Achieving the Optimal AgO Concentrations to Modulate the Anti-Trypanosoma cruzi Activity of Ag-ZnO/AgO Nanocomposites: In Vivo Investigations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanomaterials
2.2. Animals
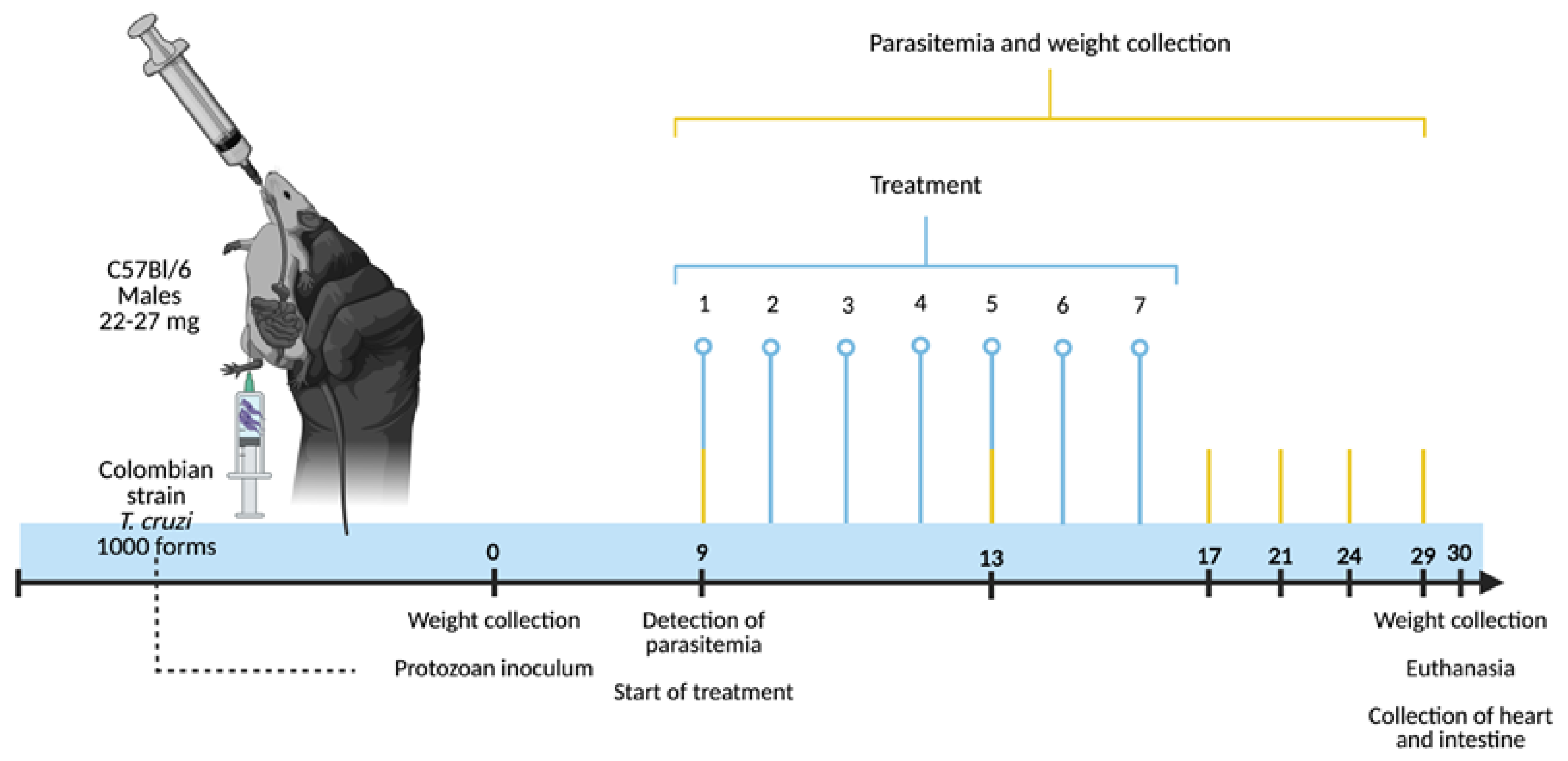
2.3. Experimental Design: Infection, Treatment, Euthanasia and Organ Harvesting
2.4. Monitoring Parasitemia, Weight and Survival
2.5. Processing of the Samples for Histopathology
2.5.1. Cardiac Tissue Parasitism
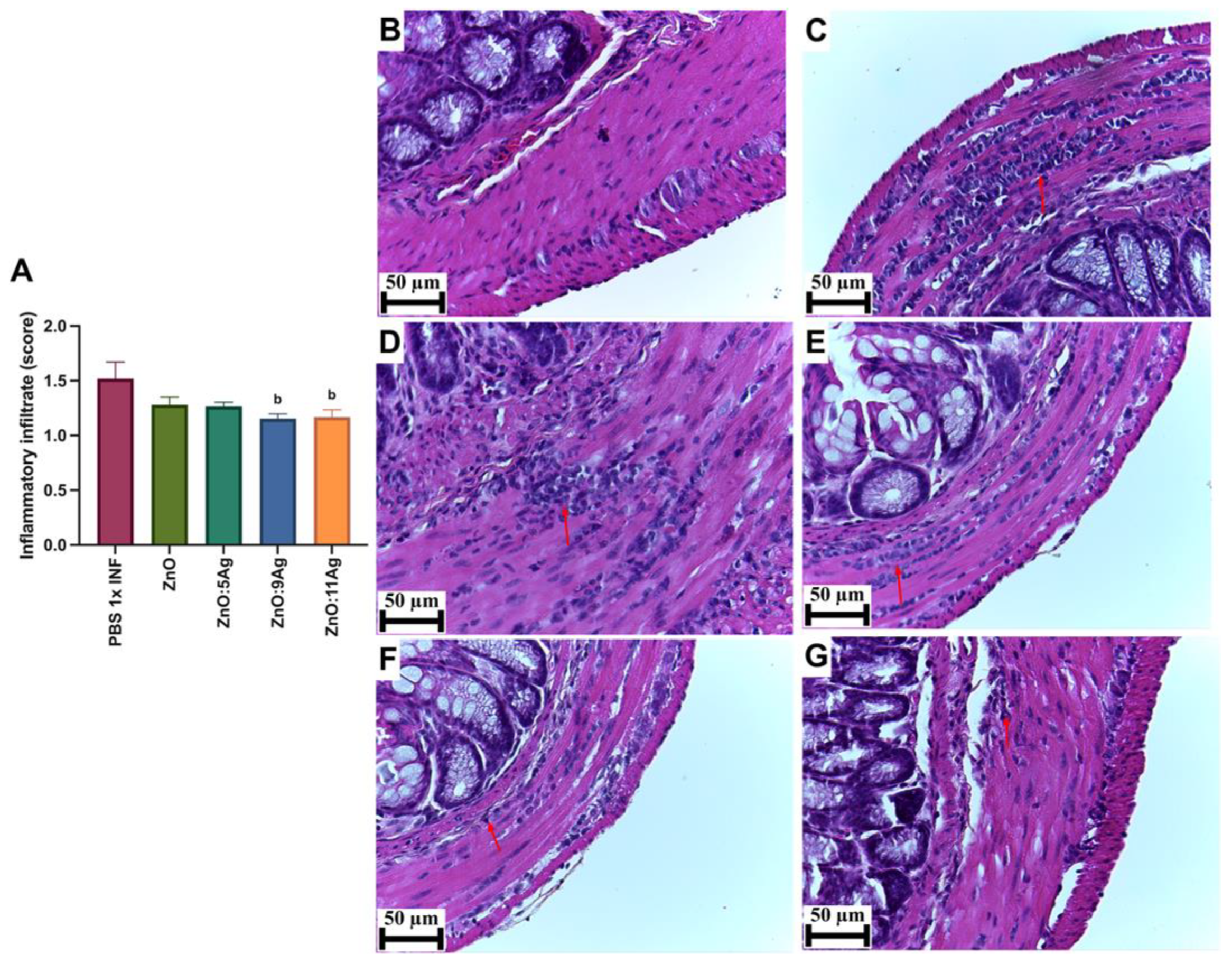
2.5.2. Quantification of Cardiac and Intestinal Inflammatory Infiltrate
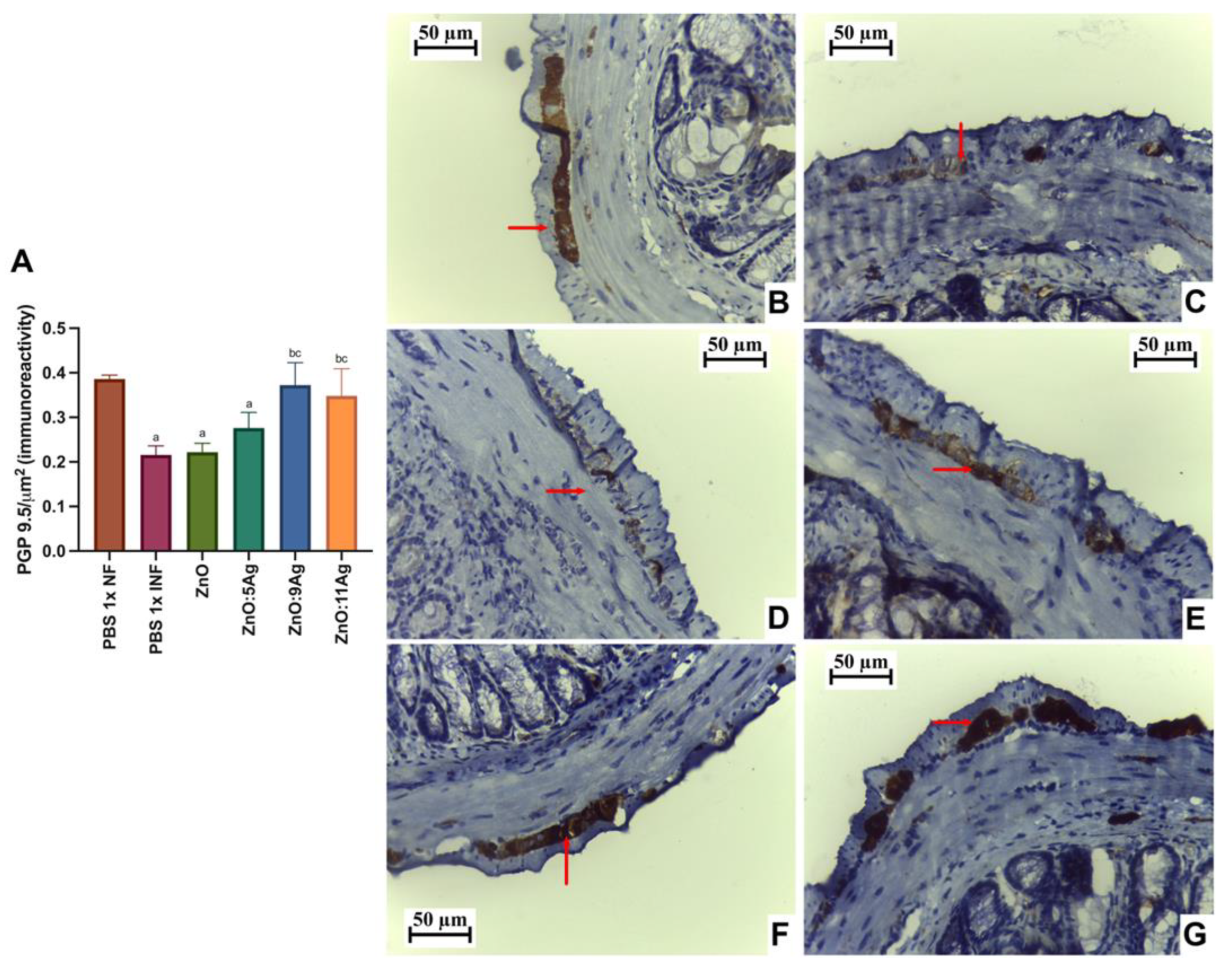
2.5.3. Quantification of PGP 9.5 Immunoreactivity in the Myenteric Plexus
2.5.4. Quantification of Cardiac and Intestinal Collagen
2.6. Obtaining Cardiac and Intestinal Homogenates and Immunological Analysis
2.7. Statistical Analysis
3. Results
3.1. A Reduction in Parasitemia and An Increase in Animal Survival Are Dependent on the Concentration of Ag in the Nanocomposites
3.2. Cardiac Histopathological Parameters: The Reduction in Cardiac Parasitism Is Dependent on the Concentration of Ag in the Nanocomposites
3.3. Intestinal Histopathological Parameters: ZnO:9 and ZnO:11 Nanocomposites Reduce Intestinal Inflammatory Infiltrate and Induce Neuroprotection
3.4. Treatment with ZnO:9 and ZnO:11 Nanocomposites Reduces Inflammatory Cytokines in the Heart and Intestine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chao, C.; Leone, J.L.; Vigliano, C.A. Chagas Disease: Historic Perspective. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165689. [Google Scholar] [CrossRef] [PubMed]
- Conteh, L.; Engels, T.; Molyneux, D.H. Socioeconomic Aspects of Neglected Tropical Diseases. Lancet 2010, 375, 239–247. [Google Scholar] [CrossRef]
- Lee, B.Y.; Bacon, K.M.; Bottazzi, M.E.; Hotez, P.J. Global Economic Burden of Chagas Disease: A Computational Simulation Model. Lancet Infect. Dis. 2013, 13, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Molina, J.A.; Molina, I. Chagas Disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Coura, J.R.; De Castro, S.L. A Critical Review on Chagas Disease Chemotherapy. Mem. Inst. Oswaldo Cruz 2002, 97, 3–24. [Google Scholar] [CrossRef]
- Coura, J.R.; Borges-Pereira, J. Chagas Disease: 100 Years After Its Discovery. A Systemic Review. Acta Trop. 2010, 115, 5–13. [Google Scholar] [CrossRef]
- Wilkinson, S.R.; Taylor, M.C.; Horn, D.; Kelly, J.M.; Cheeseman, I. A Mechanism for Cross-Resistance to Nifurtimox and Benznidazole in Trypanosomes. Proc. Natl. Acad. Sci. USA 2008, 105, 5022–5027. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.; Dias, N.; Paiva, T.; Hagström-Bex, L.; Nitz, N.; Pratesi, R.; Hecht, M. Current Trends in the Pharmacological Management of Chagas Disease. Int. J. Parasitol. Drugs Drug Resist. 2020, 12, 7–17. [Google Scholar] [CrossRef]
- Sales, P.A.; Molina, I.; Murta, S.M.F.; Sánchez-Montalvá, A.; Salvador, F.; Corrêa-Oliveira, R.; Carneiro, C.M. Experimental and Clinical Treatment of Chagas Disease: A Review. Am. J. Trop. Med. Hyg. 2017, 97, 1289–1303. [Google Scholar] [CrossRef]
- Morillo, C.; Marin-Neto, J.; Avezum, A.; Sosa-Estani, S.; Rassi, A.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; et al. Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy. N. Engl. J. Med. 2015, 373, e124–e125. [Google Scholar] [CrossRef]
- Jackson, Y.; Wyssa, B.; Chappuis, F. Tolerance to Nifurtimox and Benznidazole in Adult Patients with Chronic Chagas’ Disease. J. Antimicrob. Chemother. 2020, 75, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, C.; García-Vázquez, E.; Carrilero, B.; Simón, M.; Franco, F.; Iborra, M.A.; Gil-Gallardo, L.J.; Segovia, M. Tolerance and Adherence of Patients with Chronic Chagas Disease Treated with Benznidazole. Rev. Soc. Bras. Med. Trop. 2023, 56, 2023. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.L.d.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.L.R.; Chiavacci, L.A. Relationship Between Structure and Antimicrobial Activity of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef]
- Baghbani, Z.; Esmaeilnejad, B.; Asri-Rezaei, S. Assessment of Oxidative/Nitrosative Stress Biomarkers and DNA Damage in Teladorsagia circumcincta Following Exposure to Zinc Oxide Nanoparticles. J. Helminthol. 2020, 94, e115. [Google Scholar] [CrossRef]
- Singh, S. Zinc Oxide Nanoparticles Impacts: Cytotoxicity, Genotoxicity, Developmental Toxicity, and Neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.A.; Iqbal, J.; Ahmad, R.; Zia, L.; Kanwal, S.; Mahmood, T.; Wang, C.; Chen, J.T. Bioactivities of Geranium Wallichianum Leaf Extracts Conjugated with Zinc Oxide Nanoparticles. Biomolecules 2020, 10, 38. [Google Scholar] [CrossRef]
- do Carmo Neto, J.R.; Guerra, R.O.; Machado, J.R.; Silva, A.C.A.; da Silva, M.V. Antiprotozoal and Anthelmintic Activity of Zinc Oxide Nanoparticles. Curr. Med. Chem. 2022, 29, 2127–2141. [Google Scholar] [CrossRef]
- Guerra, R.O.; do Carmo Neto, J.R.; de Albuquerque Martins, T.; Farnesi de-Assunção, T.S.; Junior, V.R.; de Oliveira, C.J.F.; Silva, A.C.A.; da Silva, M.V. Metallic Nanoparticles: A New Frontier in the Fight Against Leishmaniasis. Curr. Med. Chem. 2022, 29, 4547–4573. [Google Scholar] [CrossRef]
- Barbosa, R.M.; Obata, M.M.S.; Neto, J.R.d.C.; Guerra, R.O.; Borges, A.V.B.e.; Trevisan, R.O.; Ruiz, L.C.; Bernardi, J.d.M.; Oliveira-Scussel, A.C.d.M.; Tanaka, S.C.S.V.; et al. Development of Ag-ZnO/AgO Nanocomposites Effectives for Leishmania Braziliensis Treatment. Pharmaceutics 2022, 14, 2642. [Google Scholar] [CrossRef]
- Fonseca, B.B.; Silva, P.L.A.P.A.; Silva, A.C.A.; Dantas, N.O.; De Paula, A.T.; Olivieri, O.C.L.; Beletti, M.E.; Rossi, D.A.; Goulart, L.R. Nanocomposite of Ag-Doped ZnO and AgO Nanocrystals as a Preventive Measure to Control Biofilm Formation in Eggshell and Salmonella spp. Entry Into Eggs. Front. Microbiol. 2019, 10, 217. [Google Scholar] [CrossRef]
- BRENER, Z. Therapeutic Activity and Criterion of Cure on Mice Experimentally Infected with Trypanosoma cruzi. Rev. Inst. Med. Trop. 1962, 4, 389–396. [Google Scholar]
- Brener, Z. Comparative studies of different strains of trypanosoma cruzi. Ann. Trop. Med. Parasitol. 1965, 59, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Jorge, T.C.d.; Castro, S.L.D. Doença de Chagas: Manual Para Experimentação Animal; Editora FIOCRUZ: Rio de Janeiro, Brazil, 2000. [Google Scholar]
- do Carmo Neto, J.R.; Vinicius da Silva, M.; Braga, Y.L.L.; Florencio da Costa, A.W.; Fonseca, S.G.; Nagib, P.R.A.; Nunes Celes, M.R.; Oliveira, M.A.P.; Machado, J.R. Correlation between Intestinal BMP2, IFNγ, and Neural Death in Experimental Infection with Trypanosoma cruzi. PLoS ONE 2021, 16, e0246692. [Google Scholar] [CrossRef]
- Braga, Y.L.L.; Neto, J.R.C.; Costa, A.W.F.; Silva, M.V.T.; Silva, M.V.; Celes, M.R.N.; Oliveira, M.A.P.; Joosten, L.A.B.; Ribeiro-Dias, F.; Gomes, R.S.; et al. Interleukin-32γ in the Control of Acute Experimental Chagas Disease. J. Immunol. Res. 2022, 2022, 7070301. [Google Scholar] [CrossRef]
- Wesley, M.; Moraes, A.; Rosa, A.d.C.; Carvalho, J.L.; Shiroma, T.; Vital, T.; Dias, N.; de Carvalho, B.; Rabello, D.D.A.; Borges, T.K.D.S.; et al. Correlation of Parasite Burden, Kdna Integration, Autoreactive Antibodies, and Cytokine Pattern in the Pathophysiology of Chagas Disease. Front. Microbiol. 2019, 10, 1856. [Google Scholar] [CrossRef]
- Do Carmo Neto, J.R.; Da Costa, A.W.F.; Braga, Y.L.L.; Lucio, F.H.; Dos Santos Martins, A.L.M.; Dos Reis, M.A.; De Oliveira, F.A.; Celes, M.R.N.; Da Silva, M.V.; Oliveira, M.A.P.; et al. The Colombian Strain of Trypanosoma cruzi Induces a Proinflammatory Profile, Neuronal Death, and Collagen Deposition in the Intestine of C57BL/6 Mice Both during the Acute and Early Chronic Phase. Mediat. Inflamm. 2022, 2022, 7641357. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.F.; Cangussú, S.D.; Duz, A.L.C.; Cartelle, C.T.; De Lourdes Noviello, M.; Veloso, V.M.; Bahia, M.T.; Almeida-Leite, C.M.; Arantes, R.M.E. Enteric Neuronal Damage, Intramuscular Denervation and Smooth Muscle Phenotype Changes as Mechanisms of Chagasic Megacolon: Evidence from a Long-Term Murine Model of Tripanosoma Cruzi Infection. PLoS ONE 2016, 11, e0153038. [Google Scholar] [CrossRef]
- Reis, É.d.M.; de Rezende, A.A.A.; Santos, D.V.; de Oliveria, P.F.; Nicolella, H.D.; Tavares, D.C.; Silva, A.C.A.; Dantas, N.O.; Spanó, M.A. Assessment of the Genotoxic Potential of Two Zinc Oxide Sources (Amorphous and Nanoparticles) Using the in Vitro Micronucleus Test and the in Vivo Wing Somatic Mutation and Recombination Test. Food Chem. Toxicol. 2015, 84, 55–63. [Google Scholar] [CrossRef]
- Alsamman, A.M.; Khedr, M.; Kabary, H.A.; El-Sehrawy, M.H. Elimination of Pathogenic Multidrug Resistant Isolates through Different Metal Oxide Nanoparticles Synthesized from Organic Plant and Microbial Sources. Microb. Pathog. 2023, 178, 106055. [Google Scholar] [CrossRef]
- Cho, W.S.; Kang, B.C.; Lee, J.K.; Jeong, J.; Che, J.H.; Seok, S.H. Comparative Absorption, Distribution, and Excretion of Titanium Dioxide and Zinc Oxide Nanoparticles after Repeated Oral Administration. Part. Fibre Toxicol. 2013, 10, 9. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Amara, S.; Slama, I.B.; Mrad, I.; Rihane, N.; Khemissi, W.; El Mir, L.; Rhouma, K.B.; Abdelmelek, H.; Sakly, M. Effects of Zinc Oxide Nanoparticles and/or Zinc Chloride on Biochemical Parameters and Mineral Levels in Rat Liver and Kidney. Hum. Exp. Toxicol. 2014, 33, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; Chung, H.E.; Yu, J.; Lee, J.A.; Kim, T.H.; Oh, J.M.; Lee, W.J.; Paek, S.M.; Lee, J.K.; Jeong, J.; et al. Pharmacokinetics, Tissue Distribution, and Excretion of Zinc Oxide Nanoparticles. Int. J. Nanomed. 2012, 7, 3081. [Google Scholar] [CrossRef]
- Liang, C.; Fang, J.; Hu, J.; Geng, X.; Liu, H.; Feng, Y.; Wang, W.; Cui, W.; Yu, Z.; Jia, X. Toxicokinetics of Zinc Oxide Nanoparticles and Food Grade Bulk-Sized Zinc Oxide in Rats After Oral Dosages. NanoImpact 2022, 25, 100368. [Google Scholar] [CrossRef]
- Paek, H.J.; Lee, Y.J.; Chung, H.E.; Yoo, N.H.; Lee, J.A.; Kim, M.K.; Lee, J.K.; Jeong, J.; Choi, S.J. Modulation of the Pharmacokinetics of Zinc Oxide Nanoparticles and Their Fates In Vivo. Nanoscale 2013, 5, 11416–11427. [Google Scholar] [CrossRef]
- Yang, P.; Hong, W.; Zhou, P.; Chen, B.; Xu, H. Nano and Bulk ZnO Trigger Diverse Zn-Transport-Related Gene Transcription in Distinct Regions of the Small Intestine in Mice After Oral Exposure. Biochem. Biophys. Res. Commun. 2017, 493, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2017, 9, 1–16. [Google Scholar] [CrossRef]
- Elje, E.; Mariussen, E.; Moriones, O.H.; Bastús, N.G.; Puntes, V.; Kohl, Y.; Dusinska, M.; Rundén-Pran, E. Hepato(Geno)Toxicity Assessment of Nanoparticles in a HepG2 Liver Spheroid Model. Nanomaterials 2020, 10, 545. [Google Scholar] [CrossRef]
- Ivask, A.; Juganson, K.; Bondarenko, O.; Mortimer, M.; Aruoja, V.; Kasemets, K.; Blinova, I.; Heinlaan, M.; Slaveykova, V.; Kahru, A. Mechanisms of Toxic Action of Ag, ZnO and CuO Nanoparticles to Selected Ecotoxicological Test Organisms and Mammalian Cells in Vitro: A Comparative Review. Nanotoxicology 2014, 8 (Suppl. S1), 57–71. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc Oxide Nanoparticles: Synthesis, Antiseptic Activity and Toxicity Mechanism. Adv. Colloid. Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- McShan, D.; Ray, P.C.; Yu, H. Molecular Toxicity Mechanism of Nanosilver. J. Food Drug Anal. 2014, 22, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial Properties and Toxicity from Metallic Nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef] [PubMed]
- Roberge, S.; Roussel, J.; Andersson, D.C.; Meli, A.C.; Vidal, B.; Blandel, F.; Lanner, J.T.; Le Guennec, J.Y.; Katz, A.; Westerblad, H.; et al. TNF-α-Mediated Caspase-8 Activation Induces ROS Production and TRPM2 Activation in Adult Ventricular Myocytes. Cardiovasc. Res. 2014, 103, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.P.S.; Andrieux, P.; Brochet, P.; Almeida, R.R.; Kitano, E.; Honda, A.K.; Iwai, L.K.; Andrade-Silva, D.; Goudenège, D.; Alcântara Silva, K.D.; et al. Co-Exposure of Cardiomyocytes to IFN-γ and TNF-α Induces Mitochondrial Dysfunction and Nitro-Oxidative Stress: Implications for the Pathogenesis of Chronic Chagas Disease Cardiomyopathy. Front. Immunol. 2021, 12, 755862. [Google Scholar] [CrossRef]
- Queiroga, T.B.D.; Pereira, N.d.S.; Silva, D.D.d.; Andrade, C.d.M.; Araújo Júnior, R.F.d.; Brito, C.R.d.N.; Galvão, L.M.d.C.; Câmara, A.C.J.d.; Nascimento, M.S.L.; Guedes, P.M.M. Virulence of Trypanosoma cruzi Strains Is Related to the Differential Expression of Innate Immune Receptors in the Heart. Front. Cell Infect. Microbiol. 2021, 11, 696719. [Google Scholar] [CrossRef]
- Talvani, A.; Ribeiro, C.S.; Aliberti, J.C.S.; Michailowsky, V.; Santos, P.V.A.; Murta, S.M.F.; Romanha, A.J.; Almeida, I.C.; Farber, J.; Lannes-Vieira, J.; et al. Kinetics of Cytokine Gene Expression in Experimental Chagasic Cardiomyopathy: Tissue Parasitism and Endogenous IFN-Gamma as Important Determinants of Chemokine MRNA Expression during Infection with Trypanosoma cruzi. Microbes Infect. 2000, 2, 851–866. [Google Scholar] [CrossRef]
- Truyens, C.; Torrico, F.; Angelo-Barrios, A.; Lucas, R.; Heremans, H.; Baetselier, P.D.; Carlier, Y. The Cachexia Associated with Trypanosoma cruzi Acute Infection in Mice Is Attenuated by Anti-TNF-Alpha, but Not by Anti-IL-6 or Anti-IFN-Gamma Antibodies. Parasite Immunol. 1995, 17, 561–568. [Google Scholar] [CrossRef]
- González, F.B.; Villar, S.R.; Toneatto, J.; Pacini, M.F.; Márquez, J.; D’Attilio, L.; Bottasso, O.A.; Piwien-Pilipuk, G.; Pérez, A.R. Immune Response Triggered by Trypanosoma cruzi Infection Strikes Adipose Tissue Homeostasis Altering Lipid Storage, Enzyme Profile and Adipokine Expression. Med. Microbiol. Immunol. 2019, 208, 651–666. [Google Scholar] [CrossRef]
- Cooley, A.; Rayford, K.J.; Arun, A.; Villalta, F.; Lima, M.F.; Pratap, S.; Nde, P.N. Trypanosoma cruzi Dysregulates PiRNAs Computationally Predicted to Target IL-6 Signaling Molecules During Early Infection of Primary Human Cardiac Fibroblasts. Immune Netw. 2022, 22, e51. [Google Scholar] [CrossRef]
- Herrera, R.N.; De Amaya, E.I.D.; Aguilar, R.C.P.; Turoni, C.J.; Marañón, R.; Berman, S.G.; Luciardi, H.L.; Coviello, A.; De Bruno, M.P. Inflammatory and Prothrombotic Activation with Conserved Endothelial Function in Patients with Chronic, Asymptomatic Chagas Disease. Clin. Appl. Thromb./Hemost. 2011, 17, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.R.; Gomes, J.A.S.; Fares, R.C.G.; Damásio, M.P.D.S.; Chaves, A.T.; Ferreira, K.S.; Nunes, M.C.P.; Medeiros, N.I.; Valente, V.A.A.; Corrêa-Oliveira, R.; et al. Plasma Cytokine Expression Is Associated with Cardiac Morbidity in Chagas Disease. PLoS ONE 2014, 9, e87082. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Olarte, S.; Bolaños, N.I.; Echeverry, M.; Rodríguez, A.N.; Cuéllar, A.; Puerta, C.J.; Mariño, A.; González, J.M. Intermediate Monocytes and Cytokine Production Associated with Severe Forms of Chagas Disease. Front. Immunol. 2019, 10, 1671. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.M.; Deng, X.; Fernandes, F.; Cunha-Neto, E.; Ribeiro, A.L.; Adesina, B.; Beyer, A.I.; Contestable, P.; Custer, B.; Busch, M.P.; et al. Inflammatory and Cardiac Biomarkers Are Differentially Expressed in Clinical Stages of Chagas Disease. Int. J. Cardiol. 2015, 199, 451–459. [Google Scholar] [CrossRef]
- Saavedra, E.; Herrera, M.; Gao, W.; Uemura, H.; Pereira, M.A. The Trypanosoma cruzi Trans-Sialidase, through Its COOH-Terminal Tandem Repeat, Upregulates Interleukin 6 Secretion in Normal Human Intestinal Microvascular Endothelial Cells and Peripheral Blood Mononuclear Cells. J. Exp. Med. 1999, 190, 1825–1836. [Google Scholar] [CrossRef]
- Michailowsky, V.; Silva, N.M.; Rocha, C.D.; Vieira, L.Q.; Lannes-Vieira, J.; Gazzinelli, R.T. Pivotal Role of Interleukin-12 and Interferon-Gamma Axis in Controlling Tissue Parasitism and Inflammation in the Heart and Central Nervous System during Trypanosoma cruzi Infection. Am. J. Pathol. 2001, 159, 1723–1733. [Google Scholar] [CrossRef]
- Castaños-Velez, E.; Maerlan, S.; Osorio, L.M.; Åberg, F.; Biberfeld, P.; Örn, A.; Rottenberg, M.E. Trypanosoma cruzi Infection in Tumor Necrosis Factor Receptor P55-Deficient Mice. Infect. Immun. 1998, 66, 2960–2968. [Google Scholar] [CrossRef]
- Adad, S.J.; Andrade, D.C.; Lopes, E.R.; Chapadeiro, E. Pathological Anatomy of Chagasic Megaesophagus. Rev. Inst. Med. Trop. Sao Paulo 1991, 33, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Adad, S.J.; Cançado, C.G.; Etchebehere, R.M.; Teixeira, V.P.A.; Gomes, U.A.; Chapadeiro, E.; Lopes, E.R. Neuron Count Reevaluation in the Myenteric Plexus of Chagasic Megacolon After Morphometric Neuron Analysis. Virchows Arch. 2001, 438, 254–258. [Google Scholar] [CrossRef]
- da Silveira, A.B.M.; Arantes, R.M.E.; Vago, A.R.; Lemos, E.M.; Adad, S.J.; Correa-Oliveira, R.; D’avila Reis, D. Comparative Study of the Presence of Trypanosoma cruzi KDNA, Inflammation and Denervation in Chagasic Patients with and without Megaesophagus. Parasitology 2005, 131, 627–634. [Google Scholar] [CrossRef]
- Machado, E.M.M.; Camilo Júnior, D.J.; Pinheiro, S.W.; Lopes, E.R.; Fernandes, A.J.; Dias, J.C.P.; Adad, S.J. Morphometry of Submucous and Myenteric Esophagic Plexus of Dogs Experimentally Reinfected with Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz 2001, 96, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.M.; Santos, F.N.; Jean, M.; Toledo, O.; Moraes, S.M.F.; Araujo, E.J.A.; Ana, M.G.S.; Araujo, S.M. Moderate Physical Exercise Reduces Parasitaemia and Protects Colonic Myenteric Neurons in Mice Infected with Trypanosoma cruzi. Int. J. Exp. Pathol. 2013, 94, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Oda, J.Y.; Belém, M.O.; Carlos, T.M.; Gouveia, R.; Luchetti, B.F.C.; Moreira, N.M.; Massocatto, C.L.; Araújo, S.M.; Sant´Ana, D.M.G.; Buttow, N.C.; et al. Myenteric Neuroprotective Role of Aspirin in Acute and Chronic Experimental Infections with Trypanosoma cruzi. Neurogastroenterol. Motil. 2017, 29, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Souza, N.D.D.; Belin, B.S.; Massocatto, C.L.; Araújo, S.M.D.; Sant’ana, D.M.G.; Araújo, E.J.A.; Filho, P.P.; Nihei, O.K.; Moreira, N.M. Effect of Acetylsalicylic Acid on Total Myenteric Neurons in Mice Experimentally Infected with Trypanosoma cruzi. An. Acad. Bras. Cienc. 2019, 91, e20180389. [Google Scholar] [CrossRef]
- Arantes, R.M.E.; Marche, H.H.F.; Bahia, M.T.; Cunha, F.Q.; Rossi, M.A.; Silva, J.S. Interferon-γ-Induced Nitric Oxide Causes Intrinsic Intestinal Denervation in Trypanosoma cruzi-Infected Mice. Am. J. Pathol. 2004, 164, 1361–1368. [Google Scholar] [CrossRef]
- Megale de Almeida-Leite, C.; da Cunha Galvão, L.M.; Afonso, L.C.C.; de Queiróz Cunha, F.; Arantes, R.M.E. Interferon-γ Induced Nitric Oxide Mediates in Vitro Neuronal Damage by Trypanosoma cruzi-Infected Macrophages. Neurobiol. Dis. 2007, 25, 170–178. [Google Scholar] [CrossRef]
- de Almeida-Leite, C.M.; Silva, I.C.C.; da Cunha Galvão, L.M.; Arantes, R.M.E. Sympathetic Glial Cells and Macrophages Develop Different Responses to Trypanosoma cruzi Infection or Lipopolysaccharide Stimulation. Mem. Inst. Oswaldo Cruz 2014, 109, 459–465. [Google Scholar] [CrossRef]
- Ricci, M.F.; Béla, S.R.; Moraes, M.M.; Bahia, M.T.; Mazzeti, A.L.; Oliveira, A.C.S.; Andrade, L.O.; Radí, R.; Piacenza, L.; Arantes, R.M.E. Neuronal Parasitism, Early Myenteric Neurons Depopulation and Continuous Axonal Networking Damage as Underlying Mechanisms of the Experimental Intestinal Chagas’ Disease. Front. Cell Infect. Microbiol. 2020, 10, 583899. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Maier, O. Interrelation of Oxidative Stress and Inflammation in Neurodegenerative Disease: Role of TNF. Oxid. Med. Cell Longev. 2015, 2015, 610813. [Google Scholar] [CrossRef]
- Neniskyte, U.; Vilalta, A.; Brown, G.C. Tumour Necrosis Factor Alpha-Induced Neuronal Loss Is Mediated by Microglial Phagocytosis. FEBS Lett. 2014, 588, 2952. [Google Scholar] [CrossRef]
- Olmos, G.; Lladó, J. Tumor Necrosis Factor Alpha: A Link Between Neuroinflammation and Excitotoxicity. Mediat. Inflamm. 2014, 2014, 861231. [Google Scholar] [CrossRef] [PubMed]
- Sasidharakurup, H.; Diwakar, S. Computational Modelling of TNFα Related Pathways Regulated by Neuroinflammation, Oxidative Stress and Insulin Resistance in Neurodegeneration. Appl. Netw. Sci. 2020, 5, 72. [Google Scholar] [CrossRef]
- Chen, B.; Deng, X.; Wang, B.; Liu, H. Etanercept, an Inhibitor of TNF-a, Prevents Propofol-Induced Neurotoxicity in the Developing Brain. Int. J. Dev. Neurosci. 2016, 55, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Dai, Q.; Jiang, P.; Zhu, L.; Dai, H.; Yao, Z.; Liu, H.; Ma, X.; Qu, L.; Jiang, J. Manganese Exposure Facilitates Microglial JAK2-STAT3 Signaling and Consequent Secretion of TNF-a and IL-1β to Promote Neuronal Death. Neurotoxicology 2018, 64, 195–203. [Google Scholar] [CrossRef]





| Composition (XRD) | Size (SEM) | |||
|---|---|---|---|---|
| ZnO | AgO | Ag-ZnO | ||
| ZnO | 100% | - | - | ~260 nm |
| ZnO:5Ag | 49% | 51% | ~250 nm | |
| ZnO:9Ag | 65% | 35% | ~345 nm | |
| ZnO:11Ag | 68% | 38% | ~290 nm | |
| Groups | Trypanosoma cruzi Strain | Condition | Days of Infection/Maintenance | Treatment Dose | Days of Treatment | Route of Administration | Number of Animals |
|---|---|---|---|---|---|---|---|
| Healthy control (NF) | - | Vehicle | 30 | - | 7 consecutive days | Gavage | 6 |
| Infection control (INF) | Colombian | Vehicle | 30 | - | 7 consecutive days | Gavage | 12 |
| ZnO ZnO:5Ag ZnO:9Ag ZnO:11Ag | Colombian | Nanomaterial | 30 | 5 mg/kg | 7 consecutive days | Gavage | 12 12 12 12 |
| Nanomaterials | ||||
|---|---|---|---|---|
| ZnO | ZnO:5Ag | ZnO:9Ag | ZnO:11Ag | |
| Parameters evaluated | ||||
| Heart | ||||
| Amastigote nests | = | = | ↓ | ↓ |
| Inflammatory infiltrate | ↑ | = | = | = |
| Collagen deposition | = | = | = | = |
| Cytokines | TNF-α: = | TNF-α: = | TNF-α: ↓ | TNF-α: ↓ |
| IFN-γ: = | IFN-γ: = | IFN-γ: = | IFN-γ: = | |
| IL-6: = | IL-6: = | IL-6: ↓ | IL-6: ↓ | |
| IL-4: = | IL-4: = | IL-4: = | IL-4: = | |
| IL-10: = | IL-10: = | IL-10: = | IL-10: = | |
| Intestine | ||||
| Inflammatory infiltrate | = | = | ↓ | ↓ |
| Neuronal immunolabeling | = | = | ↓ | ↓ |
| Collagen deposition | = | = | = | ↓ |
| Cytokines | TNF-α: = | TNF-α: = | TNF-α: ↓ | TNF-α: ↓ |
| IFN-γ: = | IFN-γ: = | IFN-γ: = | IFN-γ: = | |
| IL-6: = | IL-6: = | IL-6: = | IL-6: = | |
| IL-4: = | IL-4: = | IL-4: = | IL-4: = | |
| IL-10: = | IL-10: = | IL-10: = | IL-10: = | |
| Clinical and parasitological | ||||
| Weight | ↓ | = | = | = |
| Parasitemia | = | = | ↓ | ↓ |
| Survival | ↓ | = | ↑ | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Carmo Neto, J.R.; Braga, Y.L.L.; Franco, P.I.R.; de Oliveira, J.F.; Trevisan, R.O.; Mendes, K.M.; de Oliveira, M.A.P.; Celes, M.R.N.; Silva, A.C.A.; Machado, J.R.; et al. Achieving the Optimal AgO Concentrations to Modulate the Anti-Trypanosoma cruzi Activity of Ag-ZnO/AgO Nanocomposites: In Vivo Investigations. Pharmaceutics 2024, 16, 1415. https://doi.org/10.3390/pharmaceutics16111415
do Carmo Neto JR, Braga YLL, Franco PIR, de Oliveira JF, Trevisan RO, Mendes KM, de Oliveira MAP, Celes MRN, Silva ACA, Machado JR, et al. Achieving the Optimal AgO Concentrations to Modulate the Anti-Trypanosoma cruzi Activity of Ag-ZnO/AgO Nanocomposites: In Vivo Investigations. Pharmaceutics. 2024; 16(11):1415. https://doi.org/10.3390/pharmaceutics16111415
Chicago/Turabian Styledo Carmo Neto, José Rodrigues, Yarlla Loyane Lira Braga, Pablo Igor Ribeiro Franco, Jordana Fernandes de Oliveira, Rafael Obata Trevisan, Karen Martins Mendes, Milton Adriano Pelli de Oliveira, Mara Rúbia Nunes Celes, Anielle Christine Almeida Silva, Juliana Reis Machado, and et al. 2024. "Achieving the Optimal AgO Concentrations to Modulate the Anti-Trypanosoma cruzi Activity of Ag-ZnO/AgO Nanocomposites: In Vivo Investigations" Pharmaceutics 16, no. 11: 1415. https://doi.org/10.3390/pharmaceutics16111415
APA Styledo Carmo Neto, J. R., Braga, Y. L. L., Franco, P. I. R., de Oliveira, J. F., Trevisan, R. O., Mendes, K. M., de Oliveira, M. A. P., Celes, M. R. N., Silva, A. C. A., Machado, J. R., & da Silva, M. V. (2024). Achieving the Optimal AgO Concentrations to Modulate the Anti-Trypanosoma cruzi Activity of Ag-ZnO/AgO Nanocomposites: In Vivo Investigations. Pharmaceutics, 16(11), 1415. https://doi.org/10.3390/pharmaceutics16111415











