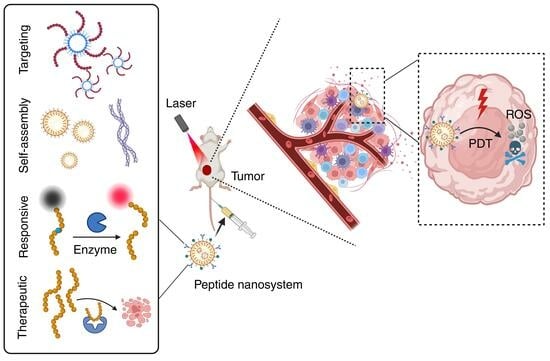
Versatile Peptide-Based Nanosystems for Photodynamic Therapy
Abstract
1. Introduction

2. Properties and Architecture of Peptides
2.1. Chemical Structures of Peptide
2.2. Function and Bioactivity of Peptide
2.3. Architecture of Peptide Nanosystems
3. Multifunctional Peptide-Based Nanosystems for PDT
3.1. Targeted Peptide-Based Nanosystems for PDT
3.2. Stimuli-Responsive Peptide-Based Nanosystems for PDT
3.3. Self-Assembled Peptide-Based Nanosystems for PDT
3.4. Therapeutic Peptide-Based Nanosystems for PDT
4. Outlook and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Kawczyk-Krupka, A.; Bugaj, A.M.; Latos, W.; Zaremba, K.; Wawrzyniec, K.; Sieron, A. Photodynamic therapy in colorectal cancer treatment: The state of the art in clinical trials. Photodiagnosis Photodyn. Ther. 2015, 12, 545–553. [Google Scholar] [CrossRef]
- Vermandel, M.; Dupont, C.; Lecomte, F.; Leroy, H.A.; Tuleasca, C.; Mordon, S.; Hadjipanayis, C.G.; Reyns, N. Standardized intraoperative 5-ALA photodynamic therapy for newly diagnosed glioblastoma patients: A preliminary analysis of the INDYGO clinical trial. J. Neurooncol. 2021, 152, 501–514. [Google Scholar] [CrossRef]
- Usuda, J.; Inoue, T.; Tsuchida, T.; Ohtani, K.; Maehara, S.; Ikeda, N.; Ohsaki, Y.; Sasaki, T.; Oka, K. Clinical trial of photodynamic therapy for peripheral-type lung cancers using a new laser device in a pilot study. Photodiagnosis Photodyn. Ther. 2020, 30, 101698. [Google Scholar] [CrossRef]
- Marmur, E.S.; Schmults, C.D.; Goldberg, D.J. A review of laser and photodynamic therapy for the treatment of nonmelanoma skin cancer. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2004, 30, 264–271. [Google Scholar]
- Frochot, C.; Mordon, S. Update of the situation of clinical photodynamic therapy in Europe in the 2003–2018 period. J. Porphyr. Phthalocyanines 2019, 23, 347–357. [Google Scholar] [CrossRef]
- Rigual, N.; Shafirstein, G.; Cooper, M.T.; Baumann, H.; Bellnier, D.A.; Sunar, U.; Tracy, E.C.; Rohrbach, D.J.; Wilding, G.; Tan, W.; et al. Photodynamic Therapy with 3-(1′-Hexyloxyethyl) Pyropheophorbide a for Cancer of the Oral Cavity. Clin. Cancer Res. 2013, 19, 6605–6613. [Google Scholar] [CrossRef]
- Huggett, M.T.; Jermyn, M.; Gillams, A.; Illing, R.; Mosse, S.; Novelli, M.; Kent, E.; Bown, S.G.; Hasan, T.; Pogue, B.W.; et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br. J. Cancer 2014, 110, 1698–1704. [Google Scholar] [CrossRef]
- Wang, I.; Bendsoe, N.; Klinteberg, C.A.; Enejder, A.M.K.; Andersson-Engels, S.; Svanberg, S.; Svanberg, K. Photodynamic therapy vs. cryosurgery of basal cell carcinomas: Results of a phase III clinical trial. Br. J. Dermatol. 2001, 144, 832–840. [Google Scholar] [CrossRef]
- Stepp, H.; Stummer, W. 5-ALA in the management of malignant glioma. Lasers Surg. Med. 2018, 50, 399–419. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Felsher, D.W. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 375–380. [Google Scholar] [CrossRef]
- Tong, R.; Kohane, D.S. Shedding light on nanomedicine. WIREs Nanomed. Nanobiotechnol. 2012, 4, 638–662. [Google Scholar] [CrossRef]
- Woodhams, J.H.; Macrobert, A.J.; Bown, S.G. The role of oxygen monitoring during photodynamic therapy and its potential for treatment dosimetry. Photochem. Photobiol. Sci. 2007, 6, 1246–1256. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Zhao, H.; Qiu, H.; Wang, Y.; Yang, J.; Gu, Y. Monitoring perfusion and oxygen saturation in port-wine stains during vascular targeted photodynamic therapy. Ann. Transl. Med. 2021, 9, 214. [Google Scholar] [CrossRef]
- Wan, Y.; Fu, L.H.; Li, C.; Lin, J.; Huang, P. Conquering the Hypoxia Limitation for Photodynamic Therapy. Adv. Mater. 2021, 33, e2103978. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Zhang, R.; Duan, Y.; Liu, B. Recent advances of AIE dots in NIR imaging and phototherapy. Nanoscale 2019, 11, 19241–19250. [Google Scholar] [CrossRef]
- Schrama, D.; Reisfeld, R.A.; Becker, J.C. Antibody targeted drugs as cancer therapeutics. Nat. Rev. Drug Discov. 2006, 5, 147–159. [Google Scholar] [CrossRef]
- Majumdar, S.; Siahaan, T.J. Peptide-mediated targeted drug delivery. Med. Res. Rev. 2012, 32, 637–658. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, W.; Zhong, T.; Zhang, S.; Huang, D.; Guo, Y.; Yao, X.; Wang, C.; Zhang, W.Q.; Zhang, X.; et al. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J. Control. Release 2016, 222, 56–66. [Google Scholar] [CrossRef]
- He, H.; Sun, L.; Ye, J.; Liu, E.; Chen, S.; Liang, Q.; Shin, M.C.; Yang, V.C. Enzyme-triggered, cell penetrating peptide-mediated delivery of anti-tumor agents. J. Control. Release 2016, 240, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Celis, E.; Tsai, V.; Crimi, C.; DeMars, R.; Wentworth, P.A.; Chesnut, R.W.; Grey, H.M.; Sette, A.; Serra, H.M. Induction of anti-tumor cytotoxic T lymphocytes in normal humans using primary cultures and synthetic peptide epitopes. Proc. Natl. Acad. Sci. USA 1994, 91, 2105–2109. [Google Scholar] [CrossRef]
- Wu, C.H.; Liu, I.J.; Lu, R.M.; Wu, H.C. Advancement and applications of peptide phage display technology in biomedical science. J. Biomed. Sci. 2016, 23, 8. [Google Scholar] [CrossRef]
- Ruoslahti, E. Tumor penetrating peptides for improved drug delivery. Adv. Drug Deliv. Rev. 2017, 110, 3–12. [Google Scholar] [CrossRef]
- Sheehan, F.; Sementa, D.; Jain, A.; Kumar, M.; Tayarani-Najjaran, M.; Kroiss, D.; Ulijn, R.V. Peptide-Based Supramolecular Systems Chemistry. Chem. Rev. 2021, 121, 13869–13914. [Google Scholar] [CrossRef]
- Tugyi, R.; Uray, K.; Ivan, D.; Fellinger, E.; Perkins, A.; Hudecz, F. Partial D-amino acid substitution: Improved enzymatic stability and preserved Ab recognition of a MUC2 epitope peptide. Proc. Natl. Acad. Sci. USA 2005, 102, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M. Solid-phase peptide synthesis: An overview focused on the preparation of biologically relevant peptides. RSC Adv. 2014, 4, 32658–32672. [Google Scholar] [CrossRef]
- Vandekerckhove, J.; Deboben, A.; Nassal, M.; Wieland, T. The phalloidin binding site of F-actin. EMBO J. 1985, 4, 2815–2818. [Google Scholar] [CrossRef]
- Melak, M.; Plessner, M.; Grosse, R. Actin visualization at a glance. J. Cell Sci. 2017, 130, 525–530. [Google Scholar] [CrossRef]
- Sheikh, A.; Alhakamy, N.A.; Md, S.; Kesharwani, P. Recent Progress of RGD Modified Liposomes as Multistage Rocket Against Cancer. Front. Pharmacol. 2021, 12, 803304. [Google Scholar] [CrossRef]
- Bogdanowich-Knipp, S.J.; Chakrabarti, S.; Williams, T.D.; Dillman, R.K.; Siahaan, T.J. Solution stability of linear vs. cyclic RGD peptides. J. Pept. Res. Off. J. Am. Pept. Soc. 1999, 53, 530–541. [Google Scholar] [CrossRef]
- Jia, S.; Ji, S.; Zhao, J.; Lv, Y.; Wang, J.; Sun, D.; Ding, D. A Fluorinated Supramolecular Self-Assembled Peptide as Nanovaccine Adjuvant for Enhanced Cancer Vaccine Therapy. Small Methods 2023, 7, e2201409. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. Small Bioactive Peptides for Biomaterials Design and Therapeutics. Chem. Rev. 2017, 117, 14015–14041. [Google Scholar] [CrossRef]
- Felicio, M.R.; Silva, O.N.; Goncalves, S.; Santos, N.C.; Franco, O.L. Peptides with Dual Antimicrobial and Anticancer Activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial Peptides as Anticancer Agents: Functional Properties and Biological Activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef]
- Singh, S.B. Discovery and Development of Dolastatin 10-Derived Antibody Drug Conjugate Anticancer Drugs. J. Nat. Prod. 2022, 85, 666–687. [Google Scholar] [CrossRef]
- Shrestha, N.; Araújo, F.; Shahbazi, M.A.; Mäkilä, E.; Gomes, M.J.; Herranz-Blanco, B.; Lindgren, R.; Granroth, S.; Kukk, E.; Salonen, J.; et al. Thiolation and Cell-Penetrating Peptide Surface Functionalization of Porous Silicon Nanoparticles for Oral Delivery of Insulin. Adv. Funct. Mater. 2016, 26, 3405–3416. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, M.; Zhu, L.; Tian, Y.; Wu, M.; Li, Y.; Deng, L.; Jiang, W.; Shen, W.; Wang, Z.; et al. Cell-penetrating Peptide-modified Targeted Drug-loaded Phase-transformation Lipid Nanoparticles Combined with Low-intensity Focused Ultrasound for Precision Theranostics against Hepatocellular Carcinoma. Theranostics 2018, 8, 1892–1910. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface Modification of Gold Nanoparticles with Small Molecules for Biochemical Analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef]
- Chong, K.C.; Hu, F.; Liu, B. AIEgen bioconjugates for specific detection of disease-related protein biomarkers. Mater. Chem. Front. 2019, 3, 12–24. [Google Scholar] [CrossRef]
- Yuan, C.; Ji, W.; Xing, R.; Li, J.; Gazit, E.; Yan, X. Hierarchically oriented organization in supramolecular peptide crystals. Nat. Rev. Chem. 2019, 3, 567–588. [Google Scholar] [CrossRef]
- Liu, K.; Xing, R.; Zou, Q.; Ma, G.; Möhwald, H.; Yan, X. Simple peptide-tuned self-assembly of photosensitizers towards anticancer photodynamic therapy. Angew. Chem. 2016, 128, 3088–3091. [Google Scholar] [CrossRef]
- Lynn, G.M.; Sedlik, C.; Baharom, F.; Zhu, Y.; Ramirez-Valdez, R.A.; Coble, V.L.; Tobin, K.; Nichols, S.R.; Itzkowitz, Y.; Zaidi, N.; et al. Peptide-TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat. Biotechnol. 2020, 38, 320–332. [Google Scholar] [CrossRef]
- Mu, J.; Lin, J.; Huang, P.; Chen, X. Development of endogenous enzyme-responsive nanomaterials for theranostics. Chem. Soc. Rev. 2018, 47, 5554–5573. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Ou, H.; Liu, Q.; Ding, D. Gathering brings strength: How organic aggregates boost disease phototheranostics. Aggregate 2021, 2, 95–113. [Google Scholar] [CrossRef]
- Zhang, N.Y.; Hu, X.J.; An, H.W.; Liang, J.X.; Wang, H. Programmable design and self-assembly of peptide conjugated AIEgens for biomedical applications. Biomaterials 2022, 287, 121655. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.B.; Gao, Y.J.; Wang, L.; Wang, H. Self-Assembled Peptide-Based Nanomaterials for Biomedical Imaging and Therapy. Adv. Mater. 2018, 30, e1703444. [Google Scholar] [CrossRef]
- Gao, J.; Zhan, J.; Yang, Z. Enzyme-Instructed Self-Assembly (EISA) and Hydrogelation of Peptides. Adv. Mater. 2020, 32, e1805798. [Google Scholar] [CrossRef]
- Sun, M.; Wang, C.; Lv, M.; Fan, Z.; Du, J. Intracellular Self-Assembly of Peptides to Induce Apoptosis against Drug-Resistant Melanoma. J. Am. Chem. Soc. 2022, 144, 7337–7345. [Google Scholar] [CrossRef]
- Sun, S.; Liang, H.-W.; Wang, H.; Zou, Q. Light-Triggered Self-Assembly of Peptide Nanoparticles into Nanofibers in Living Cells through Molecular Conformation Changes and H-Bond Interactions. ACS Nano 2022, 16, 18978–18989. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Cheng, Z.; Wang, P.; Ma, Z.; Liu, K.; Cheng, Y.; Zhou, Y.; Lin, X.; Shao, X.; et al. Cell-penetrating riboflavin conjugate for antitumor photodynamic therapy. Chin. Chem. Lett. 2022, 33, 4339–4344. [Google Scholar] [CrossRef]
- Wei, D.; Huang, Y.; Wang, B.; Ma, L.; Karges, J.; Xiao, H. Photo-Reduction with NIR Light of Nucleus-Targeting PtIV Nanoparticles for Combined Tumor-Targeted Chemotherapy and Photodynamic Immunotherapy. Angew. Chem. Int. Ed. 2022, 61, e202201486. [Google Scholar] [CrossRef]
- Tian, Y.; Cheng, Q.; Dang, H.; Qian, H.; Teng, C.; Xie, K.; Yan, L. Amino modified iodinated BODIPY photosensitizer for highly efficient NIR imaging-guided photodynamic therapy with ultralow dose. Dye. Pigment. 2021, 194, 109611. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, R.; Yu, T.; He, J. An acid-targeting peptide can be used as a carrier for photodynamic therapy (PDT). Mater. Today Commun. 2022, 31, 103659. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, G.-L.; Fan, J.-H.; Yuan, P.; Deng, F.-A.; Qiu, X.-Z.; Yu, X.-Y.; Li, S.-Y. Epigenetics-inspired photosensitizer modification for plasma membrane-targeted photodynamic tumor therapy. Biomaterials 2019, 224, 119497. [Google Scholar] [CrossRef]
- Cheng, H.; Zheng, R.R.; Fan, G.L.; Fan, J.H.; Zhao, L.P.; Jiang, X.Y.; Yang, B.; Yu, X.Y.; Li, S.Y.; Zhang, X.Z. Mitochondria and plasma membrane dual-targeted chimeric peptide for single-agent synergistic photodynamic therapy. Biomaterials 2019, 188, 1–11. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, F.; Wu, W.; Qiu, W.X.; Zhang, L.; Li, R.; Zhuang, Z.N.; Yu, W.; Cheng, H.; Zhang, X.Z. Enzyme-Driven Membrane-Targeted Chimeric Peptide for Enhanced Tumor Photodynamic Immunotherapy. ACS Nano 2019, 13, 11249–11262. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S.K.; Porter, S.L.; Rizk, N.; Sheng, Y.; McKaig, T.; Burnett, K.; White, B.; Nesbitt, H.; Matin, R.N.; McHale, A.P.; et al. Rose Bengal-Amphiphilic Peptide Conjugate for Enhanced Photodynamic Therapy of Malignant Melanoma. J. Med. Chem. 2020, 63, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Ihsanullah, K.M.; Kumar, B.N.; Zhao, Y.; Muhammad, H.; Liu, Y.; Wang, L.; Liu, H.; Jiang, W. Stepwise-activatable hypoxia triggered nanocarrier-based photodynamic therapy for effective synergistic bioreductive chemotherapy. Biomaterials 2020, 245, 119982. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Zhang, Z.; Xu, W.; Wen, H.; Zhu, W.; Wu, Q.; Wu, H.; Gong, J.; Wang, Z.; Wang, D.; et al. Good Steel Used in the Blade: Well-Tailored Type-I Photosensitizers with Aggregation-Induced Emission Characteristics for Precise Nuclear Targeting Photodynamic Therapy. Adv. Sci. 2021, 8, 2100524. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yin, Y.; Yang, L.; Lu, B.; Yang, Z.; Wang, W.; Li, R. Nucleus-Targeted Photosensitizer Nanoparticles for Photothermal and Photodynamic Therapy of Breast Carcinoma. Int. J. Nanomed. 2021, 16, 1473–1485. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, J.-H.; Zhao, L.-P.; Fan, G.-L.; Zheng, R.-R.; Qiu, X.-Z.; Yu, X.-Y.; Li, S.-Y.; Zhang, X.-Z. Chimeric peptide engineered exosomes for dual-stage light guided plasma membrane and nucleus targeted photodynamic therapy. Biomaterials 2019, 211, 14–24. [Google Scholar] [CrossRef]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic Cell Death and DAMPs in Cancer Therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting Photodynamic and Photothermal Therapy to the Endoplasmic Reticulum Enhances Immunogenic Cancer Cell Death. Nat. Commun. 2019, 10, 3349. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, H.; Liu, R.; Chen, C.; Zeng, S.; Liu, Q.; Ding, D. Endoplasmic reticulum targeted AIE bioprobe as a highly efficient inducer of immunogenic cell death. Sci. China Chem. 2020, 63, 1428–1434. [Google Scholar] [CrossRef]
- Chen, P.-L.; Huang, P.-Y.; Chen, J.-Y.; Shi, Q.-Y.; Zhu, Y.-Y.; Chen, Y.; Liu, L.-H.; Zhang, X.-Z. A self-delivery chimeric peptide for high efficient cell membrane-targeting low-temperature photothermal/photodynamic combinational therapy and metastasis suppression of tumor. Biomaterials 2022, 286, 121593. [Google Scholar] [CrossRef]
- Zheng, D.; Liu, J.; Xie, L.; Wang, Y.; Ding, Y.; Peng, R.; Cui, M.; Wang, L.; Zhang, Y.; Zhang, C.; et al. Enzyme-instructed and mitochondria-targeting peptide self-assembly to efficiently induce immunogenic cell death. Acta Pharm. Sin. B 2022, 12, 2740–2750. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chang, R.; Xing, R.; Yan, X. Bioactive Peptide Nanodrugs Based on Supramolecular Assembly for Boosting Immunogenic Cell Death-Induced Cancer Immunotherapy. Small Methods 2023, 7, 2201708. [Google Scholar] [CrossRef]
- Wang, T.; Gao, Z.; Zhang, Y.; Hong, Y.; Tang, Y.; Shan, K.; Kong, X.; Wang, Z.; Shi, Y.; Ding, D. A supramolecular self-assembled nanomaterial for synergistic therapy of immunosuppressive tumor. J. Control. Release 2022, 351, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Youssef, Z.; Yesmurzayeva, N.; Larue, L.; Jouan-Hureaux, V.; Colombeau, L.; Arnoux, P.; Acherar, S.; Vanderesse, R.; Frochot, C. New Targeted Gold Nanorods for the Treatment of Glioblastoma by Photodynamic Therapy. J. Clin. Med. 2019, 8, 2205. [Google Scholar] [CrossRef]
- Dai, G.; Chu, J.C.H.; Chan, C.K.W.; Choi, C.H.J.; Ng, D.K.P. Reactive oxygen species-responsive polydopamine nanoparticles for targeted and synergistic chemo and photodynamic anticancer therapy. Nanoscale 2021, 13, 15899–15915. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Lee, H.-I.; Kim, J.-K.; Kim, C.-H.; Kim, Y.-J. Peptide 18-4/chlorin e6-conjugated polyhedral oligomeric silsesquioxane nanoparticles for targeted photodynamic therapy of breast cancer. Colloids Surf. B 2020, 189, 110829. [Google Scholar] [CrossRef] [PubMed]
- Panikar, S.S.; Ramírez-García, G.; Banu, N.; Vallejo-Cardona, A.A.; Lugo-Fabres, P.; Camacho-Villegas, T.A.; Salas, P.; De la Rosa, E. Ligand-targeted Theranostic Liposomes combining methylene blue attached upconversion nanoparticles for NIR activated bioimaging and photodynamic therapy against HER-2 positive breast cancer. J. Lumin. 2021, 237, 118143. [Google Scholar] [CrossRef]
- Xue, E.Y.; Wong, R.C.H.; Wong, C.T.T.; Fong, W.-P.; Ng, D.K.P. Synthesis and biological evaluation of an epidermal growth factor receptor-targeted peptide-conjugated phthalocyanine-based photosensitiser. RSC Adv. 2019, 9, 20652–20662. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, Q.; Wong, R.C.H.; Zhao, S.; Ng, D.K.P.; Lo, P.-C. Synthesis and biological evaluation of phthalocyanine-peptide conjugate for EGFR-targeted photodynamic therapy and bioimaging. Dyes Pigm. 2019, 163, 197–203. [Google Scholar] [CrossRef]
- Chu, J.C.H.; Fong, W.-P.; Wong, C.T.T.; Ng, D.K.P. Facile Synthesis of Cyclic Peptide–Phthalocyanine Conjugates for Epidermal Growth Factor Receptor-Targeted Photodynamic Therapy. J. Med. Chem. 2021, 64, 2064–2076. [Google Scholar] [CrossRef]
- Yan, S.; Tang, D.; Hong, Z.; Wang, J.; Yao, H.; Lu, L.; Yi, H.; Fu, S.; Zheng, C.; He, G.; et al. CD133 peptide-conjugated pyropheophorbide-a as a novel photosensitizer for targeted photodynamic therapy in colorectal cancer stem cells. Biomater. Sci. 2021, 9, 2020–2031. [Google Scholar] [CrossRef]
- Zahmatkeshan, M.; Gheybi, F.; Rezayat, S.M.; Jaafari, M.R. Improved drug delivery and therapeutic efficacy of PEgylated liposomal doxorubicin by targeting anti-HER2 peptide in murine breast tumor model. Eur. J. Pharm. Sci. 2016, 86, 125–135. [Google Scholar] [CrossRef]
- Panikar, S.S.; Ramírez-García, G.; Vallejo-Cardona, A.A.; Banu, N.; Patrón-Soberano, O.A.; Cialla-May, D.; Camacho-Villegas, T.A.; de la Rosa, E. Novel anti-HER2 peptide-conjugated theranostic nanoliposomes combining NaYF4:Yb,Er nanoparticles for NIR-activated bioimaging and chemo-photodynamic therapy against breast cancer. Nanoscale 2019, 11, 20598–20613. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-L.; Chou, Y.-T.; Su, B.-K.; Wu, C.-C.; Wang, C.-H.; Chang, K.-H.; Ho, J.-A.A.; Chou, P.-T. Comprehensive Thione-Derived Perylene Diimides and Their Bio-Conjugation for Simultaneous Imaging, Tracking, and Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2022, 144, 17249–17260. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Shin, Y.; Won, W.R.; Lim, C.; Kim, J.C.; Kang, K.; Husni, P.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Development of AE147 Peptide-Conjugated Nanocarriers for Targeting uPAR-Overexpressing Cancer Cells. Int. J. Nanomed. 2021, 16, 5437–5449. [Google Scholar] [CrossRef]
- Pethő, L.; Murányi, J.; Pénzes, K.; Gurbi, B.; Brauswetter, D.; Halmos, G.; Csík, G.; Mező, G. Suitability of GnRH Receptors for Targeted Photodynamic Therapy in Head and Neck Cancers. Int. J. Mol. Sci. 2019, 20, 5027. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Zhou, X.-Q.; Bretin, L.; Zeng, X.; Husiev, Y.; Polanco, E.A.; Zhao, G.; Wijaya, L.S.; Biver, T.; et al. Cyclic Ruthenium-Peptide Conjugates as Integrin-Targeting Phototherapeutic Prodrugs for the Treatment of Brain Tumors. J. Am. Chem. Soc. 2023, 145, 14963–14980. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimova, V.; González-Delgado, J.A.; Levêque, M.; Torres, T.; Garanger, E.; Lecommandoux, S. Photooxidation Responsive Elastin-Like Polypeptide Conjugates for Photodynamic Therapy Application. Bioconjug. Chem. 2021, 32, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Le, D.H.T.; Ibrahimova, V.; van den Wildenberg, S.A.H.; Wu, H.; Fonseca, A.; Torres, T.; Garanger, E.; Leenders, W.P.J.; Brock, R.; Lecommandoux, S.; et al. Light-Responsive Elastin-Like Peptide-Based Targeted Nanoparticles for Enhanced Spheroid Penetration. Angew. Chem. Int. Ed. 2023, 62, e202300511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Gao, Z.; Cui, J.; Hao, J. Dual-Stimuli-Responsive Polypeptide Nanoparticles for Photothermal and Photodynamic Therapy. ACS Appl. Bio Mater. 2019, 3, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Wu, J.; Sah, B.; Vanasse, A.; Cooper, L.N.; Ma, L.; Li, G.; Zheng, H.; Chen, W.; Antosh, M.P. X-ray induced photodynamic therapy with copper-cysteamine nanoparticles in mice tumors. Proc. Natl. Acad. Sci. USA 2019, 116, 16823–16828. [Google Scholar] [CrossRef] [PubMed]
- Ballance, W.C.; Qin, E.C.; Chung, H.J.; Gillette, M.U.; Kong, H. Reactive oxygen species-responsive drug delivery systems for the treatment of neurodegenerative diseases. Biomaterials 2019, 217, 119292. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, C.; Ma, Y.; Zhu, L.; Lu, B.; Wang, Y.; Wang, J.; Chen, T.; Dong, C.-M.; Yao, Y. pH/ROS dual-responsive supramolecular polypeptide prodrug nanomedicine based on host-guest recognition for cancer therapy. Acta Biomater. 2022, 143, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Lei, X.; Ren, H.; Zheng, S.; Qiang, J.; Zhang, Z.; Chen, Y.; Wei, T.; Wang, F.; Chen, X. PEGylated Dimeric BODIPY Photosensitizers as Nanocarriers for Combined Chemotherapy and Cathepsin B-Activated Photodynamic Therapy in 3D Tumor Spheroids. ACS Appl. Bio Mater. 2020, 3, 3835–3845. [Google Scholar] [CrossRef]
- Hu, C.; Zhuang, W.; Yu, T.; Chen, L.; Liang, Z.; Li, G.; Wang, Y. Multi-stimuli responsive polymeric prodrug micelles for combined chemotherapy and photodynamic therapy. J. Mater. Chem. B 2020, 8, 5267–5279. [Google Scholar] [CrossRef]
- Qi, G.; Liu, X.; Shi, L.; Wu, M.; Liu, J.; Liu, B. Enzyme-Mediated Intracellular Polymerization of AIEgens for Light-Up Tumor Localization and Theranostics. Adv. Mater. 2021, 34, 2106885. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, M.; Mu, Y.; Li, J.; Foda, M.F.; Zhang, W.; Han, K.; Han, H. Reasonably retard O2 consumption through a photoactivity conversion nanocomposite for oxygenated photodynamic therapy. Biomaterials 2019, 218, 119312. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, K.; Li, X.; Zhang, J.; Ma, Z.; Foda, M.F.; Mu, Y.; Dai, X.; Han, H. Au Hollow Nanorods-Chimeric Peptide Nanocarrier for NIR-II Photothermal Therapy and Real-time Apoptosis Imaging for Tumor Theranostics. Theranostics 2019, 9, 4971–4981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mu, Y.; Xu, M.; Foda, M.F.; Han, H. Sequential assembled chimeric peptide for precise synergistic phototherapy and photoacoustic imaging of tumor apoptosis. Chem. Eng. J. 2022, 427, 130775. [Google Scholar] [CrossRef]
- Fan, Y.; Li, P.; Hu, B.; Liu, T.; Huang, Z.; Shan, C.; Cao, J.; Cheng, B.; Liu, W.; Tang, Y. A Smart Photosensitizer–Cerium Oxide Nanoprobe for Highly Selective and Efficient Photodynamic Therapy. Inorg. Chem. 2019, 58, 7295–7302. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xiao, Z.; Huang, J.; Wang, Y.; An, Y.; Xiao, H.; Peng, Y.; Pang, P.; Han, S.; Zhu, K.; et al. Dual-Sensitive PEG-Sheddable Nanodrug Hierarchically Incorporating PD-L1 Antibody and Zinc Phthalocyanine for Improved Immuno-Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 12845–12856. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Tang, J.; Chen, L.; Zeng, Q.; Li, C.; Xiao, S.; Jiang, Z.; Liu, J. Tumor microenvironment triple-responsive nanoparticles enable enhanced tumor penetration and synergetic chemo-photodynamic therapy. Biomaterials 2021, 268, 120574. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Lv, C.; Chen, T.; Sun, L.; Sun, L.; Hao, J.; Ding, F.; Wang, T.; Jiang, J.; et al. Amino porphyrin-peptide assemblies induce ribosome damage and cancer stem cell inhibition for an enhanced photodynamic therapy. Biomaterials 2022, 289, 121812. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, A.; Ren, P.; Yan, X.; Bai, S. One-step co-assembly method to fabricate photosensitive peptide nanoparticles for two-photon photodynamic therapy. Chem. Commun. 2019, 55, 3191–3194. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Kuang, Y.; Jiang, Q.; Zhou, S.; Yu, J.; He, Z.; Sun, J. Arginine-peptide complex-based assemblies to combat tumor hypoxia for enhanced photodynamic therapeutic effect. Nano Res. 2022, 15, 5183–5192. [Google Scholar] [CrossRef]
- Xu, Y.; Teng, C.; Dang, H.; Yin, D.; Yan, L. Highly bright stable organic radicals encapsulated by amphiphilic polypeptide for efficient near-infrared phototheranostics. Talanta 2024, 266, 124948. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Y.; Liang, R.; Hong, G.; An, J.; Peng, X.; Zheng, W.-H.; Song, F. Self-assembly of amphiphilic peptides to construct activatable nanophotosensitizers for theranostic photodynamic therapy. Chin. Chem. Lett. 2021, 32, 3903–3906. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Chen, W.; Liu, L.; Yu, F. Light and sound to trigger the Pandora’s box against breast cancer: A combination strategy of sonodynamic, photodynamic and photothermal therapies. Biomaterials 2020, 232, 119685. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wong, R.C.H.; Yan, X.; Ng, D.K.P.; Lo, P.-C. Self-Assembled Nanophotosensitizing Systems with Zinc(II) Phthalocyanine-Peptide Conjugates as Building Blocks for Targeted Chemo-Photodynamic Therapy. ACS Appl. Bio Mater. 2020, 3, 5463–5473. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Jiao, Q.; Wang, C.; Zheng, Y.; Pan, X.; Zhong, W.; Xu, K. Nanofibrillar peptide hydrogels for self-delivery of lonidamine and synergistic photodynamic therapy. Acta Biomater. 2023, 155, 139–153. [Google Scholar] [CrossRef]
- Gao, Q.; Huang, D.; Deng, Y.; Yu, W.; Jin, Q.; Ji, J.; Fu, G. Chlorin e6 (Ce6)-loaded supramolecular polypeptide micelles with enhanced photodynamic therapy effect against Pseudomonas aeruginosa. Chem. Eng. J. 2021, 417, 129334. [Google Scholar] [CrossRef]
- Zheng, X.; Pan, D.; Chen, X.; Wu, L.; Chen, M.; Wang, W.; Zhang, H.; Gong, Q.; Gu, Z.; Luo, K. Self-Stabilized Supramolecular Assemblies Constructed from PEGylated Dendritic Peptide Conjugate for Augmenting Tumor Retention and Therapy. Adv. Sci. 2021, 8, 2102741. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Shi, B.; Shangguan, L.; Tong, W.; Yu, G.; Mao, Z.; Huang, F. Supramolecular peptide constructed by molecular Lego allowing programmable self-assembly for photodynamic therapy. Nat. Commun. 2019, 10, 2412. [Google Scholar] [CrossRef]
- Li, S.; Zhao, L.; Chang, R.; Xing, R.; Yan, X. Spatiotemporally Coupled Photoactivity of Phthalocyanine–Peptide Conjugate Self-Assemblies for Adaptive Tumor Theranostics. Chem. Eur. J. 2019, 25, 13429–13435. [Google Scholar] [CrossRef]
- Zou, Q.; Chang, R.; Xing, R.; Yuan, C.; Yan, X. Injectable self-assembled bola-dipeptide hydrogels for sustained photodynamic prodrug delivery and enhanced tumor therapy. J. Control Release 2020, 319, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, D.; Jin, Z.; Xia, C.; Xu, Q.; Fan, M.; Dai, Y.; Liu, J.; Li, Y.; He, Q. Light-triggered nitric oxide release and structure transformation of peptide for enhanced intratumoral retention and sensitized photodynamic therapy. Bioact. Mater. 2022, 12, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yu, M.; Yang, X.; Umeshappa, C.S.; Hu, C.; Yu, W.; Qin, L.; Huang, Y.; Gao, H. Linear Chimeric Triblock Molecules Self-Assembled Micelles with Controllably Transformable Property to Enhance Tumor Retention for Chemo-Photodynamic Therapy of Breast Cancer. Adv. Funct. Mater. 2019, 29, 1808462. [Google Scholar] [CrossRef]
- Cheng, Z.; Cheng, Y.; Chen, Q.; Li, M.; Wang, J.; Liu, H.; Li, M.; Ning, Y.; Yu, Z.; Wang, Y.; et al. Self-assembly of pentapeptides into morphology-adaptable nanomedicines for enhanced combinatorial chemo-photodynamic therapy. Nano Today 2020, 33, 100878. [Google Scholar] [CrossRef]
- Li, M.; Ning, Y.; Chen, J.; Duan, X.; Song, N.; Ding, D.; Su, X.; Yu, Z. Proline Isomerization-Regulated Tumor Microenvironment-Adaptable Self-Assembly of Peptides for Enhanced Therapeutic Efficacy. Nano Lett. 2019, 19, 7965–7976. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Chang, R.; Cao, S.; Yuan, C.; Zhao, L.; Yang, H.; Li, J.; Yan, X.; van Hest, J.C.M. Acid-Activatable Transmorphic Peptide-Based Nanomaterials for Photodynamic Therapy. Angew. Chem. Int. Ed. 2020, 59, 20582–20588. [Google Scholar] [CrossRef]
- Zhong, C.; Zhang, L.; Yu, L.; Huang, J.; Huang, S.; Yao, Y. A review for antimicrobial peptides with anticancer properties: Re-purposing of potential anticancer agents. BIO Integr. 2021, 1, 156–167. [Google Scholar] [CrossRef]
- Moret, F.; Gobbo, M.; Reddi, E. Conjugation of photosensitisers to antimicrobial peptides increases the efficiency of photodynamic therapy in cancer cells. Photochem. Photobiol. Sci. 2015, 14, 1238–1250. [Google Scholar] [CrossRef]
- Song, M.; Liu, G.; Liu, Y.; Cheng, Z.; Lin, H.; Liu, J.; Wu, Z.; Xue, J.; Hong, W.; Huang, M.; et al. Using porphyrins as albumin-binding molecules to enhance antitumor efficacies and reduce systemic toxicities of antimicrobial peptides. Eur. J. Med. Chem. 2021, 217, 113382. [Google Scholar] [CrossRef]
- Xu, Y.; Yao, Y.; Wang, L.; Chen, H.; Tan, N. Hyaluronic Acid Coated Liposomes Co-Delivery of Natural Cyclic Peptide RA-XII and Mitochondrial Targeted Photosensitizer for Highly Selective Precise Combined Treatment of Colon Cancer. Int. J. Nanomed. 2021, 16, 4929–4942. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, Y.; Sun, Y.; Wan, C.; Zhang, Z.; Dai, X.; Lin, Z.; He, Q.; Yang, Z.; Huang, P.; et al. Co-delivery of Bee Venom Melittin and a Photosensitizer with an Organic–Inorganic Hybrid Nanocarrier for Photodynamic Therapy and Immunotherapy. ACS Nano 2019, 13, 12638–12652. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhou, Y.; Xu, Y.; Ren, X.; Zhou, S.; Shang, Q.; Jiang, Y.; Luan, Y. Molecular engineering of anti-PD-L1 peptide and photosensitizer for immune checkpoint blockade photodynamic-immunotherapy. Chem. Eng. J. 2020, 400, 125995. [Google Scholar] [CrossRef]
- Qiu, Z.; Lu, Z.; Huang, J.; Zhong, Y.; Yan, N.; Kong, R.; Cheng, H. Self-reinforced photodynamic immunostimulator to downregulate and block PD-L1 for metastatic breast cancer treatment. Biomaterials 2023, 303, 122392. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Jia, S.; Kang, X.; Wu, X.; Hong, Y.; Shan, K.; Kong, X.; Wang, Z.; Ding, D. Semiconducting Polymer Nanoparticles with Surface-Mimicking Protein Secondary Structure as Lysosome-Targeting Chimaeras for Self-Synergistic Cancer Immunotherapy. Adv. Mater. 2022, 34, 2203309. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Hu, J.J.; Dong, X.; Chen, B.; Dong, X.; Liu, R.; Xia, F.; Lou, X. Deep Downregulation of PD-L1 by Caged Peptide-Conjugated AIEgen/miR-140 Nanoparticles for Enhanced Immunotherapy. Angew. Chem. Int. Ed. 2022, 61, e202117798. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, X.; Cheng, Y.; Chen, Z.; Li, X.; Deng, Y. Recent advances of nanomaterials for intervention in Parkinson’s disease in the context of anti-inflammation. Coord. Chem. Rev. 2024, 502, 215616. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Alam, P.; Bai, H.; Bhalla, V.; Bryce, M.R.; Cao, M.; Chen, C.; Chen, S.; Chen, X.; et al. Aggregation-Induced Emission (AIE), Life and Health. ACS Nano 2023, 17, 14347–14405. [Google Scholar] [CrossRef]










| Peptide Sequence (Name) | Targeting Receptor | Types of Cancer Cells | References |
|---|---|---|---|
| QRHKPRE (QRH) | Epidermal growth factor receptor (EGFR) | Lung cancer, colon cancer, breast cancer, kidney cancer, head and neck cancer, glioma, etc. | [67,74] |
| YHWYGYTPQNVI (GE11) | [75] | ||
| CMYIEALDKYAC (N.A.) | [76] | ||
| WxEAAYQrFL (peptide 18-4) | Keratin 1 (KRT1) | breast cancer | [72] |
| LQNAPRS (N.A.) | CD133 | Colorectal cancer | [77] |
| anti-HER2 peptide | Human epidermal growth factor receptor 2 (HER2) | HER-2 positive breast cancer | [74,78,79] |
| cyclo-[2-NaI-Gly-d-Tyr-Arg-Arg] (FC131) | CXCR4, a cell-surface chemokine receptor | Breast cancer, ovarian cancer, lung cancer, colorectal cancer, primary brain tumors, etc. | [80] |
| KSD-cha-FskYLWSSK(AE147) | Urokinase-type plasminogen activator receptor (uPAR) | Aggressive cancer cells such as breast, prostate, glioma, colorectal, endometrial, bladder, liver, and melanoma cancer | [81] |
| KDKPPR (N.A.) | NRP-1 | Glioma, acute myeloid leukemia, pancreatic cancer, lung cancer, ovarian cancer, gastrointestinal tumors, melanoma, etc. | [66] |
| EHWSYGLRPG (N.A.) | Gonadotropin-releasing hormone receptor (GnRH-R) | Head and neck squamous cell carcinomas | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Ming, R.; Huang, L.; Zhang, R. Versatile Peptide-Based Nanosystems for Photodynamic Therapy. Pharmaceutics 2024, 16, 218. https://doi.org/10.3390/pharmaceutics16020218
Li Q, Ming R, Huang L, Zhang R. Versatile Peptide-Based Nanosystems for Photodynamic Therapy. Pharmaceutics. 2024; 16(2):218. https://doi.org/10.3390/pharmaceutics16020218
Chicago/Turabian StyleLi, Qiuyan, Ruiqi Ming, Lili Huang, and Ruoyu Zhang. 2024. "Versatile Peptide-Based Nanosystems for Photodynamic Therapy" Pharmaceutics 16, no. 2: 218. https://doi.org/10.3390/pharmaceutics16020218
APA StyleLi, Q., Ming, R., Huang, L., & Zhang, R. (2024). Versatile Peptide-Based Nanosystems for Photodynamic Therapy. Pharmaceutics, 16(2), 218. https://doi.org/10.3390/pharmaceutics16020218