Antimicrobial Preservatives in Cyclodextrin-Containing Drug Formulations
Abstract
:1. Introduction
2. Classification of Preservatives and Their Physiochemical Properties
3. Preservative—Cyclodextrin Interactions
4. Studies of Antimicrobial Efficacy in Aqueous CD Solutions
5. Examples of Marketed Products
6. Examples from the Patent Literature
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cox, S.D.; Mann, C.M.; Markham, J.L. Interactions between components of the essential oil of Melaleuca alternifolia. J. Appl. Microbiol. 2001, 91, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Kurup, T.R.; Wan, L.S.; Chan, L.W. Availability and activity of preservatives in emulsified systems. Pharm. Acta Helv. 1991, 66, 76–82. [Google Scholar] [PubMed]
- Bean, H.; Heman-Ackah, S.; Thomas, J. The activity of antibacterials in two-phase systems. J. Soc. Cosmet. Chem. 1965, 16, 15–30. [Google Scholar]
- Barnes, A.R. Compatibility of a commercially available low-density polyethylene eye-drop container with antimicrobial preservatives and potassium ascorbate. J. Clin. Pharm. Ther. 1995, 20, 341. [Google Scholar] [CrossRef]
- Anurova, M.N.; Bakhrushina, E.O.; Demina, N.B.; Panteleeva, E.S. Modern Preservatives of Microbiological Stability (Review). Pharm. Chem. J. 2019, 53, 564–571. [Google Scholar] [CrossRef]
- Inaba, K.; Minami, M.; Yamaguchi, M.; Goto, R.; Otake, H.; Kotake, T.; Nagai, N. Effects of the Ophthalmic Additive Mannitol on Antimicrobial Activity and Corneal Toxicity of Various Preservatives. Chem. Pharm. Bull. 2020, 68, 1069–1073. [Google Scholar] [CrossRef]
- Allen, L.V. Rx Remington: The Science and Practice of Pharmacy, 22nd ed.; Pharmaceutical Press: London, UK, 2013; Volume 1. [Google Scholar]
- Florence, A.T.; Attwood, D. (Eds.) Physicochemical Principles of Pharmacy in Manufacture, Formulation and Clinical Use; Pharmaceutical Press: London, UK, 2015; p. 664. [Google Scholar]
- Sinko, P.J. (Ed.) Martin’s Physical Pharmacy and Pharmaceutical Sciences, 6th ed.; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Loftsson, T.; Stefánsdóttir, Ó.; Friôriksdóttir, H.; Guômundsson, Ö. Interactions between preservatives and 2-hydroxypropyl-β-cyclodextrin. Drug Dev. Ind. Pharm. 1992, 18, 1477–1484. [Google Scholar] [CrossRef]
- Miyajima, K.; Ikuto, M.; Nakagaki, M. Interaction of Short-Chain Alkylammonium Salts with Cyclodextrins in Aqueous Solutions. Chem. Pharm. Bull. 1987, 35, 389–393. [Google Scholar] [CrossRef]
- Simpson, W.J. Neutralisation of the antibacterial action of quaternary ammonium compounds with cyclodextrins. FEMS Microbiol. Lett. 1992, 90, 197–199. [Google Scholar] [CrossRef]
- Lehner, S.J.; Müller, B.W.; Seydel, J.K. Interactions between p-hydroxybenzoic acid esters and hydroxypropyl-β-cyclodextrin and their antimicrobial effect against Candida albicans. Int. J. Pharm. 1993, 93, 201–208. [Google Scholar] [CrossRef]
- Holm, R.; Olesen, N.E.; Alexandersen, S.D.; Dahlgaard, B.N.; Westh, P.; Mu, H. Thermodynamic investigation of the interaction between cyclodextrins and preservatives—Application and verification in a mathematical model to determine the needed preservative surplus in aqueous cyclodextrin formulations. Eur. J. Pharm. Sci. 2016, 87, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Lehner, S.J.; Müller, B.W.; Seydel, J.K. Effect of Hydroxypropyl-β-cyclodextrin on the Antimicrobial Action of Preservatives. J. Pharm. Pharmacol. 1994, 46, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Malaekeh-Nikouei, B.; Bazzaz, B.S.F.; Soheili, V.; Mohammadian, K. Problems in Ophthalmic Drug Delivery: Evaluation of the Interaction Between Preservatives and Cyclodextrins. Jundishapur J. Microbiol. 2013, 6, 6333. [Google Scholar] [CrossRef]
- Moraes, G.S.; Tozetto, N.M.; Pedroso, T.A.A.; de Mattos, M.A.; Urban, A.M.; Paludo, K.S.; dos Santos, F.A.; Neppelenbroek, K.H.; Urban, V.M. Anti-Candida Activity and in Vitro Toxicity Screening of Antifungals Complexed With β-cyclodextrin. J. Appl. Toxicol. 2024, 44, 747–755. [Google Scholar] [CrossRef]
- Bhargava, S.; Agrawal, G.P. Preparation and characterization of solid inclusion complex of cefpodoxime proxetil with β-cyclodextrin. Curr. Drug Deliv. 2008, 5, 1–6. [Google Scholar] [CrossRef]
- Shlar, I.; Droby, S.; Choudhary, R.; Rodov, V. The Mode of Antimicrobial Action of Curcumin Depends on the Delivery System: Monolithic Nanoparticles vs. Supramol. Incl. Complex. RSC Adv. 2017, 7, 42559–42569. [Google Scholar] [CrossRef]
- Jug, M.; Kosalec, I.; Maestrelli, F.; Mura, P. Analysis of triclosan inclusion complexes with β-cyclodextrin and its water-soluble polymeric derivative. J. Pharm. Biomed. Anal. 2011, 54, 1030–1039. [Google Scholar] [CrossRef]
- Mizera, M.; Szymanowska, D.; Stasilowicz, A.; Siakowska, D.; Lewandowska, K.; Miklaszewski, A.; Plech, T.; Tykarska, E.; Cielecka-Piontek, J. Computer-aided design of cefuroxime axetil/cyclodextrin system with enhanced solubility and antimicrobial activity. Biomolecules 2020, 10, 24. [Google Scholar] [CrossRef]
- Shi, Y.-G.; Li, D.-H.; Kong, Y.-M.; Zhang, R.-R.; Gu, Q.; Hu, M.-X.; Tian, S.-Y.; Jin, W.-G. Enhanced antibacterial efficacy and mechanism of octyl gallate/beta-cyclodextrins against Pseudomonas fluorescens and Vibrio parahaemolyticus and incorporated electrospun nanofibers for Chinese giant salamander fillets preservation. Int. J. Food Microbiol. 2022, 361, 109460. [Google Scholar] [CrossRef]
- Teixeira, K.I.R.; Denadai, A.M.L.; Sinisterra, R.D.; Cortes, M.E. Cyclodextrin modulates the cytotoxic effects of chlorhexidine on microorganisms and cells in vitro. Drug Deliv. 2015, 22, 444–453. [Google Scholar] [CrossRef]
- Sripetch, S.; Prajapati, M.; Loftsson, T. Cyclodextrins and Drug Membrane Permeation: Thermodynamic Considerations. J. Pharm. Sci. 2022, 111, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Volkova, T.; Simonova, O.; Perlovich, G. Controlling the Solubility, Release Rate and Permeation of Riluzole With Cyclodextrins. Pharmaceutics 2024, 16, 757. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, T.; Yang, W.; Xu, Y.; Wang, S.; Cai, H.; Liu, Z.; Qiang, H.; Zhang, J. Rational Formulation Engineering of Fraxinellone Utilizing 6-O-A-D-Maltosyl-Β-Cyclodextrin for Enhanced Oral Bioavailability and Hepatic Fibrosis Therapy. Drug Deliv. 2021, 28, 1890–1902. [Google Scholar] [CrossRef]
- Kis, G.L.; Fetz, A.; Schoch, C. Ophthalmic Compositions Containing Cyclodextrins and Quaternary Ammonium Compounds. WO9710805, 27 March 1997. [Google Scholar]
- Castillo, E.J.; Espino, R.L. Preservative Systems for Pharmaceutical Compositions Containing Cyclodextrins. WO9806381, 19 February 1998. [Google Scholar]
- Elder, D.; Crowley, P.J. Antimicrobial preservatives part one: Choosing a preservative system. Am. Pharm. Rev. 2017, 20, 44–52. [Google Scholar]
- Ali, Y.; Kimura, A.; Coffey, M.J.; Tyle, P. Pharmaceutical Development of Suspension Dosage Form. In Pharmaceutical Suspensions: From Formulation Development to Manufacturing; Kulshreshtha, A.K., Singh, O.N., Wall, G.M., Eds.; Springer: New York, NY, USA, 2017; pp. 103–126. [Google Scholar]
- Geier, D.A.; Sykes, L.K.; Geier, M.R. A Review of Thimerosal (Merthiolate) and its Ethylmercury Breakdown Product: Specific Historical Considerations Regarding Safety and Effectiveness. J. Toxicol. Environ. Health Part B 2007, 10, 575–596. [Google Scholar] [CrossRef]
- Cashman, A.L.; Warshaw, E.M. Parabens: A review of epidemiology, structure, allergenicity, and hormonal properties. Dermatitis 2005, 16, 57–66. [Google Scholar] [CrossRef]
- Hutchins, K.M. Functional Materials Based on Molecules with Hydrogen-Bonding Ability: Applications to Drug Co-Crystals and Polymer Complexes. R. Soc. Open Sci. 2018, 5, 180564. [Google Scholar] [CrossRef]
- Mayer, M.; Nakashima, S.; Zimmerman, S.C. Synthesis of a Soluble Ureido-Naphthyridine Oligomer That Self-Associates via Eight Contiguous Hydrogen Bonds. Org. Lett. 2005, 7, 3005–3008. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Cyclodextrins as functional excipients: Methods to enhance complexation efficiency. J. Pharm. Sci. 2012, 101, 3019–3032. [Google Scholar] [CrossRef]
- Murai, S. Inclusion into β-cyclodextrin and adsorption by β-cyclodextrin polymer for ionic surfactants. Kenkyu Hokoku-Kanagawa-Ken Sangyo Gijutsu Senta 2008, 14, 10–13. [Google Scholar]
- Matsui, Y.; Mochida, K. Binding forces contributing to the association of cyclodextrin with alcohol in an aqueous solution. Bull. Chem. Soc. Jpn. 1979, 52, 2808. [Google Scholar] [CrossRef]
- Couto, A.R.S.; Ryzhakov, A.; Larsen, K.L.; Loftsson, T. Interaction of native cyclodextrins and their hydroxypropylated derivatives with parabens in aqueous solutions. Part 1: Evaluation of inclusion complexes. J. Incl. Phenom. Macrocycl. Chem. 2019, 93, 309–321. [Google Scholar] [CrossRef]
- Qi, H.; Nishihata, T.; Rytting, J.H. Study of the interaction between β-cyclodextrin and chlorhexidine. Pharm. Res. 1994, 11, 1207. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Nishioka, T.; Fujita, T. Quantitative structure-reactivity analysis of the inclusion mechanism by cyclodextrins. Top. Curr. Chem. 1985, 128, 61–89. [Google Scholar]
- Buvari, A.; Barcza, L. Complex formation of phenol, aniline, and their nitro derivatives with β-cyclodextrin. J. Chem. Soc. Perkin Trans. 2 1988, 543–545. [Google Scholar] [CrossRef]
- Padula, C.; Pescina, S.; Lucca, L.G.; Demurtas, A.; Santi, P.; Nicoli, S. Skin Retention of Sorbates from an After Sun Formulation for a Broad Photoprotection. Cosmetics 2019, 6, 14. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdottir, D.; Masson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. The complexation efficiency. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 545–552. [Google Scholar] [CrossRef]
- Maw, P.D.; Jansook, P. Cyclodextrin-based Pickering nanoemulsions containing amphotericin B: Part I. evaluation of oil/cyclodextrin and amphotericin B/cyclodextrin inclusion complexes. J. Drug Deliv. Sci. Technol. 2022, 68, 103118. [Google Scholar] [CrossRef]
- Praphanwittaya, P.; Saokham, P.; Jansook, P.; Loftsson, T. Aqueous solubility of kinase inhibitors: I the effect of hydrophilic polymers on their γ-cyclodextrin solubilization. J. Drug Deliv. Sci. Technol. 2020, 55, 101462. [Google Scholar] [CrossRef]
- Jansook, P.; Stefánsson, E.; Thorsteinsdóttir, M.; Sigurdsson, B.B.; Kristjánsdóttir, S.S.; Bas, J.F.; Sigurdsson, H.H.; Loftsson, T. Cyclodextrin solubilization of carbonic anhydrase inhibitor drugs: Formulation of dorzolamide eye drop microparticle suspension. Eur. J. Pharm. Biopharm. 2010, 76, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Hnin, H.M.; Praphanwittaya, P.; Loftsson, T.; Stefansson, E. Effect of salt formation on γ-cyclodextrin solubilization of irbesartan and candesartan and the chemical stability of their ternary complexes. J. Drug Deliv. Sci. Technol. 2022, 67, 102980. [Google Scholar] [CrossRef]
- Jansook, P.; Kulsirachote, P.; Loftsson, T. Cyclodextrin solubilization of celecoxib: Solid and solution state characterization. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 75–88. [Google Scholar] [CrossRef]
- Khin, S.Y.; Soe, H.M.S.H.; Chansriniyom, C.; Pornputtapong, N.; Asasutjarit, R.; Loftsson, T.; Jansook, P. Development of fenofibrate/randomly methylated β-cyclodextrin-loaded eudragit RL 100 nanoparticles for ocular delivery. Molecules 2022, 27, 4755. [Google Scholar] [CrossRef]
- Malaekeh-Nikouei, B.; Tabassi, S.A.S.; Ashari, H.; Gholamzadeh, A. Evaluation the effect of cyclodextrin complexation on aqueous solubility of fluorometholone to achieve ophthalmic solution. J. Incl. Phenom. Macrocycl. Chem. 2009, 65, 335–340. [Google Scholar] [CrossRef]
- Cirri, M.; Maestrelli, F.; Corti, G.; Furlanetto, S.; Mura, P. Simultaneous effect of cyclodextrin complexation, pH, and hydrophilic polymers on naproxen solubilization. J. Pharm. Biomed. Anal. 2006, 42, 126–131. [Google Scholar] [CrossRef]
- Soe, H.M.; Kerdpol, K.; Rungrotmongkol, T.; Pruksakorn, P.; Autthateinchai, R.; Wet-osot, S.; Loftsson, T.; Jansook, P. Voriconazole Eye Drops: Enhanced Solubility and Stability through Ternary Voriconazole/Sulfobutyl Ether β-Cyclodextrin/Polyvinyl Alcohol Complexes. Int. J. Mol. Sci. 2023, 24, 2343. [Google Scholar] [CrossRef]
- Rahimian, A.; Lakzaei, M.; Askari, H.; Dostdari, S.; Khafri, A.; Aminian, M. In vitro assessment of Thimerosal cytotoxicity and antimicrobial activity. J. Trace Elem. Med. Biol. 2023, 77, 127129. [Google Scholar] [CrossRef]
- Puskás, I.; Szente, L.; Szöcs, L.; Fenyvesi, E. Recent List of Cyclodextrin-Containing Drug Products. Period. Polytech.-Chem. Eng. 2023, 67, 11–17. [Google Scholar] [CrossRef]
- Maurin, F.; Pages, B.; Coquelet, C. Ready-To-Use Eye Lotions Containing Indomethacin. EP0761217A1, 12 March 1997. [Google Scholar]
- Cox, M.; Nanda, N. Methods of Treating Pediatric Cancers. US11191766B2, 7 December 2021. [Google Scholar]
- Lopalco, A.; Lopedota, A.A.; Laquintana, V.; Denora, N.; Stella, V.J. Boric Acid, a Lewis Acid with Unique and Unusual Properties: Formulation Implications. J. Pharm. Sci. 2020, 109, 2375–2386. [Google Scholar] [CrossRef]
- Jansook, P.; Kurkov, S.V.; Loftsson, T. Cyclodextrins as solubilizers: Formation of complex aggregates. J. Pharm. Sci. 2010, 99, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Kis, G.L.; Schoch, C.; Fetz, A.; Szeman, J.; Szejtli, J. Development of a diclofenac-Na/HPγCD ophthalmic composition. In Proceedings of the Cyclodextrin: From Basic Research to Market, International Cyclodextrin Symposium, Ann Arbor, MI, USA, 21–24 May 2000; pp. 210–218. [Google Scholar]
- Loftsson, T.; Pilotaz, F. Multidose Aqueous Ophthalmic Compositions Comprising Drug/Cyclodextrin Complexes and Sorbic Acid. WO2023148231, 10 August 2023. [Google Scholar]
- Lyons, R.T.; Chang, J.N. Inhibition of Irritating Side Effects Associated with Use of a Topical Ophthalmic Medication. U.S. Patent US 6,933,289 B2, 23 August 2005. [Google Scholar]
- Feitosa, R.C.; Costa, J.S.R.; Lima, M.v.V.; Ishikawa, E.S.A.; Müller, K.C.; Okasaki, F.B.; Sabadini, E.; Garnero, C.; Longhi, M.R.; Lavayen, V.; et al. Supramolecular Arrangement of Doxycycline with Sulfobutylether-β-Cyclodextrin: Impact on Nanostructuration with Chitosan, Drug Degradation and Antimicrobial Potency. Pharmaceutics 2023, 15, 1285. [Google Scholar] [CrossRef] [PubMed]
- Skuredina, A.A.; Tychinina, A.S.; Le-Deygen, I.M.; Golyshev, S.A.; Kopnova, T.Y.; Le, N.T.; Belogurova, N.G.; Kudryashova, E.V. Cyclodextrins and Their Polymers Affect the Lipid Membrane Permeability and Increase Levofloxacin’s Antibacterial Activity In Vitro. Polymers 2022, 14, 4476. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, F.; Marino, A.; Marchetta, A.; Bongiorno, C.; Zagami, R.; Cristiano, M.C.; Paolino, D.; Pistarà, V.; Ventura, C.A. Development of Chitosan/Cyclodextrin Nanospheres for Levofloxacin Ocular Delivery. Pharmaceutics 2021, 13, 1293. [Google Scholar] [CrossRef] [PubMed]
- Esaki, N.; Pipkin, J.D. Composition Containing Sulfoalkyl Ether Cyclodextrin and Latanoprost. US10463677B2, 26 January 2012. [Google Scholar]
- Mosher, G.L.; Gayed, A.A.; Wedel, R.L. Taste-Masked Formulations Containing Sertraline and Sulfoalkyl Ether Cyclodextrin. U.S. Patent US20050250738A1, 10 November 2005. [Google Scholar]
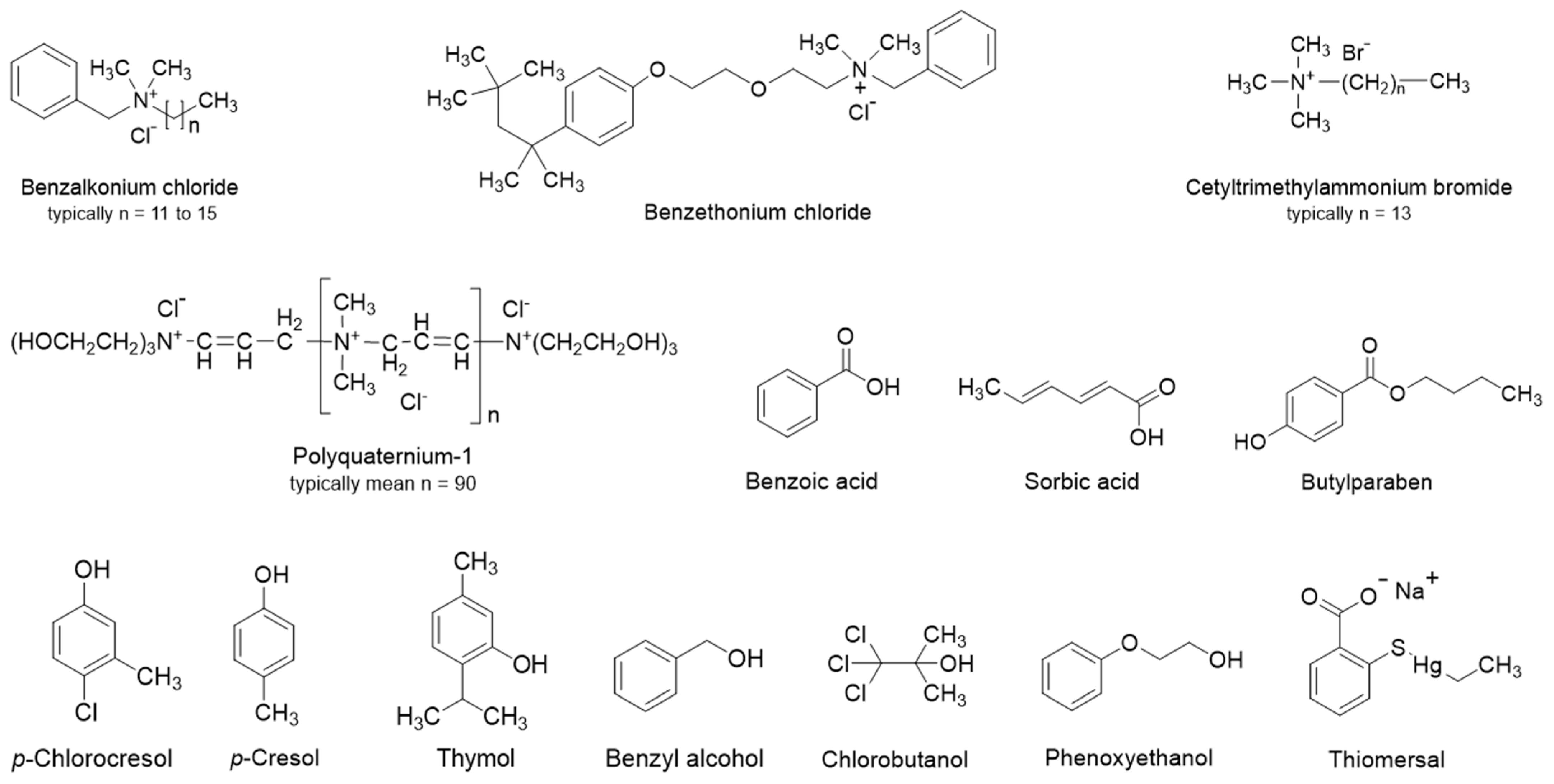

| Preservative | Molecular Weight (g/mol) | pKa | H-Bonds | LogD4 | LogD7 | Solubility (mg/mL) ** | ||
|---|---|---|---|---|---|---|---|---|
| Donors | Acceptors | pH 4 | pH 7 | |||||
| Benzethonium chloride | 448.08 | - | 0 | 3 | - | 4.0 | >10 | >10 |
| Benzoic acid | 122.12 | 4.20 | 1 | 2 | 1.35 | −1.08 | 8.8 | 1000 |
| Benzyl alcohol | 108.14 | - | 1 | 1 | 1.06 | 1.06 | 47 | 47 |
| Benzyldodecyldimethylammonium chloride * | 339.99 | - | 0 | 1 | 2.63 | 2.63 | 866 | 866 |
| Butyl paraben | 194.23 | 8.22 | 1 | 3 | 3.41 | 3.38 | 0.50 | 0.54 |
| Chlorobutanol | 177.46 | - | 1 | 1 | 1.73 | 1.73 | 10 | 10 |
| Chlorohexidine | 505.45 | 11.51 | 10 | 10 | 1.56 | 1.58 | 1.1 | 1.0 |
| m-Cresol | 108.14 | 10.07 | 1 | 1 | 2.04 | 2.04 | 23 | 23 |
| Diazolidinyl urea | 278.22 | 11.22 | 5 | 11 | −5.40 | −5.40 | 999 | 999 |
| Imidazolidinyl urea | 388.29 | 7.41 | 8 | 16 | −4.93 | −5.02 | 0.002 | 0.002 |
| Isobutyl paraben | 194.23 | 8.17 | 1 | 3 | 3.25 | 3.23 | 0.56 | 0.60 |
| Methyl paraben | 152.15 | 8.31 | 1 | 3 | 1.88 | 1.86 | 5.5 | 5.6 |
| Phenol | 94.11 | 9.86 | 1 | 1 | 1.54 | 1.54 | 96 | 96 |
| Phenoxyethanol | 138.16 | - | 1 | 2 | 1.25 | 1.25 | 17 | 17 |
| Polyquaternium-1 | >800 | - | 6 | ≥8 | −9.90 | −9.90 | *** | *** |
| Propyl paraben | 180.20 | 8.23 | 1 | 3 | 2.90 | 2.88 | 1.1 | 1.2 |
| Quaternium-15 | 251.16 | 3.7 | 0 | 4 | - | −0.1 | - | 1000 |
| Sorbic acid | 112.13 | 4.60 | 1 | 2 | 1.17 | −1.12 | 11 | 1000 |
| Thiomersal | 404.82 | 3.62 | 0 | 3 | - | −1.88 | - | 1000 |
| Preservative | Effective Conc. (% w/v) | Cyclodextrin | K1:1 (M−1) | CE | Ref. |
|---|---|---|---|---|---|
| Benzalkonium chloride | 0.004–0.02 | βCD | 1400 | 3500 | [36] |
| Benzoic acid, unionized | 0.1–0.2 | βCD | 678 | 30 | [14] |
| RMβCD | 1013 | 44 | [14] | ||
| HPβCD | 536 | 36 | [14] | ||
| SBEβCD | 924 | 41 | [14] | ||
| Benzyl alcohol | 0.5–5 | αCD | 22 | 9.5 | [37] |
| βCD | 50 | 22 | [37] | ||
| Butyl paraben | 0.02–0.4 | αCD | 701 | 0.38 | [38] |
| HPαCD | 323 | 0.18 | [38] | ||
| βCD | 4582 | 2.5 | [38] | ||
| HPβCD | 16,240 | 9.0 | [38] | ||
| Chlorohexidine | 0.1–0.2 | βCD | 268 | 0.58 | [39] |
| m-Cresol | 0.15–0.3 | βCD | 95 | 20 | [40] |
| Ethyl paraben | 0.1–0.3 | αCD | 193 | 0.84 | [38] |
| HPαCD | 149 | 0.65 | [38] | ||
| βCD | 1709 | 7.46 | [38] | ||
| Methyl paraben | 0.01–0.4 | HPαCD | 67 | 1.3 | [38] |
| βCD | 772 | 27 | [14] | ||
| RMβCD | 1453 | 52 | [14] | ||
| HPβCD | 1128 | 28 | [14] | ||
| SBEβCD | 1519 | 55 | [14] | ||
| Phenol | 0.2–0.5 | βCD | 129 | 129 | [41] |
| Phenoxyethanol | 0.25–0.5 | HPβCD | 100 | 12 | [13] |
| Propyl paraben | 0.005–0.1 | αCD | 240 | 0.42 | [38] |
| HPαCD | 230 | 0.39 | [38] | ||
| βCD | 1548 | 9.3 | [14] | ||
| RMβCD | 3544 | 21 | [14] | ||
| HPβCD | 2360 | 16 | [14] | ||
| SBEβCD | 3165 | 19 | [14] | ||
| Sorbic acid, unionized | 0.05–0.5 | αCD | [119] 1 | [2.2] 1 | [42] |
| HPβCD | [42] 1 | [0.76] 1 | [42] | ||
| Thiomersal | 0.001–0.1 | HPβCD | 1916 | 19 | [13] |
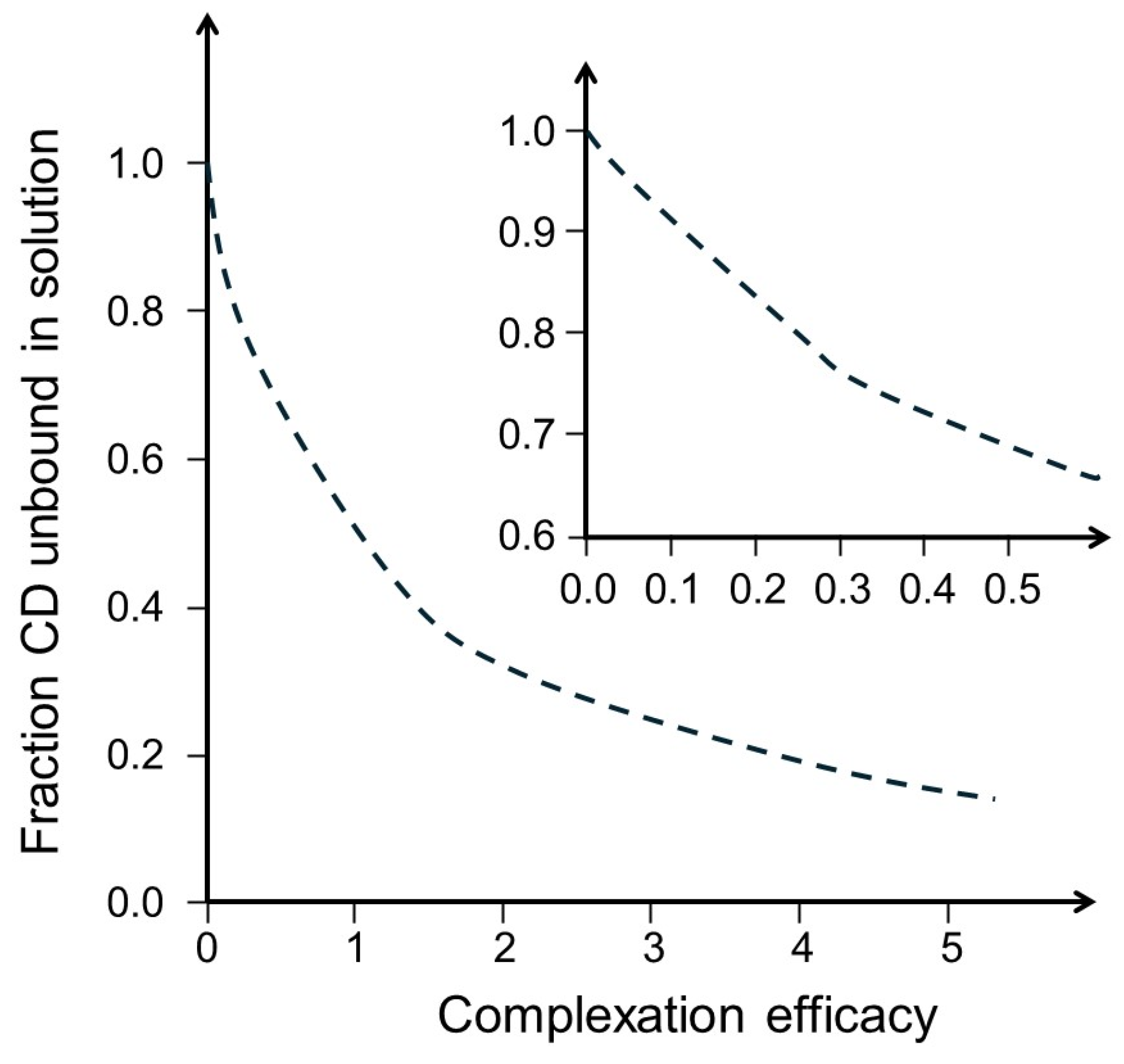
| Drug | MW (g/mol) | S0 (M) | Cyclodextrin | CE | D:CD Molar Ratio | funbound | Ref. |
|---|---|---|---|---|---|---|---|
| Acetazolamide (pKa 7.4) | 222.25 | 0.003 | HPβCD | 0.246 | 1:5 | 0.80 | [43] |
| RMβCD | 0.566 | 1:3 | 0.67 | [44] | |||
| HPγCD | 0.021 | 1:50 | 0.98 | [44] | |||
| Amphotericin B (pKa 5.7, 10.0) 1 | 924.09 | 0.000002 | αCD | 0.002 | 1:500 | 1.0 | [45] |
| βCD | 0.001 | 1:1000 | 1.0 | [45] | |||
| γCD | 0.069 | 1:16 | 0.94 | [45] | |||
| HPγCD | 0.039 | 1:27 | 0.96 | [45] | |||
| Axitinib (pKa 4.3) 2 | 386.47 | 0.000001 | γCD | 0.0002 | 1:5000 | 1.00 | [46] |
| Brinzolamide (pKa 5.9, 8.4) | 383.51 | 0.001 | γCD | 0.02 | 1:50 | 0.98 | [47] |
| HPγCD | 0.03 | 1:35 | 0.97 | [47] | |||
| Candesartan cilexetil (pKa 3.5, 5.9) 3 | 610.66 | 0.00001 | γCD | 0.0012 | 1:835 | 1.0 | [48] |
| Celecoxib (pKa 9.6) | 381.37 | 0.000003 | αCD | 0.0001 | 1:10,000 | 1.00 | [49] |
| βCD | 0.0022 | 1:500 | 1.00 | [49] | |||
| γCD | 0.0004 | 1:2500 | 1.00 | [49] | |||
| HPβCD | 0.0075 | 1:135 | 0.99 | [49] | |||
| RMβCD | 0.0089 | 1:113 | 0.99 | [49] | |||
| Cyclosporin A | 1202.61 | 0.00001 | HPβCD | 0.004 | 1:250 | 1.00 | [43] |
| Dexamethasone | 392.46 | 0.0004 | HPβCD | 0.326 | 1:4 | 0.75 | [43] |
| Dovitinib (pKa 7.7) 2 | 392.43 | 0.00002 | γCD | 0.011 | 1:92 | 0.99 | [46] |
| Fenofibrate | 360.83 | 0.00001 | αCD | 0.20 | 1:6 | 0.83 | [50] |
| βCD | 1.85 | 1:1.5 | 0.33 | [50] | |||
| γCD | 0.21 | 1:6 | 0.83 | [50] | |||
| SBEβCD | 0.63 | 1:3 | 0.67 | [50] | |||
| HPβCD | 2.62 | 1:1.4 | 0.29 | [50] | |||
| RMβCD | 4.54 | 1:1.2 | 0.17 | [50] | |||
| Fluorometholone | 376.46 | 0.00008 | SBEβCD | 1.91 | 1:1.5 | 0.33 | [51] |
| HPγCD | 0.467 | 1:3 | 0.67 | [51] | |||
| Hydrocortisone | 362.46 | 0.001 | HPβCD | 2.00 | 1:1.5 | 0.33 | [43] |
| Irbesartan (pKa 4.1, 7.4) 3 | 428.53 | 0.00001 | γCD | 0.289 | 1:5 | 0.80 | [48] |
| Methazolamide (pKa 7.3) | 236.26 | 0.004 | γCD | 0.04 | 1:26 | 0.96 | [47] |
| HPγCD | 0.05 | 1:21 | 0.95 | [47] | |||
| Naproxen (pKa 4.84) 2 | 230.26 | 0.0056 | HPβCD | 1.29 | 1:1.8 | 0.44 | [52] |
| Triamcinolone acetonide | 434.50 | 0.0003 | HPβCD | 0.063 | 1:17 | 0.94 | [43] |
| Voriconazole (pKa 1.7) | 349.31 | 0.002 | αCD | 0.066 | 1:17 | 0.94 | [53] |
| βCD | 0.658 | 1:3 | 0.67 | [53] | |||
| RMβCD | 0.545 | 1:3 | 0.67 | [53] | |||
| HPβCD | 0.668 | 1:3 | 0.67 | [53] |
| Preservative | Comment | Ref. |
|---|---|---|
| Benzalkonium chloride (BAC) | In aqueous solution, the preservative efficacy was not affected by 0.5% HPβCD but 5% HPβCD had a significant effect. | [10] |
| HPβCD and SBEβCD reduced the antimicrobial efficacy of BAC, both in the presence and absence of 0.1% EDTA, and the presence of the competing drug (0.1% fluorometholone) had no effect. Aqueous eye drops containing 0.1% fluorometholone, 5% HPβCD, 0.02% BAC, and 0.1% EDTA 1 passed the USP antimicrobial efficacy test. | [16] | |
| Benzethonium chloride | 1.1% (10 mM) βCD results in an almost 1000-fold increase in the MIC. | [12] |
| Benzoic acid (pKa 4.2) | Aqueous 1% citric acid 1 solution containing 5% HPβCD passes the European Pharmacopoeia antimicrobial efficacy test at benzoic acid concentrations ≥0.15% at pH 4.0 and ≥0.36% at pH 5.0. | [14] |
| Chlorobutanol | In aqueous solution, the preservative efficacy was not affected by 0.5% HPβCD but 5% HPβCD had significant effect. | [10] |
| m-Cresol | An exponential increase in m-cresol inactivation was observed with rising HPβCD concentration. | [15] |
| Methyl paraben | Aqueous 1% citric acid 1 solution, pH 5.0, containing 5% HPβCD passes the USP and Eur. Ph. antimicrobial efficacy test at preservative concentrations ≥ 0.46%. | [14] |
| HPβCD and SBEβCD reduced antimicrobial efficacy, both in the presence and absence of 0.1% EDTA 1, and the presence of the competing drug (0.1% fluorometholone) had no effect. | [16] | |
| Thiomersal | Thimerosal is water soluble and a very potent antimicrobial preservative. The antimicrobial activity of thimerosal is not inhibited by 4.5% HPβCD. | [15,54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansook, P.; Sigurdsson, H.H.; Pilotaz, F.; Loftsson, T. Antimicrobial Preservatives in Cyclodextrin-Containing Drug Formulations. Pharmaceutics 2024, 16, 1601. https://doi.org/10.3390/pharmaceutics16121601
Jansook P, Sigurdsson HH, Pilotaz F, Loftsson T. Antimicrobial Preservatives in Cyclodextrin-Containing Drug Formulations. Pharmaceutics. 2024; 16(12):1601. https://doi.org/10.3390/pharmaceutics16121601
Chicago/Turabian StyleJansook, Phatsawee, Hákon Hrafn Sigurdsson, Frédéric Pilotaz, and Thorsteinn Loftsson. 2024. "Antimicrobial Preservatives in Cyclodextrin-Containing Drug Formulations" Pharmaceutics 16, no. 12: 1601. https://doi.org/10.3390/pharmaceutics16121601
APA StyleJansook, P., Sigurdsson, H. H., Pilotaz, F., & Loftsson, T. (2024). Antimicrobial Preservatives in Cyclodextrin-Containing Drug Formulations. Pharmaceutics, 16(12), 1601. https://doi.org/10.3390/pharmaceutics16121601







