Recent Advances in the Treatment of Malaria
Abstract
:1. Introduction
2. Current State of the Art in Malaria Treatment Modalities
2.1. Quinine Derivatives
2.1.1. Mechanisms of Action
2.1.2. Treatment Modalities
2.2. 8-Aminoquinoline Compounds
2.2.1. Mechanisms of Action
2.2.2. Treatment Modalities
2.3. Antifolate Compounds in Malaria Treatment
Mechanisms of Action
2.4. Artemisinin Compounds
2.4.1. Mechanisms of Action
2.4.2. Current ACT Treatment Modalities
Artemether–Lumefantrine (Coartem)
Artesunate–Amodiaquine
Dihydroartemisinin–Piperaquine (DHA-PPQ)
Artesunate–Mefloquine
3. Genetic Basis for Resistance to Antimalarials
4. Innovative Approaches to the Treatment of Resistance
4.1. Triple Artemisinin-Based Combination Therapies (TACTs)
4.2. Antimalarial Vaccine Development
4.2.1. Preclinical and Clinical Studies
Pre-Erythrocytic-Stage Vaccines
Whole Sporozoite Vaccines (WSVs)
Subunit Vaccines
Blood-Stage Vaccines
5. Challenges and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Al-Awadhi, M.; Ahmad, S.; Iqbal, J. Current Status and the Epidemiology of Malaria in the Middle East Region and Beyond. Microorganisms 2021, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Aliyo, A.; Golicha, W.; Fikrie, A. Malaria and associated factors among under-five children in Borena pastoral communities, southern Ethiopia. Front. Parasitol. 2024, 3, 1438218. [Google Scholar] [CrossRef]
- Anwar, M. Introduction: An Overview of Malaria and Plasmodium. In Drug Targets for Plasmodium falciparum: Historic to Future Perspectives; Tarique, M., Ed.; Springer Nature: Singapore, 2024; pp. 1–17. [Google Scholar] [CrossRef]
- Zekar, L.; Sharman, T. Plasmodium falciparum Malaria. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK555962/ (accessed on 10 August 2024).
- Varo, R.; Chaccour, C.; Bassat, Q. Update on malaria. Med. Clínica Engl. Ed. 2020, 155, 395–402. [Google Scholar] [CrossRef]
- Kotepui, M.; Kotepui, K.U.; Milanez, G.D.J.; Masangkay, F.R. Prevalence and risk factors related to poor outcome of patients with severe Plasmodium vivax infection: A systematic review, meta-analysis, and analysis of case reports. BMC Infect. Dis. 2020, 20, 363. [Google Scholar] [CrossRef]
- Naik, D.G. Plasmodium knowlesi-mediated zoonotic malaria: A challenge for elimination. Trop. Parasitol. 2020, 10, 3. [Google Scholar]
- Oriero, E.C.; Amenga-Etego, L.; Ishengoma, D.S.; Amambua-Ngwa, A. Plasmodium malariae, current knowledge and future research opportunities on a neglected malaria parasite species. Crit. Rev. Microbiol. 2021, 47, 44–56. [Google Scholar] [CrossRef]
- Dagen, M. Chapter 1—History of malaria and its treatment. In Antimalarial Agents; Patrick, G.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–48. [Google Scholar] [CrossRef]
- Dhorda, M.; Amaratunga, C.; Dondorp, A.M. Artemisinin and multidrug-resistant Plasmodium falciparum—A threat for malaria control and elimination. Curr. Opin. Infect. Dis. 2021, 34, 432–439. [Google Scholar] [CrossRef]
- Siqueira-Neto, J.L.; Wicht, K.J.; Chibale, K.; Burrows, J.N.; Fidock, D.A.; Winzeler, E.A. Antimalarial drug discovery: Progress and approaches. Nat. Rev. Drug Discov. 2023, 22, 807–826. [Google Scholar] [CrossRef]
- Pandey, S.K.; Anand, U.; Siddiqui, W.A.; Tripathi, R. Drug Development Strategies for Malaria: With the Hope for New Antimalarial Drug Discovery—An Update. Adv. Med. 2023, 2023, 5060665. [Google Scholar] [CrossRef]
- Shibeshi, M.A.; Kifle, Z.D.; Atnafie, S.A. Antimalarial Drug Resistance and Novel Targets for Antimalarial Drug Discovery. Infect. Drug Resist. 2020, 13, 4047–4060. [Google Scholar] [CrossRef]
- Kapishnikov, S.; Hempelmann, E.; Elbaum, M.; Als-Nielsen, J.; Leiserowitz, L. Malaria Pigment Crystals: The Achilles′ Heel of the Malaria Parasite. ChemMedChem 2021, 16, 1515–1532. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Roy, R.; Ghosh, K.; Nath, D.; Samadder, A.; Nandi, S. To quest new targets of Plasmodium parasite and their potential inhibitors to combat antimalarial drug resistance. J. Parasit. Dis. 2024. [Google Scholar] [CrossRef]
- Wilkins, C.A.; du Plessis, L.H.; Viljoen, J.M. Investigating In Vitro and Ex Vivo Properties of Artemether/Lumefantrine Double-Fixed Dose Combination Lipid Matrix Tablets Prepared by Hot Fusion. Pharmaceutics 2021, 13, 922. [Google Scholar] [CrossRef]
- Popovici, J.; Tebben, K.; Witkowski, B.; Serre, D. Primaquine for Plasmodium vivax radical cure: What we do not know and why it matters. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 36–42. [Google Scholar] [CrossRef]
- Capela, R.; Moreira, R.; Lopes, F. An Overview of Drug Resistance in Protozoal Diseases. Int. J. Mol. Sci. 2019, 20, 5748. [Google Scholar] [CrossRef] [PubMed]
- Yadessa, A.M.; Zeleke, D. A Review on Synthesis of Quinoline Analogs as Antimalarial, Antibacterial and Anticancer agents. Ethiop. J. Sci. Sustain. Dev. 2021, 8, 73–95. [Google Scholar] [CrossRef]
- Chamma-Siqueira, N.N.; Negreiros, S.C.; Ballard, S.-B.; Farias, S.; Silva, S.P.; Chenet, S.M.; Santos, E.J.M.; de Sena, L.W.P.; da Costa, F.P.; Cardoso-Mello, A.G.N.; et al. Higher-Dose Primaquine to Prevent Relapse of Plasmodium vivax Malaria. N. Engl. J. Med. 2022, 386, 1244–1253. [Google Scholar] [CrossRef]
- Hanboonkunupakarn, B.; White, N.J. Advances and roadblocks in the treatment of malaria. Br. J. Clin. Pharmacol. 2022, 88, 374–382. [Google Scholar] [CrossRef]
- Lacerda, M.V.G.; Llanos-Cuentas, A.; Krudsood, S.; Lon, C.; Saunders, D.L.; Mohammed, R.; Yilma, D.; Batista Pereira, D.; Espino, F.E.J.; Mia, R.Z.; et al. Single-Dose Tafenoquine to Prevent Relapse of Plasmodium vivax Malaria. N. Engl. J. Med. 2019, 380, 215–228. [Google Scholar] [CrossRef]
- Wångdahl, A.; Sondén, K.; Wyss, K.; Stenström, C.; Björklund, D.; Zhang, J.; Hervius Askling, H.; Carlander, C.; Hellgren, U.; Färnert, A. Relapse of Plasmodium vivax and Plasmodium ovale Malaria With and Without Primaquine Treatment in a Nonendemic Area. Clin. Infect. Dis. 2022, 74, 1199–1207. [Google Scholar] [CrossRef]
- Zottig, V.E.; Carr, K.A.; Clarke, J.G.; Shmuklarsky, M.J.; Kreishman-Deitrick, M. Army Antimalarial Drug Development: An Advanced Development Case Study for Tafenoquine. Mil. Med. 2020, 185 (Suppl. S1), 617–623. [Google Scholar] [CrossRef] [PubMed]
- Pethrak, C.; Posayapisit, N.; Pengon, J.; Suwanakitti, N.; Saeung, A.; Shorum, M.; Aupalee, K.; Taai, K.; Yuthavong, Y.; Kamchonwongpaisan, S.; et al. New Insights into Antimalarial Chemopreventive Activity of Antifolates. Antimicrob. Agents Chemother. 2022, 66, e01538-21. [Google Scholar] [CrossRef] [PubMed]
- Abugri, J.; Ansah, F.; Asante, K.P.; Opoku, C.N.; Amenga-Etego, L.A.; Awandare, G.A. Prevalence of chloroquine and antifolate drug resistance alleles in Plasmodium falciparum clinical isolates from three areas in Ghana. AAS Open Res. 2018, 1, 1. [Google Scholar] [CrossRef]
- Shamshad, H.; Bakri, R.; Mirza, A.Z. Dihydrofolate reductase, thymidylate synthase, and serine hydroxy methyltransferase: Successful targets against some infectious diseases. Mol. Biol. Rep. 2022, 49, 6659–6691. [Google Scholar] [CrossRef]
- Sehrawat, R.; Rathee, P.; Khatkar, S.; Akkol, E.; Khayatkashani, M.; Nabavi, S.M.; Khatkar, A. Dihydrofolate Reductase (DHFR) Inhibitors: A Comprehensive Review. Curr. Med. Chem. 2024, 31, 799–824. [Google Scholar] [CrossRef] [PubMed]
- Nzila, A. The past, present and future of antifolates in the treatment of Plasmodium falciparum infection. J. Antimicrob. Chemother. 2006, 57, 1043–1054. [Google Scholar] [CrossRef]
- Laminou, I.M.; Issa, I.; Adehossi, E.; Maman, K.; Jackou, H.; Coulibaly, E.; Tohon, Z.B.; Ahmed, J.; Sanoussi, E.; Koko, D. Therapeutic efficacy and tolerability of artemether-lumefantrine for uncomplicated Plasmodium falciparum malaria in Niger, 2020. Malar. J. 2024, 23, 144. [Google Scholar] [CrossRef]
- L’Huillier, A.G.; Lau, R.; Bitnun, A.; Boggild, A.K. Atovaquone-proguanil treatment failure in a case of pediatric Plasmodium falciparum infection: Malabsorption and resistance. Travel Med. Infect. Dis. 2020, 37, 101829. [Google Scholar] [CrossRef]
- Kurup, N.; Rajnani, N. Chronology of Drug Development for Malaria. In Drug Development for Malaria; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 1–23. [Google Scholar] [CrossRef]
- Fernandes, V.d.S.; da Rosa, R.; Zimmermann, L.A.; Rogério, K.R.; Kümmerle, A.E.; Bernardes, L.S.C.; Graebin, C.S. Antiprotozoal agents: How have they changed over a decade? Arch. Pharm. 2022, 355, 2100338. [Google Scholar] [CrossRef]
- Mbeye, N.; ter Kuile, F.O.; Davies, M.-A.; Phiri, K.; Egger, M.; Wandeler, G. Cotrimoxazole prophylactic treatment prevents malaria in children in sub-Saharan Africa: Systematic review and meta-analysis. Trop. Med. Int. Health TM IH 2014, 19, 1057–1067. [Google Scholar] [CrossRef]
- Egwu, C.O.; Augereau, J.-M.; Reybier, K.; Benoit-Vical, F. Reactive Oxygen Species as the Brainbox in Malaria Treatment. Antioxidants 2021, 10, 1872. [Google Scholar] [CrossRef] [PubMed]
- Egwu, C.O.; Tsamesidis, I.; Pério, P.; Augereau, J.-M.; Benoit-Vical, F.; Reybier, K. Superoxide: A major role in the mechanism of action of essential antimalarial drugs. Free Radic. Biol. Med. 2021, 167, 271–275. [Google Scholar] [CrossRef]
- Gupta, Y.; Goicoechea, S.; Pearce, C.M.; Mathur, R.; Romero, J.G.; Kwofie, S.K.; Weyenberg, M.C.; Daravath, B.; Sharma, N.; Poonam; et al. The emerging paradigm of calcium homeostasis as a new therapeutic target for protozoan parasites. Med. Res. Rev. 2022, 42, 56–82. [Google Scholar] [CrossRef]
- Erhunse, N.; Sahal, D. Protecting future antimalarials from the trap of resistance: Lessons from artemisinin-based combination therapy (ACT) failures. J. Pharm. Anal. 2021, 11, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Wicht, K.J.; Mok, S.; Fidock, D.A. Molecular Mechanisms of Drug Resistance in Plasmodium falciparum Malaria. Annu. Rev. Microbiol. 2020, 74, 431–454. [Google Scholar] [CrossRef]
- Derbie, A.; Mekonnen, D.; Adugna, M.; Yeshitela, B.; Woldeamanuel, Y.; Abebe, T. Therapeutic Efficacy of Artemether-Lumefantrine (Coartem®) for the Treatment of Uncomplicated Falciparum Malaria in Africa: A Systematic Review. J. Parasitol. Res. 2020, 2020, 7371681. [Google Scholar] [CrossRef]
- Mumtaz, R.; Okell, L.C.; Challenger, J.D. Asymptomatic recrudescence after artemether–lumefantrine treatment for uncomplicated falciparum malaria: A systematic review and meta-analysis. Malar. J. 2020, 19, 453. [Google Scholar] [CrossRef] [PubMed]
- Maude, R.J.; Plewes, K.; Faiz, M.A.; Hanson, J.; Charunwatthana, P.; Lee, S.J.; Tärning, J.; Yunus, E.B.; Hoque, M.G.; Hasan, M.U.; et al. Does Artesunate Prolong the Electrocardiograph QT Interval in Patients with Severe Malaria? Am. J. Trop. Med. Hyg. 2009, 80, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Marwa, K.; Kapesa, A.; Baraka, V.; Konje, E.; Kidenya, B.; Mukonzo, J.; Kamugisha, E.; Swedberg, G. Therapeutic efficacy of artemether-lumefantrine, artesunate-amodiaquine and dihydroartemisinin-piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Sub-Saharan Africa: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0264339. [Google Scholar] [CrossRef]
- Zwang, J.; Dorsey, G.; Mårtensson, A.; d’Alessandro, U.; Ndiaye, J.-L.; Karema, C.; Djimde, A.; Brasseur, P.; Sirima, S.B.; Olliaro, P. Plasmodium falciparum clearance in clinical studies of artesunate-amodiaquine and comparator treatments in sub-Saharan Africa, 1999–2009. Malar. J. 2014, 13, 114. [Google Scholar] [CrossRef]
- Mohammed, H.; Sime, H.; Hailgiorgis, H.; Gubae, K.; Haile, M.; Solomon, H.; Etana, K.; Girma, S.; Bekele, W.; Chernet, M.; et al. Therapeutic efficacy of dihydroartemisinin–piperaquine for the treatment of uncomplicated Plasmodium vivax malaria in Seacha area, Arbaminch Zuria District, South West Ethiopia. Malar. J. 2022, 21, 351. [Google Scholar] [CrossRef] [PubMed]
- Popovici, J.; Vantaux, A.; Primault, L.; Samreth, R.; Piv, E.P.; Bin, S.; Kim, S.; Lek, D.; Serre, D.; Menard, D. Therapeutic and Transmission-Blocking Efficacy of Dihydroartemisinin/Piperaquine and Chloroquine against Plasmodium vivax Malaria, Cambodia. Emerg. Infect. Dis. 2018, 24, 1516–1519. [Google Scholar] [CrossRef] [PubMed]
- Carrara, V.I.; Sirilak, S.; Thonglairuam, J.; Rojanawatsirivet, C.; Proux, S.; Gilbos, V.; Brockman, A.; Ashley, E.A.; McGready, R.; Krudsood, S.; et al. Deployment of early diagnosis and mefloquine-artesunate treatment of falciparum malaria in Thailand: The Tak Malaria Initiative. PLoS Med. 2006, 3, e183. [Google Scholar] [CrossRef] [PubMed]
- Nosten, F.; van Vugt, M.; Price, R.; Luxemburger, C.; Thway, K.L.; Brockman, A.; McGready, R.; ter Kuile, F.; Looareesuwan, S.; White, N.J. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: A prospective study. Lancet Lond. Engl. 2000, 356, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Dondorp, A.M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A.P.; Tarning, J.; Lwin, K.M.; Ariey, F.; Hanpithakpong, W.; Lee, S.J.; et al. Artemisinin Resistance in Plasmodium falciparum Malaria. N. Engl. J. Med. 2009, 361, 455–467. [Google Scholar] [CrossRef]
- Price, R.N.; Nosten, F.; Luxemburger, C.; Kham, A.; Brockman, A.; Chongsuphajaisiddhi, T.; White, N.J. Artesunate versus artemether in combination with mefloquine for the treatment of multidrug-resistant falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 523–527. [Google Scholar] [CrossRef]
- Sirima, S.B.; Ogutu, B.; Lusingu, J.P.A.; Mtoro, A.; Mrango, Z.; Ouedraogo, A.; Yaro, J.B.; Onyango, K.O.; Gesase, S.; Mnkande, E.; et al. Comparison of artesunate–mefloquine and artemether–lumefantrine fixed-dose combinations for treatment of uncomplicated Plasmodium falciparum malaria in children younger than 5 years in sub-Saharan Africa: A randomised, multicentre, phase 4 trial. Lancet Infect. Dis. 2016, 16, 1123–1133. [Google Scholar] [CrossRef]
- Lee, S.J.; ter Kuile, F.O.; Price, R.N.; Luxemburger, C.; Nosten, F. Adverse effects of mefloquine for the treatment of uncomplicated malaria in Thailand: A pooled analysis of 19,850 individual patients. PLoS ONE 2017, 12, e0168780. [Google Scholar] [CrossRef]
- Belete, T.M. Recent Progress in the Development of New Antimalarial Drugs with Novel Targets. Drug Des. Devel. Ther. 2020, 14, 3875–3889. [Google Scholar] [CrossRef]
- Ibraheem, Z.O.; Abd Majid, R.; Noor, S.M.; Sedik, H.M.; Basir, R. Role of Different Pfcrt and Pfmdr-1 Mutations in Conferring Resistance to Antimalaria Drugs in Plasmodium falciparum. Malar. Res. Treat. 2014, 2014, 950424. [Google Scholar] [CrossRef]
- Habtamu, K.; Petros, B.; Yan, G. Plasmodium vivax: The potential obstacles it presents to malaria elimination and eradication. Trop. Dis. Travel Med. Vaccines 2022, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Nureye, D.; Salahaddin, M.; Zewudie, A. Current Medicines for Malaria Including Resistance Issues. J. Pharmacol. Pharmacother. 2020, 11, 90–99. [Google Scholar]
- Rumaseb, A.; Moraes Barros, R.R.; Sá, J.M.; Juliano, J.J.; William, T.; Braima, K.A.; Barber, B.E.; Anstey, N.M.; Price, R.N.; Grigg, M.J.; et al. No Association between the Plasmodium vivax crt-o MS334 or In9pvcrt Polymorphisms and Chloroquine Failure in a Pre-Elimination Clinical Cohort from Malaysia with a Large Clonal Expansion. Antimicrob. Agents Chemother. 2023, 67, e01610-22. [Google Scholar] [CrossRef]
- Orjuela-Sánchez, P.; de Santana Filho, F.S.; Machado-Lima, A.; Chehuan, Y.F.; Costa, M.R.F.; Alecrim, M.d.G.C.; del Portillo, H.A. Analysis of Single-Nucleotide Polymorphisms in the crt-o and mdr1 Genes of Plasmodium vivax among Chloroquine-Resistant Isolates from the Brazilian Amazon Region. Antimicrob. Agents Chemother. 2009, 53, 3561–3564. [Google Scholar] [CrossRef] [PubMed]
- Mayxay, M.; Barends, M.; Brockman, A.; Jaidee, A.; Nair, S.; Sudimack, D.; Pongvongsa, T.; Phompida, S.; Phetsouvanh, R.; Anderson, T.; et al. In Vitro Antimalarial Drug Susceptibility and Pfcrt Mutation Among Fresh Plasmodium falciparum Isolates from the Lao PDR (Laos). Am. J. Trop. Med. Hyg. 2007, 76, 245–250. [Google Scholar] [CrossRef]
- Legrand, E.; Volney, B.; Meynard, J.-B.; Mercereau-Puijalon, O.; Esterre, P. In vitro monitoring of Plasmodium falciparum drug resistance in French Guiana: A synopsis of continuous assessment from 1994 to 2005. Antimicrob. Agents Chemother. 2008, 52, 288–298. [Google Scholar] [CrossRef]
- Ogetii, G.N.; Akech, S.; Jemutai, J.; Boga, M.; Kivaya, E.; Fegan, G.; Maitland, K. Hypoglycaemia in severe malaria, clinical associations and relationship to quinine dosage. BMC Infect. Dis. 2010, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, H.A.; Kovacs, R.J.; Kingery, J.R.; Overholser, B.R.; Tisdale, J.E. High Risk of QT Interval Prolongation and Torsades de Pointes Associated with Intravenous Quinidine Used for Treatment of Resistant Malaria or Babesiosis. Antimicrob. Agents Chemother. 2012, 56, 4495–4499. [Google Scholar] [CrossRef]
- Henry, M.; Briolant, S.; Fontaine, A.; Mosnier, J.; Baret, E.; Amalvict, R.; Fusaï, T.; Fraisse, L.; Rogier, C.; Pradines, B. In vitro activity of ferroquine is independent of polymorphisms in transport protein genes implicated in quinoline resistance in Plasmodium falciparum. Antimicrob. Agents Chemother. 2008, 52, 2755–2759. [Google Scholar] [CrossRef]
- White, N.; Looareesuwan, S.; Edwards, G.; Phillips, R.; Karbwang, J.; Nicholl, D.; Bunch, C.; Warrell, D. Pharmacokinetics of intravenous amodiaquine. Br. J. Clin. Pharmacol. 1987, 23, 127–135. [Google Scholar] [CrossRef]
- Nasveld, P.E.; Edstein, M.D.; Reid, M.; Brennan, L.; Harris, I.E.; Kitchener, S.J.; Leggat, P.A.; Pickford, P.; Kerr, C.; Ohrt, C.; et al. Randomized, Double-Blind Study of the Safety, Tolerability, and Efficacy of Tafenoquine versus Mefloquine for Malaria Prophylaxis in Nonimmune Subjects. Antimicrob. Agents Chemother. 2010, 54, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Price, R.N.; Simpson, J.A.; McCarthy, J.S. Halofantrine. In Kucers’ the Use of Antibiotics, 7th ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Wattanakul, T.; Ogutu, B.; Kabanywanyi, A.M.; Asante, K.-P.; Oduro, A.; Adjei, A.; Sie, A.; Sevene, E.; Macete, E.; Compaore, G.; et al. Pooled Multicenter Analysis of Cardiovascular Safety and Population Pharmacokinetic Properties of Piperaquine in African Patients with Uncomplicated Falciparum Malaria. Antimicrob. Agents Chemother. 2020, 64, e01848-19. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, S.K.; Redhi, D.; Combrinck, J.M.; Yeo, T.; Okombo, J.; Henrich, P.P.; Cowell, A.N.; Gupta, P.; Stegman, M.L.; Hoke, J.M.; et al. A Variant PfCRT Isoform Can Contribute to Plasmodium falciparum Resistance to the First-Line Partner Drug Piperaquine. mBio 2017, 8, e00303-17. [Google Scholar] [CrossRef]
- White, N.J.; van Vugt, M.; Ezzet, F.D. Clinical Pharmacokinetics and Pharmacodynamics of Artemether-Lumefantrine. Clin. Pharmacokinet. 1999, 37, 105–125. [Google Scholar] [CrossRef]
- Stover, K.R.; King, S.T.; Robinson, J. Artemether-Lumefantrine: An Option for Malaria. Ann. Pharmacother. 2012, 46, 567–577. [Google Scholar] [CrossRef]
- Ashley, E.A.; Recht, J.; White, N.J. Primaquine: The risks and the benefits. Malar. J. 2014, 13, 418. [Google Scholar] [CrossRef]
- Ganesan, S.; Chaurasiya, N.D.; Sahu, R.; Walker, L.A.; Tekwani, B.L. Understanding the mechanisms for metabolism-linked hemolytic toxicity of primaquine against glucose 6-phosphate dehydrogenase deficient human erythrocytes: Evaluation of eryptotic pathway. Toxicology 2012, 294, 54–60. [Google Scholar] [CrossRef]
- Frampton, J.E. Tafenoquine: First Global Approval. Drugs 2018, 78, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- de Kock, M.; Tarning, J.; Workman, L.; Allen, E.N.; Tekete, M.M.; Djimde, A.A.; Bell, D.J.; Ward, S.A.; Barnes, K.I.; Denti, P. Population Pharmacokinetic Properties of Sulfadoxine and Pyrimethamine: A Pooled Analysis To Inform Optimal Dosing in African Children with Uncomplicated Malaria. Antimicrob. Agents Chemother. 2018, 62, e01370-17. [Google Scholar] [CrossRef]
- Helsby, N.; Edwards, G.; Breckenridge, A.; Ward, S. The multiple dose pharmacokinetics of proguanil. Br. J. Clin. Pharmacol. 1993, 35, 653–656. [Google Scholar] [CrossRef]
- Veenendaal, R.; Edstein, M.D.; Rieckmann, K.H. Pharmacokinetics of Chlorproguanil in Man after a Single Oral Dose of Lapudrine®. Chemotherapy 2009, 34, 277–283. [Google Scholar] [CrossRef]
- Ashton, M.; Gordi, T.; Hai, T.N.; Van Huong, N.; Sy, N.D.; Nieu, N.T.; Huong, D.X.; Johansson, M.; Công, L.D. Artemisinin pharmacokinetics in healthy adults after 250, 500 and 1000 mg single oral doses. Biopharm. Drug Dispos. 1998, 19, 245–250. [Google Scholar] [CrossRef]
- Gobbi, F.; Buonfrate, D.; Menegon, M.; Lunardi, G.; Angheben, A.; Severini, C.; Gori, S.; Bisoffi, Z. Failure of dihydroartemisinin-piperaquine treatment of uncomplicated Plasmodium falciparum malaria in a traveller coming from Ethiopia. Malar. J. 2016, 15, 525. [Google Scholar] [CrossRef]
- Naing, C.; Racloz, V.; Whittaker, M.A.; Aung, K.; Reid, S.A.; Mak, J.W.; Tanner, M. Efficacy and Safety of Dihydroartemisinin-Piperaquine for Treatment of Plasmodium vivax Malaria in Endemic Countries: Meta-Analysis of Randomized Controlled Studies. PLoS ONE 2013, 8, e78819. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, J.; Li, Q.; Hu, Y.; Ruan, Y.; Tao, Z.; Xia, H.; Qiao, J.; Meng, L.; Zeng, W.; et al. Ex vivo susceptibilities of Plasmodium vivax isolates from the China-Myanmar border to antimalarial drugs and association with polymorphisms in Pvmdr1 and Pvcrt-o genes. PLoS Negl. Trop. Dis. 2020, 14, e0008255. [Google Scholar] [CrossRef]
- Sridapan, T.; Rattanakoch, P.; Kijprasong, K.; Srisutham, S. Drug resistance markers in Plasmodium vivax isolates from a Kanchanaburi province, Thailand between January to May 2023. PLoS ONE 2024, 19, e0304337. [Google Scholar] [CrossRef] [PubMed]
- Shekalaghe, S.A.; ter Braak, R.; Daou, M.; Kavishe, R.; van den Bijllaardt, W.; van den Bosch, S.; Koenderink, J.B.; Luty, A.J.F.; Whitty, C.J.M.; Drakeley, C.; et al. In Tanzania, Hemolysis after a Single Dose of Primaquine Coadministered with an Artemisinin Is Not Restricted to Glucose-6-Phosphate Dehydrogenase-Deficient (G6PD A−) Individuals. Antimicrob. Agents Chemother. 2010, 54, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Plowe, C.V.; Kublin, J.G.; Doumbo, O.K. P. falciparum dihydrofolate reductase and dihydropteroate synthase mutations: Epidemiology and role in clinical resistance to antifolates. Drug Resist. Updat. 1998, 1, 389–396. [Google Scholar] [CrossRef]
- Nair, S.; Miller, B.; Barends, M.; Jaidee, A.; Patel, J.; Mayxay, M.; Newton, P.; Nosten, F.; Ferdig, M.T.; Anderson, T.J.C. Adaptive Copy Number Evolution in Malaria Parasites. PLOS Genet. 2008, 4, e1000243. [Google Scholar] [CrossRef]
- Kidgell, C.; Volkman, S.K.; Daily, J.; Borevitz, J.O.; Plouffe, D.; Zhou, Y.; Johnson, J.R.; Roch, K.G.L.; Sarr, O.; Ndir, O.; et al. A Systematic Map of Genetic Variation in Plasmodium falciparum. PLoS Pathog. 2006, 2, e57. [Google Scholar] [CrossRef]
- Cowell, A.N.; Winzeler, E.A. The genomic architecture of antimalarial drug resistance. Brief. Funct. Genom. 2019, 18, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A.; Dhorda, M.; Fairhurst, R.M.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Anderson, J.M.; Mao, S.; Sam, B.; et al. Spread of Artemisinin Resistance in Plasmodium falciparum Malaria. N. Engl. J. Med. 2014, 371, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, M.R.; Ng, C.L. Plasmodium falciparum Artemisinin Resistance: The Effect of Heme, Protein Damage, and Parasite Cell Stress Response. ACS Infect. Dis. 2020, 6, 1599–1614. [Google Scholar] [CrossRef]
- Rahmasari, F.V.; Asih, P.B.S.; Dewayanti, F.K.; Rotejanaprasert, C.; Charunwatthana, P.; Imwong, M.; Syafruddin, D. Drug resistance of Plasmodium falciparum and Plasmodium vivax isolates in Indonesia. Malar. J. 2022, 21, 354. [Google Scholar] [CrossRef]
- Djimde, A.A.; Makanga, M.; Kuhen, K.; Hamed, K. The emerging threat of artemisinin resistance in malaria: Focus on artemether-lumefantrine. Expert Rev. Anti-Infect. Ther. 2015, 13, 1031–1045. [Google Scholar] [CrossRef]
- Windle, S.T.; Lane, K.D.; Gadalla, N.B.; Liu, A.; Mu, J.; Caleon, R.L.; Rahman, R.S.; Sá, J.M.; Wellems, T.E. Evidence for linkage of pfmdr1, pfcrt, and pfk13 polymorphisms to lumefantrine and mefloquine susceptibilities in a Plasmodium falciparum cross. Int. J. Parasitol. Drugs Drug Resist. 2020, 14, 208–217. [Google Scholar] [CrossRef]
- Deng, C.; Huang, B.; Wang, Q.; Wu, W.; Zheng, S.; Zhang, H.; Li, D.; Feng, D.; Li, G.; Xue, L.; et al. Large-scale Artemisinin–Piperaquine Mass Drug Administration With or Without Primaquine Dramatically Reduces Malaria in a Highly Endemic Region of Africa. Clin. Infect. Dis. 2018, 67, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- van der Pluijm, R.W.; Tripura, R.; Hoglund, R.M.; Phyo, A.P.; Lek, D.; ul Islam, A.; Anvikar, A.R.; Satpathi, P.; Satpathi, S.; Behera, P.K.; et al. Triple artemisinin-based combination therapies versus artemisinin-based combination therapies for uncomplicated Plasmodium falciparum malaria: A multicentre, open-label, randomised clinical trial. Lancet 2020, 395, 1345–1360. [Google Scholar] [CrossRef]
- Ross, L.S.; Dhingra, S.K.; Mok, S.; Yeo, T.; Wicht, K.J.; Kümpornsin, K.; Takala-Harrison, S.; Witkowski, B.; Fairhurst, R.M.; Ariey, F.; et al. Emerging Southeast Asian PfCRT mutations confer Plasmodium falciparum resistance to the first-line antimalarial piperaquine. Nat. Commun. 2018, 9, 3314. [Google Scholar] [CrossRef]
- Duru, V.; Witkowski, B.; Ménard, D. Plasmodium falciparum Resistance to Artemisinin Derivatives and Piperaquine: A Major Challenge for Malaria Elimination in Cambodia. Am. J. Trop. Med. Hyg. 2016, 95, 1228–1238. [Google Scholar] [CrossRef]
- Rasmussen, C.; Alonso, P.; Ringwald, P. Current and emerging strategies to combat antimalarial resistance. Expert Rev. Anti-Infect. Ther. 2022, 20, 353–372. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, H.J.; Tajudeen, Y.A.; Oladunjoye, I.O.; Yusuff, S.I.; Yusuf, R.O.; Oluwaseyi, E.M.; AbdulBasit, M.O.; Adebisi, Y.A.; El-Sherbini, M.S. Increasing challenges of malaria control in sub-Saharan Africa: Priorities for public health research and policymakers. Ann. Med. Surg. 2022, 81, 104366. [Google Scholar] [CrossRef] [PubMed]
- Rajneesh; Tiwari, R.; Singh, V.K.; Kumar, A.; Gupta, R.P.; Singh, A.K.; Gautam, V.; Kumar, R. Advancements and Challenges in Developing Malaria Vaccines: Targeting Multiple Stages of the Parasite Life Cycle. ACS Infect. Dis. 2023, 9, 1795–1814. [Google Scholar] [CrossRef]
- Halbroth, B.R.; Draper, S.J. Chapter One—Recent Developments in Malaria Vaccinology. In Advances in Parasitology; Rollinson, D., Stothard, J.R., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 88, pp. 1–49. [Google Scholar] [CrossRef]
- Aly, A.S.I.; Vaughan, A.M.; Kappe, S.H.I. Malaria Parasite Development in the Mosquito and Infection of the Mammalian Host. Annu. Rev. Microbiol. 2009, 63, 195–221. [Google Scholar] [CrossRef]
- Nunes-Cabaço, H.; Moita, D.; Prudêncio, M. Five decades of clinical assessment of whole-sporozoite malaria vaccines. Front. Immunol. 2022, 13, 977472. [Google Scholar] [CrossRef] [PubMed]
- Seder, R.A.; Chang, L.-J.; Enama, M.E.; Zephir, K.L.; Sarwar, U.N.; Gordon, I.J.; Holman, L.A.; James, E.R.; Billingsley, P.F.; Gunasekera, A.; et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013, 341, 1359–1365. [Google Scholar] [CrossRef]
- Birkett, A.J.; Moorthy, V.S.; Loucq, C.; Chitnis, C.E.; Kaslow, D.C. Malaria vaccine R&D in the Decade of Vaccines: Breakthroughs, challenges and opportunities. Vaccine 2013, 31 (Suppl. S2), B233–B243. [Google Scholar] [CrossRef]
- Epstein, J.E.; Tewari, K.; Lyke, K.E.; Sim, B.K.L.; Billingsley, P.F.; Laurens, M.B.; Gunasekera, A.; Chakravarty, S.; James, E.R.; Sedegah, M.; et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science 2011, 334, 475–480. [Google Scholar] [CrossRef]
- Hoffman, S.L.; Goh, L.M.L.; Luke, T.C.; Schneider, I.; Le, T.P.; Doolan, D.L.; Sacci, J.; de la Vega, P.; Dowler, M.; Paul, C.; et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 2002, 185, 1155–1164. [Google Scholar] [CrossRef]
- Roestenberg, M.; McCall, M.; Hopman, J.; Wiersma, J.; Luty, A.J.F.; van Gemert, G.J.; van de Vegte-Bolmer, M.; van Schaijk, B.; Teelen, K.; Arens, T.; et al. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 2009, 361, 468–477. [Google Scholar] [CrossRef]
- Kublin, J.G.; Mikolajczak, S.A.; Sack, B.K.; Fishbaugher, M.E.; Seilie, A.; Shelton, L.; VonGoedert, T.; Firat, M.; Magee, S.; Fritzen, E.; et al. Complete attenuation of genetically engineered Plasmodium falciparum sporozoites in human subjects. Sci. Transl. Med. 2017, 9, eaad9099. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.M.; Wang, R.; Kappe, S.H.I. Genetically engineered, attenuated whole-cell vaccine approaches for malaria. Hum. Vaccin. 2010, 6, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Butler, N.S.; Vaughan, A.M.; Harty, J.T.; Kappe, S.H.I. Whole parasite vaccination approaches for prevention of malaria infection. Trends Immunol. 2012, 33, 247–254. [Google Scholar] [CrossRef]
- Stanisic, D.I.; Good, M.F. Malaria Vaccines: Progress to Date. Biodrugs 2023, 37, 737–756. [Google Scholar] [CrossRef]
- Syed, Y.Y. RTS,S/AS01 malaria vaccine (Mosquirix®): A profile of its use. Drugs Ther. Perspect. 2022, 38, 373–381. [Google Scholar] [CrossRef]
- Laurens, M.B. RTS,S/AS01 vaccine (Mosquirix™): An overview. Hum. Vaccines Immunother. 2020, 16, 480–489. [Google Scholar] [CrossRef]
- de Almeida, M.E.M.; de Vasconcelos, M.G.S.; Tarragô, A.M.; Mariúba, L.A.M. Circumsporozoite Surface Protein-based malaria vaccines: A review. Rev. Inst. Med. Trop. São Paulo 2021, 63, e11. [Google Scholar] [CrossRef] [PubMed]
- Stertman, L.; Palm, A.-K.E.; Zarnegar, B.; Carow, B.; Lunderius Andersson, C.; Magnusson, S.E.; Carnrot, C.; Shinde, V.; Smith, G.; Glenn, G.; et al. The Matrix-M™ adjuvant: A critical component of vaccines for the 21st century. Hum. Vaccines Immunother. 2023, 19, 2189885. [Google Scholar] [CrossRef]
- Datoo, M.S.; Dicko, A.; Tinto, H.; Ouédraogo, J.-B.; Hamaluba, M.; Olotu, A.; Beaumont, E.; Ramos Lopez, F.; Natama, H.M.; Weston, S.; et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: A multicentre, double-blind, randomised, phase 3 trial. Lancet Lond. Engl. 2024, 403, 533–544. [Google Scholar] [CrossRef]
- Sang, S.; Datoo, M.S.; Otieno, E.; Muiruri, C.; Bellamy, D.; Gathuri, E.; Ngoto, O.; Musembi, J.; Provstgaard-Morys, S.; Stockdale, L.; et al. Safety and immunogenicity of varied doses of R21/Matrix-M™ vaccine at three years follow-up: A phase 1b age de-escalation, dose-escalation trial in adults, children, and infants in Kilifi-Kenya. Wellcome Open Res. 2023, 8, 450. [Google Scholar] [CrossRef]
- Neafsey, D.E.; Juraska, M.; Bedford, T.; Benkeser, D.; Valim, C.; Griggs, A.; Lievens, M.; Abdulla, S.; Adjei, S.; Agbenyega, T.; et al. Genetic Diversity and Protective Efficacy of the RTS,S/AS01 Malaria Vaccine. N. Engl. J. Med. 2015, 373, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhang, C.; Joy, D.A. Host-Malaria Parasite Interactions and Impacts on Mutual Evolution. Front. Cell. Infect. Microbiol. 2020, 10, 587933. [Google Scholar] [CrossRef]
- Tajudeen, Y.A.; Oladipo, H.J.; Yusuff, S.I.; Abimbola, S.O.; Abdulkadir, M.; Oladunjoye, I.O.; Omotosho, A.O.; Egbewande, O.M.; Shittu, H.D.; Yusuf, R.O.; et al. A landscape review of malaria vaccine candidates in the pipeline. Trop. Dis. Travel Med. Vaccines 2024, 10, 19. [Google Scholar] [CrossRef]
- Duffy, P.E. Current approaches to malaria vaccines. Curr. Opin. Microbiol. 2022, 70, 102227. [Google Scholar] [CrossRef]
- Goodier, M.R.; Wolf, A.-S.; Riley, E.M. Differentiation and adaptation of natural killer cells for anti-malarial immunity. Immunol. Rev. 2020, 293, 25–37. [Google Scholar] [CrossRef]
- Patarroyo, M.A.; Molina-Franky, J.; Gómez, M.; Arévalo-Pinzón, G.; Patarroyo, M.E. Hotspots in Plasmodium and RBC Receptor-Ligand Interactions: Key Pieces for Inhibiting Malarial Parasite Invasion. Int. J. Mol. Sci. 2020, 21, 4729. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, C.E.; Sharma, A. Targeting the Plasmodium vivax Duffy-binding protein. Trends Parasitol. 2008, 24, 29–34. [Google Scholar] [CrossRef]
- Weiss, G.E.; Traore, B.; Kayentao, K.; Ongoiba, A.; Doumbo, S.; Doumtabe, D.; Kone, Y.; Dia, S.; Guindo, A.; Traore, A.; et al. The Plasmodium falciparum-Specific Human Memory B Cell Compartment Expands Gradually with Repeated Malaria Infections. PLOS Pathog. 2010, 6, e1000912. [Google Scholar] [CrossRef] [PubMed]
- White, N.J.; Pukrittayakamee, S.; Hien, T.T.; Faiz, M.A.; Mokuolu, O.A.; Dondorp, A.M. Malaria. Lancet Lond. Engl. 2014, 383, 723–735. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Rosenfeld, L.C.; Lim, S.S.; Andrews, K.G.; Foreman, K.J.; Haring, D.; Fullman, N.; Naghavi, M.; Lozano, R.; Lopez, A.D. Global malaria mortality between 1980 and 2010: A systematic analysis. Lancet Lond. Engl. 2012, 379, 413–431. [Google Scholar] [CrossRef]
- Acharya, P.; Garg, M.; Kumar, P.; Munjal, A.; Raja, K.D. Host–Parasite Interactions in Human Malaria: Clinical Implications of Basic Research. Front. Microbiol. 2017, 8, 889. [Google Scholar] [CrossRef] [PubMed]
- Frimpong, A.; Kusi, K.A.; Ofori, M.F.; Ndifon, W. Novel Strategies for Malaria Vaccine Design. Front. Immunol. 2018, 9, 2769. [Google Scholar] [CrossRef] [PubMed]
- Kogan, F. Malaria Burden. In Remote Sensing for Malaria: Monitoring and Predicting Malaria from Operational Satellites; Kogan, F., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 15–41. [Google Scholar] [CrossRef]
- Roberts, T.; Cohn, J.; Bonner, K.; Hargreaves, S. Scale-up of Routine Viral Load Testing in Resource-Poor Settings: Current and Future Implementation Challenges. Clin. Infect. Dis. 2016, 62, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Ruckstuhl, L.; Lengeler, C.; Moyen, J.M.; Garro, H.; Allan, R. Malaria case management by community health workers in the Central African Republic from 2009–2014: Overcoming challenges of access and instability due to conflict. Malar. J. 2017, 16, 388. [Google Scholar] [CrossRef]
- Ishtiaq, F. Ecology and Evolution of Avian Malaria: Implications of Land Use Changes and Climate Change on Disease Dynamics. J. Indian Inst. Sci. 2021, 101, 213–225. [Google Scholar] [CrossRef]
- Cowell, A.N.; Winzeler, E.A. Advances in omics-based methods to identify novel targets for malaria and other parasitic protozoan infections. Genome Med. 2019, 11, 63. [Google Scholar] [CrossRef]
- Carolino, K.; Winzeler, E.A. The antimalarial resistome—Finding new drug targets and their modes of action. Curr. Opin. Microbiol. 2020, 57, 49–55. [Google Scholar] [CrossRef]
- Abuga, K.M.; Jones-Warner, W.; Hafalla, J.C.R. Immune responses to malaria pre-erythrocytic stages: Implications for vaccine development. Parasite Immunol. 2021, 43, e12795. [Google Scholar] [CrossRef]
- Stutzer, C.; Richards, S.A.; Ferreira, M.; Baron, S.; Maritz-Olivier, C. Metazoan Parasite Vaccines: Present Status and Future Prospects. Front. Cell. Infect. Microbiol. 2018, 8, 67. [Google Scholar] [CrossRef]
- Franke-Fayard, B.; Marin-Mogollon, C.; Geurten, F.J.A.; Chevalley-Maurel, S.; Ramesar, J.; Kroeze, H.; Baalbergen, E.; Wessels, E.; Baron, L.; Soulard, V.; et al. Creation and preclinical evaluation of genetically attenuated malaria parasites arresting growth late in the liver. Npj Vaccines 2022, 7, 139. [Google Scholar] [CrossRef]
- Burt, A.; Coulibaly, M.; Crisanti, A.; Diabate, A.; Kayondo, J.K. Gene drive to reduce malaria transmission in sub-Saharan Africa. J. Responsible Innov. 2018, 5 (Suppl. S1), S66–S80. [Google Scholar] [CrossRef]
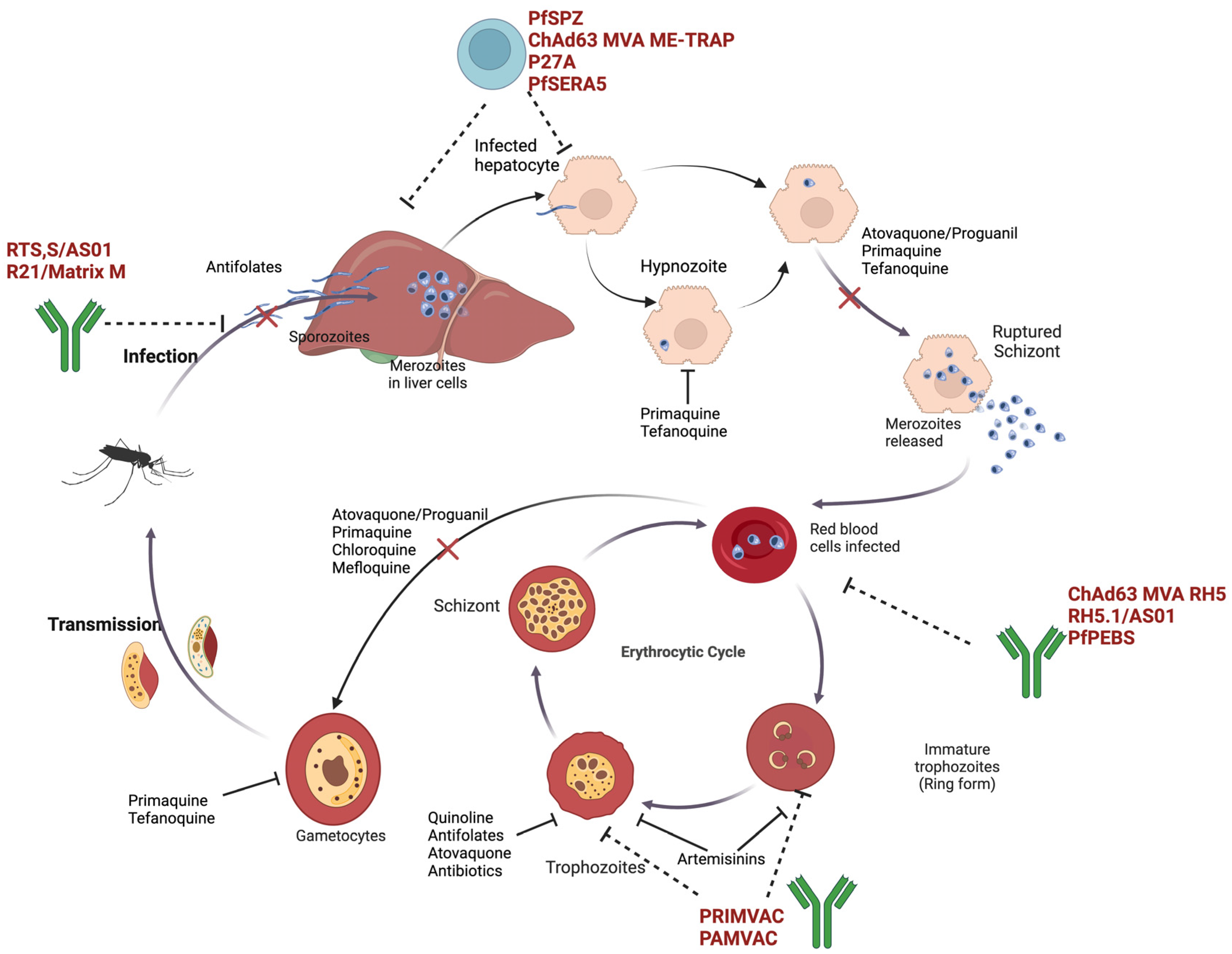
| Name | Parasite Target | Mechanism of Action | Dosage | Side Effects | Resistance | Genetics of Resistance | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Quinine derivatives | ||||||||
| Quinine | 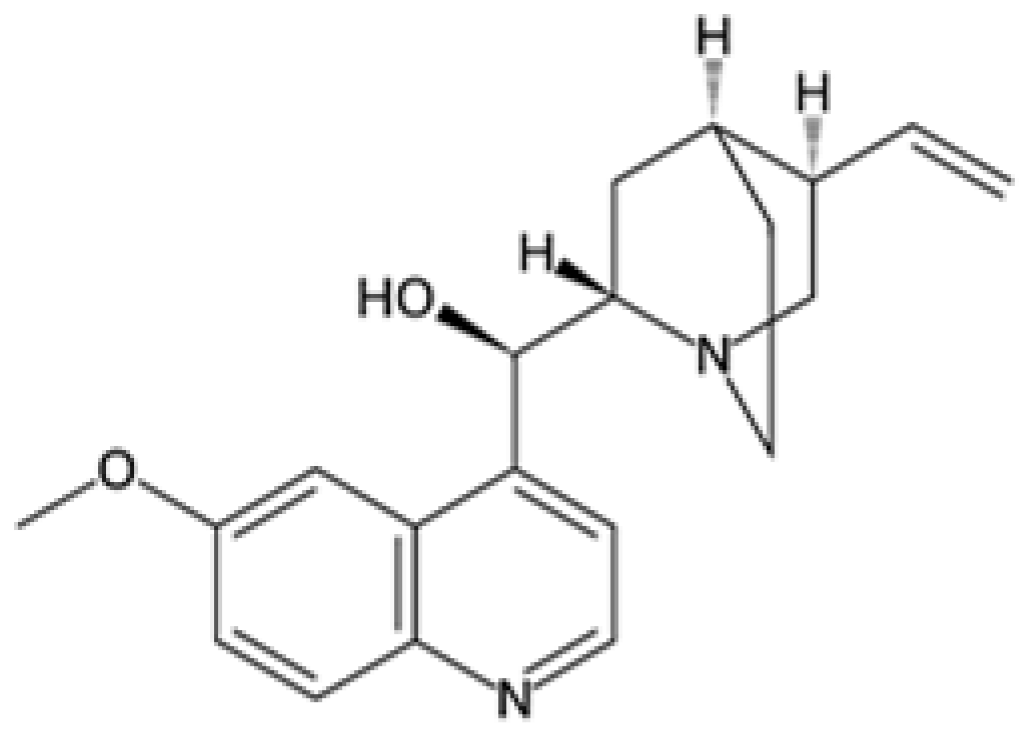 | P. falciparum, P. vivax | Interferes with parasite’s ability to digest hemoglobin | 600 mg 3 times daily for 7 days | Tinnitus, nausea, headache, and blurred vision | Resistance present in some regions | Mutations in Pfcrt (P. falciparum chloroquine resistance transporter) and Pfmdr1 (multidrug resistance gene 1) | [59,60,61] |
| Quinidine | 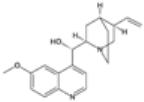 | P. falciparum | Similar to quinine, blocks DNA replication | 10 mg/kg loading dose, then 0.02 mg/kg/min | Arrhythmia, hypotension, and dizziness | Resistance reported | Similar resistance mechanisms as quinine, Pfcrt and Pfmdr1 mutations | [59,60,62] |
| Chloroquine |  | P. falciparum, P. vivax | Inhibits heme polymerase activity | 25 mg/kg over 3 days | Itching, gastrointestinal upset, and retinopathy | Widespread resistance, especially in P. falciparum | Pfcrt mutations (particularly K76T) and Pfmdr1 mutations | [57] |
| Amodiaquine | 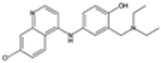 | P. falciparum | Similar to chloroquine, disrupts heme digestion | 10 mg/kg for 3 days | Agranulocytosis and hepatotoxicity | Resistance reported | Pfcrt and Pfmdr1 mutations | [63,64] |
| Mefloquine | 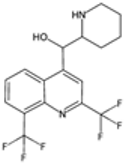 | P. falciparum | Inhibits heme polymerization | 250 mg weekly for prophylaxis | Neuropsychiatric effects and gastrointestinal upset | Resistance in Southeast Asia | Amplification and mutations in Pfmdr1 | [56,65] |
| Halofantrine |  | P. falciparum | Interferes with heme metabolism | 8 mg/kg body weight, then repeat in 6 h | Cardiotoxicity and gastrointestinal upset | Limited use due to resistance | Pfmdr1 mutations | [13,66] |
| Piperaquine |  | P. falciparum | Similar to chloroquine, disrupts heme digestion | 160–1600 mg daily for 3 days | QT prolongation and gastrointestinal upset | Emerging resistance | Amplification and mutations in Pfmdr1 and Pfpm2 (plasmepsin 2) | [67,68] |
| Lumefantrine | 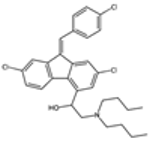 | P. falciparum | Interferes with heme metabolism | 480 mg twice daily for 3 days | Headache, dizziness, and gastrointestinal upset | Resistance emerging | Pfmdr1 mutations | [69,70] |
| 8-Aminoquinoline | ||||||||
| Primaquine | 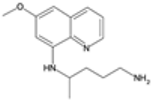 | P. vivax, P. ovale | Generates reactive oxygen species and disrupts mitochondria | 15 mg daily for 14 days | Hemolysis in G6PD-deficient patients and nausea | Some evidence of reduced efficacy | G6PD (glucose-6-phosphate dehydrogenase) deficiency affects drug efficacy and safety, no specific parasite gene mutations | [71,72] |
| Tafenoquine | 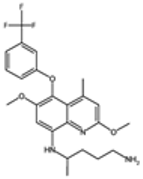 | P. vivax | Similar to primaquine, disrupts mitochondria | 100–300 mg | Hemolysis in G6PD-deficient patients and dizziness | Limited reports of resistance | G6PD deficiency impacts drug safety and efficacy, no known specific parasite resistance | [22,24,73] |
| Antifolate compounds | ||||||||
| Sulfadoxine | 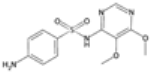 | P. falciparum | Inhibits dihydropteroate synthase | 500 mg as single dose | Rash, gastrointestinal upset, and Stevens–Johnson syndrome | Resistance common in many regions | Mutations in Pfdhps (P. falciparum dihydropteroate synthase), particularly A437G and K540E | [74] |
| Pyrimethamine |  | P. falciparum | Inhibits dihydrofolate reductase | 25 mg single dose | Anemia, rash, and gastrointestinal upset | Resistance common in many regions | Mutations in Pfdhfr (P. falciparum dihydrofolate reductase), particularly N51I, C59R, S108N, and I164L | [28,74] |
| Proguanil | 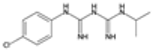 | P. falciparum | Prodrug, inhibits dihydrofolate reductase | 200 mg daily | Mouth ulcers and gastrointestinal upset | Some resistance reported | Pfdhfr mutations (similar to pyrimethamine) | [28,75] |
| Chlorproguanil | 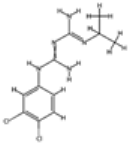 | P. falciparum | Inhibits dihydrofolate reductase | 20 mg daily | Hemolysis in G6PD-deficient patients and nausea | Withdrawn due to safety concerns | Pfdhfr mutations (similar to pyrimethamine and proguanil) | [76] |
| Artemisinin compounds | ||||||||
| Artemisinin | 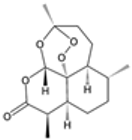 | P. falciparum | Generates reactive oxygen species and damages proteins and membranes | 250–1000 mg daily for 7 days | Nausea, dizziness, and neutropenia | Resistance emerging in Southeast Asia | Mutations in Pfk13 (Kelch 13 gene), particularly C580Y, R539T, and Y493H | [10,77] |
| Artesunate |  | P. falciparum | Similar to artemisinin, more water soluble | 200 mg daily for 7 days | Hemolysis and gastrointestinal upset | Resistance emerging in Southeast Asia | Pfk13 mutations (similar to artemisinin) | [43,44,51] |
| Artemether |  | P. falciparum | Similar to artemisinin, lipid-soluble | 160 mg daily for 3 days | Fever, nausea, and headache | Resistance emerging in Southeast Asia | Pfk13 mutations (similar to artemisinin) | [16,30,43] |
| Dihydroartemisinin | 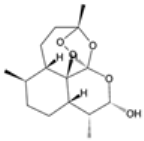 | P. falciparum | Active metabolite of artemisinin, generates reactive oxygen species | 200 mg daily for 7 days | Nausea, dizziness, and anemia | Resistance emerging in Southeast Asia | Pfk13 mutations (similar to artemisinin) | [28,45,46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, J.M.; Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Recent Advances in the Treatment of Malaria. Pharmaceutics 2024, 16, 1416. https://doi.org/10.3390/pharmaceutics16111416
Alghamdi JM, Al-Qahtani AA, Alhamlan FS, Al-Qahtani AA. Recent Advances in the Treatment of Malaria. Pharmaceutics. 2024; 16(11):1416. https://doi.org/10.3390/pharmaceutics16111416
Chicago/Turabian StyleAlghamdi, Jawaher M., Arwa A. Al-Qahtani, Fatimah S. Alhamlan, and Ahmed A. Al-Qahtani. 2024. "Recent Advances in the Treatment of Malaria" Pharmaceutics 16, no. 11: 1416. https://doi.org/10.3390/pharmaceutics16111416
APA StyleAlghamdi, J. M., Al-Qahtani, A. A., Alhamlan, F. S., & Al-Qahtani, A. A. (2024). Recent Advances in the Treatment of Malaria. Pharmaceutics, 16(11), 1416. https://doi.org/10.3390/pharmaceutics16111416






