A Novel PBM for Nanomilling of Drugs in a Recirculating Wet Stirred Media Mill: Impacts of Batch Size, Flow Rate, and Back-Mixing
Abstract
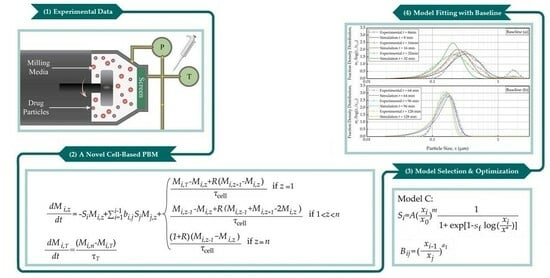
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation and Characterization Methods
3. Theoretical
3.1. Preliminaries: Residence Time Distribution
3.2. Formulation of the PBM
3.3. Functional Forms of Si and Bij
3.4. A Full Back-Calculation Method for the Estimation of PBM Parameters
3.5. Impacts of the Batch Size, Flow Rate, and Imperfect Mixing and PBM Validation
4. Results and Discussion
4.1. Estimating the Breakage Parameters and Discrimination of Models A–D
4.2. Prediction of the Impacts of Batch Size and Flow Rate Using Model C
4.2.1. Impact of Batch Size and Flow Rate
4.2.2. Milling Time vs. Effective Milling Time
4.3. Simulating the Impact of Imperfect Mixing in the Milling Chamber
4.4. On the Grinding Limit and the Transition Particle Size
4.5. Limitations of the Current PBM
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Symbols used | |
| A | apparent breakage rate constant, min–1 |
| a1 | breakage distribution exponent |
| Ac | cross-sectional area of the mill chamber perpendicular to suspension flow, m2 |
| b | breakage distribution parameter |
| B | cumulative breakage distribution parameter |
| c | volume fraction of the beads in the suspension–bead mixture |
| CFD | computational fluid dynamics |
| D | diameter of the rotor, m |
| dispersion coefficient, m2/s | |
| Dm | mill chamber diameter, m |
| DEM | discrete element method |
| K | total number of size classes in the laser diffraction measurement |
| L | length of the mill, m |
| mi | mass fraction of particles in size class i |
| i | mass fraction density of particles in size class i |
| Mi | mass concentration in size class i, kg/m3 |
| MHD | microhydrodynamic |
| n | number of cells in cell-based PBM |
| N | number of size classes used in the PBM |
| Nto | number of theoretical passes or turnovers |
| NT | number of trials |
| ODE | ordinary differential equation |
| P | total number of time samples |
| PBM | population balance model |
| Pec | Peclet number of a continuous mill in recirculation operation |
| Pes | Peclet number of a single-pass continuous mill |
| PSD | particle size distribution |
| Q | volumetric flow rate of the recirculating suspension, m3/s |
| R | back-mixing ratio |
| internal recirculation rate, m3/s | |
| RTD | residence time distribution |
| S | specific breakage rate parameter, min–1 |
| sf | shape factor |
| SI | stress intensity, Pa |
| SN | stress number |
| SSR | sum-of-squared-residuals |
| t | milling time, min |
| ua | axial interstitial velocity of the drug suspension, m/s |
| us | superficial velocity of the drug suspension, m/s |
| Vm | free volume of the milling chamber available for bead filling, m3 |
| Vs | batch volume of the suspension, m3 |
| VT | volume of the suspension in the holding tank, m3 |
| WSMM | wet stirred media milling |
| x | particle size, µm |
| x* | transition particle size, µm |
| normalizing reference particle size, µm | |
| YSZ | yttrium-stabilized zirconia |
| Greek Letters | |
| τs | average residence time in a single pass, min |
| τc | average residence time in the mill in recirculation (circuit) mode, min |
| τcell | average residence time in a cell, min |
| τT | average residence time in the holding tank, min |
| ω | rotational speed of the stirrer (rotor), rpm |
| Indices | |
| 10, 50, 90 | 10%, 50%, and 90% passing sizes of the cumulative volume PSD |
| EXP | experimental |
| i, j | size-class index |
| m | mill chamber |
| MOD | model predicted |
| n | total number of cells in the cell-based PBM |
| p | time index |
| T | holding tank |
Appendix A
References
- Tanaka, Y.; Inkyo, M.; Yumoto, R.; Nagai, J.; Takano, M.; Nagata, S. Nanoparticulation of Probucol, a Poorly Water-Soluble Drug, Using a Novel Wet-Milling Process to Improve in vitro Dissolution and in vivo Oral Absorption. Drug Dev. Ind. Pharm. 2012, 38, 1015–1023. [Google Scholar] [CrossRef]
- Li, M.; Azad, M.; Davé, R.; Bilgili, E. Nanomilling of Drugs for Bioavailability Enhancement: A Holistic Formulation-Process Perspective. Pharmaceutics 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Malamatari, M.; Taylor, K.M.G.; Malamataris, S.; Douroumis, D.; Kachrimanis, K. Pharmaceutical Nanocrystals: Production by Wet Milling and Applications. Drug Discov. Today 2018, 23, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Bhakay, A.; Merwade, M.; Bilgili, E.; Dave, R.N. Novel Aspects of Wet Milling for the Production of Microsuspensions and Nanosuspensions of Poorly Water-Soluble Drugs. Drug Dev. Ind. Pharm. 2011, 37, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Bhakay, A.; Rahman, M.; Dave, R.N.; Bilgili, E. Bioavailability Enhancement of Poorly Water-Soluble Drugs via Nanocomposites: Formulation–Processing Aspects and Challenges. Pharmaceutics 2018, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A Formulation Approach for Poorly-Water-Soluble Compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, L. Design Space and QbD Approach for Production of Drug Nanocrystals by Wet Media Milling Techniques. Pharmaceutics 2018, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yaragudi, N.; Afolabi, A.; Dave, R.; Bilgili, E. Sub-100 nm Drug Particle Suspensions Prepared via Wet Milling with Low Bead Contamination Through Novel Process Intensification. Chem. Eng. Sci. 2015, 130, 207–220. [Google Scholar] [CrossRef]
- Tuomela, A.; Hirvonen, J.; Peltonen, L. Stabilizing Agents for Drug Nanocrystals: Effect on Bioavailability. Pharmaceutics 2016, 8, 16. [Google Scholar] [CrossRef]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing—Oral Formulation Development and Biopharmaceutical Evaluation. Adv. Drug Deliv. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of Nanosuspensions in Drug Delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J. Pharmaceutical Nanocrystals by Nanomilling: Critical Process Parameters, Particle Fracturing, and Stabilization Methods. J. Pharm. Pharmacol. 2010, 62, 1569–1579. [Google Scholar] [CrossRef]
- Bitterlich, A.; Laabs, C.; Busmann, E.; Grandeury, A.; Juhnke, M.; Bunjes, H.; Kwade, A. Challenges in Nanogrinding of Active Pharmaceutical Ingredients. Chem. Eng. Technol. 2014, 37, 840–846. [Google Scholar] [CrossRef]
- Cerdeira, A.M.; Mazzotti, M.; Gander, B. Miconazole Nanosuspensions: Influence of Formulation Variables on Particle Size Reduction and Physical Stability. Int. J. Pharm. 2010, 396, 210–218. [Google Scholar] [CrossRef]
- Verma, S.; Huey, B.D.; Burgess, D.J. Scanning Probe Microscopy Method for Nanosuspension Stabilizer Selection. Langmuir 2009, 25, 12481–12487. [Google Scholar] [CrossRef]
- Kawatra, S.K. Advances in Comminution; Society for Mining, Metallurgy and Exploration: Englewood, CO, USA, 2006. [Google Scholar]
- Juhnke, M.; Märtin, D.; John, E. Generation of Wear During the Production of Drug Nanosuspensions by Wet Media Milling. Eur. J. Pharm. Biopharm. 2012, 81, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Burgess, D.J. Wet Milling Induced Physical and Chemical Instabilities of Naproxen Nano-Crystalline Suspensions. Int. J. Pharm. 2014, 466, 223–232. [Google Scholar] [CrossRef]
- Sharma, P.; Denny, W.A.; Garg, S. Effect of Wet Milling Process on the Solid State of Indomethacin and Simvastatin. Int. J. Pharm. 2009, 380, 40–48. [Google Scholar] [CrossRef]
- Bilgili, E.; Guner, G. Mechanistic Modeling of Wet Stirred Media Milling for Production of Drug Nanosuspensions. AAPS Pharm. Sci. Technol. 2020, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.; Rahman, M.; Bilgili, E. Impact of Media Material and Process Parameters on Breakage Kinetics–Energy Consumption During Wet Media Milling of Drugs. Eur. J. Pharm. Biopharm. 2020, 153, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Alvarez, P.; Bilgili, E. A Microhydrodynamic Rationale for Selection of Bead Size in Preparation of Drug Nanosuspensions via Wet Stirred Media Milling. Int. J. Pharm. 2017, 524, 178–192. [Google Scholar] [CrossRef]
- Afolabi, A.; Akinlabi, O.; Bilgili, E. Impact of Process Parameters on the Breakage Kinetics of Poorly Water-Soluble Drugs During Wet Stirred Media Milling: A Microhydrodynamic View. Eur. J. Pharm. Sci. 2014, 51, 75–86. [Google Scholar] [CrossRef]
- Singh, S.K.; Srinivasan, K.; Gowthamarajan, K.; Singare, D.S.; Prakash, D.; Gaikwad, N.B. Investigation of Preparation Parameters of Nanosuspension by Top-Down Media Milling to Improve the Dissolution of Poorly Water-Soluble Glyburide. Eur. J. Pharm. Biopharm. 2011, 78, 441–446. [Google Scholar] [CrossRef]
- Patel, D.J.; Patel, J.K.; Pandya, V.M. Improvement in the Dissolution of Poorly Water-Soluble Drug Using Media Milling Technique. Thai J. Pharm. Sci. 2010, 34, 155–164. [Google Scholar]
- Nakach, M.; Authelin, J.-R.; Agut, C. New Approach and Practical Modelling of Bead Milling Process for the Manufacturing of Nanocrystalline Suspensions. J. Pharm. Sci. 2017, 106, 1889–1904. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Schenck, D.; Bose, S.; Ruegger, C. Optimization of Formulation and Process Parameters for the Production of Nanosuspension by Wet Media Milling Technique: Effect of Vitamin E TPGS and Nanocrystal Particle Size on Oral Absorption. Eur. J. Pharm. Sci. 2012, 47, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Toneva, P.; Peukert, W. Modelling of Mills and Milling Circuits. In Handbook of Powder Technology; Salman, A.D., Ghadiri, M., Hounslow, M.J., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; Volume 12, pp. 873–911. ISBN 978-044-453-080-6. [Google Scholar]
- Winardi, S.; Widiyastuti, W.; Septiani, E.; Nurtono, T. Simulation of Solid-Liquid Flows in a Stirred Bead Mill Based on Computational Fluid Dynamics (CFD). Mater. Res. Express 2018, 5, 054002. [Google Scholar] [CrossRef]
- Gudin, D.; Turczyn, R.; Mio, H.; Kano, J.; Saito, F. Simulation of the Movement of Beads by the Discrete Element Method (DEM) with Respect to the Wet Grinding Process. AIChE J. 2006, 52, 3421–3426. [Google Scholar] [CrossRef]
- Gudin, D.; Kano, J.; Saito, F. Effect of the Friction Coefficient in the Discrete Element Method Simulation on Media Motion in a Wet Bead Mill. Adv. Powder Technol. 2007, 18, 555–565. [Google Scholar] [CrossRef]
- Annapragada, A.; Adjei, A. Numerical Simulation of Milling Processes as an Aid to Process Design. Int. J. Pharm. 1996, 136, 1–11. [Google Scholar] [CrossRef]
- Frances, C. On Modelling of Submicronic Wet Milling Processes in Bead Mills. Powder Technol. 2004, 143, 253–263. [Google Scholar] [CrossRef]
- Bilgili, E.; Hamey, R.; Scarlett, B. Nano-Milling of Pigment Agglomerates Using a Wet Stirred Media Mill: Elucidation of the Kinetics and Breakage Mechanisms. Chem. Eng. Sci. 2006, 61, 149–157. [Google Scholar] [CrossRef]
- Guner, G.; Yilmaz, D.; Bilgili, E. Kinetic and Microhydrodynamic Modeling of Fenofibrate Nanosuspension Production in a Wet Stirred Media Mill. Pharmaceutics 2021, 13, 1055. [Google Scholar] [CrossRef] [PubMed]
- Eskin, D.; Zhupanska, O.; Hamey, R.; Moudgil, B.; Scarlett, B. Microhydrodynamic Analysis of Nanogrinding in Stirred Media Mills. AIChE J. 2005, 51, 1346–1358. [Google Scholar] [CrossRef]
- Jayasundara, C.T.; Yang, R.; Guo, B.; Yu, A.; Rubenstein, J. Effect of Slurry Properties on Particle Motion in IsaMills. Miner. Eng. 2009, 22, 886–892. [Google Scholar] [CrossRef]
- Jayasundara, C.T.; Yang, R.; Yu, A. Effect of the Size of Media on Grinding Performance in Stirred Mills. Miner. Eng. 2012, 33, 66–71. [Google Scholar] [CrossRef]
- Yamada, Y.; Sakai, M. Lagrangian–Lagrangian Simulations of Solid–Liquid Flows in a Bead Mill. Powder Technol. 2013, 239, 105–114. [Google Scholar] [CrossRef]
- Beinert, S.; Schilde, C.; Gronau, G.; Kwade, A. CFD-Discrete Element Method Simulations Combined with Compression Experiments to Characterize Stirred-Media Mills. Chem. Eng. Technol. 2014, 37, 770–778. [Google Scholar] [CrossRef]
- Cerdeira, A.M.; Gander, B.; Mazzotti, M. Role of Milling Parameters and Particle Stabilization on Nanogrinding of Drug Substances of Similar Mechanical Properties. Chem. Eng. Technol. 2011, 34, 1427–1438. [Google Scholar] [CrossRef]
- Becker, M.; Kwade, A.; Schwedes, J. Stress Intensity in Stirred Media Mills and Its Effect on Specific Energy Requirement. Int. J. Miner. Process. 2001, 61, 189–208. [Google Scholar] [CrossRef]
- Randolph, A. Theory of Particulate Processes: Analysis and Techniques of Continuous Crystallization; Elsevier: New York, NY, USA, 2012. [Google Scholar]
- Ramkrishna, D. Population Balances: Theory and Applications to Particulate Systems in Engineering; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Verkoeijen, D.; Pouw, G.A.; Meesters, G.M.; Scarlett, B. Population Balances for Particulate Processes—A Volume Approach. Chem. Eng. Sci. 2002, 57, 2287–2303. [Google Scholar] [CrossRef]
- Hounslow, M. The Population Balance as a Tool for Understanding Particle Rate Processes. KONA Powder Part. J. 1998, 16, 179–193. [Google Scholar] [CrossRef]
- Varinot, C.; Berthiaux, H.; Dodds, J. Prediction of the Product Size Distribution in Associations of Stirred Bead Mills. Powder Technol. 1999, 105, 228–236. [Google Scholar] [CrossRef]
- Kwade, A. Scriptum Grinding and Dispersing with Stirred Media Mills: Research and Application. In Proceedings of the 21st iPAT, Braunsschweig, Germany, 13–14 October 2005; Institute for Particle Technology, Technical University of Braunsschweig: Braunsschweig, Germany, 2005. [Google Scholar]
- Kwade, A.; Schwedes, J. Wet Grinding in Stirred Media Mills. In Handbook of Powder Technology; Mills, S.M., Salman, A.D., Ghadiri, M., Hounslow, M.J., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2007; Volume 12, Chapter 6; pp. 251–382. ISBN 978-044-453-080-6. [Google Scholar]
- King, R.P. Modeling and Simulation of Mineral Processing Systems; Butterworth-Heinemann: Oxford, UK, 2001; 416p. [Google Scholar]
- Abouzeid, A.-Z.; Mika, T.; Sastry, K.V.; Fuerstenau, D. The Influence of Operating Variables on the Residence Time Distribution for Material Transport in a Continuous Rotary Drum. Powder Technol. 1974, 10, 273–288. [Google Scholar] [CrossRef]
- Austin, L.; Luckie, P.; Ateya, B. Residence Time Distributions in Mills. Cem. Concr. Res. 1971, 1, 241–256. [Google Scholar] [CrossRef]
- Bilgili, E.; Scarlett, B. Numerical Simulation of Open-Circuit Continuous Mills Using a Non-Linear Population Balance Framework: Incorporation of Non-First-Order Effects. Chem. Eng. Technol. Ind. Chem. Plant Equip. Process Eng. Biotechnol. 2005, 28, 153–159. [Google Scholar] [CrossRef]
- Muanpaopong, N.; Davé, R.; Bilgili, E. A Cell-Based PBM for Continuous Open-Circuit Dry Milling: Impact of Axial Mixing, Nonlinear Breakage, and Screen Size. Powder Technol. 2022, 399, 117099. [Google Scholar] [CrossRef]
- Whiten, W. A Matrix Theory of Comminution Machines. Chem. Eng. Sci. 1974, 29, 589–599. [Google Scholar] [CrossRef]
- Berthiaux, H.; Heitzmann, D.; Dodds, J.A. Validation of a Model of a Stirred Bead Mill by Comparing Results Obtained in Batch and Continuous Mode Grinding. Int. J. Miner. Process. 1996, 44, 653–661. [Google Scholar] [CrossRef]
- Fadhel, H.B.; Frances, C.; Mamourian, A. Investigations on Ultra-Fine Grinding of Titanium Dioxide in a Stirred Media Mill. Powder Technol. 1999, 105, 362–373. [Google Scholar] [CrossRef]
- Kwade, A. Wet Comminution in Stirred Media Mills—Research and Its Practical Application. Powder Technol. 1999, 105, 14–20. [Google Scholar] [CrossRef]
- Lira, B.; Kavetsky, A. Applications of a New Model-Based Method of Ball Mill Simulation and Design. Miner. Eng. 1990, 3, 149–163. [Google Scholar] [CrossRef]
- Tuzun, M.A. A Study of Comminution in a Vertical Stirred Ball Mill. Ph.D. Thesis, Chemical Engineering Department, University of Natal, Durban, South Africa, 1993. [Google Scholar]
- Austin, A.G.; Luckie, P.T.; Klimpel, R.R. Process Engineering of Size Reduction: Ball Milling; Society of Mining Engineers of the American Institute of Mining, Metallurgical and Petroleum Engineers: New York, NY, USA, 1984. [Google Scholar]
- Hasan, M.; Palaniandy, S.; Hilden, M.; Powell, M. Simulating Product Size Distribution of an Industrial Scale VertiMill® Using a Time-Based Population Balance Model. Miner. Eng. 2018, 127, 312–317. [Google Scholar] [CrossRef]
- Hasan, M.; Palaniandy, S.; Hilden, M.; Powell, M. Calculating Breakage Parameters of a Batch Vertical Stirred Mill. Miner. Eng. 2017, 111, 229–237. [Google Scholar] [CrossRef]
- Knieke, C.; Sommer, M.; Peukert, W. Identifying the Apparent and True Grinding Limit. Powder Technol. 2009, 195, 25–30. [Google Scholar] [CrossRef]
- Cho, H.; Waters, M.A.; Hogg, R. Investigation of the grind limit in stirred-media milling. Int. J. Miner. Process. 1996, 44–45, 607–615. [Google Scholar] [CrossRef]
- Knieke, C.; Steinborn, C.; Romeis, S.; Peukert, W.; Breitung-Faes, S.; Kwade, A. Nanoparticle production with stirred-media mills: Opportunities and limits. Chem. Eng. Technol. 2010, 33, 1401–1411. [Google Scholar] [CrossRef]
- Muanpaopong, N.; Davé, R.; Bilgili, E. A Comparative Analysis of Steel and Alumina Balls in Fine Milling of Cement Clinker via PBM and DEM. Powder Technol. 2023, 421, 118454. [Google Scholar] [CrossRef]
- The MathWorks, Inc. Global Optimization Toolbox User’s Guide (R2022a); The MathWorks, Inc.: Natick, MA, USA, 2022. [Google Scholar]
- Glover, F. A Template for Scatter Search and Path Relinking. In Proceedings of the European Conference on Artificial Evolution, Berlin/Heidelberg, Germany, 20–24 October 1997; pp. 1–51. [Google Scholar]
- Ullrich, T.; Fellner, D.W. Statistical Analysis on Global Optimization. In Proceedings of the 2014 International Conference on Mathematics and Computers in Sciences and in Industry, Varna, Bulgaria, 13–15 September 2014; pp. 99–106. [Google Scholar]
- Vogel, L.; Peukert, W. Breakage Behaviour of Different Materials—Construction of a Master Curve for the Breakage Probability. Powder Technol. 2003, 129, 101–110. [Google Scholar] [CrossRef]
- Boldyrev, V.; Pavlov, S.; Goldberg, E. Interrelation Between Fine Grinding and Mechanical Activation. Int. J. Miner. Process. 1996, 44, 181–185. [Google Scholar] [CrossRef]
- Schönert, K.; Steier, K. Die Grenze der Zerkleinerung bei kleinen Korngrößen. Chem. Ing. Tech. 1971, 43, 773–777. [Google Scholar] [CrossRef]
- Maar, S.; Damm, C.; Peukert, W. Wet Nanomilling of Naproxen Using a Novel Stabilization Mechanism via Zirconium Complexation. Adv. Powder Technol. 2022, 33, 103723. [Google Scholar] [CrossRef]

















| Run No. | Identifier | Batch Size, Vs (mL) | Volumetric Flow Rate, Q (mL/min) |
|---|---|---|---|
| 1 | Baseline | 236 | 126 |
| 2 | Smaller Batch Size | 118 | 126 |
| 3 | Larger Batch Size | 472 | 126 |
| 4 | Lower Flow Rate | 236 | 63 |
| 5 | Higher Flow Rate | 236 | 250 |
| Run No. | Simulated Effect | Batch Size, Vs (mL) | Volumetric Flow Rate, Q (mL/min) | No. of Cells, n (-) | Back-Mixing Ratio, R (-) |
|---|---|---|---|---|---|
| 1 | Baseline | 236 | 126 | 1 | – |
| 2 | Smaller Batch Size | 118 | 126 | 1 | – |
| 3 | Larger Batch Size | 472 | 126 | 1 | – |
| 4 | Lower Flow Rate | 236 | 63 | 1 | – |
| 5 | Higher Flow Rate | 236 | 250 | 1 | – |
| 6 | No. of Cells n, R = 0 | 236 | 126 | 2 | 0 |
| 7 | 236 | 126 | 3 | 0 | |
| 8 | 236 | 126 | 6 | 0 | |
| 9 | 236 | 126 | 8 | 0 | |
| 10 | No. of Cells n, R = 2.52 | 236 | 126 | 2 | 2.52 |
| 11 | 236 | 126 | 3 | 2.52 | |
| 12 | 236 | 126 | 6 | 2.52 | |
| 13 | 236 | 126 | 8 | 2.52 | |
| 14 | Back-mixing Ratio R, n = 3 | 236 | 126 | 3 | 0.5 |
| 15 | 236 | 126 | 3 | 1 | |
| 16 | 236 | 126 | 3 | 2.52 | |
| 17 | 236 | 126 | 3 | 4 |
| Model C | sf | |||||
|---|---|---|---|---|---|---|
| Lower Boundary | 0 | 50 | 0 | 0.038 | 5 | |
| Upper Boundary | 5 | 108 | 4 | 1 | 30 | |
| Initial Guess | 2 | 5000 | 2 | 0.1 | 20 | |
| NT = 200 | Fitted Parameters | 2.31 | 2.72 × 106 | 2.15 | 0.196 | 16.7 |
| SSR | 38.0 | |||||
| NT = 400 | Fitted Parameters | 2.31 | 2.72 × 106 | 2.15 | 0.196 | 16.7 |
| SSR | 38.0 | |||||
| NT = 800 | Fitted Parameters | 2.31 | 2.72 × 106 | 2.15 | 0.196 | 16.7 |
| SSR | 38.0 | |||||
| NT = 1000 | Fitted Parameters | 2.31 | 2.72 × 106 | 2.15 | 0.196 | 16.7 |
| SSR | 38.0 |
| Model A | |||||
| Fitted Parameters | 2.46 | 3.32 × 1011 | 3.84 | ||
| SSR | 53.5 | ||||
| Model B | |||||
| Fitted Parameters | 2.25 | 2.92 × 109 | 3.19 | 0.174 | |
| SSR | 43.4 | ||||
| Model C | sf | ||||
| Fitted Parameters | 2.31 | 2.72 × 106 | 2.15 | 0.196 | 16.7 |
| SSR | 38.0 | ||||
| Model D | |||||
| Fitted Parameters | 2.31 | 1.03 × 107 | 2.36 | 0.214 | 41.7 |
| SSR | 38.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidari, H.; Muanpaopong, N.; Guner, G.; Yao, H.F.; Clancy, D.J.; Bilgili, E. A Novel PBM for Nanomilling of Drugs in a Recirculating Wet Stirred Media Mill: Impacts of Batch Size, Flow Rate, and Back-Mixing. Pharmaceutics 2024, 16, 353. https://doi.org/10.3390/pharmaceutics16030353
Heidari H, Muanpaopong N, Guner G, Yao HF, Clancy DJ, Bilgili E. A Novel PBM for Nanomilling of Drugs in a Recirculating Wet Stirred Media Mill: Impacts of Batch Size, Flow Rate, and Back-Mixing. Pharmaceutics. 2024; 16(3):353. https://doi.org/10.3390/pharmaceutics16030353
Chicago/Turabian StyleHeidari, Hamidreza, Nontawat Muanpaopong, Gulenay Guner, Helen F. Yao, Donald J. Clancy, and Ecevit Bilgili. 2024. "A Novel PBM for Nanomilling of Drugs in a Recirculating Wet Stirred Media Mill: Impacts of Batch Size, Flow Rate, and Back-Mixing" Pharmaceutics 16, no. 3: 353. https://doi.org/10.3390/pharmaceutics16030353
APA StyleHeidari, H., Muanpaopong, N., Guner, G., Yao, H. F., Clancy, D. J., & Bilgili, E. (2024). A Novel PBM for Nanomilling of Drugs in a Recirculating Wet Stirred Media Mill: Impacts of Batch Size, Flow Rate, and Back-Mixing. Pharmaceutics, 16(3), 353. https://doi.org/10.3390/pharmaceutics16030353