Breast Cancer Treatment Using the Magneto-Hyperthermia Technique Associated with Omega-3 Polyunsaturated Fatty Acids’ Supplementation and Physical Training
Abstract
:1. Introduction
2. Materials and Methods
2.1. ION Physicalchemical Evaluation
2.1.1. ION Characterization
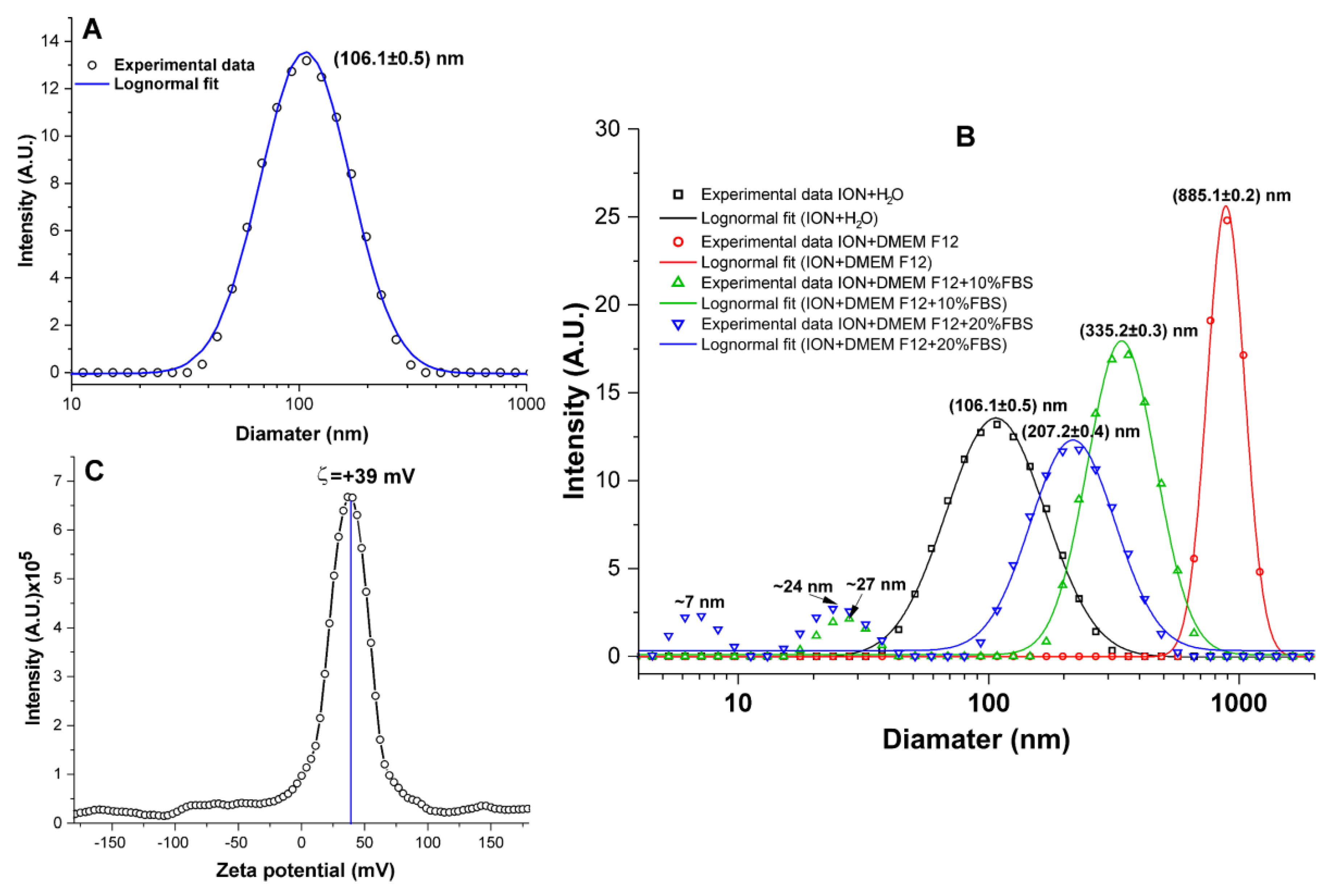
2.1.2. Evaluation of ION Size Polydispersity
2.1.3. Evaluation of the Colloidal Stability of ION
2.1.4. Evaluation of the Zeta Potential of ION
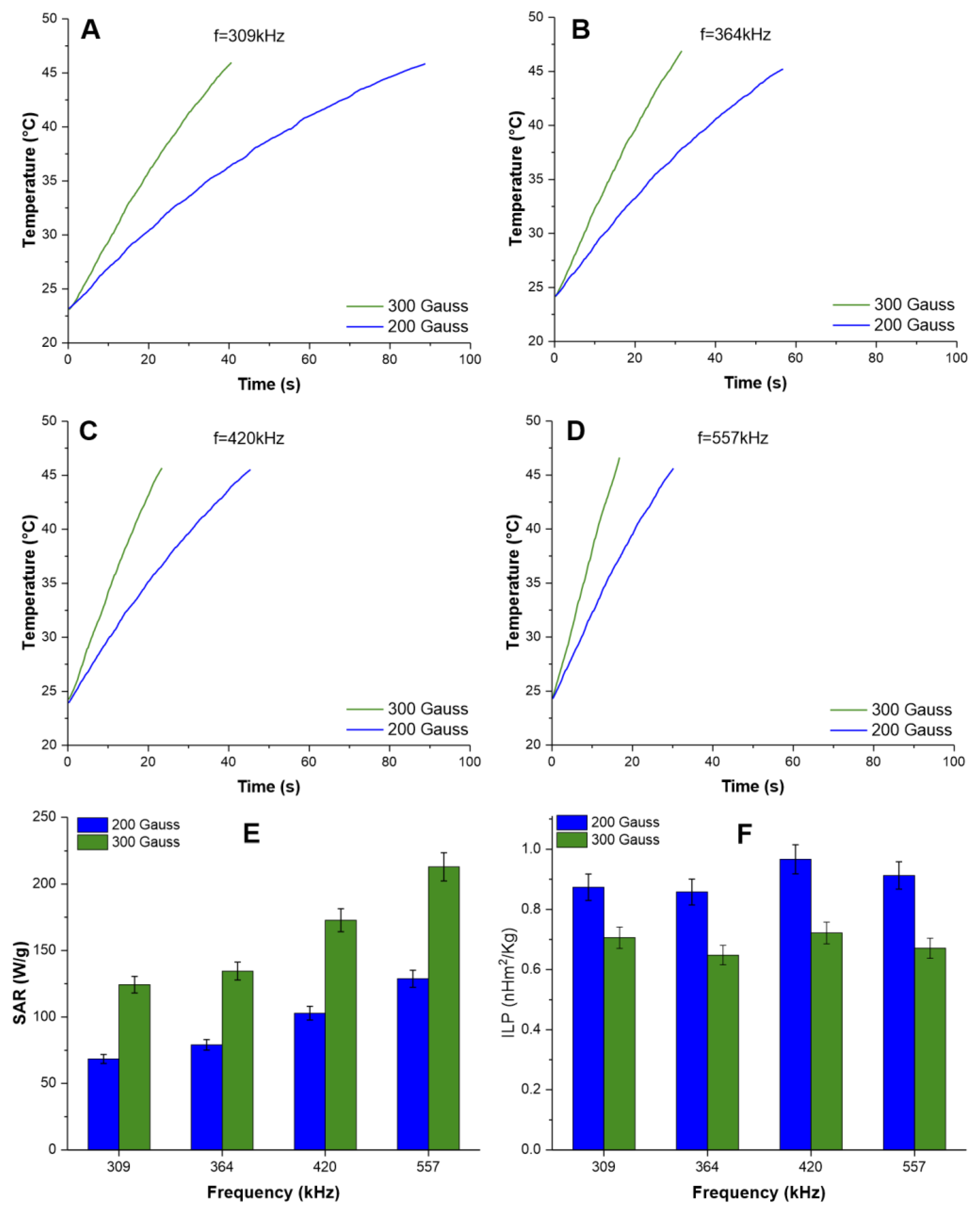
2.1.5. Evaluation of the Heating Potential of IONs for MHT Therapy
2.1.6. Specific Absorption Rate and Intrinsic Loss Power Calculation
2.2. In-Vitro Study
2.2.1. MDA-MB-231 Cell Culture
2.2.2. Evaluation of Kinetics of Bioluminescent Signal of MDA-MB-231 Cells
2.2.3. Evaluation of Labeling of MDA-MB-231 Cells with ION and Their Internalization into Cells
2.2.4. Evaluation of Nanoparticle Toxicity for MDA-MB-231
2.2.5. Evaluation of Magneto-Hyperthermia Therapy in MDA-MB-231 Cells
2.3. In-Vivo Study
2.3.1. Animals
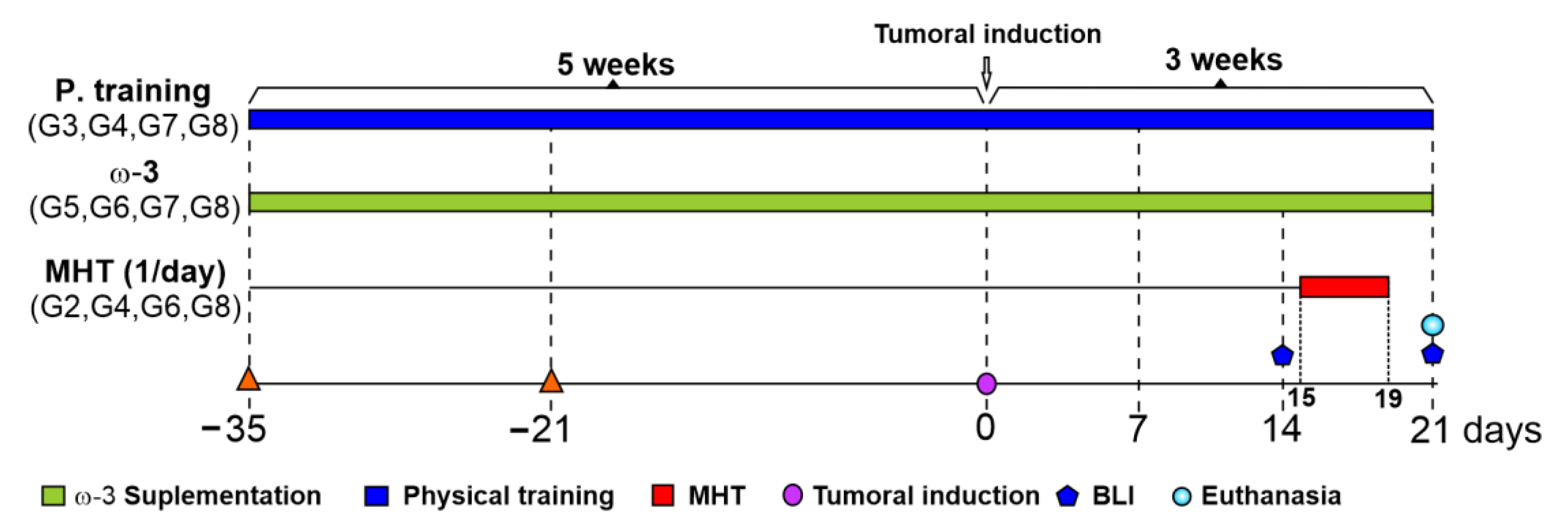
2.3.2. Experimental Design
2.3.3. Omega-3 Supplementation
2.3.4. Physical Training
2.3.5. Magneto-Hyperthermia Therapy
2.4. Statistic Analyses
3. Results
3.1. ION Physicochemical Evaluation
3.2. Evaluation of the Heating Potential of IONs for MHT Therapy
3.3. In Vitro Study
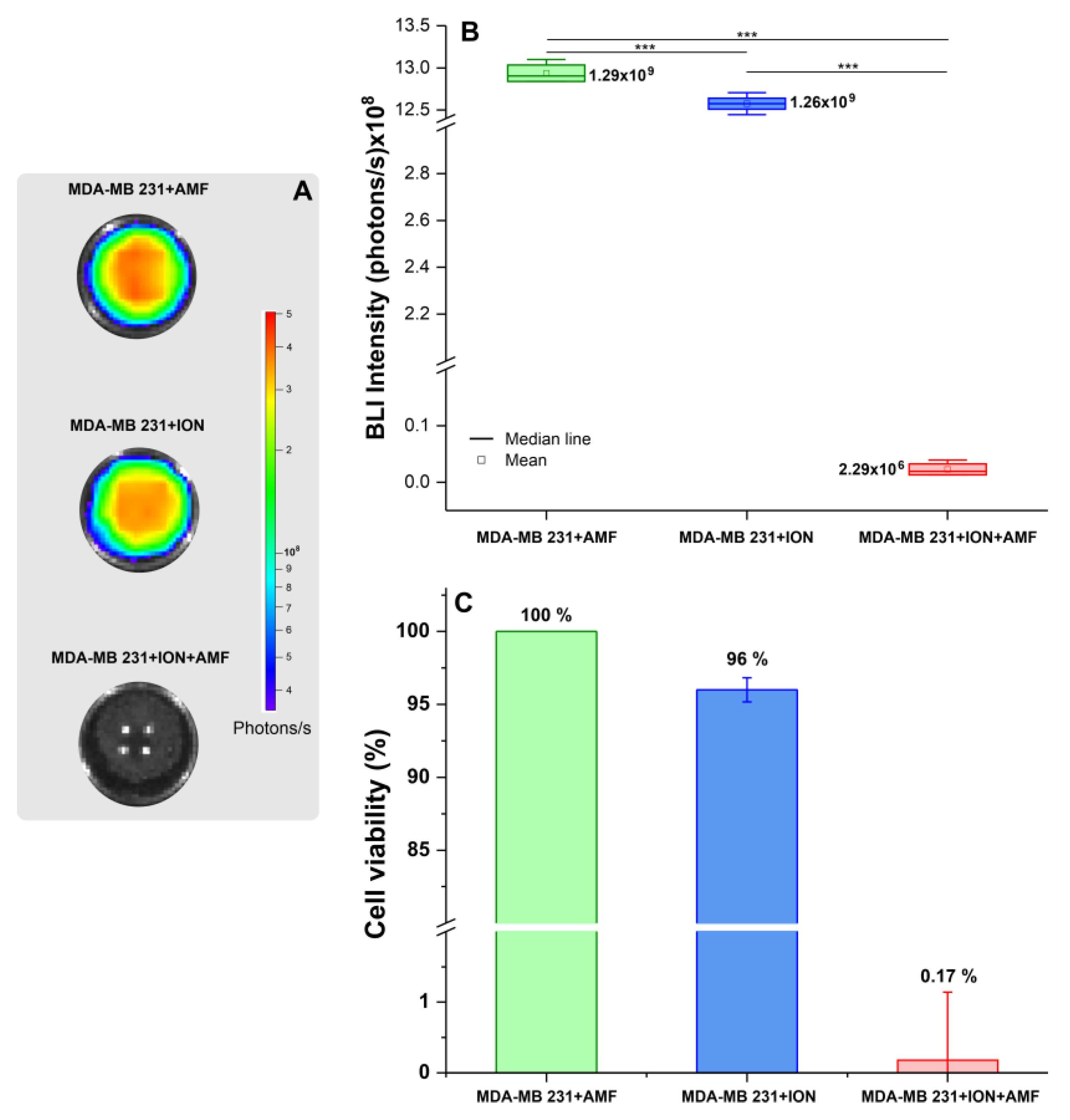
3.3.1. Evaluation of Kinetics of BLI of MDA-MB-231, and, after Labeling with ION, Their Internalization, and Cytotoxicity
3.3.2. Evaluation of Efficacy of Therapeutic Magneto-Hyperthermia
3.4. In Vivo Study
3.4.1. Evaluation of the Tumor Growth by BLI and Histology
3.4.2. Evaluation of the Efficacy of Therapeutic Combinations: Omega-3 Supplementation, Physical Training, and Magneto-Hyperthermia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 24 January 2024).
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Delort, L.; Rossary, A.; Farges, M.C.; Vasson, M.P.; Caldefie-Chézet, F. Leptin, adipocytes and breast cancer: Focus on inflammation and anti-tumor immunity. Life Sci. 2015, 140, 37–48. [Google Scholar] [CrossRef]
- Nathanson, S.D.; Detmar, M.; Padera, T.P.; Yates, L.R.; Welch, D.R.; Beadnell, T.C.; Scheid, A.D.; Wrenn, E.D.; Cheung, K. Mechanisms of breast cancer metastasis. Clin. Exp. Metastasis 2022, 39, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Câncer—INCA. Conceito e Magnitude. Definição do Câncer de Mama e Dados de Incidência e Mortalidade no Brasil. Available online: https://www.gov.br/inca/pt-br/search?SearchableText=cancer%20de%20mama (accessed on 10 August 2023).
- Le Naour, A.; Koffi, Y.; Diab, M.; Le Guennec, D.; Rougé, S.; Aldekwer, S.; Goncalves-Mendes, N.; Talvas, J.; Farges, M.C.; Caldefie-Chezet, F.; et al. EO771, the first luminal B mammary cancer cell line from C57BL/6 mice. Cancer Cell Int. 2020, 20, 328. [Google Scholar] [CrossRef] [PubMed]
- Peto, J. Cancer epidemiology in the last century and the next decade. Nature 2001, 411, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct. Target. Ther. 2019, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, R.G.; Otoni, K.M. Histological and molecular classification of breast cancer: What do we know. Mastology 2020, 30, e20200024. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahayri, Z.N.; Patrinos, G.P.; Ali, B.R. Toxicity and Pharmacogenomic Biomarkers in Breast Cancer Chemotherapy. Front. Pharmacol. 2020, 11, 445. [Google Scholar] [CrossRef]
- Türk, K.E.; Yılmaz, M. The Effect on Quality of Life and Body Image of Mastectomy Among Breast Cancer Survivors. Eur. J. Breast Health 2018, 14, 205–210. [Google Scholar] [CrossRef]
- Ilg, P.; Kröger, M. Dynamics of interacting magnetic nanoparticles: Effective behavior from competition between Brownian and Néel relaxation. Phys. Chem. Chem. Phys. 2020, 22, 22244–22259. [Google Scholar] [CrossRef]
- Peiravi, M.; Eslami, H.; Ansari, M.; Zare-Zardini, H. Magnetic hyperthermia: Potentials and limitations. J. Indian Chem. Soc. 2022, 99, 100269. [Google Scholar] [CrossRef]
- Huang, H.; Delikanli, S.; Zeng, H.; Ferkey, D.M.; Pralle, A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat. Nanotechnol. 2010, 5, 602–606. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Gorgoń, S.; Radoń, A.; Bajdak-Rusinek, K. Magnetite Nanoparticles in Magnetic Hyperthermia and Cancer Therapies: Challenges and Perspectives. Nanomaterials 2022, 12, 1807. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-A.; Hadadian, Y.; Yoon, J. A prediction model for magnetic particle imaging–based magnetic hyperthermia applied to a brain tumor model. Comput. Methods Prog. Biomed. 2023, 235, 107546. [Google Scholar] [CrossRef]
- Tang, X.; Loc, W.S.; Dong, C.; Matters, G.L.; Butler, P.J.; Kester, M.; Meyers, C.; Jiang, Y.; Adair, J.H. The use of nanoparticulates to treat breast cancer. Nanomedicine 2017, 12, 2367–2388. [Google Scholar] [CrossRef] [PubMed]
- Rego, G.N.A.; Nucci, M.P.; Mamani, J.B.; Oliveira, F.A.; Marti, L.C.; Filgueiras, I.S.; Ferreira, J.M.; Real, C.C.; Faria, D.P.; Espinha, P.L.; et al. Therapeutic Efficiency of Multiple Applications of Magnetic Hyperthermia Technique in Glioblastoma Using Aminosilane Coated Iron Oxide Nanoparticles: In Vitro and In Vivo Study. Int. J. Mol. Sci. 2020, 21, 958. [Google Scholar] [CrossRef] [PubMed]
- Albarqi, H.A.; Demessie, A.A.; Sabei, F.Y.; Moses, A.S.; Hansen, M.N.; Dhagat, P.; Taratula, O.R.; Taratula, O. Systemically Delivered Magnetic Hyperthermia for Prostate Cancer Treatment. Pharmaceutics 2020, 12, 1020. [Google Scholar] [CrossRef] [PubMed]
- Tansi, F.L.; Fröbel, F.; Maduabuchi, W.O.; Steiniger, F.; Westermann, M.; Quaas, R.; Teichgräber, U.K.; Hilger, I. Effect of Matrix-Modulating Enzymes on the Cellular Uptake of Magnetic Nanoparticles and on Magnetic Hyperthermia Treatment of Pancreatic Cancer Models In Vivo. Nanomaterials 2021, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Rego, G.N.A.; Mamani, J.B.; Souza, T.K.F.; Nucci, M.P.; Silva, H.R.D.; Gamarra, L.F. Therapeutic evaluation of magnetic hyperthermia using Fe3O4-aminosilane-coated iron oxide nanoparticles in glioblastoma animal model. Einstein 2019, 17, eAO4786. [Google Scholar] [CrossRef] [PubMed]
- Mamani, J.B.; Souza, T.K.F.; Nucci, M.P.; Oliveira, F.A.; Nucci, L.P.; Alves, A.H.; Rego, G.N.A.; Marti, L.; Gamarra, L.F. In Vitro Evaluation of Hyperthermia Magnetic Technique Indicating the Best Strategy for Internalization of Magnetic Nanoparticles Applied in Glioblastoma Tumor Cells. Pharmaceutics 2021, 13, 1219. [Google Scholar] [CrossRef] [PubMed]
- Mamani, J.B.; Marinho, B.S.; Rego, G.N.A.; Nucci, M.P.; Alvieri, F.; Santos, R.S.D.; Ferreira, J.V.M.; Oliveira, F.A.; Gamarra, L.F. Magnetic hyperthermia therapy in glioblastoma tumor on-a-Chip model. Einstein 2020, 18, eAO4954. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, T.; Matsumi, Y.; Aono, H.; Ohara, T.; Tazawa, H.; Shigeyasu, K.; Yano, S.; Takeda, S.; Komatsu, Y.; Hoffman, R.M.; et al. Immuno-hyperthermia effected by antibody-conjugated nanoparticles selectively targets and eradicates individual cancer cells. Cell Cycle 2021, 20, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Pazouki, N.; Irani, S.; Olov, N.; Atyabi, S.M.; Bagheri-Khoulenjani, S. Fe3O4 nanoparticles coated with carboxymethyl chitosan containing curcumin in combination with hyperthermia induced apoptosis in breast cancer cells. Prog. Biomater. 2022, 11, 43–54. [Google Scholar] [CrossRef]
- Newell, M.; Goruk, S.; Schueler, J.; Mazurak, V.; Postovit, L.M.; Field, C.J. Docosahexaenoic acid enrichment of tumor phospholipid membranes increases tumor necroptosis in mice bearing triple negative breast cancer patient-derived xenografts. J. Nutr. Biochem. 2022, 107, 109018. [Google Scholar] [CrossRef]
- Tehrani, M.H.H.; Soltani, M.; Kashkooli, F.M.; Mahmoudi, M.; Raahemifar, K. Computational modeling of combination of magnetic hyperthermia and temperature-sensitive liposome for controlled drug release in solid tumor. Pharmaceutics 2022, 14, 35. [Google Scholar] [CrossRef]
- Luo, H.; Chen, C.Y.; Li, X.; Zhang, X.; Su, C.W.; Liu, Y.; Cao, T.; Hao, L.; Wang, M.; Kang, J.X. Increased lipogenesis is critical for self-renewal and growth of breast cancer stem cells: Impact of omega-3 fatty acids. Stem Cells 2021, 39, 1660–1670. [Google Scholar] [CrossRef]
- Fabian, C.J.; Befort, C.A.; Phillips, T.A.; Nydegger, J.L.; Kreutzjans, A.L.; Powers, K.R.; Metheny, T.; Klemp, J.R.; Carlson, S.E.; Sullivan, D.K.; et al. Change in Blood and Benign Breast Biomarkers in Women Undergoing a Weight-Loss Intervention Randomized to High-Dose ω-3 Fatty Acids versus Placebo. Cancer Prev. Res. 2021, 14, 893–904. [Google Scholar] [CrossRef]
- Goupille, C.; Frank, P.G.; Arbion, F.; Jourdan, M.L.; Guimaraes, C.; Pinault, M.; Body, G.; Chevalier, S.; Bougnoux, P.; Ouldamer, L. Low Levels of Omega-3 Long-Chain Polyunsaturated Fatty Acids Are Associated with Bone Metastasis Formation in Premenopausal Women with Breast Cancer: A Retrospective Study. Nutrients 2020, 12, 3832. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Hou, J.; Sun, J.; Guo, N.; Wang, Z. Dietary Intake of N-3 and N-6 Polyunsaturated Fatty Acids and Risk of Cancer: Meta-Analysis of Data from 32 Studies. Nutr. Cancer 2021, 73, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Caterina Zito, M.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Giacobbe, J.; Benoiton, B.; Zunszain, P.; Pariante, C.M.; Borsini, A. The Anti-Inflammatory Role of Omega-3 Polyunsaturated Fatty Acids Metabolites in Pre-Clinical Models of Psychiatric, Neurodegenerative, and Neurological Disorders. Front. Psychiatry 2020, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Osouli-Tabrizi, S.; Mehdizadeh, A.; Naghdi, M.; Sanaat, Z.; Vahed, N.; Farshbaf-Khalili, A. The effectiveness of omega-3 fatty acids on health outcomes in women with breast cancer: A systematic review. Food Sci. Nutr. 2023, 11, 4355–4371. [Google Scholar] [CrossRef] [PubMed]
- Bekhet, A.H.; Abdallah, A.R.; Ismail, H.M.; Genena, D.M.; Osman, N.A.; El Khatib, A.; Abbas, R.L. Benefits of Aerobic Exercise for Breast Cancer Survivors: A Systematic Review of Randomized Controlled Trials. Asian Pac. J. Cancer Prev. 2019, 20, 3197–3209. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Zhang, L.; Yang, J.; Yin, X.; Zhang, T.; Wu, X.; Ma, X. Physical activity prevents tumor metastasis through modulation of immune function. Front. Pharmacol. 2022, 13, 1034129. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, Y.; Park, S.; Kim, E.; Kim, Y.; Kim, Y.; Kim, J.H. Effects of Steady Low-Intensity Exercise on High-Fat Diet Stimulated Breast Cancer Progression Via the Alteration of Macrophage Polarization. Integr. Cancer Ther. 2020, 19, 1534735420949678. [Google Scholar] [CrossRef]
- Abdelmagid, S.A.; MacKinnon, J.L.; Janssen, S.M.; Ma, D.W.L. Role of n-3 Polyunsaturated Fatty Acids and Exercise in Breast Cancer Prevention: Identifying Common Targets. Nutr. Metab. Insights 2016, 9, 71–84. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Willett, W.C.; Manson, J.E.; Dawson-Hughes, B.; Manz, M.G.; Theiler, R.; Braendle, K.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; et al. Combined Vitamin D, Omega-3 Fatty Acids, and a Simple Home Exercise Program May Reduce Cancer Risk Among Active Adults Aged 70 and Older: A Randomized Clinical Trial. Front. Aging 2022, 3, 852643. [Google Scholar] [CrossRef]
- Fabian, C.J.; Kimler, B.F.; Hursting, S.D. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 2015, 17, 62. [Google Scholar] [CrossRef]
- Dishman, R.K.; Armstrong, R.B.; Delp, M.D.; Graham, R.E.; Dunn, A.L. Open-field behavior is not related to treadmill performance in exercising rats. Physiol. Behav. 1988, 43, 541–546. [Google Scholar] [CrossRef]
- Baranova, A.; Krasnoselskyi, M.; Starikov, V.; Kartashov, S.; Zhulkevych, I.; Vlasenko, V.; Oleshko, K.; Bilodid, O.; Sadchikova, M.; Vinnyk, Y. Triple-negative breast cancer: Current treatment strategies and factors of negative prognosis. J. Med. Life 2022, 15, 153–161. [Google Scholar] [CrossRef]
- Wang, X.; Lin, J.; Wang, Z.; Li, Z.; Wang, M. Possible therapeutic targets for NLRP3 inflammasome-induced breast cancer. Discov. Oncol. 2023, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhao, Q.; Xu, Y.; Wang, L. Improving the efficacy of combined radiotherapy and immunotherapy: Focusing on the effects of radiosensitivity. Radiat. Oncol. 2023, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yao, Y.; Tang, Y.; Xin, Z.; Wu, D.; Ni, C.; Huang, J.; Wei, Q.; Zhang, T. Radiation-induced tumor immune microenvironments and potential targets for combination therapy. Signal Transduct. Target. Ther. 2023, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Klein, P.; Tiersten, A.; Sparano, J.A. An emerging generation of endocrine therapies in breast cancer: A clinical perspective. npj Breast Cancer 2023, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, R.; Sethuraman, S.; Krishnan, U.M.; Subramanian, A. Self-assembled multifunctional nanotheranostics against circulating tumor clusters in metastatic breast cancer. Acta Pharm. Sin. B 2023, 13, 1711–1725. [Google Scholar] [CrossRef] [PubMed]
- Gunakala, S.R.; Job, V.M.; Murthy, P.V.S.N.; Nagarani, P.; Seetharaman, H.; Chowdary, B.V. In-silico investigation of intratumoural magnetic hyperthermia for breast cancer therapy using FePt or FeCrNbB magnetic nanoparticles. Int. J. Therm. Sci. 2023, 192, 108405. [Google Scholar] [CrossRef]
- Mahesh, N.; Singh, N.; Talukdar, P. Investigation of a breast cancer magnetic hyperthermia through mathematical modeling of intratumoral nanoparticle distribution and temperature elevations. Therm. Sci. Eng. Prog. 2023, 40, 101756. [Google Scholar] [CrossRef]
- Sun, R.; Chen, H.; Zheng, J.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Composite Scaffolds of Gelatin and Fe3O4 Nanoparticles for Magnetic Hyperthermia-Based Breast Cancer Treatment and Adipose Tissue Regeneration. Adv. Healthc. Mater. 2023, 12, e2202604. [Google Scholar] [CrossRef] [PubMed]
- Theinel, M.H.; Nucci, M.P.; Alves, A.H.; Dias, O.F.M.; Mamani, J.B.; Garrigós, M.M.; Oliveira, F.A.; Rego, G.N.A.; Valle, N.M.E.; Cianciarullo, G.; et al. The Effects of Omega-3 Polyunsaturated Fatty Acids on Breast Cancer as a Preventive Measure or as an Adjunct to Conventional Treatments. Nutrients 2023, 15, 1310. [Google Scholar] [CrossRef]
- Guo, C.H.; Hsia, S.; Chung, C.H.; Lin, Y.C.; Shih, M.Y.; Chen, P.C.; Peng, C.L.; Henning, S.M.; Hsu, G.W.; Li, Z. Nutritional supplements in combination with chemotherapy or targeted therapy reduces tumor progression in mice bearing triple-negative breast cancer. J. Nutr. Biochem. 2021, 87, 108504. [Google Scholar] [CrossRef] [PubMed]
- Global Recommendations for EPA and DHA Intake (Rev 19 November 2014). Available online: https://www.issfal.org/assets/globalrecommendationssummary19nov2014landscape_-3-.pdf (accessed on 22 January 2024).
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n−3 fatty acids in humans 23. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef] [PubMed]
- García-Chico, C.; López-Ortiz, S.; Peñín-Grandes, S.; Pinto-Fraga, J.; Valenzuela, P.L.; Emanuele, E.; Ceci, C.; Graziani, G.; Fiuza-Luces, C.; Lista, S.; et al. Physical Exercise and the Hallmarks of Breast Cancer: A Narrative Review. Cancers 2023, 15, 324. [Google Scholar] [CrossRef]
- Ficarra, S.; Thomas, E.; Bianco, A.; Gentile, A.; Thaller, P.; Grassadonio, F.; Papakonstantinou, S.; Schulz, T.; Olson, N.; Martin, A.; et al. Impact of exercise interventions on physical fitness in breast cancer patients and survivors: A systematic review. Breast Cancer 2022, 29, 402–418. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef]
- Furmaniak, A.C.; Menig, M.; Markes, M.H. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst. Rev. 2016, 9, Cd005001. [Google Scholar] [CrossRef]
- Cormie, P.; Zopf, E.M.; Zhang, X.; Schmitz, K.H. The Impact of Exercise on Cancer Mortality, Recurrence, and Treatment-Related Adverse Effects. Epidemiol. Rev. 2017, 39, 71–92. [Google Scholar] [CrossRef]
- Maginador, G.; Lixandrão, M.E.; Bortolozo, H.I.; Vechin, F.C.; Sarian, L.O.; Derchain, S.; Telles, G.D.; Zopf, E.; Ugrinowitsch, C.; Conceição, M.S. Aerobic Exercise-Induced Changes in Cardiorespiratory Fitness in Breast Cancer Patients Receiving Chemotherapy: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 2240. [Google Scholar] [CrossRef]
- Schutz, S.; Aidar, F.J.; Souza, R.L.M.; dos Santos, J.L.; Voltarelli, F.A.; Vieira Junior, R.C.; Soares, N.M.M.; Marçal, A.C. Different Methods of Physical Training Applied to Women Breast Cancer Survivors: A Systematic Review. Front. Physiol. 2021, 12, 639406. [Google Scholar] [CrossRef]
- Vulczak, A.; Souza, A.O.; Ferrari, G.D.; Azzolini, A.; Pereira-da-Silva, G.; Alberici, L.C. Moderate Exercise Modulates Tumor Metabolism of Triple-Negative Breast Cancer. Cells 2020, 9, 628. [Google Scholar] [CrossRef]
- Vilas-Boas, V.; Carvalho, F.; Espiña, B. Magnetic Hyperthermia for Cancer Treatment: Main Parameters Affecting the Outcome of In Vitro and In Vivo Studies. Molecules 2020, 25, 2874. [Google Scholar] [CrossRef]
- Noh, S.H.; Moon, S.H.; Shin, T.H.; Lim, Y.; Cheon, J. Recent advances of magneto-thermal capabilities of nanoparticles: From design principles to biomedical applications. Nano Today 2017, 13, 61–76. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Luo, K.; Song, H.; Lan, F.; Wu, Y.; Gu, Z. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics 2013, 3, 595–615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, M.; Portney, N.G.; Cui, D.; Budak, G.; Ozbay, E.; Ozkan, M.; Ozkan, C.S. Zeta potential: A surface electrical characteristic to probe the interaction of nanoparticles with normal and cancer human breast epithelial cells. Biomed. Microdevices 2008, 10, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, L.; de la Cueva, L.; Moros, M.; Mazarío, E.; de Bernardo, S.; de la Fuente, J.M.; Morales, M.P.; Salas, G. Aggregation effects on the magnetic properties of iron oxide colloids. Nanotechnology 2019, 30, 112001. [Google Scholar] [CrossRef]
- Calatayud, M.P.; Sanz, B.; Raffa, V.; Riggio, C.; Ibarra, M.R.; Goya, G.F. The effect of surface charge of functionalized Fe3O4 nanoparticles on protein adsorption and cell uptake. Biomaterials 2014, 35, 6389–6399. [Google Scholar] [CrossRef]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef]
- Deatsch, A.E.; Evans, B.A. Heating efficiency in magnetic nanoparticle hyperthermia. J. Magn. Magn. Mater. 2014, 354, 163–172. [Google Scholar] [CrossRef]
- Das, P.; Colombo, M.; Prosperi, D. Recent advances in magnetic fluid hyperthermia for cancer therapy. Colloids Surf. B Biointerfaces 2019, 174, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Apostolov, A.; Apostolova, I.; Wesselinowa, J. Specific absorption rate in Zn-doted ferrites for self-controlled magnetic hyperthermia. Eur. Phys. J. B 2019, 92, 58. [Google Scholar] [CrossRef]
- Martinez-Boubeta, C.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Iglesias, O.; Guardia, P.; Cabot, A.; Yedra, L.; Estradé, S.; Peiró, F.; et al. Learning from nature to improve the heat generation of iron-oxide nanoparticles for magnetic hyperthermia applications. Sci. Rep. 2013, 3, 1652. [Google Scholar] [CrossRef]
- Rytov, R.A.; Bautin, V.A.; Usov, N.A. Towards optimal thermal distribution in magnetic hyperthermia. Sci. Rep. 2022, 12, 3023. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.G.D.; Ferreira, D.; Veloso, S.R.S.; Santos-Pereira, C.; Castanheira, E.M.S.; Côrte-Real, M.; Rodrigues, L.R. Synthesis and Cytotoxicity Assessment of Citrate-Coated Calcium and Manganese Ferrite Nanoparticles for Magnetic Hyperthermia. Pharmaceutics 2022, 14, 2694. [Google Scholar] [CrossRef]
- Shah, R.R.; Davis, T.P.; Glover, A.L.; Nikles, D.E.; Brazel, C.S. Impact of magnetic field parameters and iron oxide nanoparticle properties on heat generation for use in magnetic hyperthermia. J. Magn. Magn. Mater. 2015, 387, 96–106. [Google Scholar] [CrossRef]
- Iacovita, C.; Fizeșan, I.; Pop, A.; Scorus, L.; Dudric, R.; Stiufiuc, G.; Vedeanu, N.; Tetean, R.; Loghin, F.; Stiufiuc, R.; et al. In Vitro Intracellular Hyperthermia of Iron Oxide Magnetic Nanoparticles, Synthesized at High Temperature by a Polyol Process. Pharmaceutics 2020, 12, 424. [Google Scholar] [CrossRef]
- Shubitidze, F.; Kekalo, K.; Stigliano, R.; Baker, I. Magnetic nanoparticles with high specific absorption rate of electromagnetic energy at low field strength for hyperthermia therapy. J. Appl. Phys. 2015, 117, 094302. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Li, G.L.; Gao, X.; Zhang, H.; Zhang, T.B.; Jiao, W.B.; Chen, X.Y.; Zhu, W.J.; Liu, X.L.; Fan, H.M. Method for Ferrite Nanomaterials-Mediated Cellular Magnetic Hyperthermia. ACS Biomater. Sci. Eng. 2020, 6, 6652–6660. [Google Scholar] [CrossRef]
- Li, C.H.; Hodgins, P.; Peterson, G.P. Experimental study of fundamental mechanisms in inductive heating of ferromagnetic nanoparticles suspension (Fe3O4 Iron Oxide Ferrofluid). J. Appl. Phys. 2011, 110, 054303. [Google Scholar] [CrossRef]
- Sanz, B.; Calatayud, M.P.; Torres, T.E.; Fanarraga, M.L.; Ibarra, M.R.; Goya, G.F. Magnetic hyperthermia enhances cell toxicity with respect to exogenous heating. Biomaterials 2017, 114, 62–70. [Google Scholar] [CrossRef]
- Conway, M.; Xu, T.; Kirkpatrick, A.; Ripp, S.; Sayler, G.; Close, D. Real-time tracking of stem cell viability, proliferation, and differentiation with autonomous bioluminescence imaging. BMC Biol. 2020, 18, 79. [Google Scholar] [CrossRef] [PubMed]
- Cosette, J.; Ben Abdelwahed, R.; Donnou-Triffault, S.; Sautès-Fridman, C.; Flaud, P.; Fisson, S. Bioluminescence-Based Tumor Quantification Method for Monitoring Tumor Progression and Treatment Effects in Mouse Lymphoma Models. J. Vis. Exp. 2016, 113, e53609. [Google Scholar] [CrossRef]
- Rozhina, E.; Danilushkina, A.; Akhatova, F.; Fakhrullin, R.; Rozhin, A.; Batasheva, S. Biocompatibility of magnetic nanoparticles coating with polycations using A549 cells. J. Biotechnol. 2021, 325, 25–34. [Google Scholar] [CrossRef]
- Zeng, L.; Li, W.; Chen, C.S. Breast cancer animal models and applications. Zool. Res. 2020, 41, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Veta, M.; Pluim, J.P.W.; Diest, P.J.V.; Viergever, M.A. Breast Cancer Histopathology Image Analysis: A Review. IEEE Trans. Biomed. Eng. 2014, 61, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.-G.; Piao, J.-L.; Kondo, T.; Ogawa, R.; Tsuneyama, K.; Zhao, Q.-L.; Feril, L.B.; Inadera, H. Molecular mechanisms of hyperthermia-induced apoptosis enhanced by docosahexaenoic acid: Implication for cancer therapy. Chem.-Biol. Interact. 2014, 215, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Beak, S.; Ahn, S.H.; Moon, B.S.; Kim, J.; Lee, K.P. Suppressing breast cancer by exercise: Consideration to animal models and exercise protocols. Phys. Act. Nutr. 2020, 24, 22–29. [Google Scholar] [CrossRef]
- Farzanegan, Z.; Tahmasbi, M. Evaluating the applications and effectiveness of magnetic nanoparticle-based hyperthermia for cancer treatment: A systematic review. Appl. Radiat. Isot. 2023, 198, 110873. [Google Scholar] [CrossRef]
- Samanta, C.; Tewari, S.; Chakraborty, D.; Vaishnav, S. Omega-3 Fatty Acid and its Protective Effect against Cancer and Cancer-related Complication. J. Pharm. Res. Int. 2022, 34, 51–62. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Ashcraft, K.A.; Peace, R.M.; Betof, A.S.; Dewhirst, M.W.; Jones, L.W. Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer Res. 2016, 76, 4032–4050. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.W.; Eves, N.D.; Courneya, K.S.; Chiu, B.K.; Baracos, V.E.; Hanson, J.; Johnson, L.; Mackey, J.R. Effects of exercise training on antitumor efficacy of doxorubicin in MDA-MB-231 breast cancer xenografts. Clin. Cancer Res. 2005, 11, 6695–6698. [Google Scholar] [CrossRef]
- Jones, L.W.; Viglianti, B.L.; Tashjian, J.A.; Kothadia, S.M.; Keir, S.T.; Freedland, S.J.; Potter, M.Q.; Moon, E.J.; Schroeder, T.; Herndon, J.E., 2nd; et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J. Appl. Physiol. 2010, 108, 343–348. [Google Scholar] [CrossRef] [PubMed]

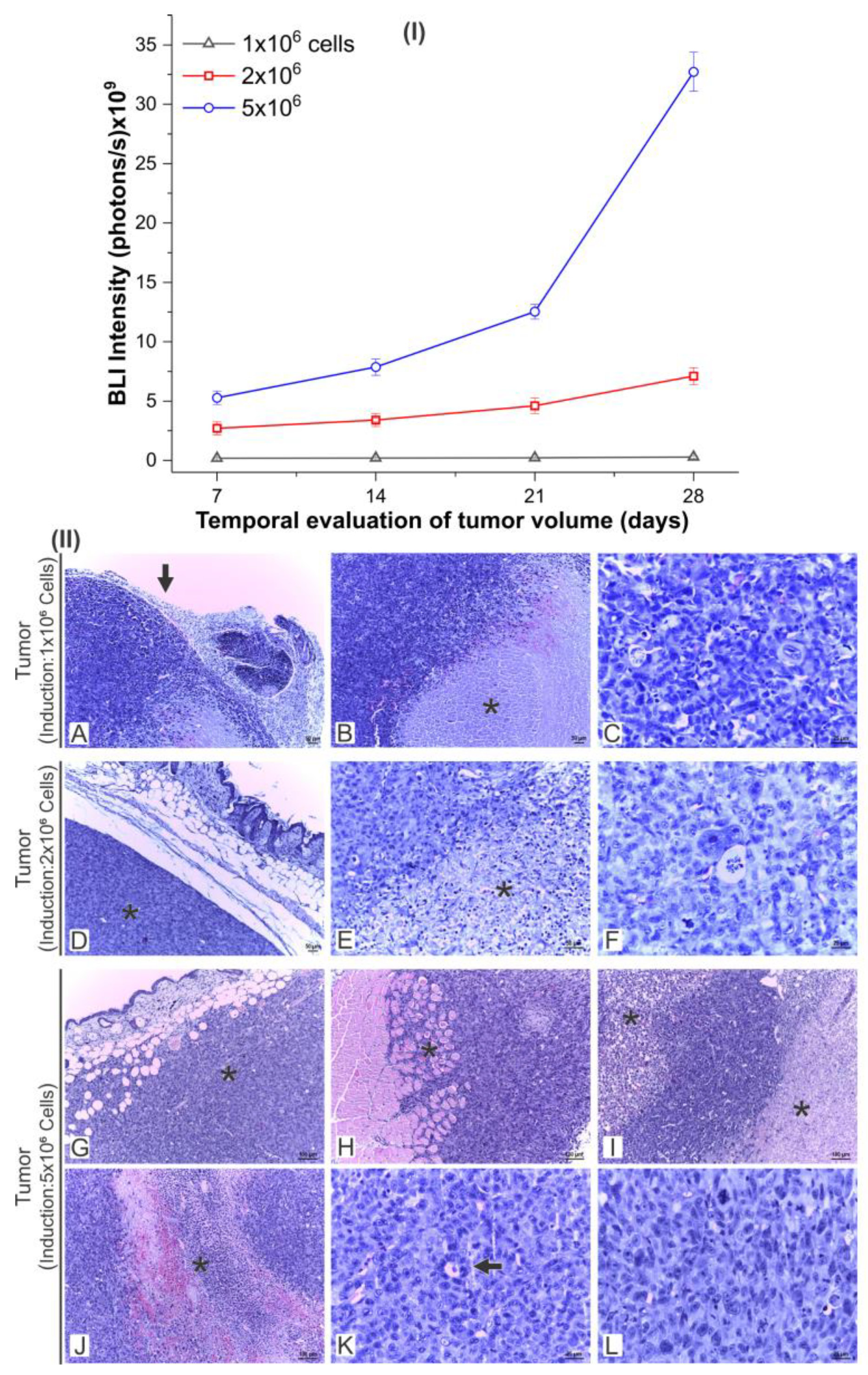
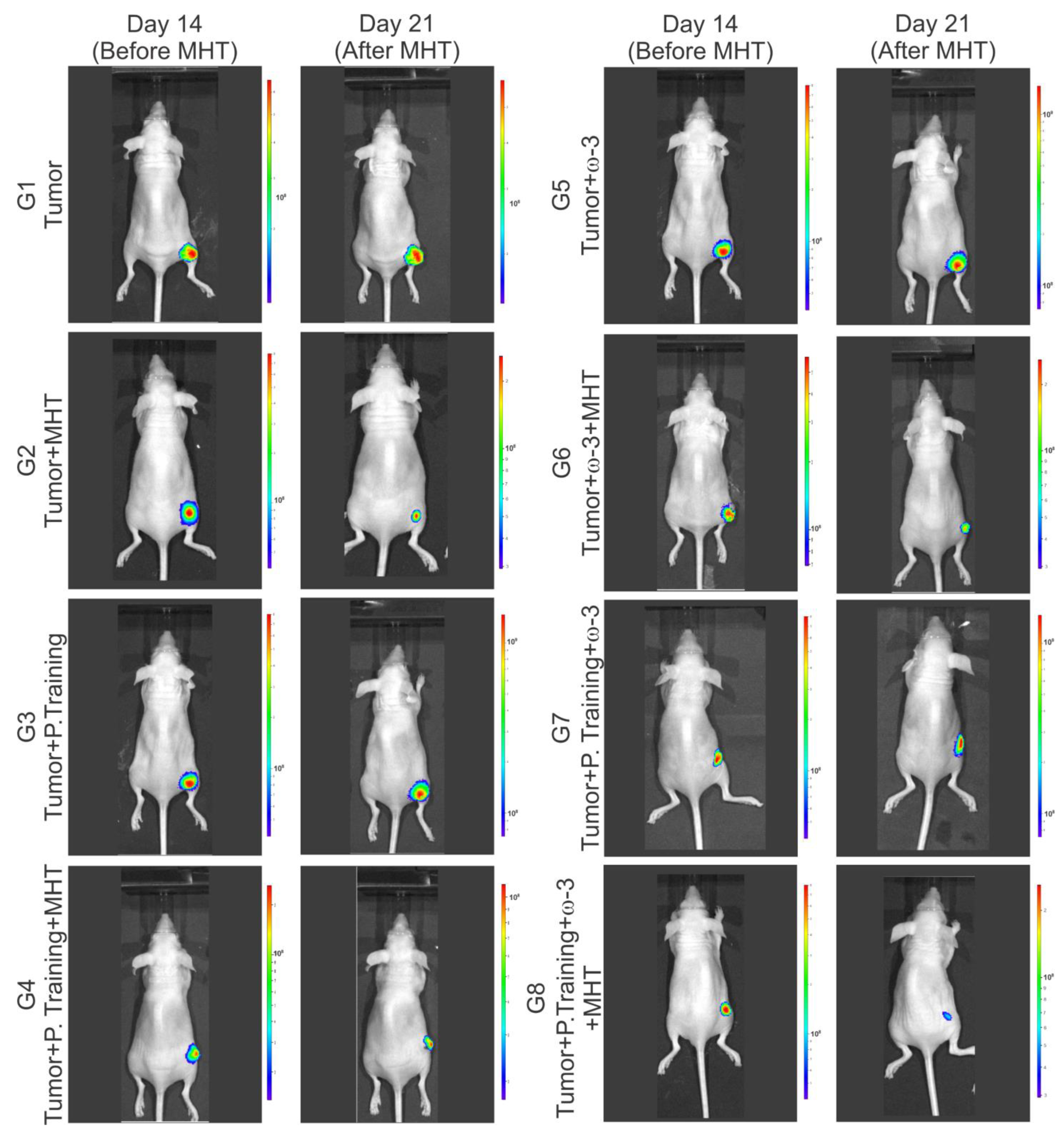
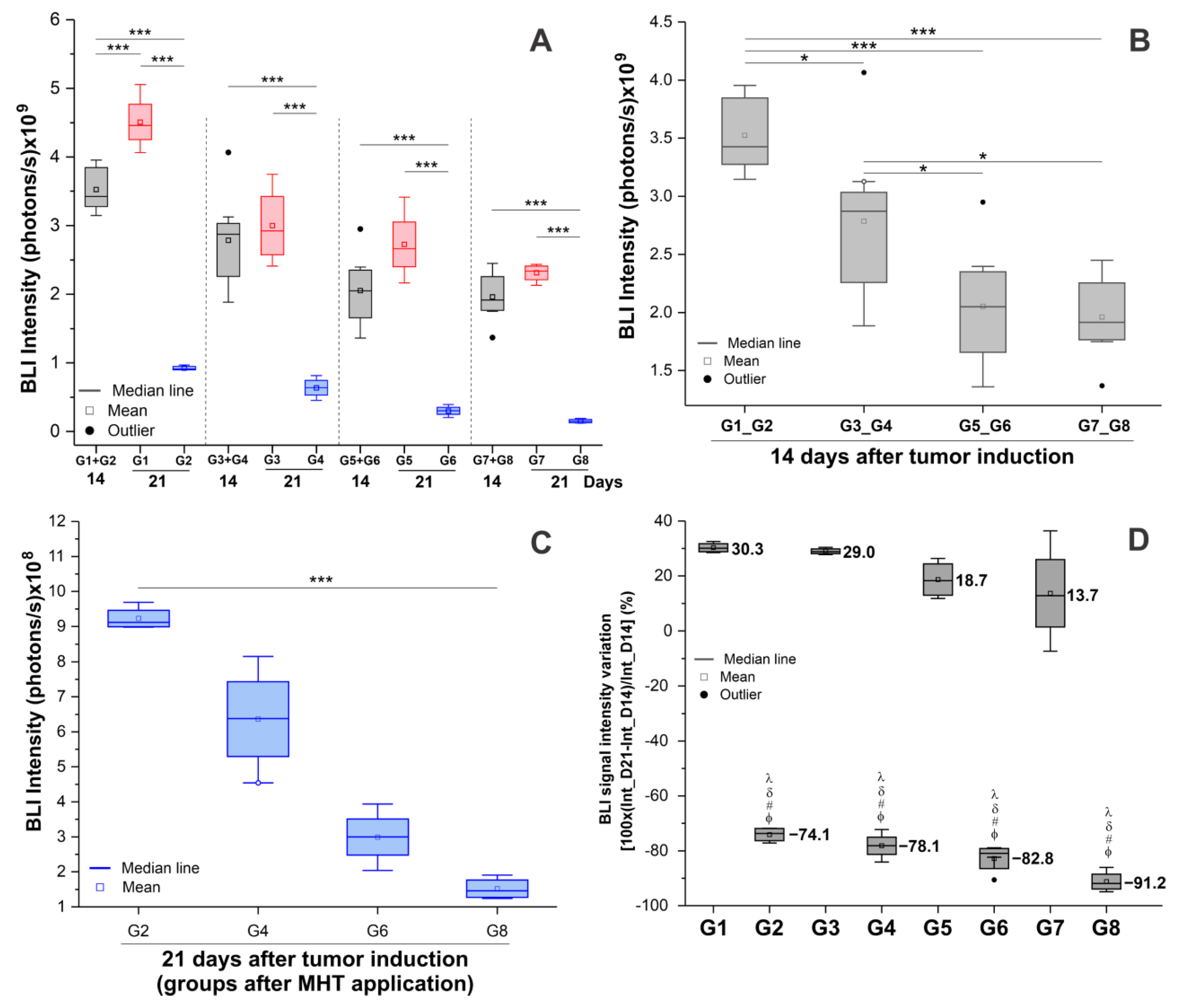
| Condition | G1 (n = 8) | G2 (n = 8) | G3 (n = 8) | G4 (n = 8) | G5 (n = 8) | G6 (n = 8) | G7 (n = 8) | G8 (n = 8) |
|---|---|---|---|---|---|---|---|---|
| Tumor Induction | ● | ● | ● | ● | ● | ● | ● | ● |
| Physical Training | ● | ● | ● | ● | ||||
| -3 supplementation | ● | ● | ● | ● | ||||
| MHT | ● | ● | ● | ● |
| Comparisons | Mean Difference | SE | t | p | |
|---|---|---|---|---|---|
| MDA-MB-231 + AMF | MDA-MB-231 + ION | 3.603 × 107 | 6.749 × 106 | 5.338 | 0.001 |
| MDA-MB-231 + ION + AMF | 1.291 × 109 | 6.749 × 106 | 191.339 | <0.001 | |
| MDA-MB-231 + ION | MDA-MB-231 + ION + AMF | 1.255 × 109 | 6.749 × 106 | 186.001 | <0.001 |
| Groups | Before MHT (14D) | After MHT (21D) | Statistic | ||
|---|---|---|---|---|---|
| Mean (Photon/s) | SD | Mean (Photon/s) | SD | p | |
| G1 | 3.524 × 109 | 3.155 × 108 | 4.508 × 109 | 4.082 × 108 | <0.001 |
| G2 | 9.228 × 108 | 3.279 × 107 | <0.001 | ||
| G3 | 2.785 × 109 | 6.769 × 108 | 3.000 × 109 | 5.729 × 108 | 0.816 |
| G4 | 6.363 × 108 | 1.498 × 108 | <0.001 | ||
| G5 | 2.055 × 109 | 5.100 × 108 | 2.723 × 109 | 5.160 × 108 | 0.074 |
| G6 | 2.995 × 108 | 7.784 × 107 | <0.001 | ||
| G7 | 1.961 × 109 | 3.495 × 108 | 2.313 × 109 | 3.495 × 108 | 0.116 |
| G8 | 1.520 × 108 | 3.080 × 107 | <0.001 | ||
| Comparisons | Mean Difference | SE | t | p | |
|---|---|---|---|---|---|
| G1 + G2-14D | G3 + G4-14D | 7.387 × 108 | 2.424 × 108 | 3.048 | 0.030 |
| G5 + G6-14D | 1.469 × 109 | 2.424 × 108 | 6.059 | <0.001 | |
| G7 + G8-14D | 1.563 × 109 | 2.424 × 108 | 6.446 | <0.001 | |
| G3 + G4-14D | G5 + G6-14D | 7.300 × 108 | 2.424 × 108 | 3.012 | 0.033 |
| G7 + G8-14D | 8.238 × 108 | 2.424 × 108 | 3.398 | 0.012 | |
| G5 + G6-14D | G7 + G8-14D | 9.375 × 107 | 2.424 × 108 | 0.387 | 1.000 |
| Comparisons | Mean Difference | SE | t | p | |
|---|---|---|---|---|---|
| G1 + G2-14D | G1-D21 | −9.837 × 108 | 1.860 × 108 | −5.288 | <0.001 |
| G2-D21 | 2.601 × 109 | 1.860 × 108 | 13.981 | <0.001 | |
| G1-D21 | G2-D21 | 3.585 × 109 | 2.148 × 108 | 16.687 | <0.001 |
| G3 + G4-D14 | G3-D21 | −2.150 × 108 | 3.507 × 108 | −0.613 | 0.816 |
| G4-D21 | 2.149 × 109 | 3.507 × 108 | 6.127 | <0.001 | |
| G3-D21 | G4-D21 | 2.364 × 109 | 4.049 × 108 | 5.837 | <0.001 |
| G5 + G6-D14 | G5-D21 | −6.675 × 108 | 2.758 × 108 | −2.420 | 0.074 |
| G6-D21 | 1.756 × 109 | 2.758 × 108 | 6.365 | <0.001 | |
| G5-D21 | G6-D21 | 2.423 × 109 | 3.185 × 108 | 7.608 | <0.001 |
| G7 + G8-D14 | G7-D21 | −3.512 × 108 | 1.624 × 108 | −2.163 | 0.116 |
| G8-D21 | 1.809 × 109 | 1.624 × 108 | 11.141 | <0.001 | |
| G7-D21 | G8-D21 | 2.161 × 109 | 1.875 × 108 | 11.522 | <0.001 |
| Comparisons | Mean Difference | SE | t | p | |
|---|---|---|---|---|---|
| G2_D21 | G4-D21 | 2.865 × 108 | 6.178 × 107 | 4.637 | 0.003 |
| G6-D21 | 6.232 × 108 | 6.178 × 107 | 10.088 | <0.001 | |
| G8-D21 | 7.707 × 108 | 6.178 × 107 | 12.476 | <0.001 | |
| G4-D21 | G6-D21 | 3.368 × 108 | 6.178 × 107 | 5.451 | <0.001 |
| G8-D21 | 4.842 × 108 | 6.178 × 107 | 7.838 | <0.001 | |
| G6-D21 | G8-D21 | 1.475 × 108 | 6.178 × 107 | 2.388 | 0.133 |
| Groups | Efficacy (%) | |
|---|---|---|
| Mean | SD | |
| G1 | +30.3 | 1.8 |
| G2 | −74.1 | 2.6 |
| G3 | +29.0 | 1.1 |
| G4 | −78.1 | 4.8 |
| G5 | +18.7 | 6.8 |
| G6 | −82.8 | 5.3 |
| G7 | +13.7 | 18.0 |
| G8 | −91.2 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theinel, M.H.; Nucci, M.P.; Cianciarullo, G.; Oliveira, F.A.; Alves, A.d.H.; Mamani, J.B.; Rego, G.N.d.A.; Valle, N.M.E.d.; Dias, O.F.M.; Costa, C.J.S.; et al. Breast Cancer Treatment Using the Magneto-Hyperthermia Technique Associated with Omega-3 Polyunsaturated Fatty Acids’ Supplementation and Physical Training. Pharmaceutics 2024, 16, 310. https://doi.org/10.3390/pharmaceutics16030310
Theinel MH, Nucci MP, Cianciarullo G, Oliveira FA, Alves AdH, Mamani JB, Rego GNdA, Valle NMEd, Dias OFM, Costa CJS, et al. Breast Cancer Treatment Using the Magneto-Hyperthermia Technique Associated with Omega-3 Polyunsaturated Fatty Acids’ Supplementation and Physical Training. Pharmaceutics. 2024; 16(3):310. https://doi.org/10.3390/pharmaceutics16030310
Chicago/Turabian StyleTheinel, Matheus Haubert, Mariana Penteado Nucci, Gabriela Cianciarullo, Fernando Anselmo Oliveira, Arielly da Hora Alves, Javier Bustamante Mamani, Gabriel Nery de Albuquerque Rego, Nicole Mastandrea Ennes do Valle, Olívia Furiama Metropolo Dias, Cícero Júlio Silva Costa, and et al. 2024. "Breast Cancer Treatment Using the Magneto-Hyperthermia Technique Associated with Omega-3 Polyunsaturated Fatty Acids’ Supplementation and Physical Training" Pharmaceutics 16, no. 3: 310. https://doi.org/10.3390/pharmaceutics16030310









