Liposome Encapsulation of the Palmitoyl–KTTKS Peptide: Structural and Functional Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liposome Preparation and Characterization
2.3. Physicochemical Characterization of Liposomes
2.3.1. Determination of Hydrodynamic Diameter and Size Distribution
2.3.2. Determination of ζ-Potential
2.3.3. Phospholipid Assay
2.3.4. Effect of Culture Medium on Vesicle Stability
2.4. Cell Culture and Cytotoxicity Assays
2.5. Sirius Red Assay for Collagen Detection
2.6. Statistical Analysis
3. Results and Discussion
3.1. Liposome Preparation and Characterization
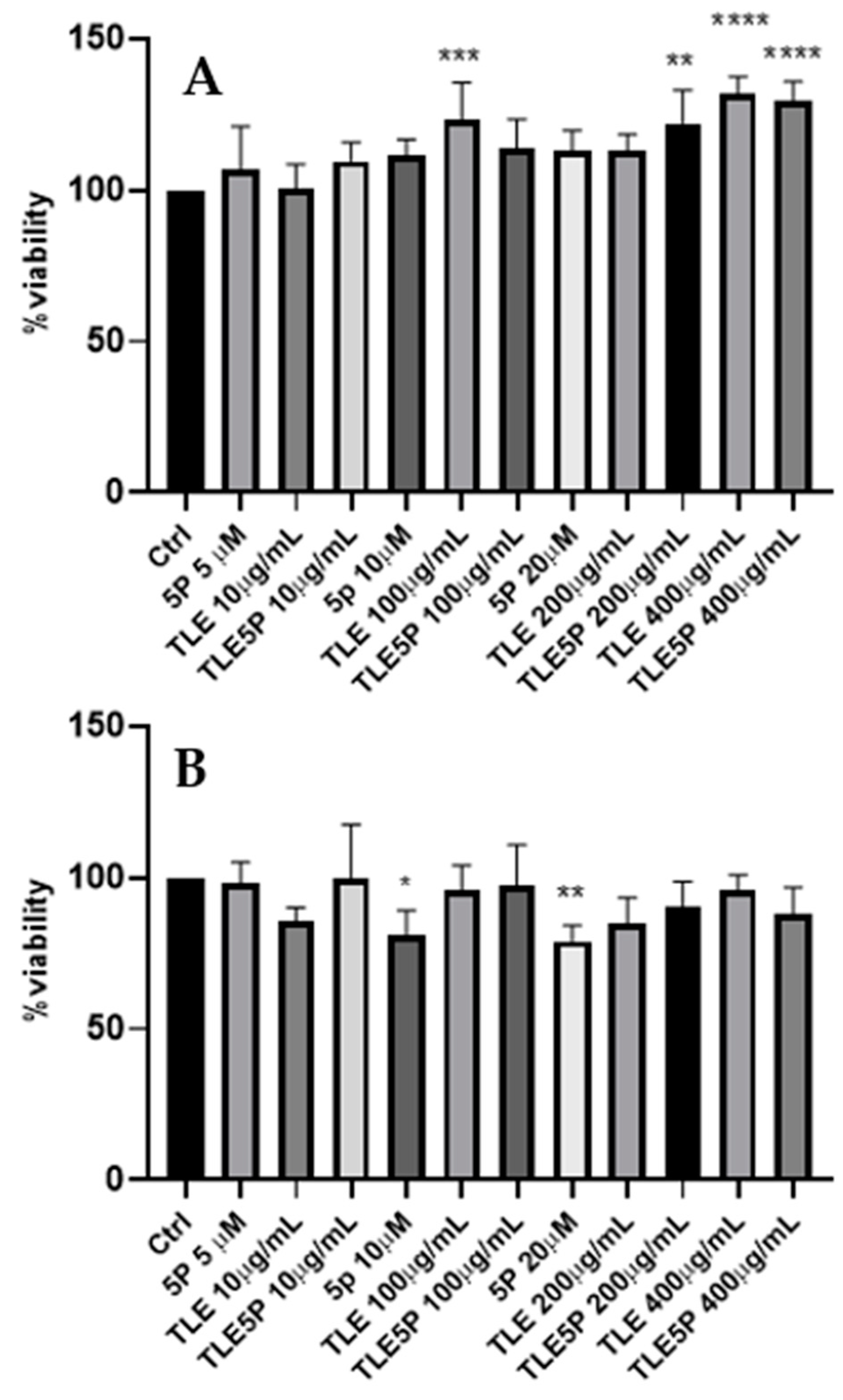
3.2. Cytotoxicity Assay
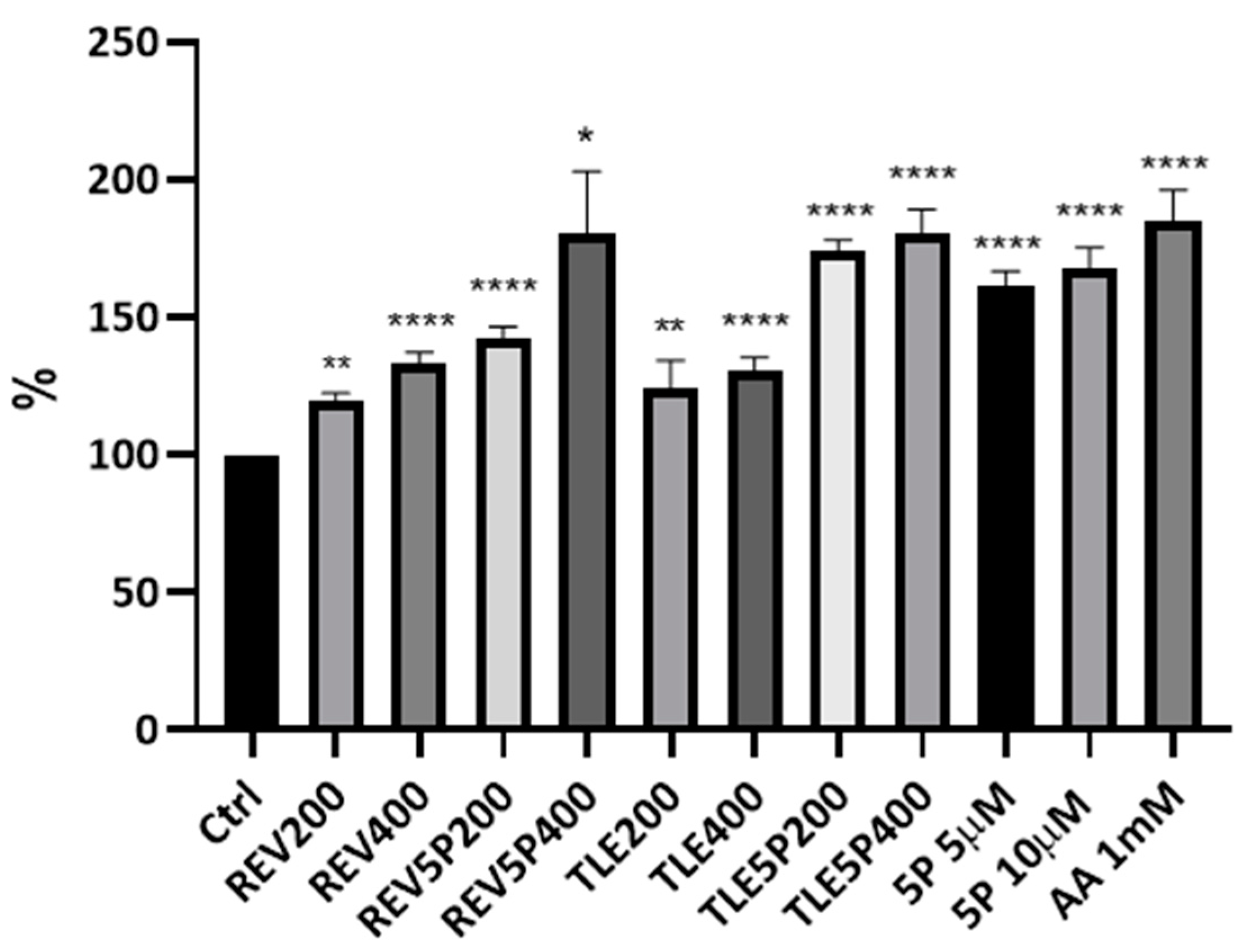
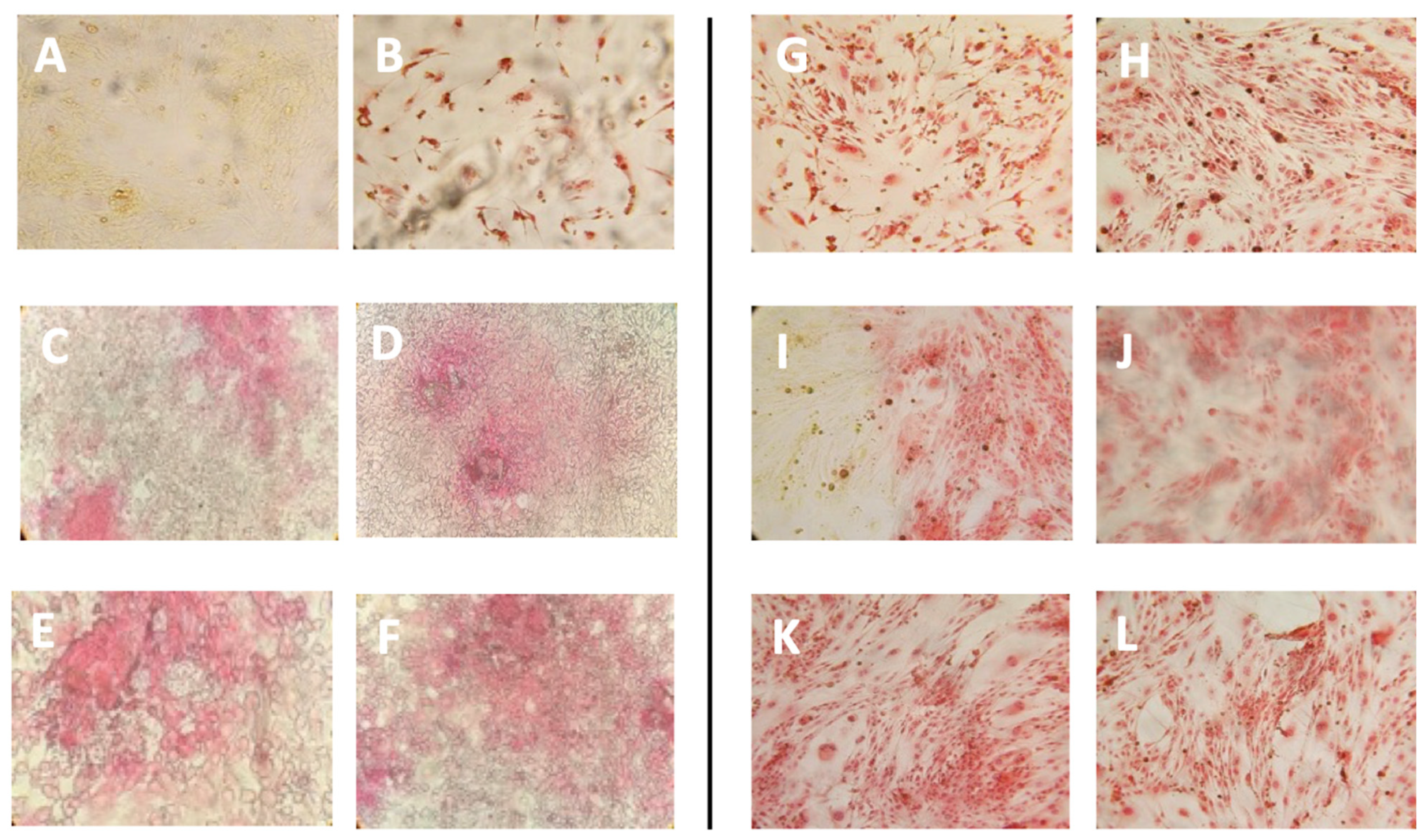
Collagen Production upon Liposome Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fligiel, S.E.G.; Varani, J.; Datta, S.C.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Collagen Degradation in Aged/Photodamaged Skin In Vivo and After Exposure to Matrix Metalloproteinase-1 In Vitro. J. Investig. Dermatol. 2003, 120, 842–848. [Google Scholar] [CrossRef]
- De Castro Brás, L.E.; Frangogiannis, N.G. Extracellular Matrix-Derived Peptides in Tissue Remodeling and Fibrosis. Matrix Biol. 2020, 91–92, 176–187. [Google Scholar] [CrossRef]
- Iavarone, F.; Desiderio, C.; Vitali, A.; Messana, I.; Martelli, C.; Castagnola, M.; Cabras, T. Cryptides: Latent Peptides Everywhere. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 246–263. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.L.; Dunn, M.K. Therapeutic Peptides: Historical Perspectives, Current Development Trends, and Future Directions. Bioorganic Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Armendariz-Borunda, J.; Raghow, R.; Kang, A.H.; Seyer, J.M. A Pentapeptide from Type I Procollagen Promotes Extracellular Matrix Production. J. Biol. Chem. 1993, 268, 9941–9944. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.; Hsu, C.; Chung, C.; Lin, M.; Li, S.; Pang, J.S. The Pentapeptide KTTKS Promoting the Expressions of Type I Collagen and Transforming Growth Factor-β of Tendon Cells. J. Orthop. Res. 2007, 25, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; An, E.; Cho Lee, A.-R. Effect of Palmitoyl-Pentapeptide (Pal-KTTKS) on Wound Contractile Process in Relation with Connective Tissue Growth Factor and α-Smooth Muscle Actin Expression. Tissue Eng. Regen. Med. 2017, 14, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Monboisse, J.C.; Oudart, J.B.; Ramont, L.; Brassart-Pasco, S.; Maquart, F.X. Matrikines from Basement Membrane Collagens: A New Anti-Cancer Strategy. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, J.; Ehrhardt, C. Liposomal Delivery of Proteins and Peptides. Expert Opin. Drug Deliv. 2012, 9, 1489–1503. [Google Scholar] [CrossRef]
- Guillot, A.J.; Martínez-Navarrete, M.; Garrigues, T.M.; Melero, A. Skin Drug Delivery Using Lipid Vesicles: A Starting Guideline for Their Development. J. Control. Release 2023, 355, 624–654. [Google Scholar] [CrossRef]
- Abu Samah, N.H.; Heard, C.M. Topically Applied KTTKS: A Review. Int. J. Cosmet. Sci. 2011, 33, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.L.; Park, E.J.; Kim, E.; Na, D.H.; Shin, Y.-H. Dermal Stability and In Vitro Skin Permeation of Collagen Pentapeptides (KTTKS and Palmitoyl-KTTKS). Biomol. Ther. 2014, 22, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.M.; Moghimi, H.R. Skin Permeability, a Dismissed Necessity for Anti-Wrinkle Peptide Performance. Int. J. Cosmet. Sci. 2022, 44, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Tałałaj, U.; Uścinowicz, P.; Bruzgo, I.; Surażyński, A.; Zaręba, I.; Markowska, A. The Effects of a Novel Series of KTTKS Analogues on Cytotoxicity and Proteolytic Activity. Molecules 2019, 24, 3698. [Google Scholar] [CrossRef] [PubMed]
- Phatale, V.; Vaiphei, K.K.; Jha, S.; Patil, D.; Agrawal, M.; Alexander, A. Overcoming Skin Barriers through Advanced Transdermal Drug Delivery Approaches. J. Control. Release 2022, 351, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kumar, A. Transfersomes: The Ultra-Deformable Carrier System for Non-Invasive Delivery of Drug. CDD 2021, 18, 408–420. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Jones, R.R.; Castelletto, V.; Connon, C.J.; Hamley, I.W. Collagen Stimulating Effect of Peptide Amphiphile C 16 –KTTKS on Human Fibroblasts. Mol. Pharm. 2013, 10, 1063–1069. [Google Scholar] [CrossRef]
- Mortazavi, S.M.; Kobarfard, F.; Maibach, H.I.; Moghimi, H.R. Effect of Palmitic Acid Conjugation on Physicochemical Properties of Peptide KTTKS: A Preformulation Study. J. Cosmet. Sci. 2019, 70, 299–312. [Google Scholar]
- Trilli, J.; Caramazza, L.; Paolicelli, P.; Casadei, M.A.; Liberti, M.; Apollonio, F.; Petralito, S. The Impact of Bilayer Rigidity on the Release from Magnetoliposomes Vesicles Controlled by PEMFs. Pharmaceutics 2021, 13, 1712. [Google Scholar] [CrossRef]
- Petralito, S.; Paolicelli, P.; Nardoni, M.; Trilli, J.; Di Muzio, L.; Cesa, S.; Relucenti, M.; Matassa, R.; Vitalone, A.; Adrover, A.; et al. Gelation of the Internal Core of Liposomes as a Strategy for Stabilization and Modified Drug Delivery I. Physico-Chemistry Study. Int. J. Pharm. 2020, 585, 119467. [Google Scholar] [CrossRef]
- Szoka, F.; Papahadjopoulos, D. Comparative Properties and Methods of Preparation of Lipid Vesicles (Liposomes). Annu. Rev. Biophys. Bioeng. 1980, 9, 467–508. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Furuya, E.; Tagawa, K. A Direct Colorimetric Method for the Determination of Phospholipids with Dithiocyanatoiron Reagent. J. Biochem. 1980, 88, 463–468. [Google Scholar] [CrossRef]
- Trackman, P.C.; Saxena, D.; Bais, M.V. TGF-Β1- and CCN2-Stimulated Sirius Red Assay for Collagen Accumulation in Cultured Cells. In CCN Proteins; Methods in Molecular Biology; Takigawa, M., Ed.; Springer: New York, NY, USA, 2017; Volume 1489, pp. 481–485. ISBN 978-1-4939-6428-4. [Google Scholar]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Wang, N.; Chen, M.; Wang, T. Liposomes Used as a Vaccine Adjuvant-Delivery System: From Basics to Clinical Immunization. J. Control. Release 2019, 303, 130–150. [Google Scholar] [CrossRef]
- Katayama, K.; Seyer, J.M.; Raghow, R.; Kang, A.H. Regulation of Extracellular Matrix Production by Chemically Synthesized Subfragments of Type I Collagen Carboxy Propeptide. Biochemistry 1991, 30, 7097–7104. [Google Scholar] [CrossRef]
- Szász, C.; Pap, D.; Szebeni, B.; Bokrossy, P.; Őrfi, L.; Szabó, A.J.; Vannay, Á.; Veres-Székely, A. Optimization of Sirius Red-Based Microplate Assay to Investigate Collagen Production In Vitro. Int. J. Mol. Sci. 2023, 24, 17435. [Google Scholar] [CrossRef] [PubMed]
- Maione-Silva, L.; De Castro, E.G.; Nascimento, T.L.; Cintra, E.R.; Moreira, L.C.; Cintra, B.A.S.; Valadares, M.C.; Lima, E.M. Ascorbic Acid Encapsulated into Negatively Charged Liposomes Exhibits Increased Skin Permeation, Retention and Enhances Collagen Synthesis by Fibroblasts. Sci. Rep. 2019, 9, 522. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.L.; Combs, S.B.; Pinnell, S.R. Effects of Ascorbic Acid on Proliferation and Collagen Synthesis in Relation to the Donor Age of Human Dermal Fibroblasts. J. Investig. Dermatol. 1994, 103, 228–232. [Google Scholar] [CrossRef]
- Gomes, A.; Bessa, L.J.; Fernandes, I.; Aguiar, L.; Ferraz, R.; Monteiro, C.; Martins, M.C.L.; Mateus, N.; Gameiro, P.; Teixeira, C.; et al. Boosting Cosmeceutical Peptides: Coupling Imidazolium-Based Ionic Liquids to Pentapeptide-4 Originates New Leads with Antimicrobial and Collagenesis-Inducing Activities. Microbiol. Spectr. 2022, 10, e02291-21. [Google Scholar] [CrossRef]
- Yang, J.; Azuar, A.; Toth, I.; Skwarczynski, M. Liposomes for the Delivery of Lipopeptide Vaccines. In Vaccine Design; Methods in Molecular Biology; Thomas, S., Ed.; Springer: New York, NY, USA, 2022; Volume 2412, pp. 295–307. ISBN 978-1-07-161891-2. [Google Scholar]
- Kato, N.; Yamada, S.; Suzuki, R.; Iida, Y.; Matsumoto, M.; Fumoto, S.; Arima, H.; Mukai, H.; Kawakami, S. Development of an Apolipoprotein E Mimetic Peptide–Lipid Conjugate for Efficient Brain Delivery of Liposomes. Drug Deliv. 2023, 30, 2173333. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.H.; Yamada, M.; Lin, L.L.; Grice, J.E.; Roberts, M.S.; Raphael, A.P.; Benson, H.A.E.; Prow, T.W. Microneedle Enhanced Delivery of Cosmeceutically Relevant Peptides in Human Skin. PLoS ONE 2014, 9, e101956. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ping, Q.; Zhang, L. Transdermal Delivery of Insulin in Mice by Using Lecithin Vesicles as a Carrier. Drug Deliv. 2000, 7, 113–116. [Google Scholar] [CrossRef] [PubMed]
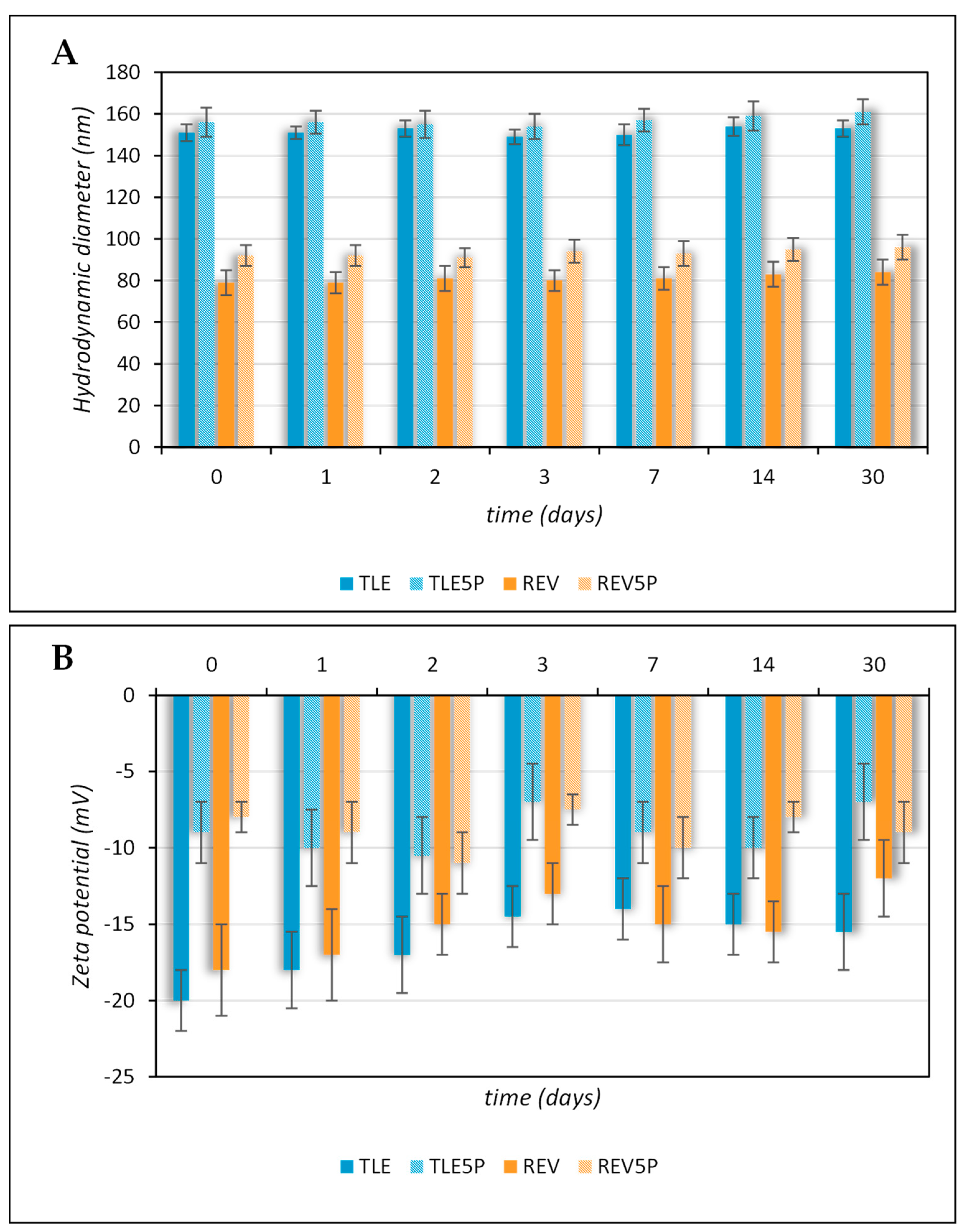
| Sample | Hydrodynamic Diameter a Mean (SD) | PdI b Mean (SD) | Zeta Potential c Mean (SD) | % of Structured Phospholipid d |
|---|---|---|---|---|
| TLE | 151 (4) nm | 0.11 (0.08) | −20.58 (1.85) mV | 94 |
| REV | 80 (6) nm | 0.19 (0.04) | −18.58 (3.00) mV | 95 |
| TLE5P | 156 (7) nm | 0.11 (0.02) | −9.08 (0.65) mV | 92 |
| REV5P | 92 (5) nm | 0.19 (0.01) | −8.58 (0.65) mV | 91 |
| Sample | t0 | t24 h | t48 h | ||||
|---|---|---|---|---|---|---|---|
| HEPES | DMEM | HEPES | DMEM | HEPES | DMEM | ||
| TLE | Hydrodynamic Diameter (nm) | 157 | 138 | 155 | 144 | 148 | 142 |
| PdI | 0.09 | 0.30 | 0.08 | 0.20 | 0.09 | 0.2 | |
| TLE5P | Hydrodynamic Diameter (nm) | 182 | 134 | 161 | 143 | 169 | 144 |
| PdI | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.10 | |
| REV | Hydrodynamic Diameter (nm) | 86 | 79 | 89 | 126 | 88 | 137 |
| PdI | 0.20 | 0.30 | 0.20 | 0.20 | 0.20 | 0.20 | |
| REV5P | Hydrodynamic Diameter (nm) | 84 | 73 | 83 | 106 | 86 | 111 |
| PdI | 0.20 | 0.30 | 0.20 | 0.20 | 0.20 | 0.20 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitali, A.; Paolicelli, P.; Bigi, B.; Trilli, J.; Di Muzio, L.; Carriero, V.C.; Casadei, M.A.; Petralito, S. Liposome Encapsulation of the Palmitoyl–KTTKS Peptide: Structural and Functional Characterization. Pharmaceutics 2024, 16, 219. https://doi.org/10.3390/pharmaceutics16020219
Vitali A, Paolicelli P, Bigi B, Trilli J, Di Muzio L, Carriero VC, Casadei MA, Petralito S. Liposome Encapsulation of the Palmitoyl–KTTKS Peptide: Structural and Functional Characterization. Pharmaceutics. 2024; 16(2):219. https://doi.org/10.3390/pharmaceutics16020219
Chicago/Turabian StyleVitali, Alberto, Patrizia Paolicelli, Barbara Bigi, Jordan Trilli, Laura Di Muzio, Vito Cosimo Carriero, Maria Antonietta Casadei, and Stefania Petralito. 2024. "Liposome Encapsulation of the Palmitoyl–KTTKS Peptide: Structural and Functional Characterization" Pharmaceutics 16, no. 2: 219. https://doi.org/10.3390/pharmaceutics16020219
APA StyleVitali, A., Paolicelli, P., Bigi, B., Trilli, J., Di Muzio, L., Carriero, V. C., Casadei, M. A., & Petralito, S. (2024). Liposome Encapsulation of the Palmitoyl–KTTKS Peptide: Structural and Functional Characterization. Pharmaceutics, 16(2), 219. https://doi.org/10.3390/pharmaceutics16020219






