Cannabinoid-Induced Stereoselective Inhibition of R-S-Oxazepam Glucuronidation: Cannabinoid–Oxazepam Drug Interactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. UGT-Overexpressing HEK293 Cell Lines (rUGT) and Microsomal Preparation
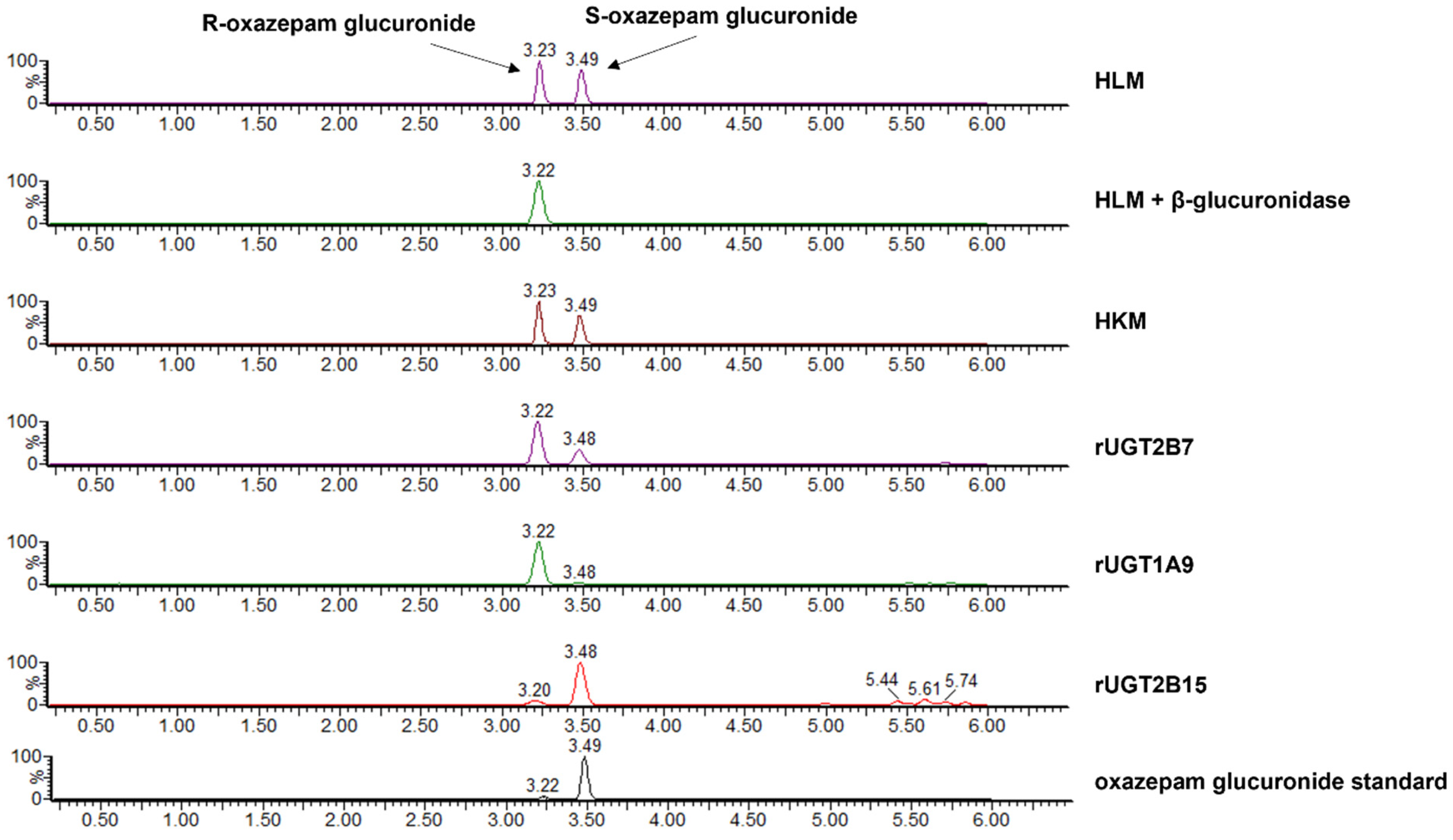
2.3. Separation of R- and S-Oxazepam Glucuronides Using LC–MS/MS
2.4. In Vitro Inhibition Assays of R- and S-Oxazepam Glucuronidation
2.5. Identification of R,S-Oxazepam Glucuronides by β-Glucuronidase Hydrolysis
2.6. Prediction of Potential In Vivo DDIs Based on In Vitro–In Vivo Extrapolation (IVIVE)
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dinis-Oliveira, R.J. Metabolic profile of oxazepam and related benzodiazepines: Clinical and forensic aspects. Drug Metab. Rev. 2017, 49, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Court, M.H.; Duan, S.X.; Guillemette, C.; Journault, K.; Krishnaswamy, S.; Von Moltke, L.L.; Greenblatt, D.J. Stereoselective conjugation of oxazepam by human UDP-glucuronosyltransferases (UGTs): S-oxazepam is glucuronidated by UGT2B15, while R-oxazepam is glucuronidated by UGT2B7 and UGT1A9. Drug Metab. Dispos. 2002, 30, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Tang, B.K.; Kalow, W. (S)oxazepam glucuronidation is inhibited by ketoprofen and other substrates of UGT2B7. Pharmacogenetics 1995, 5, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, D.R.; Greenblatt, D.J.; Divoll, M.; Shader, R.I. Enhanced glucuronide conjugation of drugs in obesity: Studies of lorazepam, oxazepam, and acetaminophen. J. Lab. Clin. Med. 1983, 101, 873–880. [Google Scholar] [PubMed]
- Uchaipichat, V.; Suthisisang, C.; Miners, J.O. The glucuronidation of R- and S-lorazepam: Human liver microsomal kinetics, UDP-glucuronosyltransferase enzyme selectivity, and inhibition by drugs. Drug Metab. Dispos. 2013, 41, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hesse, L.M.; Hazarika, S.; Masse, G.; Harmatz, J.S.; Greenblatt, D.J.; Court, M.H. Evidence for oxazepam as an in vivo probe of UGT2B15: Oxazepam clearance is reduced by UGT2B15 D85Y polymorphism but unaffected by UGT2B17 deletion. Br. J. Clin. Pharmacol. 2009, 68, 721–730. [Google Scholar] [CrossRef]
- Hok, L.; BoŽičević, L.; Sremec, H.; Šakić, D.; Vrček, V. Racemization of oxazepam and chiral 1,4-benzodiazepines. DFT study of the reaction mechanism in aqueous solution. Org. Biomol. Chem. 2019, 17, 1471–1479. [Google Scholar] [CrossRef]
- Patel, M.; Tang, B.K.; Grant, D.M.; Kalow, W. Interindividual variability in the glucuronidation of (S) oxazepam contrasted with that of (R) oxazepam. Pharmacogenetics 1995, 5, 287–297. [Google Scholar] [CrossRef]
- Schmitz, A. Benzodiazepine use, misuse, and abuse: A review. Ment. Health Clin. 2016, 6, 120–126. [Google Scholar] [CrossRef]
- Authier, N.; Balayssac, D.; Sautereau, M.; Zangarelli, A.; Courty, P.; Somogyi, A.A.; Vennat, B.; Llorca, P.M.; Eschalier, A. Benzodiazepine dependence: Focus on withdrawal syndrome. Ann. Pharm. Fr. 2009, 67, 408–413. [Google Scholar] [CrossRef]
- Kogan, N.M.; Mechoulam, R. Cannabinoids in health and disease. Dialogues Clin. Neurosci. 2007, 9, 413–430. [Google Scholar] [CrossRef]
- Bridgeman, M.B.; Abazia, D.T. Medicinal Cannabis: History, Pharmacology, and Implications for the Acute Care Setting. Pharm. Ther. 2017, 42, 180–188. [Google Scholar]
- Jones, C.M.; McAninch, J.K. Emergency Department Visits and Overdose Deaths from Combined Use of Opioids and Benzodiazepines. Am. J. Prev. Med. 2015, 49, 493–501. [Google Scholar] [CrossRef]
- Sun, E.C.; Dixit, A.; Humphreys, K.; Darnall, B.D.; Baker, L.C.; Mackey, S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: Retrospective analysis. BMJ 2017, 356, j760. [Google Scholar] [CrossRef]
- Tanaka, E. Clinically significant pharmacokinetic drug interactions with benzodiazepines. J. Clin. Pharm. Ther. 1999, 24, 347–355. [Google Scholar] [CrossRef]
- Shah, K.N.; Rana, D.A.; Patel, V.J. Study of potential drug-drug interactions between benzodiazepines and four commonly used antiepileptic drugs in mice. Int. J. Basic Clin. Pharmacol. 2017, 3, 830–835. [Google Scholar] [CrossRef]
- Citti, C.; Linciano, P.; Russo, F.; Luongo, L.; Iannotta, M.; Maione, S.; Laganà, A.; Capriotti, A.L.; Forni, F.; Vandelli, M.A.; et al. A Novel Phytocannabinoid Isolated From Cannabis sativa L. With an in vivo Cannabimimetic Activity Higher Than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Sci. Rep. 2019, 9, 20335. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S163–S171. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Murthy, P.; Bharath, M.M.S. Chemistry, metabolism, and toxicology of cannabis: Clinical implications. Iran. J. Psychiatry 2012, 7, 149–156. [Google Scholar] [PubMed]
- Bansal, S.; Paine, M.F.; Unadkat, J.D. Comprehensive Predictions of Cytochrome P450 (P450)-Mediated In Vivo Cannabinoid-Drug Interactions Based on Reversible and Time-Dependent P450 Inhibition in Human Liver Microsomes. Drug Metab. Dispos. 2022, 50, 351–360. [Google Scholar] [CrossRef]
- Meiri, E.; Jhangiani, H.; Vredenburgh, J.J.; Barbato, L.M.; Carter, F.J.; Yang, H.M.; Baranowski, V. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr. Med. Res. Opin. 2007, 23, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.N.; Dulberg, Z.; Chan, A.W.; Hare, G.M.; Mazer, C.D.; Hong, A. A randomized-controlled trial of nabilone for the prevention of acute postoperative nausea and vomiting in elective surgery. Can. J. Anaesth. 2017, 64, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.; Gunderson, E.W.; Rabkin, J.; Hart, C.L.; Vosburg, S.K.; Comer, S.D.; Foltin, R.W. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J. Acquir. Immune Defic. Syndr. 2007, 45, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.A.-O.; Navarra, G.; Coppola, L.; Avilia, G.; Bifulco, M.; Laezza, C. Cannabinoids: Therapeutic Use in Clinical Practice. Int. J. Mol. Sci. 2022, 23, 3344. [Google Scholar] [CrossRef]
- Bergamaschi, M.M.; Queiroz, R.H.; Chagas, M.H.; de Oliveira, D.C.; De Martinis, B.S.; Kapczinski, F.; Quevedo, J.; Roesler, R.; Schröder, N.; Nardi, A.E.; et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology 2011, 36, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Pennypacker, S.D.; Romero-Sandoval, E.A. CBD and THC: Do They Complement Each Other Like Yin and Yang? Pharmacotherapy 2020, 40, 1152–1165. [Google Scholar] [CrossRef] [PubMed]
- Boggs, D.L.; Nguyen, J.D.; Morgenson, D.; Taffe, M.A.; Ranganathan, M. Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Δ9-Tetrahydrocannabinol. Neuropsychopharmacology 2018, 43, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Nasrin, S.; Coates, S.; Bardhi, K.; Watson, C.; Muscat, J.E.; Lazarus, P. Inhibition of Nicotine Metabolism by Cannabidiol (CBD) and 7-Hydroxycannabidiol (7-OH-CBD). Chem. Res. Toxicol. 2023, 36, 177–187. [Google Scholar] [CrossRef]
- Bland, T.M.; Haining, R.L.; Tracy, T.S.; Callery, P.S. CYP2C-catalyzed Δ9-tetrahydrocannabinol metabolism: Kinetics, pharmacogenetics and interaction with phenytoin. Biochem. Pharmacol. 2005, 70, 1096–1103. [Google Scholar] [CrossRef]
- Watanabe, K.; Yamaori, S.; Funahashi, T.; Kimura, T.; Yamamoto, I. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci. 2007, 80, 1415–1419. [Google Scholar] [CrossRef]
- Matsunaga, T.; Iwawaki, Y.; Watanabe, K.; Yamamoto, I.; Kageyama, T.; Yoshimura, H. Metabolism of Δ9-tetrahydrocannabinol by cytochrome P450 isozymes purified from hepatic microsomes of monkeys. Life Sci. 1995, 56, 2089–2095. [Google Scholar] [CrossRef]
- Jiang, R.; Yamaori, S.; Okamoto, Y.; Yamamoto, I.; Watanabe, K. Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab. Pharmacokinet. 2013, 28, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Beers, J.L.; Fu, D.; Jackson, K.D. Cytochrome P450-Catalyzed Metabolism of Cannabidiol to the Active Metabolite 7-Hydroxy-Cannabidiol. Drug Metab. Dispos. 2021, 49, 882–891. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Gidal, B.; Blakey, G.; Tayo, B.; Morrison, G. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs 2018, 32, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Maharao, N.; Paine, M.F.; Unadkat, J.D. Predicting the Potential for Cannabinoids to Precipitate Pharmacokinetic Drug Interactions via Reversible Inhibition or Inactivation of Major Cytochromes P450. Drug Metab. Dispos. 2020, 48, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Wang, X.; Markowitz, J.S. In Vitro Inhibition of Carboxylesterase 1 by Major Cannabinoids and Selected Metabolites. Drug Metab. Dispos. 2019, 47, 465–472. [Google Scholar] [CrossRef]
- Nasrin, S.; Watson, C.J.W.; Bardhi, K.; Fort, G.; Chen, G.; Lazarus, P. Inhibition of UDP-Glucuronosyltransferase Enzymes by Major Cannabinoids and Their Metabolites. Drug Metab. Dispos. 2021, 49, 1081–1089. [Google Scholar] [CrossRef]
- Nasrin, S.; Watson, C.J.W.; Perez-Paramo, Y.X.; Lazarus, P. Cannabinoid Metabolites as Inhibitors of Major Hepatic CYP450 Enzymes, with Implications for Cannabis-Drug Interactions. Drug Metab. Dispos. 2021, 49, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.L.; Absalom, N.L.; Abelev, S.V.; Low, I.K.; Doohan, P.T.; Martin, L.J.; Chebib, M.; McGregor, I.S.; Arnold, J.C. Coadministered cannabidiol and clobazam: Preclinical evidence for both pharmacodynamic and pharmacokinetic interactions. Epilepsia 2019, 60, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N.; Szaflarski, J.P.; Gidal, B.; VanLandingham, K.; Critchley, D.; Morrison, G. Clinical implications of trials investigating drug-drug interactions between cannabidiol and enzyme inducers or inhibitors or common antiseizure drugs. Epilepsia 2020, 61, 1854–1868. [Google Scholar] [CrossRef] [PubMed]
- Geffrey, A.L.; Pollack, S.F.; Bruno, P.L.; Thiele, E.A. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015, 56, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.W.; Fang, J.L.; Chen, G.; Weinberg, R.; Lazarus, P. Importance of UDP-glucuronosyltransferase 1A10 (UGT1A10) in the detoxification of polycyclic aromatic hydrocarbons: Decreased glucuronidative activity of the UGT1A10139Lys isoform. Drug Metab. Dispos. 2006, 34, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Sharma, A.K.; Dellinger, R.W.; Blevins-Primeau, A.S.; Balliet, R.M.; Chen, G.; Boyiri, T.; Amin, S.; Lazarus, P. Glucuronidation of active tamoxifen metabolites by the human UDP glucuronosyltransferases. Drug Metab. Dispos. 2007, 35, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.; Xia, Z.; Chen, G.; Lazarus, P. Exemestane potency is unchanged by common nonsynonymous polymorphisms in CYP19A1: Results of a novel anti-aromatase activity assay examining exemestane and its derivatives. Pharmacol. Res. Perspect. 2017, 5, e00313. [Google Scholar] [CrossRef] [PubMed]
- Ruelius, H.W.; Tio, C.O.; Knowles, J.A.; McHugh, S.L.; Schillings, R.T.; Sisenwine, S.F. Diastereoisomeric glucuronides of oxazepam. Isolation and stereoselective enzymic hydrolysis. Drug Metab. Dispos. 1979, 7, 40–43. [Google Scholar]
- Cer, R.Z.; Mudunuri, U.; Stephens, R.; Lebeda, F.J. IC50-to-Ki: A web-based tool for converting IC50 to Ki values for inhibitors of enzyme activity and ligand binding. Nucleic Acids Res. 2009, 37, 441–445. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Jia, Y.; Yin, H.; Feng, Y.; Jiang, L.; Cao, J.; Liu, Y. Inhibition of human UDP-glucuronosyltransferase enzymes by midostaurin and ruxolitinib: Implications for drug-drug interactions. Biopharm. Drug Dispos. 2020, 41, 231–238. [Google Scholar] [CrossRef]
- Miners, J.O.; Polasek, T.M.; Hulin, J.A.; Rowland, A.; Meech, R. Drug-drug interactions that alter the exposure of glucuronidated drugs: Scope, UDP-glucuronosyltransferase (UGT) enzyme selectivity, mechanisms (inhibition and induction), and clinical significance. Pharmacol. Ther. 2023, 248, 108459. [Google Scholar] [CrossRef] [PubMed]
- In Vitro Drug Interaction Studies Cytochrome P450 Enzymes and Transporter Mediated Drug Interactions. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/in-vitro-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions#:~:text=Guidance%20for%20Industry-,In%20Vitro%20Drug%20Interaction%20Studies%20%E2%80%94%20Cytochrome%20P450%20Enzyme%2D%20and%20Transporter,Guidance%20for%20Industry%20January%202020&text=This%20final%20guidance%20is%20intended,of%20an%20investigational%20drug%20product (accessed on 4 October 2023).
- Docci, L.; Umehara, K.; Krähenbühl, S.; Fowler, S.; Parrott, N. Construction and Verification of Physiologically Based Pharmacokinetic Models for Four Drugs Majorly Cleared by Glucuronidation: Lorazepam, Oxazepam, Naloxone, and Zidovudine. AAPS J. 2020, 22, 128. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Iwatsubo, T.; Kanamitsu, S.; Ueda, K.; Suzuki, H.; Sugiyama, Y. Prediction of pharmacokinetic alterations caused by drug-drug interactions: Metabolic interaction in the liver. Pharmacol. Rev. 1998, 50, 387–412. [Google Scholar] [PubMed]
- Garrett, E.R.; Hunt, C.A. Physiochemical properties, solubility, and protein binding of Δ9-tetrahydrocannabinol. J. Pharm. Sci. 1974, 63, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Skopp, G.; Pötsch, L.; Mauden, M.; Richter, B. Partition coefficient, blood to plasma ratio, protein binding and short-term stability of 11-nor-Δ9-carboxy tetrahydrocannabinol glucuronide. Forensic Sci. Int. 2002, 126, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Cox, E.J.; Maharao, N.; Patilea-Vrana, G.; Unadkat, J.D.; Rettie, A.E.; McCune, J.S.; Paine, M.F. A marijuana-drug interaction primer: Precipitants, pharmacology, and pharmacokinetics. Pharmacol. Ther. 2019, 201, 25–38. [Google Scholar] [CrossRef]
- Schwilke, E.W.; Schwope, D.M.; Karschner, E.L.; Lowe, R.H.; Darwin, W.D.; Kelly, D.L.; Goodwin, R.S.; Gorelick, D.A.; Huestis, M.A. Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC. Clin. Chem. 2009, 55, 2180–2189. [Google Scholar] [CrossRef]
- Bansal, S.; Zamarripa, C.A.; Spindle, T.R.; Weerts, E.M.; Thummel, K.E.; Vandrey, R.; Paine, M.F.; Unadkat, J.D. Evaluation of Cytochrome P450-Mediated Cannabinoid-Drug Interactions in Healthy Adult Participants. Clin. Pharmacol. Ther. 2023, 114, 693–703. [Google Scholar] [CrossRef]
- Anderson, L.L.; Doohan, P.T.; Oldfield, L.; Kevin, R.C.; Arnold, J.C.; Berger, M.; Amminger, G.P.; McGregor, I.S. Citalopram and Cannabidiol: In Vitro and In Vivo Evidence of Pharmacokinetic Interactions Relevant to the Treatment of Anxiety Disorders in Young People. J. Clin. Psychopharmacol. 2021, 41, 525–533. [Google Scholar] [CrossRef]
- Kasteel, E.E.J.; Darney, K.; Kramer, N.I.; Dorne, J.L.C.M.; Lautz, L.S. Human variability in isoform-specific UDP-glucuronosyltransferases: Markers of acute and chronic exposure, polymorphisms and uncertainty factors. Arch. Toxicol. 2020, 94, 2637–2661. [Google Scholar] [CrossRef]
- Rowland, A.; Miners, J.O.; Mackenzie, P.I. The UDP-glucuronosyltransferases: Their role in drug metabolism and detoxification. Int. J. Biochem. Cell Biol. 2013, 45, 1121–1132. [Google Scholar] [CrossRef]
- Nadulski, T.; Pragst, F.; Weinberg, G.; Roser, P.; Schnelle, M.; Fronk, E.-M.; Stadelmann, A.M. Randomized, Double-Blind, Placebo-Controlled Study about the Effects of Cannabidiol (CBD) on the Pharmacokinetics of Δ9-Tetrahydrocannabinol (THC) After Oral Application of THC Verses Standardized Cannabis Extract. Ther. Drug Monit. 2005, 27, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A.; Henningfield, J.E.; Cone, E.J. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J. Anal. Toxicol. 1992, 16, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, J.E.; Ohlsson, A.; Agurell, S.; Hollister, L.; Gillespie, H. Clinical effects and plasma levels of Δ9-tetrahydrocannabinol (Δ9-THC) in heavy and light users of cannabis. Psychopharmacology 1981, 74, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Lindgren, J.E.; Andersson, S.; Agurell, S.; Gillespie, H.; Hollister, L.E. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed. Environ. Mass. Spectrom. 1986, 13, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Morrison, G.; Crockett, J.; Blakey, G.; Sommerville, K. A Phase 1, Open-Label, Pharmacokinetic Trial to Investigate Possible Drug-Drug Interactions between Clobazam, Stiripentol, or Valproate and Cannabidiol in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2019, 8, 1009–1031. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.A.; Bae, S.K.; Choi, Y.K.; Choi, C.S.; Liu, K.H.; Shin, J.G. Metabolism of 1′- and 4-hydroxymidazolam by glucuronide conjugation is largely mediated by UDP-glucuronosyltransferases 1A4, 2B4, and 2B7. Drug Metab. Dispos. 2010, 38, 2007–2013. [Google Scholar] [CrossRef]
- Yang, S.K.; Lu, X.L. N,N-dimethylcarbamyl derivatives of oxazepam. Chirality 1991, 3, 212–219. [Google Scholar] [CrossRef]



| R-Oxazepam Glucuronide | S-Oxazepam Glucuronide | ||||
|---|---|---|---|---|---|
| Cannabinoid | Enzyme a | IC50 | IC50,u b | IC50 | IC50,u b |
| rUGT1A9 | 13 ± 7.7 | 1.0 ± 0.60 | - | - | |
| 11-OH-THC | rUGT2B7 | 9.9 ± 8.2 | 0.77 ± 0.64 | 6.8 ± 3.3 | 0.53 ± 0.26 |
| rUGT2B15 | - c | - | 58 ± 27 | 4.5 ± 2.1 | |
| HKM | 18 ± 4.3 | 1.7 ± 0.40 | 19 ± 2.0 | 1.8 ± 0.19 | |
| HLM | 18 ± 5.0 | 1.7 ± 0.47 | 41 ± 25 | 3.9 ± 2.4 | |
| rUGT1A9 | 1.4 ± 0.29 | 0.053 ± 0.011 | - | - | |
| CBD | rUGT2B7 | 2.6 ± 1.2 | 0.10 ± 0.045 | 2.9 ± 2.9 | 0.11 ± 0.11 |
| rUGT2B15 | - | - | 54 ± 9.0 | 2.0 ± 0.35 | |
| HKM | 5.9 ± 1.0 | 0.36 ± 0.062 | 16 ± 7.7 | 0.98 ± 0.48 | |
| HLM | 16 ± 7.7 | 0.84 ± 0.39 | 87 ± 46 | 4.5 ± 2.4 | |
| rUGT1A9 | 11 ± 7.5 | 0.50 ± 0.31 | - | - | |
| THC | rUGT2B7 | 33 ± 16 | 1.4 ± 0.67 | 33 ± 6.6 | 1.4 ± 0.28 |
| rUGT2B15 | - | - | 20 ± 10 | 0.84 ± 0.42 | |
| HKM | 94 ± 26 | 4.5 ± 1.2 | >100 | >4.8 d | |
| HLM | 34 ± 16 | 1.6 ± 0.77 | >100 | >5.2 d | |
| R-Oxazepam Glucuronide | S-Oxazepam Glucuronide | ||||
|---|---|---|---|---|---|
| Cannabinoid | Enzyme | Ki b | Ki,u c | Ki b | Ki,u c |
| 11-OH-THC | HLM a | 18 ± 4.9 d | 1.7 ± 0.46 | 34 ± 21 | 3.2 ± 2.0 |
| CBD | HLM | 16 ± 7.4 | 0.82 ± 0.38 | 72 ± 32 | 3.7 ± 1.6 |
| THC | HLM | 33 ± 15 | 1.6 ± 0.74 | >83 e | >3.5 e |
| Cannabinoid | Dose (mg) a | Route of Administration | Cmax (μM) a | Predicted AUCR | |
|---|---|---|---|---|---|
| R-Oxazepam c | S-Oxazepam d | ||||
| CBD | 70 | Oral | 0.089 | 1.21 | 1.05 |
| 700 | Oral | 0.89 | 2.42 e | 1.44 | |
| 2000 | Oral | 2.5 | 3.45 | 2.03 | |
| 2 b | Inhalation | 0.0064 b | 1.00 | 1.00 | |
| 19 b | Inhalation | 0.35 b | 1.07 | 1.02 | |
| THC | 20 | Oral | 0.030 | 1.03 | - f |
| 130 | Oral | 0.20 | 1.20 | - | |
| 160 | Oral | 0.24 | 1.25 | - | |
| 25 | Inhalation | 0.25 | 1.04 | - | |
| 70 | Inhalation | 0.70 | 1.12 | - | |
| 100 | Inhalation | 0.99 | 1.17 | - | |
| 11-OH-THC | 20 | Oral | 0.015 | 1.03 | 1.02 |
| 130 | Oral | 0.10 | 1.18 | 1.10 | |
| 160 | Oral | 0.12 | 1.22 | 1.12 | |
| 25 | Inhalation | 0.012 | 1.04 | 1.01 | |
| 70 | Inhalation | 0.032 | 1.10 | 1.05 | |
| 100 | Inhalation | 0.046 | 1.14 | 1.08 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardhi, K.; Coates, S.; Chen, G.; Lazarus, P. Cannabinoid-Induced Stereoselective Inhibition of R-S-Oxazepam Glucuronidation: Cannabinoid–Oxazepam Drug Interactions. Pharmaceutics 2024, 16, 243. https://doi.org/10.3390/pharmaceutics16020243
Bardhi K, Coates S, Chen G, Lazarus P. Cannabinoid-Induced Stereoselective Inhibition of R-S-Oxazepam Glucuronidation: Cannabinoid–Oxazepam Drug Interactions. Pharmaceutics. 2024; 16(2):243. https://doi.org/10.3390/pharmaceutics16020243
Chicago/Turabian StyleBardhi, Keti, Shelby Coates, Gang Chen, and Philip Lazarus. 2024. "Cannabinoid-Induced Stereoselective Inhibition of R-S-Oxazepam Glucuronidation: Cannabinoid–Oxazepam Drug Interactions" Pharmaceutics 16, no. 2: 243. https://doi.org/10.3390/pharmaceutics16020243
APA StyleBardhi, K., Coates, S., Chen, G., & Lazarus, P. (2024). Cannabinoid-Induced Stereoselective Inhibition of R-S-Oxazepam Glucuronidation: Cannabinoid–Oxazepam Drug Interactions. Pharmaceutics, 16(2), 243. https://doi.org/10.3390/pharmaceutics16020243






