Excipients in Pharmaceutical Additive Manufacturing: A Comprehensive Exploration of Polymeric Material Selection for Enhanced 3D Printing
Abstract
:1. Introduction
- Binder jetting;
- Material jetting;
- Powder-bed fusion;
- Material extrusion;
- VAT photopolymerization;
- Sheet lamination;
- Directed energy deposition.
- Their origin (naturally occurring, semi-synthetically modified, or synthetically manufactured), e.g., while cellulose is the basic structural component of plant cell walls and thus the most abundant of all naturally occurring polymers, cellulose ethers such as hydroxypropyl cellulose (HPC) or hydroxyethyl cellulose (HEC) are semi-synthetic polymers that become water-soluble due to the introduction of their side chains. On the other hand, polymers such as polyvinylpyrrolidone (PVP) and copovidone (PVPVA) are synthetic polymers obtained by the radical polymerization of their monomers.
- Types of monomer(s) (homopolymer or copolymer), e.g., PVP is obtained from the polymerization of N-vinyl-pyrrolidone (NVP) solely, while PVPVA is a copolymer obtained from the polymerization of NVP with vinyl acetate.
- Gross topology of the chain structure (linear, branched, or crosslinked), e.g., whether the polymer chain or chain assembly can be mapped onto a one- (linear), two- (branched), or three- (crosslinked network) dimensional object.
- Interaction with water (water-soluble or water-insoluble), e.g., hydroxypropyl cellulose (HPC) is a water-soluble cellulose ether, while ethyl cellulose (EC) does not dissolve in water.
- Explanation of the significance of material properties in successful 3D printing.
- Introduction to the key process stages of 3D printing—feeding, deposition and adhesion—and a discussion about the importance of these key determinants of printability.
- Discussion of pharmaceutical polymers (cellulose ethers, polyvinyl polymers, and bioresorbable polymers) and their material properties (solubility, viscosity, rheology, and mechanical characteristics) for their use in different AM technologies.
- The additive manufacturing technology was clearly described and fit into the five ISO/ASTM 52900:2021 pharmaceutically relevant categories listed above.
- The polymers described were relevant for the functionality of the printed drug product and the authors provided sufficient information on the polymer types and grades used.
- The scope of the work was pharmaceutical or biomedical research with a particular focus on processability.
- There was no restriction on the publication period for manuscripts; however, more focus was put on papers published within the last 5 years.
2. Printability of Polymers—Important Material Characteristics
3. Pharmaceutical Polymers in Additive Manufacturing
3.1. Cellulosic Polymers
3.1.1. Carboxymethyl Cellulose Sodium (CMC)
3.1.2. Hydroxypropyl Methylcellulose (HPMC)
3.1.3. Hydroxypropyl Cellulose (HPC)
3.1.4. Hydroxyethyl Cellulose (HEC)
3.1.5. Ethyl Cellulose (EC)
3.2. Polyvinyl Polymers
3.2.1. Polyvinylpyrrolidone (PVP)
3.2.2. Copovidone (PVPVA)
3.3. Bioresorbable Polymers—Aliphatic Polyesters
3.3.1. Polyglycolide or Poly(glycolic acid) (PGA)
3.3.2. Polylactide or Poly(lactic acid) (PLA)
3.3.3. Polylactide-co-Glycolide or Poly(lactic-co-glycolic acid) (PLGA)
3.3.4. Polycaprolactone or Poly(ε-caprolactone) (PCL)
3.3.5. Poly(L-lactide-co-ε-caprolactone) (PLCL)
4. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bordbar-Khiabani, A.; Gasik, M. Electrochemical behavior of additively manufactured patterned titanium alloys under simulated normal, inflammatory, and severe inflammatory conditions. J. Mater. Res. Technol. 2023, 26, 356–370. [Google Scholar] [CrossRef]
- Fard, M.G.; Sharifianjazi, F.; Kazemi, S.S.; Rostamani, H.; Bathaei, M.S. Laser-Based Additive Manufacturing of Magnesium Alloys for Bone Tissue Engineering Applications: From Chemistry to Clinic. J. Manuf. Mater. Process. 2022, 6, 158. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Kovrlija, I.; Locs, J.; Loca, D.; Gasik, M. Octacalcium Phosphate-Laden Hydrogels on 3D-Printed Titanium Biomaterials Improve Corrosion Resistance in Simulated Biological Media. Int. J. Mol. Sci. 2023, 24, 13135. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, S. FDA Approves First 3D-printed epilepsy drug experts assess the benefits and caveats. Neurol. Today 2015, 15, 26–27. [Google Scholar] [CrossRef]
- Di Prima, M.; Coburn, J.; Hwang, D.; Kelly, J.; Khairuzzaman, A.; Ricles, L. Additively manufactured medical products—The FDA perspective. 3d Print. Med. 2016, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Tracy, T.; Wu, L.; Liu, X.; Cheng, S.; Li, X. 3D printing: Innovative solutions for patients and pharmaceutical industry. Int. J. Pharm. 2023, 631, 122480. [Google Scholar] [CrossRef]
- FDA. Technical Considerations for Additive Manufactured Medical Devices—Guidance for Industry and Food and Drug Administration Staff; U.S. Food & Drug Administration (FDA): Silver Spring, MD, USA, 2017.
- Awad, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Reshaping drug development using 3D printing. Drug Discov. Today 2018, 23, 1547–1555. [Google Scholar] [CrossRef]
- Boniatti, J.; Januskaite, P.; Fonseca, L.B.D.; Viçosa, A.L.; Amendoeira, F.C.; Tuleu, C.; Basit, A.W.; Goyanes, A.; Ré, M.I. Direct Powder Extrusion 3D Printing of Praziquantel to Overcome Neglected Disease Formulation Challenges in Paediatric Populations. Pharmaceutics 2021, 13, 1114. [Google Scholar] [CrossRef]
- Karavasili, C.; Eleftheriadis, G.K.; Gioumouxouzis, C.; Andriotis, E.G.; Fatouros, D.G. Mucosal drug delivery and 3D printing technologies: A focus on special patient populations. Adv. Drug Deliv. Rev. 2021, 176, 113858. [Google Scholar] [CrossRef]
- Awad, A.; Yao, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Tablets (Printlets) with Braille and Moon Patterns for Visually Impaired Patients. Pharmaceutics 2020, 12, 172. [Google Scholar] [CrossRef]
- ISO/ASTM 52900; Additive Manufacturing-General Principles-Fundamentals and Vocabulary. ISO: Geneva, Switzerland, 2021.
- Jacob, S.; Nair, A.B.; Patel, V.; Shah, J. 3D Printing Technologies: Recent Development and Emerging Applications in Various Drug Delivery Systems. AAPS PharmSciTech 2020, 21, 220. [Google Scholar] [CrossRef]
- Varghese, R.; Salvi, S.; Sood, P.; Karsiya, J.; Kumar, D. Recent advancements in additive manufacturing techniques employed in the pharmaceutical industry: A bird’s eye view. Ann. 3D Print. Med. 2022, 8, 100081. [Google Scholar] [CrossRef]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. J. Control. Release 2021, 329, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advances in powder bed fusion 3D printing in drug delivery and healthcare. Adv. Drug Deliv. Rev. 2021, 174, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Bandari, S.; Nyavanandi, D.; Dumpa, N.; Repka, M.A. Coupling hot melt extrusion and fused deposition modeling: Critical properties for successful performance. Adv. Drug Deliv. Rev. 2021, 172, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Rahman, J.; Quodbach, J. Versatility on demand—The case for semi-solid micro-extrusion in pharmaceutics. Adv. Drug Deliv. Rev. 2021, 172, 104–126. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Mehta, T.; Sansare, S.; Sharifi, L.; Ma, A.W.K.; Chaudhuri, B. Pharmaceutical applications of powder-based binder jet 3D printing process—A review. Adv. Drug Deliv. Rev. 2021, 177, 113943. [Google Scholar] [CrossRef] [PubMed]
- Govender, R.; Kissi, E.O.; Larsson, A.; Tho, I. Polymers in pharmaceutical additive manufacturing: A balancing act between printability and product performance. Adv. Drug Deliv. Rev. 2021, 177, 113923. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Brady, J.; Dürig, T.; Lee, P.I.; Li, J.X. Polymer Properties and Characterization. In Developing Solid Oral Dosage Forms, 2nd ed.; Qiu, Y., Chen, Y., Zhang, G.G.Z., Yu, L., Mantri, R.V., Eds.; Academic Press: Boston, MA, USA, 2017; Chapter 7; pp. 181–223. [Google Scholar] [CrossRef]
- Cader, H.K.; Rance, G.A.; Alexander, M.R.; Gonçalves, A.D.; Roberts, C.J.; Tuck, C.J.; Wildman, R.D. Water-based 3D inkjet printing of an oral pharmaceutical dosage form. Int. J. Pharm. 2019, 564, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Infanger, S.; Haemmerli, A.; Iliev, S.; Baier, A.; Stoyanov, E.; Quodbach, J. Powder bed 3D-printing of highly loaded drug delivery devices with hydroxypropyl cellulose as solid binder. Int. J. Pharm. 2019, 555, 198–206. [Google Scholar] [CrossRef]
- Elbadawi, M.; Nikjoo, D.; Gustafsson, T.; Gaisford, S.; Basit, A.W. Pressure-assisted microsyringe 3D printing of oral films based on pullulan and hydroxypropyl methylcellulose. Int. J. Pharm. 2021, 595, 120197. [Google Scholar] [CrossRef]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical applications of hot-melt extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.G.; Figueiredo, S.; Fernandes, A.I.; Pinto, J.F. Polymer Selection for Hot-Melt Extrusion Coupled to Fused Deposition Modelling in Pharmaceutics. Pharmaceutics 2020, 12, 795. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Li, Y.; Wang, S.; Chai, Y.; Lou, J.; Chen, F.; Li, Q.; Pan, W.; Ding, P. Exploration and Preparation of a Dose-Flexible Regulation System for Levetiracetam Tablets via Novel Semi-Solid Extrusion Three-Dimensional Printing. J. Pharm. Sci. 2019, 108, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Gomez, L.; Gonzalez-Prada, I.; Millan, R.; Da Silva-Candal, A.; Bugallo-Casal, A.; Campos, F.; Concheiro, A.; Alvarez-Lorenzo, C. 3D printed carboxymethyl cellulose scaffolds for autologous growth factors delivery in wound healing. Carbohydr. Polym. 2022, 278, 118924. [Google Scholar] [CrossRef]
- Panraksa, P.; Qi, S.; Udomsom, S.; Tipduangta, P.; Rachtanapun, P.; Jantanasakulwong, K.; Jantrawut, P. Characterization of Hydrophilic Polymers as a Syringe Extrusion 3D Printing Material for Orodispersible Film. Polymers 2021, 13, 3454. [Google Scholar] [CrossRef]
- Gottschalk, N.; Burkard, A.; Quodbach, J.; Bogdahn, M. Drop-on-powder 3D printing of amorphous high dose oral dosage forms: Process development, opportunities and printing limitations. Int. J. Pharm. X 2023, 5, 100151. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm. 2018, 541, 101–107. [Google Scholar] [CrossRef]
- Mohamed, E.M.; Barakh Ali, S.F.; Rahman, Z.; Dharani, S.; Ozkan, T.; Kuttolamadom, M.A.; Khan, M.A. Formulation Optimization of Selective Laser Sintering 3D-Printed Tablets of Clindamycin Palmitate Hydrochloride by Response Surface Methodology. AAPS PharmSciTech 2020, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.A.; Thakkar, R.; Su, Y.; Williams, R.O.; Maniruzzaman, M. Selective Laser Sintering 3-Dimensional Printing as a Single Step Process to Prepare Amorphous Solid Dispersion Dosage Forms for Improved Solubility and Dissolution Rate. J. Pharm. Sci. 2021, 110, 1432–1443. [Google Scholar] [CrossRef] [PubMed]
- Barakh Ali, S.F.; Mohamed, E.M.; Ozkan, T.; Kuttolamadom, M.A.; Khan, M.A.; Asadi, A.; Rahman, Z. Understanding the effects of formulation and process variables on the printlets quality manufactured by selective laser sintering 3D printing. Int. J. Pharm. 2019, 570, 118651. [Google Scholar] [CrossRef] [PubMed]
- Allahham, N.; Fina, F.; Marcuta, C.; Kraschew, L.; Mohr, W.; Gaisford, S.; Basit, A.W.; Goyanes, A. Selective Laser Sintering 3D Printing of Orally Disintegrating Printlets Containing Ondansetron. Pharmaceutics 2020, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Salvage, J.P.; Maniruzzaman, M.; Nokhodchi, A. Role of release modifiers to modulate drug release from fused deposition modelling (FDM) 3D printed tablets. Int. J. Pharm. 2021, 597, 120315. [Google Scholar] [CrossRef] [PubMed]
- Ayyoubi, S.; Cerda, J.R.; Fernández-García, R.; Knief, P.; Lalatsa, A.; Healy, A.M.; Serrano, D.R. 3D printed spherical mini-tablets: Geometry versus composition effects in controlling dissolution from personalised solid dosage forms. Int. J. Pharm. 2021, 597, 120336. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; Samaro, A.; Marchesini, F.H.; Shaqour, B.; Macedo, J.; Vanhoorne, V.; Vervaet, C. Extrusion-based 3D printing of oral solid dosage forms: Material requirements and equipment dependencies. Int. J. Pharm. 2021, 598, 120361. [Google Scholar] [CrossRef] [PubMed]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Solanki, N.G.; Tahsin, M.; Shah, A.V.; Serajuddin, A.T.M. Formulation of 3D Printed Tablet for Rapid Drug Release by Fused Deposition Modeling: Screening Polymers for Drug Release, Drug-Polymer Miscibility and Printability. J. Pharm. Sci. 2018, 107, 390–401. [Google Scholar] [CrossRef]
- Fanous, M.; Gold, S.; Hirsch, S.; Ogorka, J.; Imanidis, G. Development of immediate release (IR) 3D-printed oral dosage forms with focus on industrial relevance. Eur. J. Pharm. Sci. 2020, 155, 105558. [Google Scholar] [CrossRef]
- Govender, R.; Abrahmsén-Alami, S.; Larsson, A.; Borde, A.; Liljeblad, A.; Folestad, S. Independent Tailoring of Dose and Drug Release via a Modularized Product Design Concept for Mass Customization. Pharmaceutics 2020, 12, 771. [Google Scholar] [CrossRef]
- Saydam, M.; Takka, S. Improving the dissolution of a water-insoluble orphan drug through a fused deposition modelling 3-Dimensional printing technology approach. Eur. J. Pharm. Sci. 2020, 152, 105426. [Google Scholar] [CrossRef]
- Vo, A.Q.; Zhang, J.; Nyavanandi, D.; Bandari, S.; Repka, M.A. Hot melt extrusion paired fused deposition modeling 3D printing to develop hydroxypropyl cellulose based floating tablets of cinnarizine. Carbohydr. Polym. 2020, 246, 116519. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Yang, X.L.; Huang, W.D.; Liu, J.; Wang, Y.G.; Xu, H. Tablets with material gradients fabricated by three-dimensional printing. J. Pharm. Sci. 2007, 96, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Branford-White, C.; Yang, Y.C.; Zhu, L.M.; Welbeck, E.W.; Yang, X.L. A novel fast disintegrating tablet fabricated by three-dimensional printing. Drug Dev. Ind. Pharm. 2009, 35, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Branford-White, C.; Ma, Z.H.; Zhu, L.M.; Li, X.Y.; Yang, X.L. Novel drug delivery devices for providing linear release profiles fabricated by 3DP. Int. J. Pharm. 2009, 370, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Fina, F.; Goyanes, A.; Madla, C.M.; Awad, A.; Trenfield, S.J.; Kuek, J.M.; Patel, P.; Gaisford, S.; Basit, A.W. 3D printing of drug-loaded gyroid lattices using selective laser sintering. Int. J. Pharm. 2018, 547, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Fina, F.; Trenfield, S.J.; Patel, P.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Pellets (Miniprintlets): A Novel, Multi-Drug, Controlled Release Platform Technology. Pharmaceutics 2019, 11, 148. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, P.; Vo, A.Q.; Bandari, S.; Yang, F.; Durig, T.; Repka, M.A. Development and evaluation of pharmaceutical 3D printability for hot melt extruded cellulose-based filaments. J. Drug Deliv. Sci. Technol. 2019, 52, 292–302. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef]
- Adams, D.; Ounaies, Z.; Basak, A. Printability Assessment of Ethyl Cellulose Biopolymer Using Direct Ink Writing. JOM 2021, 73, 3761–3770. [Google Scholar] [CrossRef]
- Borujeni, S.H.; Mirdamadian, S.Z.; Varshosaz, J.; Taheri, A. Three-dimensional (3D) printed tablets using ethyl cellulose and hydroxypropyl cellulose to achieve zero order sustained release profile. Cellulose 2020, 27, 1573–1589. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Li, H.; Ou, Z.; Yang, G. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release. Eur. J. Pharm. Sci. 2018, 115, 11–18. [Google Scholar] [CrossRef]
- Hartzke, D.; Pössl, A.; Schlupp, P.; Runkel, F.E. Evaluation of Hydroxyethyl Cellulose Grades as the Main Matrix Former to Produce 3D-Printed Controlled-Release Dosage Forms. Pharmaceutics 2022, 14, 2103. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Rowland, M.; Gaisford, S.; Basit, A.W. 3D Printing of Tunable Zero-Order Release Printlets. Polymers 2020, 12, 1769. [Google Scholar] [CrossRef]
- Gospodinova, A.; Nankov, V.; Tomov, S.; Redzheb, M.; Petrov, P.D. Extrusion bioprinting of hydroxyethylcellulose-based bioink for cervical tumor model. Carbohydr. Polym. 2021, 260, 117793. [Google Scholar] [CrossRef]
- Elbl, J.; Gajdziok, J.; Kolarczyk, J. 3D printing of multilayered orodispersible films with in-process drying. Int. J. Pharm. 2020, 575, 118883. [Google Scholar] [CrossRef]
- Luo, J.; Xia, G.; Liu, L.; Ji, A.; Luo, Q. Fabrication of Chitosan/Hydroxyethyl Cellulose/TiO2 Incorporated Mulberry Anthocyanin 3D-Printed Bilayer Films for Quality of Litchis. Foods 2022, 11, 3286. [Google Scholar] [CrossRef]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 118471. [Google Scholar] [CrossRef] [PubMed]
- Ghanizadeh Tabriz, A.; Nandi, U.; Hurt, A.P.; Hui, H.W.; Karki, S.; Gong, Y.; Kumar, S.; Douroumis, D. 3D printed bilayer tablet with dual controlled drug release for tuberculosis treatment. Int. J. Pharm. 2021, 593, 120147. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Parietti, F.; Loreti, G.; Maroni, A.; Gazzaniga, A.; Zema, L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J. Drug Deliv. Sci. Technol. 2015, 30, 360–367. [Google Scholar] [CrossRef]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient acceptability of 3D printed medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef]
- Sjöholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123. [Google Scholar] [CrossRef]
- Cui, M.; Pan, H.; Fang, D.; Qiao, S.; Wang, S.; Pan, W. Fabrication of high drug loading levetiracetam tablets using semi-solid extrusion 3D printing. J. Drug Deliv. Sci. Technol. 2020, 57, 101683. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Goyanes, A.; Telford, R.; Wilsdon, D.; Rowland, M.; Gaisford, S.; Basit, A.W. 3D printed drug products: Non-destructive dose verification using a rapid point-and-shoot approach. Int. J. Pharm. 2018, 549, 283–292. [Google Scholar] [CrossRef]
- Kadry, H.; Al-Hilal, T.A.; Keshavarz, A.; Alam, F.; Xu, C.; Joy, A.; Ahsan, F. Multi-purposable filaments of HPMC for 3D printing of medications with tailored drug release and timed-absorption. Int. J. Pharm. 2018, 544, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Roberts, C.J. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int. J. Pharm. 2014, 461, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Yang, Y.; Jia, D.; Li, P.; Li, Q.; Chen, F.; Wang, S.; Pan, W.; Ding, P. Effect of novel internal structures on printability and drug release behavior of 3D printed tablets. J. Drug Deliv. Sci. Technol. 2019, 49, 14–23. [Google Scholar] [CrossRef]
- Conceição, J.; Farto-Vaamonde, X.; Goyanes, A.; Adeoye, O.; Concheiro, A.; Cabral-Marques, H.; Sousa Lobo, J.M.; Alvarez-Lorenzo, C. Hydroxypropyl-β-cyclodextrin-based fast dissolving carbamazepine printlets prepared by semisolid extrusion 3D printing. Carbohydr. Polym. 2019, 221, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Qin, H.; Acevedo, N.C.; Jiang, X.; Shi, X. 3D printing of extended-release tablets of theophylline using hydroxypropyl methylcellulose (HPMC) hydrogels. Int. J. Pharm. 2020, 591, 119983. [Google Scholar] [CrossRef] [PubMed]
- Abdella, S.; Afinjuomo, F.; Song, Y.; Upton, R.; Garg, S. Mucoadhesive Buccal Film of Estradiol for Hormonal Replacement Therapy: Development and In-Vivo Performance Prediction. Pharmaceutics 2022, 14, 542. [Google Scholar] [CrossRef]
- Wen, H.; He, B.; Wang, H.; Chen, F.; Li, P.; Cui, M.; Li, Q.; Pan, W.; Yang, X. Structure-Based Gastro-Retentive and Controlled-Release Drug Delivery with Novel 3D Printing. AAPS PharmSciTech 2019, 20, 68. [Google Scholar] [CrossRef]
- Fu, J.; Yu, X.; Jin, Y. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int. J. Pharm. 2018, 539, 75–82. [Google Scholar] [CrossRef]
- Liang, K.; Carmone, S.; Brambilla, D.; Leroux, J.-C. 3D printing of a wearable personalized oral delivery device: A first-in-human study. Sci. Adv. 2018, 4, eaat2544. [Google Scholar] [CrossRef]
- Wu, B.M.; Borland, S.W.; Giordano, R.A.; Cima, L.G.; Sachs, E.M.; Cima, M.J. Solid free-form fabrication of drug delivery devices. J. Control. Release 1996, 40, 77–87. [Google Scholar] [CrossRef]
- Kempin, W.; Domsta, V.; Brecht, I.; Semmling, B.; Tillmann, S.; Weitschies, W.; Seidlitz, A. Development of a dual extrusion printing technique for an acid- and thermo-labile drug. Eur. J. Pharm. Sci. 2018, 123, 191–198. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, J.H.; Ahn, C.B.; Hong, J.H.; Son, K.H.; Lee, J.W. Development of a 3D-Printed Drug-Eluting Stent for Treating Obstructive Salivary Gland Disease. ACS Biomater. Sci. Eng. 2019, 5, 3572–3581. [Google Scholar] [CrossRef] [PubMed]
- Viidik, L.; Vesala, J.; Laitinen, R.; Korhonen, O.; Ketolainen, J.; Aruväli, J.; Kirsimäe, K.; Kogermann, K.; Heinämäki, J.; Laidmäe, I.; et al. Preparation and characterization of hot-melt extruded polycaprolactone-based filaments intended for 3D-printing of tablets. Eur. J. Pharm. Sci. 2021, 158, 105619. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Nagarajan, S.; Belaid, H.; Farha, C.; Iatsunskyi, I.; Coy, E.; Soussan, L.; Huon, V.; Bares, J.; Belkacemi, K.; et al. Fabrication of 3D printed antimicrobial polycaprolactone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2021, 118, 111525. [Google Scholar] [CrossRef] [PubMed]
- Rahman-Yildir, J.; Fischer, B.; Breitkreutz, J. Development of sustained-release drug-loaded intravesical inserts via semi-solid micro-extrusion 3D-printing for bladder targeting. Int. J. Pharm. 2022, 622, 121849. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y.; Tsuji, H. Biodegradable polyesters for medical and ecological applications. Mol. Rapid Commun. 2000, 21, 117–132. [Google Scholar] [CrossRef]
- Serris, I.; Serris, P.; Frey, K.M.; Cho, H. Development of 3D-Printed Layered PLGA Films for Drug Delivery and Evaluation of Drug Release Behaviors. AAPS PharmSciTech 2020, 21, 256. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Yang, Y.; Huang, R.; Shi, X.; Chen, H.; Wang, J.; Chen, Y.; Tan, Y.; Tan, Z. E-Jet 3D-Printed Scaffolds as Sustained Multi-Drug Delivery Vehicles in Breast Cancer Therapy. Pharm. Res. 2019, 36, 182. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Holzberg, T.R.; Lim, C.G.; Gao, F.; Gargava, A.; Trachtenberg, J.E.; Mikos, A.G.; Fisher, J.P. 3D printing PLGA: A quantitative examination of the effects of polymer composition and printing parameters on print resolution. Biofabrication 2017, 9, 024101. [Google Scholar] [CrossRef] [PubMed]
- Walejewska, E.; Idaszek, J.; Heljak, M.; Chlanda, A.; Choinska, E.; Hasirci, V.; Swieszkowski, W. The effect of introduction of filament shift on degradation behaviour of PLGA- and PLCL-based scaffolds fabricated via additive manufacturing. Polym. Degrad. Stab. 2020, 171, 109030. [Google Scholar] [CrossRef]
- Bachtiar, E.O.; Ritter, V.C.; Gall, K. Structure-property relationships in 3D-printed poly(l-lactide-co-epsilon-caprolactone) degradable polymer. J. Mech. Behav. Biomed. Mater. 2021, 121, 104650. [Google Scholar] [CrossRef] [PubMed]
- Chausse, V.; Schieber, R.; Raymond, Y.; Ségry, B.; Sabaté, R.; Kolandaivelu, K.; Ginebra, M.-P.; Pegueroles, M. Solvent-cast direct-writing as a fabrication strategy for radiopaque stents. Addit. Manuf. 2021, 48, 102392. [Google Scholar] [CrossRef]
- Katstra, W.E.; Palazzolo, R.D.; Rowe, C.W.; Giritlioglu, B.; Teung, P.; Cima, M.J. Oral dosage forms fabricated by Three Dimensional Printing™. J. Control. Release 2000, 66, 1–9. [Google Scholar] [CrossRef]
- Rowe, C.W.; Katstra, W.E.; Palazzolo, R.D.; Giritlioglu, B.; Teung, P.; Cima, M.J. Multimechanism oral dosage forms fabricated by three dimensional printing™. J. Control. Release 2000, 66, 11–17. [Google Scholar] [CrossRef]
- Lee, K.-J.; Kang, A.; Delfino, J.J.; West, T.G.; Chetty, D.; Monkhouse, D.C.; Yoo, J. Evaluation of Critical Formulation Factors in the Development of a Rapidly Dispersing Captopril Oral Dosage Form. Drug Dev. Ind. Pharm. 2003, 29, 967–979. [Google Scholar] [CrossRef]
- Tian, P.; Yang, F.; Xu, Y.; Lin, M.-M.; Yu, L.-P.; Lin, W.; Lin, Q.-F.; Lv, Z.-F.; Huang, S.-Y.; Chen, Y.-Z. Oral disintegrating patient-tailored tablets of warfarin sodium produced by 3D printing. Drug Dev. Ind. Pharm. 2018, 44, 1918–1923. [Google Scholar] [CrossRef]
- Sen, K.; Mukherjee, R.; Sansare, S.; Halder, A.; Kashi, H.; Ma, A.W.K.; Chaudhuri, B. Impact of powder-binder interactions on 3D printability of pharmaceutical tablets using drop test methodology. Eur. J. Pharm. Sci. 2021, 160, 105755. [Google Scholar] [CrossRef]
- Kozakiewicz-Latała, M.; Nartowski, K.P.; Dominik, A.; Malec, K.; Gołkowska, A.M.; Złocińska, A.; Rusińska, M.; Szymczyk-Ziółkowska, P.; Ziółkowski, G.; Górniak, A.; et al. Binder jetting 3D printing of challenging medicines: From low dose tablets to hydrophobic molecules. Eur. J. Pharm. Biopharm. 2022, 170, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Li, S.W.; Kowsari, K.; Shetty, A.; Sorrells, L.; Sen, K.; Nagapudi, K.; Chaudhuri, B.; Ma, A.W.K. Binder-Jet 3D Printing of Indomethacin-laden Pharmaceutical Dosage Forms. J. Pharm. Sci. 2020, 109, 3054–3063. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, K.A.; de Wit, M.T.W.; Dickhoff, B.H.J. Evaluation of lactose based 3D powder bed printed pharmaceutical drug product tablets. Powder Technol. 2021, 390, 97–102. [Google Scholar] [CrossRef]
- Tian, P.; Yang, F.; Yu, L.-P.; Lin, M.-M.; Lin, W.; Lin, Q.-F.; Lv, Z.-F.; Huang, S.-Y.; Chen, Y.-Z. Applications of excipients in the field of 3D printed pharmaceuticals. Drug Dev. Ind. Pharm. 2019, 45, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Okwuosa, T.C.; Stefaniak, D.; Arafat, B.; Isreb, A.; Wan, K.-W.; Alhnan, M.A. A Lower Temperature FDM 3D Printing for the Manufacture of Patient-Specific Immediate Release Tablets. Pharm. Res. 2016, 33, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Okwuosa, T.C.; Pereira, B.C.; Arafat, B.; Cieszynska, M.; Isreb, A.; Alhnan, M.A. Fabricating a Shell-Core Delayed Release Tablet Using Dual FDM 3D Printing for Patient-Centred Therapy. Pharm. Res. 2017, 34, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Gioumouxouzis, C.I.; Tzimtzimis, E.; Katsamenis, O.L.; Dourou, A.; Markopoulou, C.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. Fabrication of an osmotic 3D printed solid dosage form for controlled release of active pharmaceutical ingredients. Eur. J. Pharm. Sci. 2020, 143, 105176. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Alexander, M.R.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. 3D extrusion printing of high drug loading immediate release paracetamol tablets. Int. J. Pharm. 2018, 538, 223–230. [Google Scholar] [CrossRef]
- Khaled, S.A.; Alexander, M.R.; Irvine, D.J.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. Extrusion 3D Printing of Paracetamol Tablets from a Single Formulation with Tunable Release Profiles Through Control of Tablet Geometry. AAPS PharmSciTech 2018, 19, 3403–3413. [Google Scholar] [CrossRef]
- Dores, F.; Kuźmińska, M.; Soares, C.; Bohus, M.; A Shervington, L.; Habashy, R.; Pereira, B.C.; Peak, M.; Isreb, A.; Alhnan, M.A. Temperature and solvent facilitated extrusion based 3D printing for pharmaceuticals. Eur. J. Pharm. Sci. 2020, 152, 105430. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Choudhury, D.; Yadav, V.; Murty, U.S.N.; Banerjee, S. 3D printing of nanocomposite pills through desktop vat photopolymerization (stereolithography) for drug delivery reasons. 3D Print Med. 2022, 8, 3. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Aho, J.; Bøtker, J.P.; Genina, N.; Edinger, M.; Arnfast, L.; Rantanen, J. Roadmap to 3D-Printed Oral Pharmaceutical Dosage Forms: Feedstock Filament Properties and Characterization for Fused Deposition Modeling. J. Pharm. Sci. 2019, 108, 26–35. [Google Scholar] [CrossRef]
- Hirshfield, L.; Giridhar, A.; Taylor, L.S.; Harris, M.T.; Reklaitis, G.V. Dropwise Additive Manufacturing of Pharmaceutical Products for Solvent-Based Dosage Forms. J. Pharm. Sci. 2014, 103, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Içten, E.; Giridhar, A.; Taylor, L.S.; Nagy, Z.K.; Reklaitis, G.V. Dropwise Additive Manufacturing of Pharmaceutical Products for Melt-Based Dosage Forms. J. Pharm. Sci. 2015, 104, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- McMullen, R.L.; Ozkan, S.; Gillece, T. Physicochemical Properties of Cellulose Ethers. Cosmetics 2022, 9, 52. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing: Principles and pharmaceutical applications of selective laser sintering. Int. J. Pharm. 2020, 586, 119594. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Chavan, R.B.; Rathi, S.; Jyothi, V.; Shastri, N.R. Cellulose based polymers in development of amorphous solid dispersions. Asian J Pharm Sci 2019, 14, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Funk, N.L.; Fantaus, S.; Beck, R.C.R. Immediate release 3D printed oral dosage forms: How different polymers have been explored to reach suitable drug release behaviour. Int. J. Pharm. 2022, 625, 122066. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.R.; Poudel, S.; Kim, D.W. Cellulose and its derivatives for application in 3D printing of pharmaceuticals. J. Pharm. Investig. 2021, 51, 1–22. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tukhani, M.; Hussain, C.M. Recent advancements in 3D bioprinting technology of carboxymethyl cellulose-based hydrogels: Utilization in tissue engineering. Adv. Colloid Interface Sci. 2021, 292, 102415. [Google Scholar] [CrossRef] [PubMed]
- Zennifer, A.; Senthilvelan, P.; Sethuraman, S.; Sundaramurthi, D. Key advances of carboxymethyl cellulose in tissue engineering & 3D bioprinting applications. Carbohydr. Polym. 2021, 256, 117561. [Google Scholar] [CrossRef]
- Wang, X.; Qi, J.; Zhang, W.; Pu, Y.; Yang, R.; Wang, P.; Liu, S.; Tan, X.; Chi, B. 3D-printed antioxidant antibacterial carboxymethyl cellulose/ε-polylysine hydrogel promoted skin wound repair. Int. J. Biol. Macromol. 2021, 187, 91–104. [Google Scholar] [CrossRef]
- Sarkar, N. Thermal gelation properties of methyl and hydroxypropyl methylcellulose. J. Appl. Polym. Sci. 1979, 24, 1073–1087. [Google Scholar] [CrossRef]
- Haque, A.; Morris, E.R. Thermogelation of methylcellulose. Part I: Molecular structures and processes. Carbohydr. Polym. 1993, 22, 161–173. [Google Scholar] [CrossRef]
- Haque, A.; Richardson, R.K.; Morris, E.R.; Gidley, M.J.; Caswell, D.C. Thermogelation of methylcellulose. Part II: Effect of hydroxypropyl substituents. Carbohydr. Polym. 1993, 22, 175–186. [Google Scholar] [CrossRef]
- Dürig, T.; Karan, K. Binders in Pharmaceutical Granulation. In Handbook of Pharmaceutical Granulation Technology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Khatri, P.; Katikaneni, P.; Desai, D.; Minko, T. Evaluation of Affinisol® HPMC polymers for direct compression process applications. J. Drug Deliv. Sci. Technol. 2018, 47, 461–467. [Google Scholar] [CrossRef]
- Meena, A.; Parikh, T.; Gupta, S.S.; Serajuddin, A.T. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion-II: Cellulosic polymers. J. Excip. Food Chem. 2014, 5, 46–55. [Google Scholar]
- Rials, T.G.; Glasser, W.G. Thermal and dynamic mechanical properties of hydroxypropyl cellulose films. J. Appl. Polym. Sci. 1988, 36, 749–758. [Google Scholar] [CrossRef]
- Picker-Freyer, K.M.; Dürig, T. Physical mechanical and tablet formation properties of hydroxypropylcellulose: In pure form and in mixtures. AAPS PharmSciTech 2007, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Luebbert, C.; Stoyanov, E.; Sadowski, G. Phase behavior of ASDs based on hydroxypropyl cellulose. Int. J. Pharm. X 2021, 3, 100070. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Dürig, T. Cellulose Ethers for Extrusion Applications. In Melt Extrusion: Materials, Technology and Drug Product Design; Repka, M.A., Langley, N., DiNunzio, J., Eds.; Springer: New York, NY, USA, 2013; pp. 123–144. [Google Scholar] [CrossRef]
- Rekhi, G.S.; Jambhekar, S.S. Ethylcellulose—A Polymer Review. Drug Dev. Ind. Pharm. 1995, 21, 61–77. [Google Scholar] [CrossRef]
- Kavimughil, M.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Effect of material composition and 3D printing temperature on hot-melt extrusion of ethyl cellulose based medium chain triglyceride oleogel. J. Food Eng. 2022, 329, 111055. [Google Scholar] [CrossRef]
- Haaf, F.; Sanner, A.; Straub, F. Polymers of N-Vinylpyrrolidone: Synthesis, Characterization and Uses. Polym. J. 1985, 17, 143–152. [Google Scholar] [CrossRef]
- Wen, H.; Morris, K.R.; Park, K. Study on the Interactions Between Polyvinylpyrrolidone (PVP) and Acetaminophen Crystals: Partial Dissolution Pattern Change. J. Pharm. Sci. 2005, 94, 2166–2174. [Google Scholar] [CrossRef]
- Kroschwitz, J. Kirk-Othmer Encyclopedia of Chemical Technology; Wiley-Interscience: Hoboken, NJ, USA, 1993; Volume 10. [Google Scholar] [CrossRef]
- Dürig, T. Binders in Pharmaceutical Granulation. In Handbook of Pharmaceutical Granulation Technology, 3rd ed.; Parikh, Ed.; Informa Health Care: New York, NY, USA, 2010; pp. 78–97. [Google Scholar]
- Van den Mooter, G.; Wuyts, M.; Blaton, N.; Busson, R.; Grobet, P.; Augustijns, P.; Kinget, R. Physical stabilisation of amorphous ketoconazole in solid dispersions with polyvinylpyrrolidone K25. Eur. J. Pharm. Sci. 2001, 12, 261–269. [Google Scholar] [CrossRef]
- Fikentscher, H.; Herrle, K. Polyvinylpyrrolidone. Mod. Plast. 1946, 23, 157–161, 212, 214, 216, 218. [Google Scholar]
- Bühler, V. Polyvinylpyrrolidone Excipients for Pharmaceuticals; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Rahman, M.; Ozkan, S.; Lester, J.; Farzana, I.; Bi, V.; Dürig, T. Plasticizer Compatibility and thermal and rheological properties of Plasdone povidone and copovidone polymers for hot-melt extrusion applications. In Proceedings of the Annual Meeting of the American Association of Pharmaceutical Scientists (AAPS), Chicago, IL, USA, 14–18 October 2012. [Google Scholar]
- Butreddy, A.; Sarabu, S.; Bandari, S.; Batra, A.; Lawal, K.; Chen, N.N.; Bi, V.; Durig, T.; Repka, M.A. Influence of Plasdone(™) S630 Ultra-an Improved Copovidone on the Processability and Oxidative Degradation of Quetiapine Fumarate Amorphous Solid Dispersions Prepared via Hot-Melt Extrusion Technique. AAPS PharmSciTech 2021, 22, 196. [Google Scholar] [CrossRef]
- Arndt, O.-R.; Kleinebudde, P. Influence of binder properties on dry granules and tablets. Powder Technol. 2018, 337, 68–77. [Google Scholar] [CrossRef]
- Bühler, V. Kollidon-Polyvinylpyrrolidone Excipients for the Pharmaceutical Industry; BASF SE Pharma Ingredients & Services: Ludwigshafen, Germany, 2008. [Google Scholar]
- Parikh, T.; Gupta, S.S.; Meena, A.K.; Vitez, I.; Mahajan, N.; Serajuddin, A.T. Application of film-casting technique to investigate drug-polymer miscibility in solid dispersion and hot-melt extrudate. J. Pharm. Sci. 2015, 104, 2142–2152. [Google Scholar] [CrossRef] [PubMed]
- Moroni, A. A Novel Copovidone Binder for Dry Granulation and Direct-Compression Tableting. Pharm. Technol. 2001, 1, 8. [Google Scholar]
- Duffy, P.; McMahon, S.; Wang, X.; Keaveney, S.; O’Cearbhaill, E.D.; Quintana, I.; Rodríguez, F.J.; Wang, W. Synthetic bioresorbable poly-α-hydroxyesters as peripheral nerve guidance conduits; a review of material properties, design strategies and their efficacy to date. Biomater. Sci. 2019, 7, 4912–4943. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymer 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Agrawal, C.; Niederauer, G.; Micallef, D.; Athanasiou, K. The use of PLA-PGA polymers in orthopaedics. Encycl. Handb. Biomater. Bioeng. Part A Mater. 1995, 2, 1055. [Google Scholar]
- Löfgren, A.; Albertsson, A.-C.; Dubois, P.; Jérôme, R. Recent Advances in Ring-Opening Polymerization of Lactones and Related Compounds. J. Macromol. Sci. Part C 1995, 35, 379–418. [Google Scholar] [CrossRef]
- Jérôme, C.; Lecomte, P. Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization. Adv. Drug Deliv. Rev. 2008, 60, 1056–1076. [Google Scholar] [CrossRef]
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci. 2002, 27, 87–133. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Kausch, H.H. Molecular Weight Distribution and Mechanical Properties. In Mechanical Properties and Testing of Polymers: An A–Z Reference; Springer Netherlands: Dordrecht, The Netherlands, 1999; pp. 143–150. [Google Scholar]
- Ammar, H.O.; Ghorab, M.M.; Mahmoud, A.A.; Higazy, I.M. Lamotrigine loaded poly-ε-(d,l-lactide-co-caprolactone) nanoparticles as brain delivery system. Eur. J. Pharm. Sci. 2018, 115, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, K.; Kiekens, P. Biopolymers: Overview of several properties and consequences on their applications. Polym. Test. 2002, 21, 433–442. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Zhang, Z.; He, F.; Wang, B.; Zhao, Y.; Wei, Z.; Zhang, H.; Sang, L. Biodegradable PGA/PBAT Blends for 3D Printing: Material Performance and Periodic Minimal Surface Structures. Polymers 2021, 13, 3757. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Etxeberria, A.; Sarasua, J.-R. Synthesis, structure and properties of poly(L-lactide-co-ε-caprolactone) statistical copolymers. J. Mech. Behav. Biomed. Mater. 2012, 9, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Vert, M. Polymères de fermentation: Les poly(acide lactique)s et leurs précurseurs, les acides lactiques. Actual. Chim. 2002, 11, 79–82. [Google Scholar]
- Vouyiouka, S.; Papaspyrides, C. Mechanistic aspects of solid-state polycondensation. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; Volume 4, pp. 857–874. [Google Scholar] [CrossRef]
- Mochizuki, M.; Hirami, M. Structural Effects on the Biodegradation of Aliphatic Polyesters. Polym. Adv. Technol. 1997, 8, 203–209. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.P.; Foster, R.J.; Mow, V.C. Composition and dynamics of articular cartilage: Structure, function, and maintaining healthy state. J. Orthop. Sports Phys. Ther. 1998, 28, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Bassand, C.; Siepmann, F.; Benabed, L.; Verin, J.; Freitag, J.; Charlon, S.; Soulestin, J.; Siepmann, J. 3D printed PLGA implants: How the filling density affects drug release. J. Control. Release 2023, 363, 1–11. [Google Scholar] [CrossRef]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casañas Pimentel, R.; Ige, P.P. Poly-ε-caprolactone (PCL), a promising polymer for pharmaceutical and biomedical applications: Focus on nanomedicine in cancer. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 85–126. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Teoh, S.-H.; Goh, B.-T.; Lim, J. Three-Dimensional Printed Polycaprolactone Scaffolds for Bone Regeneration Success and Future Perspective. Tissue Eng. Part A 2019, 25, 931–935. [Google Scholar] [CrossRef]
- Won, J.Y.; Kim, J.; Gao, G.; Kim, J.; Jang, J.; Park, Y.-H.; Cho, D.-W. 3D printing of drug-loaded multi-shell rods for local delivery of bevacizumab and dexamethasone: A synergetic therapy for retinal vascular diseases. Acta Biomater. 2020, 116, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Kang, Y.; Zhang, G.; Gao, C.; Lu, W.; Yang, C.; Zou, H.; Jiang, H. A comparative study on in vitro degradation behavior of PLLA-based copolymer monofilaments. Polym. Degrad. Stab. 2018, 158, 148–156. [Google Scholar] [CrossRef]
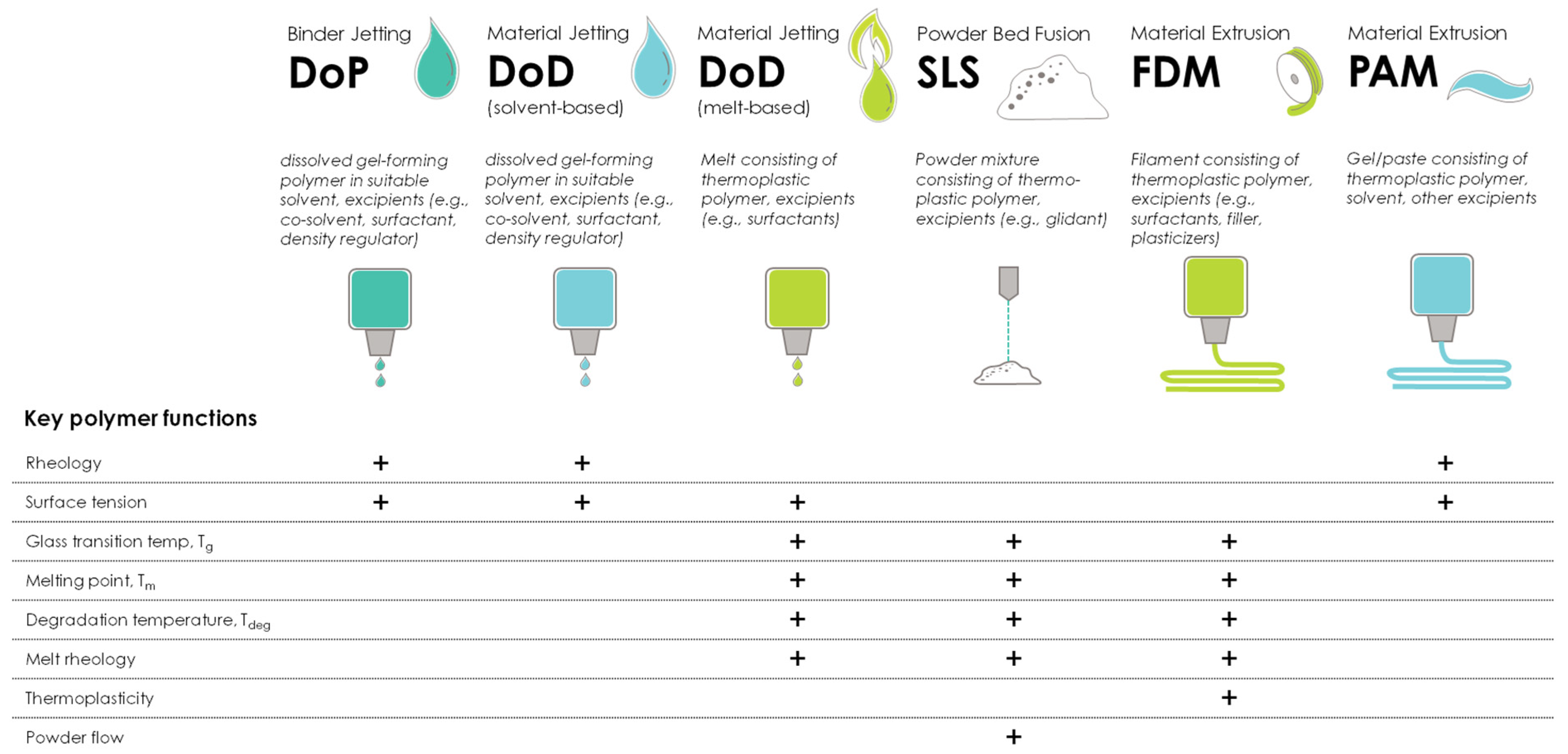




| Polymer (Alphabetical Order) | Additive Manufacturing Technology | ||||
|---|---|---|---|---|---|
| Binder Jetting | Material Jetting | Powder-Bed Fusion | Material Extrusion | ||
| DoP | DoD | SLS | FDM | PAM | |
| Carboxymethylcellulose sodium (Na-CMC) | [28,29,30] | ||||
| Copovidone (PVP/VA) | [31,32] | [11,33,34,35,36,37] | [9,38,39,40,41,42,43,44,45,46] | ||
| Ethyl cellulose (EC) | [47,48,49] | [50,51] | [38,39,52,53,54,55,56] | ||
| Hydroxyethyl cellulose (HEC) | [57,58] | [59,60,61] | |||
| Hydroxypropyl cellulose (HPC) | [24] | [39,40,43,46,52,53,55,57,62,63,64,65] | [66,67] | ||
| Hypromellose (HPMC) | [47,48,49] | [33,68] | [38,42,52,53,69] | [25,70,71,72,73,74,75,76] | |
| Polyglycolic acid (PGA) | [77,78] | ||||
| Polycaprolactone (PCL) | [79] | [77,80,81,82,83] | [84] | ||
| Polylactic acid (PLA) | [85] | [77,78] | |||
| Poly(lactic-co-glycolic) acid (PLGA) | [86,87] | [86,88] | |||
| Poly(L-lactide-co-ε-caprolactone) (PLCL) | [89] | [90] | [91] | ||
| Povidone (PVP) | [47,48,49,92,93,94,95,96,97,98,99,100] | [23] | [41,80,101,102,103] | [28,71,72,73,75,76,104,105,106] | |
| Cellulose Ether | Manufacturer | Approximate Molecular Weight (MW, Daltons) | Approximate DS/MS | Surface Tension (mN/m) |
|---|---|---|---|---|
| Hydroxypropyl cellulose | Ashland, Wilmington, DE, USA | 80,000–1,150,000 | 4.0 MS | 41.1–41.7 |
| Hydroxyethyl cellulose | Ashland, Wilmington, DE, USA | 720,000–1,300,000 | 2.6 MS | 46.4–49.4 |
| Hypromellose | Ashland, Wilmington, DE, USA | 400,000–1,200,000 | 1.5 Me DS 0.3 HP MS | 51.1–54.1 |
| Carboxy methylcellulose sodium | Ashland, Wilmington, DE, USA | 250,000–725,000 | 0.7–1.2 DS | 70.5–71.2 |
| Viscosity Grade | Weight Average Molecular Weight (Da) | Viscosity (mPa∙s) in Aqueous Solution | Solution Concentration (%) | Grade Used in 3DP Applications (Based on MW) |
|---|---|---|---|---|
| HF | 1,150,000 | 1500–3000 | 1 | [52] |
| MF | 850,000 | 4000–6500 | 2 | [46,55,67] |
| GF | 370,000 | 150–400 | 2 | |
| JF | 140,000 | 150–400 | 5 | [62] |
| LF | 95,000 | 75–150 | 5 | [39,53,64] |
| EF | 80,000 | 300–600 | 10 | [40,52,53,63,66] |
| ELF | 40,000 | 150–300 | 10 | [24,43,57,62,65] |
| Grade | Ethoxyl Substitution % | Average Molecular Weight | Viscosity | Solution Concentration (%) | Grade Used in 3DP Applications |
|---|---|---|---|---|---|
| N7 | 48.0–49.5 | 65,000 | 6–8 | 5 | [50,51] |
| N10 | 48.0–49.5 | 75,000 | 8–11 | 5 | [38,39,55,56] |
| N14 | 48.0–49.5 | 120,000 | 12–16 | 5 | [52,53] |
| N22 | 48.0–49.5 | 140,000 | 18–24 | 5 | |
| N50 | 48.0–49.5 | 160,000 | 40–52 | 5 | |
| N100 | 48.0–49.5 | 215,000 | 80–105 | 5 | |
| T10 | 49.6–51.0 | 75,000 | 8–11 | 5 |
| Grade | Nominal K-Value | K-Value Range a | Calculated Relative Viscosity of 10% (w/w) Solution (mm²/s) b | Intrinsic Viscosity (dL g−1) | MW (Dalton) | Mn (Dalton) | Tg (°C) |
|---|---|---|---|---|---|---|---|
| PVP K-12 | 12 | 10.2–13.8 | 1.48–1.8 | 0.05 | 2500 e | 1300 | 120 |
| PVP K-17 | 17 | 15.3–18.4 | 1.98–2.41 | 0.09 | 10,000 c | 2500 d | 140 |
| PVP K-25 | 25 | 22.5–27 | 3.23–4.56 | 0.16 | 25,000 c | 6000 d | 160 |
| PVP K-30 | 30 | 27–32.4 | 4.56–7.14 | 0.22 | 40,000 c | 10,000 d | 164 |
| PVP K-90 | 90 | 81–97.2 | 1075.37–7157.85 | 1.6 | 1,100,000 c | 150,000 e | 174 |
| Product Name | Manufacturer | Molecular Weight (Mn) | Tg | Particle Size (x50) |
|---|---|---|---|---|
| Plasdone™ S630 | Ashland | 14.000–18.000 | 110.69 | <100 |
| Plasdone™ S630 ultra | Ashland | 20.000 | 108.72 | <100 |
| Kollidon™ VA64 | BASF | 15.000–20.000 | 109 | 71.1 |
| Kollidon™ VA64 fine | BASF | 15.000–20.000 | 109 | 16.2 |
| Polymer | Tg (°C) | Tm (°C) | Tensile Strength (MPa) | Tensile Modulus (GPa) | Elongation at Break (%) | Degradation Time (Months) |
|---|---|---|---|---|---|---|
| PGA | 35–45 | >220 | 60–100 | 6–7 | 1.5–20 | 6–12 |
| PLLA | 55–65 | >170 | 45–70 | 2–4 | 3–10 | >24 |
| PDLLA | 45–55 | Amorphous | 15–30 | <2 | 2–10 | 12–16 |
| PLGA (50–85% DLA) | 40–55 | Amorphous | 40–55 | 1–2 | 2–10 | 1–6 |
| PCL | −60 | 60 | 20–34 | 0.2–0.35 | >700 | >24 |
| PLCL (60–90% LLA) | 35–54 | 156–163 | 23–27 | 0.44–1.6 | 300–379 | 3–12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muehlenfeld, C.; Duffy, P.; Yang, F.; Zermeño Pérez, D.; El-Saleh, F.; Durig, T. Excipients in Pharmaceutical Additive Manufacturing: A Comprehensive Exploration of Polymeric Material Selection for Enhanced 3D Printing. Pharmaceutics 2024, 16, 317. https://doi.org/10.3390/pharmaceutics16030317
Muehlenfeld C, Duffy P, Yang F, Zermeño Pérez D, El-Saleh F, Durig T. Excipients in Pharmaceutical Additive Manufacturing: A Comprehensive Exploration of Polymeric Material Selection for Enhanced 3D Printing. Pharmaceutics. 2024; 16(3):317. https://doi.org/10.3390/pharmaceutics16030317
Chicago/Turabian StyleMuehlenfeld, Christian, Patrick Duffy, Fengyuan Yang, David Zermeño Pérez, Firas El-Saleh, and Thomas Durig. 2024. "Excipients in Pharmaceutical Additive Manufacturing: A Comprehensive Exploration of Polymeric Material Selection for Enhanced 3D Printing" Pharmaceutics 16, no. 3: 317. https://doi.org/10.3390/pharmaceutics16030317
APA StyleMuehlenfeld, C., Duffy, P., Yang, F., Zermeño Pérez, D., El-Saleh, F., & Durig, T. (2024). Excipients in Pharmaceutical Additive Manufacturing: A Comprehensive Exploration of Polymeric Material Selection for Enhanced 3D Printing. Pharmaceutics, 16(3), 317. https://doi.org/10.3390/pharmaceutics16030317






