Utilizing an Ex Vivo Skin Penetration Analysis Model for Predicting Ocular Drug Penetration: A Feasibility Study with Curcumin Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Formulations
2.2.2. Characterization of the Formulations
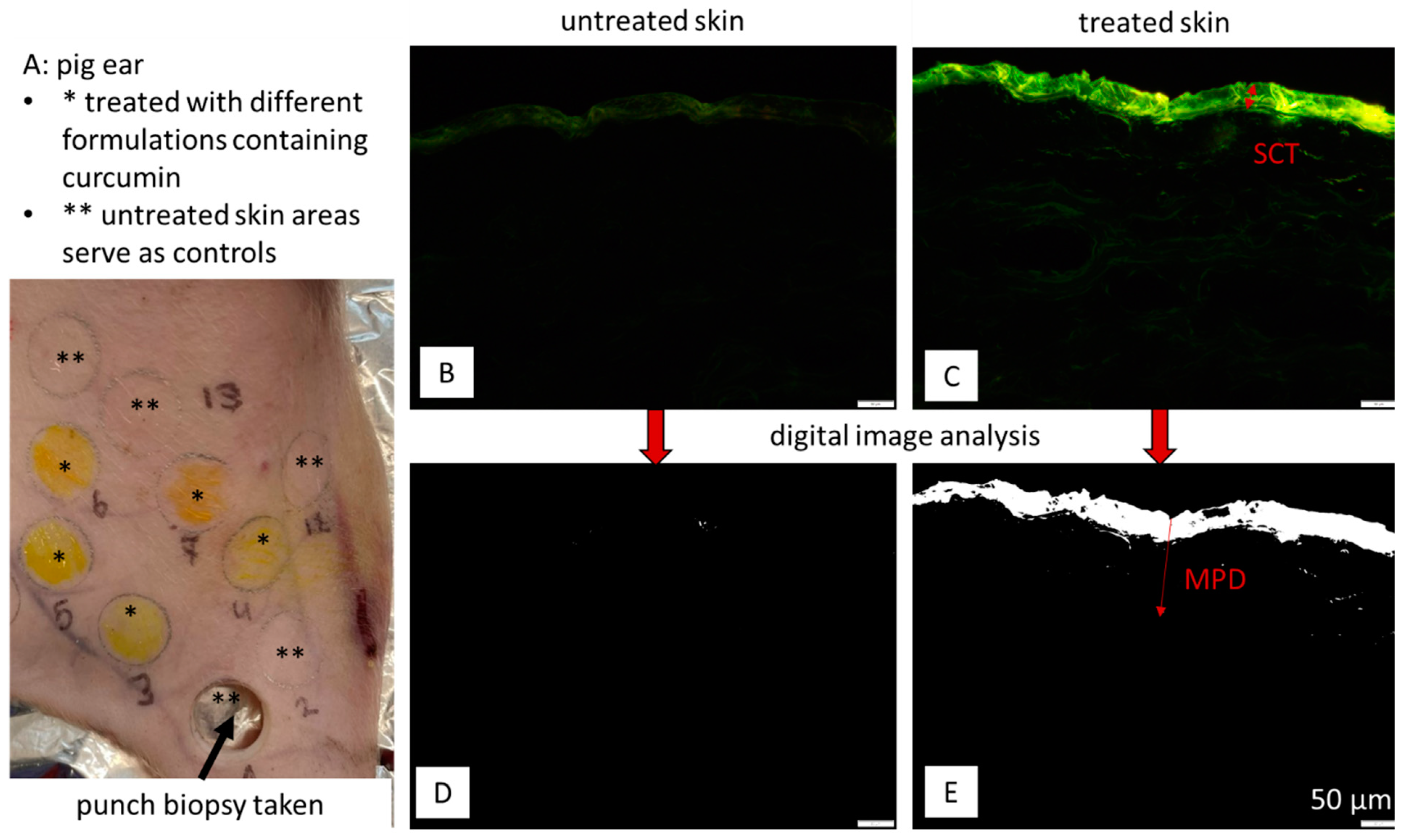
2.2.3. Dermal Penetration Studies
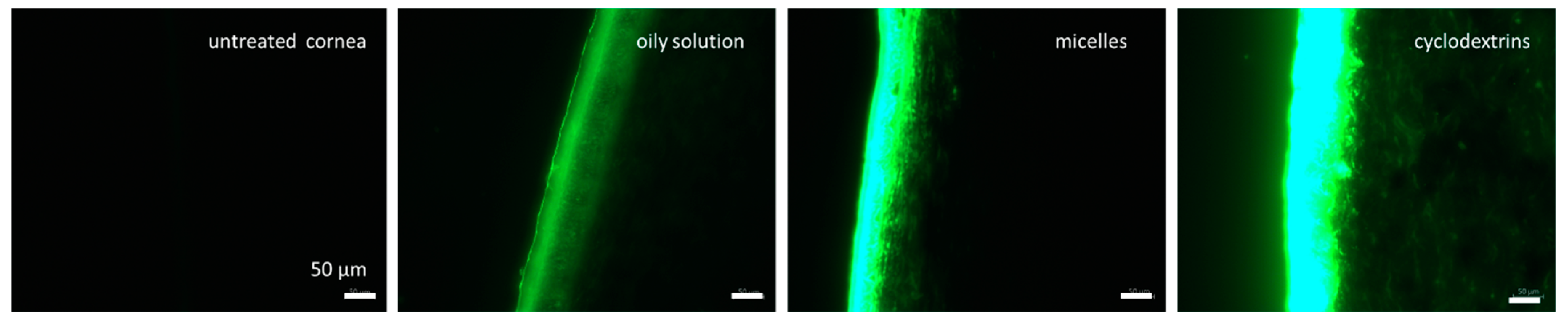
2.2.4. Corneal Penetration Studies
2.2.5. Digital Image Analysis
2.2.6. Statistical Analysis
3. Results
3.1. Formulation Preparation and Characterization
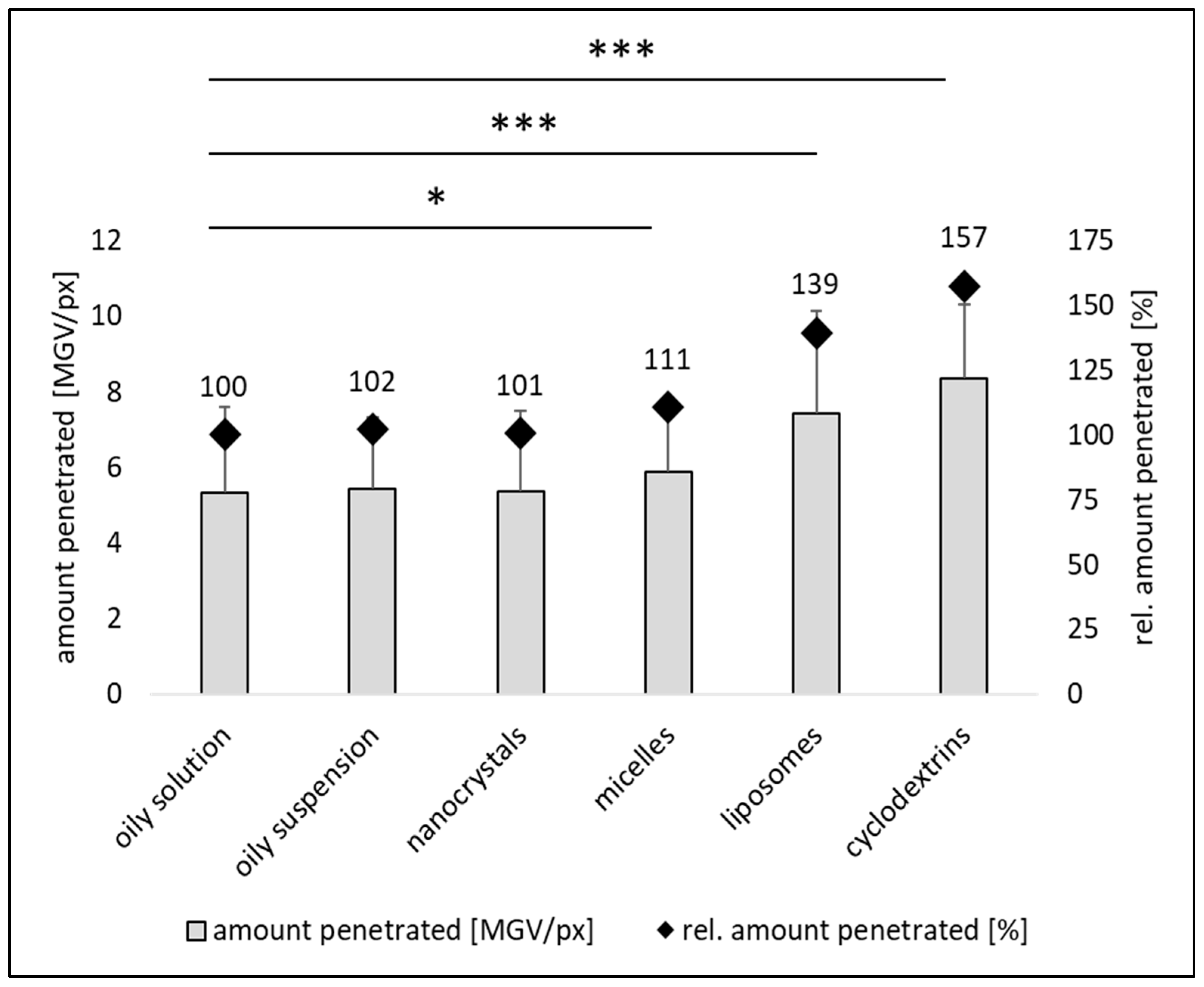
Dermal Penetration Efficacy
3.2. Influence on Stratum Corneum Thickness
3.3. Corneal Penetration Efficacy of Formulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Resnikoff, S.; Pascolini, D.; Mariotti, S.P.; Pokharel, G.P. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull. World Health Organ. 2008, 86, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef] [PubMed]
- Lanier, O.L.; Manfre, M.G.; Bailey, C.; Liu, Z.; Sparks, Z.; Kulkarni, S.; Chauhan, A. Review of Approaches for Increasing Ophthalmic Bioavailability for Eye Drop Formulations. AAPS PharmSciTech 2021, 22, 107. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Rupenthal, I.D. In vitro and ex vivo corneal penetration and absorption models. Drug Deliv. Transl. Res. 2016, 6, 634–647. [Google Scholar] [CrossRef] [PubMed]
- De Hoon, I.; Boukherroub, R.; de Smedt, S.C.; Szunerits, S.; Sauvage, F. In Vitro and Ex Vivo Models for Assessing Drug Permeation across the Cornea. Mol. Pharm. 2023, 20, 3298–3319. [Google Scholar] [CrossRef]
- Józsa, L.; Nemes, D.; Pető, Á.; Kósa, D.; Révész, R.; Bácskay, I.; Haimhoffer, Á.; Vasvári, G. Recent Options and Techniques to Assess Improved Bioavailability: In Vitro and Ex Vivo Methods. Pharmaceutics 2023, 15, 1146. [Google Scholar] [CrossRef]
- Agarwal, P.; Scherer, D.; Günther, B.; Rupenthal, I.D. Semifluorinated alkane based systems for enhanced corneal penetration of poorly soluble drugs. Int. J. Pharm. 2018, 538, 119–129. [Google Scholar] [CrossRef]
- Rimpelä, A.-K.; Garneau, M.; Baum-Kroker, K.S.; Schönberger, T.; Runge, F.; Sauer, A. Quantification of Drugs in Distinctly Separated Ocular Substructures of Albino and Pigmented Rats. Pharmaceutics 2020, 12, 1174. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Maran, J.J.; Rupenthal, I.D.; Agarwal, P. Mechanism of Ocular Penetration of Lipophilic Drugs from Lipophilic Vehicles. J. Pharm. Sci. 2024, 113, 2756–2763. [Google Scholar] [CrossRef] [PubMed]
- Mun, E.A.; Morrison, P.W.J.; Williams, A.C.; Khutoryanskiy, V.V. On the barrier properties of the cornea: A microscopy study of the penetration of fluorescently labeled nanoparticles, polymers, and sodium fluorescein. Mol. Pharm. 2014, 11, 3556–3564. [Google Scholar] [CrossRef]
- Pelikh, O.; Pinnapireddy, S.R.; Keck, C.M. Dermal Penetration Analysis of Curcumin in an ex vivo Porcine Ear Model Using Epifluorescence Microscopy and Digital Image Processing. Skin Pharmacol. Physiol. 2021, 34, 281–299. [Google Scholar] [CrossRef]
- Cholkar, K.; Dasari, S.R.; Pal, D.; Mitra, A.K. 1-Eye: Anatomy, physiology and barriers to drug delivery. In Ocular Transporters and Receptors: Their Role in Drug Delivery; Mitra, A.K., Ed.; Woodhead Publ: Oxford, UK, 2013; pp. 1–36. ISBN 978-1-907568-86-2. [Google Scholar]
- Sliney, D.; Wolbarsht, M. Review of Anatomy and Physiology of the Eye and Skin. Available online: https://link.springer.com/chapter/10.1007/978-1-4899-3596-0_3#citeas (accessed on 20 September 2024).
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Karami, T.K.; Hailu, S.; Feng, S.; Graham, R.; Gukasyan, H.J. Eyes on Lipinski’s Rule of Five: A New “Rule of Thumb” for Physicochemical Design Space of Ophthalmic Drugs. J. Ocul. Pharmacol. Ther. 2022, 38, 43–55. [Google Scholar] [CrossRef]
- Bukowiecki, A.; Hos, D.; Cursiefen, C.; Eming, S.A. Wound-Healing Studies in Cornea and Skin: Parallels, Differences and Opportunities. Int. J. Mol. Sci. 2017, 18, 1257. [Google Scholar] [CrossRef]
- Balzus, B.; Sahle, F.F.; Hönzke, S.; Gerecke, C.; Schumacher, F.; Hedtrich, S.; Kleuser, B.; Bodmeier, R. Formulation and ex vivo evaluation of polymeric nanoparticles for controlled delivery of corticosteroids to the skin and the corneal epithelium. Eur. J. Pharm. Biopharm. 2017, 115, 122–130. [Google Scholar] [CrossRef]
- Uwaezuoke, O.; Du Toit, L.C.; Kumar, P.; Ally, N.; Choonara, Y.E. Linoleic Acid-Based Transferosomes for Topical Ocular Delivery of Cyclosporine A. Pharmaceutics 2022, 14, 1695. [Google Scholar] [CrossRef] [PubMed]
- Koppa Raghu, P.; Bansal, K.K.; Thakor, P.; Bhavana, V.; Madan, J.; Rosenholm, J.M.; Mehra, N.K. Evolution of Nanotechnology in Delivering Drugs to Eyes, Skin and Wounds via Topical Route. Pharmaceuticals 2020, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, V.A.; Ribeiro, L.N.M.; Tofoli, G.R.; Franz-Montan, M.; de Paula, E.; de Jesus, M.B. Current Challenges and Future of Lipid nanoparticles formulations for topical drug application to oral mucosa, skin, and eye. Curr. Pharm. Des. 2017, 23, 17. [Google Scholar] [CrossRef]
- Gershkovich, P.; Wasan, K.M.; Barta, C.A. A review of the application of lipid-based systems in systemic, dermal/ transdermal, and ocular drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 2008, 25, 545–584. [Google Scholar] [CrossRef] [PubMed]
- Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Kos Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; Mayo, B.; et al. Safety and efficacy of turmeric extract, turmeric oil, turmeric oleoresin and turmeric tincture from Curcuma longa L. rhizome when used as sensory additives in feed for all animal species. EFSA J. 2020, 18, e06146. [Google Scholar] [CrossRef]
- Hartono, H.; Suryawati, B.; Sari, Y.; Avicena, A.; Maryani, M.; Sukmagautama, C.; Apriningsih, H.; Shofiyah, L.; Novika, R.G.; Wahidah, N.J.; et al. The Effect of Curcumin and Virgin Coconut Oil Towards Cytokines Levels in COVID-19 Patients at Universitas Sebelas Maret Hospital, Surakarta, Indonesia. Pharmacogn. J. 2022, 14, 216–225. [Google Scholar] [CrossRef]
- Buniowska-Olejnik, M.; Urbański, J.; Mykhalevych, A.; Bieganowski, P.; Znamirowska-Piotrowska, A.; Kačániová, M.; Banach, M. The influence of curcumin additives on the viability of probiotic bacteria, antibacterial activity against pathogenic microorganisms, and quality indicators of low-fat yogurt. Front. Nutr. 2023, 10, 1118752. [Google Scholar] [CrossRef] [PubMed]
- Ossikbayeva, S.; Khanin, M.; Sharoni, Y.; Trachtenberg, A.; Tuleukhanov, S.; Sensenig, R.; Rom, S.; Danilenko, M.; Orynbayeva, Z. Curcumin and Carnosic Acid Cooperate to Inhibit Proliferation and Alter Mitochondrial Function of Metastatic Prostate Cancer Cells. Antioxidants 2021, 10, 1591. [Google Scholar] [CrossRef] [PubMed]
- Stefánsson, E.; Loftsson, T. Cyclodextrins in Eye Drop Formulations. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 23–27. [Google Scholar] [CrossRef]
- Flory, S.; Sus, N.; Haas, K.; Jehle, S.; Kienhöfer, E.; Waehler, R.; Adler, G.; Venturelli, S.; Frank, J. Increasing Post-Digestive Solubility of Curcumin Is the Most Successful Strategy to Improve its Oral Bioavailability: A Randomized Cross-Over Trial in Healthy Adults and In Vitro Bioaccessibility Experiments. Mol. Nutr. Food Res. 2021, 65, e2100613. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Behera, S.; Rupenthal, I.D. Ocular Distribution of Papaverine Using Non-aqueous Vehicles. AAPS PharmSciTech 2021, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Suresh, S.; Devi, K.; Yadav, S. Effect of cyclodextrin complexation of curcumin on its solubility and antiangiogenic and anti-inflammatory activity in rat colitis model. AAPS PharmSciTech 2009, 10, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.M.; Ng, K.W. Finite and Infinite Dosing. In Percutaneous Penetration Enhancers Drug Penetration into/through the Skin: Methodology and General Considerations; Dragicevic, N.I., Maibach, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 35–44. ISBN 978-3-662-53268-3. [Google Scholar]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W. ImageJ: Image Processing and Analysis in Java. 2012. Available online: https://www.researchgate.net/publication/258729119_ImageJ_-_Image_Processing_and_Analysis_in_Java (accessed on 12 July 2024).
- JASP Team. JASP (Version 0.13.1) [Computer Software]. Available online: https://jasp-stats.org/ (accessed on 12 July 2024).
- Klang, V.; Haberfeld, S.; Hartl, A.; Valenta, C. Effect of γ-cyclodextrin on the in vitro skin permeation of a steroidal drug from nanoemulsions: Impact of experimental setup. Int. J. Pharm. 2012, 423, 535–542. [Google Scholar] [CrossRef]
- Chaiprateep, E.-O.; Wiemann, S.; Eckert, R.W.; Raab, C.; Sengupta, S.; Keck, C.M. Influence of Dose, Particle Size and Concentration on Dermal Penetration Efficacy of Curcumin. Pharmaceutics 2023, 15, 2645. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.S. Etodolac-liquid-filled dispersion into hard gelatin capsules: An approach to improve dissolution and stability of etodolac formulation. Drug Dev. Ind. Pharm. 2006, 32, 865–876. [Google Scholar] [CrossRef]
- Day, C.P.F.; Miloserdov, A.; Wildish-Jones, K.; Pearson, E.; Carruthers, A.E. Quantifying the hygroscopic properties of cyclodextrin containing aerosol for drug delivery to the lungs. Phys. Chem. Chem. Phys. 2020, 22, 11327–11336. [Google Scholar] [CrossRef]
- Wiemann, S.; Keck, C.M. Particle-Assisted Dermal Penetration—A Simple Formulation Strategy to Foster the Dermal Penetration Efficacy. Pharmaceutics 2022, 14, 1039. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Maibach, H.I. Occlusion vs. skin barrier function. Skin Res. Technol. 2002, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, V.; Ganashalingam, Y.; Schesny, R.; Raab, C.; Sengupta, S.; Keck, C.M. Influence of Massage and Skin Hydration on Dermal Penetration Efficacy of Nile Red from Petroleum Jelly-An Unexpected Outcome. Pharmaceutics 2021, 13, 2190. [Google Scholar] [CrossRef] [PubMed]
- Khanji, A.N.; Michaux, F.; Petit, J.; Salameh, D.; Rizk, T.; Jasniewski, J.; Banon, S. Structure, gelation, and antioxidant properties of curcumin-doped casein micelle powder produced by spray-drying. Food Funct. 2018, 9, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Craig, J.P.; Krösser, S.; Eickhoff, K.; Swift, S.; Rupenthal, I.D. Topical semifluorinated alkane-based azithromycin suspension for the management of ocular infections. Eur. J. Pharm. Biopharm. 2019, 142, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, Y.; Yang, Z.; Li, M.; Li, F.; Cui, F.; Liu, T.; Shi, W.; Wu, X. Nanomicelle formulation for topical delivery of cyclosporine A into the cornea: In vitro mechanism and in vivo permeation evaluation. Sci. Rep. 2015, 5, 12968. [Google Scholar] [CrossRef]
- Lorenzo-Veiga, B.; Sigurdsson, H.H.; Loftsson, T.; Alvarez-Lorenzo, C. Cyclodextrin–Amphiphilic Copolymer Supramolecular Assemblies for the Ocular Delivery of Natamycin. Nanomaterials 2019, 9, 745. [Google Scholar] [CrossRef]
- Huang, H.S.; Schoenwald, R.D.; Lach, J.L. Corneal penetration behavior of beta-blocking agents II: Assessment of barrier contributions. J. Pharm. Sci. 1983, 72, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Chetoni, P.; Burgalassi, S.; Najarro, M.; Saettone, M.F. Increased corneal hydration induced by potential ocular penetration enhancers: Assessment by differential scanning calorimetry (DSC) and by desiccation. Int. J. Pharm. 2002, 232, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Edelhauser, H.F. The balance between corneal transparency and edema: The Proctor Lecture. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Noonan, J.S. Permeability of cornea, sclera, and conjunctiva: A literature analysis for drug delivery to the eye. J. Pharm. Sci. 1998, 87, 1479–1488. [Google Scholar] [CrossRef]
- Srinivas, S.P. Dynamic regulation of barrier integrity of the corneal endothelium. Optom. Vis. Sci. 2010, 87, E239–E254. [Google Scholar] [CrossRef]

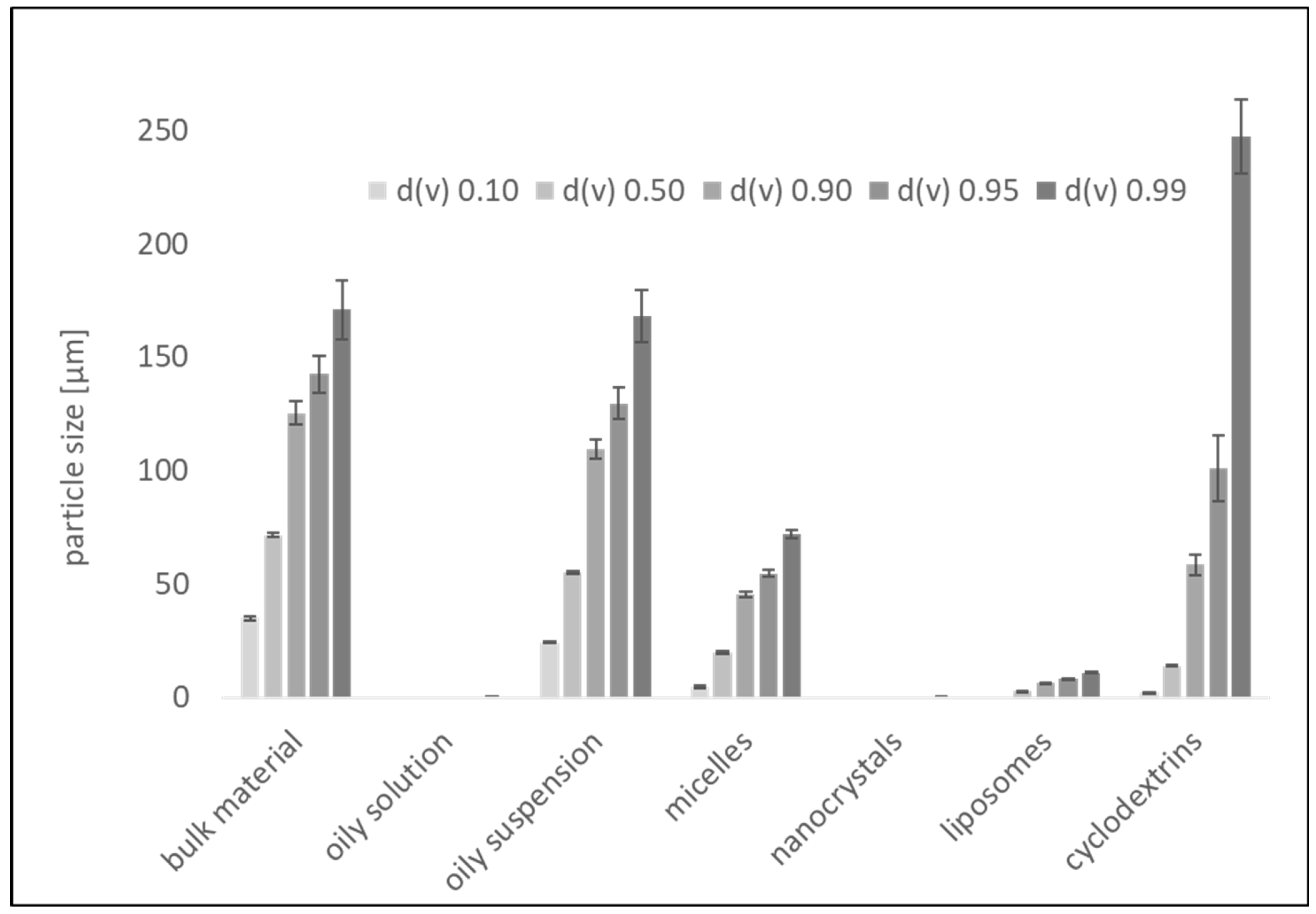



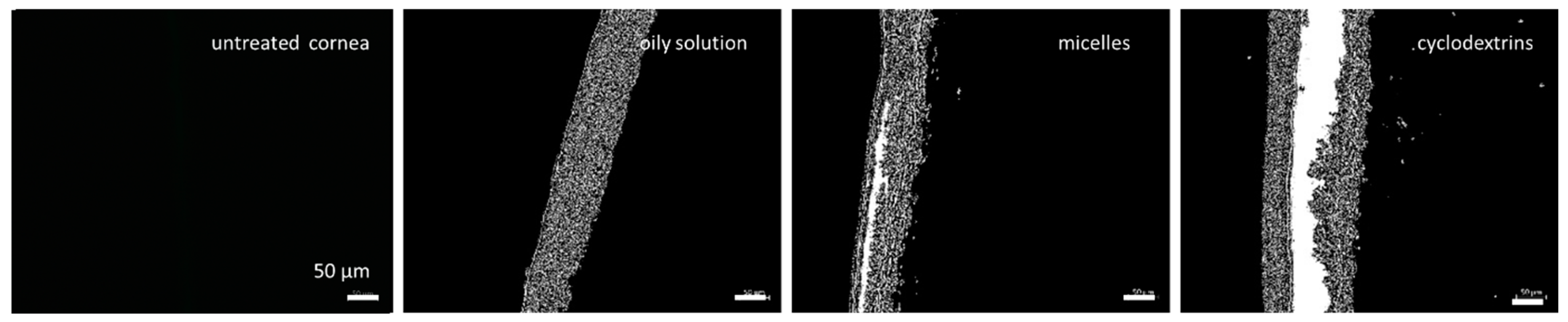
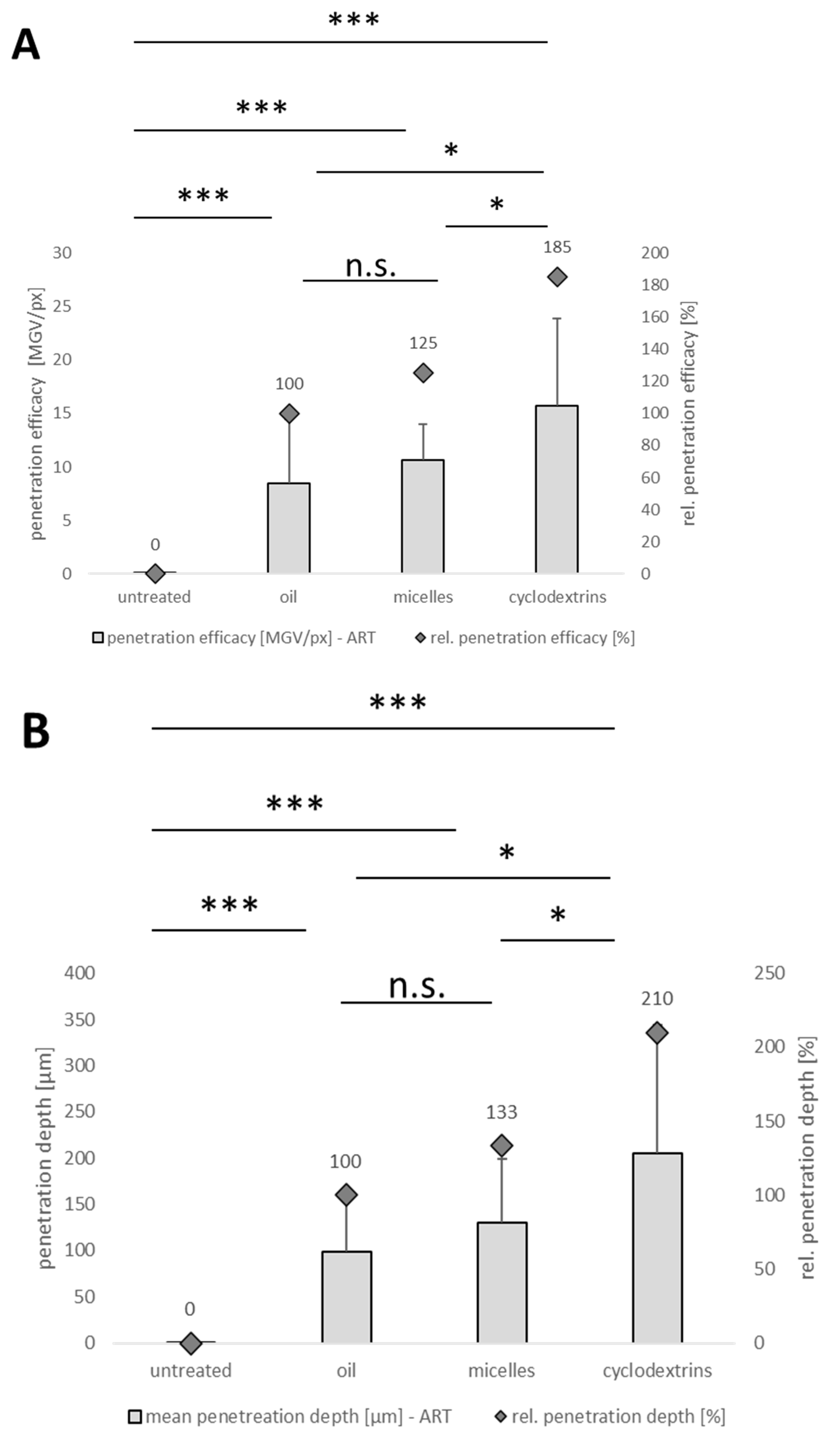

| Formulation | Amount of Curcumin [% w/w] | Other Excipients [% w/w] |
|---|---|---|
| oily solution | 0.1 | MCT 99.9 |
| oily suspension | 1.0 | MCT 99.0 |
| nanocrystals | 1.0 | TPGS 1.0, water 98.0 |
| micelles | 1.0 | Tween 80 1.0, water 98.0 |
| liposomes | 1.0 | phospholipid 1.0, water 98.0 |
| cyclodextrins | 1.0 | ß-cyclodextrins 1.0, water 98.0 |
| Formulation | MPD [µm] ± SD | SCT [µm] ± SD | Δ MPD-SCT [µm] |
|---|---|---|---|
| oily solution | 39 ± 2 | 29 ± 6 | −10 |
| oily suspension | 37 ± 2 | 29 ± 6 | −8 |
| nanocrystals | 33 ± 2 | 27 ± 4 | −7 |
| micelles | 38 ± 2 | 27 ± 4 | −11 |
| liposomes | 57 ± 3 | 30 ± 5 | −27 |
| cyclodextrins | 62 ± 2 | 30 ± 6 | −32 |
| (A) | (B) | (C) | ||||
|---|---|---|---|---|---|---|
| SCT of Unloaded Vehicle to SCT of Untreated Skin | SCT of Formulation to SCT of Unloaded Vehicle | SCT of Formulation to SCT of Untreated Skin | ||||
| untreated skin | 100 | 100 | 100 | |||
| oily solution | 83 | *** | 133 | *** | 109 | ** |
| oily suspension | 83 | *** | 121 | *** | 100 | n.s. |
| nanocrystals | 93 | * | 98 | n.s. | 91 | *** |
| micelles | 86 | *** | 108 | ** | 93 | ** |
| liposomes | 97 | n.s. | 106 | * | 103 | n.s. |
| cyclodextrin | 92 | ** | 111 | *** | 103 | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raab, C.; Brugger, S.; Lechner, J.-S.; Barbalho, G.N.; Gratieri, T.; Agarwal, P.; Rupenthal, I.D.; Keck, C.M. Utilizing an Ex Vivo Skin Penetration Analysis Model for Predicting Ocular Drug Penetration: A Feasibility Study with Curcumin Formulations. Pharmaceutics 2024, 16, 1302. https://doi.org/10.3390/pharmaceutics16101302
Raab C, Brugger S, Lechner J-S, Barbalho GN, Gratieri T, Agarwal P, Rupenthal ID, Keck CM. Utilizing an Ex Vivo Skin Penetration Analysis Model for Predicting Ocular Drug Penetration: A Feasibility Study with Curcumin Formulations. Pharmaceutics. 2024; 16(10):1302. https://doi.org/10.3390/pharmaceutics16101302
Chicago/Turabian StyleRaab, Christian, Stefan Brugger, Jara-Sophie Lechner, Geisa Nascimento Barbalho, Taís Gratieri, Priyanka Agarwal, Ilva D. Rupenthal, and Cornelia M. Keck. 2024. "Utilizing an Ex Vivo Skin Penetration Analysis Model for Predicting Ocular Drug Penetration: A Feasibility Study with Curcumin Formulations" Pharmaceutics 16, no. 10: 1302. https://doi.org/10.3390/pharmaceutics16101302
APA StyleRaab, C., Brugger, S., Lechner, J.-S., Barbalho, G. N., Gratieri, T., Agarwal, P., Rupenthal, I. D., & Keck, C. M. (2024). Utilizing an Ex Vivo Skin Penetration Analysis Model for Predicting Ocular Drug Penetration: A Feasibility Study with Curcumin Formulations. Pharmaceutics, 16(10), 1302. https://doi.org/10.3390/pharmaceutics16101302









