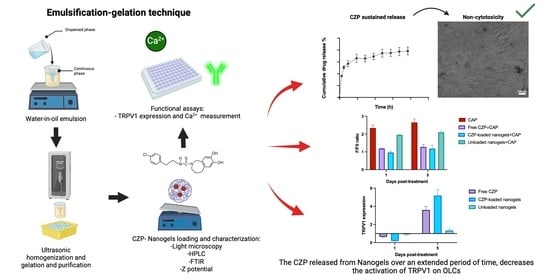
Modulation of TRPV1 on Odontoblast-like Cells Using Capsazepine-Loaded Nanogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Prototype Nanogel Formulation
2.2. Preparation of CZP-Loaded Nanogels and Loading Efficiency
2.3. High-Performance Liquid Chromatography
2.4. Nanogel Size Measurement
2.5. Fourier Transform Infrared Spectroscopy (FTIR) and Zeta Potential
2.6. In Vitro Release of CZP-Loaded Nanogels
2.7. Cell Culture
2.8. Cytotoxicity Evaluation
2.9. Biological Activity Assays of Nanogels in OLCs
2.10. Statistical Analysis
3. Results
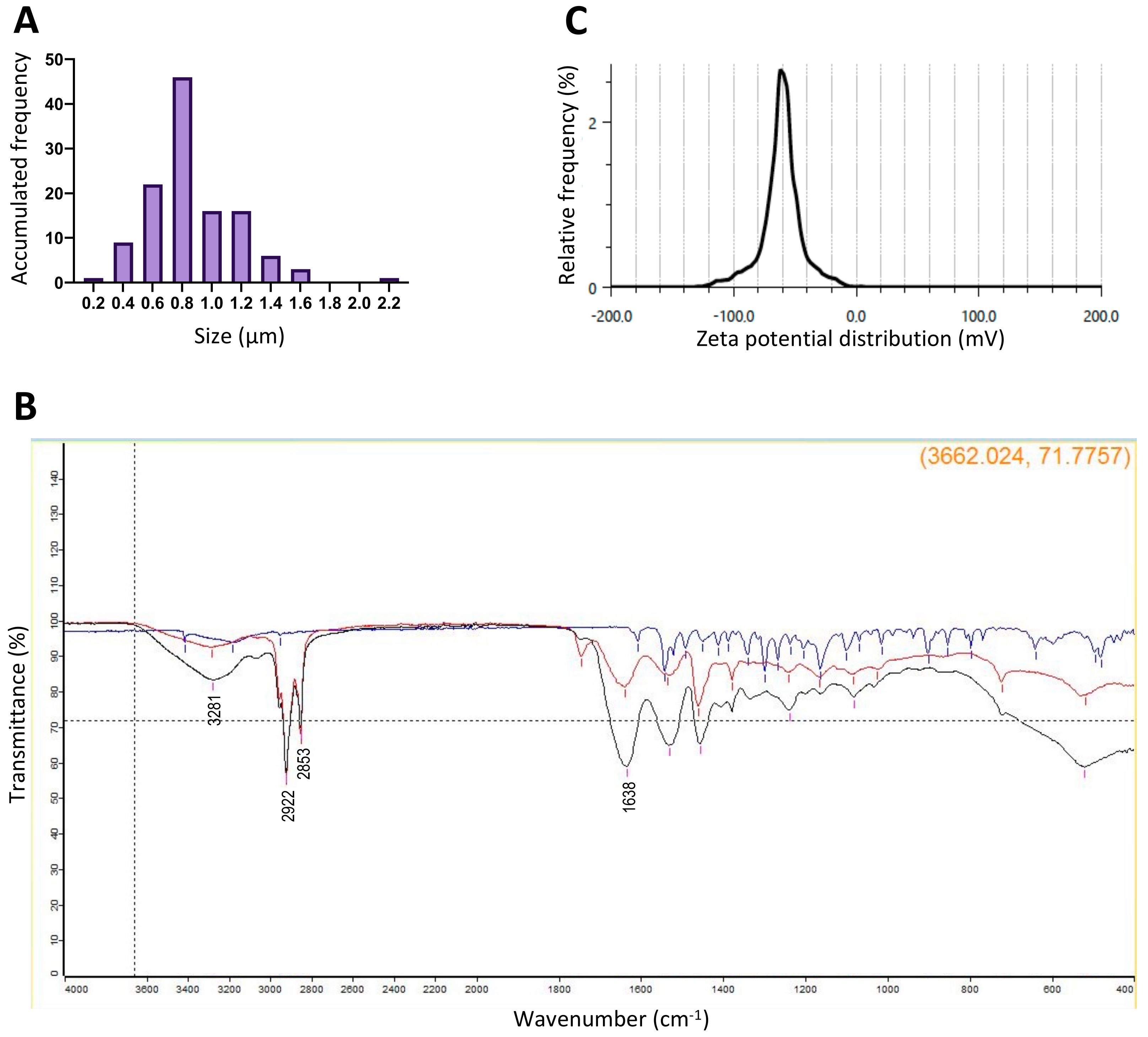
3.1. Nanogels Have Desirable Characteristics
3.2. Loaded Nanogels Showed Sustained Release of CZP
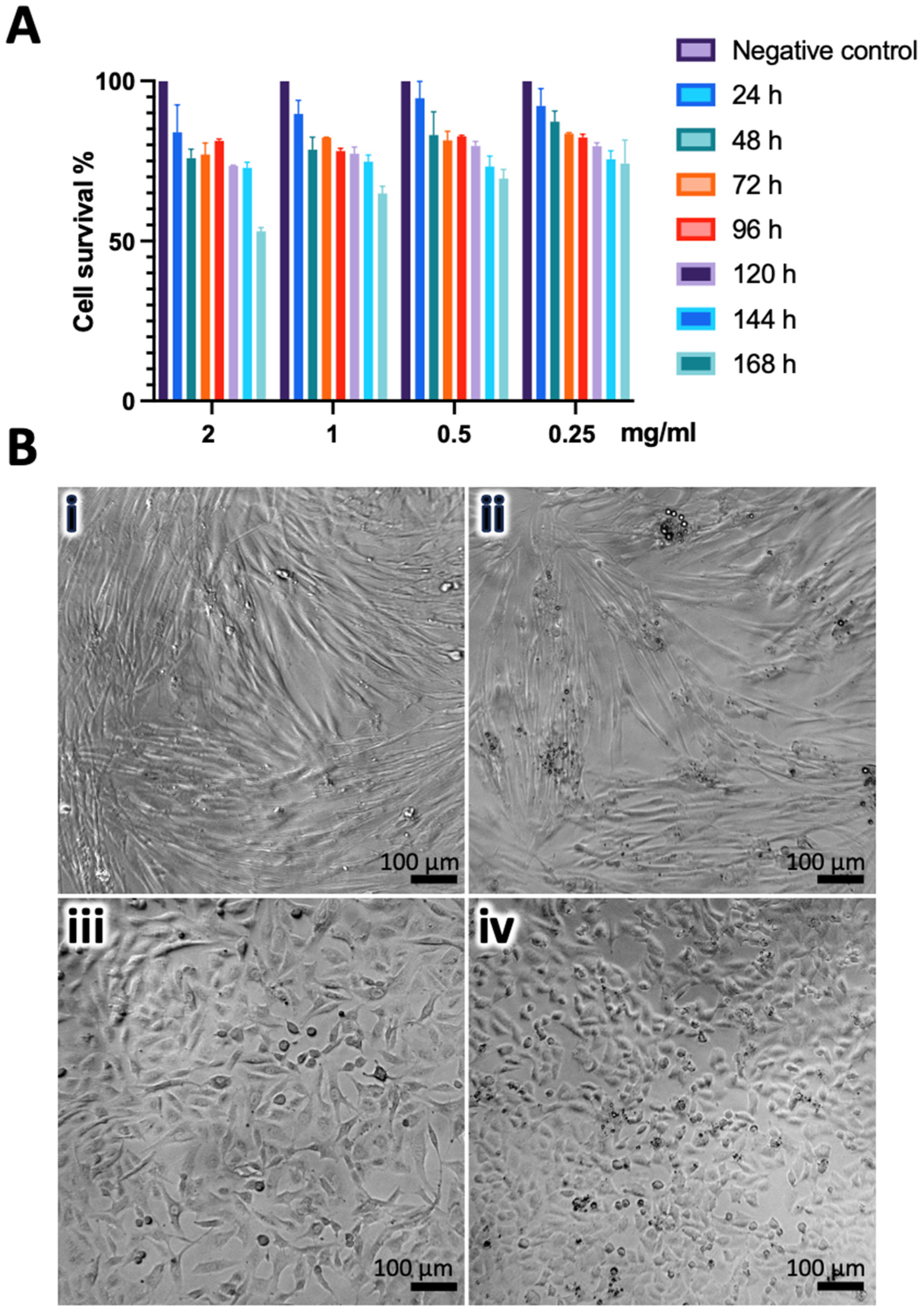
3.3. CZP-Loaded and Unloaded Nanogels Induce Low Cytotoxicity
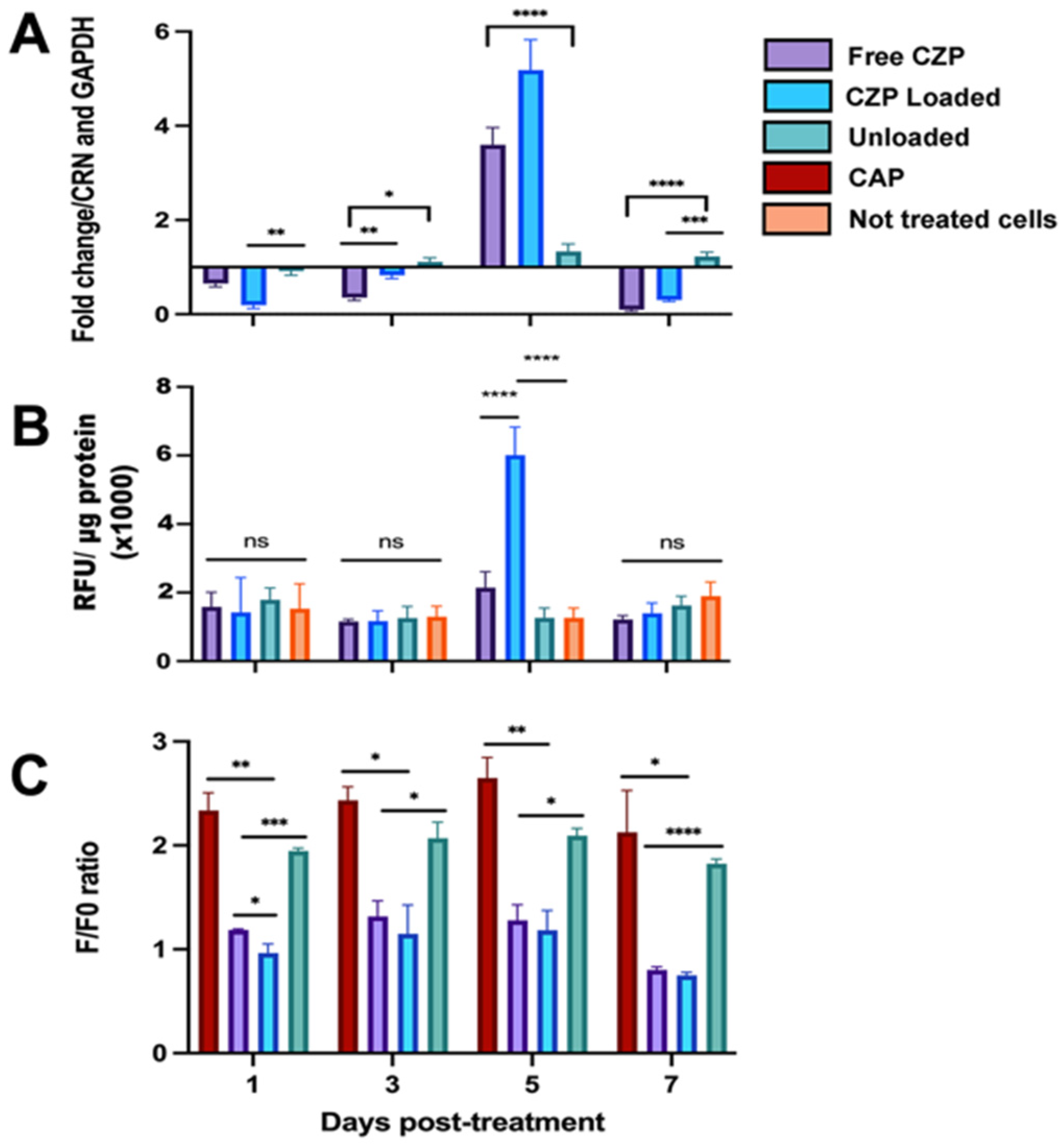
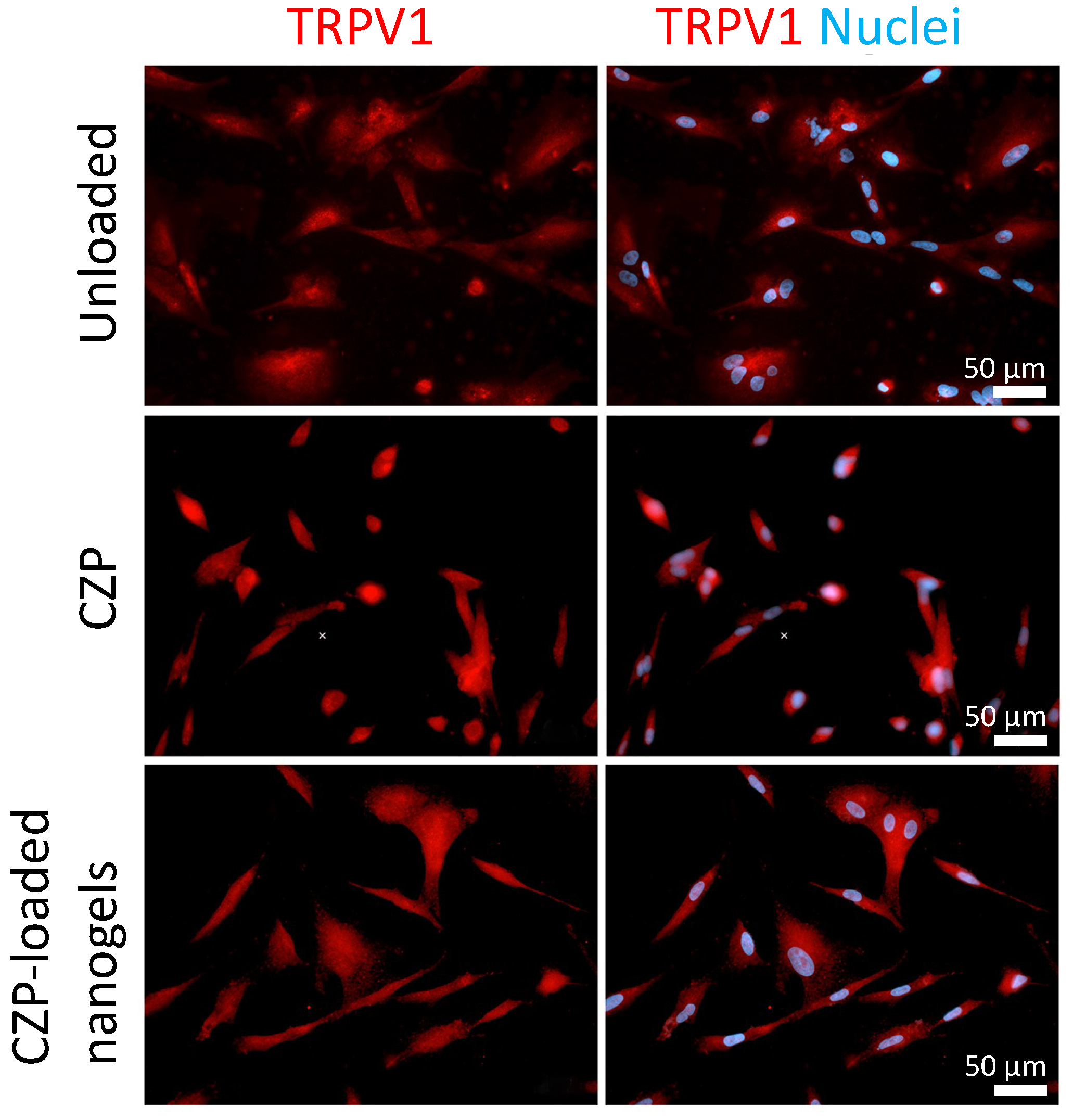
3.4. CZP Released from Nanogels Modulated the Expression and Reduced TRPV1 Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nermo, H.; Hadler-Olsen, E. Are Dental Visiting Patterns and Oral Pain Associated with Dental Disease among Norwegian Adults? A Cross-Sectional Study Based on the Tromsø Study. Clin. Exp. Dent. Res. 2023, 9, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Bakri, M.M.; Yahya, F.; Ando, H.; Unno, S.; Kitagawa, J. The Role of Transient Receptor Potential (TRP) Channels in the Transduction of Dental Pain. Int. J. Mol. Sci. 2019, 20, 526. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, A.; Sakakibara, S.; Kusumoto, J.; Takeda, D.; Hasegawa, T.; Akashi, M.; Minamikawa, T.; Hashikawa, K.; Terashi, H.; Komori, T. Upregulated Expression of Transient Receptor Potential Cation Channel Subfamily v Receptors in Mucosae of Patients with Oral Squamous Cell Carcinoma and Patients with a History of Alcohol Consumption or Smoking. PLoS ONE 2017, 12, e0169723. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.R. Therapeutic Targeting of TRP Channels-The TR(i)P to Pain Relief. Curr. Top. Med. Chem. 2011, 11, 2118–2130. [Google Scholar] [CrossRef]
- Fernández-Carvajal, A.; González-Muñiz, R.; Fernández-Ballester, G.; Ferrer-Montiel, A. Investigational Drugs in Early Phase Clinical Trials Targeting Thermotransient Receptor Potential (thermoTRP) Channels. Expert. Opin. Investig. Drugs 2020, 29, 1209–1222. [Google Scholar] [CrossRef]
- Shinoda, M.; Ogino, A.; Ozaki, N.; Urano, H.; Hironaka, K.; Yasui, M.; Sugiura, Y. Involvement of TRPV1 in Nociceptive Behavior in a Rat Model of Cancer Pain. J. Pain. 2008, 9, 687–699. [Google Scholar] [CrossRef]
- Fakih, D.; Guerrero-Moreno, A.; Baudouin, C.; Réaux-Le Goazigo, A.; Parsadaniantz, S.M. Capsazepine Decreases Corneal Pain Syndrome in Severe Dry Eye Disease. J. Neuroinflamm. 2021, 18, 111. [Google Scholar] [CrossRef]
- Yang, M.H.; Jung, S.H.; Sethi, G.; Ahn, K.S. Pleiotropic Pharmacological Actions of Capsazepine, a Synthetic Analogue of Capsaicin, against Various Cancers and Inflammatory Diseases. Molecules 2019, 24, 995. [Google Scholar] [CrossRef]
- Wang, X.; Bao, C.; Li, Z.; Yue, L.; Hu, L. Side Effects of Opioids Are Ameliorated by Regulating TRPV1 Receptors. Int. J. Environ. Res. Public Health 2022, 19, 2387. [Google Scholar] [CrossRef]
- Jager, J.; Obst, K.; Lohan, S.B.; Viktorov, J.; Staufenbiel, S.; Renz, H.; Unbehauen, M.; Haag, R.; Hedtrich, S.; Teutloff, C.; et al. Characterization of Hyperbranched Core-Multishell Nanocarriers as an Innovative Drug Delivery System for the Application at the Oral Mucosa. J. Periodontal Res. 2018, 53, 57–65. [Google Scholar] [CrossRef]
- Moraes, G.; Zambom, C.; Siqueira, W.L. Nanoparticles in Dentistry: A Comprehensive Review. Pharmaceuticals 2021, 14, 752. [Google Scholar] [CrossRef] [PubMed]
- Molavi, F.; Barzegar-Jalali, M.; Hamishehkar, H. Polyester Based Polymeric Nano and Microparticles for Pharmaceutical Purposes: A Review on Formulation Approaches. J. Control. Release 2020, 320, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Khatoon, F. Different Types of Smart Nanogel for Targeted Delivery. J. Sci. Adv. Mater. Devices 2019, 4, 201–212. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A Versatile Nano-Delivery System for Biomedical Applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef]
- Yasmin, R.; Shah, M.; Khan, S.A.; Ali, R. Gelatin Nanoparticles: A Potential Candidate for Medical Applications. Nanotechnol. Rev. 2017, 6, 191–207. [Google Scholar] [CrossRef]
- Lee, E.J.; Khan, S.A.; Lim, K.H. Gelatin Nanoparticle Preparation by Nanoprecipitation. J. Biomater. Sci. Polym. Ed. 2011, 22, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yan, J.; Mujtaba, B.M.; Li, Y.; Wei, H.; Huang, S. The Dual Anti-Caries Effect of Carboxymethyl Chitosan Nanogel Loaded with Chimeric Lysin ClyR and Amorphous Calcium Phosphate. Eur. J. Oral. Sci. 2021, 129, e12784. [Google Scholar] [CrossRef]
- Song, J.; Li, T.; Gao, J.; Li, C.; Jiang, S.; Zhang, X. Building an Aprismatic Enamel-like Layer on a Demineralized Enamel Surface by Using Carboxymethyl Chitosan and Lysozyme-Encapsulated Amorphous Calcium Phosphate Nanogels. J. Dent. 2021, 107, 103599. [Google Scholar] [CrossRef] [PubMed]
- Aminu, N.; Yam, M.F.; Chan, S.Y.; Bello, I.; Umar, N.M.; Nuhu, T.; Toh, S.M. The Evaluation of Healing Effect of Triclosan and Flurbiprofen-Loaded Nanogels in Experimental Periodontitis in Rats by Morphometric Analysis. Saudi Dent. J. 2021, 33, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, R.A.; Millán, D.; Sosnik, A.; Fontanilla, M.R. Aloe Vera–Eluting Collagen I Microgels: Physicochemical Characterization and in Vitro Biological Performance. Mater. Today Chem. 2022, 23, 100722. [Google Scholar] [CrossRef]
- Fernández Lozano, M.; Millan, D.; Jiménez Cruz, R.A. Aporte al Desarrollo de Un Nanogel Para La Liberación Controlada de Latanoprost. Rev. Colomb. Cienc. Quím. Farm. 2023, 52, 1039–1057. [Google Scholar] [CrossRef]
- Hoare, T.; Sivakumaran, D.; Stefanescu, C.F.; Lawlor, M.W.; Kohane, D.S. Nanogel Scavengers for Drugs: Local Anesthetic Uptake by Thermoresponsive Nanogels. Acta Biomater. 2012, 8, 1450–1458. [Google Scholar] [CrossRef]
- Bernal-Cepeda, L.J.; Velandia-Romero, M.L.; Castellanos, J.E. Capsazepine Antagonizes TRPV1 Activation Induced by Thermal and Osmotic Stimuli in Human Odontoblast-like Cells. J. Oral. Biol. Craniofac. Res. 2023, 13, 71–77. [Google Scholar] [CrossRef]
- Gao, Z.G.; Lee, M.K.; Oh, U.; Suh, Y.G.; Kim, C.K. Determination of Capsazepine in Rat Plasma by High Performance Liquid Chromatography. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 1865–1872. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 369–400. ISBN 9780128179093. [Google Scholar]
- Dionysopoulos, D.; Strakas, D.; Koliniotou-Koumpia, E.; Koumpia, E. Effect of Er,Cr:YSGG Laser Irradiation on Bovine Enamel Surface during in-Office Tooth Bleaching Ex Vivo. Odontology 2017, 105, 320–328. [Google Scholar] [CrossRef]
- Vos, M.H.; Neelands, T.R.; McDonald, H.A.; Choi, W.; Kroeger, P.E.; Puttfarcken, P.S.; Faltynek, C.R.; Moreland, R.B.; Han, P. TRPV1b Overexpression Negatively Regulates TRPV1 Responsiveness to Capsaicin, Heat and Low pH in HEK293 Cells. J. Neurochem. 2006, 99, 1088–1102. [Google Scholar] [CrossRef]
- Schefe, J.H.; Lehmann, K.E.; Buschmann, I.R.; Unger, T.; Funke-Kaiser, H. Quantitative Real-Time RT-PCR Data Analysis: Current Concepts and the Novel “Gene Expression’s C T Difference” Formula. J. Mol. Med. 2006, 84, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Saiz, L.M.; Bernal-Cepeda, L.J.; García-Jiménez, F.; Abril, D.; Castellanos, J.E. Reference Gene Validation for the Relative Quantification of Cannabinoid Receptor Expression in Human Odontoblasts via Quantitative Polymerase Chain Reaction. J. Oral. Biol. Craniofac. Res. 2022, 12, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP Channel Drug Discovery: From Target Validation to Clinical Studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef]
- Costa, J.V.; Portugal, J.; Neves, C.B.; Bettencourt, A.F. Should Local Drug Delivery Systems Be Used in Dentistry? Drug Deliv. Transl. Res. 2022, 12, 1395–1407. [Google Scholar] [CrossRef]
- Sarangi, D.; Pattanaik, S. Nanoparticles in Dentistry. In Advanced Nanomaterials for Point of Care Diagnosis and Therapy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 335–358. ISBN 9780323857253. [Google Scholar]
- Makvandi, P.; Josic, U.; Delfi, M.; Pinelli, F.; Jahed, V.; Kaya, E.; Ashrafizadeh, M.; Zarepour, A.; Rossi, F.; Zarrabi, A.; et al. Drug Delivery (Nano)Platforms for Oral and Dental Applications: Tissue Regeneration, Infection Control, and Cancer Management. Adv. Sci. 2021, 8, 2004014. [Google Scholar] [CrossRef]
- Charelli, L.E.; de Mattos, G.C.; de Jesus Sousa-Batista, A.; Pinto, J.C.; Balbino, T.A. Polymeric Nanoparticles as Therapeutic Agents against Coronavirus Disease. J. Nanoparticle Res. 2022, 24, 12. [Google Scholar] [CrossRef]
- Shewan, H.M.; Stokes, J.R. Review of Techniques to Manufacture Micro-Hydrogel Particles for the Food Industry and Their Applications. J. Food Eng. 2013, 119, 781–792. [Google Scholar] [CrossRef]
- Nekkanti, V.; Vabalaboina, V.; Pillai, R. Drug Nanoparticles—An Overview. In The Delivery of Nanoparticles; InTech: Rijeka, Croatia, 2012; pp. 111–132. ISBN 9789535106159. [Google Scholar]
- ChEMBL-EBI Compound Report Card-Capsazepine. Available online: https://www.ebi.ac.uk/chembl/compound_report_card/CHEMBL391997/ (accessed on 21 December 2023).
- Favatela, F.; Horst, M.F.; Bracone, M.; Gonzalez, J.; Alvarez, V.; Lassalle, V. Gelatin/Cellulose Nanowhiskers Hydrogels Intended for the Administration of Drugs in Dental Treatments: Study of Lidocaine as Model Case. J. Drug Deliv. Sci. Technol. 2021, 61, 101886. [Google Scholar] [CrossRef]
- Sahoo, N.; Sahoo, R.K.; Biswas, N.; Guha, A.; Kuotsu, K. Recent Advancement of Gelatin Nanoparticles in Drug and Vaccine Delivery. Int. J. Biol. Macromol. 2015, 81, 317–331. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. Understanding the Burst Release Phenomenon: Toward Designing Effective Nanoparticulate Drug-Delivery Systems. Ther. Deliv. 2021, 12, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.; Dang, L.H.; Truong, M.D.; Nguyen, T.H.; Le, L.; Le, V.T.; Nam, N.D.; Bach, L.G.; Nguyen, V.T.; Tran, N.Q. A Dual Synergistic of Curcumin and Gelatin on Thermal-Responsive Hydrogel Based on Chitosan-P123 in Wound Healing Application. Biomed. Pharmacother. 2019, 117, 109183. [Google Scholar] [CrossRef] [PubMed]
- Baldion, P.A.; Velandia-Romero, M.L.; Castellanos, J.E. Dental Resin Monomers Induce Early and Potent Oxidative Damage on Human Odontoblast-like Cells. Chem. Biol. Interact. 2021, 333, 109336. [Google Scholar] [CrossRef] [PubMed]
- Alqutub, A.W. Pain Experience after Dental Implant Placement Compared to Tooth Extraction. Int. J. Dent. 2021, 2021, 4134932. [Google Scholar] [CrossRef]
- Hersh, E.V.; Kenna, G.A.; Moore, P.A. Prescribing Recommendations for the Treatment of Acute Pain in Dentistry. Compendium 2011, 32, 22–31. [Google Scholar]
- Jiménez-López, J.; El-Hammadi, M.M.; Ortiz, R.; Cayero-Otero, M.D.; Cabeza, L.; Perazzoli, G.; Martin-Banderas, L.; Baeyens, J.M.; Prados, J.; Melguizo, C. A Novel Nanoformulation of PLGA with High Non-Ionic Surfactant Content Improves in Vitro and in Vivo PTX Activity against Lung Cancer. Pharmacol. Res. 2019, 141, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Ulmansky, R.; Turjeman, K.; Baru, M.; Katzavian, G.; Harel, M.; Sigal, A.; Naparstek, Y.; Barenholz, Y. Glucocorticoids in Nano-Liposomes Administered Intravenously and Subcutaneously to Adjuvant Arthritis Rats Are Superior to the Free Drugs in Suppressing Arthritis and Inflammatory Cytokines. J. Control Release 2012, 160, 299–305. [Google Scholar] [CrossRef]
- Gray, J.A.; Roth, B.L. Paradoxical Trafficking and Regulation of 5-HT2A Receptors by Agonists and Antagonists. Brain Res. Bull. 2001, 56, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Bang, T.H.; Van, T.T.T.; Hung, L.X.; Ly, B.M.; Nhut, N.D.; Thuy, T.T.T.; Huy, B.T. Nanogels of Acetylated Ulvan Enhance the Solubility of Hydrophobic Drug Curcumin. Bull. Mater. Sci. 2019, 42, 1–7. [Google Scholar] [CrossRef]
- Babaie, S.; Taghvimi, A.; Hong, J.H.; Hamishehkar, H.; An, S.; Kim, K.H. Recent Advances in Pain Management Based on Nanoparticle Technologies. J. Nanobiotechnol. 2022, 20, 290. [Google Scholar] [CrossRef]
- Logothetidis, S. Nanodentistry. In Nanomedicine and Nanobiotechnology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 61, pp. 95–107. ISBN 9783642241802. [Google Scholar]


| Prototype | Disperse Phase * | Continuous Phase (Surfactant) | Homogenization Method | ||
|---|---|---|---|---|---|
| Magnetic Agitation | Ultrasonic Homogenization (Amplitude, Time) | High-Shear Homogenization | |||
| 1 | 0.25 g gelatin topped with H2O to 5 g | 25 g mineral oil (Sorbitan 1%) | 1000 rpm | 50%, 3 min | NA |
| 2 | 0.25 g gelatin, topped with H2O to 5 g | 25 g mineral oil (Sorbitan 1%) | 1000 rpm | 50%, 6 min | NA |
| 3 | 0.25 g gelatin, topped with H2O to 7.5 g | 25 g mineral oil (Sorbitan 2.5%) | 1000 rpm | 70%, 10 min | NA |
| 4 | 0.25 g gelatin, topped with H2O to 7.5 g | 25 g mineral oil (Sorbitan 7.5%) | 1000 rpm | 70%, 10 min | 13,800 rpm, 15 min |
| 5 | 0.25 g gelatin, topped with H2O to 7.5 g | 25 g mineral oil (Sorbitan 10%) | 1000 rpm | 70%, 10 min | 13,800 rpm, 15 min |
| Gene | Primer Sequences (5′-3′) | Fragment Size |
|---|---|---|
| TRPV1 | Forward-GTGCACTCCTCGCTGTACGA Reverse-CACCTCCAGCACCGAGTTCT | (66 bp) |
| GAPDH CRN | Forward-CACTAGGCGCTCACTGTTCTC Reverse-AAATCCGTTGACTCCGACCT Forward-CAATGCTGACGGCATGTACGA Reverse-CACGAACGGAACTTCATGGTG | (90 bp) (165 bp) |
| Prototype Number | Average Size (µm) | PDI | Yield (%) |
|---|---|---|---|
| 1 | 8.41 ± 1.65 | 0.31 | 28.11 ± 1.40 |
| 2 | 4.22 ± 1.44 | 0.12 | 43.37 ± 4.34 |
| 3 | 2.97 ± 1.15 | 0.15 | 57.91 ± 3.25 |
| 4 | 1.07 ± 0.45 | 0.18 | 59.60 ± 4.47 |
| 5 | 0.85 ± 0.11 | 0.11 | 69.23 ± 8.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal-Cepeda, L.J.; Jiménez, R.A.; Velandia-Romero, M.L.; Acosta-Guzmán, P.; Castellanos, J.E. Modulation of TRPV1 on Odontoblast-like Cells Using Capsazepine-Loaded Nanogels. Pharmaceutics 2024, 16, 355. https://doi.org/10.3390/pharmaceutics16030355
Bernal-Cepeda LJ, Jiménez RA, Velandia-Romero ML, Acosta-Guzmán P, Castellanos JE. Modulation of TRPV1 on Odontoblast-like Cells Using Capsazepine-Loaded Nanogels. Pharmaceutics. 2024; 16(3):355. https://doi.org/10.3390/pharmaceutics16030355
Chicago/Turabian StyleBernal-Cepeda, Lilia Jadith, Ronald Andrés Jiménez, Myriam L. Velandia-Romero, Paola Acosta-Guzmán, and Jaime E. Castellanos. 2024. "Modulation of TRPV1 on Odontoblast-like Cells Using Capsazepine-Loaded Nanogels" Pharmaceutics 16, no. 3: 355. https://doi.org/10.3390/pharmaceutics16030355