Designing a Placebo Microneedle Stamp: Modeling and Validation in a Clinical Control Trial
Abstract
:1. Introduction
2. Materials and Methods
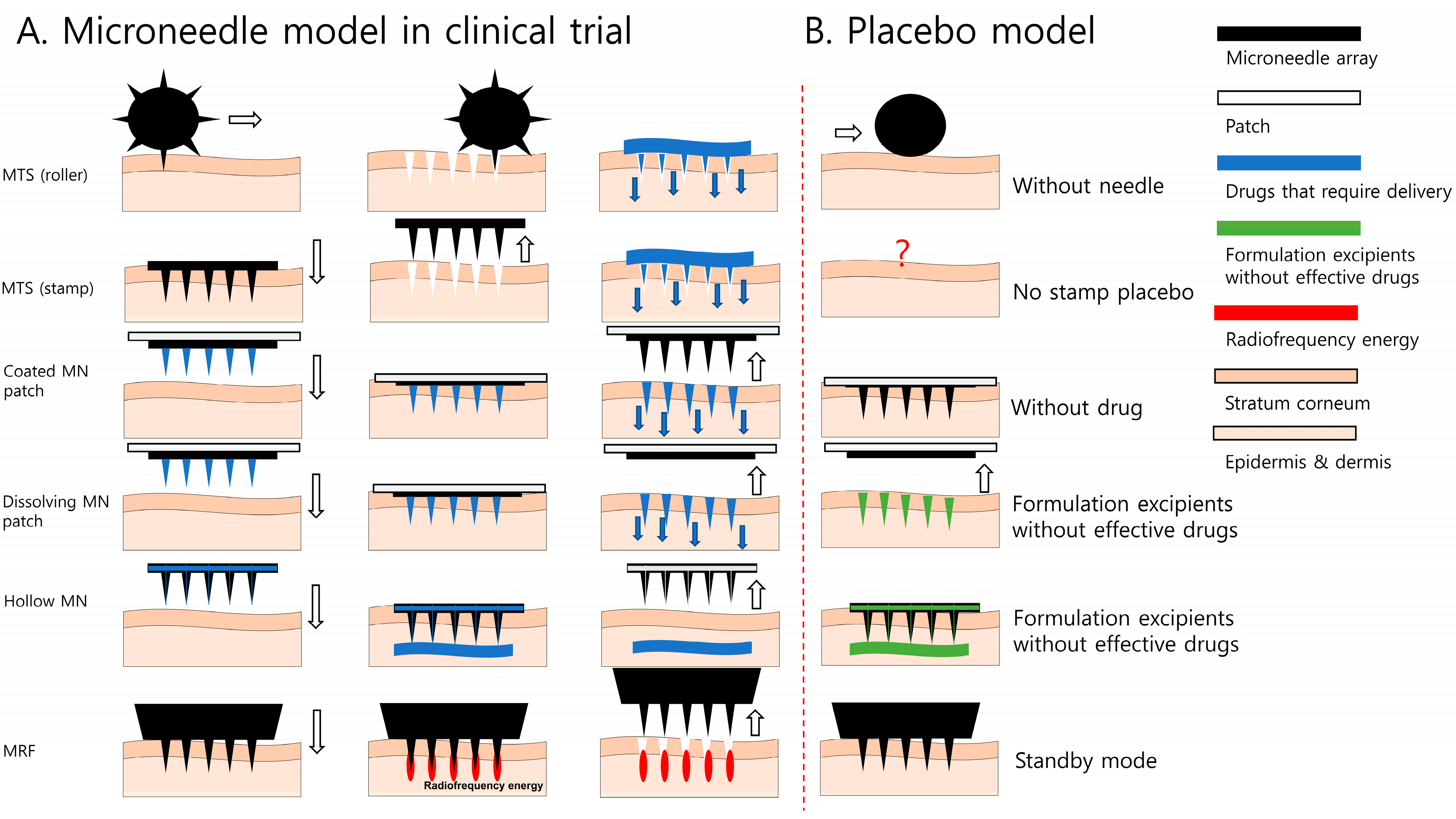
2.1. Modeling Placebo Stamp
2.1.1. Placebo Stamp Concept
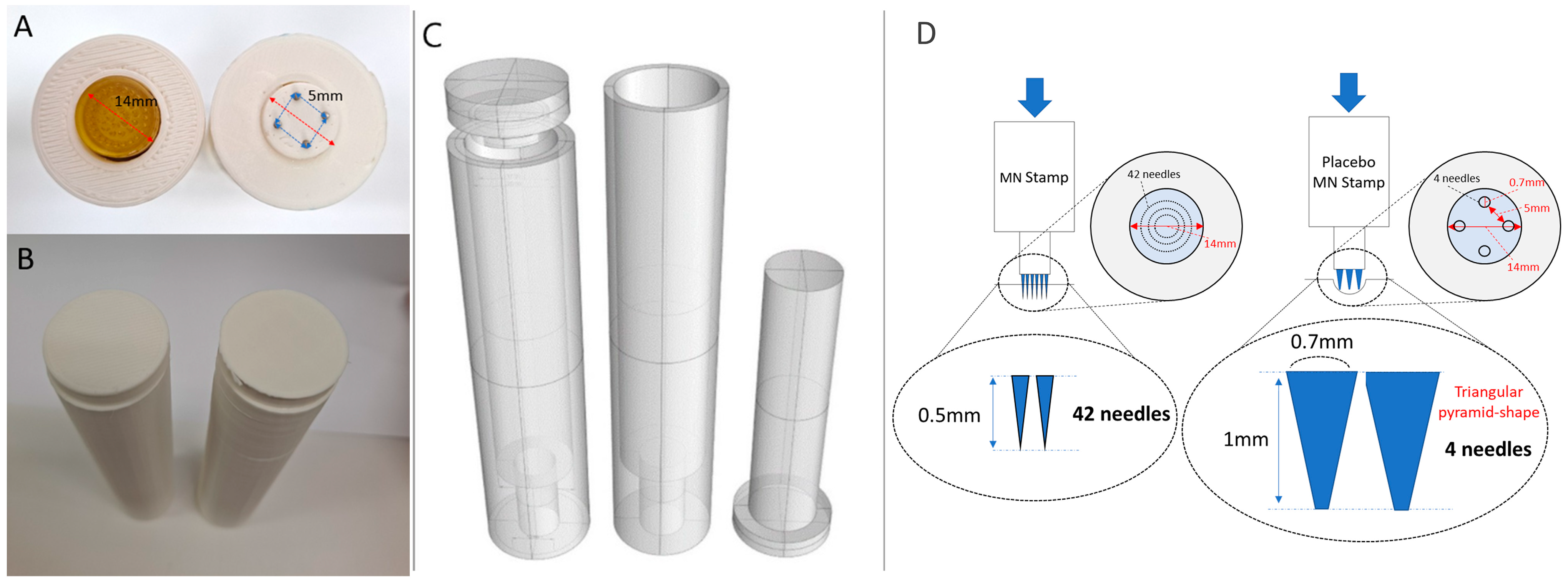
2.1.2. Configuration of Placebo MN Stamp Design
2.1.3. Confirmation of Skin Penetration of Placebo MN Stamp and Setting of Experimental Conditions for Blind Testing
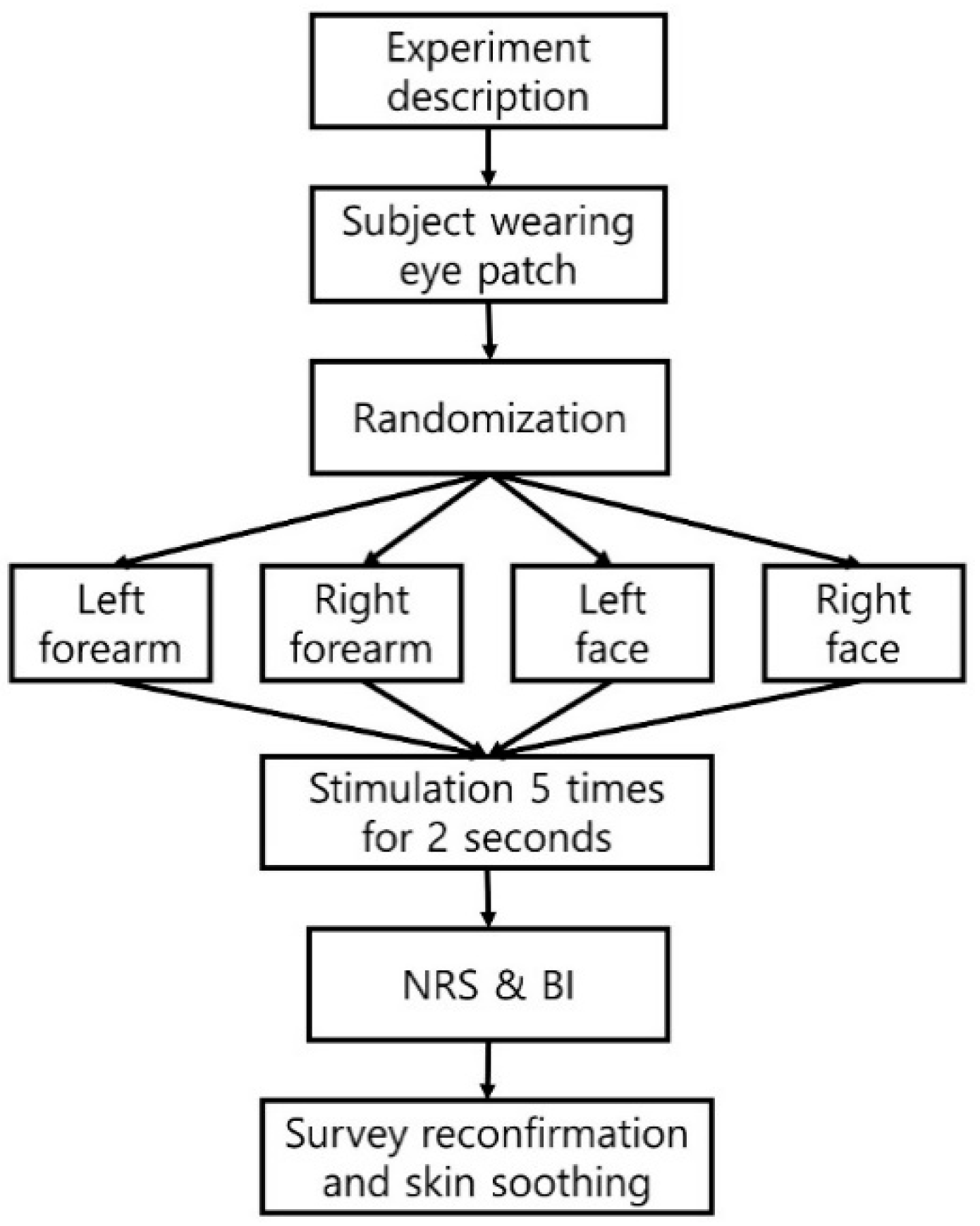
2.2. Validation Test for Placebo MN Stamp
2.2.1. Baseline Characteristics and Skin Sensitivity by Two-Point Discrimination (TPD)
2.2.2. Blinding Assessment of Placebo MN Stamp
2.2.3. Statistical Analysis
3. Results
3.1. Invasion Comparison and Stimulation Intensity Setting
3.2. Validation Test
3.2.1. Patients’ Characteristics
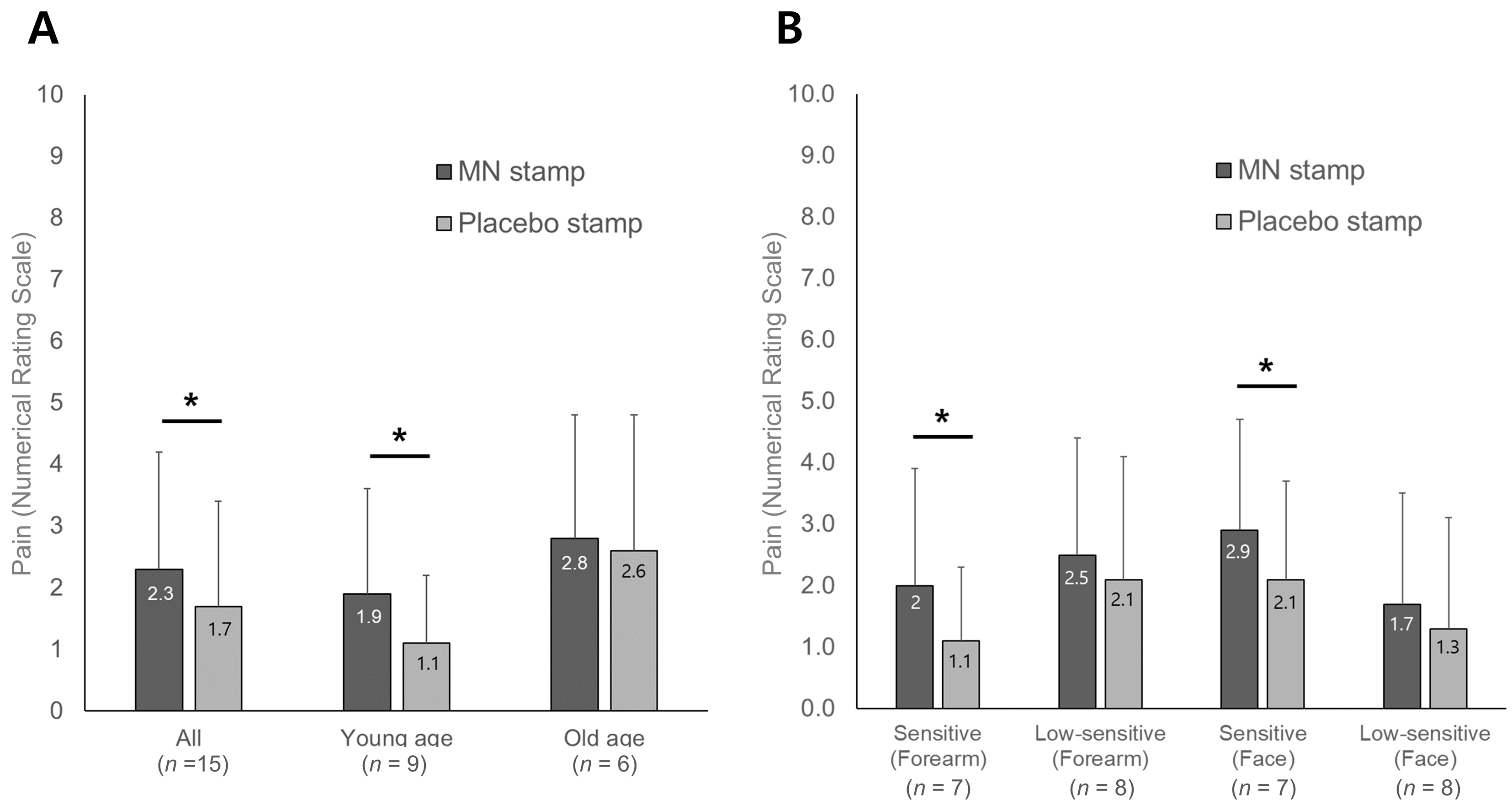
3.2.2. Numerical Rating Scale for Pain Caused by Stimulation
3.2.3. Blinding Indices
3.3. Needle Drawing of the Skin in Contact with the Stimulation Device
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donnelly, R.F.; Singh, T.R.R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Park, J.H.; Lee, Y.S.; Kim, Y.S.; Park, J.Y.; Kim, S.Y. The Current Status of Clinical Research Involving Microneedles: A Systematic Review. Pharmaceutics 2020, 12, 1113. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, J.H. Trend of Microneedle Industry; KoreaBio: Seongnam, Republic of Korea, 2019. [Google Scholar]
- Tasca, F.; Tortolini, C.; Bollella, P.; Antiochia, R. Microneedle-based electrochemical devices for transdermal biosensing: A review. Curr. Opin. Electrochem. 2019, 16, 42–49. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Park, J.H. Human studies with microneedles for evaluation of their efficacy and safety. Expert Opin. Drug Deliv. 2018, 15, 235–245. [Google Scholar] [CrossRef] [PubMed]
- McCrudden, M.T.; McAlister, E.; Courtenay, A.J.; Gonzalez-Vazquez, P.; Singh, T.R.; Donnelly, R.F. Microneedle applications in improving skin appearance. Exp. Dermatol. 2015, 24, 561–566. [Google Scholar] [CrossRef]
- Kim, S.J.; Cho, G.R.; Lee, K.S. Review Articles: Skin Effect and Mechanism Using Microneedle Therapy System (MTS). J. Ski. Barrier Res. 2013, 15, 57–64. [Google Scholar]
- Rouphael, N.G.; Paine, M.; Mosley, R.; Henry, S.; McAllister, D.V.; Kalluri, H.; Pewin, W.; Frew, P.M.; Yu, T.; Thornburg, N.J. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): A randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 2017, 390, 649–658. [Google Scholar] [CrossRef]
- Hantash, B.M.; Ubeid, A.A.; Chang, H.; Kafi, R.; Renton, B. Bipolar fractional radiofrequency treatment induces neoelastogenesis and neocollagenesis. Lasers Surg. Med. 2009, 41, 1–9. [Google Scholar] [CrossRef]
- McVey, E.; Hirsch, L.; Sutter, D.E.; Kapitza, C.; Dellweg, S.; Clair, J.; Rebrin, K.; Judge, K.; Pettis, R.J. Pharmacokinetics and postprandial glycemic excursions following insulin lispro delivered by intradermal microneedle or subcutaneous infusion. J. Diabetes Sci. Technol. 2012, 6, 743–754. [Google Scholar] [CrossRef]
- Back, Y.H.; Park, D.S.; Kang, S.K. The Review on the Evidence—Based Study of Acupuncture. J. Korean Acupunct. Moxibustion Soc. 2002, 19, 138–155. [Google Scholar]
- Khater, M.H.; Khattab, F.M.; Abdelhaleem, M.R. Treatment of striae distensae with needling therapy versus CO2 fractional laser. J. Cosmet. Laser Ther. 2016, 18, 75–79. [Google Scholar] [CrossRef]
- Ryu, H.R.; Jeong, H.R.; Seon-Woo, H.S.; Kim, J.S.; Lee, S.K.; Kim, H.J.; Baek, J.O.; Park, J.H.; Roh, J.Y. Efficacy of a bleomycin microneedle patch for the treatment of warts. Drug Deliv. Transl. Res. 2018, 8, 273–280. [Google Scholar] [CrossRef]
- Hong, J.Y.; Ko, E.J.; Choi, S.Y.; Li, K.; Kim, A.R.; Park, J.O.; Kim, B.J. Efficacy and safety of a novel, soluble microneedle patch for the improvement of facial wrinkle. J. Cosmet. Dermatol. 2018, 17, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Troy, S.B.; Kouiavskaia, D.; Siik, J.; Kochba, E.; Beydoun, H.; Mirochnitchenko, O.; Levin, Y.; Khardori, N.; Chumakov, K.; Maldonado, Y. Comparison of the immunogenicity of various booster doses of inactivated polio vaccine delivered intradermally versus intramuscularly to HIV-infected adults. J. Infect. Dis. 2015, 211, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Levin, Y.; Kochba, E.; Kenney, R. Clinical evaluation of a novel microneedle device for intradermal delivery of an influenza vaccine: Are all delivery methods the same? Vaccine 2014, 32, 4249–4252. [Google Scholar] [CrossRef] [PubMed]
- Kochba, E.; Levin, Y.; Raz, I.; Cahn, A. Improved insulin pharmacokinetics using a novel microneedle device for intradermal delivery in patients with type 2 diabetes. Diabetes Technol. Ther. 2016, 18, 525–531. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Chae, Y. Powerful effects of placebo needles. Acupunct. Med. 2018, 36, 197–199. [Google Scholar] [CrossRef]
- Streitberger, K.; Kleinhenz, J. Introducing a placebo needle into acupuncture research. Lancet 1998, 352, 364–365. [Google Scholar] [CrossRef]
- Fatemi Naeini, F.; Abtahi-Naeini, B.; Pourazizi, M.; Nilforoushzadeh, M.A.; Mirmohammadkhani, M. Fractionated microneedle radiofrequency for treatment of primary axillary hyperhidrosis: A sham control study. Australas. J. Dermatol. 2015, 56, 279–284. [Google Scholar] [CrossRef]
- Petukhova, T.A.; Hassoun, L.A.; Foolad, N.; Barath, M.; Sivamani, R.K. Effect of expedited microneedle-assisted photodynamic therapy for field treatment of actinic keratoses: A randomized clinical trial. JAMA Dermatol. 2017, 153, 637–643. [Google Scholar] [CrossRef]
- Thevarajah, S.; Huston, T.L.; Simmons, R.M. A comparison of the adverse reactions associated with isosulfan blue versus methylene blue dye in sentinel lymph node biopsy for breast cancer. Am. J. Surg. 2005, 189, 236–239. [Google Scholar] [CrossRef]
- O’Mahony, C.; Pini, F.; Vereschagina, L.; Blake, A.; O’Brien, J.; Webster, C.; Galvin, P.; McCarthy, K.G. Skin insertion mechanisms of microneedle-based dry electrodes for physiological signal monitoring. In Proceedings of the 2013 IEEE Biomedical Circuits and Systems Conference (BioCAS), Rotterdam, The Netherlands, 31 October–2 November 2013; pp. 69–72. [Google Scholar]
- Haq, M.I.; Smith, E.; John, D.N.; Kalavala, M.; Edwards, C.; Anstey, A.; Morrissey, A.; Birchall, J.C. Clinical administration of microneedles: Skin puncture, pain and sensation. Biomed Microdevices 2009, 11, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Lenzenweger, M.F. Two-point discrimination thresholds and schizotypy: Illuminating a somatosensory dysfunction. Schizophr. Res. 2000, 42, 111–124. [Google Scholar] [CrossRef]
- Vriens, J.; Van der Glas, H. Extension of normal values on sensory function for facial areas using clinical tests on touch and two-point discrimination. Int. J. Oral Maxillofac. Surg. 2009, 38, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.F. Two-point discrimination assessment in the upper limb in young adult men and women. Phys. Ther. 1982, 62, 965–969. [Google Scholar] [CrossRef]
- Waite, J.C. Assessing Blinding in Randomized Clinical Trials; California State Polytechnic University: Pomona, CA, USA, 2017. [Google Scholar]
- Park, J.; Bang, H.; Canette, I. Blinding in clinical trials, time to do it better. Complement. Ther. Med. 2008, 16, 121–123. [Google Scholar] [CrossRef]
- Arya, J.; Henry, S.; Kalluri, H.; McAllister, D.V.; Pewin, W.P.; Prausnitz, M.R. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials 2017, 128, 1–7. [Google Scholar] [CrossRef]
- To, M.; Alexander, C. The effects of Park sham needles: A pilot study. J. Integr. Med. 2015, 13, 20–24. [Google Scholar] [CrossRef] [PubMed]
- White, P.; Lewith, G.; Hopwood, V.; Prescott, P. The placebo needle, is it a valid and convincing placebo for use in acupuncture trials? A randomised, single-blind, cross-over pilot trial. Pain 2003, 106, 401–409. [Google Scholar] [CrossRef]
- Chae, Y. The dilemma of placebo needles in acupuncture research. Acupunct. Med. 2017, 35, 382. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.; Park, H.J.; Chae, Y.; Lee, H. Does different information disclosure on placebo control affect blinding and trial outcomes? A case study of participant information leaflets of randomized placebo-controlled trials of acupuncture. BMC Med. Res. Methodol. 2018, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Jeong, S.S.; Roh, D.H.; Kim, D.Y.; Choi, H.K.; Lee, E.H. A practical guide to the development of microneedle systems—In clinical trials or on the market. Int. J. Pharm. 2020, 573, 118778. [Google Scholar] [CrossRef]
- Ahmed Saeed Al-Japairai, K.; Mahmood, S.; Hamed Almurisi, S.; Reddy Venugopal, J.; Rebhi Hilles, A.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, J.; Wen, D.; Kahkoska, A.R.; Gu, Z. Polymeric microneedles for transdermal protein delivery. Adv. Drug Deliv. Rev. 2018, 127, 106–118. [Google Scholar] [CrossRef]
- Moreira, A.F.; Rodrigues, C.F.; Jacinto, T.A.; Miguel, S.P.; Costa, E.C.; Correia, I.J. Microneedle-based delivery devices for cancer therapy: A review. Pharmacol. Res. 2019, 148, 104438. [Google Scholar] [CrossRef]
- Wing, D.; Prausnitz, M.R.; Buono, M.J. Skin pretreatment with microneedles prior to pilocarpine iontophoresis increases sweat production. Clin. Physiol. Funct. Imaging 2013, 33, 436–440. [Google Scholar] [CrossRef]
- Spencer, J.M.; Freeman, S.A. Microneedling Prior to Levulan PDT for the Treatment of Actinic Keratoses: A Split-Face, Blinded Trial. J. Drugs Dermatol. 2016, 15, 1072–1074. [Google Scholar]
- Singh, A.; Yadav, S. Microneedling: Advances and widening horizons. Indian Dermatol. Online J. 2016, 7, 244. [Google Scholar]
- Davis, S.P.; Landis, B.J.; Adams, Z.H.; Allen, M.G.; Prausnitz, M.R. Insertion of microneedles into skin: Measurement and prediction of insertion force and needle fracture force. J. Biomech. 2004, 37, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Gittard, S.D.; Chen, B.; Xu, H.; Ovsianikov, A.; Chichkov, B.N.; Monteiro-Riviere, N.A.; Narayan, R.J. The Effects of Geometry on Skin Penetration and Failure of Polymer Microneedles. J. Adhes. Sci. Technol. 2013, 27, 227–243. [Google Scholar] [CrossRef] [PubMed]
- van der Maaden, K.; Sekerdag, E.; Jiskoot, W.; Bouwstra, J. Impact-insertion applicator improves reliability of skin penetration by solid microneedle arrays. AAPS J. 2014, 16, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain 2008, 24, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.J.; Arya, J.M.; McClain, M.A.; Frew, P.M.; Meltzer, M.I.; Prausnitz, M.R. Microneedle patches: Usability and acceptability for self-vaccination against influenza. Vaccine 2014, 32, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Fernando, G.J.P.; Hickling, J.; Jayashi Flores, C.M.; Griffin, P.; Anderson, C.D.; Skinner, S.R.; Davies, C.; Witham, K.; Pryor, M.; Bodle, J.; et al. Safety, tolerability, acceptability and immunogenicity of an influenza vaccine delivered to human skin by a novel high-density microprojection array patch (Nanopatch). Vaccine 2018, 36, 3779–3788. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.K.; Chang, S.E.; Park, G.H.; Roh, M.R. Comparison of microneedle fractional radiofrequency therapy with intradermal botulinum toxin a injection for periorbital rejuvenation. Dermatology 2013, 227, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, H.; Kim, S.; Kim, M.; Kang, H.; Kim, N.; An, S.; Koh, J.; Jung, H. Evaluation of the anti-wrinkle effect of an ascorbic acid-loaded dissolving microneedle patch via a double-blind, placebo-controlled clinical study. Int. J. Cosmet. Sci. 2016, 38, 375–381. [Google Scholar] [CrossRef]
- Choi, Y.-E.; Ahn, H.-S. The Effect of Microneedle Therapy System to Improve the Facial Skin Conditions for Mid-aged Women. Korean J. Aesthet. Cosmetol. 2012, 10, 611–618. [Google Scholar]
- Park, K.Y.; Kwon, H.J.; Lee, C.; Kim, D.; Yoon, J.J.; Kim, M.N.; Kim, B.J. Efficacy and safety of a new microneedle patch for skin brightening: A Randomized, split-face, single-blind study. J. Cosmet. Dermatol. 2017, 16, 382–387. [Google Scholar] [CrossRef]
- Bao, L.; Gong, L.; Guo, M.; Liu, T.; Shi, A.; Zong, H.; Xu, X.; Chen, H.; Gao, X.; Li, Y. Randomized trial of electrodynamic microneedle combined with 5% minoxidil topical solution for the treatment of Chinese male Androgenetic alopecia. J. Cosmet. Laser Ther. 2020, 22, 1–7. [Google Scholar] [CrossRef]
- Yoo, K.H.; Lee, J.W.; Li, K.; Kim, B.J.; Kim, M.N. Photodynamic therapy with methyl 5-aminolevulinate acid might be ineffective in recalcitrant alopecia totalis regardless of using a microneedle roller to increase skin penetration. Dermatol. Surg. 2010, 36, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.J.; Brown, M.R.; Raviele, N.A.; Prausnitz, M.R.; Felner, E.I. Faster pharmacokinetics and increased patient acceptance of intradermal insulin delivery using a single hollow microneedle in children and adolescents with type 1 diabetes. Pediatr. Diabetes 2013, 14, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; Oosterhuis-Kafeja, F.; Van der Wielen, M.; Almagor, Y.; Sharon, O.; Levin, Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine 2009, 27, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Zaman, K.; Estivariz, C.F.; Yunus, M.; Gary, H.E.; Weldon, W.C.; Bari, T.I.; Steven Oberste, M.; Wassilak, S.G.; Luby, S.P.; et al. Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: A randomized controlled trial. Vaccine 2015, 33, 6816–6822. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yi, S.H.; Cho, J.H.; Yin, C.S.; Lee, H.; Park, H.J. Heat stimulation on the skin for medical treatment: Can it be controlled? J. Altern. Complement. Med. 2011, 17, 497–504. [Google Scholar] [CrossRef]
- Aust, M.C.; Fernandes, D.; Kolokythas, P.; Kaplan, H.M.; Vogt, P.M. Percutaneous collagen induction therapy: An alternative treatment for scars, wrinkles, and skin laxity. Plast. Reconstr. Surg. 2008, 121, 1421–1429. [Google Scholar] [CrossRef]


| All Age (n = 15) | Young Age Group (n = 9) | Old Age Group (n = 6) | Differences by Age Group (p-Value) | |
|---|---|---|---|---|
| Age [mean ± SD, (range)] | 42.3 ± 20.2 (23–71) | 26.6 ± 1.6 (23–28) | 65.8 ± 4.8 (60–71) | 0.027 * |
| Male [n (%)] | 6.0 (40) | 5.0 (55.6) | 1.0 (16.7) | 0.046 * |
| Height (mean ± SD, cm) | 165.2 ± 7.3 | 167.8 ± 6.8 | 161.3 ± 6.7 | 0.249 |
| Weight (mean ± SD, kg) | 63.4 ± 14.6 | 65.8 ± 17.5 | 59.8 ± 9.1 | 0.600 |
| BMI (mean ± SD, kg/m2) | 23.1 ± 4.2 | 23.2 ± 5.3 | 22.9 ± 2.2 | 0.917 |
| Drinking [%, (na/wk)] | 0.5 (0.6 ± 0.8) | 0.7 (1.0 ± 0.9) | 0.1 (0.2 ± 0.4) | 0.157 |
| Smoking [%, (nb/day)] | 0.1 (10) | 0.1 (10) | 0.1 (10) | 1.000 |
| TPD arm (mm, mean ± SD) | 38.7 ± 29.2 # | 21.7 ± 9.8 # | 64.2 ± 30.4 # | 0.003 * |
| TPD face (mm, mean ± SD) | 10.9 ± 3.7 | 9.9 ± 3.5 | 12.4 ± 3.6 | 0.135 |
| TPD total (mm, mean ± SD) | 24.8 ± 25.0 | 15.8 ± 9.4 | 38.3 ± 33.9 | 0.013 * |
| Stimulation Site | Types of Stimulated Stamp | Guessed MN Stamp | Guessed Placebo Stamp | Don’t Know | Total |
|---|---|---|---|---|---|
| Face site | MN | 7 (23.3%) * | 6 (20.0%) | 2 | 15 |
| Placebo | 4 (13.3%) | 8 (26.7%) * | 3 | 15 | |
| Total | 11 | 14 | 5 | 30 | |
| Forearm site | MN | 6 (20.0%) * | 7 (23.3%) | 2 | 15 |
| Placebo | 4 (13.3%) | 9 (30.0%) * | 2 | 15 | |
| Total | 10 | 16 | 4 | 30 | |
| All site | MN | 13 (21.7%) * | 13 (21.6%) | 4 | 30 |
| Placebo | 8 (13.3%) | 17 (28.3%) * | 5 | 30 | |
| Total | 21 | 30 | 9 | 60 |
| Stimulation Site | Types of Stimulated Stamp | All Age (n = 15) | Young Age Group (n = 9) | Old Age Group (n = 6) | |||
|---|---|---|---|---|---|---|---|
| BI (95% CI) | Blinding Status | BI (95% CI) | Blinding Status | BI (95% CI) | Blinding Status | ||
| Face site | MN | 0.07 (−0.4 to 0.54) | Random guess | −0.13 (−0.77 to 0.52) | Random guess | 0.50 (−0.11 to 1.11) | Unblinded |
| Placebo | 0.27 (−0.17 to 0.7) | Unblinded | 0.38 (−0.22 to 0.97) | Unblinded | 0.00 (−0.65 to 0.65) | Unblinded | |
| Forearm site | MN | −0.07 (−0.54% to 0.4) | Random guess | −0.30 (−0.86 to 0.26) | Opposite guesses | 0.29 (−0.37 to 0.94) | Unblinded |
| Placebo | 0.33 (−0.11 to 0.77) | Unblinded | 0.30 (−0.26 to 0.86) | Unblinded | 0.60 (−0.10 to 1.30) | Unblinded | |
| All site | MN | 0.00 (−0.33 to 0.33) | Random guess | −0.20 (−0.65 to 0.20) | Random guess | 0.33 (−0.15 to 0.81) | Unblinded |
| Placebo | 0.30 (−0.01 to 0.61) | Unblinded | 0.33 (−0.07 to 0.74) | Unblinded | 0.25 (−0.22 to 0.72) | Unblinded | |
| Groups | Stimulation Site | Types of Stimulated Stamp | Low-Sensitive Group (n = 8) | Sensitive Group (n = 7) | ||
|---|---|---|---|---|---|---|
| BI (95% CI) | Blinding Status | BI (95% CI) | Blinding Status | |||
| Based on sensory of forearm | Forearm site | MN | 0.00 (−0.69 to 0.69) | Random guess | −0.13 (−0.77 to 0.52) | Random guess |
| Placebo | 0.00 (−0.69 to 0.69) | Random guess | 0.63 (0.14 to 1.11) | Unblinded | ||
| All site | MN | −0.14 (−0.62 to 0.34) | Random guess | 0.13 (−0.33 to 0.58) | Random guess | |
| Placebo | 0.14 (−0.34 to 0.62) | Random guess | 0.44 (0.05 to 0.82) | Unblinded | ||
| Based on sensory of face | Face site | MN | 0.13 (−0.52 to 0.77) | Random guess | 0.00 (−0.69 to 0.69) | Random guess |
| Placebo | −0.25 (−0.82 to 0.32) | Opposite guesses | 0.86 (0.60 to 1.12) | Unblinded | ||
| All site | MN | 0.00 (−0.46 to 0.46) | Random guess | 0.00 (−0.48 to 0.48) | Random guess | |
| Placebo | −0.06 (0.50 to 0.38) | Random guess | 0.71 (0.41 to 1.02) | Unblinded | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-Y.; Lee, Y.-S.; Park, J.-Y.; Park, J.-H.; Park, H.-J.; Kim, S.-Y. Designing a Placebo Microneedle Stamp: Modeling and Validation in a Clinical Control Trial. Pharmaceutics 2024, 16, 395. https://doi.org/10.3390/pharmaceutics16030395
Jeong S-Y, Lee Y-S, Park J-Y, Park J-H, Park H-J, Kim S-Y. Designing a Placebo Microneedle Stamp: Modeling and Validation in a Clinical Control Trial. Pharmaceutics. 2024; 16(3):395. https://doi.org/10.3390/pharmaceutics16030395
Chicago/Turabian StyleJeong, Seung-Yeon, Ye-Seul Lee, Ji-Yeun Park, Jung-Hwan Park, Hi-Joon Park, and Song-Yi Kim. 2024. "Designing a Placebo Microneedle Stamp: Modeling and Validation in a Clinical Control Trial" Pharmaceutics 16, no. 3: 395. https://doi.org/10.3390/pharmaceutics16030395
APA StyleJeong, S.-Y., Lee, Y.-S., Park, J.-Y., Park, J.-H., Park, H.-J., & Kim, S.-Y. (2024). Designing a Placebo Microneedle Stamp: Modeling and Validation in a Clinical Control Trial. Pharmaceutics, 16(3), 395. https://doi.org/10.3390/pharmaceutics16030395






