Alginate and Chitosan-Based Delivery Systems for Improving the Bioavailability and Therapeutic Efficacy of Curcumin
Abstract
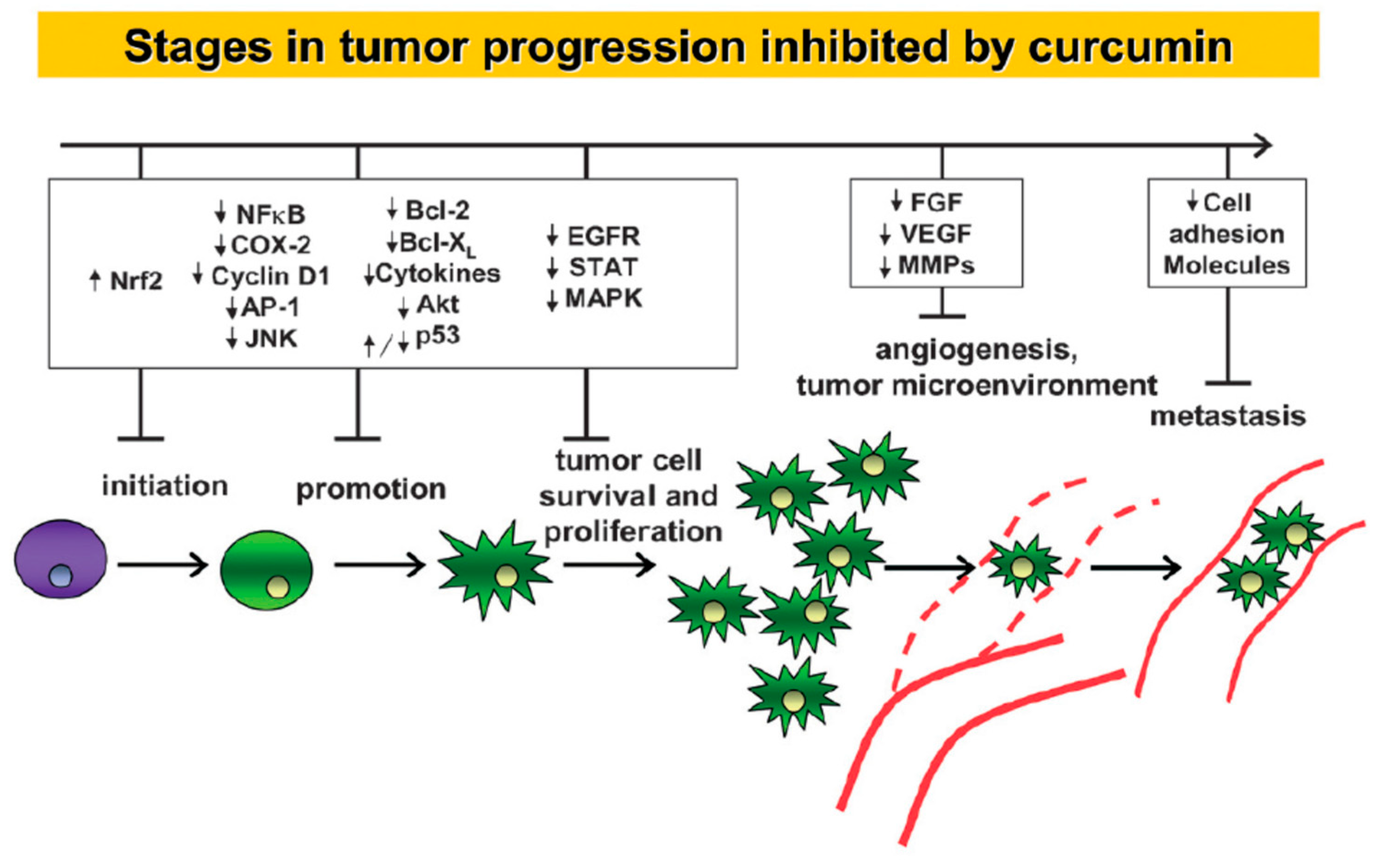
:1. Curcumin and Its Limitations as a Drug Molecule
2. Drug Delivery System (DDS)
3. Carbohydrate as Excipient for DDS
4. Alginate Based DDS for Curcumin
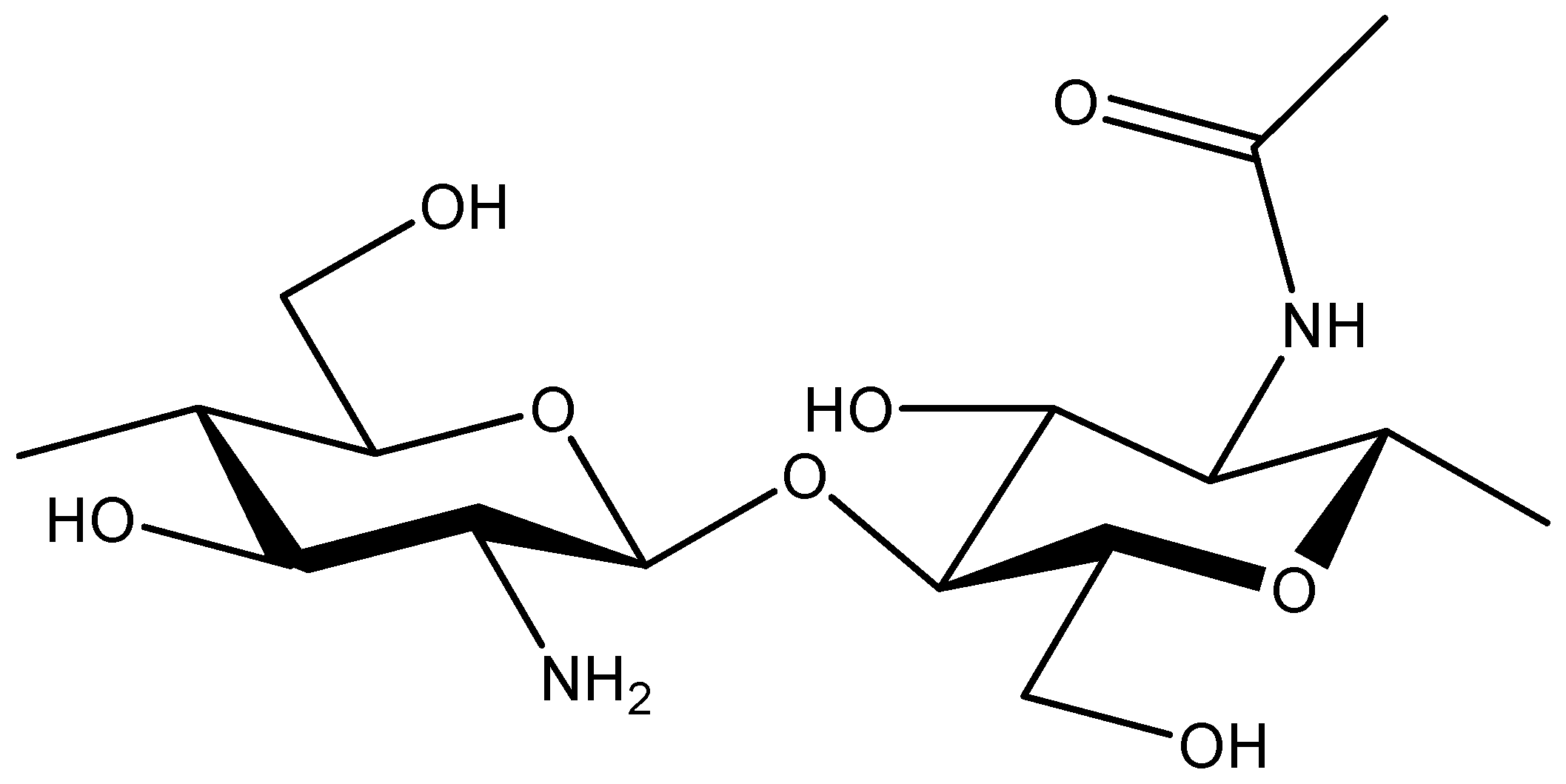
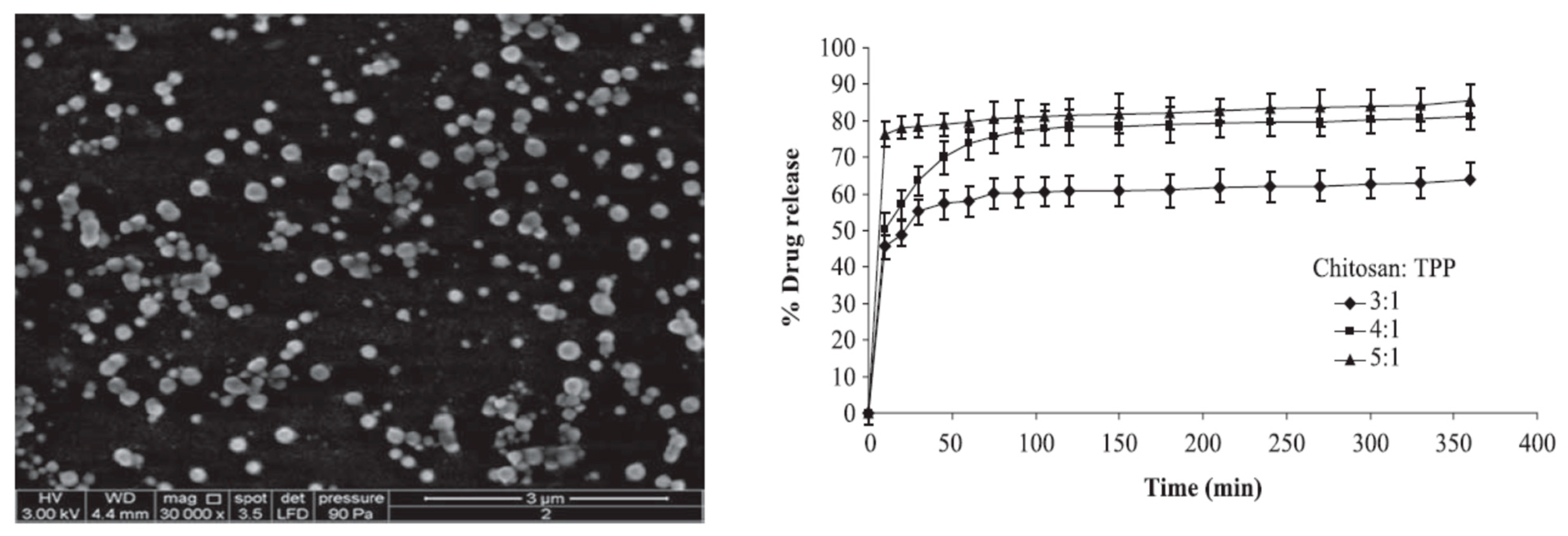
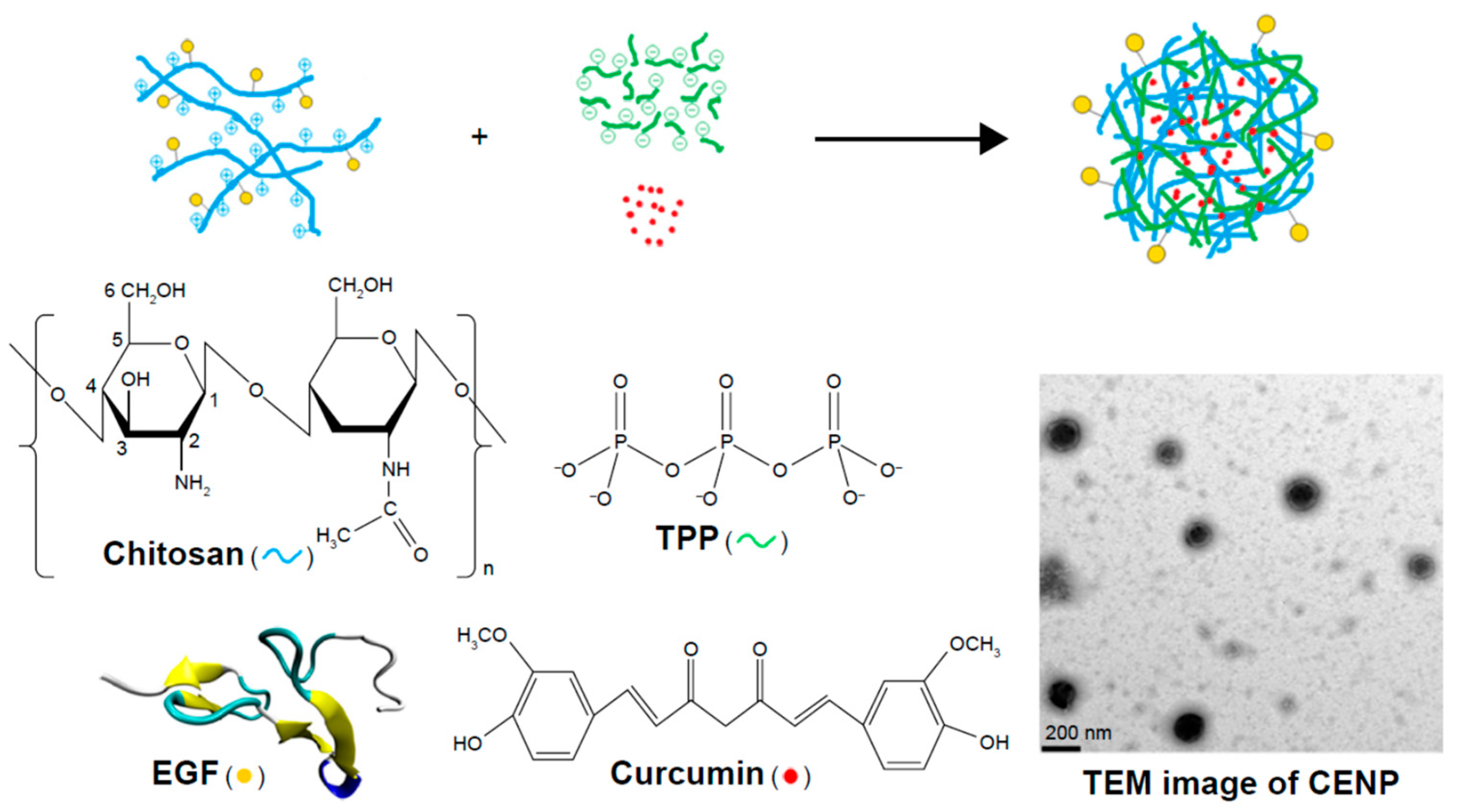
5. Chitosan Based DDS for Curcumin
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [PubMed]
- Kaur, A. Historical background of usage of turmeric: A review. J. Pharmacogn. Phytochem. 2019, 8, 2769–2771. [Google Scholar]
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.Á.; Carmena-Bargueno, M.; Pérez-Sánchez, H.; Espín, J.C.; González-Sarrías, A. Physiologically relevant curcuminoids inhibit angiogenesis via VEGFR2 in human aortic endothelial cells. Food Chem. Toxicol. 2022, 166, 113254. [Google Scholar] [CrossRef] [PubMed]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Parveen, N.; Khan, N.U.; Hadi, S.M. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdeme-thoxycurcumin. Chem. Biol. Interact. 1999, 121, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Jaganmohan, R.L.; Sakariah, K.K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Yixuan, L.; Qaria, M.A.; Sivasamy, S.; Jianzhong, S.; Daochen, Z. Curcumin production and bioavailability: A comprehensive review of curcumin extraction, synthesis, biotransformation and delivery systems. Ind. Crops. Prod. 2021, 172, 114050. [Google Scholar] [CrossRef]
- Hewling, S.J.; Kalman, D.S. Curcumin: A Review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Lin, J.-K. Molecular targets of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Aggarwal, B.B., Surh, Y.-J., Shishodia, S., Eds.; Springer US: Boston, MA, USA, 2007; pp. 227–243. [Google Scholar]
- Priyadarsini, K.I.; Gandhi, V.V.; Kunwar, A. Important chemical structural features of curcumin and its derivatives: How do they influence their anticancer activity? Indian J. Biochem. Biophys. 2020, 57, 228–235. [Google Scholar]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef]
- Suhr, Y.-J. Molecular mechanisms of chemo preventive effects of selected dietary and medicinal phenolic substances. Mutat. Res. 1999, 428, 305–327. [Google Scholar]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.-F. The pharmacology of curcumin: Is it the degradation products? Trends Mol. Med. 2012, 18, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled drug delivery systems: Current status and future directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Chamundeeswari, M.; Jeslin, J.; Verma, M.L. Nanocarriers for drug delivery applications. Environ. Chem. Lett. 2019, 17, 849–865. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotech. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liu, R.; Zhou, Y.; Gao, H. Size-tunable strategies for a tumor targeted drug delivery system. ACS Cent. Sci. 2020, 6, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Mirzaie, Z.; Barati, M.; Asadi Tokmedash, M.A. Anticancer drug delivery systems based on curcumin nanostructures: A review. Pharm. Chem. J. 2020, 54, 353–360. [Google Scholar] [CrossRef]
- Moballegh, M.N.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin delivery mediated by bio-based nanoparticles: A Review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A. Curcumin formulations for better bioavailability: What we learned from clinical trials thus far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Banat, F.; Sivamani, S.; Sivakumar, N.; Bandegharaei, A.H.; Show, P.L. Biopolymers and composites: Properties, characterization and their applications in food, medical and pharmaceutical industries. J. Environ. Chem. Eng. 2021, 9, 105322. [Google Scholar] [CrossRef]
- Blanco, A.; Blanco, G. Chapter 4—Carbohydrates. In Medical Biochemistry; Blanco, A., Blanco, G., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 73–97. [Google Scholar]
- Schlemmer, W.; Selinger, J.; Hobisch, M.A.; Spirk, S. Polysaccharides for sustainable energy storage—A review. Carbohydr. Polym. 2021, 265, 118063. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of polymer hydrogels and nanoparticulate systems for biomedical and pharmaceutical applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef]
- Stylianopoulos, C. Carbohydrates: Chemistry and classification. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 265–271. [Google Scholar]
- Jiang, M.; Gan, Y.; Li, Y.; Qi, Y.; Zhou, Z.; Fang, X.; Jiao, J.; Han, X.; Gao, W.; Zhao, J. Protein-polysaccharide- based delivery systems for enhancing the bioavailability of curcumin: A review. Int. J. Biol. Macromol. 2023, 250, 126153. [Google Scholar] [CrossRef]
- Severino, P.; da Silva Classius, F.; Andrade, L.N.; de Lima Oliveira, D.; Joana, C.; Souto, E.B. Alginate nanoparticles for drug delivery and targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Alberti, S.; Gaggero, G.; Ferretti, M.; Botter, R.; Vicini, S.; Castellano, M. An up-to-date review on alginate nanoparticles and nanofibers for biomedical and pharmaceutical applications. Adv. Mater. Interfaces 2021, 8, 2100809. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a promising biopolymer in drug delivery and wound healing: A review of the state-of-the-art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Rehman, A.; Feng, J.; Noreen, A.; Assadpour, E.; Kharazmi, M.S.; Lianfu, Z.; Jafari, S.M. Alginate-based nanocarriers for the delivery and controlled-release of bioactive compounds. Adv. Colloid. Interface Sci. 2022, 307, 102744. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wei, Z.; Xue, C. Alginate-based delivery systems for food bioactive ingredients: An overview of recent advances and future trends. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5345–5369. [Google Scholar] [CrossRef] [PubMed]
- Malektaj, H.; Drozdov, A.D.; deClaville Christiansen, J. Mechanical properties of alginate hydrogels cross-linked with multivalent cations. Polymers 2023, 15, 3012. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Xia, Q. Nanostructured lipid carriers incorporated in alginate hydrogel: Enhanced stability and modified behavior in gastrointestinal tract. Colloids Surf. A Physicochem. Eng. 2019, 574, 197–206. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Ohta, S.; Taniguchi, M.; Yoshida, H.; Tanaka, D.; Omichi, K.; Shimizu, A.; Isaji, M.; Hasegawa, K.; Ito, T. Balance of antiperitoneal adhesion, hemostasis, and operability of compressed bilayer ultrapure alginate sponges. Biomater. Adv. 2022, 137, 212825. [Google Scholar]
- Ohta, S.; Toda, T.; Inagaki, F.; Omichi, K.; Shimizu, A.; Kokudo, N.; Hasegawa, K.; Ito, T. The prevention of hepatectomy-induced adhesions by bilayer sponge composed of ultrapure alginate. J. Surg. Res. 2019, 242, 286–295. [Google Scholar] [CrossRef]
- Severino, P.; Chaud, M.V.; Shimojo, A.; Antonini, D.; Lancelloti, M.; Santana, M.H.A.; Souto, E.B. Sodium alginate-cross-linked polymyxin B sulphate-loaded solid lipid nanoparticles: Antibiotic resistance tests and HaCat and NIH/3T3 cell viability studies. Colloids Surf. B. 2015, 129, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Dev, A.; Das, S.S.; Kim, H.J.; Kumar, A.; Thakur, V.K.; Han, S.S. Curcumin-loaded alginate hydrogels for cancer therapy and wound healing applications: A review. Int. J. Biol. Macromol. 2023, 232, 123283. [Google Scholar] [CrossRef]
- Hegde, V.; Uthappa, U.T.; Altalhi, T.; Jung, H.Y.; Han, S.S.; Kurkuri, M.D. Alginate based polymeric systems for drug delivery, antibacterial/microbial, and wound dressing applications. Mater. Today Commun. 2023, 33, 104813. [Google Scholar] [CrossRef]
- Tan, J.; Luo, Y.; Guo, Y.; Zhou, Y.; Liao, X.; Li, D.; Lai, X.; Liu, Y. Development of alginate-based hydrogels: Crosslinking strategies and biomedical applications. Int. J. Biol. Macromol. 2023, 239, 124275. [Google Scholar] [CrossRef] [PubMed]
- Sookkasem, A.; Chatpun, S.; Yuenyongsawad, S.; Wiwattanapatapee, R. Alginate beads for colon specific delivery of self-emulsifying curcumin. J. Drug Deliv. Sci. Technol. 2015, 29, 159–166. [Google Scholar] [CrossRef]
- Shaikh, S.A.M.; Barik, A. Encapsulation of curcumin in alginate microbeads (AMB) for control release of curcumin. J. Chem. Sci. 2023, 135, 39. [Google Scholar] [CrossRef]
- Ding, R.; Zhang, M.; Zhu, Q.; Qu, Y.; Jia, X.; Yin, L. Curcumin loaded Zein-alginate nanogels with “core-shell” structure: Formation, characterization and simulated digestion. Int. J. Biol. Macromol. 2023, 251, 126201. [Google Scholar] [CrossRef]
- Xu, W.; Huang, L.; Jin, W.; Ge, P.; Shah, B.R.; Zhu, D.; Jing, J. Encapsulation and release behavior of curcumin based on nanoemulsions-filled alginate hydrogel beads. Int. J. Biol. Macromol. 2019, 134, 210–215. [Google Scholar] [CrossRef]
- Paswan, M.; Chandel, A.K.S.; Malek, N.I.; Dholakiya, B.Z. Preparation of sodium alginate/Cur-PLA hydrogel beads for curcumin encapsulation. Int. J. Biol. Macromol. 2024, 254, 128005. [Google Scholar] [CrossRef]
- Kumavat, S.; Chaudhari, Y.; Borole, P.; Mishra, P.; Shenghani, K.; Duvvuri, P. Degradation studies of curcumin. Int. J. Pharm. Res. 2013, 3, 50–55. [Google Scholar]
- Govindaraju, R.; Karki, R.; Chandrashekarappa, J.; Santhanam, M.; Shankar, A.K.K.; Joshi, H.K.; Divakar, G. Enhanced water dispersibility of curcumin encapsulated in alginate-polysorbate 80 nano particles and bioavailability in healthy human volunteers. Pharm. Nanotechnol. 2019, 7, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Sarika, P.R.; James, N.R.; Anil Kumar, P.R.; Raj, D.K. Preparation, characterization and biological evaluation of curcumin loaded alginate aldehyde–gelatin nanogels. Mater. Sci. Eng. C 2016, 68, 251–257. [Google Scholar]
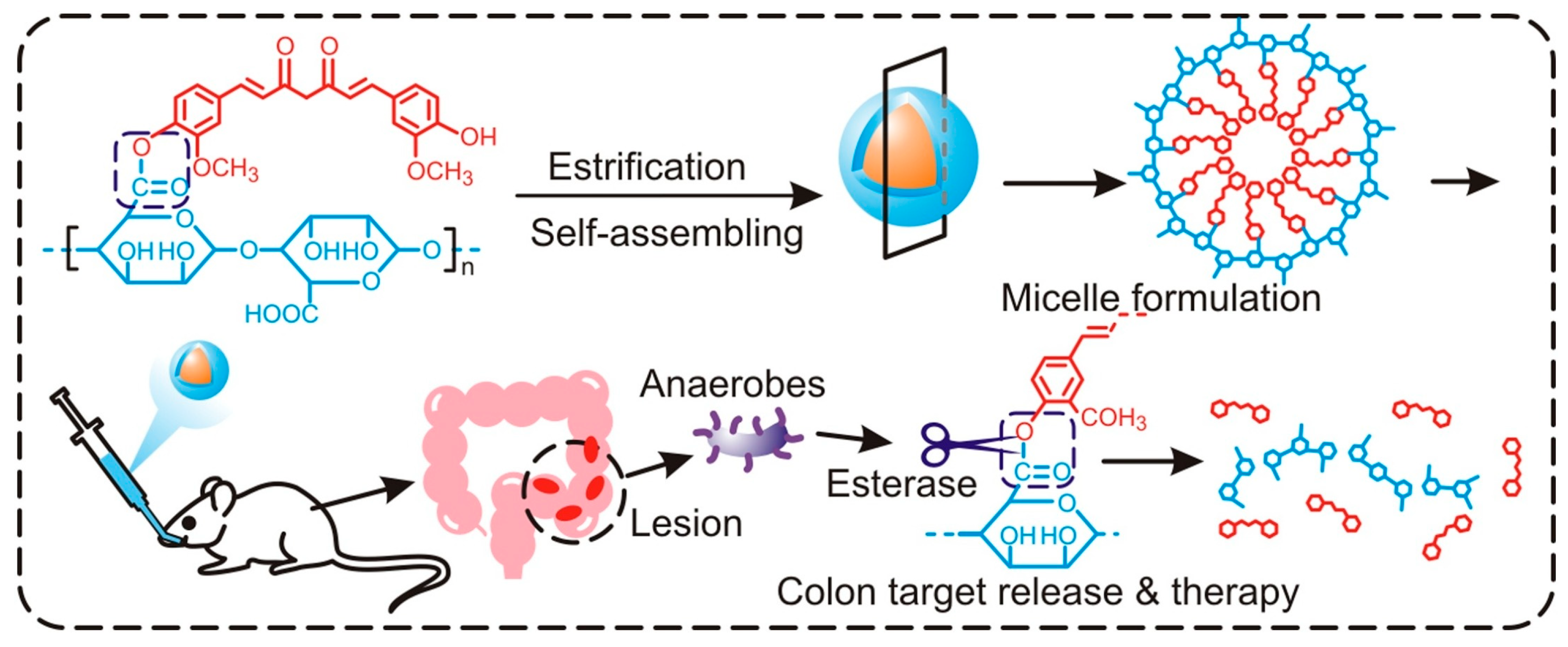
- Wang, Y.; Li, Y.; He, L.; Mao, B.; Chen, S.; Martinez, V.; Guo, X.; Shen, X.; Liu, B.; Li, C. Commensal flora triggered target anti-inflammation of alginate-curcumin micelle for ulcerative colitis treatment. Colloids Surf. B 2021, 203, 111756. [Google Scholar] [CrossRef]
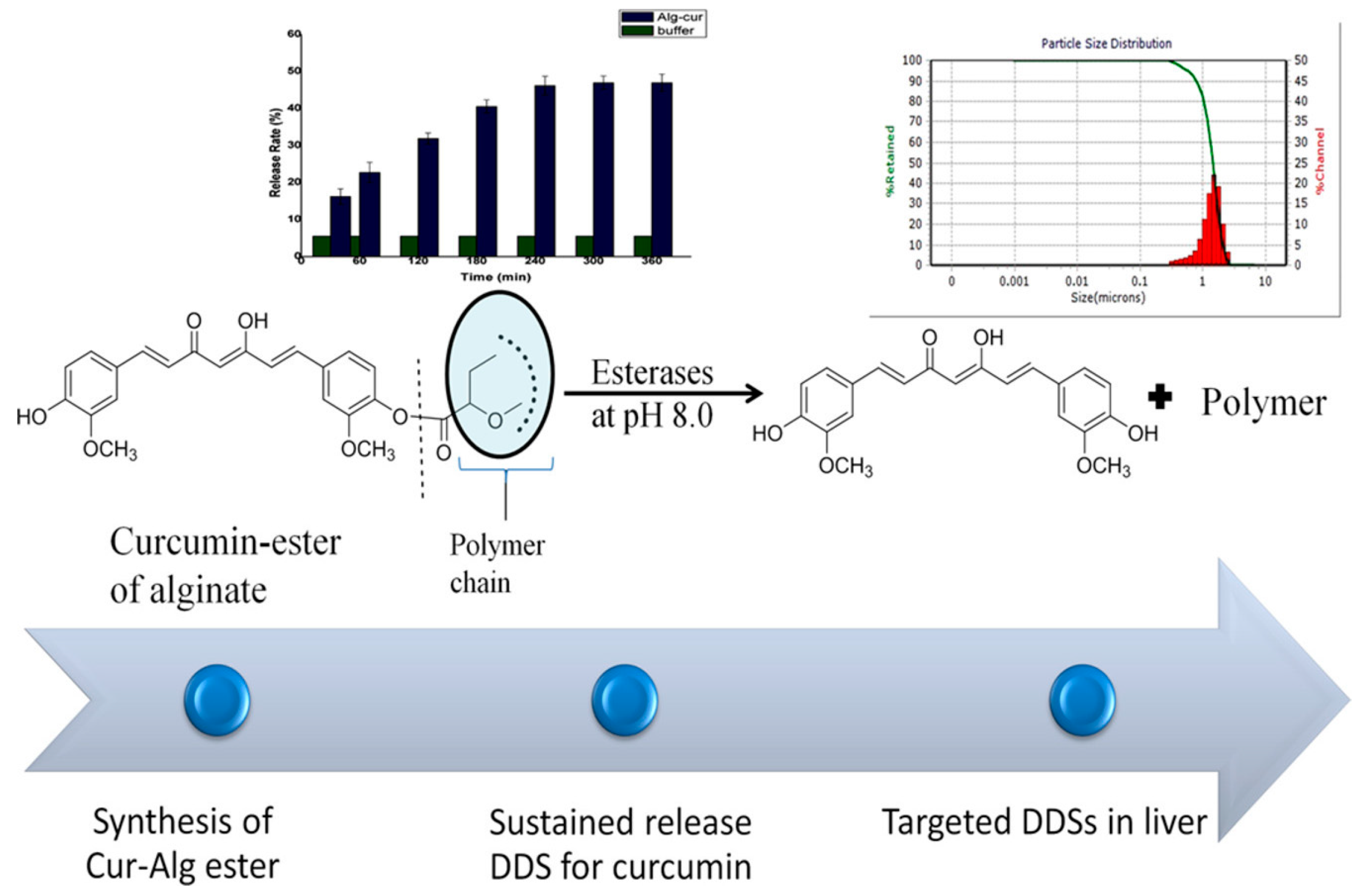
- Mor, N.; Raghav, N. In-vitro simulation of modified-alginate ester as sustained release delivery system for curcumin. J. Mol. Struct. 2023, 1283, 135307. [Google Scholar] [CrossRef]
- Lachowicz, D.; Karabasz, A.; Bzowska, M.; Szuwarzyński, M.; Karewicz, A.; Nowakowska, M. Blood-compatible, stable micelles of sodium alginate—Curcumin bioconjugate for anti-cancer applications. Eur. Polym. J. 2019, 113, 208–219. [Google Scholar] [CrossRef]
- Karabasz, A.; Lachowicz, D.; Karewicz, A.; Mezyk-Kopec, R.; Stalińska, K.; Werner, E.; Cierniak, A.; Dyduch, G.; Bereta, J.; Bzowska, M. Analysis of toxicity and anticancer activity of micelles of sodium alginate-curcumin. Int. J. Nanomed. 2019, 14, 7249–7262. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Federer, C.; Kurpiers, M.; Bernkop-Schnürch, A. Thiolated chitosans: A multi-talented class of polymers for various applications. Biomacromolecules 2021, 22, 24–56. [Google Scholar] [CrossRef]
- M Ways, T.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan based self-assembled nanoparticles in drug delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef]
- Saha, P.; Singh, P.; Kathuria, H.; Chitkara, D.; Pandey, M.M. Self-assembled lecithin-chitosan nanoparticles improved rotigotine nose-to-brain delivery and brain targeting efficiency. Pharmaceutics 2023, 15, 851. [Google Scholar] [CrossRef]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Chaiwarit, T.; Sommano, S.R.; Rachtanapun, P.; Kantrong, N.; Ruksiriwanich, W.; Kumpugdee-Vollrath, M.; Jantrawut, P. Development of carboxymethyl chitosan nanoparticles prepared by ultrasound-assisted technique for a Clindamycin HCl carrier. Polymers 2022, 14, 1736. [Google Scholar] [CrossRef] [PubMed]
- Birch, N.P.; Schiffman, J.D. Characterization of self-assembled polyelectrolyte complex nanoparticles formed from chitosan and pectin. Langmuir 2014, 30, 3441–3447. [Google Scholar] [CrossRef] [PubMed]
- Muñana-González, S.; Veloso-Fernández, A.; Ruiz-Rubio, L.; Pérez-Álvarez, L.; Vilas-Vilela, J.L. Covalent cross-linking as a strategy to prepare water-dispersible chitosan nanogels. Polymers 2023, 15, 434. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Qian, J.; Zhao, C.; Yang, H.; Zhao, X.; Guo, H. Study on the relationship between crosslinking degree and properties of TPP crosslinked chitosan nanoparticles. Carbohydr. Polym. 2020, 241, 116349. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, M.C.; D’ Antoni, C.L.; Domínguez Rubio, A.P.; Alaimo, A.; Pérez, O.E. Chitosan-tripolyphosphate nanoparticles designed to encapsulate polyphenolic compounds for biomedical and pharmaceutical applications—A review. Biomed. Pharmacother. 2021, 142, 111970. [Google Scholar] [CrossRef]
- Chuah, L.H.; Billa, N.; Roberts, C.J.; Burley, J.C.; Manickam, S. Curcumin-containing chitosan nanoparticles as a potential mucoadhesive delivery system to the colon. Pharm. Dev. Technol. 2013, 18, 591–599. [Google Scholar] [CrossRef]
- Keerthikumar; Jalalpure, S.S.; Malleshwara Rao, P.V.S.N. Chitosan encapsulated curcumin nanoparticles as an effective drug delivery system for oral cancer treatment. Indian Drugs 2015, 52, 40–48. [Google Scholar] [CrossRef]
- Reddy, D.N.K.; Huang, F.; Wang, S.; Kumar, R. Synergistic Antioxidant and antibacterial activity of curcumin-C3 encapsulated chitosan nanoparticles. Curr. Pharm. Des. 2020, 26, 5021–5029. [Google Scholar] [CrossRef]
- Yadav, A.; Lomash, V.; Samim, M.; Flora, S.J. Curcumin encapsulated in chitosan nanoparticles: A novel strategy for the treatment of arsenic toxicity. Chem. Biol. Interact. 2012, 199, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wilkinson, J., 4th; Christine Pietsch, E.; Buss, J.L.; Wang, W.; Planalp, R.; Torti, F.M.; Torti, S.V. Iron chelation in the biological activity of curcumin. Free Radic. Biol. Med. 2006, 40, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Mishra, B.; Kunwar, A.; Kadam, R.M.; Shen, L.; Dutta, S.; Padhye, S.; Satpati, A.K.; Zhang, H.-Y.; Priyadarsini, K.I. Comparative study of copper(II)–curcumin complexes as superoxide dismutase mimics and free radical scavengers. Eur. J. Med. Chem. 2007, 42, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.H.; Yu, K.H.; Huang, Y.C.; Lee, C.I. EGFR-targeted photodynamic therapy by curcumin-encapsulated chitosan/TPP nanoparticles. Int. J. Nanomed. 2018, 13, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Arozal, W.; Louisa, M.; Rahmat, D.; Chendrana, P.; Sandhiutami, N.M.D. Development, Characterization and pharmacokinetic profile of chitosan-sodium tripolyphosphate nanoparticles based drug delivery systems for curcumin. Adv. Pharm. Bull. 2021, 11, 77–85. [Google Scholar] [CrossRef]
- Sampathi, S.; Haribhau, C.J.; Kuchana, V.; Junnuthula, V.; Dyawanapelly, S. Nanosuspension encapsulated chitosan-pectin microbeads as a novel delivery platform for enhancing oral bioavailability. Carbohydr. Polym. 2023, 319, 121177. [Google Scholar] [CrossRef]
- Vijayakurup, V.; Thulasidasan, A.T.; Shankar, G.M.; Retnakumari, A.P.; Nandan, C.D.; Somaraj, J.; Antony, J.; Alex, V.V.; Vinod, B.S.; Liju, V.B.; et al. Chitosan encapsulation enhances the bioavailability and tissue retention of curcumin and improves its efficacy in preventing B[a]P-induced lung carcinogenesis. Cancer Prev. Res. 2019, 12, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.K.; Akhtar, F.; Ray, G.; Pandey, A.K. Curcumin Nanoparticles and Methods of Producing the Same. World Patent WO 2010/013224 A2, 4 February 2010. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sable, A.A.; Kunwar, A.; Barik, A. Alginate and Chitosan-Based Delivery Systems for Improving the Bioavailability and Therapeutic Efficacy of Curcumin. Pharmaceutics 2024, 16, 423. https://doi.org/10.3390/pharmaceutics16030423
Sable AA, Kunwar A, Barik A. Alginate and Chitosan-Based Delivery Systems for Improving the Bioavailability and Therapeutic Efficacy of Curcumin. Pharmaceutics. 2024; 16(3):423. https://doi.org/10.3390/pharmaceutics16030423
Chicago/Turabian StyleSable, Anand A., Amit Kunwar, and Atanu Barik. 2024. "Alginate and Chitosan-Based Delivery Systems for Improving the Bioavailability and Therapeutic Efficacy of Curcumin" Pharmaceutics 16, no. 3: 423. https://doi.org/10.3390/pharmaceutics16030423
APA StyleSable, A. A., Kunwar, A., & Barik, A. (2024). Alginate and Chitosan-Based Delivery Systems for Improving the Bioavailability and Therapeutic Efficacy of Curcumin. Pharmaceutics, 16(3), 423. https://doi.org/10.3390/pharmaceutics16030423





