Impact of Drug-Mediated Inhibition of Intestinal Transporters on Nutrient and Endogenous Substrate Disposition…an Afterthought?
Abstract
1. Introduction
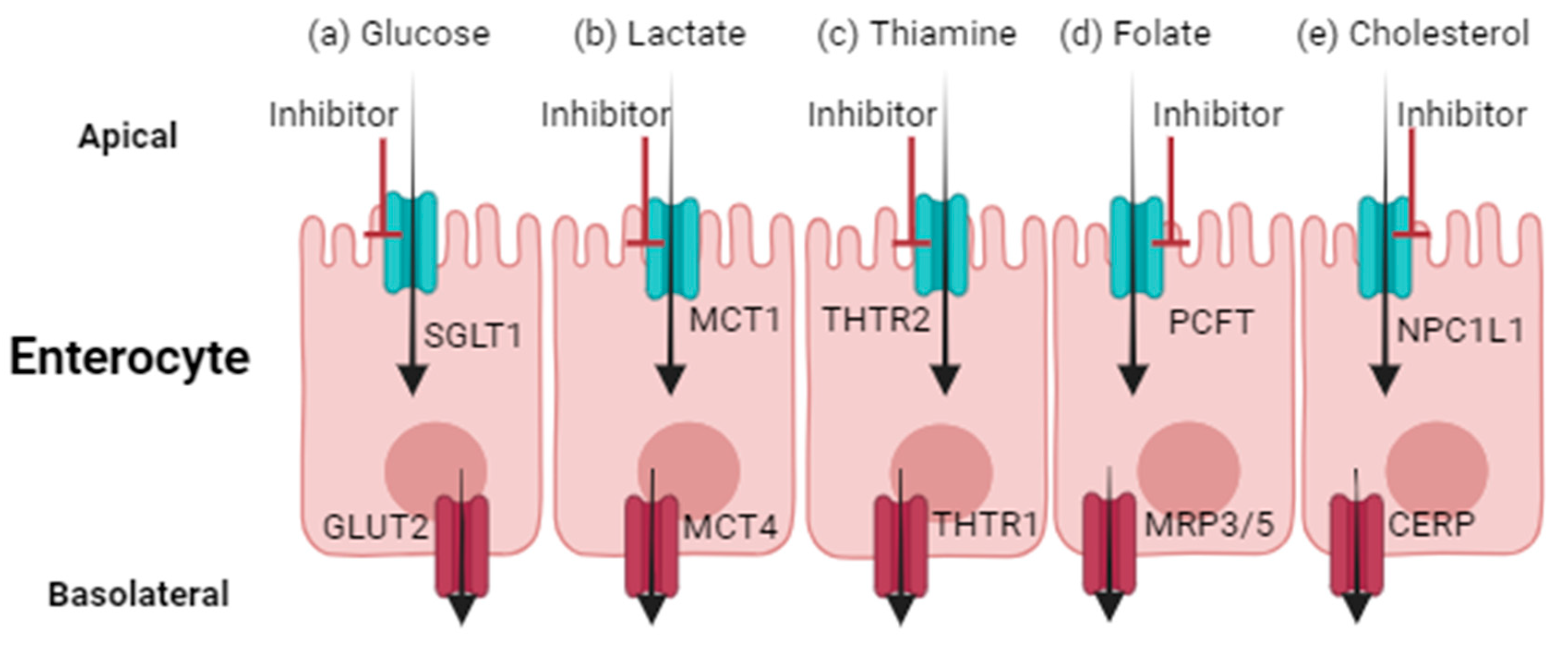
2. Examples of Altered Nutrient and Endogenous Substrate Disposition due to Drugs
3. Disruption of Glucose Disposition
4. Disruption of Thiamine Absorption
5. Disruption of Folate Disposition
6. Disruption of Lactate Disposition
7. Disruption of Cholesterol Disposition
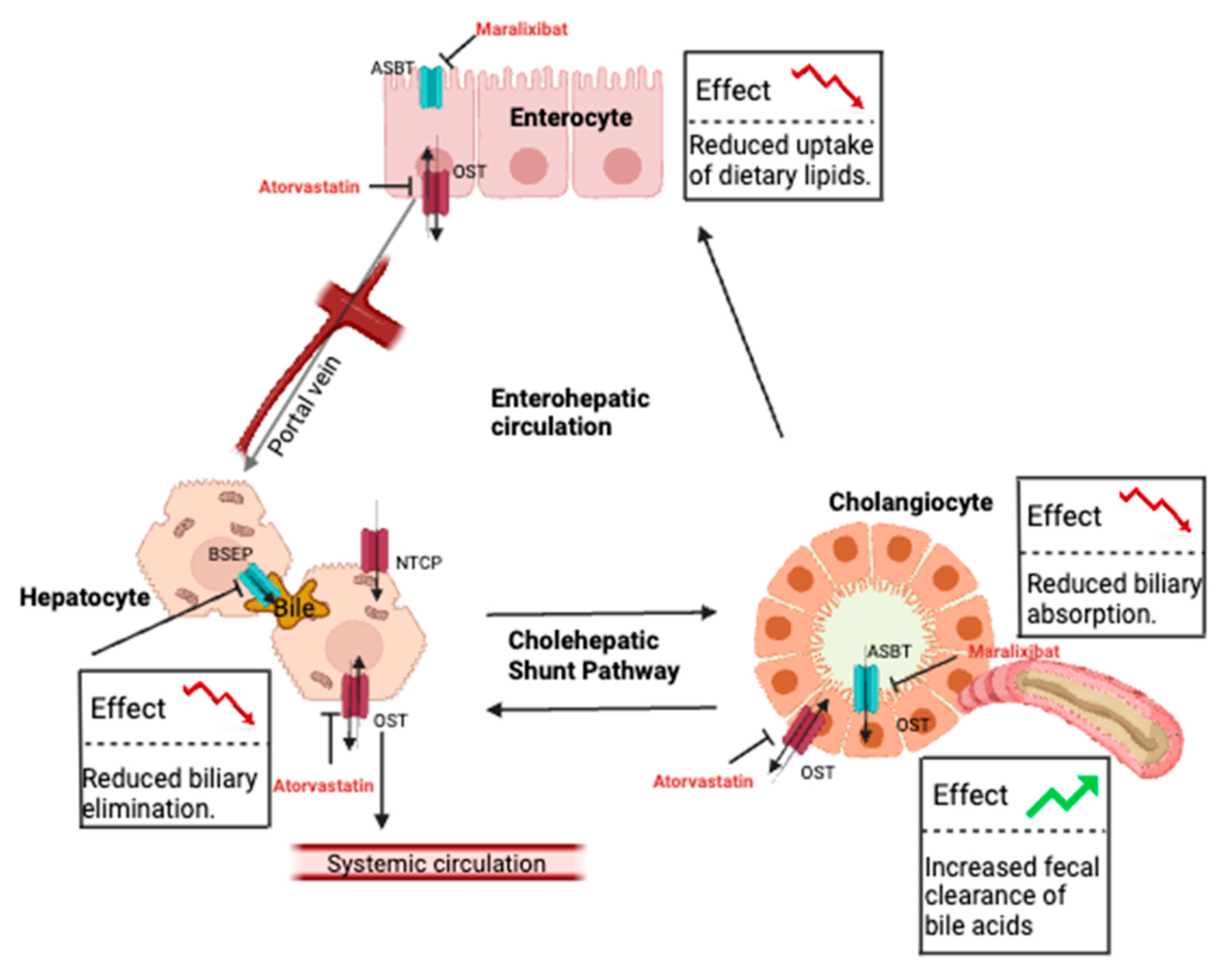
8. Pathological Alteration of Bile Acid Recirculation
9. Conclusions and Future Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, X.; Smith, J.C. Biological Membrane Organization and Cellular Signaling. Chem. Rev. 2019, 119, 5849–5880. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Chan, G.; Hu, Y.; Hu, H.; Ouyang, D. A Comprehensive Map of FDA-Approved Pharmaceutical Products. Pharmaceutics 2018, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A. Role of the intestinal bile acid transporters in bile acid and drug disposition. Drug Transp. 2011, 201, 169–203. [Google Scholar] [CrossRef]
- Estudante, M.; Morais, J.G.; Soveral, G.; Benet, L.Z. Intestinal drug transporters: An overview. Adv. Drug Deliv. Rev. 2013, 65, 1340–1356. [Google Scholar] [CrossRef] [PubMed]
- Zamek-Gliszczynski, M.J.; Sangha, V.; Shen, H.; Feng, B.; Wittwer, M.B.; Varma, M.V.S.; Liang, X.; Sugiyama, Y.; Zhang, L.; Bendayan, R.; et al. Transporters in Drug Development: International Transporter Consortium Update on Emerging Transporters of Clinical Importance. Clin. Pharmacol. Ther. 2022, 112, 485–500. [Google Scholar] [CrossRef] [PubMed]
- McFeely, S.J.; Wu, L.; Ritchie, T.K.; Unadkat, J. Organic anion transporting polypeptide 2B1—More than a glass-full of drug interactions. Pharmacol. Ther. 2019, 196, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Keiser, M.; Drozdzik, M.; Oswald, S. Expression, regulation and function of intestinal drug transporters: An update. Biol. Chem. 2017, 398, 175–192. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Lagaron, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef]
- Hayden, E.; Chen, M.; Pasquariello, K.Z.; Gibson, A.A.; Petti, J.J.; Shen, S.; Qu, J.; Ong, S.S.; Chen, T.; Jin, Y.; et al. Regulation of OATP1B1 function by tyrosine kinase-mediated phosphorylation. Clin. Cancer Res. 2021, 27, 4301–4310. [Google Scholar] [CrossRef]
- Hove, V.N.; Anderson, K.; Hayden, E.R.; Pasquariello, K.Z.; Gibson, A.A.; Shen, S.; Qu, J.; Jin, Y.; Miecznikowski, J.C.; Hu, S.; et al. Influence of Tyrosine Kinase Inhibition on OATP1B3-mediated Uptake. Mol. Pharmacol. 2022, 101, 381–389. [Google Scholar] [CrossRef]
- Lieder, B.; Hoi, J.K.; Holik, A.-K.; Geissler, K.; Hans, J.; Friedl, B.; Liszt, K.; Krammer, G.E.; Ley, J.P.; Somoza, V. The flavanone homoeriodictyol increases SGLT-1-mediated glucose uptake but decreases serotonin release in differentiated Caco-2 cells. PLoS ONE 2017, 12, e0171580. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Liang, X.; Chien, H.C.; Yee, S.W.; Giacomini, M.M.; Chen, E.C.; Piao, M.; Hao, J.; Twelves, J.; Lepist, E.I.; Ray, A.S.; et al. Metformin Is a Substrate and Inhibitor of the Human Thiamine Transporter, THTR-2 (SLC19A3). Mol. Pharm. 2015, 12, 4301–4310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Matherly, L.H.; Goldman, I.D. Membrane transporters and folate homeostasis: Intestinal absorption and transport into systemic compartments and tissues. Expert. Rev. Mol. Med. 2009, 11, e4. [Google Scholar] [CrossRef]
- Halestrap, A.P. The SLC16 gene family—structure, role and regulation in health and disease. Mol. Aspects Med. 2013, 34, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Betters, J.L.; Yu, L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu. Rev. Physiol. 2011, 73, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Durnik, R.; Sindlerova, L.; Babica, P.; Jurcek, O. Bile Acids Transporters of Enterohepatic Circulation for Targeted Drug Delivery. Molecules 2022, 27, 2961. [Google Scholar] [CrossRef]
- Lam, J.T.; Martin, M.G.; Turk, E.; Hirayama, B.A.; Bosshard, N.U.; Steinmann, B.; Wright, E.M. Missense mutations in SGLT1 cause glucose-galactose malabsorption by trafficking defects. Biochim. Biophys. Acta 1999, 1453, 297–303. [Google Scholar] [CrossRef]
- Gorboulev, V.; Schurmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R.; et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012, 61, 187–196. [Google Scholar] [CrossRef]
- Sands, A.T.; Zambrowicz, B.P.; Rosenstock, J.; Lapuerta, P.; Bode, B.W.; Garg, S.K.; Buse, J.B.; Banks, P.; Heptulla, R.; Rendell, M.; et al. Sotagliflozin, a Dual SGLT1 and SGLT2 Inhibitor, as Adjunct Therapy to Insulin in Type 1 Diabetes. Diabetes Care 2015, 38, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Zambrowicz, B.; Freiman, J.; Brown, P.M.; Frazier, K.S.; Turnage, A.; Bronner, J.; Ruff, D.; Shadoan, M.; Banks, P.; Mseeh, F.; et al. LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial. Clin. Pharmacol. Ther. 2012, 92, 158–169. [Google Scholar] [CrossRef]
- Sha, S.; Devineni, D.; Ghosh, A.; Polidori, D.; Chien, S.; Wexler, D.; Shalayda, K.; Demarest, K.; Rothenberg, P. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes. Metab. 2011, 13, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Henry, R.R.; Banks, P.; Buse, J.B.; Davies, M.J.; Fulcher, G.R.; Pozzilli, P.; Gesty-Palmer, D.; Lapuerta, P.; Simo, R.; et al. Effects of Sotagliflozin Added to Insulin in Patients with Type 1 Diabetes. N. Engl. J. Med. 2017, 377, 2337–2348. [Google Scholar] [CrossRef]
- Huber, S.M.; Misovic, M.; Mayer, C.; Rodemann, H.P.; Dittmann, K. EGFR-mediated stimulation of sodium/glucose cotransport promotes survival of irradiated human A549 lung adenocarcinoma cells. Radiother. Oncol. 2012, 103, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, M.; Weidow, B.; Koehler, E.; Bakane, N.; Garbett, S.; Shyr, Y.; Quaranta, V. Development of high-throughput quantitative assays for glucose uptake in cancer cell lines. Mol. Imaging Biol. 2011, 13, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Wang, B.W.; Hsiao, Y.C.; Wu, C.Y.; Cheng, F.J.; Hsia, T.C.; Chen, C.Y.; Wang, Y.; Weihua, Z.; Chou, R.H.; et al. PKCdelta-mediated SGLT1 upregulation confers the acquired resistance of NSCLC to EGFR TKIs. Oncogene 2021, 40, 4796–4808. [Google Scholar] [CrossRef] [PubMed]
- Buffier, P.; Bouillet, B.; Smati, S.; Archambeaud, F.; Cariou, B.; Verges, B. Expert opinion on the metabolic complications of new anticancer therapies: Tyrosine kinase inhibitors. Ann. Endocrinol. 2018, 79, 574–582. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Diamond, S.; Boer, J.; Harris, J.J.; Li, Y.; Rupar, M.; Behshad, E.; Gardiner, C.; Collier, P.; et al. The Janus kinase 2 inhibitor fedratinib inhibits thiamine uptake: A putative mechanism for the onset of Wernicke’s encephalopathy. Drug Metab. Dispos. 2014, 42, 1656–1662. [Google Scholar] [CrossRef]
- Giacomini, M.M.; Hao, J.; Liang, X.; Chandrasekhar, J.; Twelves, J.; Whitney, J.A.; Lepist, E.I.; Ray, A.S. Interaction of 2,4-Diaminopyrimidine-Containing Drugs Including Fedratinib and Trimethoprim with Thiamine Transporters. Drug Metab. Dispos. 2017, 45, 76–85. [Google Scholar] [CrossRef]
- Vora, B.; Green, E.A.E.; Khuri, N.; Ballgren, F.; Sirota, M.; Giacomini, K.M. Drug-nutrient interactions: Discovering prescription drug inhibitors of the thiamine transporter ThTR-2 (SLC19A3). Am. J. Clin. Nutr. 2020, 111, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Vora, B.; Wen, A.; Yee, S.W.; Trinh, K.; Azimi, M.; Green, E.A.E.; Sirota, M.; Greenberg, A.S.; Newman, J.W.; Giacomini, K.M. The Effect of Trimethoprim on Thiamine Absorption: A Transporter-Mediated Drug-Nutrient Interaction. Clin. Pharmacol. Ther. 2023, 114, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Nakai, Y.; Ueda, S.; Kamigaso, S.; Ohta, K.Y.; Hatakeyama, M.; Hayashi, Y.; Otagiri, M.; Yuasa, H. Functional characterization of PCFT/HCP1 as the molecular entity of the carrier-mediated intestinal folate transport system in the rat model. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G660–G668. [Google Scholar] [CrossRef]
- Morris, M.E.; Felmlee, M.A. Overview of the proton-coupled MCT (SLC16A) family of transporters: Characterization, function and role in the transport of the drug of abuse gamma-hydroxybutyric acid. AAPS J. 2008, 10, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Cundy, K.C.; Branch, R.; Chernov-Rogan, T.; Dias, T.; Estrada, T.; Hold, K.; Koller, K.; Liu, X.; Mann, A.; Panuwat, M.; et al. XP13512 [(+/-)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: I. Design, synthesis, enzymatic conversion to gabapentin, and transport by intestinal solute transporters. J. Pharmacol. Exp. Ther. 2004, 311, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Halford, S.; Veal, G.J.; Wedge, S.R.; Payne, G.S.; Bacon, C.M.; Sloan, P.; Dragoni, I.; Heinzmann, K.; Potter, S.; Salisbury, B.M.; et al. A Phase I Dose-escalation Study of AZD3965, an Oral Monocarboxylate Transporter 1 Inhibitor, in Patients with Advanced Cancer. Clin. Cancer Res. 2023, 29, 1429–1439. [Google Scholar] [CrossRef]
- Guan, X.; Morris, M.E. In Vitro and In Vivo Efficacy of AZD3965 and Alpha-Cyano-4-Hydroxycinnamic Acid in the Murine 4T1 Breast Tumor Model. AAPS J. 2020, 22, 84. [Google Scholar] [CrossRef]
- Drazic, T.; Molcanov, K.; Sachdev, V.; Malnar, M.; Hecimovic, S.; Patankar, J.V.; Obrowsky, S.; Levak-Frank, S.; Habus, I.; Kratky, D. Novel amino-beta-lactam derivatives as potent cholesterol absorption inhibitors. Eur. J. Med. Chem. 2014, 87, 722–734. [Google Scholar] [CrossRef]
- Sudhop, T.; Lutjohann, D.; Kodal, A.; Igel, M.; Tribble, D.L.; Shah, S.; Perevozskaya, I.; von Bergmann, K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation 2002, 106, 1943–1948. [Google Scholar] [CrossRef]
- Gonzales, E.; Hardikar, W.; Stormon, M.; Baker, A.; Hierro, L.; Gliwicz, D.; Lacaille, F.; Lachaux, A.; Sturm, E.; Setchell, K.D.R.; et al. Efficacy and safety of maralixibat treatment in patients with Alagille syndrome and cholestatic pruritus (ICONIC): A randomised phase 2 study. Lancet 2021, 398, 1581–1592. [Google Scholar] [CrossRef]
- Shneider, B.L.; Spino, C.A.; Kamath, B.M.; Magee, J.C.; Ignacio, R.V.; Huang, S.; Horslen, S.P.; Molleston, J.P.; Miethke, A.G.; Kohli, R.; et al. Impact of long-term administration of maralixibat on children with cholestasis secondary to Alagille syndrome. Hepatol. Commun. 2022, 6, 1922–1933. [Google Scholar] [CrossRef] [PubMed]
- Shneider, B.L.; Spino, C.; Kamath, B.M.; Magee, J.C.; Bass, L.M.; Setchell, K.D.; Miethke, A.; Molleston, J.P.; Mack, C.L.; Squires, R.H.; et al. Placebo-Controlled Randomized Trial of an Intestinal Bile Salt Transport Inhibitor for Pruritus in Alagille Syndrome. Hepatol. Commun. 2018, 2, 1184–1198. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.J.; Artan, R.; Baumann, U.; Calvo, P.L.; Czubkowski, P.; Dalgic, B.; D’Antiga, L.; Di Giorgio, A.; Durmaz, O.; Gonzales, E.; et al. Interim results from an ongoing, open-label, single-arm trial of odevixibat in progressive familial intrahepatic cholestasis. JHEP Rep. 2023, 5, 100782. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Odevixibat: First Approval. Drugs 2021, 81, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- Malinen, M.M.; Kauttonen, A.; Beaudoin, J.J.; Sjostedt, N.; Honkakoski, P.; Brouwer, K.L.R. Novel in Vitro Method Reveals Drugs That Inhibit Organic Solute Transporter Alpha/Beta (OSTalpha/beta). Mol. Pharm. 2019, 16, 238–246. [Google Scholar] [CrossRef] [PubMed]
- van de Wiel, S.M.W.; de Waart, D.R.; Oude Elferink, R.P.J.; van de Graaf, S.F.J. Intestinal Farnesoid X Receptor Activation by Pharmacologic Inhibition of the Organic Solute Transporter alpha-beta. Cell Mol. Gastroenterol. Hepatol. 2018, 5, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Sprowl, J.A.; Ong, S.S.; Gibson, A.A.; Hu, S.; Du, G.; Lin, W.; Li, L.; Bharill, S.; Ness, R.A.; Stecula, A.; et al. A phosphotyrosine switch regulates organic cation transporters. Nat. Commun. 2016, 7, 10880. [Google Scholar] [CrossRef]
- Reidling, J.C.; Lambrecht, N.; Kassir, M.; Said, H.M. Impaired intestinal vitamin B1 (thiamin) uptake in thiamin transporter-2-deficient mice. Gastroenterology 2010, 138, 1802–1809. [Google Scholar] [CrossRef]
- Marce-Grau, A.; Marti-Sanchez, L.; Baide-Mairena, H.; Ortigoza-Escobar, J.D.; Perez-Duenas, B. Genetic defects of thiamine transport and metabolism: A review of clinical phenotypes, genetics, and functional studies. J. Inherit. Metab. Dis. 2019, 42, 581–597. [Google Scholar] [CrossRef]
- Ott, M.; Werneke, U. Wernicke’s encephalopathy—From basic science to clinical practice. Part 1: Understanding the role of thiamine. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320978106. [Google Scholar] [CrossRef]
- Salojin, K.V.; Cabrera, R.M.; Sun, W.; Chang, W.C.; Lin, C.; Duncan, L.; Platt, K.A.; Read, R.; Vogel, P.; Liu, Q.; et al. A mouse model of hereditary folate malabsorption: Deletion of the PCFT gene leads to systemic folate deficiency. Blood 2011, 117, 4895–4904. [Google Scholar] [CrossRef]
- Zhao, R.; Min, S.H.; Qiu, A.; Sakaris, A.; Goldberg, G.L.; Sandoval, C.; Malatack, J.J.; Rosenblatt, D.S.; Goldman, I.D. The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood 2007, 110, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Goldman, I.D. The proton-coupled folate transporter: Physiological and pharmacological roles. Curr. Opin. Pharmacol. 2013, 13, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, C.R. Is folate absorption impaired by high dose methotrexate? Br. J. Cancer 1983, 47, 303–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Urquhart, B.L.; Gregor, J.C.; Chande, N.; Knauer, M.J.; Tirona, R.G.; Kim, R.B. The human proton-coupled folate transporter (hPCFT): Modulation of intestinal expression and function by drugs. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G248–G254. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; Saksena, S.; Alrefai, W.A.; Sarwar, Z.; Goldstein, J.L.; Carroll, R.E.; Ramaswamy, K.; Dudeja, P.K. Expression and membrane localization of MCT isoforms along the length of the human intestine. Am. J. Physiol. Cell Physiol. 2005, 289, C846–C852. [Google Scholar] [CrossRef] [PubMed]
- Payen, V.L.; Mina, E.; Van Hee, V.F.; Porporato, P.E.; Sonveaux, P. Monocarboxylate transporters in cancer. Mol. Metab. 2020, 33, 48–66. [Google Scholar] [CrossRef]
- van Hasselt, P.M.; Ferdinandusse, S.; Monroe, G.R.; Ruiter, J.P.; Turkenburg, M.; Geerlings, M.J.; Duran, K.; Harakalova, M.; van der Zwaag, B.; Monavari, A.A.; et al. Monocarboxylate transporter 1 deficiency and ketone utilization. N. Engl. J. Med. 2014, 371, 1900–1907. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Zhao, J.; Bai, M.; Lin, Y.; Chu, Q.; Gong, J.; Qiu, J.; Chen, Y. Intestinal monocarboxylate transporter 1 mediates lactate transport in the gut and regulates metabolic homeostasis of mouse in a sex-dimorphic pattern. Life Metab. 2023, 3, load041. [Google Scholar] [CrossRef]
- Altmann, S.W.; Davis, H.R., Jr.; Zhu, L.J.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 2004, 303, 1201–1204. [Google Scholar] [CrossRef]
- Fahmi, S.; Yang, C.; Esmail, S.; Hobbs, H.H.; Cohen, J.C. Functional characterization of genetic variants in NPC1L1 supports the sequencing extremes strategy to identify complex trait genes. Hum. Mol. Genet. 2008, 17, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Calvo, M.; Lisnock, J.; Bull, H.G.; Hawes, B.E.; Burnett, D.A.; Braun, M.P.; Crona, J.H.; Davis, H.R., Jr.; Dean, D.C.; Detmers, P.A.; et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc. Natl. Acad. Sci. USA 2005, 102, 8132–8137. [Google Scholar] [CrossRef] [PubMed]
- Strilchuk, L.; Tocci, G.; Fogacci, F.; Cicero, A.F.G. An overview of rosuvastatin/ezetimibe association for the treatment of hypercholesterolemia and mixed dyslipidemia. Expert. Opin. Pharmacother. 2020, 21, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Simbrunner, B.; Trauner, M.; Reiberger, T. Review article: Therapeutic aspects of bile acid signalling in the gut-liver axis. Aliment. Pharmacol. Ther. 2021, 54, 1243–1262. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Haywood, J.; Craddock, A.L.; Wilson, M.; Tietjen, M.; Kluckman, K.; Maeda, N.; Parks, J.S. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J. Biol. Chem. 2003, 278, 33920–33927. [Google Scholar] [CrossRef]
- Rao, A.; Haywood, J.; Craddock, A.L.; Belinsky, M.G.; Kruh, G.D.; Dawson, P.A. The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc. Natl. Acad. Sci. USA 2008, 105, 3891–3896. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Fantin, A.C.; Scheurer, U.; Fried, M.; Kullak-Ublick, G.A. Human ileal bile acid transporter gene ASBT (SLC10A2) is transactivated by the glucocorticoid receptor. Gut 2004, 53, 78–84. [Google Scholar] [CrossRef]
- Gao, E.; Cheema, H.; Waheed, N.; Mushtaq, I.; Erden, N.; Nelson-Williams, C.; Jain, D.; Soroka, C.J.; Boyer, J.L.; Khalil, Y.; et al. Organic Solute Transporter Alpha Deficiency: A Disorder with Cholestasis, Liver Fibrosis, and Congenital Diarrhea. Hepatology 2020, 71, 1879–1882. [Google Scholar] [CrossRef]
- Li, M.; Wang, Q.; Li, Y.; Cao, S.; Zhang, Y.; Wang, Z.; Liu, G.; Li, J.; Gu, B. Apical sodium-dependent bile acid transporter, drug target for bile acid related diseases and delivery target for prodrugs: Current and future challenges. Pharmacol. Ther. 2020, 212, 107539. [Google Scholar] [CrossRef]
- Lu, Z.N.; He, H.W.; Zhang, N. Advances in understanding the regulatory mechanism of organic solute transporter alpha-beta. Life Sci. 2022, 310, 121109. [Google Scholar] [CrossRef]
- Viennois, E.; Pujada, A.; Sung, J.; Yang, C.; Gewirtz, A.T.; Chassaing, B.; Merlin, D. Impact of PepT1 deletion on microbiota composition and colitis requires multiple generations. NPJ Biofilms Microbiomes 2020, 6, 27. [Google Scholar] [CrossRef]
- Wilk, J.N.; Bilsborough, J.; Viney, J.L. The mdr1a−/− mouse model of spontaneous colitis: A relevant and appropriate animal model to study inflammatory bowel disease. Immunol. Res. 2005, 31, 151–159. [Google Scholar] [CrossRef]
| Transporter | Membrane Localization within Enterocytes | Measure of Expression * | Adipose Tissue | Adrenal Gland | Appendix | ||||
|---|---|---|---|---|---|---|---|---|---|
| SGLT1 (SLC5A1) | Apical [11] | mRNA [12] | Low | ND | Low | ||||
| Protein [13] | ND | ND | ND | ||||||
| THTR2 (SLC19A3) | Apical [14] | mRNA [12] | High | Low | Low | ||||
| Protein [13] | ND | Low | Low | ||||||
| PCFT (SLC46A1) | Apical [15] | mRNA [12] | Low | High | Low | ||||
| Protein [13] | ND | Low | Low | ||||||
| MCT1 (SLC16A1) | Apical [16] | mRNA [12] | Low | Medium | High | ||||
| Protein [13] | ND | Medium | High | ||||||
| NPC1L1 (SLC65A2) | Apical [17] | mRNA [12] | Low | Low | Low | ||||
| Protein [13] | ND | ND | Medium | ||||||
| OSTα (SLC51A) | Basolateral [18] | mRNA [12] | Low | Medium | Low | ||||
| Protein [13] | ND | ND | ND | ||||||
| OSTβ (SLC51B) | Basolateral [18] | mRNA [12] | Low | Low | Low | ||||
| Protein [13] | ND | ND | High | ||||||
| ASBT (SLC10A2) | Apical [18] | mRNA [12] | Low | Low | ND | ||||
| Protein [13] | ND | ND | ND | ||||||
| Bone Marrow | Brain | Colon | Duodenum | Endometrium | Esophagus | Gall Bladder | Heart | Kidney | Liver |
| Low | Low | Medium | High | Low | Low | High | High | Low | Low |
| ND | ND | ND | High | ND | ND | Low | ND | Medium | ND |
| Low | Low | Low | High | Low | Low | Medium | Low | Low | Medium |
| Low | Medium | Medium | Medium | Low | Medium | Medium | Medium | Medium | Medium |
| Low | Low | Low | High | Low | Low | Low | Low | Low | Low |
| ND | ND | Medium | Low | ND | ND | Low | Low | ND | ND |
| Low | High | High | High | High | Medium | High | High | Low | High |
| Low | Low | High | High | High | Medium | Medium | Medium | Medium | Medium |
| Low | Low | Low | High | Low | Low | Low | Low | Low | High |
| ND | ND | Low | High | ND | ND | Low | ND | Low | Medium |
| Medium | Low | High | High | Low | Low | Low | Low | Medium | High |
| ND | ND | ND | High | ND | ND | ND | ND | High | ND |
| ND | Low | High | High | Low | Low | Low | Low | Medium | Low |
| ND | ND | High | High | ND | ND | ND | ND | Medium | ND |
| ND | ND | Low | High | Low | ND | Low | ND | Medium | Low |
| ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Lung | Lymph Node | Ovary | Pancreas | Placenta | Prostate Gland | Salivary Gland | Skin | Small Intestine | |
| Low | Low | Low | Low | Low | Low | Low | Low | High | |
| ND | ND | ND | ND | ND | ND | ND | ND | High | |
| Low | Low | Low | Low | High | Low | Low | Low | Medium | |
| Low | ND | ND | Medium | High | ND | Medium | Low | Low | |
| Low | Low | Low | Low | Low | Medium | Medium | Low | High | |
| Low | ND | ND | Low | Low | ND | ND | ND | Low | |
| Low | Low | Low | Low | High | Medium | Low | Medium | High | |
| Medium | Medium | ND | ND | Medium | High | ND | Medium | Medium | |
| Low | Low | Low | Low | Low | Low | Low | Low | High | |
| ND | ND | ND | ND | ND | ND | ND | ND | High | |
| Low | Low | Low | Low | Low | Low | Low | Low | High | |
| ND | ND | ND | ND | ND | ND | ND | ND | High | |
| Low | Low | Low | ND | Low | Low | Low | Low | High | |
| ND | ND | ND | ND | ND | ND | ND | ND | High | |
| Low | Low | ND | ND | ND | ND | ND | Low | High | |
| ND | ND | ND | ND | ND | ND | ND | ND | High | |
| Spleen | Stomach | Testis | Thyroid Gland | Urinary Bladder | |||||
| Low | Low | Low | Low | Low | |||||
| ND | ND | ND | ND | ND | |||||
| Low | Low | Low | Low | Low | |||||
| ND | Medium | Medium | Medium | ND | |||||
| Medium | Low | Low | Low | Low | |||||
| ND | Low | High | Low | ND | |||||
| Low | High | High | Medium | Medium | |||||
| Low | High | High | ND | Low | |||||
| Low | Low | Low | Low | Low | |||||
| ND | ND | ND | ND | ND | |||||
| Low | Low | High | Low | Low | |||||
| ND | ND | ND | ND | ND | |||||
| Low | Low | Low | Low | Low | |||||
| ND | Medium | High | ND | ND | |||||
| ND | Low | ND | ND | ND | |||||
| ND | ND | ND | ND | ND | |||||
| Nutrient/Endogenous Substrate and Transport Process | Inhibitor (In Vitro IC50) | Level of Evidence | Reference(s) |
|---|---|---|---|
| Glucose uptake by SGLT1 | Sotagliflozin (0.036 μM)—direct inhibitor | Reduced plasma glucose concentration in patients | [21,22] |
| Erlotinib * (NA)—indirect inhibitor | Reduced glucose uptake in A549, MCF10A, H322, or H292 cells | [25,26,27] | |
| Lapatinib * (NA)—indirect inhibitor | Reduced glucose uptake in A549 or MCF10A2 cells | [26] | |
| Sorafenib * (NA)—indirect inhibitor | Reduced plasma glucose concentration in patients | [28] | |
| Dasatinib * (NA)—indirect inhibitor | Reduced plasma glucose concentration in patients | [28] | |
| Sunitinib * (NA)—indirect inhibitor | Reduced plasma glucose concentration in patients | [28] | |
| Imatinib * (NA)—indirect inhibitor | Reduced plasma glucose concentration in patients | [28] | |
| Thiamine uptake by THTR2 | Fedratinib (0.94–1.36 μM)—direct inhibitor | Onset of Wernicke’s encephalopathy in patients | |
| Reduced thiamine uptake in Caco-2 and THTR2-overexpressing HEK293 cells | [29,30,31] | ||
| Trimethoprim (5.6 μM)—direct inhibitor | Increased plasma thiamine concentration in patients | [32] | |
| Reduced thiamine uptake in Caco-2 and THTR2-overexpressing HEK293 cells | [30] | ||
| Metformin (680 μM)—direct inhibitor | Reduced thiamine uptake in THTR2-overexpressing HEK293 cells | [31] | |
| Hydroxychloroquine (17 μM)—unknown if direct/indirect inhibitor | Reduced thiamine uptake in THTR2-overexpressing HEK293 cells | [31] | |
| Verapamil (141 μM)—unknown if direct/indirect inhibitor | Reduced thiamine uptake in THTR2-overexpressing HEK293 cells | [31] | |
| Folate uptake by PCFT | Sulfasalazine (60 μM)—direct inhibitor | Reduced folate and methotrexate uptake in PCFT-overexpressing oocytes | [33] |
| Lactate uptake by MCT1 | Phloretin (NA)—direct inhibitor | Reduced lactate uptake in MCT1-overexpressing oocytes | [16,34] |
| Gabapentin enacarbil/XP-13512 (0.62 μM)—direct inhibitor | Reduced lactate uptake in Caco-2 cells and MCT1-overexpressing HEK293 cells and oocytes | [34,35] | |
| Quercetin (NA)—direct inhibitor | Reduced lactate uptake in MCT1-overexpressing oocytes | [16,34] | |
| AR-C155858 (NA)—direct inhibitor | Reduced lactate uptake in MCT1-overexpressing oocytes | [16] | |
| ADZ3965 (17 nM)—direct inhibitor | Metabolic acidosis risk; increased urinary elimination of lactate and ketone; no changes in lactate plasma concentrations in patients | [36,37] | |
| Cholesterol uptake by NPC1L1 | Ezetimibe (24 μM)—direct inhibitor | Reduced cholesterol uptake in NPC1L1-overexpressing MDCKII cells | [38] |
| Reduced dietary cholesterol absorption in patients | [39] | ||
| Bile acid transport by ASBT | Maralixibat (0.3 nM)—direct inhibitor | Reduced serum bile acid concentrations in patients | [40,41,42] |
| Odevixibat (0.10 nM)—direct inhibitor | Reduced serum bile acid concentrations in patients | [43,44] | |
| Elobixibat (0.53 nM)—direct inhibitor | Reduced complete spontaneous bowel movements per week in patients | NCT01007123 | |
| Bile acid transport by OSTα/β | Atorvastatin (NA)—unknown if direct/indirect inhibitor | Reduced dehydroepiandrosterone sulfate in OSTα/β-overexpressing HEK293 cells | [45] |
| Ethinylestradiol (53 μM)—unknown if direct/indirect inhibitor | Reduced dehydroepiandrosterone sulfate in OSTα/β-overexpressing HEK293 cells | [45] | |
| Fidaxomicin (169 μM)—unknown if direct/indirect inhibitor | Reduced dehydroepiandrosterone sulfate in OSTα/β-overexpressing HEK293 cells | [45] | |
| Indomethacin (NA)—unknown if direct/indirect inhibitor | Reduced dehydroepiandrosterone sulfate in OSTα/β-overexpressing HEK293 cells | [45] | |
| Spironolactone (NA)—unknown if direct/indirect inhibitor | Reduced dehydroepiandrosterone sulfate in OSTα/β-overexpressing HEK293 cells | [45] | |
| Troglitazone (NA)—unknown if direct/indirect inhibitor | Reduced dehydroepiandrosterone sulfate in OSTα/β-overexpressing HEK293 cells | [45] | |
| Clofazimine (30–50 μM)—unknown if direct/indirect inhibitor | Reduced taurocholic acid transport across OSTα/β-overexpressing MDCK cells | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharve, K.; Engley, A.S.; Paine, M.F.; Sprowl, J.A. Impact of Drug-Mediated Inhibition of Intestinal Transporters on Nutrient and Endogenous Substrate Disposition…an Afterthought? Pharmaceutics 2024, 16, 447. https://doi.org/10.3390/pharmaceutics16040447
Kharve K, Engley AS, Paine MF, Sprowl JA. Impact of Drug-Mediated Inhibition of Intestinal Transporters on Nutrient and Endogenous Substrate Disposition…an Afterthought? Pharmaceutics. 2024; 16(4):447. https://doi.org/10.3390/pharmaceutics16040447
Chicago/Turabian StyleKharve, Kshitee, Andrew S. Engley, Mary F. Paine, and Jason A. Sprowl. 2024. "Impact of Drug-Mediated Inhibition of Intestinal Transporters on Nutrient and Endogenous Substrate Disposition…an Afterthought?" Pharmaceutics 16, no. 4: 447. https://doi.org/10.3390/pharmaceutics16040447
APA StyleKharve, K., Engley, A. S., Paine, M. F., & Sprowl, J. A. (2024). Impact of Drug-Mediated Inhibition of Intestinal Transporters on Nutrient and Endogenous Substrate Disposition…an Afterthought? Pharmaceutics, 16(4), 447. https://doi.org/10.3390/pharmaceutics16040447





