Influence of Aza-Glycine Substitution on the Internalization of Penetratin
Abstract
:1. Introduction
2. Materials and Methods
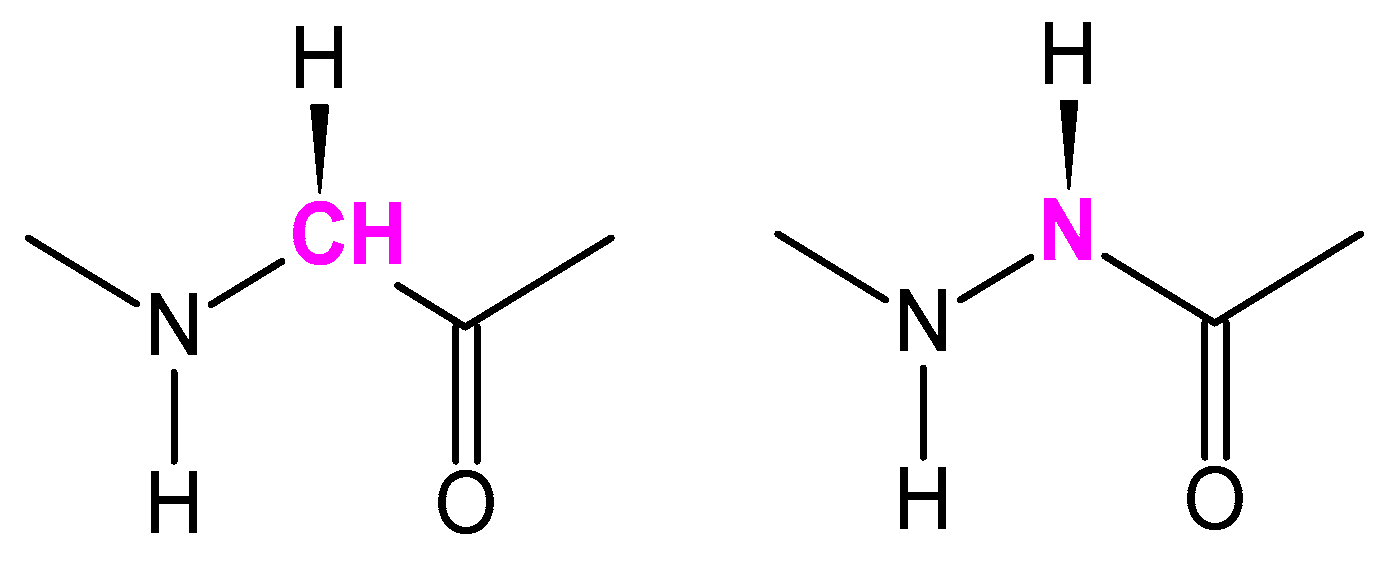
2.1. Synthesis of Peptides
2.2. Determination of Cellular Uptake by Flow Cytometry
2.3. Calculations
2.4. Statistical Analysis
3. Results
3.1. Design and Synthesis of Peptides
3.2. Cellular Uptake
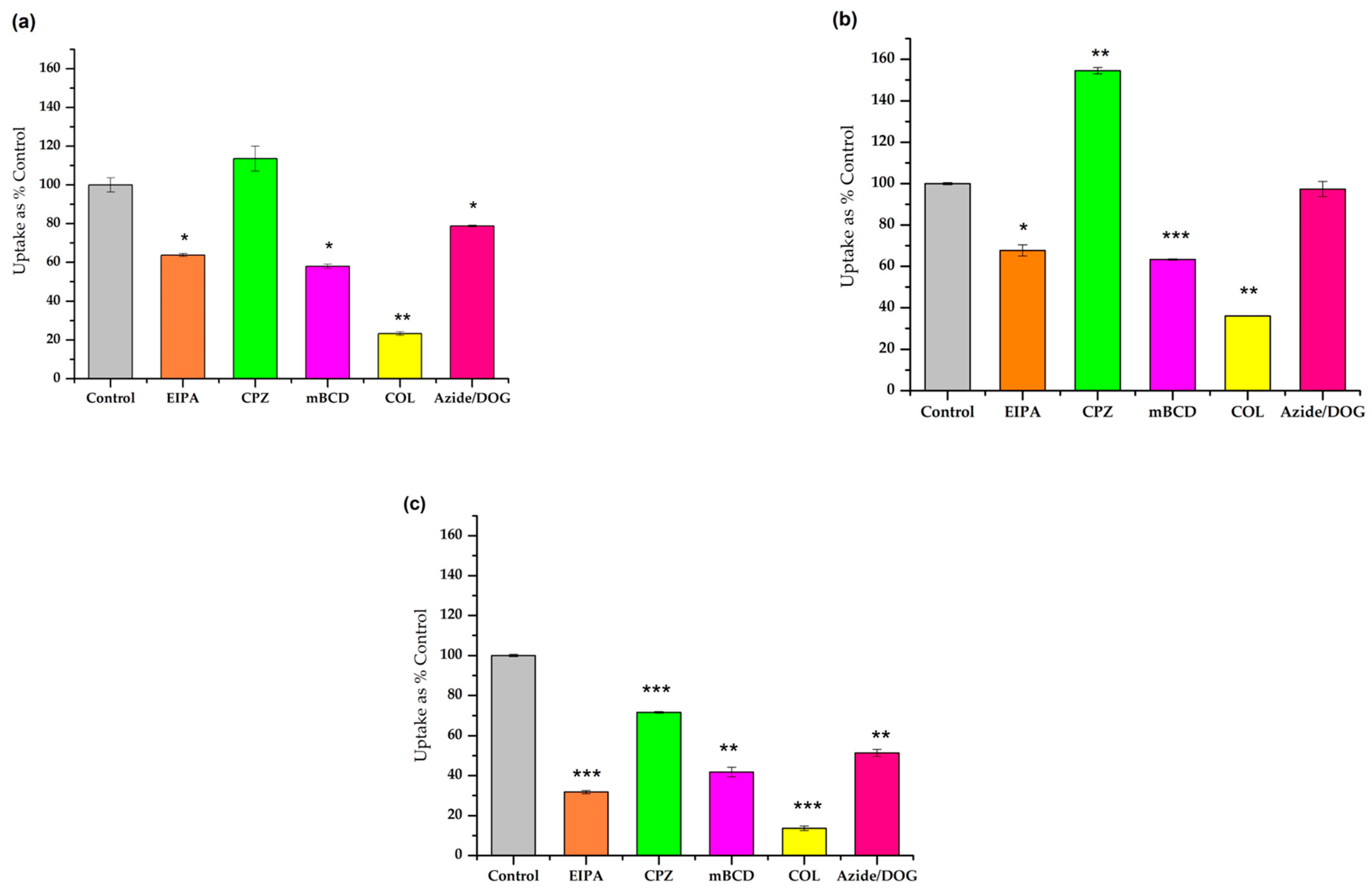
3.3. Study of the Different Endocytic Pathways of Internalization
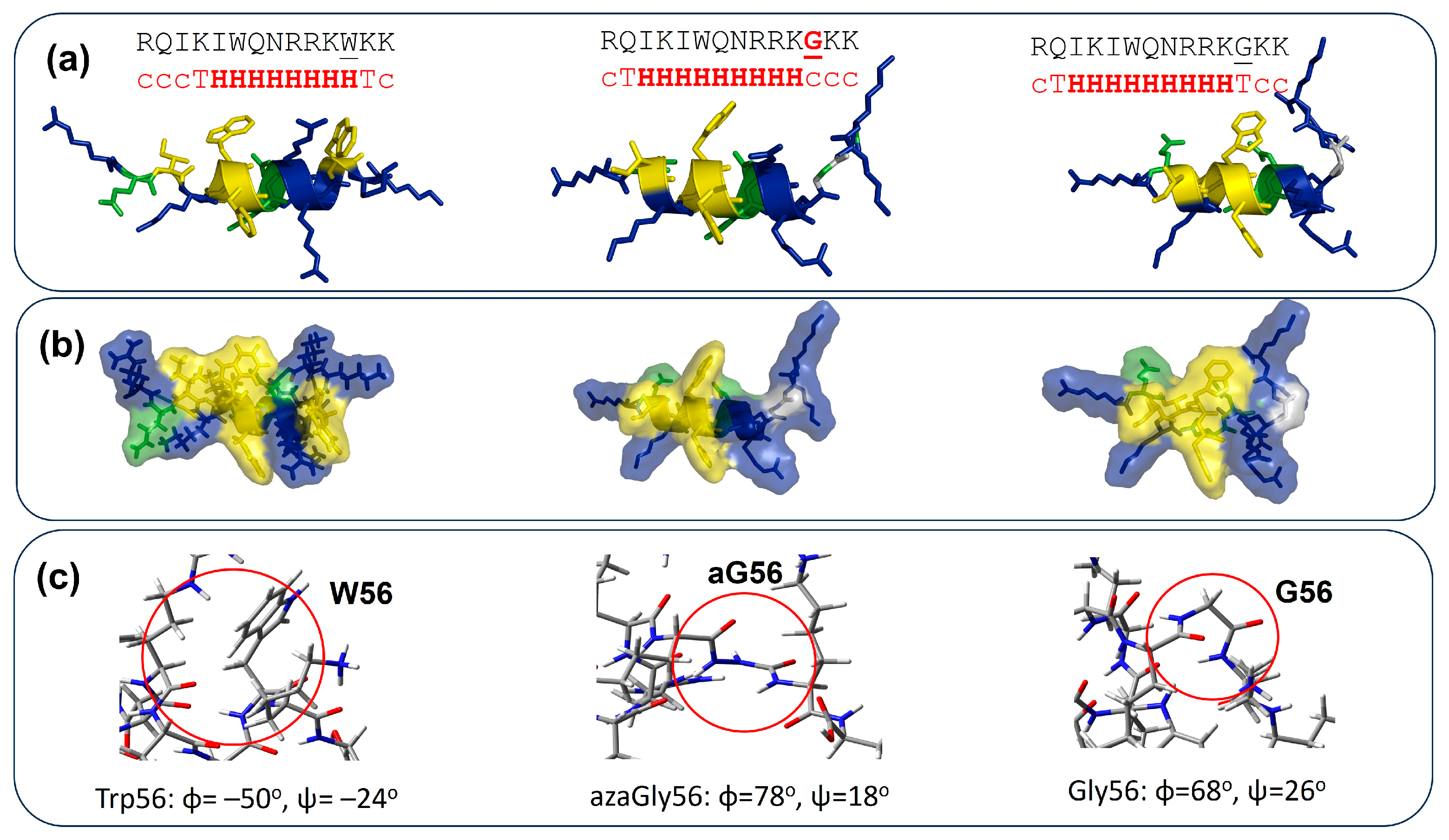
3.4. Calculation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vorherr, T. Modifying Peptides to Enhance Permeability. Future Med. Chem. 2015, 7, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Raj, N.; Verma, H.; Patel, M.; Chakraborti, S.; Khatri, B.; Doreswamy, C.M.; Anandakumar, S.R.; Seekallu, S.; Dinesh, M.B.; et al. An Amide to Thioamide Substitution Improves the Permeability and Bioavailability of Macrocyclic Peptides. Nat. Commun. 2023, 14, 6050. [Google Scholar] [CrossRef] [PubMed]
- Chiarpotti, M.V.; Longo, G.S.; Del Pópolo, M.G. Nanoparticles Modified with Cell Penetrating Peptides: Assessing Adsorption on Membranes Containing Acidic Lipids. Colloids Surf. B Biointerfaces 2021, 197, 111373. [Google Scholar] [CrossRef] [PubMed]
- Wickline, S.A.; Hou, K.K.; Pan, H. Peptide-Based Nanoparticles for Systemic Extrahepatic Delivery of Therapeutic Nucleotides. Int. J. Mol. Sci. 2023, 24, 9455. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, G.; Rosa, E.; Gallo, E.; Diaferia, C.; Morelli, G.; Stornaiuolo, M.; Accardo, A. Caveolin-Mediated Internalization of Fmoc-FF Nanogels in Breast Cancer Cell Lines. Pharmaceutics 2023, 15, 1026. [Google Scholar] [CrossRef] [PubMed]
- Hudecz, F.; Bánóczi, Z.; Csík, G. Medium-Sized Peptides as Built in Carriers for Biologically Active Compounds. Med. Res. Rev. 2005, 25, 679–736. [Google Scholar] [CrossRef] [PubMed]
- Szabó, I.; Yousef, M.; Soltész, D.; Bató, C.; Mező, G.; Bánóczi, Z. Redesigning of Cell-Penetrating Peptides to Improve Their Efficacy as a Drug Delivery System. Pharmaceutics 2022, 14, 907. [Google Scholar] [CrossRef] [PubMed]
- Bánóczi, Z.; Alexa, A.; Farkas, A.; Friedrich, P.; Hudecz, F. Novel Cell-Penetrating Calpain Substrate. Bioconjug Chem. 2008, 19, 1375–1381. [Google Scholar] [CrossRef]
- Alexa, A.; Ember, O.; Szabó, I.; Mo’ath, Y.; Póti, Á.L.; Reményi, A.; Bánóczi, Z. Peptide Based Inhibitors of Protein Binding to the Mitogen-Activated Protein Kinase Docking Groove. Front. Mol. Biosci. 2021, 8, 629. [Google Scholar] [CrossRef]
- Futaki, S.; Arafiles, J.V.V.; Hirose, H. Peptide-Assisted Intracellular Delivery of Biomacromolecules. Chem. Lett. 2020, 49, 1088–1094. [Google Scholar] [CrossRef]
- Bánóczi, Z.; Keglevich, A.; Szabó, I.; Ranđelović, I.; Hegedüs, Z.; Regenbach, F.L.; Keglevich, P.; Lengyel, Z.; Gorka-Kereskényi, Á.; Dubrovay, Z.; et al. The Effect of Conjugation on Antitumor Activity of Vindoline Derivatives with Octaarginine, a Cell-Penetrating Peptide. J. Pept. Sci. 2018, 24, e3118. [Google Scholar] [CrossRef] [PubMed]
- McClorey, G.; Banerjee, S. Cell-Penetrating Peptides to Enhance Delivery of Oligonucleotide-Based Therapeutics. Biomedicines 2018, 6, 51. [Google Scholar] [CrossRef]
- Boeglin, D.; Lubell, W.D. Aza-Amino Acid Scanning of Secondary Structure Suited for Solid-Phase Peptide Synthesis with Fmoc Chemistry and Aza-Amino Acids with Heteroatomic Side Chains. J. Comb. Chem. 2005, 7, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Bató, C.; Szabó, I.; Bánóczi, Z. Enhancing Cell Penetration Efficiency of Cyclic Oligoarginines Using Rigid Scaffolds. Pharmaceutics 2023, 15, 1736. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.; Szabó, I.; Murányi, J.; Illien, F.; Soltész, D.; Bató, C.; Tóth, G.; Batta, G.; Nagy, P.; Sagan, S.; et al. Cell-Penetrating Dabcyl-Containing Tetraarginines with Backbone Aromatics as Uptake Enhancers. Pharmaceutics 2022, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Tarchoun, K.; Yousef, M.; Bánóczi, Z. Azapeptides as an Efficient Tool to Improve the Activity of Biologically Effective Peptides. Future Pharmacol. 2022, 2, 293–305. [Google Scholar] [CrossRef]
- Lee, H.J.; Ahn, I.A.; Ro, S.; Choi, K.H.; Choi, Y.S.; Lee, K.B. Role of Azaamino Acid Residue in β-Turn Formation and Stability in Designed Peptide. J. Pept. Res. 2000, 56, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Zouikri, M.; Vicherat, A.; Aubry, A.; Marraud, M.; Boussard, G. Azaproline as a Beta-Turn-Inducer Residue Opposed to Proline. J. Pept. Res. 1998, 52, 19–26. [Google Scholar] [CrossRef]
- Zhang, W.J.; Berglund, A.; Kao, J.L.F.; Couty, J.P.; Gershengorn, M.C.; Marshall, G.R. Impact of Azaproline on Amide Cis-Trans Isomerism: Conformational Analyses and NMR Studies of Model Peptides Including TRH Analogues. J. Am. Chem. Soc. 2003, 125, 1221–1235. [Google Scholar] [CrossRef]
- Lecoq, A.; Boussard, G.; Marraud, M.; Aubry, A. Crystal State Conformation of Three Azapeptides Containing the Azaproline Residue, a β-Turn Regulator. Biopolymers 1993, 33, 1051–1059. [Google Scholar] [CrossRef]
- André, F.; Boussard, G.; Bayeul, D.; Didierjean, C.; Aubry, A.; Marraud, M. Aza-Peptides. II. X-Ray Structures of Aza-Alanine and Aza-Asparagine-Containing Peptides. J. Pept. Res. 1997, 49, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Zega, A. Azapeptides as Pharmacological Agents. Curr. Med. Chem. 2012, 12, 589–597. [Google Scholar] [CrossRef]
- Eustache, S.; Leprince, J.; Tufféry, P. Progress with Peptide Scanning to Study Structure-Activity Relationships: The Implications for Drug Discovery. Expert Opin. Drug Discov. 2016, 11, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Proulx, C.; Sabatino, D.; Hopewell, R.; Spiegel, J.; García Ramos, Y.; Lubell, W.D. Azapeptides and Their Therapeutic Potential. Future Med. Chem. 2011, 3, 1139–1164. [Google Scholar] [CrossRef] [PubMed]
- Von Hentig, N. Atazanavir/Ritonavir: A Review of Its Use in HIV Therapy. Drugs Today 2008, 44, 103–132. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.S.; Furr, B.J.A.; Giles, M.B.; Valcaccia, B. Synthesis and Biological Activity of Highly Active Alpha-Aza Analogues of Luliberin. J. Med. Chem. 1978, 21, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Ahmann, F.R.; Citrin, D.L.; DeHaan, H.A.; Guinan, P.; Jordan, V.C.; Kreis, W.; Scott, M.; Trump, D.L. Zoladex: A Sustained-Release, Monthly Luteinizing Hormone-Releasing Hormone Analogue for the Treatment of Advanced Prostate Cancer. J. Clin. Oncol. 1987, 5, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.; Goodman, S.L.; Hahn, D.; Hölzemann, G.; Kessler, H. Novel Solid-Phase Synthesis of Azapeptides and Azapeptoides via Fmoc- Strategy and Its Application in the Synthesis of RGD-Mimetics. J. Org. Chem. 1999, 64, 7388–7394. [Google Scholar] [CrossRef]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The Third Helix of the Antennapedia Homeodomain Translocates through Biological Membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef]
- Letoha, T.; Gaál, S.; Somlai, C.; Venkei, Z.; Glavinas, H.; Kusz, E.; Duda, E.; Czajlik, A.; Peták, F.; Penke, B. Investigation of Penetratin Peptides. Part 2. In Vitro Uptake of Penetratin and Two of Its Derivatives. J. Pept. Sci. 2005, 11, 805–811. [Google Scholar] [CrossRef]
- Walrant, A.; Correia, I.; Jiao, C.Y.; Lequin, O.; Bent, E.H.; Goasdoué, N.; Lacombe, C.; Chassaing, G.; Sagan, S.; Alves, I.D. Different Membrane Behaviour and Cellular Uptake of Three Basic Arginine-Rich Peptides. Biochim. Biophys. Acta Biomembr. 2011, 1808, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.; Szabó, I.; Biri-Kovács, B.; Szeder, B.; Illien, F.; Sagan, S.; Bánóczi, Z. Modification of Short Non-Permeable Peptides to Increase Cellular Uptake and Cytostatic Activity of Their Conjugates. ChemistrySelect 2021, 6, 10111–10120. [Google Scholar] [CrossRef]
- Soltész, D.; Szabó, I.; Bánóczi, Z. The Balance between Hydrophobicity/Aromaticity and Positively Charged Residues May Influence the Cell Penetration Ability. Pharmaceutics 2023, 15, 1267. [Google Scholar] [CrossRef] [PubMed]
- Derossi, D.; Chassaing, G.; Prochiantz, A. Trojan Peptides: The Penetratin System for Intracellular Delivery. Trends Cell Biol. 1998, 8, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Bánoczi, Z.; Tantos, Á.; Farkas, A.; Tompa, P.; Friedrich, P.; Hudecz, F. Synthesis of Cell-Penetrating Conjugates of Calpain Activator Peptides. Bioconjug Chem. 2007, 18, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.M.; Kulkarni, K.; Brandt, R.; Del Borgo, M.P.; Aguilar, M.I.; Wilce, J.A. Shortened Penetratin Cell-Penetrating Peptide Is Insufficient for Cytosolic Delivery of a Grb7 Targeting Peptide. ACS Omega 2017, 2, 670–677. [Google Scholar] [CrossRef]
- Letoha, T.; Gaá, S.; Somlai, C.; Czajlik, A.; Perczel, A.; Penke, B. Membrane Translocation of Penetratin and Its Derivatives in Different Cell Lines. J. Mol. Recognit. 2003, 16, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, B.; Symoens, S.; Vanderheyden, S.; Engelborghs, Y.; Joliot, A.; Prochiantz, A.; Vandekerckhove, J.; Rosseneu, M.; Vanloo, B. Tryptophan Fluorescence Study of the Interaction of Penetratin Peptides with Model Membranes. Eur. J. Biochem. 2002, 269, 2918–2926. [Google Scholar] [CrossRef]
- Dom, G.; Shaw-Jackson, C.; Matis, C.; Bouffioux, O.; Picard, J.J.; Prochiantz, A.; Mingeot-Leclerq, M.P.; Brasseur, R.; Rezsohazy, R. Cellular Uptake of Antennapedia Penetratin Peptides Is a Two-Step Process in Which Phase Transfer Precedes a Tryptophan-Dependent Translocation. Nucleic Acids Res. 2003, 31, 556–561. [Google Scholar] [CrossRef]
- Zhang, W.; Smith, S.O. Mechanism of Penetration of Antp(43–58) into Membrane Bilayers. Biochemistry 2005, 44, 10110–10118. [Google Scholar] [CrossRef]
- Esbjörner, E.K.; Lincoln, P.; Nordén, B. Counterion-Mediated Membrane Penetration: Cationic Cell-Penetrating Peptides Overcome Born Energy Barrier by Ion-Pairing with Phospholipids. Biochim. Biophys. Acta 2007, 1768, 1550–1558. [Google Scholar] [CrossRef]
- Magzoub, M.; Eriksson, L.E.G.; Gräslund, A. Comparison of the Interaction, Positioning, Structure Induction and Membrane Perturbation of Cell-Penetrating Peptides and Non-Translocating Variants with Phospholipid Vesicles. Biophys. Chem. 2003, 103, 271–288. [Google Scholar] [CrossRef]
- Fischer, P.M.; Zhelev, N.Z.; Wang, S.; Melville, J.E.; Fåhraeus, R.; Lane, D.P. Structure–Activity Relationship of Truncated and Substituted Analogues of the Intracellular Delivery Vector Penetratin. J. Pept. Res. 2000, 55, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Asami, T.; Nishizawa, N.; Ishibashi, Y.; Nishibori, K.; Nakayama, M.; Horikoshi, Y.; Matsumoto, S.I.; Yamaguchi, M.; Matsumoto, H.; Tarui, N.; et al. Serum Stability of Selected Decapeptide Agonists of KISS1R Using Pseudopeptides. Bioorg. Med. Chem. Lett. 2012, 22, 6391–6396. [Google Scholar] [CrossRef] [PubMed]
- Wieczerzak, E.; Drabik, P.; Łankiewicz, L.; Ołdziej, S.; Grzonka, Z.; Abrahamson, M.; Grubb, A.; Brömme, D. Azapeptides Structurally Based upon Inhibitory Sites of Cystatins as Potent and Selective Inhibitors of Cysteine Proteases. J. Med. Chem. 2002, 45, 4202–4211. [Google Scholar] [CrossRef]
- Koivusalo, M.; Welch, C.; Hayashi, H.; Scott, C.C.; Kim, M.; Alexander, T.; Touret, N.; Hahn, K.M.; Grinstein, S. Amiloride Inhibits Macropinocytosis by Lowering Submembranous PH and Preventing Rac1 and Cdc42 Signaling. J. Cell Biol. 2010, 188, 547–563. [Google Scholar] [CrossRef]
- Gomes dos Reis, L.; Lee, W.H.; Svolos, M.; Moir, L.M.; Jaber, R.; Engel, A.; Windhab, N.; Young, P.M.; Traini, D. Delivery of PDNA to Lung Epithelial Cells Using PLGA Nanoparticles Formulated with a Cell-Penetrating Peptide: Understanding the Intracellular Fate. Drug Dev. Ind. Pharm. 2020, 46, 427–442. [Google Scholar] [CrossRef]
- Fittipaldi, A.; Ferrari, A.; Zoppé, M.; Arcangeli, C.; Pellegrini, V.; Beltram, F.; Giacca, M. Cell Membrane Lipid Rafts Mediate Caveolar Endocytosis of HIV-1 Tat Fusion Proteins. J. Biol. Chem. 2003, 278, 34141–34149. [Google Scholar] [CrossRef] [PubMed]
- Starling, D.; Duncan, R.; Lloyd, J.B. The Role of Microtubules in Pinocytosis. Inhibition of Fluid-Phase Pinocytosis in the Rat Visceral Yolk Sac by Mitoclasic and Related Agents. Cell Biol. Int. Rep. 1983, 7, 593–602. [Google Scholar] [CrossRef]
- Rey, J.; Murail, S.; De Vries, S.; Derreumaux, P.; Tuffery, P. PEP-FOLD4: A PH-Dependent Force Field for Peptide Structure Prediction in Aqueous Solution. Nucleic Acids Res. 2023, 51, W432–W437. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chai, J.D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Touw, W.G.; Baakman, C.; Black, J.; Te Beek, T.A.H.; Krieger, E.; Joosten, R.P.; Vriend, G. A Series of PDB-Related Databanks for Everyday Needs. Nucleic Acids Res. 2015, 43, D364–D368. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Song, J.W.; Choi, Y.S.; Park, H.M.; Lee, K.B. A Theoretical Study of Conformational Properties of N-Methyl Azapeptide Derivatives. J. Am. Chem. Soc. 2002, 124, 11881–11893. [Google Scholar] [CrossRef] [PubMed]
- Magzoub, M.; Eriksson, L.E.G.; Gräslund, A. Conformational States of the Cell-Penetrating Peptide Penetratin When Interacting with Phospholipid Vesicles: Effects of Surface Charge and Peptide Concentration. Biochim. Biophys. Acta (BBA)-Biomembr. 2002, 1563, 53–63. [Google Scholar] [CrossRef]
- Binder, H.; Lindblom, G. Charge-Dependent Translocation of the Trojan Peptide Penetratin across Lipid Membranes. Biophys. J. 2003, 85, 982. [Google Scholar] [CrossRef] [PubMed]
- Binder, H.; Lindblom, G. A Molecular View on the Interaction of the Trojan Peptide Penetratin with the Polar Interface of Lipid Bilayers. Biophys. J. 2004, 87, 332–343. [Google Scholar] [CrossRef]
- Khemaissa, S.; Walrant, A.; Sagan, S. Tryptophan, More than Just an Interfacial Amino Acid in the Membrane Activity of Cationic Cell-Penetrating and Antimicrobial Peptides. Q. Rev. Biophys. 2022, 55, e10. [Google Scholar] [CrossRef] [PubMed]
- Fan Cheng, K.; VanPatten, S.; He, M.; Al-Abed, Y. Azapeptides -A History of Synthetic Milestones and Key Examples. Curr. Med. Chem. 2022, 29, 6336–6358. [Google Scholar] [CrossRef] [PubMed]
- Chingle, R.; Proulx, C.; Lubell, W.D. Azapeptide Synthesis Methods for Expanding Side-Chain Diversity for Biomedical Applications. Acc. Chem. Res. 2017, 50, 1541–1556. [Google Scholar] [CrossRef]
- Szabó, I.; Orbán, E.; Schlosser, G.; Hudecz, F.; Bánóczi, Z. Cell-Penetrating Conjugates of Pentaglutamylated Methotrexate as Potential Anticancer Drugs against Resistant Tumor Cells. Eur. J. Med. Chem. 2016, 115, 361–368. [Google Scholar] [CrossRef]
- Balayssac, S.; Burlina, F.; Convert, O.; Bolbach, G.; Chassaing, G.; Lequin, O. Comparison of Penetratin and Other Homeodomain-Derived Cell-Penetrating Peptides: Interaction in a Membrane-Mimicking Environment and Cellular Uptake Efficiency. Biochemistry 2006, 45, 1408–1420. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, I.; Joliot, A.H.; Bloch-Gallego, E.; Prochiantz, A.; Volovitch, M. Neurotrophic Activity of the Antennapedia Homeodomain Depends on Its Specific DNA-Binding Properties. Proc. Natl. Acad. Sci. USA 1993, 90, 9120. [Google Scholar] [CrossRef] [PubMed]
- Thorén, P.E.G.; Persson, D.; Isakson, P.; Goksör, M.; Önfelt, A.; Nordén, B. Uptake of Analogs of Penetratin, Tat(48–60) and Oligoarginine in Live Cells. Biochem. Biophys. Res. Commun. 2003, 307, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Drin, G.; Mazel, M.; Clair, P.; Mathieu, D.; Kaczorek, M.; Temsamani, J. Physico-Chemical Requirements for Cellular Uptake of PAntp Peptide. Eur. J. Biochem. 2001, 268, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.; Ramakrishnan, V. Circular Dichroism (CD) Spectroscopy. In Biophysical Characterization of Functional Peptides. Springer Protocols Handbooks.; Ramakrishnan, V., Ed.; Humana: New York, NY, USA, 2023; pp. 45–50. [Google Scholar]
- Åmand, H.L.; Fant, K.; Nordén, B.; Esbjörner, E.K. Stimulated Endocytosis in Penetratin Uptake: Effect of Arginine and Lysine. Biochem. Biophys. Res. Commun. 2008, 371, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Niwa, M.; Takeuchi, T.; Sonomura, K.; Kawabata, N.; Koike, Y.; Takehashi, M.; Tanaka, S.; Ueda, K.; Simpson, J.C.; et al. Cellular Uptake of Arginine-Rich Peptides: Roles for Macropinocytosis and Actin Rearrangement. Mol. Ther. 2004, 10, 1011–1022. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, H.; Cao, W.; Fang, Y.; Chen, Z.; Qi, X.; Luo, D.; Chen, C. Transcytosis Mechanisms of Cell-Penetrating Peptides: Cation-Independent CC12 and Cationic Penetratin. J. Pept. Sci. 2022, 28, e3408. [Google Scholar] [CrossRef]
- Kosuge, M.; Takeuchi, T.; Nakase, I.; Jones, A.T.; Futaki, S. Cellular Internalization and Distribution of Arginine-Rich Peptides as a Function of Extracellular Peptide Concentration, Serum, and Plasma Membrane Associated Proteoglycans. Bioconjug Chem. 2008, 19, 656–664. [Google Scholar] [CrossRef]

| Sequence | Code | Rt a | Mcalc | Mmeas b |
|---|---|---|---|---|
| Cf-RQIKIWFQNRRKWKK | Pen(desMet) c | 15.1 | 2472.9 | 2472.2 |
| Cf-RQIKIWFQNRRK-azaGly-KK | Trp56aGlyPen(desMet) | 14.8 | 2344.7 | 2344.2 |
| Cf-RQIKI-azaGly-FQNRRKWKK | Trp48aGlyPen(desMet) | 13.4 | 2344.7 | 2344.1 |
| Cf-RQIKI-azaGly-FQNRRK-azaGly-KK | Trp48,56aGlyPen(desMet) | 12.0 | 2216.5 | 2216.2 |
| Cf-RQIKIWFQNRRKGKK | Trp56GlyPen(desMet) | 15.0 | 2343.7 | 2343.2 |
| Cf-RQIKIGFQNRRKWKK | Trp48GlyPen(desMet) | 13.6 | 2343.7 | 2343.2 |
| Cf-RQIKIGFQNRRKGKK | Trp48,56GlyPen(desMet) | 13.2 | 2214.5 | 2214.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarchoun, K.; Soltész, D.; Farkas, V.; Lee, H.-J.; Szabó, I.; Bánóczi, Z. Influence of Aza-Glycine Substitution on the Internalization of Penetratin. Pharmaceutics 2024, 16, 477. https://doi.org/10.3390/pharmaceutics16040477
Tarchoun K, Soltész D, Farkas V, Lee H-J, Szabó I, Bánóczi Z. Influence of Aza-Glycine Substitution on the Internalization of Penetratin. Pharmaceutics. 2024; 16(4):477. https://doi.org/10.3390/pharmaceutics16040477
Chicago/Turabian StyleTarchoun, Karima, Dóra Soltész, Viktor Farkas, Ho-Jin Lee, Ildikó Szabó, and Zoltán Bánóczi. 2024. "Influence of Aza-Glycine Substitution on the Internalization of Penetratin" Pharmaceutics 16, no. 4: 477. https://doi.org/10.3390/pharmaceutics16040477
APA StyleTarchoun, K., Soltész, D., Farkas, V., Lee, H.-J., Szabó, I., & Bánóczi, Z. (2024). Influence of Aza-Glycine Substitution on the Internalization of Penetratin. Pharmaceutics, 16(4), 477. https://doi.org/10.3390/pharmaceutics16040477







