Multiple Regulatory Signals and Components in the Modulation of Bicarbonate Transporters
Abstract
:1. Homeostatic Role of Bicarbonate Transporters
2. Modulatory Signals in the Regulation of Bicarbonate Transporters
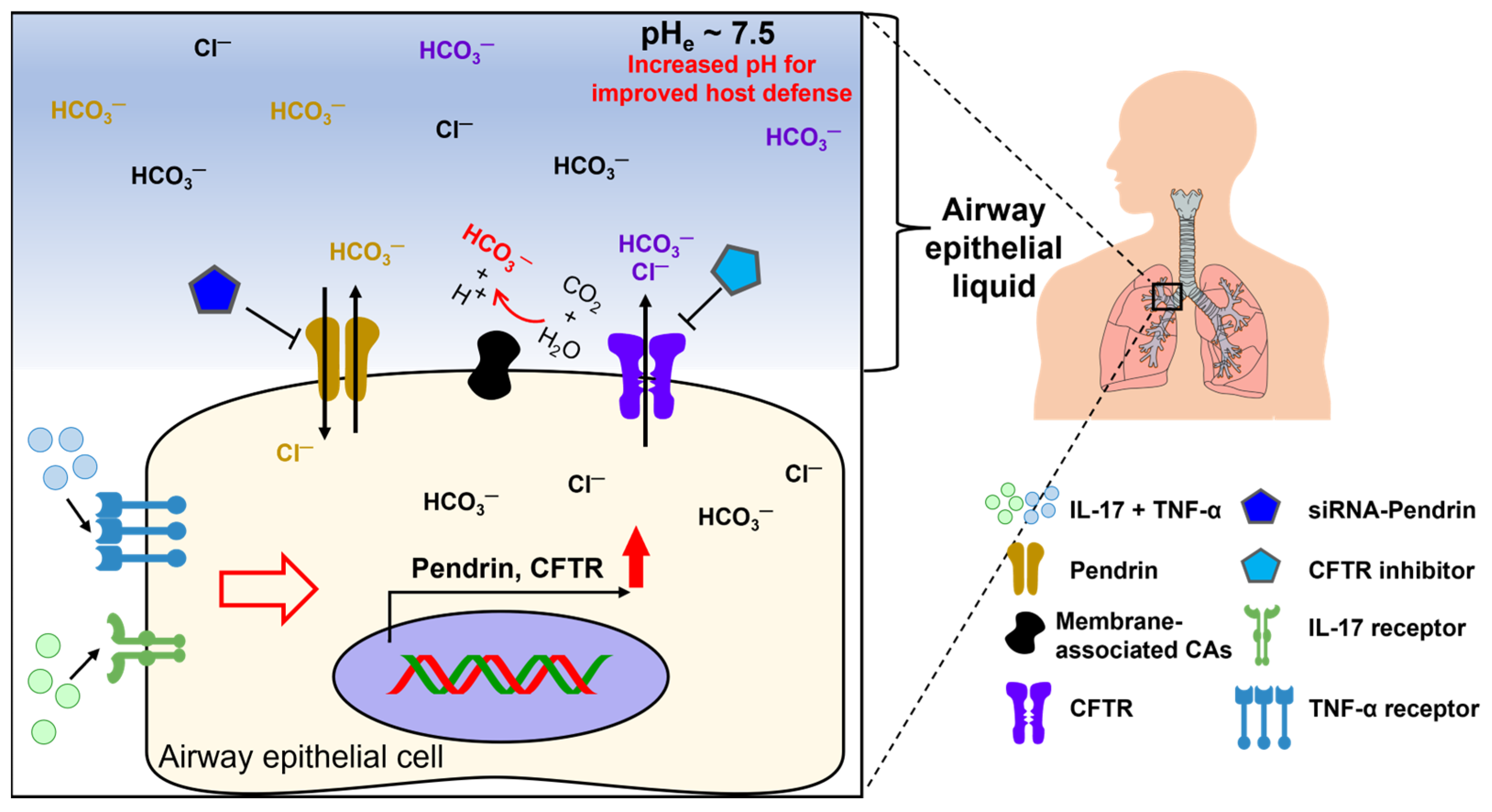
2.1. Inflammatory Disease-Associated Cytokines
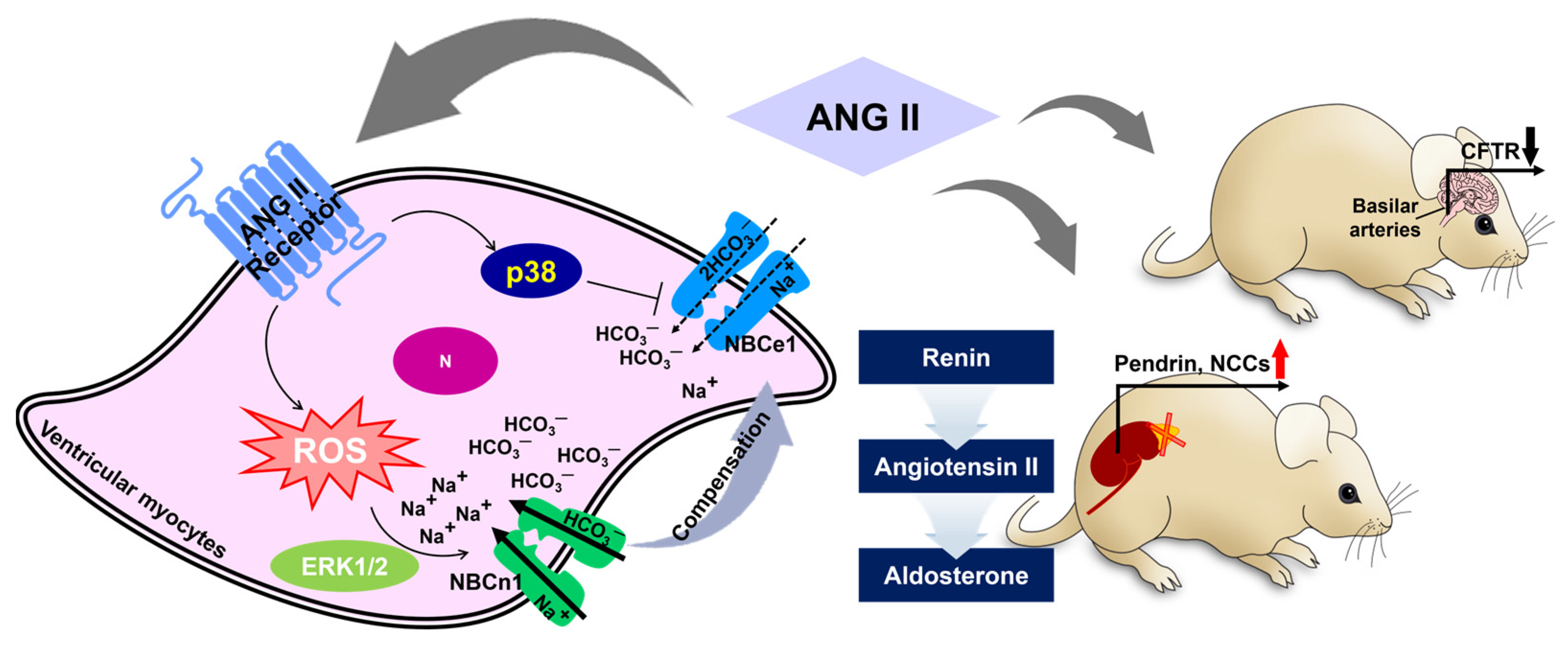
2.2. Angiotensin II-Mediated Modulation of Bicarbonate Transport
2.3. Regulation of Bicarbonate Transport by Endogenous Peptides
2.3.1. Neuropeptide Vasoactive Intestinal Peptide
2.3.2. Neuropeptide Y
2.4. Intracellular Calcium-Associated Modulation of Bicarbonate Transport
3. Host Defense Mechanisms
4. Bicarbonate-Associated Carbonic Anhydrases
5. Acidic Microenvironment and Proliferation
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borowitz, D. CFTR, bicarbonate, and the pathophysiology of cystic fibrosis. Pediatr. Pulmonol. 2015, 50 (Suppl. S40), S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Boron, W.F.; Fong, P.; Hediger, M.A.; Boulpaep, E.L.; Romero, M.F. The electrogenic Na/HCO3 cotransporter. Wien. Klin. Wochenschr. 1997, 109, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Kopito, R.R. Molecular biology of the anion exchanger gene family. Int. Rev. Cytol. 1990, 123, 177–199. [Google Scholar] [CrossRef] [PubMed]
- Alper, S.L.; Sharma, A.K. The SLC26 gene family of anion transporters and channels. Mol. Aspects Med. 2013, 34, 494–515. [Google Scholar] [CrossRef]
- Yang, O.C.Y.; Loh, S.H. Acidic Stress Triggers Sodium-Coupled Bicarbonate Transport and Promotes Survival in A375 Human Melanoma Cells. Sci. Rep. 2019, 9, 6858. [Google Scholar] [CrossRef]
- Hall, J.E.; Guyton, A.C. Guyton and Hall Textbook of Medical Physiology, 12th ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2011; 1091p. [Google Scholar]
- Cordat, E.; Casey, J.R. Bicarbonate transport in cell physiology and disease. Biochem. J. 2009, 417, 423–439. [Google Scholar] [CrossRef]
- Hamm, L.L.; Nakhoul, N.; Hering-Smith, K.S. Acid-Base Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 2232–2242. [Google Scholar] [CrossRef]
- Wang, H.; An, J.X.; Jin, H.; He, S.Y.; Liao, C.C.; Wang, J.; Tuo, B.G. Roles of Cl−/HCO3− anion exchanger 2 in the physiology and pathophysiology of the digestive system (Review). Mol. Med. Rep. 2021, 24, 491. [Google Scholar] [CrossRef]
- Martinez-Crespo, L.; Valkenier, H. Transmembrane Transport of Bicarbonate by Anion Receptors. Chempluschem 2022, 87, e202200266. [Google Scholar] [CrossRef]
- Boedtkjer, E. Ion Channels, Transporters, and Sensors Interact with the Acidic Tumor Microenvironment to Modify Cancer Progression. Rev. Physiol. Biochem. Pharmacol. 2022, 182, 39–84. [Google Scholar] [CrossRef]
- Kreindler, J.L.; Bertrand, C.A.; Lee, R.J.; Karasic, T.; Aujla, S.; Pilewski, J.M.; Frizzell, R.A.; Kolls, J.K. Interleukin-17A induces bicarbonate secretion in normal human bronchial epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L257–L266. [Google Scholar] [CrossRef] [PubMed]
- Gorrieri, G.; Scudieri, P.; Caci, E.; Schiavon, M.; Tomati, V.; Sirci, F.; Napolitano, F.; Carrella, D.; Gianotti, A.; Musante, I.; et al. Goblet Cell Hyperplasia Requires High Bicarbonate Transport To Support Mucin Release. Sci. Rep. 2016, 6, 36016. [Google Scholar] [CrossRef] [PubMed]
- Rehman, T.; Thornell, I.M.; Pezzulo, A.A.; Thurman, A.L.; Ibarra, G.S.R.; Karp, P.H.; Tan, P.; Duffey, M.E.; Welsh, M.J. TNF alpha and IL-17 alkalinize airway surface liquid through CFTR and pendrin. Am. J. Physiol.-Cell Physiol. 2020, 319, C331–C344. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jiang, W.; Furth, E.E.; Wen, X.; Katz, J.P.; Sellon, R.K.; Silberg, D.G.; Antalis, T.M.; Schweinfest, C.W.; Wu, G.D. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am. J. Physiol. 1998, 275, G1445–G1453. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Juric, M.; Li, J.; Riederer, B.; Yeruva, S.; Singh, A.K.; Zheng, L.; Glage, S.; Kollias, G.; Dudeja, P.; et al. Loss of downregulated in adenoma (DRA) impairs mucosal HCO3− secretion in murine ileocolonic inflammation. Inflamm. Bowel Dis. 2012, 18, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- Kumar, A.; Chatterjee, I.; Gujral, T.; Alakkam, A.; Coffing, H.; Anbazhagan, A.N.; Borthakur, A.; Saksena, S.; Gill, R.K.; Alrefai, W.A.; et al. Activation of Nuclear Factor-κB by Tumor Necrosis Factor in Intestinal Epithelial Cells and Mouse Intestinal Epithelia Reduces Expression of the Chloride Transporter SLC26A3. Gastroenterology 2017, 153, 1338–1350.e3. [Google Scholar] [CrossRef]
- Schweinfest, C.W.; Spyropoulos, D.D.; Henderson, K.W.; Kim, J.H.; Chapman, J.M.; Barone, S.; Worrell, R.T.; Wang, Z.; Soleimani, M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J. Biol. Chem. 2006, 281, 37962–37971. [Google Scholar] [CrossRef]
- Ding, X.M.; Li, D.X.; Li, M.K.; Tian, D.; Yu, H.B.; Yu, Q. Tumor necrosis factor-α acts reciprocally with solute carrier family 26, member 3, (downregulated-in-adenoma) and reduces its expression, leading to intestinal inflammation. Int. J. Mol. Med. 2018, 41, 1224–1232. [Google Scholar] [CrossRef]
- Sasaki, M.; Sato, Y.; Nakanuma, Y. An impaired biliary bicarbonate umbrella may be involved in dysregulated autophagy in primary biliary cholangitis. Lab. Investig. 2018, 98, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Erice, O.; Munoz-Garrido, P.; Vaquero, J.; Perugorria, M.J.; Fernandez-Barrena, M.G.; Saez, E.; Santos-Laso, A.; Arbelaiz, A.; Jimenez-Aguero, R.; Fernandez-Irigoyen, J.; et al. MicroRNA-506 promotes primary biliary cholangitis-like features in cholangiocytes and immune activation. Hepatology 2018, 67, 1420–1440. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C.; Go, S.; de Waart, D.R.; Munoz-Garrido, P.; Beuers, U.; Paulusma, C.C.; Oude Elferink, R. Soluble Adenylyl Cyclase Regulates Bile Salt-Induced Apoptosis in Human Cholangiocytes. Hepatology 2016, 64, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.M.; Abraham, V.; Spielman, D.; Kolls, J.K.; Rubenstein, R.C.; Conner, G.E.; Cohen, N.A.; Kreindler, J.L. IL-17A induces Pendrin expression and chloride-bicarbonate exchange in human bronchial epithelial cells. PLoS ONE 2014, 9, e103263. [Google Scholar] [CrossRef] [PubMed]
- Wheat, V.J.; Shumaker, H.; Burnham, C.; Shull, G.E.; Yankaskas, J.R.; Soleimani, M. CFTR induces the expression of DRA along with Cl−/HCO3− exchange activity in tracheal epithelial cells. Am. J. Physiol. Cell Physiol. 2000, 279, C62–C71. [Google Scholar] [CrossRef]
- Poulsen, J.H.; Fischer, H.; Illek, B.; Machen, T.E. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc. Natl. Acad. Sci. USA 1994, 91, 5340–5344. [Google Scholar] [CrossRef]
- Garnett, J.P.; Hickman, E.; Burrows, R.; Hegyi, P.; Tiszlavicz, L.; Cuthbert, A.W.; Fong, P.; Gray, M.A. Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells. J. Biol. Chem. 2011, 286, 41069–41082. [Google Scholar] [CrossRef]
- Rehman, T.; Karp, P.H.; Tan, P.; Goodell, B.J.; Pezzulo, A.A.; Thurman, A.L.; Thornell, I.M.; Durfey, S.L.; Duffey, M.E.; Stoltz, D.A.; et al. Inflammatory cytokines TNF-α and IL-17 enhance the efficacy of cystic fibrosis transmembrane conductance regulator modulators. J. Clin. Investig. 2021, 131, e150398. [Google Scholar] [CrossRef]
- Geibel, J.; Giebisch, G.; Boron, W.F. Angiotensin II stimulates both Na(+)-H+ exchange and Na+/HCO3− cotransport in the rabbit proximal tubule. Proc. Natl. Acad. Sci. USA 1990, 87, 7917–7920. [Google Scholar] [CrossRef]
- Romero, M.F.; Hopfer, U.; Madhun, Z.T.; Zhou, W.; Douglas, J.G. Angiotensin II actions in the rabbit proximal tubule. Angiotensin II mediated signaling mechanisms and electrolyte transport in the rabbit proximal tubule. Ren. Physiol. Biochem. 1991, 14, 199–207. [Google Scholar] [CrossRef]
- Coppola, S.; Fromter, E. An electrophysiological study of angiotensin II regulation of Na-HCO3 cotransport and K conductance in renal proximal tubules. I. Effect of picomolar concentrations. Pflugers Arch. 1994, 427, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Giebisch, G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am. J. Physiol. 1996, 271, F143–F149. [Google Scholar] [CrossRef] [PubMed]
- Kohout, T.A.; Rogers, T.B. Angiotensin-Ii Activates the Na+/HCO3- Symport through a Phosphoinositide-Independent Mechanism in Cardiac-Cells. J. Biol. Chem. 1995, 270, 20432–20438. [Google Scholar] [CrossRef] [PubMed]
- Skelton, L.A.; Boron, W.F.; Zhou, Y. Acid-base transport by the renal proximal tubule. J. Nephrol. 2010, 23 (Suppl. S16), S4–S18. [Google Scholar] [PubMed]
- Romero, M.F.; Chen, A.P.; Parker, M.D.; Boron, W.F. The SLC4 family of bicarbonate (HCO3−) transporters. Mol. Aspects Med. 2013, 34, 159–182. [Google Scholar] [CrossRef]
- Horita, S.; Zheng, Y.; Hara, C.; Yamada, H.; Kunimi, M.; Taniguchi, S.; Uwatoko, S.; Sugaya, T.; Goto, A.; Fujita, T.; et al. Biphasic regulation of Na+-HCO3− cotransporter by angiotensin II type 1A receptor. Hypertension 2002, 40, 707–712. [Google Scholar] [CrossRef]
- Baetz, D.; Haworth, R.S.; Avkiran, M.; Feuvray, D. The ERK pathway regulates Na+-HCO3− cotransport activity in adult rat cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2102–H2109. [Google Scholar] [CrossRef]
- De Giusti, V.C.; Garciarena, C.D.; Aiello, E.A. Role of reactive oxygen species (ROS) in angiotensin II-induced stimulation of the cardiac Na+/HCO3− cotransport. J. Mol. Cell. Cardiol. 2009, 47, 716–722. [Google Scholar] [CrossRef]
- Orlowski, A.; Ciancio, M.C.; Caldiz, C.I.; De Giusti, V.C.; Aiello, E.A. Reduced sarcolemmal expression and function of the NBCe1 isoform of the NaHCO3− cotransporter in hypertrophied cardiomyocytes of spontaneously hypertensive rats: Role of the reninangiotensin system. Cardiovasc. Res. 2014, 101, 211–219. [Google Scholar] [CrossRef]
- De Giusti, V.C.; Orlowski, A.; Aiello, E.A. Angiotensin II inhibits the electrogenic Na+/HCO3− cotransport of cat cardiac myocytes. J. Mol. Cell. Cardiol. 2010, 49, 812–818. [Google Scholar] [CrossRef]
- Bhullar, S.K.; Dhalla, N.S. Angiotensin II-Induced Signal Transduction Mechanisms for Cardiac Hypertrophy. Cells 2022, 11, 3336. [Google Scholar] [CrossRef] [PubMed]
- Camilion de Hurtado, M.C.; Alvarez, B.V.; Perez, N.G.; Ennis, I.L.; Cingolani, H.E. Angiotensin II activates Na+-independent Cl−-HCO3− exchange in ventricular myocardium. Circ. Res. 1998, 82, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, B.V.; Fujinaga, J.; Casey, J.R. Molecular basis for angiotensin II-induced increase of chloride/bicarbonate exchange in the myocardium. Circ. Res. 2001, 89, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, N.; Perna, A.; van der Wijst, J.; Becker, H.M.; Capasso, G.; Wagner, C.A. Regulation of two renal chloride transporters, AE1 and pendrin, by electrolytes and aldosterone. PLoS ONE 2013, 8, e55286. [Google Scholar] [CrossRef]
- Hirohama, D.; Ayuzawa, N.; Ueda, K.; Nishimoto, M.; Kawarazaki, W.; Watanabe, A.; Shimosawa, T.; Marumo, T.; Shibata, S.; Fujita, T. Aldosterone Is Essential for Angiotensin II-Induced Upregulation of Pendrin. J. Am. Soc. Nephrol. 2018, 29, 57–68. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, F.; Pan, N.; Yu, Y.; Yang, H.; Liu, Y.; Wang, R.; Zhang, B.; Wang, G. CFTR deficiency aggravates Ang II induced vasoconstriction and hypertension by regulating Ca2+ influx and RhoA/Rock pathway in VSMCs. Front. Biosci. 2021, 26, 1396–1410. [Google Scholar] [CrossRef]
- Henning, R.J.; Sawmiller, D.R. Vasoactive intestinal peptide: Cardiovascular effects. Cardiovasc. Res. 2001, 49, 27–37. [Google Scholar] [CrossRef]
- Iwasaki, M.; Akiba, Y.; Kaunitz, J.D. Recent advances in vasoactive intestinal peptide physiology and pathophysiology: Focus on the gastrointestinal system. F1000Research 2019, 8, 1629. [Google Scholar] [CrossRef]
- Leceta, J.; Gomariz, R.P.; Martinez, C.; Carrion, M.; Arranz, A.; Juarranz, Y. Vasoactive intestinal peptide regulates Th17 function in autoimmune inflammation. Neuroimmunomodulation 2007, 14, 134–138. [Google Scholar] [CrossRef]
- Hogan, D.L.; Crombie, D.L.; Isenberg, J.I.; Svendsen, P.; Schaffalitzky de Muckadell, O.B.; Ainsworth, M.A. Acid-stimulated duodenal bicarbonate secretion involves a CFTR-mediated transport pathway in mice. Gastroenterology 1997, 113, 533–541. [Google Scholar] [CrossRef]
- Wine, J.J.; Joo, N.S. Submucosal glands and airway defense. Proc. Am. Thorac. Soc. 2004, 1, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Maggi, C.A.; Giachetti, A.; Dey, R.D.; Said, S.I. Neuropeptides as Regulators of Airway Function—Vasoactive-Intestinal-Peptide and the Tachykinins. Physiol. Rev. 1995, 75, 277–322. [Google Scholar] [CrossRef] [PubMed]
- Derand, R.; Montoni, A.; Bulteau-Pignoux, L.; Janet, T.; Moreau, B.; Muller, J.M.; Becq, F. Activation of VPAC1 receptors by VIP and PACAP-27 in human bronchial epithelial cells induces CFTR-dependent chloride secretion. Br. J. Pharmacol. 2004, 141, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Laburthe, M.; Couvineau, A.; Tan, V. Class II G protein-coupled receptors for VIP and PACAP: Structure, models of activation and pharmacology. Peptides 2007, 28, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Liu, H.J.; Xiang, Y.; Tan, Y.R.; Liu, C.; Zhu, X.L.; Qin, X.Q. Activation of CFTR trafficking and gating by vasoactive intestinal peptide in human bronchial epithelial cells. J. Cell. Biochem. 2011, 112, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Rumalla, K.; Yarbrough, C.K.; Pugely, A.J.; Koester, L.; Dorward, I.G. Spinal fusion for pediatric neuromuscular scoliosis: National trends, complications, and in-hospital outcomes. J. Neurosurg. Spine 2016, 25, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Ianowski, J.P.; Choi, J.Y.; Wine, J.J.; Hanrahan, J.W. Mucus secretion by single tracheal submucosal glands from normal and cystic fibrosis transmembrane conductance regulator knockout mice. J. Physiol. 2007, 580, 301–314. [Google Scholar] [CrossRef]
- Chappe, F.; Loewen, M.E.; Hanrahan, J.W.; Chappe, V. Vasoactive intestinal peptide increases cystic fibrosis transmembrane conductance regulator levels in the apical membrane of Calu-3 cells through a protein kinase C-dependent mechanism. J. Pharmacol. Exp. Ther. 2008, 327, 226–238. [Google Scholar] [CrossRef]
- Cantin, A.M.; Bilodeau, G.; Ouellet, C.; Liao, J.; Hanrahan, J.W. Oxidant stress suppresses CFTR expression. Am. J. Physiol. Cell Physiol. 2006, 290, C262–C270. [Google Scholar] [CrossRef]
- Cantin, A.M.; Hanrahan, J.W.; Bilodeau, G.; Ellis, L.; Dupuis, A.; Liao, J.; Zielenski, J.; Durie, P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am. J. Respir. Crit. Care 2006, 173, 1139–1144. [Google Scholar] [CrossRef]
- Qu, F.; Qin, X.Q.; Cui, Y.R.; Xiang, Y.; Tan, Y.R.; Liu, H.J.; Peng, L.H.; Zhou, X.Y.; Liu, C.; Zhu, X.L. Ozone stress down-regulates the expression of cystic fibrosis transmembrane conductance regulator in human bronchial epithelial cells. Chem. Biol. Interact. 2009, 179, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Alshafie, W.; Chappe, F.G.; Li, M.S.; Anini, Y.; Chappe, V.M. VIP regulates CFTR membrane expression and function in Calu-3 cells by increasing its interaction with NHERF1 and P-ERM in a VPAC1-and PKC ε-dependent manner. Am. J. Physiol.-Cell Physiol. 2014, 307, C107–C119. [Google Scholar] [CrossRef] [PubMed]
- Short, D.B.; Trotter, K.W.; Reczek, D.; Kreda, S.M.; Bretscher, A.; Boucher, R.C.; Stutts, M.J.; Milgram, S.L. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J. Biol. Chem. 1998, 273, 19797–19801. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Moyer, B.D.; Milewski, M.; Loffing, J.; Ikeda, M.; Mickle, J.E.; Cutting, G.R.; Li, M.; Stanton, B.A.; Guggino, W.B. A Golgi-associated PDZ domain protein modulates cystic fibrosis transmembrane regulator plasma membrane expression. J. Biol. Chem. 2002, 277, 3520–3529. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhu, X.; Yang, X.; Jin, L.; Xu, J.; Ma, T.; Yang, H. Plumbagin Prevents Secretory Diarrhea by Inhibiting CaCC and CFTR Channel Activities. Front. Pharmacol. 2019, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.B.; Carey, R.M.; Kohanski, M.A.; Tong, C.C.L.; Papagiannopoulos, P.; Adappa, N.D.; Palmer, J.N.; Lee, R.J. Neuropeptide regulation of secretion and inflammation in human airway gland serous cells. Eur. Respir. J. 2020, 55, 1901386. [Google Scholar] [CrossRef] [PubMed]
- Oak, A.A.; Chhetri, P.D.; Rivera, A.A.; Verkman, A.S.; Cil, O. Repurposing calcium-sensing receptor agonist cinacalcet for treatment of CFTR-mediated secretory diarrheas. JCI Insight 2021, 6, e146823. [Google Scholar] [CrossRef]
- Rodrat, M.; Wongdee, K.; Teerapornpuntakit, J.; Thongbunchoo, J.; Tanramluk, D.; Aeimlapa, R.; Thammayon, N.; Thonapan, N.; Wattano, P.; Charoenphandhu, N. Vasoactive intestinal peptide and cystic fibrosis transmembrane conductance regulator contribute to the transepithelial calcium transport across intestinal epithelium-like Caco-2 monolayer. PLoS ONE 2022, 17, e0277096. [Google Scholar] [CrossRef]
- Nichols, K.; Staines, W.; Krantis, A. Neural sites of the human colon colocalize nitric oxide synthase-related NADPH diaphorase activity and neuropeptide Y. Gastroenterology 1994, 107, 968–975. [Google Scholar] [CrossRef]
- Sandgren, K.; Larsson, L.T.; Ekblad, E. Widespread changes in neurotransmitter expression and number of enteric neurons and interstitial cells of Cajal in lethal spotted mice—An explanation for persisting dysmotility after operation for Hirschsprung’s disease? Dig. Dis. Sci. 2002, 47, 1049–1064. [Google Scholar] [CrossRef]
- Ekblad, E.; Ekman, R.; Hakanson, R.; Sundler, F. Projections of peptide-containing neurons in rat colon. Neuroscience 1988, 27, 655–674. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Reichmann, F.; Farzi, A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 2012, 46, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.P.; Adrian, T.E.; Carolan, G.; Chatterjee, V.K.; Bloom, S.R. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut 1987, 28, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Floyd, J.C., Jr.; Fajans, S.S.; Pek, S.; Chance, R.E. A newly recognized pancreatic polypeptide; plasma levels in health and disease. Recent. Prog. Horm. Res. 1976, 33, 519–570. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, K. Neuropeptide Y: Complete amino acid sequence of the brain peptide. Proc. Natl. Acad. Sci. USA 1982, 79, 5485–5489. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, K. Isolation and characterization of peptide YY (PYY), a candidate gut hormone that inhibits pancreatic exocrine secretion. Proc. Natl. Acad. Sci. USA 1982, 79, 2514–2518. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.M.J.; Green, P.; Tapoulal, N.; Lewandowski, A.J.; Leeson, P.; Herring, N. The Role of Neuropeptide Y in Cardiovascular Health and Disease. Front. Physiol. 2018, 9, 1281. [Google Scholar] [CrossRef] [PubMed]
- Saksena, S.; Tyagi, S.; Goyal, S.; Gill, R.K.; Alrefai, W.A.; Ramaswamy, K.; Dudeja, P.K. Stimulation of apical Cl−/HCO3−(OH−) exchanger, SLC26A3 by neuropeptide Y is lipid raft dependent. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 299, G1334–G1343. [Google Scholar] [CrossRef]
- Magnani, F.; Tate, C.G.; Wynne, S.; Williams, C.; Haase, J. Partitioning of the serotonin transporter into lipid microdomains modulates transport of serotonin. J. Biol. Chem. 2004, 279, 38770–38778. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Cell biology—How cells handle cholesterol. Science 2000, 290, 1721–1726. [Google Scholar] [CrossRef]
- Fang, S.Y.; Shen, C.L.; Ohyama, M. Presence of neuropeptides in human nasal polyps. Acta Otolaryngol. 1994, 114, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Makinde, T.O.; Steininger, R.; Agrawal, D.K. NPY and NPY receptors in airway structural and inflammatory cells in allergic asthma. Exp. Mol. Pathol. 2013, 94, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, B.; Nezami, B.G.; Srinivasan, S. Emerging neuropeptide targets in inflammation: NPY and VIP. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G949–G957. [Google Scholar] [CrossRef] [PubMed]
- Barry, W.H.; Bridge, J.H. Intracellular calcium homeostasis in cardiac myocytes. Circulation 1993, 87, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Orkand, R.K.; Kettenmann, H. Glial calcium: Homeostasis and signaling function. Physiol. Rev. 1998, 78, 99–141. [Google Scholar] [CrossRef]
- Chen, Z.H.; Chen, L.; Chen, K.T.; Lin, H.R.; Shen, M.J.; Chen, L.; Zhu, H.L.; Zhu, Y.Q.; Wang, Q.C.; Xi, F.; et al. Overexpression of Na+-HCO3- cotransporter contributes to the exacerbation of cardiac remodeling in mice with myocardial infarction by increasing intracellular calcium overload. BBA-Mol. Basis Dis. 2020, 1866, 165623. [Google Scholar] [CrossRef]
- Ji, M.J.; Son, K.H.; Hong, J.H. Addition of oh8dG to Cardioplegia Attenuated Myocardial Oxidative Injury through the Inhibition of Sodium Bicarbonate Cotransporter Activity. Antioxidants 2022, 11, 1641. [Google Scholar] [CrossRef]
- Lamprecht, G.; Hsieh, C.J.; Lissner, S.; Nold, L.; Heil, A.; Gaco, V.; Schafer, J.; Turner, J.R.; Gregor, M. Intestinal Anion Exchanger Down-regulated in Adenoma (DRA) Is Inhibited by Intracellular Calcium. J. Biol. Chem. 2009, 284, 19744–19753. [Google Scholar] [CrossRef]
- Ando, H.; Mizutani, A.; Matsu-ura, T.; Mikoshiba, K. IRBIT, a novel inositol 1,4,5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. J. Biol. Chem. 2003, 278, 10602–10612. [Google Scholar] [CrossRef]
- Ando, H.; Mizutani, A.; Kiefer, H.; Tsuzurugi, D.; Michikawa, T.; Mikoshiba, K. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol. Cell 2006, 22, 795–806. [Google Scholar] [CrossRef]
- Yang, D.; Li, Q.; So, I.; Huang, C.L.; Ando, H.; Mizutani, A.; Seki, G.; Mikoshiba, K.; Thomas, P.J.; Muallem, S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J. Clin. Investig. 2011, 121, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Kawaai, K.; Mizutani, A.; Shoji, H.; Ogawa, N.; Ebisui, E.; Kuroda, Y.; Wakana, S.; Miyakawa, T.; Hisatsune, C.; Mikoshiba, K. IRBIT regulates CaMKII α activity and contributes to catecholamine homeostasis through tyrosine hydroxylase phosphorylation. Proc. Natl. Acad. Sci. USA 2015, 112, 5515–5520. [Google Scholar] [CrossRef] [PubMed]
- Shirakabe, K.; Priori, G.; Yamada, H.; Ando, H.; Horita, S.; Fujita, T.; Fujimoto, I.; Mizutani, A.; Seki, G.; Mikoshiba, K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3− cotransporter 1 (pNBC1). Proc. Natl. Acad. Sci. USA 2006, 103, 9542–9547. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Boron, W.F.; Parker, M.D. Relief of autoinhibition of the electrogenic Na-HCO3 cotransporter NBCe1-B: Role of IRBIT vs.amino-terminal truncation. Am. J. Physiol. Cell Physiol. 2012, 302, C518–C526. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Yang, D.; Shcheynikov, N.; Ohana, E.; Shin, D.M.; Muallem, S. Convergence of IRBIT, phosphatidylinositol (4,5) bisphosphate, and WNK/SPAK kinases in regulation of the Na+-HCO3− cotransporters family. Proc. Natl. Acad. Sci. USA 2013, 110, 4105–4110. [Google Scholar] [CrossRef]
- Su, P.; Wu, H.; Wang, M.; Cai, L.; Liu, Y.; Chen, L.M. IRBIT activates NBCe1-B by releasing the auto-inhibition module from the transmembrane domain. J. Physiol. 2021, 599, 1151–1172. [Google Scholar] [CrossRef]
- Wang, M.; Wu, H.; Liu, Y.; Chen, L.M. Activation of mouse NBCe1-B by Xenopus laevis and mouse IRBITs: Role of the variable Nt appendage of IRBITs. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183240. [Google Scholar] [CrossRef]
- Itoh, R.; Hatano, N.; Murakami, M.; Mitsumori, K.; Kawasaki, S.; Wakagi, T.; Kanzaki, Y.; Kojima, H.; Kawaai, K.; Mikoshiba, K.; et al. Both IRBIT and long-IRBIT bind to and coordinately regulate Cl−/HCO3− exchanger AE2 activity through modulating the lysosomal degradation of AE2. Sci. Rep. 2021, 11, 5990. [Google Scholar] [CrossRef]
- Hwang, S.; Shin, D.M.; Hong, J.H. Protective Role of IRBIT on Sodium Bicarbonate Cotransporter-n1 for Migratory Cancer Cells. Pharmaceutics 2020, 12, 816. [Google Scholar] [CrossRef]
- Blacklock, N.J.; Beavis, J.P. The response of prostatic fluid pH in inflammation. Br. J. Urol. 1974, 46, 537–542. [Google Scholar] [CrossRef]
- He, L.; Wang, Y.; Long, Z.; Jiang, C. Clinical significance of IL-2, IL-10, and TNF-α in prostatic secretion of patients with chronic prostatitis. Urology 2010, 75, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Cafferata, E.G.; Gonzalez-Guerrico, A.M.; Giordano, L.; Pivetta, O.H.; Santa-Coloma, T.A. Interleukin-1β regulates CFTR expression in human intestinal T84 cells. Biochim. Biophys. Acta 2000, 1500, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Tang, X.; Xu, W.; Diao, R.; Cai, Z.; Chan, H.C. A host defense mechanism involving CFTR-mediated bicarbonate secretion in bacterial prostatitis. PLoS ONE 2010, 5, e15255. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.X.; Ostedgaard, L.S.; Hoegger, M.J.; Moninger, T.O.; Karp, P.H.; McMenimen, J.D.; Choudhury, B.; Varki, A.; Stoltz, D.A.; Welsh, M.J. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J. Clin. Investig. 2016, 126, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Simonin, J.; Bille, E.; Crambert, G.; Noel, S.; Dreano, E.; Edwards, A.; Hatton, A.; Pranke, I.; Villeret, B.; Cottart, C.H.; et al. Airway surface liquid acidification initiates host defense abnormalities in Cystic Fibrosis. Sci. Rep. 2019, 9, 6516. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.R.; Deng, S.L.; Song, W.F.; Jin, H.; Xu, J.Y.; Liu, X.; Xie, R.M.; Song, P.H.; Tuo, B.G. Helicobacter pylori infection downregulates duodenal CFTR and SLC26A6 expressions through TGF beta signaling pathway. Bmc Microbiol. 2018, 18, 87. [Google Scholar] [CrossRef]
- Thompson, K.D.; Welykyj, S.; Massa, M.C. Antibacterial activity of lidocaine in combination with a bicarbonate buffer. J. Dermatol. Surg. Oncol. 1993, 19, 216–220. [Google Scholar] [CrossRef]
- Drake, D.R.; Vargas, K.; Cardenzana, A.; Srikantha, R. Enhanced bactericidal activity of Arm and Hammer Dental Care. Am. J. Dent. 1995, 8, 308–312. [Google Scholar]
- Craig, S.B.; Concannon, M.J.; McDonald, G.A.; Puckett, C.L. The antibacterial effects of tumescent liposuction fluid. Plast. Reconstr. Surg. 1999, 103, 666–670. [Google Scholar] [CrossRef]
- Jarvis, G.N.; Fields, M.W.; Adamovich, D.A.; Arthurs, C.E.; Russell, J.B. The mechanism of carbonate killing of Escherichia coli. Lett. Appl. Microbiol. 2001, 33, 196–200. [Google Scholar] [CrossRef]
- Priyamvada, S.; Gomes, R.; Gill, R.K.; Saksena, S.; Alrefai, W.A.; Dudeja, P.K. Mechanisms Underlying Dysregulation of Electrolyte Absorption in Inflammatory Bowel Disease-Associated Diarrhea. Inflamm. Bowel Dis. 2015, 21, 2926–2935. [Google Scholar] [CrossRef] [PubMed]
- Farkas, K.; Yeruva, S.; Rakonczay, Z., Jr.; Ludolph, L.; Molnar, T.; Nagy, F.; Szepes, Z.; Schnur, A.; Wittmann, T.; Hubricht, J.; et al. New therapeutic targets in ulcerative colitis: The importance of ion transporters in the human colon. Inflamm. Bowel Dis. 2011, 17, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, S.; Anbazhagan, A.N.; Kumar, A.; Chatterjee, I.; Borthakur, A.; Saksena, S.; Gill, R.K.; Alrefai, W.A.; Dudeja, P.K. All-trans Retinoic Acid Counteracts Diarrhea and Inhibition of Downregulated in Adenoma Expression in Gut Inflammation. Inflamm. Bowel Dis. 2020, 26, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases--an overview. Curr. Pharm. Des. 2008, 14, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Tashian, R.E. The carbonic anhydrases: Widening perspectives on their evolution, expression and function. Bioessays 1989, 10, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Sly, W.S.; Hu, P.Y. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu. Rev. Biochem. 1995, 64, 375–401. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef]
- Supuran, C.T.; Scozzafava, A.; Conway, J. Carbonic Anhydrase: Its Inhibitors and Activators; CRC Press: Boca Raton, FL, USA, 2004; pp. 363–364. [Google Scholar]
- Imtaiyaz Hassan, M.; Shajee, B.; Waheed, A.; Ahmad, F.; Sly, W.S. Structure, function and applications of carbonic anhydrase isozymes. Bioorg Med. Chem. 2013, 21, 1570–1582. [Google Scholar] [CrossRef]
- Waheed, A.; Zhu, X.L.; Sly, W.S. Membrane-associated carbonic anhydrase from rat lung. Purification, characterization, tissue distribution, and comparison with carbonic anhydrase IVs of other mammals. J. Biol. Chem. 1992, 267, 3308–3311. [Google Scholar] [CrossRef]
- Becker, H.M.; Deitmer, J.W. Carbonic anhydrase II increases the activity of the human electrogenic Na+/HCO3− cotransporter. J. Biol. Chem. 2007, 282, 13508–13521. [Google Scholar] [CrossRef]
- Schueler, C.; Becker, H.M.; McKenna, R.; Deitmer, J.W. Transport activity of the sodium bicarbonate cotransporter NBCe1 is enhanced by different isoforms of carbonic anhydrase. PLoS ONE 2011, 6, e27167. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Sly, W.S. Membrane associated carbonic anhydrase IV (CA IV): A personal and historical perspective. Subcell. Biochem. 2014, 75, 157–179. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.E.; Pastorekova, S.; Stuart-Tilley, A.K.; Alper, S.L.; Casey, J.R. Interactions of transmembrane carbonic anhydrase, CAIX, with bicarbonate transporters. Am. J. Physiol. Cell Physiol. 2007, 293, C738–C748. [Google Scholar] [CrossRef]
- Svastova, E.; Witarski, W.; Csaderova, L.; Kosik, I.; Skvarkova, L.; Hulikova, A.; Zatovicova, M.; Barathova, M.; Kopacek, J.; Pastorek, J.; et al. Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and increases cell migration via its catalytic domain. J. Biol. Chem. 2012, 287, 3392–3402. [Google Scholar] [CrossRef] [PubMed]
- Hynninen, P.; Vaskivuo, L.; Saarnio, J.; Haapasalo, H.; Kivela, J.; Pastorekova, S.; Pastorek, J.; Waheed, A.; Sly, W.S.; Puistola, U.; et al. Expression of transmembrane carbonic anhydrases IX and XII in ovarian tumours. Histopathology 2006, 49, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Kopecka, J.; Campia, I.; Jacobs, A.; Frei, A.P.; Ghigo, D.; Wollscheid, B.; Riganti, C. Carbonic anhydrase XII is a new therapeutic target to overcome chemoresistance in cancer cells. Oncotarget 2015, 6, 6776–6793. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Sly, W.S. Carbonic anhydrase XII functions in health and disease. Gene 2017, 623, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Muhammad, E.; Zheng, C.; Hershkovitz, E.; Alkrinawi, S.; Loewenthal, N.; Parvari, R.; Muallem, S. Essential role of carbonic anhydrase XII in secretory gland fluid and HCO3− secretion revealed by disease causing human mutation. J. Physiol. 2015, 593, 5299–5312. [Google Scholar] [CrossRef]
- Maueroder, C.; Mahajan, A.; Paulus, S.; Gosswein, S.; Hahn, J.; Kienhofer, D.; Biermann, M.H.; Tripal, P.; Friedrich, R.P.; Munoz, L.E.; et al. Menage-a-Trois: The Ratio of Bicarbonate to CO2 and the pH Regulate the Capacity of Neutrophils to Form NETs. Front. Immunol. 2016, 7, 583. [Google Scholar] [CrossRef]
- Ady, J.W.; Desir, S.; Thayanithy, V.; Vogel, R.I.; Moreira, A.L.; Downey, R.J.; Fong, Y.; Manova-Todorova, K.; Moore, M.A.; Lou, E. Intercellular communication in malignant pleural mesothelioma: Properties of tunneling nanotubes. Front. Physiol. 2014, 5, 400. [Google Scholar] [CrossRef]
- Stock, C.; Gassner, B.; Hauck, C.R.; Arnold, H.; Mally, S.; Eble, J.A.; Dieterich, P.; Schwab, A. Migration of human melanoma cells depends on extracellular pH and Na+/H+ exchange. J. Physiol. 2005, 567, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhong, R.; Ming, J.; Zou, L.; Zhu, B.B.; Lu, X.Z.; Ke, J.T.; Zhang, Y.; Liu, L.; Miao, X.; et al. The SLC4A7 variant rs4973768 is associated with breast cancer risk: Evidence from a case-control study and a meta-analysis. Breast Cancer Res. Treat. 2012, 136, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Axelsen, T.V.; Jesse, N.; Pedersen, S.F.; Vahi, P.; Boedtkjer, E. Na+,HCO3−-cotransporter NBCn1 (Slc4a7) accelerates ErbB2-induced breast cancer development and tumor growth in mice. Oncogene 2018, 37, 5569–5584. [Google Scholar] [CrossRef] [PubMed]
- Cappellesso, F.; Orban, M.P.; Shirgaonkar, N.; Berardi, E.; Serneels, J.; Neveu, M.A.; Di Molfetta, D.; Piccapane, F.; Caroppo, R.; Debellis, L.; et al. Targeting the bicarbonate transporter SLC4A4 overcomes immunosuppression and immunotherapy resistance in pancreatic cancer. Nat. Cancer 2022, 3, 1464–1483. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, H.J.; Lee, S.; Kim, Y.H.; Choi, I. The sodium-driven chloride/bicarbonate exchanger NDCBE in rat brain is upregulated by chronic metabolic acidosis. Brain Res. 2011, 1377, 13–20. [Google Scholar] [CrossRef]
- Ji, M.; Ryu, H.J.; Baek, H.M.; Shin, D.M.; Hong, J.H. Dynamic synovial fibroblasts are modulated by NBCn1 as a potential target in rheumatoid arthritis. Exp. Mol. Med. 2022, 54, 503–517. [Google Scholar] [CrossRef]
- Ali, E.S.; Liponska, A.; O’Hara, B.P.; Amici, D.R.; Torno, M.D.; Gao, P.; Asara, J.M.; Yap, M.F.; Mendillo, M.L.; Ben-Sahra, I. The mTORC1-SLC4A7 axis stimulates bicarbonate import to enhance de novo nucleotide synthesis. Mol. Cell. 2022, 82, 3284–3298.e3287. [Google Scholar] [CrossRef]

| Isoform Type | Transporters | Related Mechanisms | Species | Ref |
|---|---|---|---|---|
| CA1 (Microinjection) | NBCe1 | Increased NBC activity and catalytic activity | Oocytes | [123] |
| CA2 (Microinjection) | NBCe1 | Elevated NBC activity | Oocytes | [122] |
| CA2 (Transfection) | AE2 NBCe1 | Upregulated AE2 activities No effect on NBC activity | HeLa cells | [130] |
| CA4 (Transfection) | AE2, NBCe1 | Regulation of intracellular pH | HEK293T | [125] |
| CA12 (Transfection) | NBCe1, AE2 | Upregulated NBC and AE2 activities | HeLa cells | [130] |
| CA12 (E143K) (Transfection) | NBCe1, AE2 | Downregulated NBC and AE2 activities, reduced fluid secretion | HeLa and primary parotid gland cells |
| Type | BT | Ions | Expression Tissues | Related Diseases | Stimulants | Ref |
|---|---|---|---|---|---|---|
| CBE; SLC26A family | DRA (SLC26A3) | Cl−, HCO3− | Colon, ileal, and intestine | Bowel diseases, Inflammatory | Inflammatory cytokines (IL-17 and TNF-α), Caffeic acid phenethyl ester, NPY, MβCD, 4Br-A23187, and ATRA | [19,21,25,79,89,114] |
| Pendrin (SLC26A4) | Respiratory tract, kidney | Inflammatory, Dysregulation of airway surface liquid pH | TNF-α, Ang II, IL-17 | [14,25,46] | ||
| PAT-1 | Duodenal | H. pylori-associated duodenal ulcer | H. pylori | [107] | ||
| CBE; Anion exchanger family | AE1 | Cl−, HCO3− | Kidney | Maintenance of electrolyte and acid-base disturbances | Acidic stress, alkaloid stress | [45] |
| AE2 | Skin, kidney, and liver | Melanoma, Primary biliary cholangitis. | L-IRBIT K/O, IRBIT/L-IRBIT double K/O, and pro-inflammatory cytokines (IL-8, 12, 17, 18, and TNF-α) | [23,99] | ||
| AE3 | Heart | Cardiac hypertrophy | Ang II | [44] | ||
| NBC | NBCe1 | Na+, HCO3− | Heart, airway, pancreatic ducts, parotid ducts, and oocytes | Hypertrophy, heart failure | IL-17 + TNF-α, ANG II, IRBIT, CA1, and CA2 | [14,41,92,122,123] |
| NBCn1 | Airway, heart, lung, and cervix | Hypertrophy, heart failure, carcinoma, adenocarcinoma | IL-17 + TNF- α, ANG II, IRBIT, Insulin, and growth factor | [14,41,100,139] | ||
| NDCBE | Cl−, HCO3− | Brain, skin | Chronic metabolic acidosis, melanoma | Acidic stress | [5,137] | |
| ATP-binding cassette transporter | CFTR | Cl−, HCO3− | Duodenal, airway, brain, lung, and prostate | H. pylori-associated duodenal ulcer, hypertension, adenocarcinoma | H. pylori, TNF-a + IL17, ANG II, VIP, LPS, E. coli | [14,47,59,104,107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Hong, J.H. Multiple Regulatory Signals and Components in the Modulation of Bicarbonate Transporters. Pharmaceutics 2024, 16, 78. https://doi.org/10.3390/pharmaceutics16010078
Kim HJ, Hong JH. Multiple Regulatory Signals and Components in the Modulation of Bicarbonate Transporters. Pharmaceutics. 2024; 16(1):78. https://doi.org/10.3390/pharmaceutics16010078
Chicago/Turabian StyleKim, Hyeong Jae, and Jeong Hee Hong. 2024. "Multiple Regulatory Signals and Components in the Modulation of Bicarbonate Transporters" Pharmaceutics 16, no. 1: 78. https://doi.org/10.3390/pharmaceutics16010078
APA StyleKim, H. J., & Hong, J. H. (2024). Multiple Regulatory Signals and Components in the Modulation of Bicarbonate Transporters. Pharmaceutics, 16(1), 78. https://doi.org/10.3390/pharmaceutics16010078







