Abstract
Background: Specifically designed peptide mimetics offer higher selectivity regarding their toxicity to mammalian cells. In addition to the α-helix conformation, the specific activity is related to the peptide’s ability to penetrate the cell membrane. The alterations in lipid membrane properties were addressed in the presence of the peptide KLAKLAK-NH2 and analogs containing β-alanine, strengthening the antibacterial activity and/or naphtalimide with proven anticancer properties. Methods: The molecular interactions of the peptide mimetics with POPC bilayers were studied using FTIR-ATR spectroscopy. The thermal shape fluctuation analysis of quasispherical unilamellar vesicles was applied to probe the membrane bending elasticity. The impedance characteristics of bilayer lipid membranes were measured using fast Fourier-transform electrochemical impedance spectroscopy. Results: A lateral peptide association with the membrane is reported for β-alanine-containing peptides. The most pronounced membrane softening is found for the NphtG-KLβAKLβAK-NH2 analog containing both active groups that corroborate with the indications for 1,8-naphthalimide penetration in the lipid hydrophobic area obtained from the FTIR-ATR spectra analysis. The β-alanine substitution induces strong membrane-rigidifying properties even at very low concentrations of both β-alanine-containing peptides. Conclusions: The reported results are expected to advance the progress in tailoring the pharmacokinetic properties of antimicrobial peptides with strengthened stability towards enzymatic degradation. The investigation of the nonspecific interactions of peptides with model lipid membranes is featured as a useful tool to assess the antitumor and antimicrobial potential of new peptide mimetics.
1. Introduction
Nowadays, problems with bacterial resistance are reaching pandemic proportions. Many multidrug-resistant bacterial strains such as Escherichia coli (E. coli), Pseudomonas, and Staphylococcus, etc. have already proved their ability to find new pathways to overcome antibiotic action, and they are responsible for difficult-to-control bacterial infections. Thus, the problem with bacterial resistance against conventional medicaments becomes a serious challenge for medicine [1,2]. Currently, searching for new alternatives to using antibiotics to fight bacterial resistance is the main task of many scientific groups. Medical practice gradually began to rediscover the unused and not well-studied potential of natural biomolecules like amino acids, peptides, carbohydrates, etc. contained in different natural kingdoms. Specific and very useful features of peptide molecules are their natural mechanisms of elimination, small sizes and low or no side effects, etc. [3]. But, one of the most valuable properties is their ability to penetrate cell membranes. Thus, currently, they attract the attention of many scientific groups as a possible alternative to overcome bacterial resistance.
Many years ago, the discovery of magainins, a group of antimicrobial peptides (AMPs), initiated a deep search in the world of these natural molecules [4]. It was found that AMPs have a key modulatory role in the initial immune response of many organisms including plants, animals, insects, and a lot of bacteria [5,6,7,8], and they exhibit direct antimicrobial activity [9]. AMPs are characterized by a short amino acid chain including around 10 to 50 residues [10,11]. They are cationic, characterized generally by a lack of a stable conformation in water as well as by the ability to form amphipathic α-helixes in membranes. This specific feature helps them to disrupt the cell membranes of a large spectrum of bacteria, protozoa, and fungi [12]. Several mechanisms of penetration in the cell membrane are currently reported in the literature, such as the barrel-stave [13], toroid-pore, and the carpet-like model [14], etc. [15,16].
Javadpour et al. [17] reported the discovery of a new potential AMP named (KLAKLAK)2-NH2. Their investigations revealed that the peptide has both antimicrobial and anticancer properties due to a similar mechanism of penetration in both normal and tumor cells [17]. A conclusion made by Javadpour’s group is that the minimum-double repeating of the sequenced KLAKLAK is necessary for antibacterial activity. However, our previous studies on (KLAKLAK)2-NH2 analogs revealed that a single KLAKLAK-NH2 sequence has moderate activity against the Gram-negative test microorganism E. coli K12 407 at a 20 µM concentration, but its analogs containing unnatural amino acid β-Ala and nor-Leu in the primary structure do not show any activity [18]. In addition, if unnatural amino acids β-Ala and nor-Leu are added to the primary structure of double-sequenced analogues (KLβAKLβAK)2-NH2 and (Knor-LAKnor-LAK)2-NH2, antibacterial activity is enlarged against the Gram-positive microorganisms of the strain Bacillus subtilis 3562 [19,20]. Often, peptide molecules are used as vehicles in order to transport active molecules in the cell due to their ability to penetrate the membrane [21,22]. In addition, our studies showed that KLAKLAK-NH2 analogs could be successfully used as such as vehicles to transport a second pharmacophore, a very well-known naphtalimide with proven anticancer properties [23]. Moreover, both parts of the molecule showed a synergic antiproliferative effect [18,19].
Here, we study the ability of both shortened analogs KLAKLAK-NH2 and KLβAKLβAK-NH2 as well as those modified with the unnatural amino acid β-Ala doubled-sequenced analog (KLβAKLβAK)2-NH2 to influence the important mechanical and impedance parameters of model cell membranes and to predict their ability to penetrate the cell. We explore the alteration of membrane properties by the 1,8-naphtalimide- KLβAKLβAK-NH2 mimetic in order to unravel the membrane-related anticancer effects of this shortened analog. Apart from assuring cell integrity and compartmentalization, biomembranes are also involved in cellular processes and are related to strong membrane deformations and morphological changes. The investigation of the structural, mechanical and impedance properties of lipid bilayers builds the necessary background for revealing important functional parameters of membranes. We probed the mechanical and electrical characteristics of phosphatidylcholine membrane models to shed light on the effect of the studied peptide molecules. The bending elasticity was measured using the flicker spectroscopy of quasispherical giant unilamellar vesicles (GUVs) modelling the basic physical properties of biomembranes [24,25,26]. With characteristic diameters comparable to the typical cell sizes (5–100 µm), GUVs serve as a versatile platform for the direct visualization of numerous membrane-related phenomena [27]. FTIR-ATR measurements of liposome suspensions were carried out to unravel the molecular interactions of the studied peptide sequences with POPC bilayers [28,29,30,31].
2. Materials and Methods
2.1. Materials
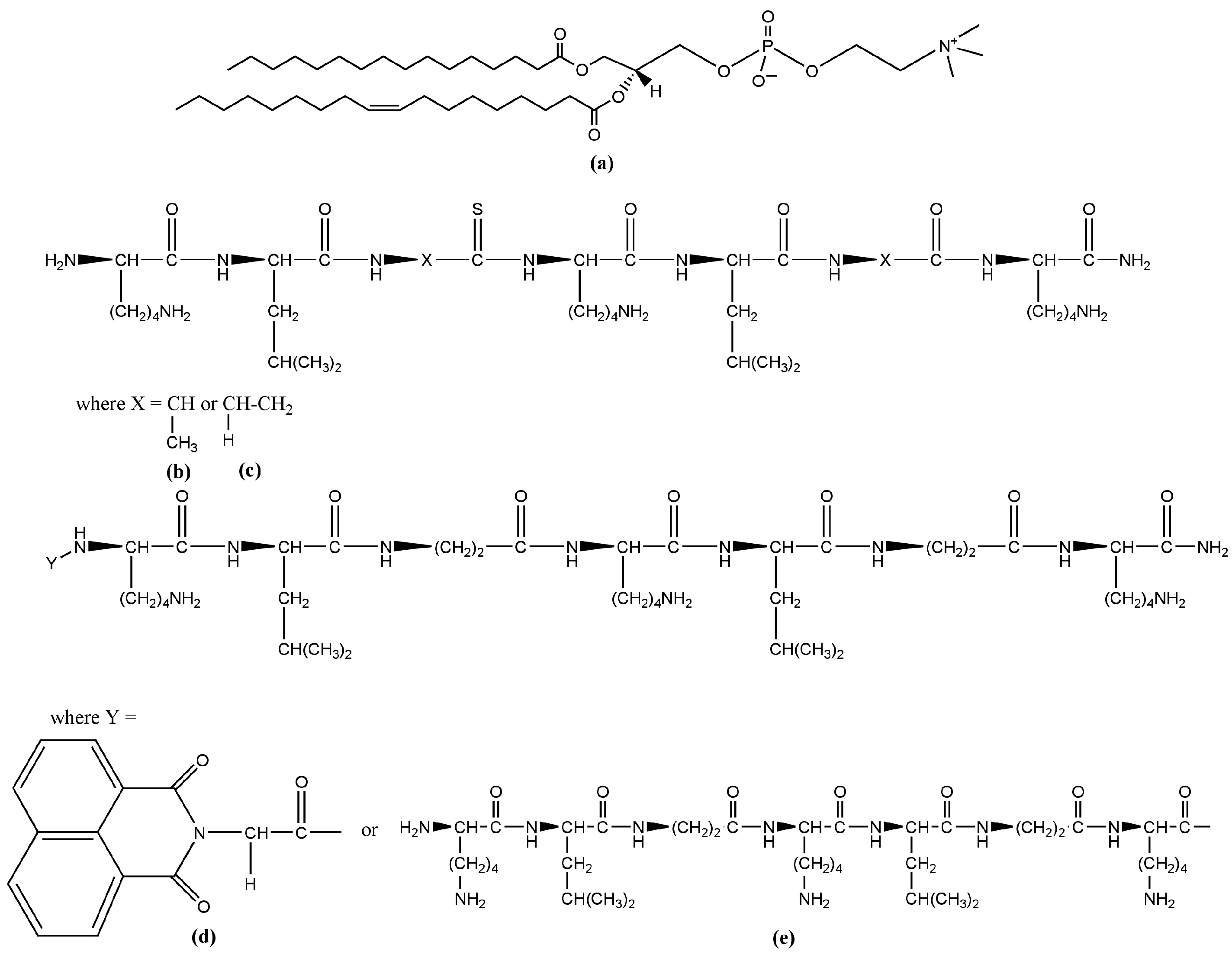
The analytical-grade chemicals, which were necessary to prepare the membrane lipid systems studied here, were used as purchased. Vesicles and suspended lipid bilayers were produced from the synthetic phosphatidylcholine (Figure 1a) 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC, C42H82NO8P, 760.08 g/mol), provided by Avanti Polar Lipids (Alabaster, AL, USA). Organic solvents methanol and chloroform purchased from Sigma-Aldrich (Darmstadt, Germany) were used for the preparation of lipid solutions. For all suspensions, bi-distilled water from a quartz distiller, filtered through 0.2 µm sterile syringe filters (Corning Incorporated, Wiesbaden, Germany), was used. The four peptide sequences (Table 1 and Figure 1b–e) studied here, namely KLAKLAK-NH2 (C36H71N11O7) and its analogues NphtG-KLβAKLβAK-NH2 (C50H78N12O10), KLβAKLβAKNH2 (C36H71N11O7), and (KLβAKLβAK)2-NH2 (C71H135N21O15), were obtained by solid phase synthesis (SPPS) [9].
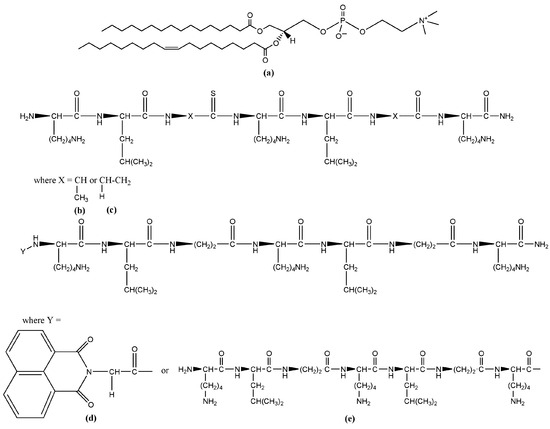
Figure 1.
Chemical structures of the lipids and peptides used in the study: (a) 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (Avanti Polar Lipids, Alabaster, AL, USA); (b) KLAKLAK-NH2; (c) KLβAKLβAK-NH2; (d) NphtGKLβAKLβAK-NH2; and (e) (KLβAKLβAK)2-NH2.

Table 1.
Description of the peptide sequences.
2.2. Methods
2.2.1. Peptide Synthesis
KLAKLAK-NH2 and its analogues were obtained by solid phase synthesis (SPPS) as described in detail elsewhere [18]. Briefly, the Fmoc/Ot-Bu strategy was used to bond subsequently all amino acids from the primary structure of the peptide, starting from C- to N-terminus by means of HBTU/DIPEA as condensation agents. PyBOP/DIPEA was used to introduce NphtG in the N-terminus of peptide P2. αN-Fmoc-group was removed at every step by treatment with 20% piperidine in DMF. The reactions of coupling and deprotection were monitored by a standard Kaiser test [32]. The final product was removed from the resin by treatment with a mixture of 95% trifluoroacetic acid (TFA), 2.5% triisopropylsilane (TIS) and 2.5% water for 4 h. At the end of the reaction time, liquids were removed under vacuum, and crude oil was precipitated in cold ether. The purity of obtained peptides and their structure were analyzed using HPLC-MS. The chromatographic purity was 100% for peptides P1, P2 and P3-long and 99.14% for the peptide P3 [18,19].
2.2.2. Vesicle Preparation
Giant unilamellar vesicles (GUVs) were produced from POPC and POPC-peptide mixtures using the electroformation method [33]. Two indium tin oxide (ITO)-coated glass plates with a polydimethylsiloxane (PDMS, Dow Corning, Wiesbaden, Germany) spacer with 5 mm-thickness comprised the electroformation cell. After spreading out 50 μg of POPC or POPC–peptide solution with total concentration of 1 g/L in chloroform-methanol solvent (9:1 volume parts) on the ITO-coated surface of each electrode, both were placed in a desiccator for a couple of hours under vacuum until the organic solvents completely evaporated. The electrodes were connected to a functional generator and the electroformation protocol [34] was applied immediately after filling the electroformation chamber with 0.2 µm-filtered bi-distilled water. The application of AC electric field (10 Hz; 0.4 V/mm peak-to-peak) was applied to the electroformation cell for a couple of hours to produce a high yield of quasispherical single-wall giant vesicles without membrane defects and appropriate for thermal shape fluctuation analysis.
Multilamellar vesicles (MLVs) were produced from POPC or POPC–peptide mixtures in bi-distilled water following the mechanical dispersion method [35,36,37]. Lipid or lipid–peptide mixtures dissolved in chloroform/methanol (9:1 volume parts) at 1 mg/mL were evaporated under vacuum for 5 h in order to obtain a solvent-free dry lipid film formed on the bottom of a glass vial. The lipid or lipid-peptide film so-obtained was hydrated using bi-distilled water to achieve 5 mg/mL as a final concentration of lipid or lipid–peptide mixture in the liposome suspension. After vortexing for 2 min, we placed each sample in an ultrasonic bath and left them to hydrate overnight. All peptide-containing samples were prepared with peptide-to-lipid molar ratio = 0.185.
2.2.3. Formation of Bilayer Lipid Membranes (BLMs)
We studied the influence of KLAKLAK-peptides on the impedance parameters of bilayer lipid membranes (BLMs) from POPC obtained by the Montal–Mueller approach [38,39]. The lipid was dissolved in HPLC-grade pentane CH3(CH2)3CH3 and hexane CH3(CH2)4CH3 (99:1, volume). Eastern Scientific LLC (Rockville, MD, USA) supplied us with the Montal–Mueller chamber (model BC-20A), in which we produced solvent-free bilayers suspended on the circular 100 µm aperture in a Teflon membrane with thickness of 0.025 mm following the established protocol [34,38]. The final bilayer formation was controlled electrically [38].
2.2.4. Thermal Shape Fluctuation Analysis (TSFA) of GUVs
Two mechanical constants and , corresponding to the resistance to stretching and bending, respectively, describe mechanically a lipid bilayer in its liquid state above the main transition temperature. The relative bilayer’s area changes and the membrane stretching modulus determine the mechanical tension . In the present study, we measure the mechanical properties of symmetrical membranes bathed by identical aqueous solutions on both sides of the bilayer. Therefore, the bending modulus relates to the density of the bending energy by the equation , where stands for the saddle-splay (Gaussian) modulus and and denote the total curvature and the Gaussian curvature, respectively [40].
We measured by thermal shape fluctuation analysis (TSFA) of quasispherical vesicles [41], based on Legendre analysis of the time-averaged angular autocorrelation function of the vesicle radius, [42] with an amplitude of the -th mode in its decomposition with respect to Legendre polynomials expressed with the bending constant and the reduced membrane tension , where denotes the vesicle radius. A stroboscopic illumination of the observed vesicles, synchronized with the camera, is implemented in our TSFA set-up, which makes possible the recording and analysis of the fast fluctuation modes [41]. All measurements of the membrane-bending elasticity were conducted at (37 ± 0.1) °C with temperature control via a two-channel system operating both with the oil-immersed objective (100×, numerical aperture 1.25; 0.106 μm/pixel) and the plate of an inverted microscope Zeiss Axiovert 100 (Zeiss, Jena, Germany) equipped with a CCD camera (C3582, Hamamatsu Photonics, Shizuoka, Japan) connected to a frame grabber (DT3155, Data Translation, Marlborough, MA, USA, 768 × 576 8-bit pixels, 0.172 μm/pixel) for digitization and analysis. Only flaccid nearly spherical vesicles with diameters larger than 10 μm were recorded and subjected to TSFA. Several hundred images were captured once per second for the calculation of the membrane-bending constant and tension of every studied vesicle using an in-house non-commercial software as described in [41]. The contour corresponding to the equatorial cross-section of the membrane of a quasispherical vesicle observed by phase-contrast microscopy was digitized using a procedure reported in detail in [42,43]. The values of and for each studied vesicle were obtained by a fitting procedure performed as in [41,42,43]. Resulting from the stochastic background of vesicle formation, the membrane tension is different for each vesicle, whilst the value of quantifying the bending rigidity of membranes with the same composition and structure characterizes the entire vesicle collection in the sample. The statistical relevance of this assumption was determined by -goodness of fit test. The below-reported values of the membrane-bending modulus were obtained over an ensemble of not less than six GUVs collected from at least two different preparations.
2.2.5. Fast Fourier-Transform Electrochemical Impedance Spectroscopy (FFT-EIS) of Lipid Bilayers
The effect of KLAKLAK-peptides on the impedance characteristics of solvent-free POPC BLMs was investigated by fast Fourier transform electrochemical impedance spectroscopy (FFT-EIS) immediately after BLM formation using equivalent circuit modelling as described in [34]. The measurement consisted of the application of a small-amplitude (~10 mV) multisine perturbation signal in the frequency range of 1.5 Hz–50 kHz. The acquisition procedure comprised the simultaneous recording of the perturbation and the response signals followed by fast Fourier transformation (FFT) to the frequency domain. Hence, the impedance spectrum was acquired in seconds by simultaneously monitoring the stationarity [43,44]. The latter excluded possible artifacts influencing the calculated impedance values. The capacitance and resistance of planar lipid bilayers were obtained from the analysis of the FFT-EIS data recorded and the equivalent circuit modeling the Montal–Mueller BLM configuration [34]. The specific capacitance and resistance of bilayers were deduced from the values measured for the capacitance and resistance of the bilayer and the surface area 8 × 10−5 cm2 of the 100 µm circle aperture on which BLMs were suspended. Further, we represent the weighted average of at least 3 independent measurements averaged over 10 repetitions each.
2.2.6. Fourier-Transform Infrared Spectroscopy by Attenuated Total Reflectance (FTIR-ATR)
The development of Fourier-transform infrared (FTIR) spectroscopy in both technological and digital aspects gives an increase in its applications. The attenuated total reflectance (ATR) technique adds at least one order in the sensitivity of the signal and eliminates the need for thick samples with sufficient content of the active substance for the transmittance measurements. Moreover, the large and intensive IR spectral bands of the water overlap with the bands of interest, and a distraction of the buffer spectrum is necessary. This diminishes drastically the photometric scale of the resulting effective spectrum. Therefore, we perform many scales to avoid the noise. All of these problems were met and overcome during the experiment reported here. Also, in all spectral sets, the spectrum of the pure POPC suspension with subtracted water spectrum is included for a comparison. FTIR spectra of the samples under investigation were collected with a Bruker, Vertex 70 spectrophotometer (Bruker, Rosenheim, Germany) in ATR geometry. A standard PIKE MIRacle ATR accessory equipped with a ZnSe reflection prism yielding 3 internal reflections and covered with a diamond plate was used. The range of interest 4000–600 cm−1 was limited at low frequencies by the transmission edge-cut of the ZnSe crystal. The spectra were obtained by averaging 100 interferograms with a resolution of 2 cm−1 at a temperature of 30 ± 0.3 °C maintained inside the instrument. The samples of water suspensions were filled into an o-ring (diameter 8 mm, thickness 1 mm) and put on the diamond plate, thus forming a basin.
3. Results and Discussion
3.1. FTIR-ATR Spectra Analysis
A relatively selective “observation” of the hydrophobic chain dynamics and the perturbation on the polar hydrophilic heads was possible due to the spectral distance between the vibrational bands of the different parts of the lipid molecules [45,46,47]. The picture became clearer after removing the strong absorbing bands of the bulk water. The role of lipid hydration in the suspension is important for the bilayer state when interacting with the peptides, and this has been discussed widely [48,49,50]. The hydrogen-bond network of membrane-bound water shows a different bond orientation, which influences the place and the nature of the possible peptide–lipid interaction. Therefore, the spectra were carried out in situ with samples such as aqueous suspensions.
Concerning the frequencies of the lipid vibrational modes, the authors are commonly in agreement [51,52]. Several distinct spectral regions are assigned to the specific vibrations in the different parts of POPC molecules. The spectrum at the highest frequencies of 2800–3600 cm−1 characterizes the C-H symmetric and asymmetric stretching vibrations in the hydrophobic chains, but the CH3 modes of the chains’ ends and the choline groups can contribute, too. Also, in this region, possible traces of the H-bond vibrations of the hydrated lipid have to be taken into account. In the spectral range of 1300–1600 cm−1 are the bands assigned to the numerous bending CH2 and CH3 modes in POPC. The lower frequencies 800–1300 cm−1 are characteristic of the vibrations in the headgroups at the membrane interface, as well as for rocking modes in the CH2 chains.
The measured spectra are presented and discussed in particular parts as they are mentioned above. These spectral domains avoid the ranges of the bending and libration modes of the bulk water molecules. Because of the small photometric values after the distraction of the water spectrum, just a noise was registered in the mentioned regions. An exception is the area, where the distraction reveals the specific spectral features of each sample, giving an idea about the mechanisms of molecular hydration.
3.1.1. Spectral Region 3600–2800 cm−1
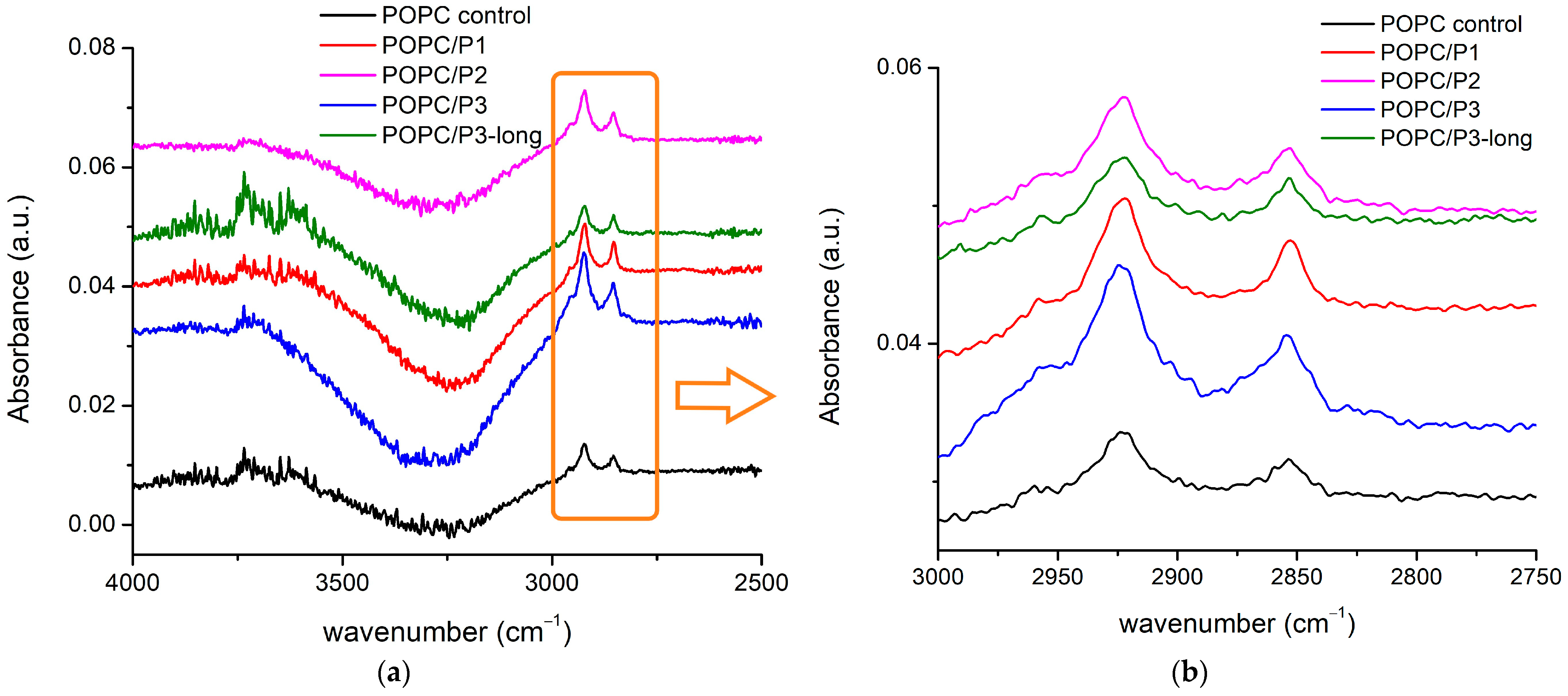
The FTIR-ATR spectra of all aqueous suspensions in this zone of the C-H, C-N, H-N and OH vibrational modes are presented in Figure 2. Looking at the higher frequencies in the spectral region of the OH vibrations, a very large band was registered (Figure 2a). This part of the spectrum gives information about the real molecular interactions in the aqueous suspension.
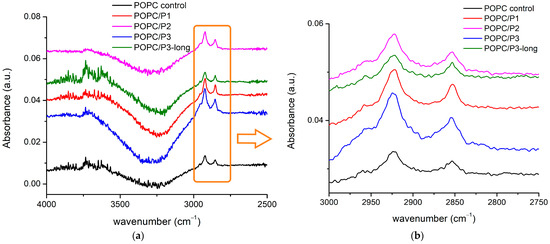
Figure 2.
(a) FTIR-ATR spectra in the range of 4000–2500 cm−1 corresponding to C-H and OH vibrations; (b) absorbance in the spectral region of 3000–2750 cm−1 corresponding to CH2 and CH3 stretching vibrations (see text).
The components arise as a result of the water’s interaction with the ingredients’ molecules. They can be assigned to the H-bond or to the OH-stretching vibrations in the vicinity of the different lipid molecular parts [49,50,51,52]. The intensive and structured band centered at 3247–3320 cm−1 most likely is a result of the intermolecular H-bonding near the lipid headgroups [50,51]. At the anionic phosphate group with a higher negative charge density than the oxygen atom of water, the negatively charged oxygen atoms directly form H-bonds with water which are stronger than that between water molecules. Replacing the alanine in the K by β-alanine yields some extra positively charged side chains in the peptide molecule. They can contribute to the formation of extra OH groups around the positive choline headgroups because of the hydrophobic character of the lipid–peptide interaction which leads to a break of the H-bond network in bulk water, thus giving rise to free OH vibrations in this spectral region. Indeed, the blue side of the broad band is most pronounced for the sample P3-long. A few weak components were registered around 3600–3720 cm−1, probably caused by the free OH vibration in the bulk water [50,52]. The most distinguished and relatively well-established bands are those of the CH2- and CH3-stretching vibrations in the hydrocarbon chains (Figure 2b). As the lipid membrane bilayer has a closely packed structure, an external perturbation can cause a detectable reflection, thus providing information about the motions of the hydrophobic tails. A conformational order–disorder state has been found to lead, respectively, to a downshift or upshift in the peak positions of the symmetric CH2 mode, while its half-width has been used as an indication of the degree of freedom of the tail motions [29,53,54]. The obtained peak positions and the bands’ half-widths of the corresponding symmetric and asymmetric CH2-stretching vibrations are summarized in Table 2. The addition of KLAKLAK-NH2 almost does not disturb the hydrocarbon backbone except for the slight narrowing of the band at 2853 cm−1. As the dipole moment of the CH2 symmetric vibration is in the C-C-chain plane [52], a negligible “hardening” can be assumed in this direction. All bands of CH2 vibrations for the POPC/P2 and POPC/P3 samples are significantly broadened by the rise of the CH3 symmetric and asymmetric vibrations at 2865 cm−1 and 2954 cm−1, respectively. The activation of the latter modes is probably caused mainly by the excitation of the CH3 choline groups. A possible explanation is given below. The end CH3 groups of the hydrocarbon chains also contribute as identified by the bands’ widening corresponding to tail agitating. Moreover, the deconvolution of both structured bands revealed that the CH2 sub-bands were broadened themselves (Table 2), which sustains the assumption of intensified tail motion. The doubled chain P3-long peptide samples do not exhibit considerable alteration in the hydrocarbon core movement but differ from the other spectra in the regions of the POPC polar group modes.

Table 2.
Spectral regions of the stretching C-H vibrations.
3.1.2. Spectral Region 1750–1700 cm−1
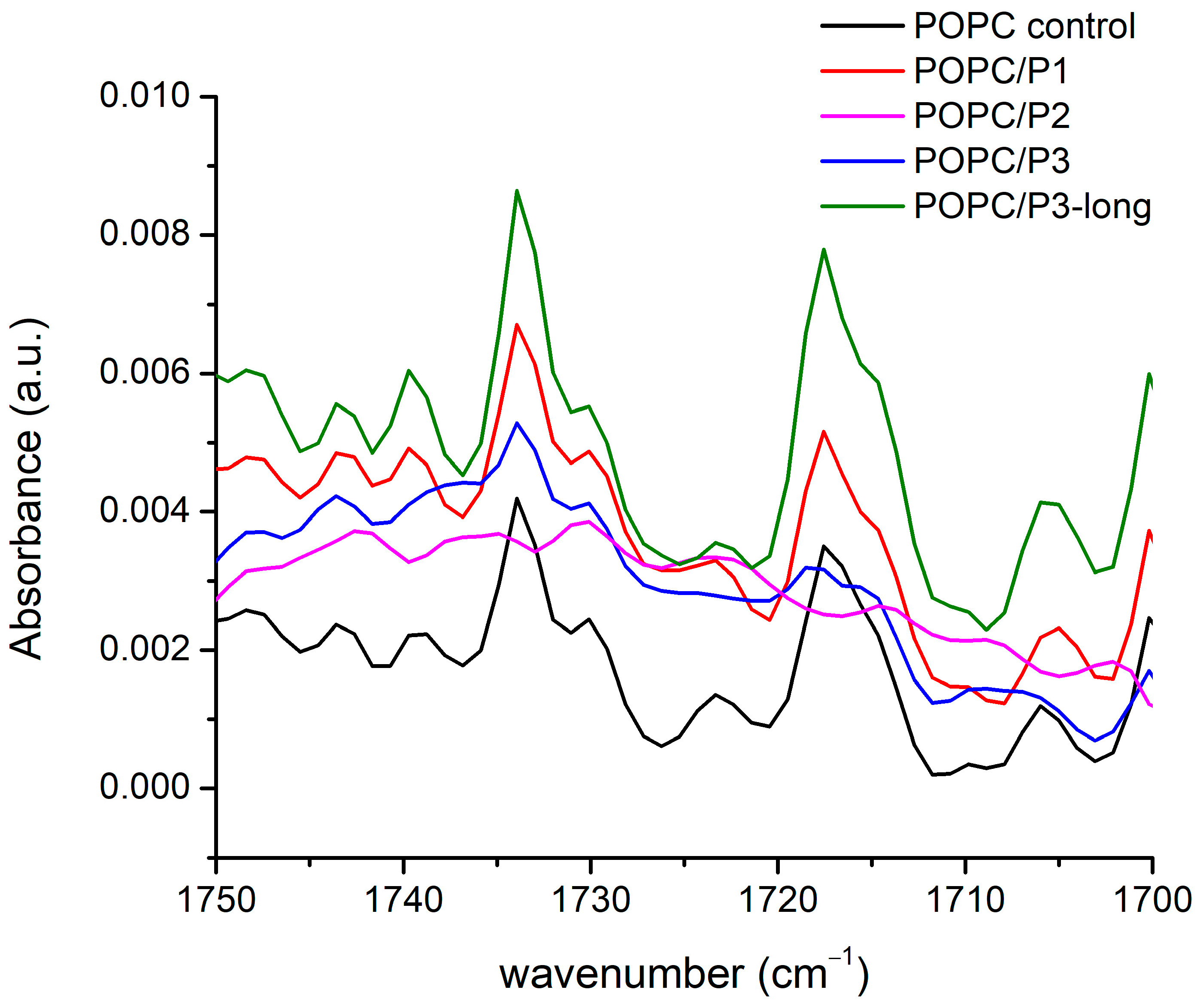
This region consists of at least two bands originating from the two ester carbonyl groups in the lipid aggregate. They are usually revealed at high resolution [29,45,46,53,54]. The C=O-stretching modes are the most useful infrared bands for probing the membrane interface. The structural arrangement and environment of the lipid interfacial carbonyl groups are responsive to structural changes located at sites remote from the bilayer surface. Thus, the sensitive location of the C=O bonds between the polar headgroup and the nonpolar hydrocarbon chain gives a possibility for obtaining information about the changes in the chemical structure of lipid molecule parts.
In Figure 3, the FTIR-ATR spectra of all the samples in the vicinity of the two ester carbonyl groups’ C=O-stretching vibrations are given. More than two constituent bands were registered, but the main doublets were seen around 1733 cm−1 and 1717 cm−1, and their possible splitting depends on their hydration status [29]. The two bands are informative also because they are associated with the two lipid tails. It has been established that the bands at lower wave numbers are attributed to the hydrogen-bonded ester group, i.e., to the unsaturated lipid chain (sn2), while the band at a higher wave number indicates a lack of H-bonding and is associated with the saturated chain (sn1) [29,55]. Both modes have their polarization along the double bond [51], i.e., they have to be sensitive to external interactions along this bond. The spectra in Figure 3 certify the strongest P2 influence on POPC molecules in the polar regions surrounding the lipid carbonyls (most likely via protonation [52]); the two C=O bands completely vanish. A possible interpretation can be that the peptide’s cyclic rings are situated near the acyl chains, being sufficiently bulky to create steric disruption in the glycerol group, hence providing the acyl chains with more space to move but without inducing any indicative order or disorder in the core. Moreover, the increase in the bandwidth of the C-H-stretching vibrations registered and described in the previous subchapter indicates an increase in the fluidity of the bilayer [30]. A comparable model has been suggested in [53,56,57], investigating an intercalation of tricyclic antidepressants into phospholipid membranes by NMR. Some phenylalanines have been reported to be located in the lipid core, significantly disordering the hydrocarbon chains [58] but not disturbing the glycerol group, while other peptides point to the headgroups [59,60].
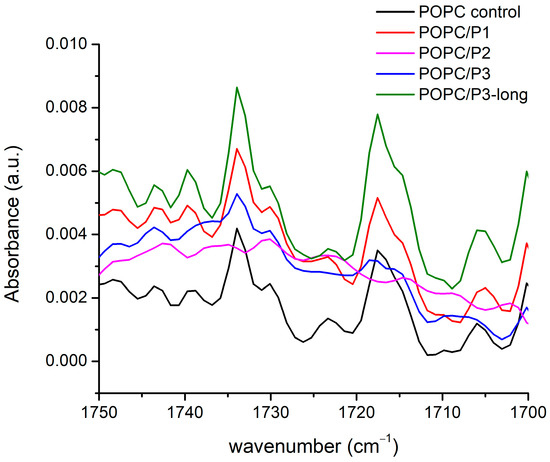
Figure 3.
FTIR-ATR spectra in the range of 1750–1700 cm−1 corresponding to C=O-stretching vibrations in the glycerol esters.
3.1.3. Spectral Region 1600–1300 cm−1
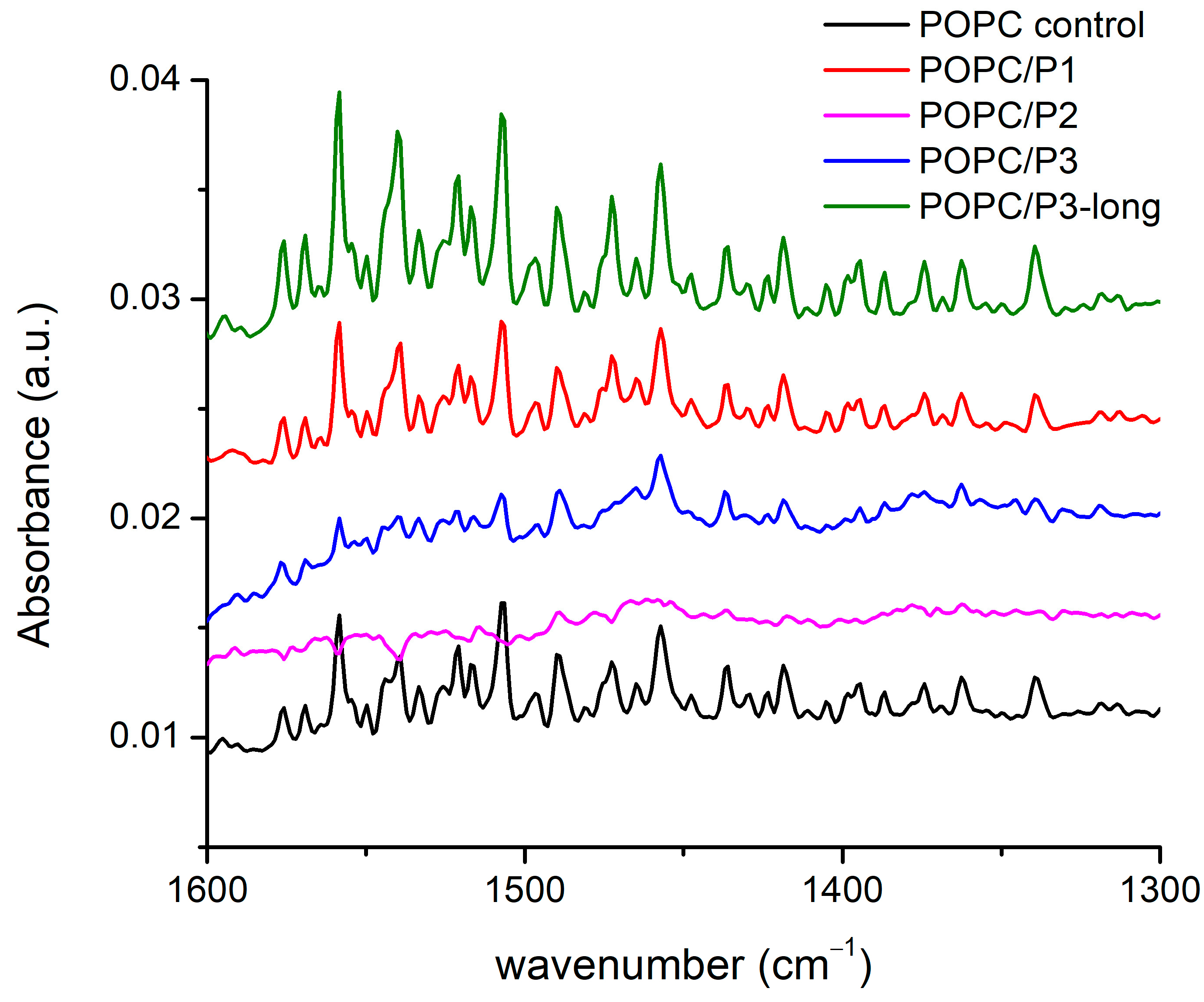
There are more than 20 registered modes in this region (Figure 4) arising from the CH2, CH3 and N-H-bending vibrations of different conformations including symmetric and asymmetric bond oscillations in and out of a specified plane, scissoring and waging, etc. [28,29,51,52,53,54]. Most of the bands overlap with one another, thus making difficult the exact assignment. Nevertheless, there are clear differences between the spectra: the lines are almost equivalent for the samples of pure POPC and POPC/P1, while for POPC/P3-long the modes become visibly more intensive and slightly narrower. The bands in the spectrum of the sample POPC/P3 diminish in intensity and are enlarged. The spectrum of POPC/P2 is almost flat in this region.
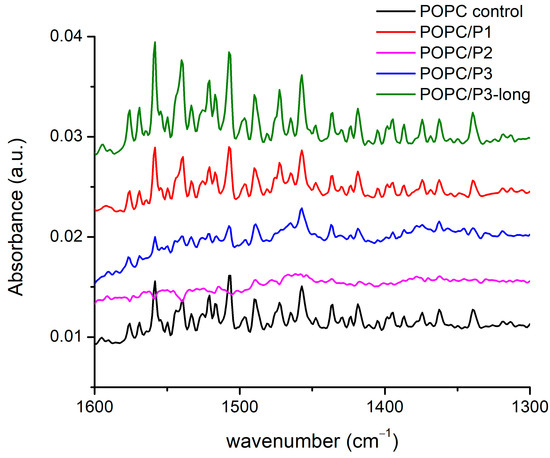
Figure 4.
FTIR-ATR spectra in the range of 1670–1600 cm−1 corresponding to CH bending in the acyl chains’ and choline groups’ vibrations.
As the modes originate mainly from the bonds in the lipid chain, they characterize their state in the various environments, although at these frequencies the umbrella-like vibrations of the CH3 of the choline group are active too. The polarization of the bending vibrations in the chain is perpendicular to the backbone; the polarization of the choline-end vibrations depends on the symmetry and is ⊥ or || to the local C3 axis for the A1 or E type, respectively [51]. Consequently, the properties of the CH2 bending are determined by the lateral packing interactions between the chains. Deviations of the hydrocarbon chain conformation from all-trans lead to marked alterations in the spectrum, such as loss of intensity, band broadening and finally the disappearance of the modes and vice versa—any parallel coupling with this kind of vibration increases the resulting oscillating dipole moment, i.e., the intensity of the absorption bands. The results in Figure 4 identify three samples to fit these trends in the spectra. Moreover, again as pointed out above, the P2 analog alters the lipid bilayer relatively in the highest degree. Probably, the bulky cyclic rings disturb the chains up to the loose of the symmetry of this type of vibration, which are usually weak. It is noteworthy that the band at 1472 cm−1 completely disappears in the spectra of the samples POPC/P2 and POPC/P3. This mode is assigned to the asymmetric N-CH3 vibrations in the choline group [51] but overlaps also with the N-H-bend and C-N-stretching vibrations of the peptide’s Amide II [52].
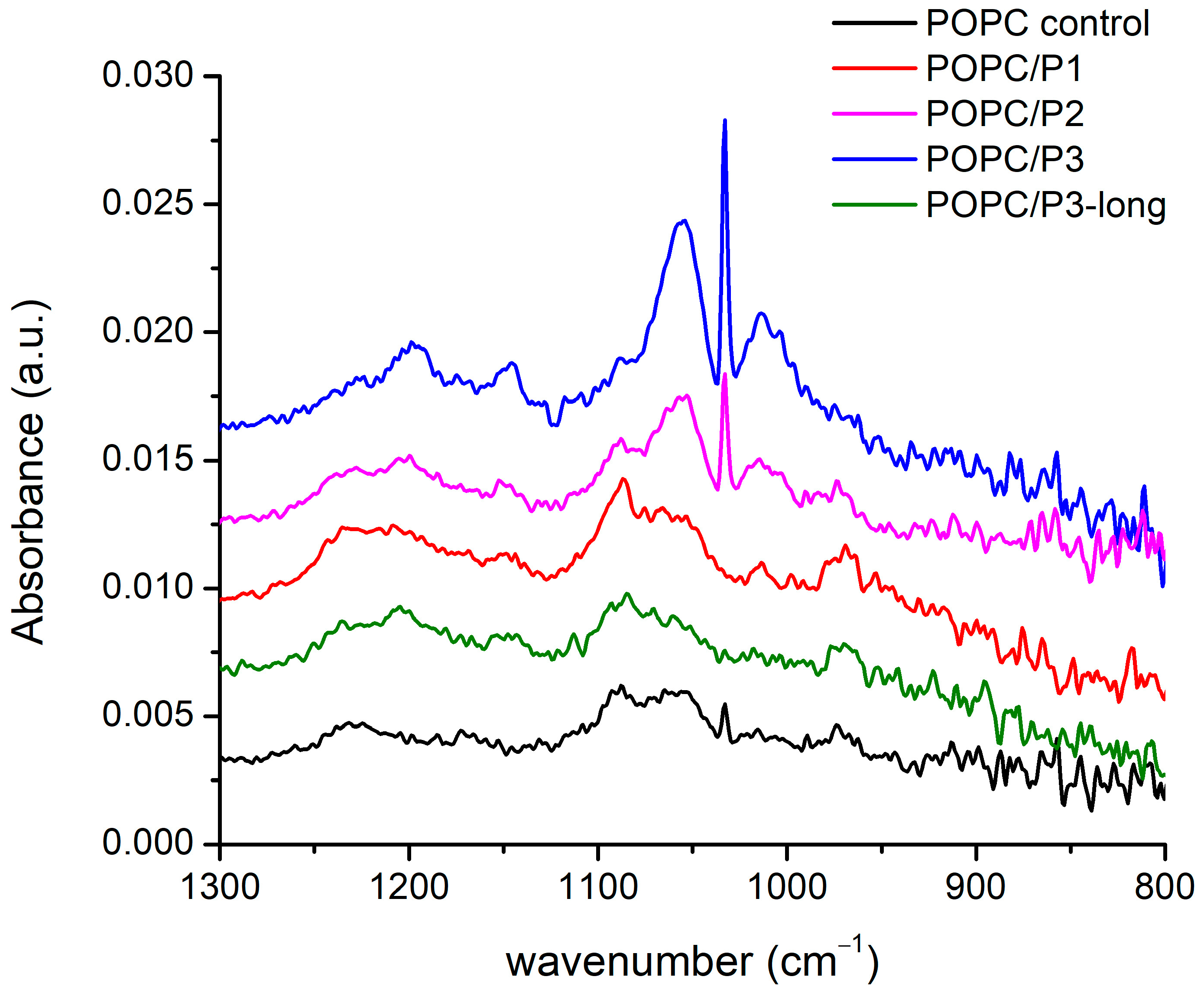
3.1.4. Spectral Region 1300–800 cm−1
The spectral alterations as a result of POPC–peptide interactions are displayed in Figure 5 in the range of the active vibrations in the lipid headgroup. The modes include the usual low-frequency modes of oscillation in phosphate and choline groups: twisting, rocking, etc. The phosphate group is generally monitored via the O-P-O antisymmetric stretching mode, which is seen as a very broad band of about 1250–1150 cm−1. The phosphate group provides hydrogen-bonding acceptors, leading to a dependence of the spectral position on the hydration state of the headgroup: upon hydration, the asymmetric O-P-O stretching frequency decreases [29,51], whereas the broadening of the band is an indicator of polar headgroup mobility in the presence of the peptide [47]. In the complicated structure of this large band, a frequency decrease in the main peak of the asymmetric double-bond stretching O-P-O, adopting a maximum from 1229 cm−1 to 1197 cm−1, is seen through the samples. The lowest frequency is obtained for the most active peptide with β-alanine in the structure (P3).
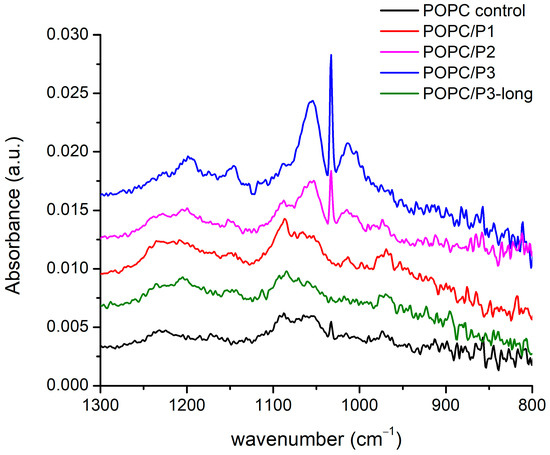
Figure 5.
FTIR-ATR spectra in the range of 1300–800 cm−1 corresponding to phosphate and choline groups’ vibrations.
The band at 1086 cm−1 arising from the symmetric O-P-O double-bond stretching vibrations is narrower and does not alter in frequency. The dipole moment of oscillations is along the bisector of the angle of deviation, and a possible coupling with the positively charged side chains of P1 leads to an increase in the intensity of this band. For the P3 analog, the mode vanishes while it remains active for the asymmetric vibrations with perpendicular polarization towards which the protonation process probably occurs. The few near bands at 1056 cm−1, 1033 cm−1, 1014 cm−1 and 971 cm−1 concern the C-O, CNC and P-O single-bond stretching vibrations in the headgroup. The best polar coupling with the first three modes in the direction perpendicular to their bonds is observed for P2 and P3. The last band with an opposite dipole moment vanishes in the spectrum of POPC/P3. It has to be underlined that the mode of the asymmetric N-CH3-bending vibration in the choline group discussed in the previous subsection becomes inactive in the spectra of both mentioned samples.
In the presented region, the strongest influence of the P3 peptide on the POPC headgroup was registered. Like in Section 3.1.1, the same possible model of molecular relations can be adopted. The substitution by β-alanine of the alanine in KLAKLAK-NH2 supplies the peptide with some extra positively charged side chains which sustains the idea that longer molecules assume a different orientation in the membrane, presumably lateral, thus decreasing the interaction activity. Indeed, the spectra of P3 and P3-long are almost identical to that of the pure POPC in all the considered regions.
3.1.5. Spectral Region 1600–1670 cm−1
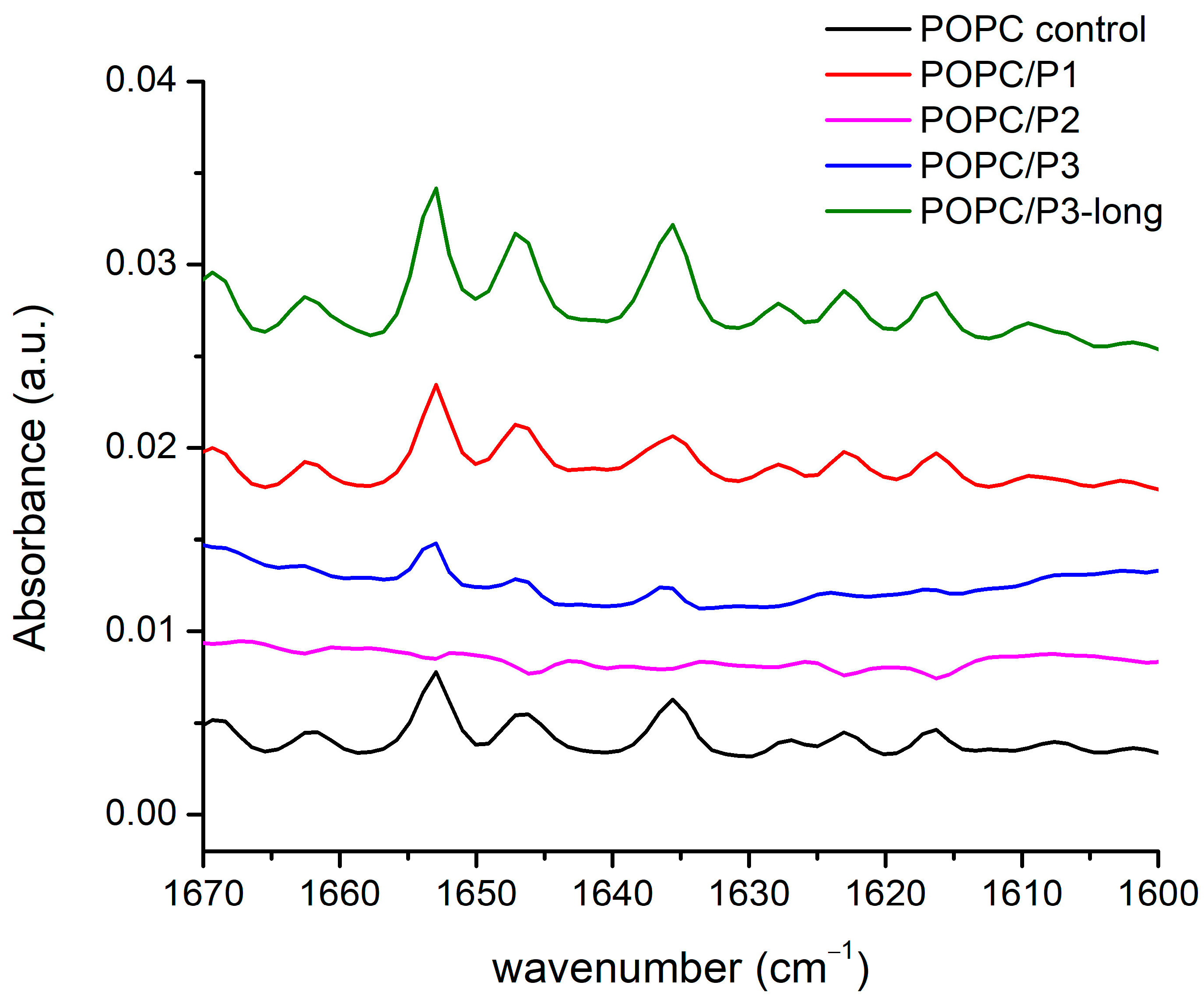
Lipid–peptide interactions result also in alterations in the peptide spectra measured. When in contact with membranes, most of the peptides adopt an amphipathic arrangement that contains α-helical, β-sheet, or both structures. Examining the interactions between the peptides and host membranes, infrared spectra show possible conformational changes in the different samples. The clarifying of the problem becomes difficult because of the very low peptide concentrations, which results in very weak bands. Moreover, the amide modes often coincide with those of the complex lipid aggregate in the region of the bending vibrations. Nevertheless, a few bands were found to be informative about the form of the mutual interactions. The amide I band indicates a strong hydrogen bonding in the α-helical peptide, which is sensitive to membrane fluidity, i.e., to the lipid chain mobility discussed above [52]. Several distinct structures of the peptide–lipid binding have been observed, suggesting that the peptides undergo functionally relevant changes in their conformation and membrane alignment [53,61,62,63,64]. More generally, surface S-states and transmembrane T-states have been reported, respectively, with a parallel and oblique direction of the helix axis toward the normal to the bilayer surface. It is useful to have in mind the difference in the abilities of the interaction of the complete helix and of the peptide local side chains. The capacity of the latter to hydrogenate the lipid headgroups is discussed largely in the previous subchapters.
FTIR-ATR spectra of the aqueous suspensions according to the legend in the zone of the peptides’ Amide I, α-helix and β-sheets’ modes are presented in Figure 6: the doublet 1660–1640 cm−1 is assigned to Amide I of the α-helix, and the band at 1635 cm−1 is assigned to the Amide I of the β-sheets [52]. As it is seen, all three bands vanish completely for the sample POPC/P2. The analysis of the spectra shown above suggests a “soft” penetration of the phenyl rings of 1,8-naphthalimide in the lipid hydrophobic area thus presuming the oblique orientation of the helix correlating with the vibrational polarity in the choline group leading to the strong narrow peak at 1033 cm−1 (see Figure 5). This peak is maximal for the POPC/P3 sample, in which the maximal mobility in the lipid chains is registered, and it is not caused by peptides penetrating the core through the lipid interfacial carbonyl groups but probably by the strong electrostatic interaction in the headgroup vicinity. In Figure 6, this sample exhibits diminishing amide vibrations like in Figure 3 and Figure 4. In the POPC/P3-long samples, the spectral regions in Figure 3, Figure 4 and Figure 6 testify to a probable lateral association to lipid molecules without altering significantly the main lipid bilayer organization.
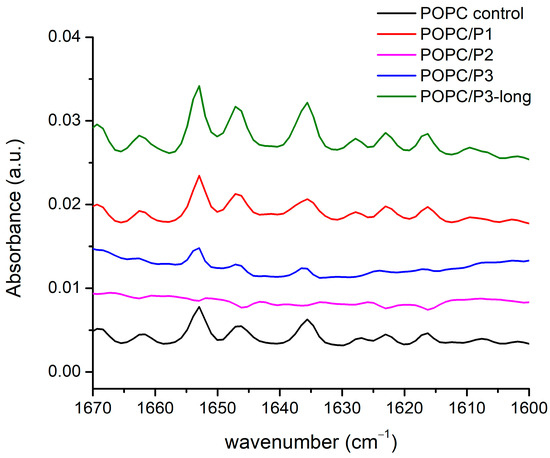
Figure 6.
FTIR-ATR spectra in the range of 1670–1600 cm−1 corresponding to the peptides’ Amide I, α-helix and β-sheets modes.
3.2. Bending Elasticity of POPC Membranes in the Presence of KLAKLAK-NH2 and Its Analogs
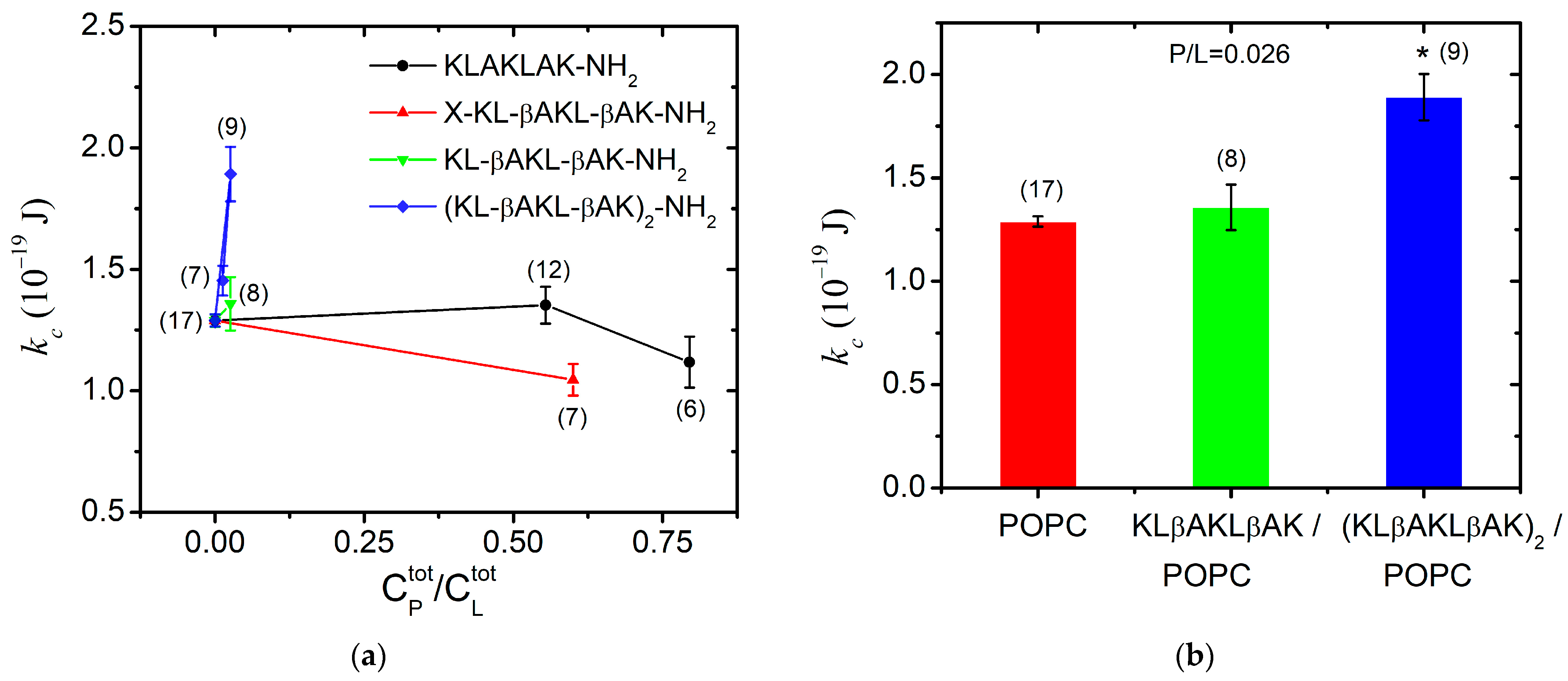
For each peptide studied, we determined the total peptide-to-lipid ratio () at which fluctuating GUVs without membrane defects were formed to satisfy all TSFA criteria discussed in detail previously [41,65]. The total lipid concentration in the experiments with GUVs ranged between = 33 µM and 37 µM. The highest that allows for producing flaccid quasispherical GUVs was achieved for KLAKLAK-NH2 (P1) and for NphtG-KLβAKLβAK-NH2 (P2)-containing samples. At very high KLAKLAK-NH2 (P1) concentrations (~30 µmol/L) and a total peptide-to-lipid molar ratio 0.90 mol/mol, we observed only aggregates after electroformation. The lipid stacking originates from the combination of the apparent negative charge of the phosphatidylcholine bilayers due to the surrounding water dipole arrangement [66] in water and the presence of two positively charged lysine residues in P1, engendering electrostatic interactions between the peptide molecules and the POPC membranes.
The bending rigidity of the POPC bilayers was obtained at two P1 concentrations near the lowest concentration at which the peptide has exhibited in vitro antiproliferative and cytotoxic activity [18]. At P1 content 0.80, we measured membrane softening with a nearly 15% reduction in the bending modulus in comparison to POPC membranes as shown in Figure 7a. This finding suggests the surface orientation of the peptide at the bilayer as reported in [67] for alamethicin, which is an antimicrobial peptide much more hydrophobic than KLAKLAK-NH2. Hence, very low total concentrations of alamethicin such as mol/mol produce significant membrane softening [67]. In the case of P1, we report only a slight reduction in the bending rigidity of POPC bilayers at nearly-three-orders-of-magnitude-higher P1 content in the sample, which we attribute to the significantly lower partition coefficient of P1 between the bilayer and the surrounding water [68]. The primary structure of P1 contains a doubled sequence of three amino acids, including the charged lysine and the non-polar leucine and alanine. This amphipathicity underlies the peptide partitioning between the lipid bilayer and the aqueous environment [69]. As obtained from the FTIR-ATR spectra analysis of POPC/P1 samples at 0.2, the non-modified peptide only negligibly disturbs the hydrocarbon backbone, which corresponds to our findings from TSFA about the unaltered bending elasticity of POPC bilayers in the same concentration range of P1 (Figure 7a).
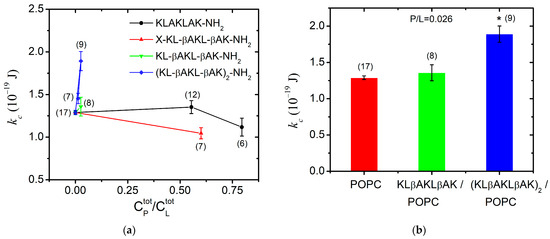
Figure 7.
Bending elastic modulus (weighed mean ± SD) of POPC membranes in the presence of (a) KLAKLAK-NH2 and its analogues at different total peptide-to-lipid molar ratios in the sample; (b) β-alanine containing peptides KLβAKLβAK-NH2 and (KLβAKLβAK)2-NH2 at 0.026 total peptide-to-lipid molar ratio (the number of GUVs given in brackets above each data point/column; * p < 0.005, -test of independence from the control).
The KLAKLAK-NH2 analog P2 containing 1,8-naphtalimide and β-alanines in place of alanines also induced membrane softening. Fluctuating quasispherical GUVs were readily obtained from POPC/P3 mixtures at a relatively high peptide concentration, 0.6 in the sample. The bending modulus was measured to decrease by nearly 20% as shown in Figure 7a. Statistical models developed recently [70,71] have considered the interactions of membrane-incorporated peptides, mediated by membrane elastic deformations. The bending elasticity changes are related to the lateral redistribution of the peptide molecules, incorporated in the lipid matrix as well as to their ability to induce spontaneous curvature in the bilayer [72,73,74,75]. The most considerable alteration of the membrane-bending rigidity reported for P2 is in agreement with the FTIR-ATR spectroscopy findings in the spectral region 1750–1700 cm−1 about the P2 influence on POPC molecules in the polar region surrounding the lipid carbonyls. The latter is attributed to the peptide’s cyclic rings, which are sufficiently bulky to induce steric disruption in the glycerol group. Evidence indicating increased bilayer fluidity is provided by the spectral characteristics in the region of 1600–1300 cm−1 as discussed in the previous subsection. The reported membrane softening is further corroborated by the increased fluidity of the bilayer detected from the bandwidth of the C-H-stretching vibrations’ signature in the spectra of POPC/P2 samples.
For KLβAKLβAK-NH2 (P3), the GUV formation was hindered at much lower total peptide-to-lipid ratios 0.33, producing stuck lipid–peptide structures and foam-like aggregates. As reported above from FTIR-ATR spectroscopy data, the hydrophobic lipid tails of POPC/P3 samples (0.2) exhibit high mobility with no evidence of peptides penetration into the core through the lipid interfacial carbonyl groups but suggesting strong electrostatic interaction in the headgroup vicinity (spectral region 1600–1670 cm−1). After decreasing the peptide content in the sample, small non-fluctuating GUVs started forming at = 0.11. Further reducing down to 0.026 led to the formation of flaccid quasispherical GUVs with diameters above 10 µm, allowing the thermal shape fluctuation analysis to be successfully applied for the determination of the bending modulus, which remains unaltered compared to its value for pure POPC membranes (Figure 7a).
The presence of the doubled P3-long sequence rigidified the lipid bilayer even at very low total concentrations of 0.03. A steep growth of the bending constant is reported with the increase in peptide concentration in the sample as shown in Figure 7a. This finding is in accordance with the results from the FTIR-ATR spectra analysis (spectral region 1300–800 cm−1), revealing the strong effect of the P3 peptide on lipid headgroups. The substitution by β-alanine of the alanine in KLAKLAK-NH2 provides some extra positively charged side chains, suggesting a peripheral orientation on the membrane. The effects of P3 and P3-long on the membrane’s mechanical properties are juxtaposed in Figure 7b for 0.03. The doubled P3-long analog rigidifies more strongly the bilayer in agreement with the findings from FTIR-ATR spectroscopy data, suggesting a probable lateral P3-long association to lipid molecules without significant alteration of the main lipid bilayer organization.
3.3. Impedance Characteristics of Lipid Bilayers in the Presence of KLAKLAK-NH2 and Analogues
The electrical parameters of suspended planar POPC bilayers, including their capacitance and resistance, were measured using FFT-EIS to probe for non-specific interactions of KLAKLAK peptides with the lipid matrix of the membrane.
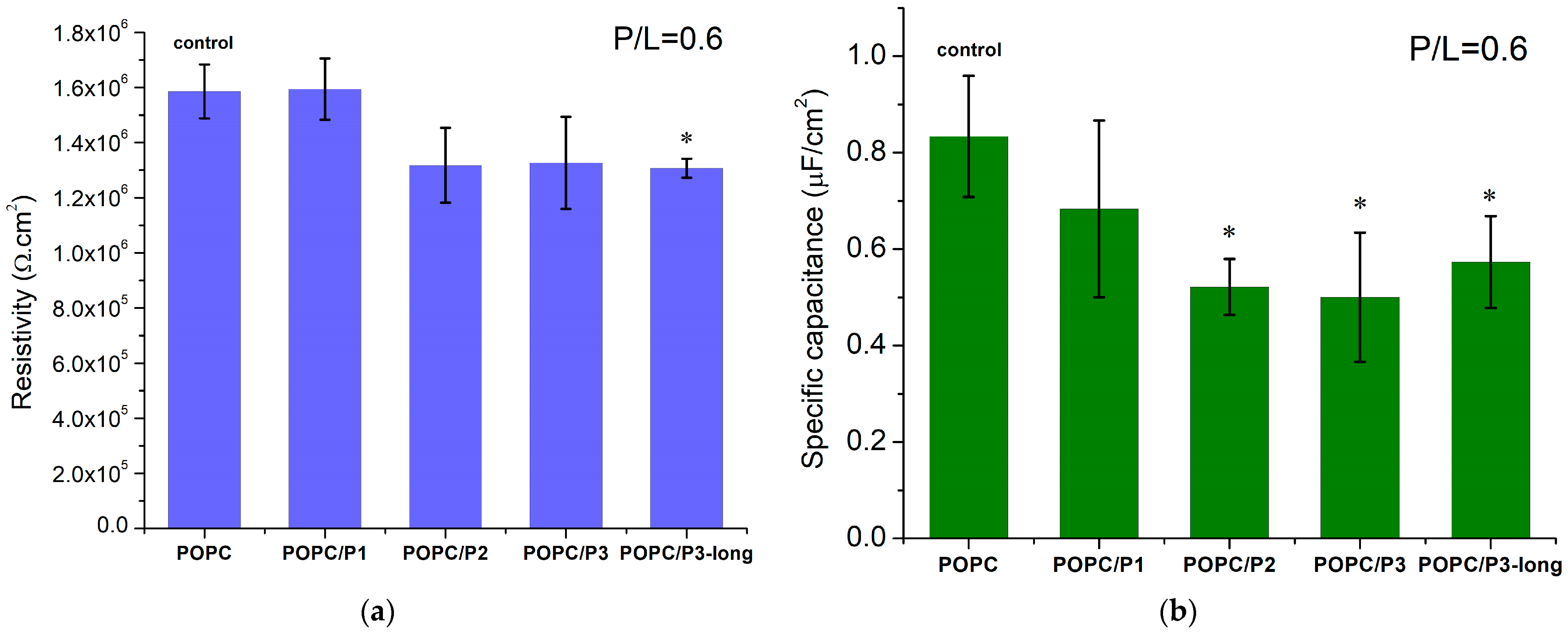
For a given peptide analog, at least three BLMs were prepared and impedance measurements were performed, with each of them averaged in over 10 repetitions. The weighted mean values with the corresponding standard deviations are presented in Figure 8. The POPC membrane resistivity obtained from the equivalent circuit fitting of the experimental data is coherent with the values for phosphatidylcholine bilayers, measured so far, ~106 Ω cm2 [34,76,77]. KLAKLAK-NH2 does not exert a measurable effect on the membrane resistivity, whereas all β-alanine-containing sequences induce a slight reduction in this parameter (Figure 8a). The substitution of alanines by β-alanines imparts additional positively charged side chains in the peptide molecule. Therefore, the formation of extra OH groups is expected around the positive choline headgroups as discussed above. A lower specific capacitance of KLAKLAK-treated POPC bilayers was measured for all peptide analogs at 0.6 peptide-to-lipid molar ratios in the samples (Figure 8b). While the obtained decrease for P3- and P3-long-treated membranes might be related to bilayer thickening, the reduced capacitance of P1- and P2-containing samples is likely due to lower dielectric permittivity. As reported from FTIR-ATR spectra, the bulky naphtalimid cyclic ring in the P2 molecule alters the aliphatic chain order in the hydrophobic core of the bilayer, which likely changes the dielectric permittivity of the lipid membrane.
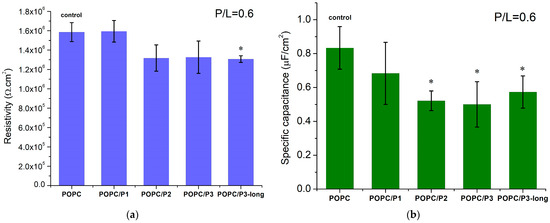
Figure 8.
(a) Resistivity and (b) specific capacitance of POPC bilayers in the presence of KLAKLAK-NH2 and its analogues at 0.6 total peptide-to-lipid molar ratios in the samples; values presented as weighted mean ± SD; at least three independent measurements (averaged over 10 repetitions each; * p < 0.005, -test).
4. Conclusions
The findings reported above elucidate the effect of the KLAKLAK-NH2 peptide family on important physicochemical parameters of lipid membrane models. Among others, the membrane-mediated processes in the biological cell are strongly related to the membrane-bending rigidity, one of the properties studied here. The specific capacitance and resistivity of lipid bilayers were explored in the presence of the antimicrobial peptide mimetics in view of unravelling their impact on membrane impedance characteristics. The latter are relevant to deciphering the effect on the electrical properties of lipid bilayers in order to shed light on the governing mechanisms involved in the transmission of electrical impulses or electroporation-based applications. The Fourier-transform infrared spectra with attenuated total reflectance revealed the interaction of KLAKLAK-NH2 and KLβAKLβAK-NH2 analogs with the polar head region of lipid bilayers. A similar effect on the mechanical properties of POPC membranes is obtained upon treatment with β-alanine-substituted short and long KLAKLAK-derivatives. The FTIR-ATR spectra of POPC bilayers in the presence of the long amino acid sequence with the β-alanine substitution of alanine lead to the conclusion of a probable lateral peptide association to the membrane. This finding is consistent with the results from the thermal shape fluctuation analysis, which reveal the concentration-dependent higher bending rigidity of (KLβAKLβAK)2-NH2-treated membranes. The membrane thickening amounts to lowering the specific electrical capacitance of KLβAKLβAK-NH2 and (KLβAKLβAK)2-NH2 containing samples. The bonding of 1,8-naphthalimide to the KLβAKLβAK-sequence induces the relatively most-pronounced structural alteration, which corroborates with the reported membrane softening.
The introduction of the β-amino analog of Ala into the primary structure of KLAKLAK-NH2 peptides producing more rigid and conformationally confined amphiphilic structures (P2, P3 and P3-long peptides) results here in the most important alterations of the physicochemical properties of the lipid membrane. These alterations are related to the antiproliferative and antimicrobial activity of the studied peptides reported so far [18,19]. The short P3-analog has been shown to exhibit a high antiproliferative effect against mammary gland type A adenocarcinoma cells (MCF-7) [18], while the doubled P3-long peptide combines low cytotoxicity and high antibacterial activity [19]. The slight nonselective increase in the biological activity of the P2 analog upon the bonding of the additional group 1,8-naphtalimideG [18] is coherent with the above findings about the structural and mechanical alterations of the lipid bilayer.
The obtained results present comprehensive information on the molecular interactions of the studied peptide mimetics on lipid membrane models. The reported influence on the structural, mechanical and impedance features of lipid membranes provides a useful groundwork for the development of liposome-based formulations for drug delivery and biomedical applications.
Author Contributions
Conceptualization, V.V.; methodology, V.V.; software, V.V.; validation, V.V.; formal analysis, V.V.; investigation, V.V., K.A., O.P., A.S.-I. and V.I.; synthesis of aimed molecules S.J. and E.N.; writing—original draft preparation, V.V., K.A., V.I. and D.D.; writing—review and editing, V.V. and D.D.; visualization, K.A. and V.V.; supervision, V.V., E.N. and D.D.; project administration, V.V. and D.D.; funding acquisition, V.V. and D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study is funded by the European Union-NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No. BG-RRP-2.004-0002, “BiOrgaMCT”. Funding by the Bulgarian National Science Fund, grant number KP-06-N58/6/2021, is acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
This study does not contain any experiments with humans or animals.
Data Availability Statement
Small amounts of the studied compounds are available from the corresponding author. All raw experimental data and protocols are also available from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Samreen; Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Thayer, A.M. Improving Peptides. Chem. Eng. News 2011, 89, 13–20. [Google Scholar] [CrossRef]
- Zasloff, M. Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G. Peptide Antibiotics and Their Role in Innate Immunity. Annu. Rev. Immunol. 1995, 13, 61–92. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G. Innate immunity and the normal microflora. Immunol. Rev. 2000, 173, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Pushpanathan, M.; Gunasekaran, P.; Rajendhran, J. Antimicrobial Peptides: Versatile Biological Properties. Int. J. Pept. 2013, 2013, 675391. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Barra, D.; Simmaco, M. Amphibian skin: A promising resource for antimicrobial peptides. Trends Biotechnol. 1995, 13, 205–209. [Google Scholar] [CrossRef]
- Yeung, A.T.; Gellatly, S.L.; Hancock, R.E. Multifunctional cationic host defence peptides and their clinical applications. Cell. Mol. Life Sci. CMLS 2011, 68, 2161–2176. [Google Scholar] [CrossRef]
- Medina, L.; Guzmán, F.; Álvarez, C.; Delgado, J.P.; Carbonell-M, B. Ramosin: The First Antibacterial Peptide Identified on Bolitoglossa ramosi Colombian Salamander. Pharmaceutics 2022, 14, 2579. [Google Scholar] [CrossRef] [PubMed]
- Michael Conlon, J.; Mechkarska, M.; King, J.D. Host-defense peptides in skin secretions of African clawed frogs (Xenopodinae, Pipidae). Gen. Comp. Endocrinol. 2012, 176, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.E.; Howard, A.; Kasprzak, A.B.; Gordon, K.H.; East, P.D. A peptidomics study reveals the impressive antimicrobial peptide arsenal of the wax moth Galleria mellonella. Insect Biochem. Mol. Biol. 2009, 39, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W. Cationic peptides: Effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 2001, 1, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Hale, J.D.F.; Hancock, R.E.W. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev. Anti-Infect. Ther. 2007, 5, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Javadpour, M.M.; Juban, M.M.; Lo, W.-C.J.; Bishop, S.M.; Alberty, J.B.; Cowell, S.M.; Becker, C.L.; McLaughlin, M.L. De Novo Antimicrobial Peptides with Low Mammalian Cell Toxicity. J. Med. Chem. 1996, 39, 3107–3113. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.; Iliev, I.; Angelova, T.; Nemska, V.; Sulikovska, I.; Naydenova, E.; Georgieva, N.; Givechev, I.; Grabchev, I.; Danalev, D. Synthesis, Antitumor and Antibacterial Studies of New Shortened Analogues of (KLAKLAK)2-NH2 and Their Conjugates Containing Unnatural Amino Acids. Molecules 2021, 26, 898. [Google Scholar] [CrossRef]
- Jaber, S.; Nemska, V.; Iliev, I.; Ivanova, E.; Foteva, T.; Georgieva, N.; Givechev, I.; Naydenova, E.; Karadjova, V.; Danalev, D. Synthesis and Biological Studies on (KLAKLAK)2-NH2 Analog Containing Unnatural Amino Acid β-Ala and Conjugates with Second Pharmacophore. Molecules 2021, 26, 7321. [Google Scholar] [CrossRef]
- Jaber, S.; Nemska, V.; Iliev, I.; Ivanova, E.; Foteva, T.; Georgieva, N.; Givechev, I.; Tanev, D.; Naydenova, E.; Danalev, D. Synthesis, antiproliferative and antimicrobial activities of (KLAKLAK)2-NH2 analogue containing nor-Leu and its conjugates with a second pharmacophore. Biotechnol. Biotechnol. Equip. 2023, 37, 151–158. [Google Scholar] [CrossRef]
- Mäe, M.; Langel, U. Cell-penetrating peptides as vectors for peptide, protein and oligonucleotide delivery. Curr. Opin. Pharmacol. 2006, 6, 509–514. [Google Scholar] [CrossRef]
- Deb, P.K.; Al-Attraqchi, O.; Chandrasekaran, B.; Paradkar, A.; Tekade, R.K. Chapter 16—Protein/Peptide Drug Delivery Systems: Practical Considerations in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 651–684. [Google Scholar] [CrossRef]
- Marinov, M.N.; Naydenova, E.D.; Momekov, G.T.; Prodanova, R.Y.; Markova, N.V.; Voynikov, Y.T.; Stoyanov, N.M. Synthesis, Characterization, Quantum-Chemical Calculations and Cytotoxic Activity of 1,8-Naphthalimide Derivatives with Non-Protein Amino Acids. Anti-Cancer Agents Med. Chem. 2019, 19, 1276–1284. [Google Scholar] [CrossRef]
- Luisi, P.L.; Walde, P. (Eds.) Giant Vesicles; John Wiley & Sons, Ltd.: Chichester, UK, 2000. [Google Scholar]
- Dimova, R.; Aranda, S.; Bezlyepkina, N.; Nikolov, V.; Riske, K.A.; Lipowsky, R. A practical guide to giant vesicles: Probing the membrane nanoregime via optical microscopy. J. Phys. Condens. Matter 2006, 18, S1151–S1176. [Google Scholar] [CrossRef]
- Dimova, R.; Marques, C. (Eds.) The Giant Vesicle Book, 1st ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Bagatolli, L.A. To see or not to see: Lateral organization of biological membranes and fluorescence microscopy. Biochim. Biophys. Acta 2006, 1758, 1541–1556. [Google Scholar] [CrossRef]
- Peng, B.; Ding, X.-Y.; Sun, C.; Yang, Y.-N.; Gao, Y.-J.; Zhao, X. The chain order of binary unsaturated lipid bilayers modulated by aromatic-residue-containing peptides: An ATR-FTIR spectroscopy study. RSC Adv. 2017, 7, 29386–29394. [Google Scholar] [CrossRef]
- Korkmaz, F.; Köster, S.; Yildiz, Ö.; Mäntele, W. The Role of Lipids for the Functional Integrity of Porin: An FTIR Study Using Lipid and Protein Reporter Groups. Biochemistry 2008, 47, 12126–12134. [Google Scholar] [CrossRef] [PubMed]
- Ergun, S.; Demir, P.; Uzbay, T.; Severcan, F. Agomelatine strongly interacts with zwitterionic DPPC and charged DPPG membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 2798–2806. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, E.; Dawgul, M.; Greber, K.; Iłowska, E.; Pogorzelska, A.; Kamysz, W. Self-assembly and interactions of short antimicrobial cationic lipopeptides with membrane lipids: ITC, FTIR and molecular dynamics studies. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 2625–2634. [Google Scholar] [CrossRef]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P.I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.; Dimitrov, D. Liposome electroformation. Faraday Discuss. Chem. Soc. 1986, 81, 303–311. [Google Scholar] [CrossRef]
- Vitkova, V.; Yordanova, V.; Staneva, G.; Petkov, O.; Stoyanova-Ivanova, A.; Antonova, K.; Popkirov, G. Dielectric Properties of Phosphatidylcholine Membranes and the Effect of Sugars. Membranes 2021, 11, 847. [Google Scholar] [CrossRef]
- Szoka, F., Jr.; Papahadjopoulos, D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu. Rev. Biophys. Bioeng. 1980, 9, 467–508. [Google Scholar] [CrossRef]
- Lasic, D.D. The mechanism of vesicle formation. Biochem. J. 1988, 256, 1–11. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Gutsmann, T.; Heimburg, T.; Keyser, U.; Mahendran, K.R.; Winterhalter, M. Protein reconstitution into freestanding planar lipid membranes for electrophysiological characterization. Nat. Protoc. 2015, 10, 188–198. [Google Scholar] [CrossRef]
- Montal, M.; Mueller, P. Formation of Bimolecular Membranes from Lipid Monolayers and a Study of Their Electrical Properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef]
- Helfrich, W. Elastic properties of lipid bilayers: Theory and possible experiments. Z. Naturforsch. 1973, 28, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Genova, J.; Vitkova, V.; Bivas, I. Registration and analysis of the shape fluctuations of nearly spherical lipid vesicles. Phys. Rev. E 2013, 88, 022707. [Google Scholar] [CrossRef] [PubMed]
- Bivas, I.; Hanusse, P.; Bothorel, P.; Lalanne, J.; Aguerre-Chariol, O. An application of the optical microscopy to the determination of the curvature elastic modulus of biological and model membranes. J. Phys. 1987, 48, 855–867. [Google Scholar] [CrossRef]
- Popkirov, G.S.; Schindler, R.N. Validation of experimental data in electrochemical impedance spectroscopy. Electrochim. Acta 1993, 38, 861–867. [Google Scholar] [CrossRef]
- Popkirov, G.S.; Schindler, R.N. A new approach to the problem of “good” and “bad” impedance data in electrochemical impedance spectroscopy. Electrochim. Acta 1994, 39, 2025–2030. [Google Scholar] [CrossRef]
- Casal, H.L.; Mantsch, H.H. Polymorphic phase behaviour of phospholipid membranes studied by infrared spectroscopy. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 1984, 779, 381–401. [Google Scholar] [CrossRef]
- Güler, G.; Gärtner, R.M.; Ziegler, C.; Mäntele, W. Lipid-Protein Interactions in the Regulated Betaine Symporter BetP Probed by Infrared Spectroscopy. J. Biol. Chem. 2016, 291, 4295–4307. [Google Scholar] [CrossRef] [PubMed]
- Arrondo, J.L.R.; Goñi, F.M.; Macarulla, J.M. Infrared spectroscopy of phosphatidylcholines in aqueous suspension a study of the phosphate group vibrations. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1984, 794, 165–168. [Google Scholar] [CrossRef]
- Nagata, Y.; Mukamel, S. Vibrational Sum-Frequency Generation Spectroscopy at the Water/Lipid Interface: Molecular Dynamics Simulation Study. J. Am. Chem. Soc. 2010, 132, 6434–6442. [Google Scholar] [CrossRef]
- Mondal, J.A.; Nihonyanagi, S.; Yamaguchi, S.; Tahara, T. Three Distinct Water Structures at a Zwitterionic Lipid/Water Interface Revealed by Heterodyne-Detected Vibrational Sum Frequency Generation. J. Am. Chem. Soc. 2012, 134, 7842–7850. [Google Scholar] [CrossRef] [PubMed]
- Ohto, T.; Backus, E.H.G.; Hsieh, C.-S.; Sulpizi, M.; Bonn, M.; Nagata, Y. Lipid Carbonyl Groups Terminate the Hydrogen Bond Network of Membrane-Bound Water. J. Phys. Chem. Lett. 2015, 6, 4499–4503. [Google Scholar] [CrossRef]
- Fringeli, U.P.; Günthard, H.H. Infrared Membrane Spectroscopy. In Membrane Spectroscopy; Grell, E., Ed.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 270–332. [Google Scholar] [CrossRef]
- Schultz, Z.D.; Levin, I.W. Vibrational Spectroscopy of Biomembranes. Annu. Rev. Anal. Chem. 2011, 4, 343–366. [Google Scholar] [CrossRef]
- Mantsch, H.H.; McElhaney, R.N. Phospholipid phase transitions in model and biological membranes as studied by infrared spectroscopy. Chem. Phys. Lipids 1991, 57, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.N.A.H.; McElhaney, R.N. Membrane lipid phase transitions and phase organization studied by Fourier transform infrared spectroscopy. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 2347–2358. [Google Scholar] [CrossRef]
- Mushayakarara, E.; Levin, I.W. Determination of acyl chain conformation at the lipid interface region: Raman spectroscopic study of the carbonyl stretching mode region of dipalmitoyl phosphatidylcholine and structurally related molecules. J. Phys. Chem. 1982, 86, 2324–2327. [Google Scholar] [CrossRef]
- Fox, C.B.; Harris, J.M. Confocal Raman microscopy for simultaneous monitoring of partitioning and disordering of tricyclic antidepressants in phospholipid vesicle membranes. J. Raman Spectrosc. 2010, 41, 498–507. [Google Scholar] [CrossRef]
- Lohner, K. Effects of small organic molecules on phospholidpid phase transitions. Chem. Phys. Lipids 1991, 57, 341–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-P.; Lewis, R.N.A.H.; Hodges, R.S.; McElhaney, R.N. Interaction of a Peptide Model of a Hydrophobic Transmembrane alpha-Helical Segment of a Membrane Protein with Phosphatidylethanolamine Bilayers: Differential Scanning Calorimetric and Fourier Transform Infrared Spectroscopic Studies. Biophys. J. 1995, 68, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Deo, N.; Somasundaran, T.; Somasundaran, P. Solution properties of amitriptyline and its partitioning into lipid bilayers. Colloids Surf. B Biointerfaces 2004, 34, 155–159. [Google Scholar] [CrossRef]
- Fišar, Z.; Fuksová, K.; Velenovská, M. Binding of Imipramine to Phospholipid Bilayers Using Radioligand Binding Assay. Gen. Physiol. Biophys. 2004, 23, 77–99. [Google Scholar]
- Qiao, Z.-Y.; Hou, C.-Y.; Zhang, D.; Liu, Y.; Lin, Y.-X.; An, H.-W.; Li, X.-J.; Wang, H. Self-assembly of cytotoxic peptide conjugated poly(β-amino ester)s for synergistic cancer chemotherapy. J. Mater. Chem. B 2015, 3, 2943–2953. [Google Scholar] [CrossRef]
- Strandberg, E.; Tiltak, D.; Ehni, S.; Wadhwani, P.; Ulrich, A.S. Lipid shape is a key factor for membrane interactions of amphipathic helical peptides. Biochim. Biophys. Acta (BBA) Biomembr. 2012, 1818, 1764–1776. [Google Scholar] [CrossRef]
- Lee, J.; Oh, E.-T.; Lee, H.-J.; Lee, E.; Kim, H.G.; Park, H.J.; Kim, C. Tuning of Peptide Cytotoxicity with Cell Penetrating Motif Activatable by Matrix Metalloproteinase-2. ACS Omega 2022, 7, 29684–29691. [Google Scholar] [CrossRef]
- Malina, A.; Shai, Y. Conjugation of fatty acids with different lengths modulates the antibacterial and antifungal activity of a cationic biologically inactive peptide. Biochem. J. 2005, 390, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Vitkova, V.; Misbah, C. Dynamics of lipid vesicles—From thermal fluctuations to rheology. In Advances in Planar Lipid Bilayers and Liposomes; Iglic, A., Ed.; Academic Press: Burlington, NJ, USA, 2011; Volume 14, pp. 258–292. [Google Scholar]
- Dreier, L.B.; Wolde-Kidan, A.; Bonthuis, D.J.; Netz, R.R.; Backus, E.H.G.; Bonn, M. Unraveling the Origin of the Apparent Charge of Zwitterionic Lipid Layers. J. Phys. Chem. Lett. 2019, 10, 6355–6359. [Google Scholar] [CrossRef]
- Vitkova, V.; Meleard, P.; Pott, T.; Bivas, I. Alamethicin influence on the membrane bending elasticity. Eur. Biophys. J. 2006, 35, 281–286. [Google Scholar] [CrossRef]
- Vitkova, V.; Stoyanova-Ivanova, A.; Jaber, S.; Naydenova, E.; Danalev, D. Bending Elasticity of Phospholipid Bilayers Containing an Amphipathic Peptide with Low Mammalian Cytotoxicity. Proc. Bulg. Acad. Sci. 2022, 75, 1428–1436. [Google Scholar] [CrossRef]
- Ben-Shaul, A.; Ben-Tal, N.; Honig, B. Statistical thermodynamic analysis of peptide and protein insertion into lipid membranes. Biophys. J. 1996, 71, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Kondrashov, O.V.; Akimov, S.A. Regulation of Antimicrobial Peptide Activity via Tuning Deformation Fields by Membrane-Deforming Inclusions. Int. J. Mol. Sci. 2022, 23, 326. [Google Scholar] [CrossRef] [PubMed]
- Kondrashov, O.V.; Rokitskaya, T.I.; Batishchev, O.V.; Kotova, E.A.; Antonenko, Y.N.; Akimov, S.A. Peptide-induced membrane elastic deformations decelerate gramicidin dimer-monomer equilibration. Biophys. J. 2021, 120, 5309–5321. [Google Scholar] [CrossRef] [PubMed]
- Bivas, I.; Meleard, P. Bending elasticity of a lipid bilayer containing an additive. Phys. Rev. E 2003, 67, 012901. [Google Scholar] [CrossRef] [PubMed]
- Shchelokovskyy, P.; Tristram-Nagle, S.; Dimova, R. Effect of the HIV-1 fusion peptide on the mechanical properties and leaflet coupling of lipid bilayers. New J. Phys. 2011, 13, 025004. [Google Scholar] [CrossRef] [PubMed]
- Dimova, R. Recent developments in the field of bending rigidity measurements on membranes. Adv. Colloid Interface Sci. 2014, 208, 225–234. [Google Scholar] [CrossRef]
- Dimova, R. Giant Vesicles and Their Use in Assays for Assessing Membrane Phase State, Curvature, Mechanics, and Electrical Properties. Annu. Rev. Biophys. 2019, 48, 93–119. [Google Scholar] [CrossRef]
- Naumowicz, M.; Zając, M.; Kusaczuk, M.; Gál, M.; Kotyńska, J. Electrophoretic Light Scattering and Electrochemical Impedance Spectroscopy Studies of Lipid Bilayers Modified by Cinnamic Acid and Its Hydroxyl Derivatives. Membranes 2020, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Velikonja, A.; Kramar, P.; Miklavčič, D.; Maček Lebar, A. Specific electrical capacitance and voltage breakdown as a function of temperature for different planar lipid bilayers. Bioelectrochemistry 2016, 112, 132–137. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).