Skin Structure, Physiology, and Pathology in Topical and Transdermal Drug Delivery
Abstract
:1. Introduction
2. Fundamental Notions
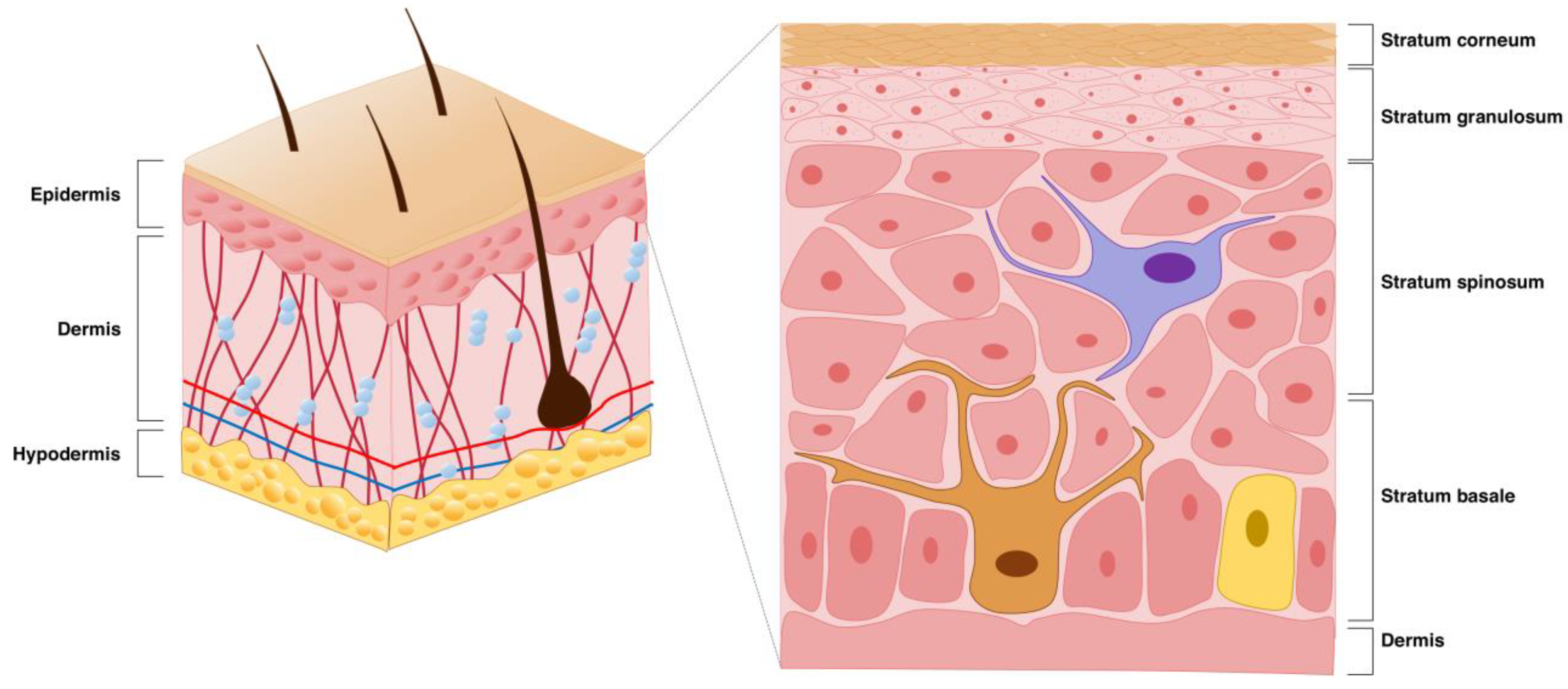
2.1. Basic Skin Structure
2.2. Drug Diffusion Through the Skin
2.2.1. Transepidermal Route
2.2.2. Transappendageal Route
2.3. Types of Permeation Enhancers for Drug Delivery Through the Skin
| Technology | Definition | Mechanism/Advantages | Ref. |
|---|---|---|---|
| Passive Strategies | |||
| Permeation Enhancers | Molecules that increase the permeability of the stratum corneum |
| [22] |
| Hydrogels | Highly flexible three-dimensional polymeric matrixes with ability to carry drugs |
| [23,24] |
| Nanocarriers | Nano-sized particle systems designed to encapsulate and transport therapeutic agents |
| [25,26,27] |
| Nanocrystals | Nanosized crystals (100–1000 nm) composed of a pharmaceutical drug |
| [28,29] |
| Microemulsions (100–400 μm) and Nanoemulsions (1–100 nm) | Colloidal systems composed of oil and water |
| [30] |
| Active Strategies | |||
| Electrical Enhancers | Application of current to generate transient modifications in the stratum corneum |
| [31,32,33] |
| Ultrasound | Utilization of low-frequency ultrasound to improve drug delivery |
| [34,35,36] |
| Microneedles | Micrometer-sized arrays of needles arranged on a small patch to creates micropores in the stratum corneum |
| [37,38,39] |
| Needleless Injections | Injection of a high-speed liquid medication jet to the skin |
| [40] |
| Thermal Ablation | Utilization of high heat to disrupt or remove the stratum corneum |
| [41,42] |
3. Considerations of Skin Structure for Drug Delivery and Strategies for Enhancement
3.1. Hair Follicles
3.2. Facial Skin
3.3. Glabrous Skin (Palmo-Plantar Regions)
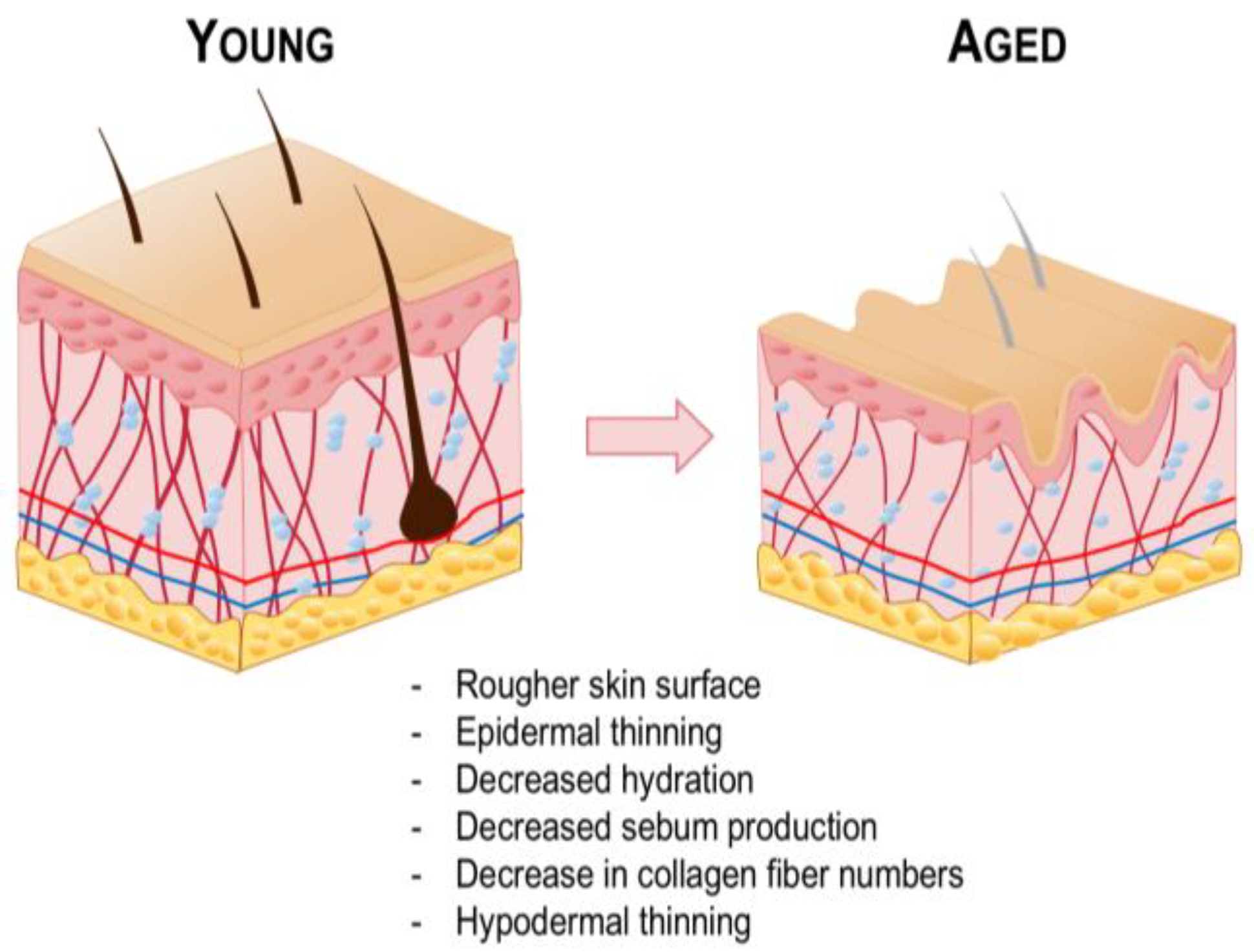
3.4. Aged Skin
4. Considerations of Skin Pathology for Drug Delivery and Strategies for Enhancement
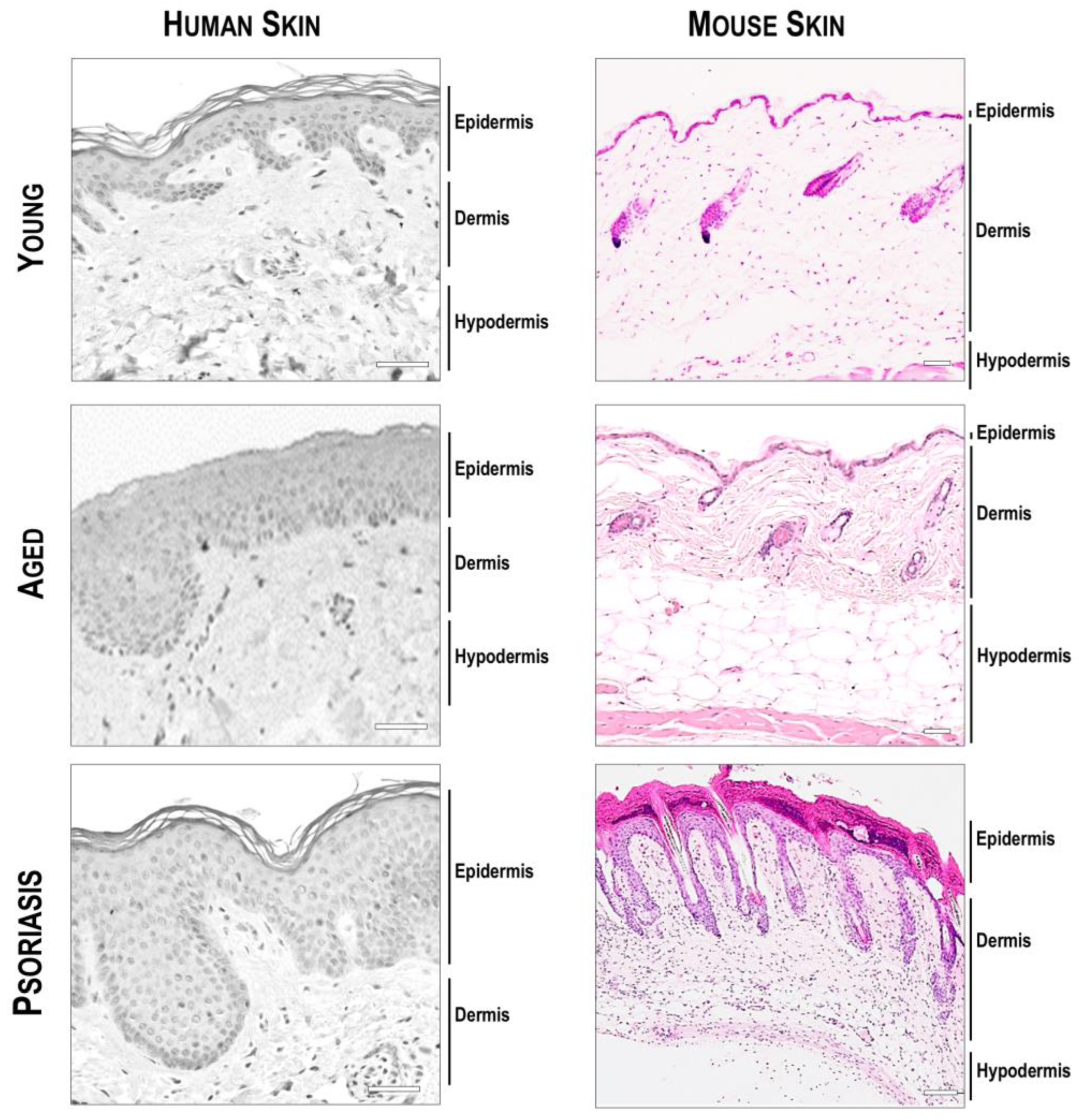
4.1. Atopic and Psoriatic Skin
4.2. Wounded Skin
4.3. Burn Injury
4.4. Diabetic Skin
4.5. Melasma
4.6. Melanoma
4.7. Hypopigmentation
5. Considerations Regarding Skin Models for Dermatological Research
| Human | Mouse | Rat | Porcine | Zebrafish | |
| Stratum corneum | Thick (10–20 μm) | Thin (~5 μm) | Thick (~25 μm) | Thick (20–26 μm) | Absent |
| Epidermis | Thick (60–100 μm) | Thin (~30 μm) | Thin (~60 μm) | Thick (30–140 μm) | Thin |
| Dermis | Thick (1–4 mm) | Thin (~400 μm) | Thick (~3 mm) | Thick (~2 mm) | Thin (<200 μm) |
| Hair follicles | Sparse | Dense | Dense | Sparse | Absent |
| Panniculus carnosus | Vestigial | Present | Present | Present | Absent |
| Permeability | Moderate | High | High | Moderate | High |
| Healing mechanism | Re-epithelization | Contraction | Contraction | Re-epithelization | Re-epithelization |
| Main advantages | - |
|
|
|
|
| Main disadvantages | - |
|
|
|
|
| References | [189,190] | [190,191] | [185,186] | [187] | [188,192,193] |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, D.H.; Lim, S.; Kwak, S.S.; Kim, J. Advancements in Skin-Mediated Drug Delivery: Mechanisms, Techniques, and Applications. Adv. Healthc. Mater. 2024, 13, 2302375. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal delivery systems in cosmetics. Biomed. Dermatol. 2020, 4, 10. [Google Scholar] [CrossRef]
- Schafer, N.; Balwierz, R.; Biernat, P.; Ochędzan-Siodłak, W.; Lipok, J. Natural ingredients of transdermal drug delivery systems as permeation enhancers of active substances through the stratum corneum. Mol. Pharm. 2023, 20, 3278–3297. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and opportunities in dermal/transdermal delivery. Ther. Deliv. 2010, 1, 109–131. [Google Scholar] [CrossRef]
- Waters, C. The development of the rotigotine transdermal patch: A historical perspective. Neurol. Clin. 2013, 31, S37–S50. [Google Scholar] [CrossRef]
- Christie, J. The use of transdermal rotigotine in a patient with advanced Parkinson’s disease and dysphagia. Palliat. Med. 2007, 21, 163–164. [Google Scholar]
- Makaroff, L.; Gunn, A.; Gervasoni, C.; Richy, F. Gastrointestinal disorders in Parkinson’s disease: Prevalence and health outcomes in a US claims database. J. Park. Dis. 2011, 1, 65–74. [Google Scholar] [CrossRef]
- Yu, Y.-Q.; Yang, X.; Wu, X.-F.; Fan, Y.-B. Enhancing permeation of drug molecules across the skin via delivery in nanocarriers: Novel strategies for effective transdermal applications. Front. Bioeng. Biotechnol. 2021, 9, 646554. [Google Scholar] [CrossRef]
- Baker, P.; Huang, C.; Radi, R.; Moll, S.B.; Jules, E.; Arbiser, J.L. Skin Barrier Function: The Interplay of Physical, Chemical, and Immunologic Properties. Cells 2023, 12, 2745. [Google Scholar] [CrossRef]
- Montagna, W. The Structure and Function of Skin; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis; StatPearls Publishing: Treasure Island, FL, USA, 2017. [Google Scholar]
- Al-Khafaji, Z.; Brito, S.; Bin, B.-H. Zinc and zinc transporters in dermatology. Int. J. Mol. Sci. 2022, 23, 16165. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Brito, S.; Kwak, B.M.; Park, S.; Lee, M.-G.; Bin, B.-H. Applications of mesenchymal stem cells in skin regeneration and rejuvenation. Int. J. Mol. Sci. 2021, 22, 2410. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, C.; Fuchs, E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009, 10, 207–217. [Google Scholar] [CrossRef]
- Bin, B.-H.; Joo, Y.H.; Lee, A.-Y.; Shin, S.S.; Cho, E.-G.; Lee, T.R. Novel inhibitory effect of N-(2-hydroxycyclohexyl) valiolamine on melanin production in a human skin model. Int. J. Mol. Sci. 2014, 15, 12188–12195. [Google Scholar] [CrossRef]
- Bin, B.-H.; Kim, S.T.; Bhin, J.; Lee, T.R.; Cho, E.-G. The development of sugar-based anti-melanogenic agents. Int. J. Mol. Sci. 2016, 17, 583. [Google Scholar] [CrossRef]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine 2021, 49, 337–342. [Google Scholar] [CrossRef]
- Jabłonska, S.; Majewski, S.; Obalek, S.; Orth, G. Cutaneous warts. Clin. Dermatol. 1997, 15, 309–319. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2021, 12, 758–791. [Google Scholar] [CrossRef]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Ahsan, A.; Tian, W.-X.; Farooq, M.A.; Khan, D.H. An overview of hydrogels and their role in transdermal drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 574–584. [Google Scholar] [CrossRef]
- Labie, H.; Blanzat, M. Hydrogels for dermal and transdermal drug delivery. Biomater. Sci. 2023, 11, 4073–4093. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Lim, H.; Park, H.; Yu, J.; An, J.; Yoo, H.Y.; Lee, T. Recent progress of lipid nanoparticles-based lipophilic drug delivery: Focus on surface modifications. Pharmaceutics 2023, 15, 772. [Google Scholar] [CrossRef]
- Yu, Z.; Meng, X.; Zhang, S.; Chen, Y.; Zhang, Z.; Zhang, Y. Recent progress in transdermal nanocarriers and their surface modifications. Molecules 2021, 26, 3093. [Google Scholar] [CrossRef]
- Lim, H.; Kwon, D.-E.; Park, P.J.; Bin, B.-H.; Kim, S.-B.; Park, S.Y.; Cho, K.H.; Jang, D.-J.; Kim, S.T. Topical Delivery of Nanosized Hydroxycitric Acid Enriched Extract-Loaded Ethosomes for Suppression of Lipid Droplet Deposition. J. Nanosci. Nanotechnol. 2021, 21, 4089–4092. [Google Scholar] [CrossRef]
- Shegokar, R.; Müller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef]
- Mohammad, I.S.; Hu, H.; Yin, L.; He, W. Drug nanocrystals: Fabrication methods and promising therapeutic applications. Int. J. Pharm. 2019, 562, 187–202. [Google Scholar] [CrossRef]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Bose, V.G.; Langer, R.; Weaver, J.C. Electroporation of mammalian skin: A mechanism to enhance transdermal drug delivery. Proc. Natl. Acad. Sci. USA 1993, 90, 10504–10508. [Google Scholar] [CrossRef]
- Dhote, V.; Bhatnagar, P.; Mishra, P.K.; Mahajan, S.C.; Mishra, D.K. Iontophoresis: A potential emergence of a transdermal drug delivery system. Sci. Pharm. 2012, 80, 1–28. [Google Scholar] [CrossRef]
- Kougkolos, G.; Laudebat, L.; Dinculescu, S.; Simon, J.; Golzio, M.; Valdez-Nava, Z.; Flahaut, E. Skin electroporation for transdermal drug delivery: Electrical measurements, numerical model and molecule delivery. J. Control. Release 2024, 367, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Byl, N.N. The use of ultrasound as an enhancer for transcutaneous drug delivery: Phonophoresis. Phys. Ther. 1995, 75, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Machet, L.; Boucaud, A. Phonophoresis: Efficiency, mechanisms and skin tolerance. Int. J. Pharm. 2002, 243, 1–15. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Aldawood, F.K.; Andar, A.; Desai, S. A comprehensive review of microneedles: Types, materials, processes, characterizations and applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef]
- Al-Japairai, K.A.S.; Mahmood, S.; Almurisi, S.H.; Venugopal, J.R.; Hilles, A.R.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef]
- Avcil, M.; Çelik, A. Microneedles in drug delivery: Progress and challenges. Micromachines 2021, 12, 1321. [Google Scholar] [CrossRef]
- Barolet, D.; Benohanian, A. Current trends in needle-free jet injection: An update. Clin. Cosmet. Investig. Dermatol. 2018, 11, 231–238. [Google Scholar] [CrossRef]
- Parhi, R.; Mandru, A. Enhancement of skin permeability with thermal ablation techniques: Concept to commercial products. Drug Deliv. Transl. Res. 2021, 11, 817–841. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Heat: A highly efficient skin enhancer for transdermal drug delivery. Front. Bioeng. Biotechnol. 2018, 6, 15. [Google Scholar] [CrossRef]
- Hodge, B.D.; Sanvictores, T.; Brodell, R.T. Anatomy, Skin Sweat Glands; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Martel, J.L.; Miao, J.H.; Badri, T. Anatomy, Hair Follicle; StatPearls Publishing: Treasure Island, FL, USA, 2017. [Google Scholar]
- Yang, G.; Chen, G.; Gu, Z. Transdermal drug delivery for hair regrowth. Mol. Pharm. 2020, 18, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Richter, H.; Schaefer, U.; Blume-Peytavi, U.; Teichmann, A.; Otberg, N.; Sterry, W. Hair follicles–a long-term reservoir for drug delivery. Ski. Pharmacol. Physiol. 2006, 19, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Pelikh, O.; Keck, C.M. Hair follicle targeting and dermal drug delivery with curcumin drug nanocrystals—Essential influence of excipients. Nanomaterials 2020, 10, 2323. [Google Scholar] [CrossRef] [PubMed]
- Oaku, Y.; Abe, A.; Sasano, Y.; Sasaki, F.; Kubota, C.; Yamamoto, N.; Nagahama, T.; Nagai, N. Minoxidil nanoparticles targeting hair follicles enhance hair growth in C57BL/6 mice. Pharmaceutics 2022, 14, 947. [Google Scholar] [CrossRef]
- Lopedota, A.; Denora, N.; Laquintana, V.; Cutrignelli, A.; Lopalco, A.; Tricarico, D.; Maqoud, F.; Curci, A.; Mastrodonato, M.; la Forgia, F. Alginate-based hydrogel containing minoxidil/hydroxypropyl-β-cyclodextrin inclusion complex for topical alopecia treatment. J. Pharm. Sci. 2018, 107, 1046–1054. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, J.; Cang, Z.; Pei, J.; Zhang, X.; Song, B.; Fan, X.; Ma, X.; Li, Y. Hair follicle stem cells promote epidermal regeneration under expanded condition. Front. Physiol. 2024, 15, 1306011. [Google Scholar] [CrossRef]
- Yuan, A.; Gu, Y.; Bian, Q.; Wang, R.; Xu, Y.; Ma, X.; Zhou, Y.; Gao, J. Conditioned media-integrated microneedles for hair regeneration through perifollicular angiogenesis. J. Control. Release 2022, 350, 204–214. [Google Scholar] [CrossRef]
- Xiao, S.-E.; Miao, Y.; Wang, J.; Jiang, W.; Fan, Z.-X.; Liu, X.-M.; Hu, Z.-Q. As a carrier–transporter for hair follicle reconstitution, platelet-rich plasma promotes proliferation and induction of mouse dermal papilla cells. Sci. Rep. 2017, 7, 1125. [Google Scholar] [CrossRef]
- Xiao, T.; Li, B.; Lai, R.; Liu, Z.; Xiong, S.; Li, X.; Zeng, Y.; Jiao, S.; Tang, Y.; Lu, Y. Active pharmaceutical ingredient-ionic liquids assisted follicular co-delivery of ferulic acid and finasteride for enhancing targeted anti-alopecia. Int. J. Pharm. 2023, 648, 123624. [Google Scholar] [CrossRef]
- Ding, Y.-W.; Li, Y.; Zhang, Z.-W.; Dao, J.-W.; Wei, D.-X. Hydrogel forming microneedles loaded with VEGF and Ritlecitinib/polyhydroxyalkanoates nanoparticles for mini-invasive androgenetic alopecia treatment. Bioact. Mater. 2024, 38, 95–108. [Google Scholar] [CrossRef]
- Guan, Y.; Yan, A.; Qiang, W.; Ruan, R.; Yang, C.; Ma, K.; Sun, H.; Liu, M.; Zhu, H. Selective delivery of tofacitinib citrate to hair follicles using lipid-coated calcium carbonate nanocarrier controls chemotherapy-induced alopecia areata. Int. J. Mol. Sci. 2023, 24, 8427. [Google Scholar] [CrossRef] [PubMed]
- Matos, B.N.; Lima, A.L.; Cardoso, C.O.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. Follicle-Targeted Delivery of Betamethasone and Minoxidil Co-Entrapped in Polymeric and Lipid Nanoparticles for Topical Alopecia Areata Treatment. Pharmaceuticals 2023, 16, 1322. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-C.; Li, C.-Z.; Lu, C.-T.; Zhao, M.-H.; Lai, S.-M.; Liao, M.-H.; Peng, C.-L.; Liu, H.-T.; Lai, P.-S. Microcurrent Cloth-Assisted Transdermal Penetration and Follicular Ducts Escape of Curcumin-Loaded Micelles for Enhanced Wound Healing. Int. J. Nanomed. 2023, 18, 8077–8097. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S.; Noorizadeh, K.; Zadmehr, O.; Rasekh, S.; Mohammadi-Samani, S.; Dehghan, D. Novel topical drug delivery systems in acne management: Molecular mechanisms and role of targeted delivery systems for better therapeutic outcomes. J. Drug Deliv. Sci. Technol. 2022, 74, 103595. [Google Scholar] [CrossRef]
- Farah, H.; Brown, M.; McAuley, W.J. Heat enhanced follicular delivery of Isotretinoin to the skin. Pharm. Res. 2019, 36, 124. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Moradishooli, F.; Daneshamouz, S.; Heidari, R.; Niroumand, U.; Mohammadi-Samani, S. Optimization, characterization, and follicular targeting assessment of tretinoin and bicalutamide loaded niosomes. Sci. Rep. 2023, 13, 20023. [Google Scholar] [CrossRef]
- Kshirsagar, S.M.; Shrestha, N.; Kipping, T.; Banga, A.K. Formulation development of tazarotene-loaded PLGA nanoparticles for follicular delivery in the treatment of inflammatory skin diseases. Eur. J. Pharm. Biopharm. 2024, 200, 114346. [Google Scholar] [CrossRef]
- Ji, Y.; Li, H.; Li, J.; Yang, G.; Zhang, W.; Shen, Y.; Xu, B.; Liu, J.; Wen, J.; Song, W. Hair Follicle-Targeted Delivery of Azelaic Acid Micro/Nanocrystals Promote the Treatment of Acne Vulgaris. Int. J. Nanomed. 2024, 19, 5173–5191. [Google Scholar] [CrossRef]
- Tagami, H. Location-related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int. J. Cosmet. Sci. 2008, 30, 413–434. [Google Scholar] [CrossRef]
- Jeong, K.M.; Seo, J.Y.; Kim, A.; Kim, Y.C.; Baek, Y.S.; Oh, C.H.; Jeon, J. Ultrasonographic analysis of facial skin thickness in relation to age, site, sex, and body mass index. Ski. Res. Technol. 2023, 29, e13426. [Google Scholar] [CrossRef]
- Ezure, T.; Kosaka, N.; Yagi, E.; Hosoi, J.; Amano, S.; Matsuzaki, K.; Ochiya, T. A novel approach to anti-aging facial skin care through reconstruction of “dermal anchoring structures” to improve facial morphology. IFSCC Mag. 2015, 18, 3–16. [Google Scholar]
- Nkengne, A.; Pellacani, G.; Ciardo, S.; De Carvalho, N.; Vié, K. Visible characteristics and structural modifications relating to enlarged facial pores. Ski. Res. Technol. 2021, 27, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Boyle, C.J.; Plotczyk, M.; Villalta, S.F.; Patel, S.; Hettiaratchy, S.; Masouros, S.D.; Masen, M.A.; Higgins, C.A. Morphology and composition play distinct and complementary roles in the tolerance of plantar skin to mechanical load. Sci. Adv. 2019, 5, eaay0244. [Google Scholar] [CrossRef] [PubMed]
- Bariya, M.; Li, L.; Ghattamaneni, R.; Ahn, C.H.; Nyein, H.Y.Y.; Tai, L.-C.; Javey, A. Glove-based sensors for multimodal monitoring of natural sweat. Sci. Adv. 2020, 6, eabb8308. [Google Scholar] [CrossRef]
- Machado-Moreira, C.A.; Taylor, N.A. Thermogenic and psychogenic recruitment of human eccrine sweat glands: Variations between glabrous and non-glabrous skin surfaces. J. Therm. Biol. 2017, 65, 145–152. [Google Scholar] [CrossRef]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef]
- Martic, I.; Jansen-Dürr, P.; Cavinato, M. Effects of air pollution on cellular senescence and skin aging. Cells 2022, 11, 2220. [Google Scholar] [CrossRef]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The impact of ultraviolet radiation on skin photoaging—Review of in vitro studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Bin, B.-H.; Kim, W.; Lee, E.; Lee, T.R.; Cho, E.-G. Exposure of human melanocytes to UVB twice and subsequent incubation leads to cellular senescence and senescence-associated pigmentation through the prolonged p53 expression. J. Dermatol. Sci. 2018, 90, 303–312. [Google Scholar] [CrossRef]
- Bin, B.-H.; Kim, D.-K.; Kim, N.-H.; Choi, E.-J.; Bhin, J.; Kim, S.T.; Gho, Y.S.; Lee, A.-Y.; Lee, T.R.; Cho, E.-G. Fibronectin-containing extracellular vesicles protect melanocytes against ultraviolet radiation-induced cytotoxicity. J. Investig. Dermatol. 2016, 136, 957–966. [Google Scholar] [CrossRef]
- Choi, K.H.; Kim, S.T.; Bin, B.H.; Park, P.J. Effect of konjac glucomannan (Kgm) on the reconstitution of the dermal environment against uvb-induced condition. Nutrients 2020, 12, 2779. [Google Scholar] [CrossRef] [PubMed]
- Rübe, C.E.; Bäumert, C.; Schuler, N.; Isermann, A.; Schmal, Z.; Glanemann, M.; Mann, C.; Scherthan, H. Human skin aging is associated with increased expression of the histone variant H2A. J in the epidermis. npj Aging Mech. Dis. 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Manuskiatti, W.; Schwindt, D.; Maibach, H. Influence of age, anatomic site and race on skin roughness and scaliness. Dermatology 1998, 196, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Waller, J.M.; Maibach, H.I. Age and skin structure and function, a quantitative approach (I): Blood flow, pH, thickness, and ultrasound echogenicity. Ski. Res. Technol. 2005, 11, 221–235. [Google Scholar] [CrossRef]
- Kang, H.Y.; Lee, J.W.; Papaccio, F.; Bellei, B.; Picardo, M. Alterations of the pigmentation system in the aging process. Pigment Cell Melanoma Res. 2021, 34, 800–813. [Google Scholar] [CrossRef]
- Kim, J.C.; Park, T.J.; Kang, H.Y. Skin-aging pigmentation: Who is the real enemy? Cells 2022, 11, 2541. [Google Scholar] [CrossRef]
- Passeron, T.; Picardo, M. Melasma, a photoaging disorder. Pigment Cell Melanoma Res. 2018, 31, 461–465. [Google Scholar] [CrossRef]
- Yoon, J.E.; Kim, Y.; Kwon, S.; Kim, M.; Kim, Y.H.; Kim, J.-H.; Park, T.J.; Kang, H.Y. Senescent fibroblasts drive ageing pigmentation: A potential therapeutic target for senile lentigo. Theranostics 2018, 8, 4620. [Google Scholar] [CrossRef]
- Brito, S.; Baek, J.-M.; Cha, B.; Heo, H.; Lee, S.-H.; Lei, L.; Jung, S.Y.; Lee, S.M.; Lee, S.H.; Kwak, B.-M. Nicotinamide mononucleotide reduces melanin production in aged melanocytes by inhibiting cAMP/Wnt signaling. J. Dermatol. Sci. 2022, 106, 159–169. [Google Scholar] [CrossRef]
- Jacobson, T.M.; Yüksel, K.Ü.; Geesin, J.C.; Gordon, J.S.; Lane, A.T.; Gracy, R.W. Effects of aging and xerosis on the amino acid composition of human skin. J. Investig. Dermatol. 1990, 95, 296–300. [Google Scholar] [CrossRef]
- Pochi, P.E.; Strauss, J.S.; Downing, D.T. Age-related changes in sebaceous gland activity. J. Investig. Dermatol. 1979, 73, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular mechanisms of dermal aging and antiaging approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Quan, T.; Fisher, G.J. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A mini-review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Yasui, T.; Yonetsu, M.; Tanaka, R.; Tanaka, Y.; Fukushima, S.-i.; Yamashita, T.; Ogura, Y.; Hirao, T.; Murota, H.; Araki, T. In vivo observation of age-related structural changes of dermal collagen in human facial skin using collagen-sensitive second harmonic generation microscope equipped with 1250-nm mode-locked Cr: Forsterite laser. J. Biomed. Opt. 2013, 18, 031108. [Google Scholar]
- Bernstein, E.; Underhill, C.; Hahn, P.; Brown, D.; Uitto, J. Chronic sun exposure alters both the content and distribution of dermal glycosaminoglycans. Br. J. Dermatol. 1996, 135, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Roskos, K.V.; Maibach, H.I. Percutaneous absorption and age: Implications for therapy. Drugs Aging 1992, 2, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lu, F.; Feng, J. Aging and homeostasis of the hypodermis in the age-related deterioration of skin function. Cell Death Dis. 2024, 15, 443. [Google Scholar] [CrossRef]
- Kuk, J.L.; Saunders, T.J.; Davidson, L.E.; Ross, R. Age-related changes in total and regional fat distribution. Ageing Res. Rev. 2009, 8, 339–348. [Google Scholar] [CrossRef]
- Cheng, T.; Tai, Z.; Shen, M.; Li, Y.; Yu, J.; Wang, J.; Zhu, Q.; Chen, Z. Advance and challenges in the treatment of skin diseases with the transdermal drug delivery system. Pharmaceutics 2023, 15, 2165. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, D.; Yang, Y.; Huang, Y.; Wang, L.; Yao, X.; Lu, Q. Global epidemiology of atopic dermatitis: A comprehensive systematic analysis and modelling study. Br. J. Dermatol. 2024, 190, 55–61. [Google Scholar] [CrossRef]
- Sewerin, P.; Brinks, R.; Schneider, M.; Haase, I.; Vordenbäumen, S. Prevalence and incidence of psoriasis and psoriatic arthritis. Ann. Rheum. Dis. 2019, 78, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Chuah, L.-H.; Loo, H.-L.; Goh, C.F.; Fu, J.-Y.; Ng, S.-F. Chitosan-based drug delivery systems for skin atopic dermatitis: Recent advancements and patent trends. Drug Deliv. Transl. Res. 2023, 13, 1436–1455. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, B.Z.; Zhang, X.P.; Zheng, H.; Li, Z.; Zhang, C.Y.; Guo, X.D. Conductive microneedle patch with electricity-triggered drug release performance for atopic dermatitis treatment. ACS Appl. Mater. Interfaces 2022, 14, 31645–31654. [Google Scholar] [CrossRef]
- Song, L.; Chi, J.; Li, Z.; Tao, Y.; Sun, Y.; Zhou, Q.; Lu, S.; Huang, Q.; Huang, S.; Lu, X. An inflammation-responsive double-layer microneedle patch for recurrent atopic dermatitis therapy. Int. J. Pharm. 2023, 643, 123215. [Google Scholar] [CrossRef]
- Kim, Y.E.; Choi, S.W.; Kim, M.K.; Nguyen, T.L.; Kim, J. Therapeutic hydrogel patch to treat atopic dermatitis by regulating oxidative stress. Nano Lett. 2022, 22, 2038–2047. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, X.; Cai, L.; Gan, J.; Wang, J.; Wang, Y.; Zhao, Y. Black phosphorus hydrogel inverse opal microneedle patches for psoriasis treatment. Nano Today 2024, 54, 102072. [Google Scholar] [CrossRef]
- Dai, P.; Ge, X.; Sun, C.; Jiang, H.; Zuo, W.; Wu, P.; Liu, C.; Deng, S.; Yang, J.; Dai, J. A Novel Methacryloyl Chitosan Hydrogel Microneedles Patch with Sustainable Drug Release Property for Effective Treatment of Psoriasis. Macromol. Biosci. 2023, 23, 2300194. [Google Scholar] [CrossRef]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Skin barrier abnormalities and immune dysfunction in atopic dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef]
- Dhabale, A.; Nagpure, S. Types of psoriasis and their effects on the immune system. Cureus 2022, 14, e29536. [Google Scholar] [CrossRef]
- Magill, E.; Demartis, S.; Gavini, E.; Permana, A.D.; Thakur, R.R.S.; Adrianto, M.F.; Waite, D.; Glover, K.; Picco, C.J.; Korelidou, A. Solid implantable devices for sustained drug delivery. Adv. Drug Deliv. Rev. 2023, 199, 114950. [Google Scholar] [CrossRef]
- Dasht Bozorg, B.; Bhattaccharjee, S.A.; Somayaji, M.R.; Banga, A.K. Topical and transdermal delivery with diseased human skin: Passive and iontophoretic delivery of hydrocortisone into psoriatic and eczematous skin. Drug Deliv. Transl. Res. 2022, 12, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Kantor, R.; Silverberg, J.I. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev. Clin. Immunol. 2017, 13, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Mailepessov, D.; Ong, J.; Nasir, M.Z.M.; Aik, J.; Woo, M.; Zhao, X.; Tey, H.L.; Yew, Y.W. Association between exposure to ambient air pollution, meteorological factors and atopic dermatitis consultations in Singapore—A stratified nationwide time-series analysis. Sci. Rep. 2024, 14, 10320. [Google Scholar] [CrossRef]
- Barrea, L.; Nappi, F.; Di Somma, C.; Savanelli, M.C.; Falco, A.; Balato, A.; Balato, N.; Savastano, S. Environmental risk factors in psoriasis: The point of view of the nutritionist. Int. J. Environ. Res. Public Health 2016, 13, 743. [Google Scholar] [CrossRef]
- Zeng, J.; Luo, S.; Huang, Y.; Lu, Q. Critical role of environmental factors in the pathogenesis of psoriasis. J. Dermatol. 2017, 44, 863–872. [Google Scholar] [CrossRef]
- Farci, F.; Mahabal, G.D. Hyperkeratosis; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Whittam, A.J.; Maan, Z.N.; Duscher, D.; Wong, V.W.; Barrera, J.A.; Januszyk, M.; Gurtner, G.C. Challenges and opportunities in drug delivery for wound healing. Adv. Wound Care 2016, 5, 79–88. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Ski. Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef]
- Mu, X.; Gu, R.; Tang, M.; Wu, X.; He, W.; Nie, X. IL-17 in wound repair: Bridging acute and chronic responses. Cell Commun. Signal. 2024, 22, 288. [Google Scholar] [CrossRef]
- Abdul Latif, M.; Mustafa, A.; Keong, L.C.; Hamid, A. Chromolaena odorata layered-nitrile rubber polymer transdermal patch enhanced wound healing in vivo. PLoS ONE 2024, 19, e0295381. [Google Scholar] [CrossRef]
- Mo, R.; Zhang, H.; Xu, Y.; Wu, X.; Wang, S.; Dong, Z.; Xia, Y.; Zheng, D.; Tan, Q. Transdermal drug delivery via microneedles to mediate wound microenvironment. Adv. Drug Deliv. Rev. 2023, 195, 114753. [Google Scholar] [CrossRef]
- Lee, J.Y.; Min, D.-J.; Kim, W.; Bin, B.-H.; Kim, K.; Cho, E.-G. Non pharmacological high-intensity ultrasound treatment of human dermal fibroblasts to accelerate wound healing. Sci. Rep. 2021, 11, 2465. [Google Scholar] [CrossRef] [PubMed]
- Kataria, K.; Gupta, A.; Rath, G.; Mathur, R.; Dhakate, S. In vivo wound healing performance of drug loaded electrospun composite nanofibers transdermal patch. Int. J. Pharm. 2014, 469, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Muchová, M.; Münster, L.; Capáková, Z.; Mikulcová, V.; Kuřitka, I.; Vícha, J. Design of dialdehyde cellulose crosslinked poly (vinyl alcohol) hydrogels for transdermal drug delivery and wound dressings. Mater. Sci. Eng. C 2020, 116, 111242. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Mony, M.P.; Harmon, K.A.; Hess, R.; Dorafshar, A.H.; Shafikhani, S.H. An updated review of hypertrophic scarring. Cells 2023, 12, 678. [Google Scholar] [CrossRef]
- Mbituyimana, B.; Bukatuka, C.F.; Qi, F.; Ma, G.; Shi, Z.; Yang, G. Microneedle-mediated drug delivery for scar prevention and treatment. Drug Discov. Today 2023, 28, 103801. [Google Scholar] [CrossRef]
- De Decker, I.; Szabó, A.; Hoeksema, H.; Speeckaert, M.; Delanghe, J.R.; Blondeel, P.; Van Vlierberghe, S.; Monstrey, S.; Claes, K.E. Treatment of hypertrophic scars with corticoid-embedded dissolving microneedles. J. Burn Care Res. 2023, 44, 158–169. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Wang, Y.; Chen, D.; Huang, J.; Dai, W.; Peng, P.; Guo, L.; Lei, Y. Intradermal delivery of an angiotensin II receptor blocker using a personalized microneedle patch for treatment of hypertrophic scars. Biomater. Sci. 2023, 11, 583–595. [Google Scholar] [CrossRef]
- Yang, Z.-R.; Suo, H.; Fan, J.-W.; Lv, N.; Du, K.; Ma, T.; Qin, H.; Li, Y.; Yang, L.; Zhou, N. Endogenous stimuli-responsive separating microneedles to inhibit hypertrophic scar through remodeling the pathological microenvironment. Nat. Commun. 2024, 15, 2038. [Google Scholar] [CrossRef]
- Tan, C.W.; Tan, W.D.; Srivastava, R.; Yow, A.P.; Wong, D.W.; Tey, H.L. Dissolving triamcinolone-embedded microneedles for the treatment of keloids: A single-blinded intra-individual controlled clinical trial. Dermatol. Ther. 2019, 9, 601–611. [Google Scholar] [CrossRef]
- Wu, T.; Hou, X.; Li, J.; Ruan, H.; Pei, L.; Guo, T.; Wang, Z.; Ci, T.; Ruan, S.; He, Y. Microneedle-mediated biomimetic cyclodextrin metal organic frameworks for active targeting and treatment of hypertrophic scars. ACS Nano 2021, 15, 20087–20104. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, H.; Zhang, W.; Cao, M.; Yu, L.; Cheng, X. Betamethasone transdermal administration combined with fractional Er: YAG lasers or microplasma radiofrequency technology improved hypertrophic scars: A retrospective study. J. Cosmet. Dermatol. 2024, 23, 2563–2573. [Google Scholar] [CrossRef] [PubMed]
- Wo, Y.; Zhang, Z.; Zhang, Y.; Zhang, Z.; Wang, K.; Mao, X.; Su, W.; Li, K.; Cui, D.; Chen, J. Enhanced in vivo delivery of 5-fluorouracil by ethosomal gels in rabbit ear hypertrophic scar model. Int. J. Mol. Sci. 2014, 15, 22786–22800. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V. Burn wound: How it differs from other wounds? Indian J. Plast. Surg. 2012, 45, 364–373. [Google Scholar] [CrossRef]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Pal, D.; Rath, P.; Das, P.; Kundu, B.; Nandi, S.K. Local delivery systems of drugs/biologicals for the management of burn wounds. J. Drug Deliv. Sci. Technol. 2023, 85, 104556. [Google Scholar] [CrossRef]
- Goh, M.; Du, M.; Peng, W.R.; Saw, P.E.; Chen, Z. Advancing burn wound treatment: Exploring hydrogel as a transdermal drug delivery system. Drug Deliv. 2024, 31, 2300945. [Google Scholar] [CrossRef]
- Carta, T.; Gawaziuk, J.; Diaz-Abele, J.; Liu, S.; Jeschke, M.; Logsetty, S. Properties of an ideal burn dressing: A survey of burn survivors and front-line burn healthcare providers. Burns 2019, 45, 364–368. [Google Scholar] [CrossRef]
- Al-Anshori, A.A.; Putri, A.N.; Ismi, A.N.; Suhud, M.K.; Plumeriastuti, H.; Maslachah, L. Efficacy of Transdermal Delivery Nano Ethosomal Gel from Ashitaba Leaves on In-vivo Burn Wound Healing in Albino Rats. J. Med. Vet. 2023, 6, 145–154. [Google Scholar] [CrossRef]
- Tungadi, R.; Susanty, W.; Wicita, P.; Pido, E. Transdermal delivery of snakehead fish (Ophiocephalus striatus) nanoemulgel containing hydrophobic powder for burn wound. Pharm. Sci. 2018, 24, 313–323. [Google Scholar] [CrossRef]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Moraes, V.R.; Melo, M.O.; Maia Campos, P.M. Evaluation of Morphological and Structural Skin Alterations on Diabetic Subjects by Biophysical and Imaging Techniques. Life 2023, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Labib, A.; Rosen, J.; Yosipovitch, G. Skin manifestations of diabetes mellitus. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK481900/ (accessed on 16 August 2024).
- Hosseini, A.; Abdollahi, M. Diabetic neuropathy and oxidative stress: Therapeutic perspectives. Oxidative Med. Cell. Longev. 2013, 2013, 168039. [Google Scholar] [CrossRef] [PubMed]
- Strecker-McGraw, M.K.; Jones, T.R.; Baer, D.G. Soft tissue wounds and principles of healing. Emerg. Med. Clin. N. Am. 2007, 25, 1–22. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics 2019, 9, 65. [Google Scholar] [CrossRef]
- Zhou, W.; Duan, Z.; Zhao, J.; Fu, R.; Zhu, C.; Fan, D. Glucose and MMP-9 dual-responsive hydrogel with temperature sensitive self-adaptive shape and controlled drug release accelerates diabetic wound healing. Bioact. Mater. 2022, 17, 1–17. [Google Scholar] [CrossRef]
- Tan, W.; Long, T.; Wan, Y.; Li, B.; Xu, Z.; Zhao, L.; Mu, C.; Ge, L.; Li, D. Dual-drug loaded polysaccharide-based self-healing hydrogels with multifunctionality for promoting diabetic wound healing. Carbohydr. Polym. 2023, 312, 120824. [Google Scholar] [CrossRef]
- Guan, G.; Zhang, Q.; Jiang, Z.; Liu, J.; Wan, J.; Jin, P.; Lv, Q. Multifunctional silk fibroin methacryloyl microneedle for diabetic wound healing. Small 2022, 18, 2203064. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, X.; Zhao, X.; Cai, Y.; Zhang, X.; Xu, K.; Weng, J.; Li, J.; Chen, X. Multifunctional Double-Layer and Dual Drug-Loaded Microneedle Patch Promotes Diabetic Wound Healing. Adv. Healthc. Mater. 2023, 12, 2300297. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, W.; Zhai, X.; Zhao, X.; Wang, J.; Weng, J.; Li, J.; Chen, X. An antibacterial and proangiogenic double-layer drug-loaded microneedle patch for accelerating diabetic wound healing. Biomater. Sci. 2023, 11, 533–541. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, H.; Shi, Z.; Lin, L.; Li, Y.; Wang, M.; Pan, G.; Lei, Y.; Xue, L. Responsive hydrogel-based microneedle dressing for diabetic wound healing. J. Mater. Chem. B 2022, 10, 3501–3511. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, D.; Li, W.; Lin, H.; Ding, C.; Liu, Q.; Wang, L.; Li, Z.; Mei, L.; Chen, H. Biofilm microenvironment triggered self-enhancing photodynamic immunomodulatory microneedle for diabetic wound therapy. Nat. Commun. 2023, 14, 7658. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Wu, J.; Deng, M.; Wang, P.; Ji, G.; Wang, M.; Zhou, C.; Blum, N.T.; Zhang, W.; Shi, H. Multifunctional magnesium organic framework-based microneedle patch for accelerating diabetic wound healing. ACS Nano 2021, 15, 17842–17853. [Google Scholar] [CrossRef]
- Hu, Y.; Tao, R.; Chen, L.; Xiong, Y.; Xue, H.; Hu, L.; Yan, C.; Xie, X.; Lin, Z.; Panayi, A.C. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 150. [Google Scholar] [CrossRef]
- Ogbechie-Godec, O.A.; Elbuluk, N. Melasma: An up-to-date comprehensive review. Dermatol. Ther. 2017, 7, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Phansuk, K.; Vachiramon, V.; Jurairattanaporn, N.; Chanprapaph, K.; Rattananukrom, T. Dermal pathology in melasma: An update review. Clin. Cosmet. Investig. Dermatol. 2022, 15, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Jiryis, B.; Toledano, O.; Avitan-Hersh, E.; Khamaysi, Z. Management of Melasma: Laser and Other Therapies—Review Study. J. Clin. Med. 2024, 13, 1468. [Google Scholar] [CrossRef]
- Bin, B.H.; Seo, J.; Yang, S.H.; Lee, E.; Choi, H.; Kim, K.H.; Cho, E.G.; Lee, T.R. Novel inhibitory effect of the antidiabetic drug voglibose on melanogenesis. Exp. Dermatol. 2013, 22, 541–546. [Google Scholar] [CrossRef]
- Srivastava, R.; John, A.M.; Tam, A.; Firoz, B.F. The Tam formula: A pilot study for a new treatment for melasma. J. Cosmet. Dermatol. 2021, 20, 2168–2171. [Google Scholar] [CrossRef]
- Arellano, I.; Cestari, T.; Ocampo-Candiani, J.; Azulay-Abulafia, L.; Bezerra Trindade Neto, P.; Hexsel, D.; Machado-Pinto, J.; Muñoz, H.; Rivitti-Machado, M.; Sittart, J. Preventing melasma recurrence: Prescribing a maintenance regimen with an effective triple combination cream based on long-standing clinical severity. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 611–618. [Google Scholar] [CrossRef]
- Taylor, S.C.; Torok, H.; Jones, T.; Lowe, N.; Rich, P.; Tschen, E.; Menter, A.; Baumann, L.; Wieder, J.J.; Jarratt, M.M. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis 2003, 72, 67–73. [Google Scholar] [PubMed]
- Machekposhti, S.; Soltani, M.; Najafizadeh, P.; Ebrahimi, S.; Chen, P. Biocompatible polymer microneedle for topical/dermal delivery of tranexamic acid. J. Control. Release 2017, 261, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Geng, R.; Liu, Y.; Zhu, J. Advanced nanocarrier-and microneedle-based transdermal drug delivery strategies for skin diseases treatment. Theranostics 2022, 12, 3372. [Google Scholar] [CrossRef]
- Fabbrocini, G.; De Vita, V.; Fardella, N.; Pastore, F.; Annunziata, M.; Mauriello, M.; Monfrecola, A.; Cameli, N. Skin needling to enhance depigmenting serum penetration in the treatment of melasma. Plast. Surg. Int. 2011, 2011, 158241. [Google Scholar] [CrossRef]
- Shaji, J.; Parab, S.S. Formulation development of tranexamic acid loaded transethosomal patch for melasma. Res. J. Pharm. Dos. Forms Technol. 2022, 14, 7–16. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, L.; Tao, W.; Yang, X.; Li, J.; Wang, R.; Zhao, Y.; Peng, C.; Zhang, C. Preparation of paeoniflorin-glycyrrhizic acid complex transethosome gel and its preventive and therapeutic effects on melasma. Eur. J. Pharm. Sci. 2024, 192, 106664. [Google Scholar] [CrossRef]
- Xing, M.; Wang, X.; Zhao, L.; Zhou, Z.; Liu, H.; Wang, B.; Cheng, A.; Zhang, S.; Gao, Y. Novel dissolving microneedles preparation for synergistic melasma therapy: Combined effects of tranexamic acid and licorice extract. Int. J. Pharm. 2021, 600, 120406. [Google Scholar] [CrossRef]
- Aboul-Einien, M.H.; Kandil, S.M.; Abdou, E.M.; Diab, H.M.; Zaki, M.S. Ascorbic acid derivative-loaded modified aspasomes: Formulation, in vitro, ex vivo and clinical evaluation for melasma treatment. J. Liposome Res. 2020, 30, 54–67. [Google Scholar] [CrossRef]
- Schadendorf, D.; Fisher, D.E.; Garbe, C.; Gershenwald, J.E.; Grob, J.-J.; Halpern, A.; Herlyn, M.; Marchetti, M.A.; McArthur, G.; Ribas, A. Melanoma. Nat. Rev. Dis. Primers 2015, 1, 15003. [Google Scholar] [CrossRef]
- Swetter, S.; Geller, A. Melanoma: Clinical features and diagnosis. UpToDate. 2018. Available online: https://medilib.ir/uptodate/show/15806 (accessed on 22 October 2024).
- Kim, H.J.; Kim, Y.H. Molecular Frontiers in Melanoma: Pathogenesis, Diagnosis, and Therapeutic Advances. Int. J. Mol. Sci. 2024, 25, 2984. [Google Scholar] [CrossRef]
- Wunderlich, K.; Suppa, M.; Gandini, S.; Lipski, J.; White, J.; Del Marmol, V. Risk Factors and Innovations in Risk Assessment for Melanoma, Basal Cell Carcinoma, and Squamous Cell Carcinoma. Cancers 2024, 16, 1016. [Google Scholar] [CrossRef] [PubMed]
- Kozar, I.; Margue, C.; Rothengatter, S.; Haan, C.; Kreis, S. Many ways to resistance: How melanoma cells evade targeted therapies. Biochim. Biophys. Acta (BBA) Rev. Cancer 2019, 1871, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.M.; Paiva, K.L.; de Souza, I.F.; Mello, V.C.; Martins da Silva, I.G.; Souza, P.E.N.; Muehlmann, L.A.; Báo, S.N. The Potential of Photodynamic Therapy Using Solid Lipid Nanoparticles with Aluminum Phthalocyanine Chloride as a Nanocarrier for Modulating Immunogenic Cell Death in Murine Melanoma In Vitro. Pharmaceutics 2024, 16, 941. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Ramzy, A.; Sawy, A.M.; Nabil, M.; Gad, M.Z.; El-Shazly, M.; Aboul-Soud, M.A.; Azzazy, H.M.E.-S. Ozonated olive oil: Enhanced cutaneous delivery via niosomal nanovesicles for melanoma treatment. Antioxidants 2022, 11, 1318. [Google Scholar] [CrossRef]
- Nagaraja, S.; Basavarajappa, G.M.; Attimarad, M.; Pund, S. Topical nanoemulgel for the treatment of skin cancer: Proof-of-technology. Pharmaceutics 2021, 13, 902. [Google Scholar] [CrossRef]
- Albuquerque, L.F.; Lins, F.V.; Bispo, E.C.; Borges, E.N.; Silva, M.T.; Gratieri, T.; Cunha-Filho, M.; Alonso, A.; Carvalho, J.L.; Saldanha-Araujo, F. Ibrutinib topical delivery for melanoma treatment: The effect of nanostructured lipid carriers’ composition on the controlled drug skin deposition. Colloids Surf. B Biointerfaces 2024, 237, 113875. [Google Scholar] [CrossRef]
- Brito, S.; Heo, H.; Cha, B.; Lee, S.-H.; Chae, S.; Lee, M.-G.; Kwak, B.-M.; Bin, B.-H. A systematic exploration reveals the potential of spermidine for hypopigmentation treatment through the stabilization of melanogenesis-associated proteins. Sci. Rep. 2022, 12, 14478. [Google Scholar] [CrossRef]
- Bolognia, J.L.; Pawelek, J.M. Biology of hypopigmentation. J. Am. Acad. Dermatol. 1988, 19, 217–255. [Google Scholar] [CrossRef]
- Dina, Y.; McKesey, J.; Pandya, A.G. Disorders of hypopigmentation. J. Drugs Dermatol. JDD 2019, 18, s115–s116. [Google Scholar]
- Bin, B.-H.; Bhin, J.; Yang, S.H.; Choi, D.-H.; Park, K.; Shin, D.W.; Lee, A.-Y.; Hwang, D.; Cho, E.-G.; Lee, T.R. Hyperosmotic stress reduces melanin production by altering melanosome formation. PLoS ONE 2014, 9, e105965. [Google Scholar] [CrossRef]
- Kliegman, R.M.; Toth, H.; Bordini, B.J.; Basel, D. Nelson Pediatric Symptom-Based Diagnosis; Elsevier Health Sciences: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Hesham, H.; Rady, M.; Hathout, R.M.; Abdel-Halim, M.; Mansour, S. The skin delivery of tofacitinib citrate using transethosomes and hybridized ethosomes/nanostructured lipid carriers for vitiligo therapy: Dermatopharmacokinetics and in vivo assays. Int. J. Pharm. 2022, 629, 122387. [Google Scholar] [CrossRef] [PubMed]
- Elhalmoushy, P.M.; Elsheikh, M.A.; Matar, N.A.; El-Hadidy, W.F.; Kamel, M.A.; Omran, G.A.; Elnaggar, Y.S. Elaboration of novel gel-core oleosomes encapsulating phytoconstituent for targeted topical delivery in a vitiligo-induced mouse model: Focus on antioxidant and anti-inflammatory pathways. J. Drug Deliv. Sci. Technol. 2023, 80, 104119. [Google Scholar] [CrossRef]
- Wang, J.V.; Friedman, P.M.; Agron, S.; Konda, A.; Parker, C.; Geronemus, R.G. Enhanced uptake and retention of 0.03% bimatoprost, 0.5% 5-fluorouracil, and 5% minoxidil after 1,550-nm or 1,927-nm nonablative laser pretreatment. Dermatol. Surg. 2022, 48, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Weshahy, R.; Abdelhamid, M.F.; Sayed, K.S.; El Desouky, E.D.; Ramez, S.A. Efficacy and safety of combined fractional ablative CO2 laser and 5 fluorouracil in the treatment of acral vitiligo: An open, uncontrolled study. J. Cosmet. Dermatol. 2022, 21, 5636–5641. [Google Scholar] [CrossRef]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. Adv. Appl. 2016, 8, 163–176. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin wound healing: An update on the current knowledge and concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Marquet, F.; Grandclaude, M.-C.; Ferrari, E.; Champmartin, C. Capacity of an in vitro rat skin model to predict human dermal absorption: Influences of aging and anatomical site. Toxicol. In Vitro 2019, 61, 104623. [Google Scholar] [CrossRef]
- Niczyporuk, M. Rat skin as an experimental model in medicine. Prog. Health Sci. 2018, 8, 223–228. [Google Scholar] [CrossRef]
- Branski, L.K.; Mittermayr, R.; Herndon, D.N.; Norbury, W.B.; Masters, O.E.; Hofmann, M.; Traber, D.L.; Redl, H.; Jeschke, M.G. A porcine model of full-thickness burn, excision and skin autografting. Burns 2008, 34, 1119–1127. [Google Scholar] [CrossRef]
- Martínez-Navarro, F.J.; Martínez-Menchón, T.; Mulero, V.; Galindo-Villegas, J. Models of human psoriasis: Zebrafish the newly appointed player. Dev. Comp. Immunol. 2019, 97, 76–87. [Google Scholar] [CrossRef]
- Naldaiz-Gastesi, N.; Bahri, O.A.; Lopez de Munain, A.; McCullagh, K.J.; Izeta, A. The panniculus carnosus muscle: An evolutionary enigma at the intersection of distinct research fields. J. Anat. 2018, 233, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Todo, H. Transdermal permeation of drugs in various animal species. Pharmaceutics 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.C.; Edwards, G.A.; Martin, D.J.; Huang, H.; Crichton, M.L.; Kendall, M.A. Allometric scaling of skin thickness, elasticity, viscoelasticity to mass for micro-medical device translation: From mice, rats, rabbits, pigs to humans. Sci. Rep. 2017, 7, 15885. [Google Scholar] [CrossRef]
- Richardson, R.; Slanchev, K.; Kraus, C.; Knyphausen, P.; Eming, S.; Hammerschmidt, M. Adult zebrafish as a model system for cutaneous wound-healing research. J. Investig. Dermatol. 2013, 133, 1655–1665. [Google Scholar] [CrossRef]
- Le Guellec, D.; Morvan-Dubois, G.; Sire, J.-Y. Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio). Int. J. Dev. Biol. 2004, 48, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Gajula, K.; Gupta, R.; Sridhar, D.; Rai, B. In-silico skin model: A multiscale simulation study of drug transport. J. Chem. Inf. Model. 2017, 57, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.; Cho, W.-W.; Park, W.; Lee, J.-S.; Choi, M.-J.; Gao, Q.; Gao, G.; Cho, D.-W.; Kim, B.S. 3D biofabrication of diseased human skin models in vitro. Biomater. Res. 2023, 27, 80. [Google Scholar] [CrossRef]
- Olejnik, A.; Semba, J.A.; Kulpa, A.; Danczak-Pazdrowska, A.; Rybka, J.D.; Gornowicz-Porowska, J. 3D bioprinting in skin related research: Recent achievements and application perspectives. ACS Synth. Biol. 2021, 11, 26–38. [Google Scholar] [CrossRef]
- Bom, S.; Martins, A.M.; Ribeiro, H.M.; Marto, J. Diving into 3D (bio) printing: A revolutionary tool to customize the production of drug and cell-based systems for skin delivery. Int. J. Pharm. 2021, 605, 120794. [Google Scholar] [CrossRef]
- Risueño, I.; Valencia, L.; Jorcano, J.; Velasco, D. Skin-on-a-chip models: General overview and future perspectives. APL Bioeng. 2021, 5, 030901. [Google Scholar] [CrossRef]
- Varga-Medveczky, Z.; Kocsis, D.; Naszlady, M.B.; Fónagy, K.; Erdő, F. Skin-on-a-chip technology for testing transdermal drug delivery—Starting points and recent developments. Pharmaceutics 2021, 13, 1852. [Google Scholar] [CrossRef] [PubMed]
- Suthar, D.; Raut, R.; Bajaj, A. Advances in Skin-Mimetic Platforms: A Comprehensive Review of Drug Permeation Models. J. Drug Deliv. Sci. Technol. 2024, 98, 105887. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, S.; Baek, M.; Bin, B.-H. Skin Structure, Physiology, and Pathology in Topical and Transdermal Drug Delivery. Pharmaceutics 2024, 16, 1403. https://doi.org/10.3390/pharmaceutics16111403
Brito S, Baek M, Bin B-H. Skin Structure, Physiology, and Pathology in Topical and Transdermal Drug Delivery. Pharmaceutics. 2024; 16(11):1403. https://doi.org/10.3390/pharmaceutics16111403
Chicago/Turabian StyleBrito, Sofia, Moonki Baek, and Bum-Ho Bin. 2024. "Skin Structure, Physiology, and Pathology in Topical and Transdermal Drug Delivery" Pharmaceutics 16, no. 11: 1403. https://doi.org/10.3390/pharmaceutics16111403
APA StyleBrito, S., Baek, M., & Bin, B.-H. (2024). Skin Structure, Physiology, and Pathology in Topical and Transdermal Drug Delivery. Pharmaceutics, 16(11), 1403. https://doi.org/10.3390/pharmaceutics16111403






