Beyond One-Size-Fits-All: Tailoring Teicoplanin Regimens for Normal Renal Function Patients Using Population Pharmacokinetics and Monte Carlo Simulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Drug Assay
2.4. Population Pharmacokinetic Analysis
2.5. Noncompartmental Analysis
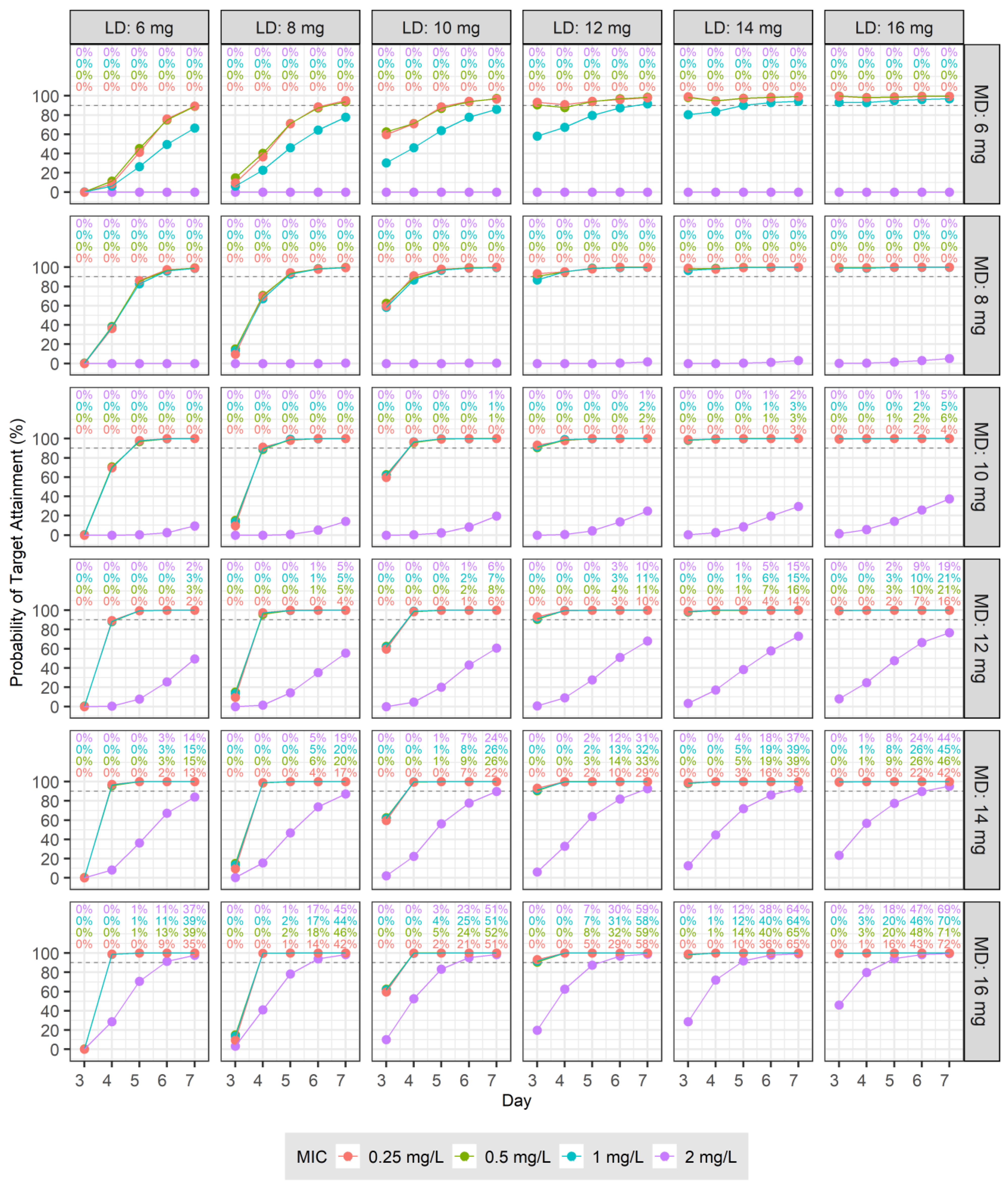
2.6. Dosage Simulations
3. Results
3.1. Subjects
3.2. Population Pharmacokinetic Analysis
3.3. Comparing Noncompartmental Analysis and Population Pharmacokinetics Results
3.4. Dosage Simulations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowland, M. Clinical pharmacokinetics of teicoplanin. Clin. Pharmacokinet. 1990, 18, 184–209. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.P. Clinical pharmacokinetics of teicoplanin. Clin. Pharmacokinet. 2000, 39, 167–183. [Google Scholar] [CrossRef]
- Targocid and Associated Names—Referral. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/targocid-associated-names (accessed on 8 March 2024).
- Craig, W.A. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 2003, 17, 479–501. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, N.; Matsumoto, K.; Ikawa, K.; Fukamizu, T.; Shigemi, A.; Yaji, K.; Shimodozono, Y.; Morikawa, N.; Takeda, Y.; Yamada, K. An initial dosing method for teicoplanin based on the area under the serum concentration time curve required for MRSA eradication. J. Infect. Chemother. 2011, 17, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, M.; Umemura, T.; Kimura, M.; Mori, T.; Hasegawa, T.; Mikamo, H. Exploration of optimal teicoplanin dosage based on pharmacokinetic parameters for the treatment of intensive care unit patients infected with methicillin-resistant Staphylococcus aureus. J. Infect. Chemother. 2012, 18, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Watanabe, E.; Kanazawa, N.; Fukamizu, T.; Shigemi, A.; Yokoyama, Y.; Ikawa, K.; Morikawa, N.; Takeda, Y. Pharmacokinetic/pharmacodynamic analysis of teicoplanin in patients with MRSA infections. Clin. Pharmacol. 2016, 8, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Martín, V.; Johnson, A.; McEntee, L.; Farrington, N.; Padmore, K.; Cojutti, P.; Pea, F.; Neely, M.N.; Hope, W.W. Pharmacodynamics of teicoplanin against MRSA. J. Antimicrob. Chemother. 2017, 72, 3382–3389. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2020, 77, 835–864. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; version 4.3.2. [Google Scholar]
- Bae, K.-S. NonCompart: Noncompartmental Analysis for Pharmacokinetic Data; The Comprehensive R Archive Network: Seoul, Republic of Korea, 2023; version 0.7.0. [Google Scholar]
- Antimicrobial Wild Type Distributions of Microorganisms. Available online: https://mic.eucast.org/ (accessed on 14 February 2024).
- Tobin, C.M.; Lovering, A.M.; Sweeney, E.; MacGowan, A.P. Analyses of teicoplanin concentrations from 1994 to 2006 from a UK assay service. J. Antimicrob. Chemother. 2010, 65, 2155–2157. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, D.; Shu, Z.; Duan, Z.; Liu, Y.; Du, Q.; Zhang, Y.; Dong, Y.; Wang, T.; Hu, S.; et al. Population pharmacokinetics and model-based dosing optimization of teicoplanin in pediatric patients. Front. Pharmacol. 2020, 11, 594562. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.Q.; Tian, T.T.; Zhang, M.X.; Song, H.T.; Zhang, L.L. Population pharmacokinetics and dosing optimization of unbound teicoplanin in Chinese adult patients. Front. Pharmacol. 2022, 13, 1045895. [Google Scholar] [CrossRef] [PubMed]
- Cazaubon, Y.; Venisse, N.; Mimoz, O.; Maire, P.; Ducher, M.; Bourguignon, L.; Goutelle, S. Population pharmacokinetics of teicoplanin administered by subcutaneous or intravenous route and simulation of optimal loading dose regimen. J. Antimicrob. Chemother. 2017, 72, 2804–2812. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Jo, H.G.; Kim, D.; Jeong, K.; Lee, J.; Lee, H.J.; Yang, S.; Park, S.; Rhie, S.J.; Chung, E.K. Population pharmacokinetics and model-based dosing optimization of teicoplanin in elderly critically ill patients with pneumonia. J. Crit. Care 2023, 78, 154402. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.J.; Roberts, J.A.; McWhinney, B.; Ryder, S.A.; Fennell, J.P.; O’Byrne, P.; Deasy, E.; Egan, S.; Desmond, R.; Enright, H.; et al. Population pharmacokinetics of teicoplanin and attainment of pharmacokinetic/pharmacodynamic targets in adult patients with haematological malignancy. Clin. Microbiol. Infect. 2017, 23, 674.e7–674.e13. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.J.; Parton, T.; McWhinney, B.; Fennell, J.P.; O’Byrne, P.; Deasy, E.; Egan, S.; Enright, H.; Desmond, R.; Ryder, S.A.; et al. Population pharmacokinetics of total and unbound teicoplanin concentrations and dosing simulations in patients with haematological malignancy. J. Antimicrob. Chemother. 2018, 73, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, D.J.; Zang, D.Y.; Lee, D.H. Effect of pharmacokinetic model misspecification on antibiotic probability of target attainment predicted by Monte Carlo simulation. Int. J. Clin. Pharmacol. Ther. 2019, 57, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Lee, J.H.; Jang, H.J.; Zang, D.Y.; Lee, D.H. Predicting antibiotic Effect of vancomycin Using pharmacokinetic/Pharmacodynamic Modeling and Simulation: Dense Sampling versus Sparse Sampling. Antibiotics 2022, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Mimoz, O.; Rolland, D.; Adoun, M.; Marchand, S.; Breilh, D.; Brumpt, I.; Debaene, B.; Couet, W. Steady-state trough serum and epithelial lining fluid concentrations of teicoplanin 12 mg/kg per day in patients with ventilator-associated pneumonia. Intensive Care Med. 2006, 32, 775–779. [Google Scholar] [CrossRef]
- Wang, J.T.; Liao, H.I.; Wu Lin, F.L.; Chang, S.C. Loading dose required to achieve rapid therapeutic teicoplanin trough plasma concentration in patients with multidrug-resistant gram-positive infections. Basic Clin. Pharmacol. Toxicol. 2012, 110, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Takesue, Y.; Nakajima, K.; Ichki, K.; Wada, Y.; Komatsu, M.; Tsuchida, T.; Takahashi, Y.; Ishihara, M.; Kimura, T.; et al. High-dose regimen to achieve novel target trough concentration in teicoplanin. J. Infect. Chemother. 2014, 20, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Takasu, O.; Sakai, Y.; Sakamoto, T.; Yamashita, N.; Mori, S.; Morita, T.; Nabeta, M.; Hirayu, N.; Yoshiyama, N.; et al. Development of a teicoplanin loading regimen that rapidly achieves target serum concentrations in critically ill patients with severe infections. J. Infect. Chemother. 2015, 21, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Hamada, Y.; Hagihara, M.; Hirai, J.; Nishiyama, N.; Koizumi, Y.; Yamagishi, Y.; Matsuura, K.; Mikamo, H. Retrospective study of teicoplanin loading regimen that rapidly achieves target 15–30 μg/mL serum trough concentration. J. Infect. Chemother. 2016, 22, 308–313. [Google Scholar] [CrossRef]




| Parameters | Mean (CV%.) | Median (IQR) |
|---|---|---|
| Demographic characteristics | ||
| Age, years | 34.8 (17.2%) | 32.0 (30.0–40.3) |
| Height, cm | 165 (4.91%) | 164 (158–169) |
| Weight, kg | 64.8 (19.9%) | 67.9 (51.3–73.4) |
| Body surface area, m2 | 1.71 (11.3%) | 1.77 (1.52–1.85) |
| Body mass index (BSA), kg/m2 | 23.6 (15.1%) | 24.8 (20.6–25.5) |
| Laboratory characteristics | ||
| Protein, g/dL | 7.52 (5.07%) | 7.35 (7.30–7.70) |
| Albumin, g/dL | 4.96 (4.90%) | 4.90 (4.80–5.03) |
| Cystatin C, mg/dL | 0.808 (14.6%) | 0.760 (0.710–0.928) |
| Creatinine, mg/dL | 0.867 (15.2%) | 0.875 (0.793–0.925) |
| Blood urea nitrogen, mg/dL | 11.2 (26.0%) | 11.2 (10.1–12.0) |
| Alanine aminotransferase, U/L | 23.2 (79.7%) | 19.5 (8.50–29.0) |
| Aspartate aminotransferase, U/L | 25.9 (41.3%) | 22.5 (19.5–27.5) |
| Gamma-glutamyl transferase, U/L | 23.8 (46.6%) | 22.5 (17.0–28.0) |
| Renal functions | ||
| CLCR by Cockcroft–Gault (mL/min) a | 102 (19.5%) | 104 (94.3–113) |
| eGFR by MDRD (mL/min/1.73 m2) b | 88.6 (13.4%) | 89.4 (81.8–96.1) |
| eGFR by CKD-EPICR (mL/min/1.73 m2) c | 104 (10.7%) | 105 (97.7–115) |
| eGFR by CKD-EPICR-CYS (mL/min/1.73 m2) d | 108 (11.6%) | 108 (98.8–120) |
| Adjusted eGFR by MDRD for BSA (mL/min) e | 87.5 (16.3%) | 89.7 (80.1–96.0) |
| Adjusted eGFR by CKD-EPICR for BSA (mL/min) e | 103 (15.5%) | 105 (96.7–113) |
| Adjusted eGFR by CKD-EPICR-CYS for BSA (mL/min) e | 106 (11.8%) | 105 (101–112) |
| Parameter | Estimates | RSE(%) | Bootstrap Median (95% CI) |
|---|---|---|---|
| Structural model | |||
| CL = θ1 × (CE/105.27) θ2 | |||
| θ1 (L/h) | 0.693 | 2.97 | 0.693 (0.653–0.74) |
| θ2 | 0.785 | 16.2 | 0.789 (0.422–1.16) |
| V1 = θ3 (L) | 3.96 | 8.41 | 3.97 (3.15–4.62) |
| Q2 = θ4 (L/h) | 4.45 | 11.6 | 4.45 (3.63–5.86) |
| V2 = θ5 (L) | 8.24 | 8.32 | 8.33 (7.07–9.85) |
| Q3 = θ6 (L/h) | 1.76 | 9.7 | 1.75 (1.44–2.13) |
| V3 = θ7 × (WT/67.85) θ8 | |||
| θ7 (L) | 69.8 | 8.74 | 69.7 (55.8–82.6) |
| θ8 | 1.73 | 22.6 | 1.73 (0.67–2.44) |
| Interindividual variability | |||
| CL (%) | 8.83 f | ||
| V1 (%) | 23.8 f | ||
| Q2 (%) | 32.7 | 20.2 | 30.7 (14.2–42.2) |
| V2 (%) | 23.9 f | ||
| Q3 (%) | 31.0 | 18.9 | 29.6 (16.7–41.4) |
| V3 (%) | 7.54 f | ||
| Residual variability | |||
| Proportional error (%) | 6.33 | 13.1 | 6.22 (4.63–7.94) |
| Parameters | Unit | Mean (CV%) | Median (IQR) |
|---|---|---|---|
| NCA results | |||
| Cmax | mg/L | 32.1 (12.9%) | 30.9 (29.5–35.6) |
| Tlast | h | 187 (11.0%) | 192 (169–194) |
| Clast | mg/L | 0.431 (24.9%) | 0.422 (0.348–0.470) |
| AUClast | mg/L·h | 273 (16.6%) | 270 (247–302) |
| AUCinf | mg/L·h | 307 (15.4%) | 305 (279–332) |
| AUMClast | mg/L·h2 | 13,800 (16.0%) | 13,800 (12,300–15,000) |
| AUMCinf | mg/L·h2 | 22,700 (14.8%) | 22,300 (20,200–23,200) |
| MRTinf | h | 74.0 (12.8%) | 76.7 (68.0–80.7) |
| CLNCA | L/h/kg | 0.0105 (15.2%) | 0.0103 (0.00896–0.0117) |
| VzZNCA | L/kg | 0.825 (21.3%) | 0.761 (0.693–0.891) |
| VssNCA | L/kg | 0.776 (19.6%) | 0.734 (0.661–0.859) |
| t1/2λz | h | 54.6 (13.2%) | 55.0 (51.9–58.6) |
| Population PK results | |||
| CL | L/h/kg | 0.0107 (14.0%) | 0.0104 (0.00961–0.0122) |
| VC | L/kg | 0.0644 (28.9%) | 0.0649 (0.0526–0.0733) |
| Vss | L/kg | 1.21 (23.0%) | 1.24 (1.06–1.34) |
| AUC | mg/L·h | 299 (16.0%) | 300 (263–316) |
| 1st t1/2 | h | 0.345 (36.3%) | 0.317 (0.289–0.417) |
| 2nd t1/2 | h | 4.12 (22.2%) | 4.23 (3.78–4.83) |
| 3rd t1/2 | h | 103 (29.9%) | 97.9 (83.1–116) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-K.; Jo, K.-M.; Lee, J.-H.; Jang, J.-H.; Choe, E.-J.; Kang, G.; Zang, D.-Y.; Lee, D.-H. Beyond One-Size-Fits-All: Tailoring Teicoplanin Regimens for Normal Renal Function Patients Using Population Pharmacokinetics and Monte Carlo Simulation. Pharmaceutics 2024, 16, 499. https://doi.org/10.3390/pharmaceutics16040499
Kim Y-K, Jo K-M, Lee J-H, Jang J-H, Choe E-J, Kang G, Zang D-Y, Lee D-H. Beyond One-Size-Fits-All: Tailoring Teicoplanin Regimens for Normal Renal Function Patients Using Population Pharmacokinetics and Monte Carlo Simulation. Pharmaceutics. 2024; 16(4):499. https://doi.org/10.3390/pharmaceutics16040499
Chicago/Turabian StyleKim, Yong-Kyun, Kyeong-Min Jo, Jae-Ha Lee, Ji-Hoon Jang, Eun-Jun Choe, Gaeun Kang, Dae-Young Zang, and Dong-Hwan Lee. 2024. "Beyond One-Size-Fits-All: Tailoring Teicoplanin Regimens for Normal Renal Function Patients Using Population Pharmacokinetics and Monte Carlo Simulation" Pharmaceutics 16, no. 4: 499. https://doi.org/10.3390/pharmaceutics16040499





