Evaluation of Dutasteride-Loaded Liposomes and Transfersomes for Follicular-Targeting for Androgenic Alopecia Topical Treatment
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Liposome Formation
2.3. Characterization of DUT-Loaded Liposomes
2.3.1. Liposome Size and Zeta Potential
2.3.2. Encapsulation Efficiency
2.3.3. Morphological Analyses
2.4. Drug Release
2.5. In Vitro Skin Penetration Tests
2.6. Analytical Method
2.7. Statistical Analysis
3. Results and Discussion
3.1. Liposome Characterization
3.2. In Vitro Drug Release
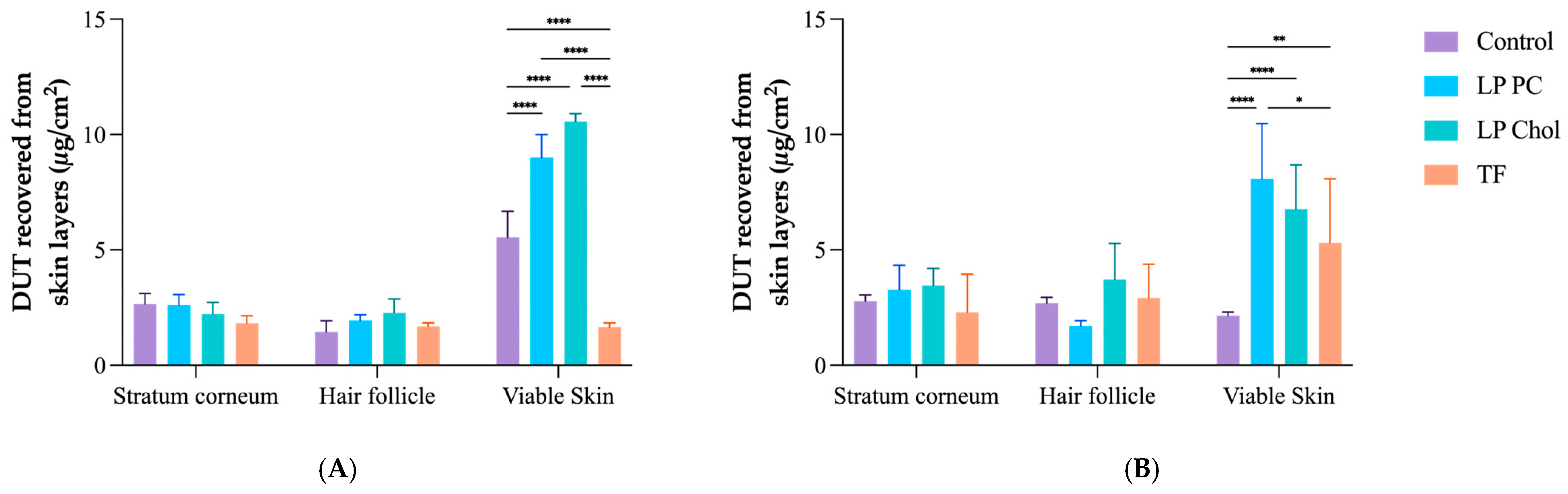
3.3. In Vitro Skin Penetration Experiment and Follicular Targeting Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alsantali, A.; Shapiro, J. Androgens and Hair Loss. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Price, V.H. Treatment of Hair Loss. N. Engl. J. Med. 1999, 341, 964–973. [Google Scholar] [CrossRef]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic Alopecia: A Review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.; McHale, S. The Psychological Impact of Alopecia. BMJ 2005, 331, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.G.; Slomiany, W.P.; Allison, R. Hair Loss: Common Causes and Treatment. Am. Fam. Physician 2017, 96, 371–378. [Google Scholar] [PubMed]
- Madheswaran, T.; Baskaran, R.; Thapa, R.K.; Rhyu, J.Y.; Choi, H.Y.; Kim, J.O.; Yong, C.S.; Yoo, B.K. Design and In Vitro Evaluation of Finasteride-Loaded Liquid Crystalline Nanoparticles for Topical Delivery. AAPS PharmSciTech 2013, 14, 45–52. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kim, S.; Lee, S.Y.; Lee, H.; Han, D.W.; Yang, S.Y.; Kim, K.S. Transdermal Delivery of Minoxidil Using HA-PLGA Nanoparticles for the Treatment in Alopecia. Biomater. Res. 2019, 23, 16. [Google Scholar] [CrossRef]
- Sharma, A.; Goren, A.; Dhurat, R.; Agrawal, S.; Sinclair, R.; Trüeb, R.M.; Vañó-Galván, S.; Chen, G.; Tan, Y.; Kovacevic, M.; et al. Tretinoin Enhances Minoxidil Response in Androgenetic Alopecia Patients by Upregulating Follicular Sulfotransferase Enzymes. Dermatol. Ther. 2019, 32, e12915. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Huang, X.; Shao, A. Preparation and Characterization of Minoxidil Loaded Nanostructured Lipid Carriers. AAPS PharmSciTech 2017, 18, 509–516. [Google Scholar] [CrossRef]
- Pereira, M.N.; Schulte, H.L.; Duarte, N.; Lima, E.M.; Sá-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M.S.S. Solid Effervescent Formulations as New Approach for Topical Minoxidil Delivery. Eur. J. Pharm. Sci. 2017, 96, 411–419. [Google Scholar] [CrossRef]
- Angelo, T.; Barbalho, G.N.; Gelfuso, G.M.; Gratieri, T. Minoxidil Topical Treatment May Be More Efficient If Applied on Damp Scalp in Comparison with Dry Scalp: Minoxidil Topical Treatment. Dermatol. Ther. 2016, 29, 330–333. [Google Scholar] [CrossRef]
- Rathnayake, D.; Sinclair, R. Male Androgenetic Alopecia. Expert Opin. Pharmacother. 2010, 11, 1295–1304. [Google Scholar] [CrossRef]
- Olsen, E.A.; Hordinsky, M.; Whiting, D.; Stough, D.; Hobbs, S.; Ellis, M.L.; Wilson, T.; Rittmaster, R.S. The Importance of Dual 5α-Reductase Inhibition in the Treatment of Male Pattern Hair Loss: Results of a Randomized Placebo-Controlled Study of Dutasteride versus Finasteride. J. Am. Acad. Dermatol. 2006, 55, 1014–1023. [Google Scholar] [CrossRef]
- Gubelin Harcha, W.; Barboza Martínez, J.; Tsai, T.-F.; Katsuoka, K.; Kawashima, M.; Tsuboi, R.; Barnes, A.; Ferron-Brady, G.; Chetty, D. A Randomized, Active- and Placebo-Controlled Study of the Efficacy and Safety of Different Doses of Dutasteride versus Placebo and Finasteride in the Treatment of Male Subjects with Androgenetic Alopecia. J. Am. Acad. Dermatol. 2014, 70, 489–498.e3. [Google Scholar] [CrossRef]
- Eun, H.C.; Kwon, O.S.; Yeon, J.H.; Shin, H.S.; Kim, B.Y.; Ro, B.I.; Cho, H.K.; Sim, W.Y.; Lew, B.L.; Lee, W.-S.; et al. Efficacy, Safety, and Tolerability of Dutasteride 0.5 Mg Once Daily in Male Patients with Male Pattern Hair Loss: A Randomized, Double-Blind, Placebo-Controlled, Phase III Study. J. Am. Acad. Dermatol. 2010, 63, 252–258. [Google Scholar] [CrossRef]
- Shanshanwal, S.J.; Dhurat, R. Superiority of Dutasteride over Finasteride in Hair Regrowth and Reversal of Miniaturization in Men with Androgenetic Alopecia: A Randomized Controlled Open-Label, Evaluator-Blinded Study. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 47. [Google Scholar] [CrossRef]
- Hirshburg, J.M.; Kelsey, P.A.; Therrien, C.A.; Gavino, A.C.; Reichenberg, J.S. Adverse Effects and Safety of 5-Alpha Reductase Inhibitors (Finasteride, Dutasteride): A Systematic Review. J. Clin. Aesthet. Dermatol. 2016, 9, 56–62. [Google Scholar]
- Ushirobira, C.Y.; Afiune, L.A.F.; Pereira, M.N.; Cunha-Filho, M.; Gelfuso, G.M.; Gratieri, T. Dutasteride Nanocapsules for Hair Follicle Targeting: Effect of Chitosan-Coating and Physical Stimulus. Int. J. Biol. Macromol. 2020, 151, 56–61. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Jha, S.; Sharma, P.K.; Malviya, R. Liposomal Drug Delivery System for Cancer Therapy: Advancement and Patents. Recent Pat. Drug Deliv. Formul. 2016, 10, 177–183. [Google Scholar] [CrossRef]
- Pepic, I.; Hafner, A.; Lovric, J.; Perina Lakos, G. Nanotherapeutics in the EU: An Overview on Current State and Future Directions. Int. J. Nanomed. 2014, 9, 1005–1023. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- dos Santos, G.A.; Ferreira-Nunes, R.; Dalmolin, L.F.; dos Santos Ré, A.C.; Anjos, J.L.V.; Mendanha, S.A.; Aires, C.P.; Lopez, R.F.V.; Cunha-Filho, M.; Gelfuso, G.M.; et al. Besifloxacin Liposomes with Positively Charged Additives for an Improved Topical Ocular Delivery. Sci. Rep. 2020, 10, 19285. [Google Scholar] [CrossRef]
- dos Santos, G.A.; Angelo, T.; Andrade, L.M.; Silva, S.M.M.; Magalhães, P.O.; Cunha-Filho, M.; Gelfuso, G.M.; Taveira, S.F.; Gratieri, T. The Role of Formulation and Follicular Pathway in Voriconazole Cutaneous Delivery from Liposomes and Nanostructured Lipid Carriers. Colloids Surf. B Biointerfaces 2018, 170, 341–346. [Google Scholar] [CrossRef]
- Andrade, L.M.; de Fátima Reis, C.; Maione-Silva, L.; Anjos, J.L.V.; Alonso, A.; Serpa, R.C.; Marreto, R.N.; Lima, E.M.; Taveira, S.F. Impact of Lipid Dynamic Behavior on Physical Stability, in Vitro Release and Skin Permeation of Genistein-Loaded Lipid Nanoparticles. Eur. J. Pharm. Biopharm. 2014, 88, 40–47. [Google Scholar] [CrossRef]
- Camera, E.; Ludovici, M.; Galante, M.; Sinagra, J.-L.; Picardo, M. Comprehensive Analysis of the Major Lipid Classes in Sebum by Rapid Resolution High-Performance Liquid Chromatography and Electrospray Mass Spectrometry. J. Lipid Res. 2010, 51, 3377–3388. [Google Scholar] [CrossRef]
- Tolentino, S.; Pereira, M.N.; de Sousa, M.C.; Cunha-Filho, M.; Gelfuso, G.M.; Gratieri, T. The Influence of Sebaceous Content on the Performance of Nanosystems Designed for the Treatment of Follicular Diseases. J. Drug Deliv. Sci. Technol. 2020, 59, 101895. [Google Scholar] [CrossRef]
- Olszewska, M.; Rudnicka, L. Effective Treatment of Female Androgenic Alopecia with Dutasteride. J. Drugs Dermatol. 2005, 4, 637–640. [Google Scholar]
- Sharma, P.; Jain, D.; Maithani, M.; Kumar Mishra, S.; Khare, P.; Jain, V.; Singh, R. Development and Characterization of Dutasteride Bearing Liposomal Systems for Topical Use. Curr. Drug Discov. Technol. 2011, 8, 136–145. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A Review on Phospholipids and Their Main Applications in Drug Delivery Systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Labhasetwar, V. Nanotech Approaches to Drug Delivery and Imaging. Drug Discov. Today 2003, 8, 1112–1120. [Google Scholar] [CrossRef]
- Crook, K.; Stevenson, B.; Dubouchet, M.; Porteous, D. Inclusion of Cholesterol in DOTAP Transfection Complexes Increases the Delivery of DNA to Cells in Vitro in the Presence of Serum. Gene Ther. 1998, 5, 137–143. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of Cationic Lipids and Cationic Polymers in Gene Delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Kaddah, S.; Khreich, N.; Kaddah, F.; Charcosset, C.; Greige-Gerges, H. Cholesterol Modulates the Liposome Membrane Fluidity and Permeability for a Hydrophilic Molecule. Food Chem. Toxicol. 2018, 113, 40–48. [Google Scholar] [CrossRef]
- Garg, V.; Singh, H.; Bimbrawh, S.; Singh, S.K.; Gulati, M.; Vaidya, Y.; Kaur, P. Ethosomes and Transfersomes: Principles, Perspectives and Practices. Curr. Drug Deliv. 2017, 14, 613–633. [Google Scholar] [CrossRef]
- Oyarzún, P.; Gallardo-Toledo, E.; Morales, J.; Arriagada, F. Transfersomes as Alternative Topical Nanodosage Forms for The Treatment of Skin Disorders. Nanomedicine 2021, 16, 2465–2489. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Balanč, B.D.; Ota, A.; Ahlin Grabnar, P.; Djordjević, V.B.; Šavikin, K.P.; Bugarski, B.M.; Nedović, V.A.; Poklar Ulrih, N. Comparative Effects of Cholesterol and β-Sitosterol on the Liposome Membrane Characteristics. Eur. J. Lipid Sci. Technol. 2018, 120, 1800039. [Google Scholar] [CrossRef]
- Siqueira, N.M.; Contri, R.V.; Paese, K.; Beck, R.C.R.; Pohlmann, A.R.; Guterres, S.S. Innovative Sunscreen Formulation Based on Benzophenone-3-Loaded Chitosan-Coated Polymeric Nanocapsules. Skin. Pharmacol. Physiol. 2011, 24, 166–174. [Google Scholar] [CrossRef]
- De Sá, F.A.P.; Taveira, S.F.; Gelfuso, G.M.; Lima, E.M.; Gratieri, T. Liposomal Voriconazole (VOR) Formulation for Improved Ocular Delivery. Colloids Surf. B Biointerfaces 2015, 133, 331–338. [Google Scholar] [CrossRef]




| Liposomes | DUT 28 mM | PC 200 mM | Cholesterol 10 mM | Tween®80 200 mM |
|---|---|---|---|---|
| LP PC | 0.25 mL | 2 mL | - | - |
| LP Chol | 0.25 mL | 2 mL | 0.25 mL | - |
| TF | 0.25 mL | 2 mL | - | 0.225 mL |
| Liposomes | Size (nm) | PdI | Zeta Potential (mV) | EE (%) | DUT Concentration (mg/mL) |
|---|---|---|---|---|---|
| LP PC | 347 ± 12.5 | 0.28 ± 0.04 | −0.61 ± 0.25 | 73.5 ± 12.9 | 0.29 ± 0.03 |
| LP Chol | 285.7 ± 4.67 | 0.22 ± 0.02 | 1.63 ± 0.13 | 70.2 ± 10.5 | 0.28 ± 0.05 |
| TF | 212.3 ± 12.2 | 0.22 ± 0.02 | 1.27 ± 0.03 | 88.1 ± 1.0 | 0.35 ± 0.09 |
| LP PC Unfiltered | LP PC Filtered | LP Chol Unfiltered | LP Chol Filtered | TF Unfiltered | TF Filtered | |
|---|---|---|---|---|---|---|
| Size (nm) | 863.4 ± 86.7 | 347 ± 12.5 | 2382.7 ± 271.0 | 285.7 ± 4.67 | 272.1 ± 28.3 | 212.3 ± 12.2 |
| PdI | 0.52 ± 0.10 | 0.28 ± 0.04 | 0.81 ± 0.16 | 0.22 ± 0.02 | 0.18 ± 0.03 | 0.22 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, J.F.M.; Matos, B.N.; Rocho, R.V.; Barbalho, G.N.; Cunha-Filho, M.; Gelfuso, G.M.; Gratieri, T. Evaluation of Dutasteride-Loaded Liposomes and Transfersomes for Follicular-Targeting for Androgenic Alopecia Topical Treatment. Pharmaceutics 2024, 16, 1524. https://doi.org/10.3390/pharmaceutics16121524
Andrade JFM, Matos BN, Rocho RV, Barbalho GN, Cunha-Filho M, Gelfuso GM, Gratieri T. Evaluation of Dutasteride-Loaded Liposomes and Transfersomes for Follicular-Targeting for Androgenic Alopecia Topical Treatment. Pharmaceutics. 2024; 16(12):1524. https://doi.org/10.3390/pharmaceutics16121524
Chicago/Turabian StyleAndrade, Jayanaraian F. M., Breno N. Matos, Rafael V. Rocho, Geisa N. Barbalho, Marcilio Cunha-Filho, Guilherme M. Gelfuso, and Taís Gratieri. 2024. "Evaluation of Dutasteride-Loaded Liposomes and Transfersomes for Follicular-Targeting for Androgenic Alopecia Topical Treatment" Pharmaceutics 16, no. 12: 1524. https://doi.org/10.3390/pharmaceutics16121524
APA StyleAndrade, J. F. M., Matos, B. N., Rocho, R. V., Barbalho, G. N., Cunha-Filho, M., Gelfuso, G. M., & Gratieri, T. (2024). Evaluation of Dutasteride-Loaded Liposomes and Transfersomes for Follicular-Targeting for Androgenic Alopecia Topical Treatment. Pharmaceutics, 16(12), 1524. https://doi.org/10.3390/pharmaceutics16121524







