Emerging Treatments and New Vehicle Formulations for Atopic Dermatitis
Abstract
:1. Introduction
2. Treatment of AD
2.1. Topical Treatments
2.2. Phototherapy
2.3. Biologic Drugs
2.4. JAK-Inhibitors
2.5. Antimetabolites and Immunosuppressants
3. Novel Vehicles and Formulations
3.1. Topical JAK Inhibitors
3.2. Recent Nanotechnologies
3.3. Natural Biopolymers
4. Emerging Advanced Technologies and Molecules
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Laughter, M.; Maymone, M.; Mashayekhi, S.; Arents, B.; Karimkhani, C.; Langan, S.; Dellavalle, R.; Flohr, C. The global burden of atopic dermatitis: Lessons from the Global Burden of Disease Study 1990–2017. Br. J. Dermatol. 2021, 184, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, M.; Iancu, G.M.; Matran, I.M. Importance of food in the control of inflammation in atopic dermatitis. Exp. Ther. Med. 2020, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Schuler, C.F., 4th; Billi, A.C.; Maverakis, E.; Tsoi, L.C.; Gudjonsson, J.E. Novel insights into atopic dermatitis. J. Allergy Clin Immunol. 2023, 151, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Torres, T.; Ferreira, E.O.; Gonçalo, M.; Mendes-Bastos, P.; Selores, M.; Filipe, P. Update on Atopic Dermatitis. Acta Med. Port. 2019, 32, 606–613. [Google Scholar] [CrossRef] [PubMed]
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 2017, 1027, 21–37. [Google Scholar] [CrossRef]
- Borowczyk, J.; Shutova, M.; Brembilla, N.C.; Boehncke, W.H. IL-25 (IL-17E) in epithelial immunology and pathophysiology. J. Allergy Clin. Immunol. 2021, 148, 40–52. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Waldman, A.; Ahluwalia, J.; Ong, P.Y.; Eichenfield, L.F. Atopic dermatitis: Pathogenesis. Semin. Cutan. Med. Surg. 2017, 36, 100–103. [Google Scholar] [CrossRef]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef]
- Larkin, C.; Chen, W.; Szabó, I.L.; Shan, C.; Dajnoki, Z.; Szegedi, A.; Buhl, T.; Fan, Y.; O’Neill, S.; Walls, D.; et al. Novel insights into the TRPV3-mediated itch in atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 1110–1114.e5. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, J.S.; Seo, H.M. Exposure to air pollution and incidence of atopic dermatitis in the general population: A national population-based retrospective cohort study. J. Am. Acad. Dermatol. 2022, 87, 1321–1327. [Google Scholar] [CrossRef]
- Fadadu, R.P.; Chee, E.; Jung, A.; Chen, J.Y.; Abuabara, K.; Wei, M.L. Air pollution and global healthcare use for atopic dermatitis: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1958–1970. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Kinberger, M.; Arents, B.; Aszodi, N.; Valle, G.A.; Barbarot, S.; Bieber, T.; Brough, H.; Pinton, P.C.; Christen-Zäch, S.; et al. European guideline (EuroGuiDerm) on atopic eczema—Part II: Non-systemic treatments and treatment recommendations for special AE patient populations. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1904–1926. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, A.; Boguniewicz, M.; Ong, P.Y.; Chu, D.K.; Schneider, L.C. Atopic Dermatitis (Eczema) Guidelines 2023: Highlights. J. Allergy Clin. Immunol. Pract. 2024, in press. [CrossRef] [PubMed]
- Xu, A.Z.; Alexander, J.T. Topical Therapies for Atopic Dermatitis. JAMA 2023, 330, 1791–1792. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Parmar, N.V. Topical Side Effects of Topical Corticosteroids. In A Treatise on Topical Corticosteroids in Dermatology; Springer: Singapore, 2017; pp. 251–260. [Google Scholar] [CrossRef]
- Mehta, A.B.; Nadkarni, N.J.; Patil, S.P.; Godse, K.V.; Gautam, M.; Agarwal, S. Topical corticosteroids in dermatology. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Arana, A.; Pottegård, A.; Kuiper, J.G.; Booth, H.; Reutfors, J.; Calingaert, B.; Lund, L.C.; Crellin, E.; Schmitt-Egenolf, M.; A Kaye, J.; et al. Long-Term Risk of Skin Cancer and Lymphoma in Users of Topical Tacrolimus and Pimecrolimus: Final Results from the Extension of the Cohort Study Protopic Joint European Longitudinal Lymphoma and Skin Cancer Evaluation (JOELLE). Clin. Epidemiol. 2021, 13, 1141–1153. [Google Scholar] [CrossRef]
- Darch, K.; Sterne, S.; Spelman, L. P68: JAK Inhibitors in Atopic Dermatitis. A Review of the Emerging Role of Inhibitors of the JAK-STAT Pathway in the Management of Atopic Dermatitis. Intern. Med. J. 2021, 51, 24. [Google Scholar] [CrossRef]
- Chovatiya, R.; Paller, A.S. JAK inhibitors in the treatment of atopic dermatitis. J. Allergy Clin. Immunol. 2021, 148, 927–940. [Google Scholar] [CrossRef]
- Papp, K.; Szepietowski, J.C.; Kircik, L.; Toth, D.; Eichenfield, L.F.; Forman, S.B.; Kuligowski, M.E.; Kallender, H.; Sun, K.; Ren, H.; et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: Results from two phase 3 studies. J. Am. Acad. Dermatol. 2023, 88, 1008–1016. [Google Scholar] [CrossRef]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Murata, R.; Kaino, H.; Nagata, T. Long-term safety and efficacy of delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with atopic dermatitis. J. Dermatol. 2020, 47, 114–120. [Google Scholar] [CrossRef]
- Lax, S.J.; Van Vogt, E.; Candy, B.; Steele, L.; Reynolds, C.; Stuart, B.; Parker, R.; Axon, E.; Roberts, A.; Doyle, M.; et al. Topical anti-inflammatory treatments for eczema: Network meta-analysis. Cochrane Database Syst. Rev. 2024, 8, CD015064. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.M.; Drucker, A.M.; Alikhan, A.; Bercovitch, L.; Cohen, D.E.; Darr, J.M.; Eichenfield, L.F.; Frazer-Green, L.; Paller, A.S.; Schwarzenberger, K.; et al. Guidelines of care for the management of atopic dermatitis in adults with phototherapy and systemic therapies. J. Am. Acad. Dermatol. 2024, 90, e43–e56. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Thaçi, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E.; et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T.; Heratizadeh, A.; Aberer, W.; Augustin, M.; Biedermann, T.; Bauer, A.; Fölster-Holst, R.; Kahle, J.; Kinberger, M.; Nemat, K.; et al. S3 guideline Atopic dermatitis: Part 2—Systemic treatment. J. Dtsch. Dermatol. Ges. 2024, 22, 307–320. [Google Scholar] [CrossRef]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br. J. Dermatol. 2021, 184, 437–449. [Google Scholar] [CrossRef]
- Keam, S.J. Lebrikizumab: First Approval. Drugs 2024, 84, 347–353. [Google Scholar] [CrossRef]
- Blauvelt, A.; Guttman-Yassky, E.; Lynde, C.; Khattri, S.; Schlessinger, J.; Imafuku, S.; Tada, Y.; Morita, A.; Wiseman, M.; Kwiek, B.; et al. Cendakimab in Patients With Moderate to Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2024, 160, 856–864. [Google Scholar] [CrossRef]
- Blauvelt, A.; Thyssen, J.P.; Guttman-Yassky, E.; Bieber, T.; Serra-Baldrich, E.; Simpson, E.; Rosmarin, D.; Elmaraghy, H.; Meskimen, E.; Natalie, C.R.; et al. Efficacy and safety of lebrikizumab in moderate-to-severe atopic dermatitis: 52-week results of two randomized double-blinded placebo-controlled phase III trials. Br. J. Dermatol. 2023, 188, 740–748. [Google Scholar] [CrossRef]
- Rodenbeck, D.L.; Silverberg, J.I.; Silverberg, N.B. Phototherapy for atopic dermatitis. Clin. Dermatol. 2016, 34, 607–613. [Google Scholar] [CrossRef]
- Siedlikowski, S.; Sandhu, V.; Lynde, C. Treatment of Atopic Dermatitis Using JAK Inhibitors: A Systematic Review. EMJ Deramtol. 2019, 7, 89–100. [Google Scholar] [CrossRef]
- Dhar, S.; Datta, S.; De, A. Use of Janus kinase inhibitors in atopic dermatitis—An update. Indian J. Dermatol. Venereol. Leprol. 2023, 90, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Simon, D. Systemic therapy of atopic dermatitis in children and adults. Curr. Probl. Dermatol. 2011, 41, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.; Penedones, A.; Mendes, D.; Batel Marques, F. The safety of systemic Janus kinase inhibitors in atopic dermatitis: A systematic review and network meta-analysis. Eur. J. Clin. Pharmacol. 2022, 78, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Schmitt, N.; Meurer, M. Cyclosporin in the treatment of patients with atopic eczema—A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.M.; Chiricozzi, A.; Calabrese, L.; Mannino, M.; Peris, K. Novel Therapeutic Strategies in the Topical Treatment of Atopic Dermatitis. Pharmaceutics 2022, 14, 2767. [Google Scholar] [CrossRef]
- Landis, M.N.; Arya, M.; Smith, S.; Draelos, Z.; Usdan, L.; Tarabar, S.; Pradhan, V.; Aggarwal, S.; Banfield, C.; Peeva, E.; et al. Efficacy and safety of topical brepocitinib for the treatment of mild-to-moderate atopic dermatitis: A phase IIb, randomized, double-blind, vehicle-controlled, dose-ranging and parallel-group study. Br. J. Dermatol. 2022, 187, 878–887. [Google Scholar] [CrossRef]
- Pires, P.C.; Damiri, F.; Zare, E.N.; Hasan, A.; Neisiany, R.E.; Veiga, F.; Makvandi, P.; Paiva-Santos, A.C. A review on natural biopolymers in external drug delivery systems for wound healing and atopic dermatitis. Int. J. Biol. Macromol. 2024, 263 Pt 1, 130296. [Google Scholar] [CrossRef]
- Sguizzato, M.; Esposito, E.; Cortesi, R. Lipid-Based Nanosystems as a Tool to Overcome Skin Barrier. Int. J. Mol. Sci. 2021, 22, 8319. [Google Scholar] [CrossRef]
- Lee, Y.I.; Lee, S.G.; Kim, J.; Choi, S.; Jung, I.; Lee, J.H. Proteoglycan Combined with Hyaluronic Acid and Hydrolyzed Collagen Restores the Skin Barrier in Mild Atopic Dermatitis and Dry, Eczema-Prone Skin: A Pilot Study. Int. J. Mol. Sci. 2021, 22, 10189. [Google Scholar] [CrossRef]
- Slavkova, M.; Lazov, C.; Spassova, I.; Kovacheva, D.; Tibi, I.P.-E.; Stefanova, D.; Tzankova, V.; Petrov, P.D.; Yoncheva, K. Formulation of Budesonide-Loaded Polymeric Nanoparticles into Hydrogels for Local Therapy of Atopic Dermatitis. Gels 2024, 10, 79. [Google Scholar] [CrossRef]
- Chuah, L.H.; Loo, H.L.; Goh, C.F.; Fu, J.Y.; Ng, S.F. Chitosan-based drug delivery systems for skin atopic dermatitis: Recent advancements and patent trends. Drug Deliv. Transl. Res. 2023, 13, 1436–1455. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Lee, J.S.; Oh, E.; Oh, H.; Kim, S.; Ok, S.; Sa, J.; Lee, J.-H.; Shin, Y.C.; Bae, Y.-S.; Choi, C.Y.; et al. Tacrolimus-loaded chitosan-based nanoparticles as an efficient topical therapeutic for the effective treatment of atopic dermatitis symptoms. Int. J. Biol. Macromol. 2024, 273 Pt 1, 133005. [Google Scholar] [CrossRef] [PubMed]
- Frei, G.; Haimhoffer; Csapó, E.; Bodnár, K.; Vasvári, G.; Nemes, D.; Lekli, I.; Gyöngyösi, A.; Bácskay, I.; Fehér, P.; et al. In Vitro and In Vivo Efficacy of Topical Dosage Forms Containing Self-Nanoemulsifying Drug Delivery System Loaded with Curcumin. Pharmaceutics 2023, 15, 2054. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, A.R.; Branum, A.; Sivamani, R.K. Effects of Turmeric (Curcuma longa) on Skin Health: A Systematic Review of the Clinical Evidence. Phytother. Res. 2016, 30, 1243–1264. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Sharma, A.; Maheshwari, R.K. Beneficial role of curcumin in skin diseases. Adv. Exp. Med. Biol. 2007, 595, 343–357. [Google Scholar] [CrossRef]
- Lim, C.; Lee, S.; Shin, Y.; Cho, S.; Park, C.; Shin, Y.; Song, E.C.; Kim, W.K.; Ham, C.; Kim, S.B.; et al. Development and application of novel peptide-formulated nanoparticles for treatment of atopic dermatitis. J. Mater. Chem. B 2023, 11, 10131–10146. [Google Scholar] [CrossRef]
- Keam, S.J. Tapinarof Cream 1%: First Approval. Drugs 2022, 82, 1221–1228, Erratum in Drugs 2022, 82, 1345; Erratum in Drugs 2022, 82, 1433. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Eichenfield, L.F.; Hebert, A.A.; Simpson, E.L.; Gold, L.S.; Bissonnette, R.; Papp, K.A.; Browning, J.; Kwong, P.; Korman, N.J.; et al. Tapinarof cream 1% once daily: Significant efficacy in the treatment of moderate to severe atopic dermatitis in adults and children down to 2 years of age in the pivotal phase 3 ADORING trials. J. Am. Acad. Dermatol. 2024, 91, 457–465. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, B.J.; Lee, Y.W.; Won, C.; Park, C.O.; Chung, B.Y.; Lee, D.H.; Jung, K.; Nam, H.-J.; Choi, G.; et al. Asivatrep, a TRPV1 antagonist, for the topical treatment of atopic dermatitis: Phase 3, randomized, vehicle-controlled study (CAPTAIN-AD). J. Allergy Clin. Immunol. 2022, 149, 1340–1347.e4. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, J.H.; Roh, K.H.; Jun, H.J.; Kang, K.S.; Kim, T.Y. Clinical Trial of Human Umbilical Cord Blood-Derived Stem Cells for the Treatment of Moderate-to-Severe Atopic Dermatitis: Phase I/IIa Studies. Stem Cells 2017, 35, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Praestegaard, M.; Steele, F.; Crutchley, N. Polyaphron Dispersion Technology, A Novel Topical Formulation and Delivery System Combining Drug Penetration, Local Tolerability and Convenience of Application. Dermatol. Ther. 2022, 12, 2217–2231. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y. Efficacy of Topical Application of a Skin Moisturizer Containing Pseudo-Ceramide and a Eucalyptus Leaf Extract on Atopic Dermatitis: A Review. J. Clin. Med. 2024, 13, 1749. [Google Scholar] [CrossRef]
- Wu, J.; Li, L.; Zhu, Q.; Zhang, T.; Miao, F.; Cui, Z.; Dong, G.; Tai, Z.; Chen, Z. JAK1/JAK2 degraders based on PROTAC for topical treatment of atopic dermatitis. Biomed. Pharmacother. 2024, 171, 116167. [Google Scholar] [CrossRef]
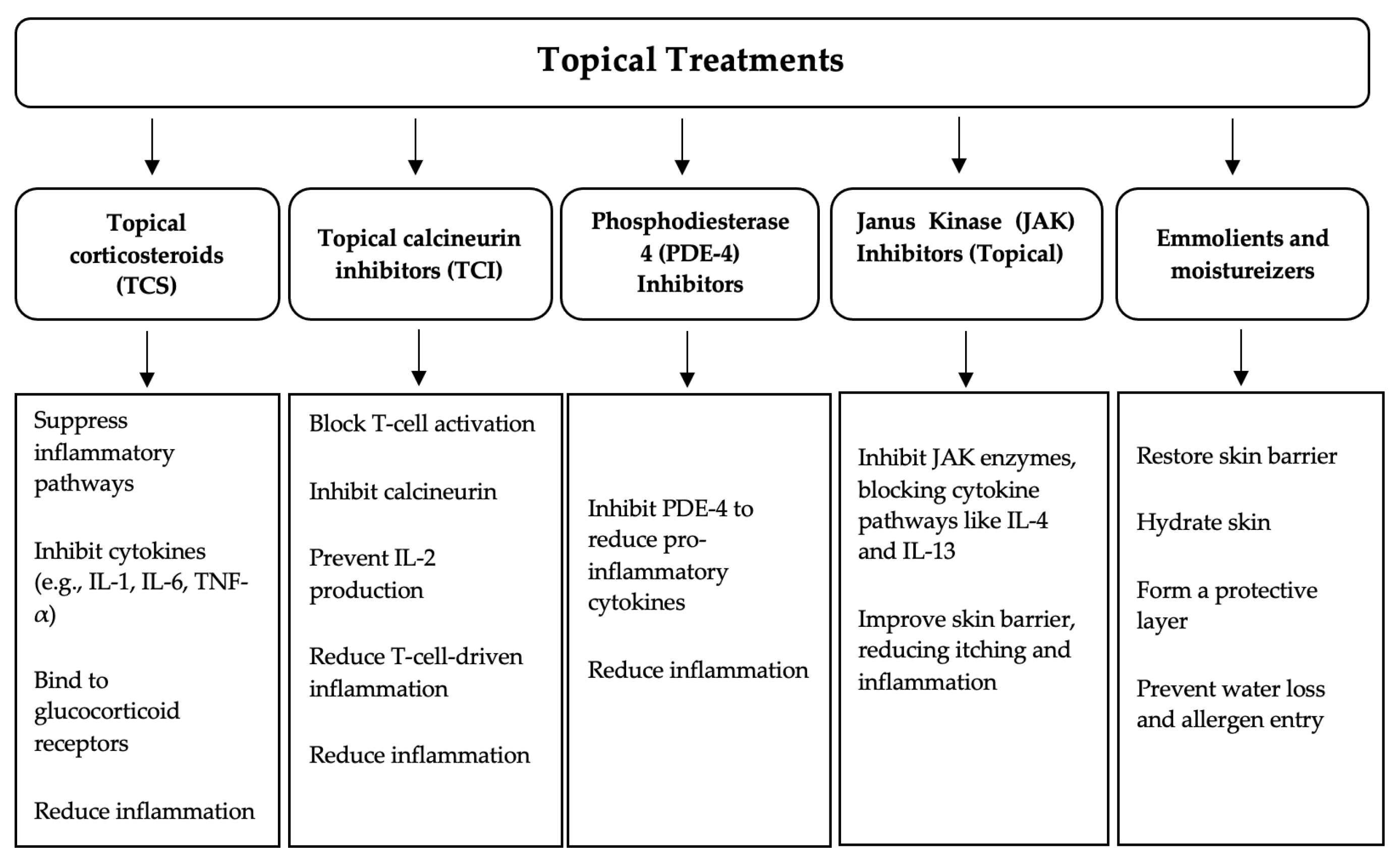
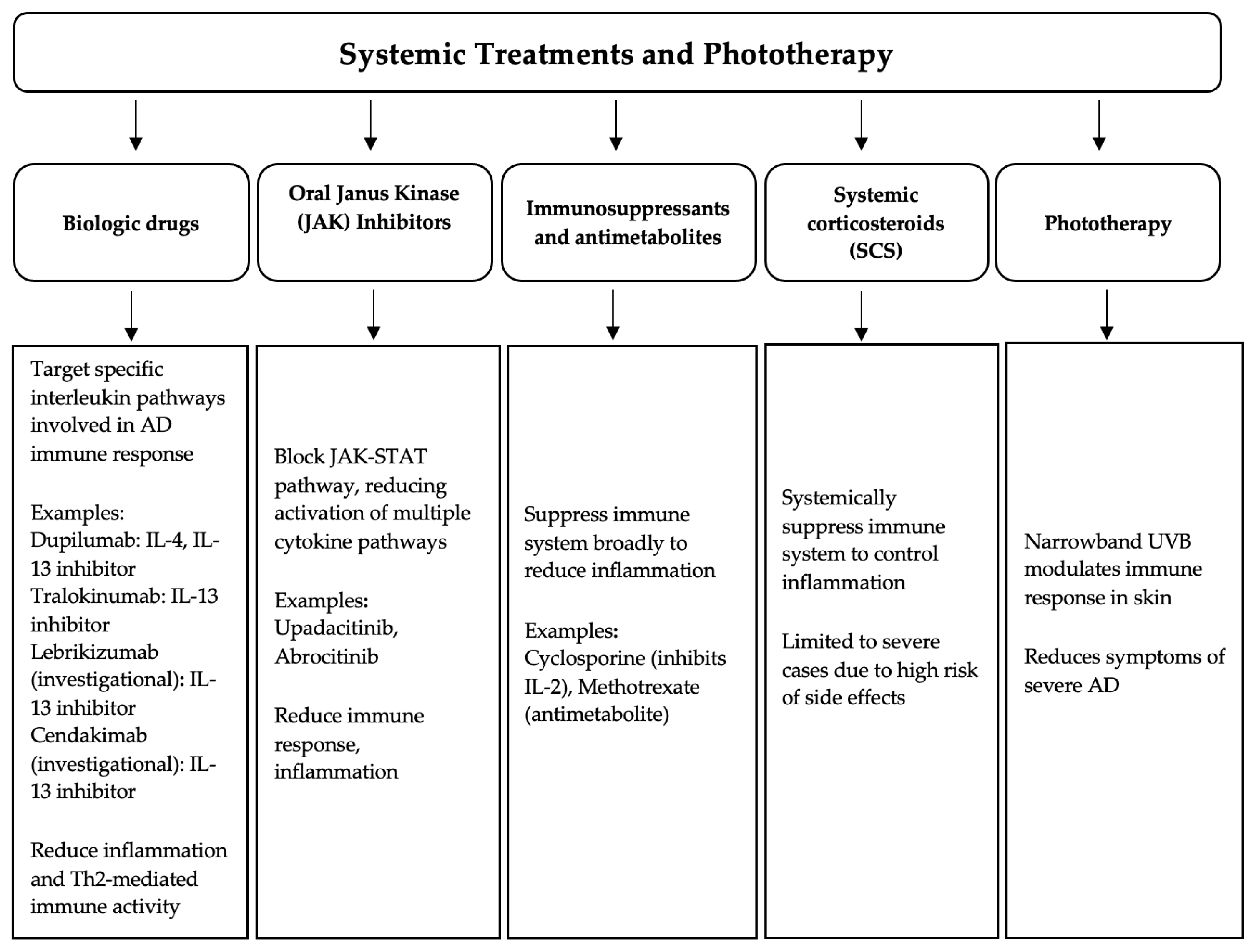
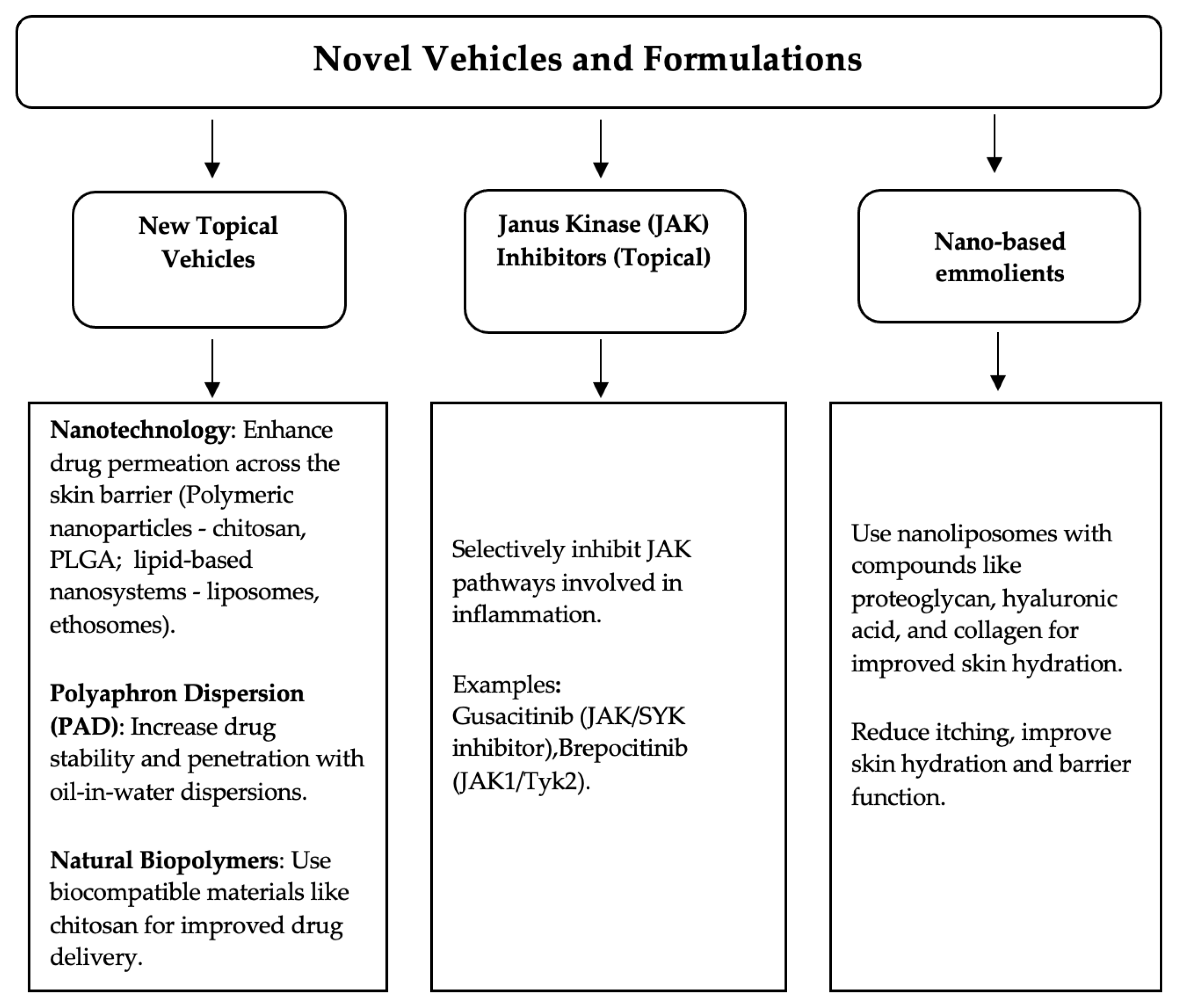
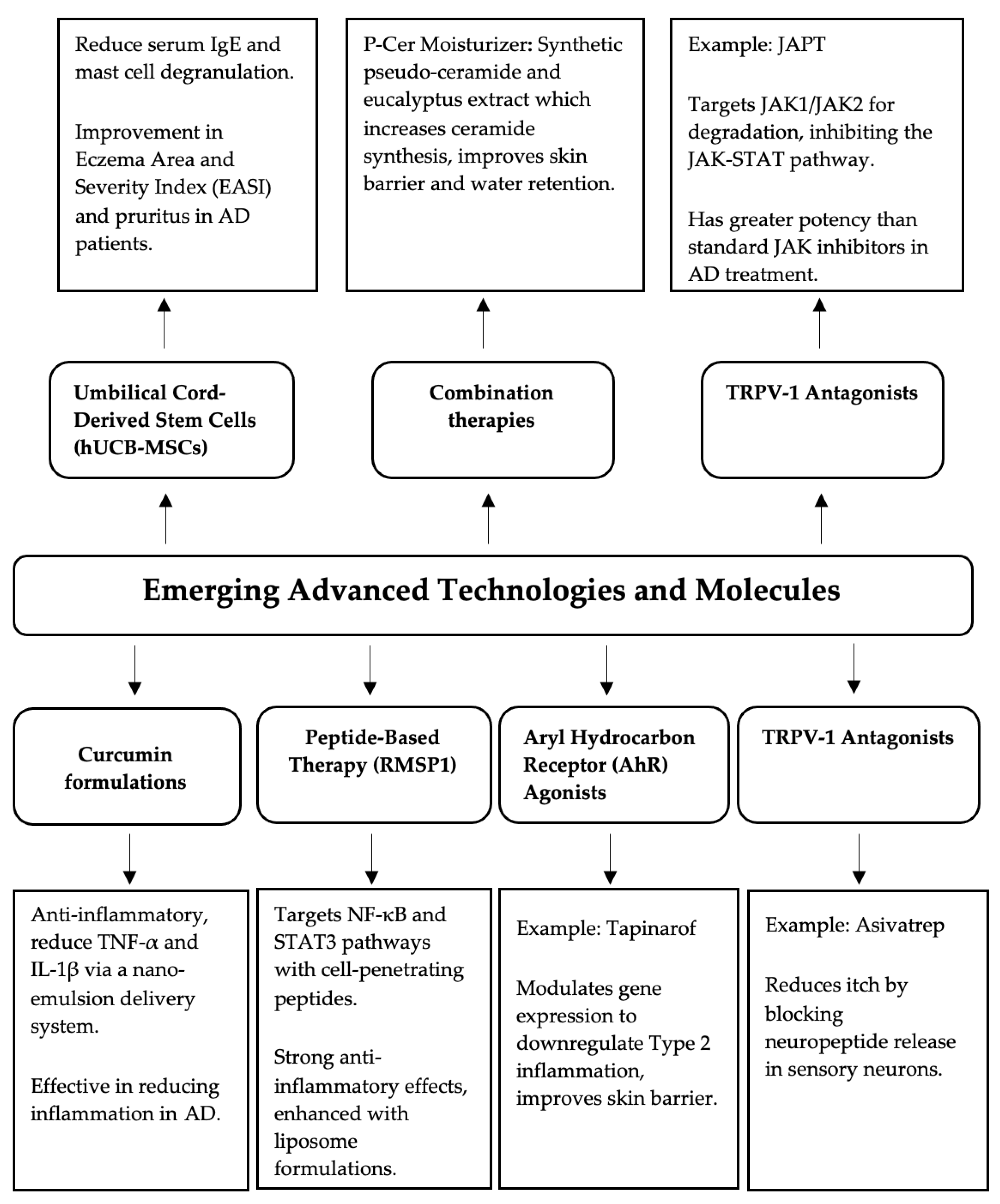
| Topical Treatment | Type | Common Adverse Reactions |
|---|---|---|
| Topical corticosteroids (TCSs) | Anti-inflammatory agent | Skin atrophy, striae, rosacea, telangiectasia, purpura, adrenal suppression (with prolonged use) |
| Topical calcineurin inhibitors (TCIs), e.g., tacrolimus, pimecrolimus | Anti-inflammatory agent | Burning and stinging at the application site, potential malignancy risk (FDA boxed warning) |
| Crisaborole (PDE-4 inhibitor) | Anti-inflammatory agent | Burning and stinging at the application site |
| Topical ruxolitinib (JAK 1/2 inhibitor) | JAK inhibitor | Application site reactions (e.g., burning, itching, redness), acne, nasopharyngitis, headache |
| Systemic Treatment | Type | Common Adverse Reactions |
|---|---|---|
| Dupilumab | Biologic (anti-IL-4, IL-13) | Conjunctivitis, injection site reactions, transient eosinophilia, recurrence of IL-17 diseases (e.g., psoriasis) |
| Tralokinumab | Biologic (anti-IL-13) | Fewer ocular complications than dupilumab, injection site reactions |
| Lebrikizumab | Biologic (anti-IL-13) | Injection site reactions, headache, upper respiratory infections |
| Upadacitinib | JAK-1 inhibitor | Acneiform skin eruptions, increased risk of herpes zoster, upper respiratory infections, headache, nausea |
| Abrocitinib | JAK-1 inhibitor | Nausea, upper-respiratory-tract infections, herpes zoster reactivation |
| Baricitinib | JAK-1/2 inhibitor | Increased risk of infections, including herpes zoster, elevated creatinine kinase, headache |
| Cyclosporine | Immunosuppressant | Nephrotoxicity, hypertension, increased risk of infections, gingival hyperplasia, hypertrichosis |
| Methotrexate | Antimetabolite | Liver toxicity, bone marrow suppression, gastrointestinal disturbances, lung toxicity |
| Azathioprine | Immunosuppressant | Bone marrow suppression, increased risk of infections, gastrointestinal disturbances |
| Mycophenolate mofetil | Immunosuppressant | Bone marrow suppression, gastrointestinal issues, increased risk of infections |
| Systemic corticosteroids | Corticosteroid | Weight gain, hypertension, hyperglycemia, osteoporosis, adrenal suppression, increased risk of infections |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Ion, A.; Orzan, O.A.; Bălăceanu-Gurău, B. Emerging Treatments and New Vehicle Formulations for Atopic Dermatitis. Pharmaceutics 2024, 16, 1425. https://doi.org/10.3390/pharmaceutics16111425
Ali S, Ion A, Orzan OA, Bălăceanu-Gurău B. Emerging Treatments and New Vehicle Formulations for Atopic Dermatitis. Pharmaceutics. 2024; 16(11):1425. https://doi.org/10.3390/pharmaceutics16111425
Chicago/Turabian StyleAli, Sibel, Ana Ion, Olguța Anca Orzan, and Beatrice Bălăceanu-Gurău. 2024. "Emerging Treatments and New Vehicle Formulations for Atopic Dermatitis" Pharmaceutics 16, no. 11: 1425. https://doi.org/10.3390/pharmaceutics16111425
APA StyleAli, S., Ion, A., Orzan, O. A., & Bălăceanu-Gurău, B. (2024). Emerging Treatments and New Vehicle Formulations for Atopic Dermatitis. Pharmaceutics, 16(11), 1425. https://doi.org/10.3390/pharmaceutics16111425








