [Google Scholar] [CrossRef]
- Kondo, S.; Kuroyanagi, Y. Development of a wound dressing composed of hyaluronic acid and collagen sponge with epidermal growth factor. J. Biomater. Sci. Polym. Ed. 2012, 23, 629–643. [Google Scholar] [CrossRef]
- Nyamweya, N.N. Applications of polymer blends in drug delivery. Futur. J. Pharm. Sci. 2021, 7, 18. [Google Scholar] [CrossRef]
- Yusova, A.A.; Lipatova, I.M. Rheological and film-forming properties of mixed sodium alginate and hyaluronate solutions. Fibre Chem. 2014, 46, 143–146. [Google Scholar] [CrossRef]
- Suriyaamporn, P.; Opanasopit, P.; Ngawhirunpat, T.; Rangsimawong, W. Computer-aided rational design for optimally Gantrez® S-97 and hyaluronic acid-based dissolving microneedles as a potential ocular delivery system. J. Drug Deliv. Sci. Technol. 2021, 61, 102319. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, J.-A.; Kim, J.C.; Park, H.; Yang, E.; Park, J.S.; Song, M.; Park, J.-H. Microneedles with dual release pattern for improved immunological efficacy of Hepatitis B vaccine. Int. J. Pharm. 2020, 591, 119928. [Google Scholar] [CrossRef] [PubMed]
- Rezaeeyazdi, M.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable hyaluronic acid-co-gelatin cryogels for tissue-engineering applications. Materials 2018, 11, 1374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, Z.; Kong, L.; Liu, L.; Liu, Q.; Zhao, Y.; Zeng, W.; Yi, Q.; Cao, D. Preparation and characterization of hyaluronic acid hydrogel blends with gelatin. J. Macromol. Sci. B 2012, 51, 2392–2400. [Google Scholar] [CrossRef]
- Sionkowska, A.; Gadomska, M.; Musiał, K.; Piątek, J. Hyaluronic acid as a component of natural polymer blends for biomedical applications: A review. Molecules 2020, 25, 4035. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, C.; Chen, T.; Jiang, Z.; Lu, C.; Wu, C.; Pan, X.; Huang, Z.; Peng, T. State-of-the-art strategies to enhance the mechanical properties of microneedles. Int. J. Pharm. 2024, 663, 124547. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, S.H.; Lee, B.Y.; Kim, S.J.; Sung, C.Y.; Jang, N.K.; Kim, J.D.; Jeong, D.H.; Ryu, H.Y.; Lee, S. A comparative study of dissolving hyaluronic acid microneedles with trehalose and poly(vinyl pyrrolidone) for efficient peptide drug delivery. Biomater. Sci. 2018, 6, 2566–2570. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, R.; Qureshi, O.S.; Yousaf, A.M.; Raza, S.A.; Sarwar, H.S.; Shahnaz, G.; Saleem, U.; Sohail, M.F. Enhanced solubility and biopharmaceutical performance of atorvastatin and metformin via electrospun polyvinylpyrrolidone-hyaluronic acid composite nanoparticles. Eur. J. Pharm. Sci. 2021, 161, 105817. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Wodo, O.; Ganapathysubramanian, B. How do evaporating thin films evolve? Unravelling phase-separation mechanisms during solvent-based fabrication of polymer blends. Appl. Phys. Lett. 2014, 105, 153104. [Google Scholar] [CrossRef]
- Shingel, K.I.; Selyanin, M.; Filion, M.C.; Polyak, F. Solid dispersions of drugs in hyaluronan matrix: The role of the biopolymer in modulating drug activity in vivo. J. Drug Deliv. Sci. Technol. 2017, 39, 140–146. [Google Scholar] [CrossRef]
- Choi, H.J.; Yoo, D.G.; Bondy, B.J.; Quan, F.S.; Compans, R.W.; Kang, S.M.; Prausnitz, M.R. Stability of influenza vaccine coated onto microneedles. Biomaterials 2012, 33, 3756–3769. [Google Scholar] [CrossRef] [PubMed]
- Picard, J.; Giraudier, S.; Larreta-Garde, V. Controlled remodeling of a protein-polysaccharide mixed gel: Examples of gelatin-hyaluronic acid mixtures. Soft Matter 2009, 5, 4198–4205. [Google Scholar] [CrossRef]
- Liu, S.; Jin, M.N.; Quan, Y.S.; Kamiyama, F.; Katsumi, H.; Sakane, T.; Yamamoto, A. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J. Control. Release 2012, 161, 933–941. [Google Scholar] [CrossRef]
- Zhou, Z.; He, S.; Huang, T.; Peng, C.; Zhou, H.; Liu, Q.; Zeng, W.; Liu, L.; Huang, H.; Xiang, L.; et al. Preparation of gelatin/hyaluronic acid microspheres with different morphologies for drug delivery. Polym. Bull. 2015, 72, 713–723. [Google Scholar] [CrossRef]
- Lewandowska, K. Miscibility studies of hyaluronic acid and poly(vinyl alcohol) blends in various solvents. Materials 2020, 13, 4750. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, K.; Yui, N. Regulated insulin release from biodegradable dextran hydrogels containing poly(ethylene glycol). J. Control. Release 1996, 42, 237–248. [Google Scholar] [CrossRef]
- Broguiere, N.; Husch, A.; Palazzolo, G.; Bradke, F.; Madduri, S.; Zenobi-Wong, M. Macroporous hydrogels derived from aqueous dynamic phase separation. Biomaterials 2019, 200, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.A.; Taylor, L.S. Evaluation of amorphous solid dispersion properties using thermal analysis techniques. Adv. Drug Deliv. Rev. 2012, 64, 396–421. [Google Scholar] [CrossRef] [PubMed]
- Padilla, A.M.; Ivanisevic, I.; Yang, Y.; Engers, D.; Bogner, R.H.; Pikal, M.J. The study of phase separation in amorphous freeze-dried systems. Part I: Raman mapping and computational analysis of XRPD data in model polymer systems. J. Pharm. Sci. 2011, 100, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.A.; Rembert, K.B.; Poyton, M.F.; Okur, H.I.; Kale, A.R.; Yang, T.; Zhang, J.; Cremer, P.S. A stepwise mechanism for aqueous two-phase system formation in concentrated antibody solutions. Proc. Natl. Acad. Sci. USA 2019, 116, 15784–15791. [Google Scholar] [CrossRef]
- Titus, A.R.; Madeira, P.P.; Ferreira, L.A.; Chernyak, V.Y.; Uversky, V.N.; Zaslavsky, B.Y. Mechanism of phase separation in aqueous two-phase systems. Int. J. Mol. Sci. 2022, 23, 14366. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Hsieh, C.-Y.; Yeh, C.-Y.; Lin, F.-H. The development of gelatin/hyaluronate copolymer mixed with calcium sulfate, hydroxyapatite, and stromal-cell-derived factor-1 for bone regeneration enhancement. Polymers 2019, 11, 1454. [Google Scholar] [CrossRef]
- Meng, F.; Dave, V.; Chauhan, H. Qualitative and quantitative methods to determine miscibility in amorphous drug-polymer systems. Eur. J. Pharm. Sci. 2015, 77, 106–111. [Google Scholar] [CrossRef]
- Her, L.M.; Nail, S.L. Measurement of glass transition temperatures of freeze-concentrated solutes by differential scanning calorimetry. Pharm. Res. 1994, 11, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Izutsu, K.; Heller, M.C.; Randolph, T.W.; Carpenter, J.F. Effect of salts and sugars on phase separation of polyvinylpyrrolidone-dextran solutions induced by freeze-concentration. J. Chem. Soc. Faraday Trans. 1998, 94, 411–417. [Google Scholar] [CrossRef]
- Sacha, G.A.; Nail, S.L. Thermal analysis of frozen solutions: Multiple glass transitions in amorphous systems. J. Pharm. Sci. 2009, 98, 3397–3405. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.S.; Randall, C.S. Use of subambient thermal analysis to optimize protein lyophilization. Cryobiology 1992, 29, 632–656. [Google Scholar] [CrossRef]
- Levine, H.; Slade, L. Thermomechanical properties of small-carbohydrate–water glasses and ‘rubbers’: Kinetically metastable systems at sub-zero temperatures. J. Chem. Soc. Faraday Trans. 1 1988, 84, 2619–2633. [Google Scholar] [CrossRef]
- Randolph, T.W. Phase separation of excipients during lyophilization: Effects on protein stability. J. Pharm. Sci. 1997, 86, 1198–1203. [Google Scholar] [CrossRef]
- Ando, D.; Miyazaki, T.; Yamamoto, E.; Koide, T.; Izutsu, K. Chemical imaging analysis of active pharmaceutical ingredient in dissolving microneedle arrays by Raman spectroscopy. Drug Deliv. Transl. Res. 2022, 12, 426–434. [Google Scholar] [CrossRef]
- Ando, D.; Ozawa, A.; Sakaue, M.; Yamamoto, E.; Miyazaki, T.; Sato, Y.; Koide, T.; Izutsu, K. Fabrication and characterization of dissolving microneedles for transdermal drug delivery of apomorphine hydrochloride in Parkinson’s disease. Pharm. Res. 2024, 41, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Le, H.V.; Dulong, V.; Picton, L.; Cerf, D.L. Polyelectrolyte complexes of hyaluronic acid and diethylaminoethyl dextran: Formation, stability and hydrophobicity. Colloid Surf. A 2021, 629, 127485. [Google Scholar] [CrossRef]
- Kayitmazer, A.B.; Koksal, A.F.; Iyilik, E.K. Complex coacervation of hyaluronic acid and chitosan: Effects of pH, ionic strength, charge density, chain length and the charge ratio. Soft Matter 2015, 11, 8605–8612. [Google Scholar] [CrossRef]
- Telang, C.; Yu, L.; Suryanarayanan, R. Effective inhibition of mannitol crystallization in frozen solutions by sodium chloride. Pharm. Res. 2003, 20, 660–667. [Google Scholar] [CrossRef]
- Akers, M.J.; Milton, N.; Byrn, S.R.; Nail, S.L. Glycine crystallization during freezing: The effects of salt form, pH, and ionic strength. Pharm. Res. 1995, 12, 1457–1461. [Google Scholar] [CrossRef]
- Izutsu, K.; Yoshioka, S.; Terao, T. Decreased protein-stabilizing effects of cryoprotectants due to crystallization. Pharm. Res. 1993, 10, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, R.; Auriemma, F.; Gaillet, C.; Rosa, C.D.; Lauprêtre, F. Investigation of the crystallinity of freeze/thaw poly(vinyl alcohol) hydrogels by different techniques. Macromolecules 2004, 37, 9510–9516. [Google Scholar] [CrossRef]
- Stauffer, S.R.; Peppast, N.A. Poly(vinyl alcohol) hydrogels prepared by freezing-thawing cyclic processing. Polymer 1992, 33, 3932–3936. [Google Scholar] [CrossRef]
- Kodavaty, J.; Deshpande, A.P. Self-assembly and drying assisted microstructural domain formation in poly(vinyl alcohol) and hyaluronic acid gels. Polym. Bull. 2017, 74, 3605–3617. [Google Scholar] [CrossRef]
- Izutsu, K.; Yoshioka, S.; Kojima, S.; Randolph, T.W.; Carpenter, J.F. Effects of sugars and polymers on crystallization of poly(ethylene glycol) in frozen solutions: Phase separation between incompatible polymers. Pharm. Res. 1996, 13, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Zaslavsky, B.Y. Aqueous Two-Phase Partitioning; Marcel Dekker: New York, NY, USA, 1995. [Google Scholar]
- Albertsson, P.-Å.; Cajarville, A.; Brooks, D.E.; Tjerneld, F. Partition of proteins in aqueous polymer two-phase systems and the effect of molecular weight of the polymer. Biochim. Biophys. Acta 1987, 926, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.A.; Melchels, F.P.W.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T.J. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 2014, 10, 214–223. [Google Scholar] [CrossRef]
- Hellebust, S.; Nilsson, S.; Blokhus, A.M. Phase behavior of anionic polyelectrolyte mixtures in aqueous solution. Effects of molecular weights, polymer charge density, and ionic strength of solution. Macromolecules 2003, 36, 5372–5382. [Google Scholar] [CrossRef]
- Le, H.V.; Cerf, D.L. Colloidal Polyelectrolyte Complexes from Hyaluronic Acid: Preparation and Biomedical Applications. Small 2022, 18, 2204283. [Google Scholar] [CrossRef]
- Nyamweya, N.; Hoag, S.W. Assessment of polymer-polymer interactions in blends of HPMC and film forming polymers by modulated temperature differential scanning calorimetry. Pharm. Res. 2000, 17, 625–631. [Google Scholar] [CrossRef]
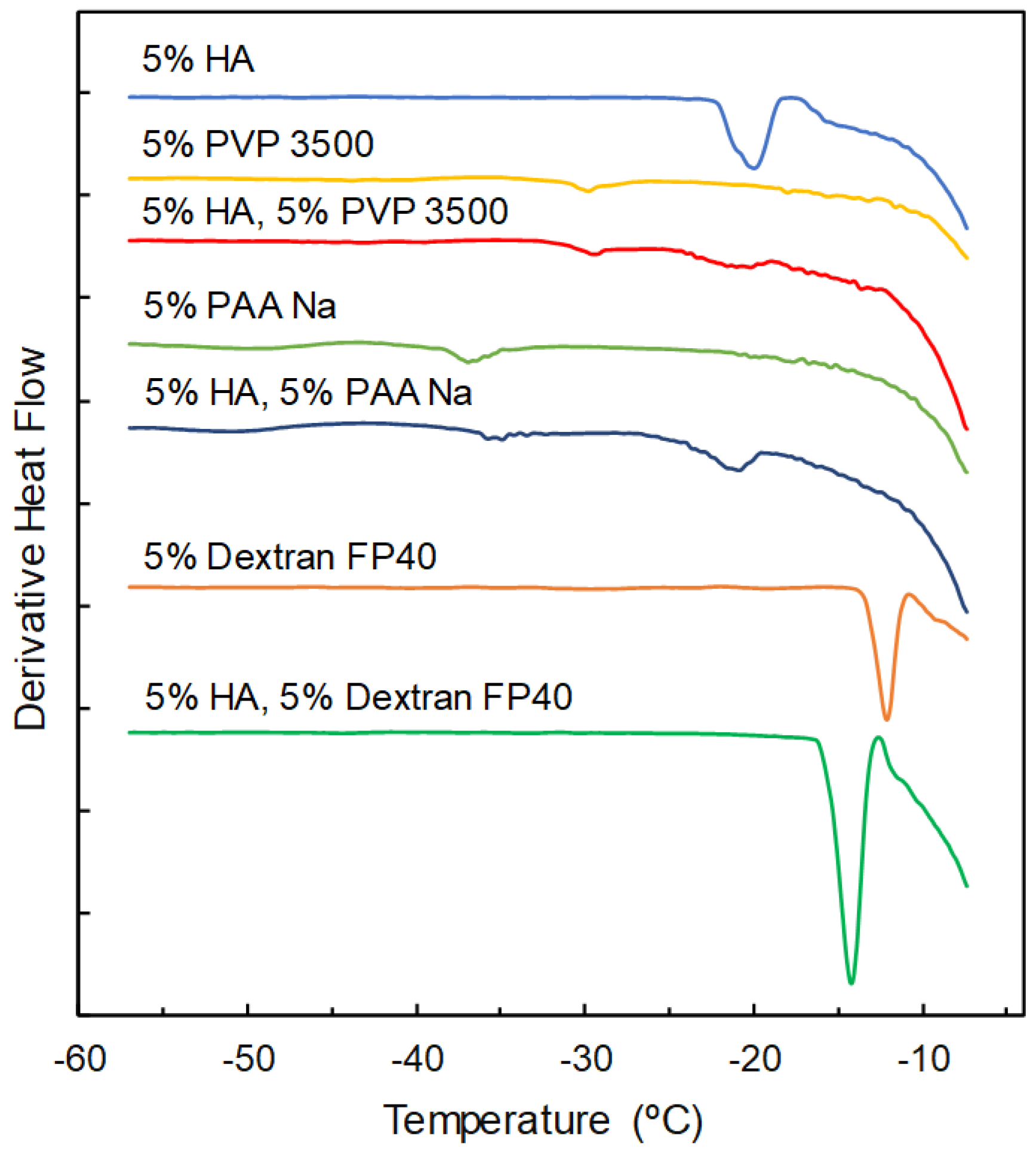
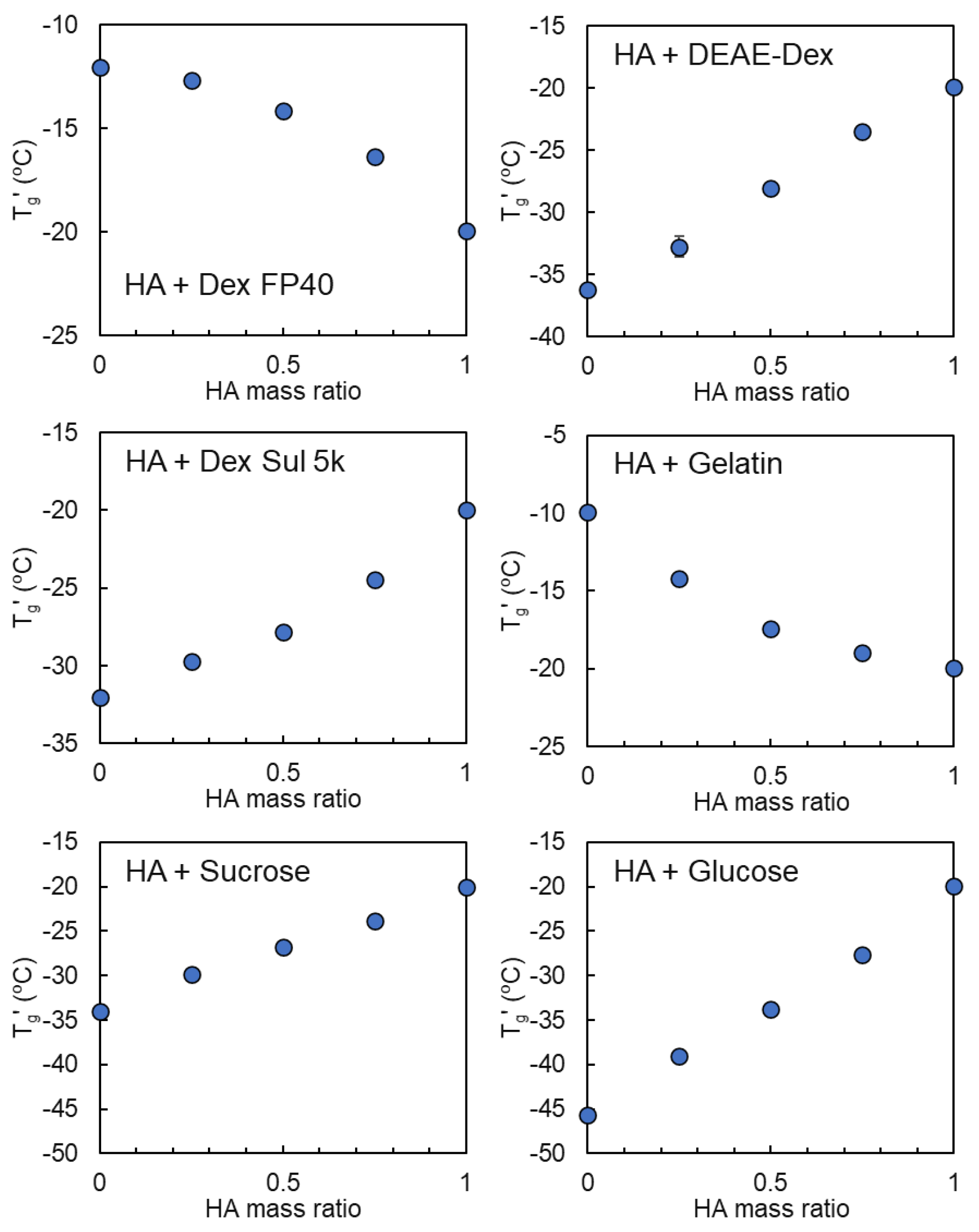
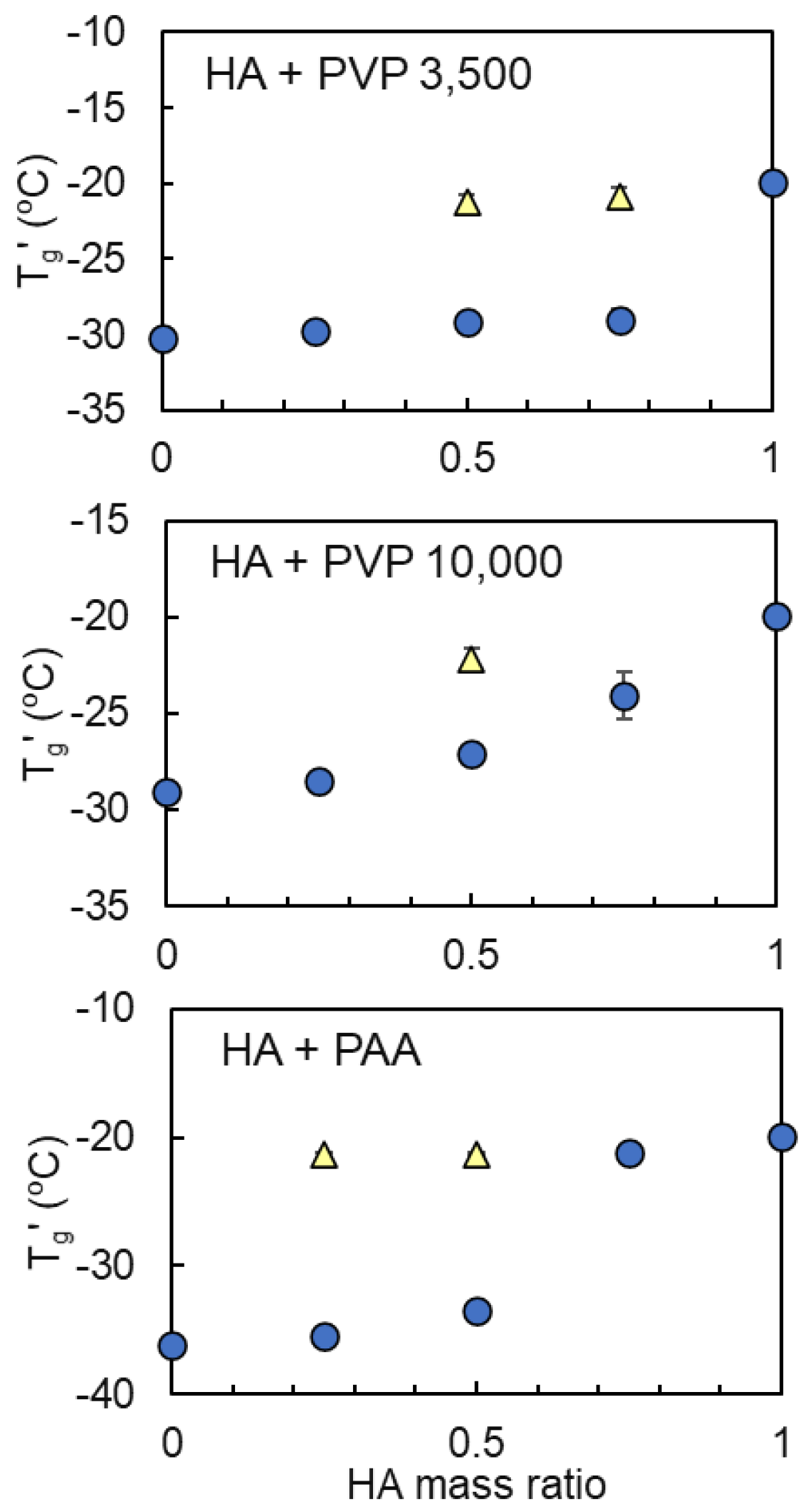


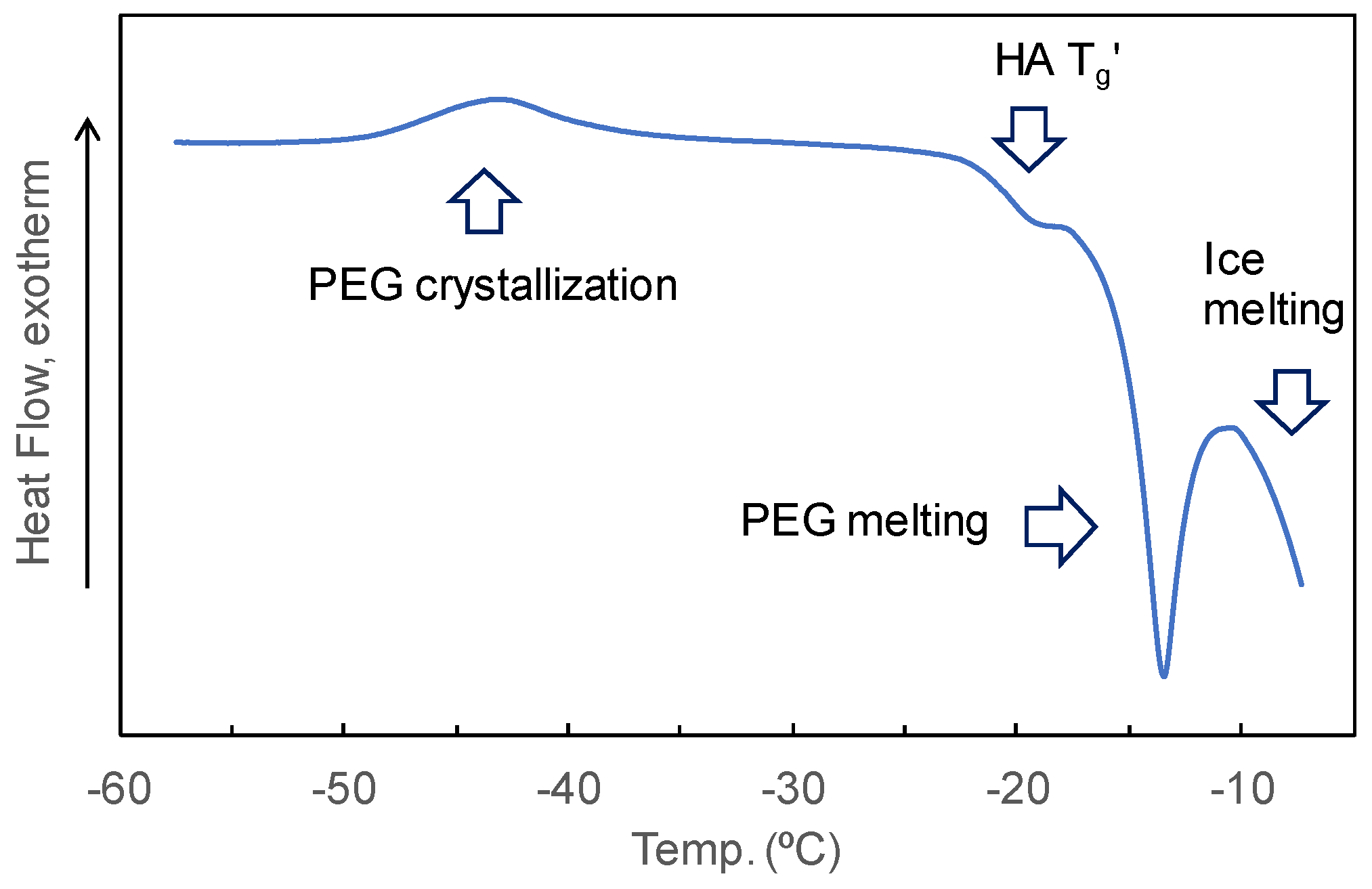
| Sodium Hyaluronate | Transition Temperature | ||
|---|---|---|---|
| Conc. | Name/Size | 1st Scan | 2nd Scan |
| % (w/w) | °C | °C | |
| 1 | FCH-SU 50–110 kDa | −20.01 ± 0.25 | −19.63 ± 0.32 |
| 1 | FCH-60 500–700 kDa | −20.40 ± 0.15 | −19.86 ± 0.17 |
| 1 | FCH-80 600–1000 kDa | −20.51 ± 0.17 | −20.03 ± 0.18 |
| 1 | FCH-120 1000–1400 kDa | −20.54 ± 0.08 | −19.86 ± 0.22 |
| 1.5 | FCH-SU | −20.15 ± 0.11 | −19.64 ± 0.07 |
| 3 | FCH-SU | −20.31 ± 0.07 | −19.65 ± 0.08 |
| 4.5 | FCH-SU | −20.03 ± 0.08 | −19.50 ± 0.14 |
| 5 | FCH-SU | −19.95 ± 0.06 | −19.42 ± 0.08 |
| 6 | FCH-SU | −19.85 ± 0.17 | −19.41 ± 0.12 |
| Transition Temperature | |||
|---|---|---|---|
| Conc. | Name/Size | 1st Scan | 2nd Scan |
| % (w/w) | °C | °C | |
| 6 | Dextran 4 | −18.24 ± 0.09 | −18.15 ± 0.12 |
| 6 | Dextran FP40 | −12.06 ± 0.13 | −11.84 ± 0.11 |
| 6 | PVP 3500 | −30.27 ± 0.27 | −29.81 ± 0.13 |
| 6 | PVP 10,000 | −29.10 ± 0.16 | −28.82 ± 0.16 |
| 6 | PVP 29,000 | −22.85 ± 0.12 | −22.69 ± 0.06 |
| 6 | PVP 40,000 | −22.40 ± 0.19 | −22.13 ± 0.14 |
| 6 | PAA Na | −36.22 ± 0.35 | −35.44 ± 0.23 |
| 6 | DEAE-dextran 10,000 | −36.16 ± 0.07 | −35.73 ± 0.04 |
| 6 | DEAE-dextran HCl | −25.69 ± 0.27 | −25.61 ± 0.06 |
| 6 | Dextran sulfate Na, 7000–20,000 | −23.38 ± 0.01 | −23.03 ± 0.22 |
| 6 | Dextran sulfate Na, 5000 | −32.05 ± 0.34 | −31.64 ± 0.13 |
| 6 | Gelatin | −9.93 ± 0.21 | −9.25 ± 0.11 |
| 6 | Glucose | −45.65 ± 0.33 | −44.34 ± 0.58 |
| 6 | Sucrose | −34.06 ± 0.12 | −33.22 ± 0.31 |
| 6 | Mannitol | Crystallization | n.d. |
| 6 | Glycine | n.d. | n.d. |
| 6 | PEG 6000 | Crystallization/Melting | Crystallization/Melting |
| 6 | Na Alginate | n.d. | n.d. |
| 6 | PVA 10,000 | n.d. | n.d. |
| 6 | CMC Na | n.d. | n.d. |
| 6 | HEC | n.d. | n.d. |
| Polymers | Miscibility in Frozen Solution * | Film Appearance ** | |
|---|---|---|---|
| 6% HA | M | - | |
| 3% HA | 3% Dextran 4 | M | - |
| 3% HA | 3% Dextran FP40 | M | - |
| 3% HA | 3% PVP 3500 | PS | ++ |
| 3% HA | 3% PVP 10,000 | PS | ++ |
| 3% HA | 3% PVP 29,000 | PS | + |
| 3% HA | 3% PVP 40,000 | PS | + |
| 3% HA | 3% PAA Na | PS | ++ |
| 3% HA | 3% DEAE-dextran 10,000 | M | ++ |
| 3% HA | 3% Dextran sulfate Na, 5000 | M | ++ |
| 3% HA | 3% Gelatin | M | - |
| 3% HA | 3% PEG 6000 | PS (crystallized) | +++ |
| 3% HA | 3% Na Alginate | PS | ++ |
| 3% HA | 3% PVA 10,000 | PS | + |
| 3% HA | 3% HEC | M | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izutsu, K.-i.; Yoshida, H.; Abe, Y.; Yamamoto, E.; Sato, Y.; Ando, D. Application of the Thermal Analysis of Frozen Aqueous Solutions to Assess the Miscibility of Hyaluronic Acid and Polymers Used for Dissolving Microneedles. Pharmaceutics 2024, 16, 1280. https://doi.org/10.3390/pharmaceutics16101280
Izutsu K-i, Yoshida H, Abe Y, Yamamoto E, Sato Y, Ando D. Application of the Thermal Analysis of Frozen Aqueous Solutions to Assess the Miscibility of Hyaluronic Acid and Polymers Used for Dissolving Microneedles. Pharmaceutics. 2024; 16(10):1280. https://doi.org/10.3390/pharmaceutics16101280
Chicago/Turabian StyleIzutsu, Ken-ichi, Hiroyuki Yoshida, Yasuhiro Abe, Eiichi Yamamoto, Yoji Sato, and Daisuke Ando. 2024. "Application of the Thermal Analysis of Frozen Aqueous Solutions to Assess the Miscibility of Hyaluronic Acid and Polymers Used for Dissolving Microneedles" Pharmaceutics 16, no. 10: 1280. https://doi.org/10.3390/pharmaceutics16101280
APA StyleIzutsu, K.-i., Yoshida, H., Abe, Y., Yamamoto, E., Sato, Y., & Ando, D. (2024). Application of the Thermal Analysis of Frozen Aqueous Solutions to Assess the Miscibility of Hyaluronic Acid and Polymers Used for Dissolving Microneedles. Pharmaceutics, 16(10), 1280. https://doi.org/10.3390/pharmaceutics16101280







