Pharmacogenomic Studies of Antiviral Drug Favipiravir
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Chemicals
2.3. Preparation of Modified Electrode
3. Results and Discussion
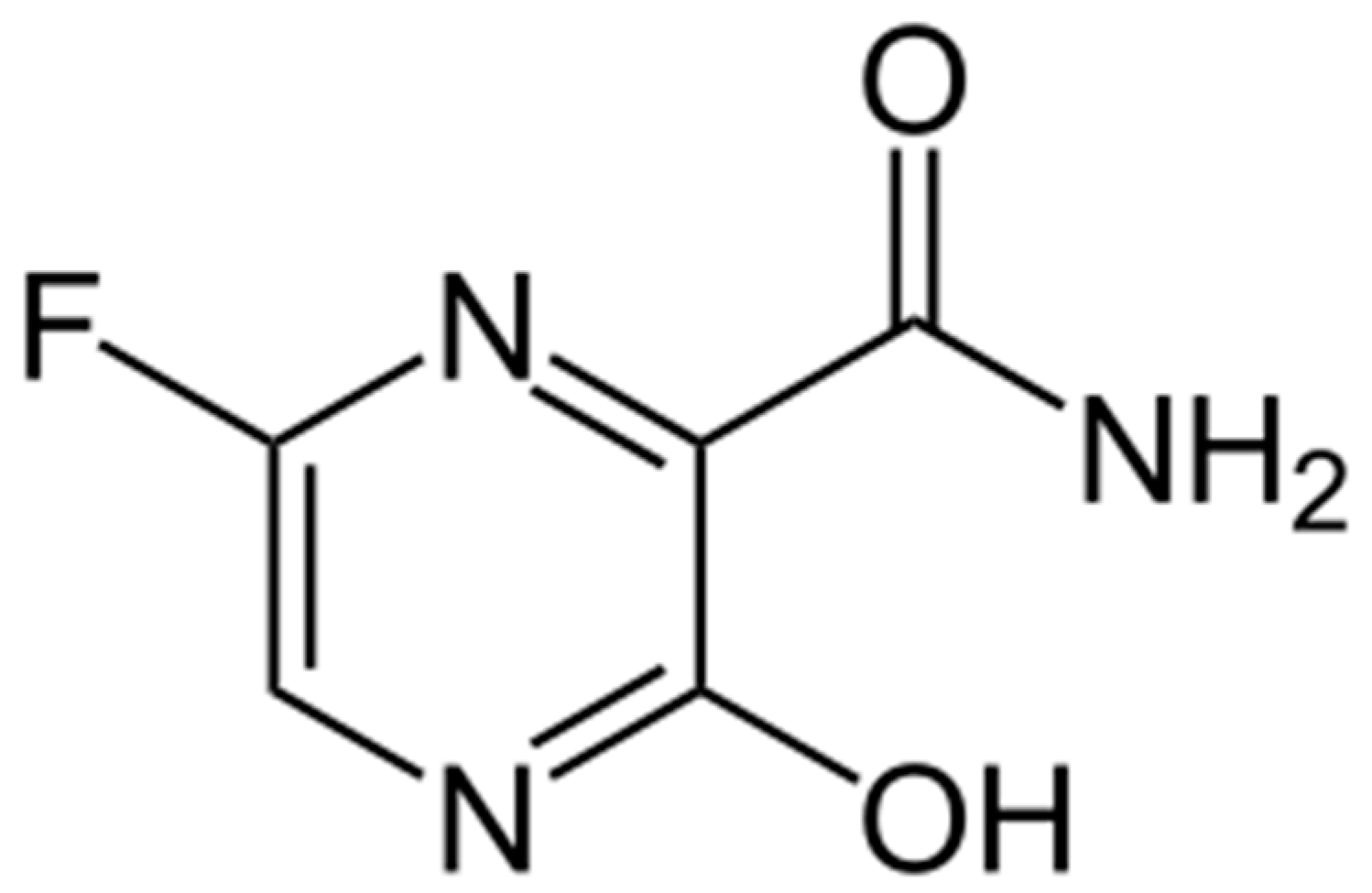
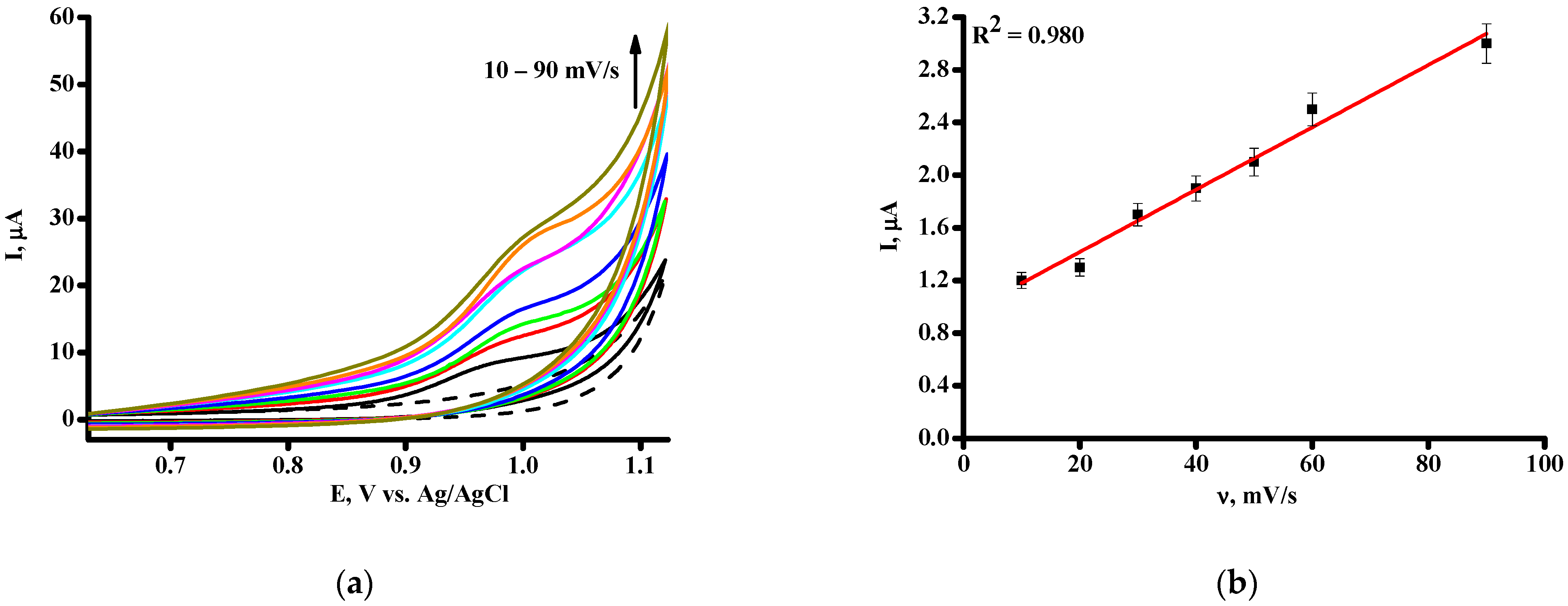
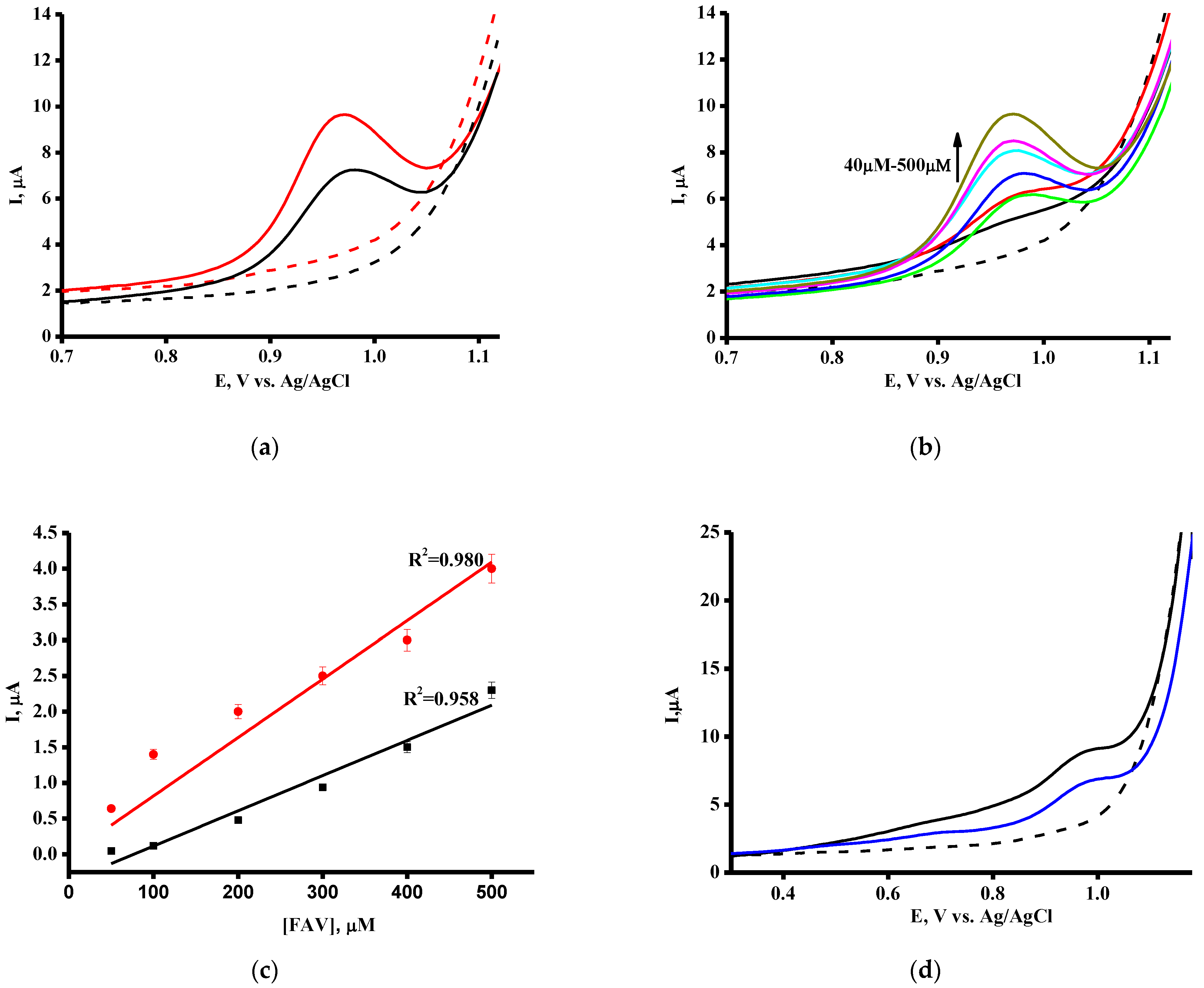
3.1. Electrochemical Profiling of Favipiravir on SPE/CNT and SPE/CNT/TiO2
3.2. Investigation of the Interaction between Favipiravir and dsDNA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madelain, V.; Mentré, F.; Baize, S.; Anglaret, X.; Laouénan, C.; Oestereich, L.; Nguyen, T.H.T.; Malvy, D.; Piorkowski, G.; Graw, F.; et al. Modeling favipiravir antiviral efficacy against emerging viruses: From animal studies to clinical trials. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Takahashi, K.; Fukuda, Y.; Kuno, M.; Kamiyama, T.; Kozaki, K.; Nomura, N.; Egawa, H.; Minami, S.; Watanabe, Y.; et al. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob. Agents Chemother. 2002, 46, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Takahashi, K.; Kuno-Maekawa, M.; Sangawa, H.; Uehara, S.; Kozaki, K.; Nomura, N.; Egawa, H.; Shiraki, K. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 2005, 49, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Kiso, M.; Takahashi, K.; Sakai-Tagawa, Y.; Shinya, K.; Sakabe, S.; Le, Q.M.; Ozawa, M.; Furuta, Y.; Kawaoka, Y. T-705 (favipiravir) activity against lethal H5N1 influenza A viruses. Proc. Natl. Acad. Sci. USA 2010, 107, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceuticals and Medical Devices Agency, Report on the Deliberation Results—Avigan; Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau: Tokyo, Japan, 2011. Available online: www.pmda.go.jp/files/000210319.pdf (accessed on 30 October 2023).
- Fang, Q.Q.; Huang, W.J.; Li, X.Y.; Cheng, Y.H.; Tan, M.J.; Liu, J.; Wei, H.J.; Meng, Y.; Wang, D.Y. Effectiveness of favipiravir (T-705) against wild-type and oseltamivir-resistant influenza B virus in mice. Virology 2020, 545, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pires de Mello, C.P.; Tao, X.; Kim, T.H.; Bulitta, J.B.; Rodriquez, J.L.; Pomeroy, J.J.; Brown, A.N. Zika virus replication is substantially inhibited by novel favipiravir and interferon alpha combination regimens. Antimicrob. Agents Chemother. 2017, 62, e01983-17. [Google Scholar] [CrossRef] [PubMed]
- Madelain, V.; Guedj, J.; Mentré, F.; Nguyen, T.H.; Jacquot, F.; Oestereich, L.; Kadota, T.; Yamada, K.; Taburet, A.M.; de Lamballerie, X.; et al. Favipiravir pharmacokinetics in nonhuman primates and insights for future efficacy studies of hemorrhagic fever viruses. Antimicrob. Agents Chemother. 2016, 61, e01305-16. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Romero, E.; Jiménez de Oya, N.; Domingo, E.; Saiz, J.C. Extinction of West Nile virus by favipiravir through lethal mutagenesis. Antimicrob. Agents Chemother. 2017, 61, e01400-17. [Google Scholar] [CrossRef] [PubMed]
- Borrego, B.; de Ávila, A.I.; Domingo, E.; Brun, A. Lethal mutagenesis of Rift Valley Fever Virus Induced by Favipiravir. Antimicrob. Agents Chemother. 2019, 63, e00669-19. [Google Scholar] [CrossRef] [PubMed]
- Oestereich, L.; Rieger, T.; Neumann, M.; Bernreuther, C.; Lehmann, M.; Krasemann, S.; Wurr, S.; Emmerich, P.; de Lamballerie, X.; Ölschläger, S.; et al. Evaluation of antiviral efficacy of ribavirin, arbidol, and T-705 (favipiravir) in a mouse model for Crimean-Congo hemorrhagic fever. PLoS Negl. Trop. Dis. 2014, 8, 2804. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, M.; Russell, A.; Smee, D.F.; Hall, J.O.; Skirpstunas, R.; Furuta, Y.; Gowen, B.B. Effective oral favipiravir (T-705) therapy initiated after the onset of clinical disease in a model of arenavirus hemorrhagic fever. PLoS Negl. Trop. Dis. 2011, 5, e1342. [Google Scholar] [CrossRef] [PubMed]
- Smyk, J.M.; Majewska, A. Favipiravir in the battle with respiratory viruses. Mini Rev. Med. Chem. 2022, 22, 2224–2236. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, D.; van Nieuwkoop, S.; Smits, S.L.; Neyts, J.; Fouchier, R.A.; van den Hoogen, B.G. Antiviral activity of favipiravir (T-705) against a broad range of paramyxoviruses in vitro and against human metapneumovirus in hamsters. Antimicrob. Agents Chemother. 2016, 60, 4620–4629. [Google Scholar] [CrossRef] [PubMed]
- Kerber, R.; Lorenz, E.; Duraffour, S.; Sissoko, D.; Rudolf, M.; Jaeger, A.; Cisse, S.D.; Camara, A.M.; Miranda, O.; Castro, C.M.; et al. Laboratory findings, compassionate use of favipiravir, and outcome in patients with Ebola virus disease, Guinea, 2015-A Retrospective observational study. J. Infect. Dis. 2019, 220, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Zhou, Y.; Tu, T.; Jiang, D.; Pang, M.; Li, Y.; Luo, Y.; Yao, X.; Yang, Z.; Wang, Y. RVG peptide-functionalized favipiravir nanoparticle delivery system facilitates antiviral therapy of neurotropic virus infection in a mouse model. Int. J. Mol. Sci. 2023, 24, 5851. [Google Scholar] [CrossRef] [PubMed]
- Takashita, E.; Morita, H.; Nagata, S.; Shirakura, M.; Fujisaki, S.; Miura, H.; Takayama, I.; Arita, T.; Suzuki, Y.; Yamaoka, M.; et al. Influenza Virus Surveillance Group of Japan. Antiviral susceptibilities of avian influenza A(H5), A(H7), and A(H9) viruses isolated in Japan. Jpn. J. Infect. Dis. 2022, 75, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Zaraket, H.; Saito, R. Japanese surveillance systems and treatment for influenza. Curr. Treat. Options Infect. Dis. 2016, 8, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Huang, J.; Yin, P.; Cheng, Z.; Wu, J.; Chen, S.; Zhang, Y.; Chen, B.; Lu, M.; et al. Favipiravir versus arbidol for clinical recovery rate in moderate and severe adult COVID-19 patients: A prospective, multicenter, open-label, randomized controlled clinical trial. Front. Pharmacol. 2021, 12, 683296. [Google Scholar] [CrossRef] [PubMed]
- Ivashchenko, A.A.; Dmitriev, K.A.; Vostokova, N.V.; Azarova, V.N.; Blinow, A.A.; Egorova, A.N.; Gordeev, I.G.; Ilin, A.P.; Karapetian, R.N.; Kravchenko, D.V.; et al. AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): Interim results of a phase II/III multicenter randomized clinical trial. Clin. Infect. Dis. 2021, 73, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Ruzhentsova, T.A.; Oseshnyuk, R.A.; Soluyanova, T.N.; Dmitrikova, E.P.; Mustafaev, D.M.; Pokrovskiy, K.A.; Markova, T.N.; Rusanova, M.G.; Kostina, N.E.; Agafina, A.S.; et al. Phase 3 trial of coronavir (favipiravir) in patients with mild to moderate COVID-19. Am. J. Transl. Res. 2021, 13, 12575–12587. [Google Scholar] [CrossRef] [PubMed]
- Hung, D.T.; Ghula, S.; Aziz, J.M.A.; Makram, A.M.; Tawfik, G.M.; Abozaid, A.A.; Pancharatnam, R.A.; Ibrahim, A.M.; Shabouk, M.B.; Turnage, M.; et al. The efficacy and adverse effects of favipiravir on patients with COVID-19: A systematic review and meta-analysis of published clinical trials and observational studies. Int. J. Infect. Dis. 2022, 120, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin-Akyildiz, A.; Aksoy, N.; Boran, T.; Ilhan, E.N.; Ozhan, G. Favipiravir induces oxidative stress and genotoxicity in cardiac and skin cells. Toxicol. Lett. 2022, 371, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A.O.; El-Readi, M.Z.; Althubiti, M.; Alhindi, Y.Z.; Ayoub, N.; Alzahrani, A.R.; Al-Ghamdi, S.S.; Eid, S.Y. Liver injury in favipiravir-treated COVID-19 patients: Retrospective single-center cohort study. Trop. Med. Infect. Dis. 2023, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Smith, L.K.; Rajwanshi, V.K.; Kim, B.; Deval, J. The ambiguous base-pairing and high substrate efficiency of T-705 (Favipiravir) Ribofuranosyl 5′-triphosphate towards influenza A virus polymerase. PLoS ONE 2013, 8, e68347. [Google Scholar] [CrossRef] [PubMed]
- Naesens, L.; Guddat, L.W.; Keough, D.T.; van Kuilenburg, A.B.P.; Meijer, J.; Voorde, J.V.; Balzarini, J. Role of human hypoxanthine guanine phosphoribosyltransferase in activation of the antiviral agent T-705 (favipiravir). Mol. Pharmacol. 2013, 84, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Huchting, J.; Vanderlinden, E.; Winkler, M.; Nasser, H.; Naesens, L.; Meier, C. Prodrugs of the phosphoribosylated forms of hydroxypyrazinecarboxamide pseudobase T-705 and its de-fluoro analogue T-1105 as potent influenza virus inhibitors. J. Med. Chem. 2018, 61, 6193–6210. [Google Scholar] [CrossRef] [PubMed]
- Baranovich, T.; Wong, S.-S.; Armstrong, J.; Marjuki, H.; Webby, R.J.; Webster, R.G.; Govorkova, E.A. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J. Virol. 2013, 87, 3741–3751. [Google Scholar] [CrossRef] [PubMed]
- Jena, N.R. Role of different tautomers in the base-pairing abilities of some of the vital antiviral drugs used against COVID-19. Phys. Chem. Chem. Phys. 2022, 22, 28115–28122. [Google Scholar] [CrossRef] [PubMed]
- de Ávila, A.I.; Gallego, I.; Soria, M.E.; Gregori, J.; Quer, J.; Esteban, J.I.; Rice, C.M.; Domingo, E.; Perales, C. Lethal mutagenesis of hepatitis C virus induced by favipiravir. PLoS ONE 2016, 11, e0164691. [Google Scholar] [CrossRef] [PubMed]
- Vanderlinden, E.; Vrancken, B.; Van Houdt, J.; Rajwanshi, V.K.; Gillemot, S.; Andrei, G.; Lemey, P.; Naesens, L. Distinct effects of T-705 (favipiravir) and ribavirin on influenza virus replication and viral RNA synthesis. Antimicrob. Agents Chemother. 2016, 60, 6679–6691. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, C.; Xu, X.; Chong, T.H.; Zhang, L.; Cheung, P.P.-H.; Huang, X. The mechanism of action of T-705 as a unique delayed chain terminator on influenza viral polymerase transcription. Biophys. Chem. 2021, 277, 106652. [Google Scholar] [CrossRef] [PubMed]
- Huchting, J.; Vanderlinden, E.; Van Berwaer, R.; Meier, C.; Naesens, L. Cell line-dependent activation and antiviral activity of T-1105, the non-fluorinated analogue of T-705 (favipiravir). Antivir. Res. 2019, 167, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sumitha, M.S.; Xavier, T.S. Recent advances in electrochemical biosensors—A brief review. Hybrid Adv. 2023, 2, 100023. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Dogan-Topal, B.; Rodriguez, E.P.; Bozal-Palabiyik, B.; Ozkan, S.A.; Uslu, B. Advances in electrochemical DNA biosensors and their interaction mechanism with pharmaceuticals. J. Electroanalyt. Chem. 2016, 775, 8–26. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N. Pharmacogenomic study using bio- and nanobioelectrochemistry: Drug–DNA interaction. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 1002–1017. [Google Scholar] [CrossRef] [PubMed]
- Ramotowska, S.; Ciesielska, A.; Makowski, M. What can electrochemical methods offer in determining DNA–drug interactions? Molecules 2021, 26, 3478. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Sarwar, T.; Husain, M.; Ishqi, H.; Tabish, M. Studying non-covalent drug–DNA interactions. Arch. Biochem. Biophys. 2015, 576, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Held, P.G. Nucleic Acid Purity Assessment Using A260/A280 Ratios; BioTek Instruments Incorporation: Winooski, VT, USA, 2006; pp. 1–5. [Google Scholar]
- Shumyantseva, V.V.; Bulko, T.V.; Agafonova, L.E.; Pronina, V.V.; Kostryukova, L.V. Comparative analysis of the interaction between the antiviral drug umifenovir and umifenovir encapsulated in phospholipids micelles (nanosome/umifenovir) with dsDNA as a model for pharmacogenomic analysis by electrochemical methods. Processes 2023, 11, 922. [Google Scholar] [CrossRef]
- Pronina, V.V.; Kostryukova, L.V.; Bulko, T.V.; Shumyantseva, V.V. Interaction of doxorubicin embedded into phospholipid nanoparticles and targeted peptide-modified phospholipid nanoparticles with DNA. Molecules 2023, 28, 5317. [Google Scholar] [CrossRef] [PubMed]
- Shumyantseva, V.V.; Bulko, T.V.; Kuzikov, A.V.; Khan, R.; Archakov, A.I. Development of methods for functionalization of screen-printed electrodes with biocompatible organic-inorganic hybrid nanocomposites for biosensing applications. Biochem. (Mos.) Sup. Ser. B Biomed. Chem. 2014, 8, 237–242. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Bulko, T.V.; Kuzikov, A.V.; Masamrekh, R.; Archakov, A.I. Analysis of L-tyrosine based on electrocatalytic oxidative reactions via screen-printed electrodes modified with multi-walled carbon nanotubes and nanosized titanium oxide (TiO2). Amino Acids 2018, 50, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 7rd ed; Pearson: London, UK, 2020. [Google Scholar]
- Shankar, S.S.; Kumara Swamy, B.E.; Mahanthesha, K.R.; Sathisha, T.V.; Vishwanath, C.C. Acetanilide modified carbon paste electrode for the electrochemical detection of dopamine: A cyclic voltammetric study. Anal. Bioanal. Electrochem. 2013, 5, 19–31. [Google Scholar]
- Konstantinova, I.D.; Andronova, V.L.; Fateev, I.V.; Esipov, R.S. Favipiravir and its structural analogs: Antiviral activity and synthesis methods. Acta Nat. 2022, 14, 16–38. [Google Scholar] [CrossRef] [PubMed]
- Mehmandoust, M.; Khoshnavaz, Y.; Tuzen, M.; Erk, N. Voltammetric sensor based on bimetallic nanocomposite for determination of favipiravir as an antiviral drug. Microchim. Acta 2021, 188, 434. [Google Scholar] [CrossRef]
- Allahverdiyeva, S.; Yunusoğlu, O.; Yardım, Y.; Şenturk, Z. First electrochemical evaluation of favipiravir used as an antiviral option in the treatment of COVID-19: A study of its enhanced voltammetric determination in cationic surfactant media using a boron-doped diamond electrode. Anal. Chim. Acta 2021, 1159, 338418. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Eldin, G.M.; Ismail, S.M.; Zine, N.; Elaissari, A.; Jaffrezic-Renault, N.; Errachid, A. Innovative electrochemical sensor for the precise determination of the new antiviral COVID-19 treatment Favipiravir in the presence of coadministered drugs. J. Electroanal. Chem. 2021, 895, 115422. [Google Scholar] [CrossRef] [PubMed]
- Erşan, T.; Dilgin, D.G.; Kumrulu, E.; Kumrulu, U.; Dilgin, Y. Voltammetric determination of favipiravir used as an antiviral drug for the treatment of COVID-19 at pencil graphite electrode. Electroanalytical 2023, 35, e202200295. [Google Scholar] [CrossRef]
- García-Miranda Ferrari, A.; Rowley-Neale, S.J.; Banks, C.E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open 2021, 3, 100032. [Google Scholar] [CrossRef]
- Paimard, G.; Ghasali, E.; Baeza, M. Screen-printed electrodes: Fabrication, modification, and biosensing applications. Chemosensors 2023, 11, 113. [Google Scholar] [CrossRef]
- Carrara, S.; Baj-Rossi, C.; Boero, C.; De Micheli, G. Do Carbon Nanotubes contribute to Electrochemical Biosensing? Electrochim. Acta 2014, 128, 102–112. [Google Scholar] [CrossRef]
- Alim, S.; Vejayan, J.; Yusoff, M.M.; Kafi, A.K.M. Recent uses of carbon nanotubes & gold nanoparticles in electrochemistry with application in biosensing: A review. Biosens. Bioelectron. 2018, 121, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Fine, D.H.; Tasciotti, E.; Bouamrani, A.; Ferrari, M. Nanodevices in diagnostics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. Nanostructured titanium oxide hybrids-based electrochemical biosensors for healthcare applications. Colloids Surf. B Biointerfaces 2019, 178, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons Inc.: New York, NY, USA, 2001. [Google Scholar]
- Gosser, D.K. Cyclic Voltammetry; VCH: New York, NY, USA, 1994. [Google Scholar]
- Compton, B.R.G.; Banks, C.E. Understanding Voltammetry; Imperial College Press: London, UK, 2011. [Google Scholar]
- Nicholson, R.S.; Shain, I. Theory of stationary electrode polarography. Single scan and cyclic methods applied to reversible, irreversible, and kinetic systems. Analyt. Chem. 1964, 36, 706–723. [Google Scholar] [CrossRef]
- Manna, S.; Sharma, A.; Satpati, A.K. Electrochemical methods in understanding the redox processes of drugs and biomolecules and their sensing. Curr. Opin. Electrochem. 2022, 32, 100886. [Google Scholar] [CrossRef]
- Mohamed, R.M.K.; Mohamed, S.H.; Asran, A.M.; Alsohaimi, I.H.; Hassan, H.M.A.; Ibrahim, H.; El-Wekil, M.M. Synergistic effect of gold nanoparticles anchored on conductive carbon black as an efficient electrochemical sensor for sensitive detection of anti-COVID-19 drug Favipiravir in absence and presence of co-administered drug Paracetamol. Microchem. J. 2023, 190, 108696. [Google Scholar] [CrossRef] [PubMed]
- Dindar, K.C.; Bozal-Palabiyik, B.; Uslu, B. Development of a Diamond Nanoparticles-based Nanosensor for Detection and Determination of Antiviral Drug Favipiravir. Electroanalytical 2022, 34, 1174–1186. [Google Scholar] [CrossRef]
- Akça, Z.; Özok, H.İ.; Yardım, Y.; Şenturk, Z. Electroanalytical investigation and voltammetric quantification of antiviral drug favipiravir in the pharmaceutical formulation and urine sample using a glassy carbon electrode in anionic surfactant media. Turk. J. Chem. 2022, 46, 869–880. [Google Scholar] [CrossRef] [PubMed]
- You, X.-H.; Liu, Y.; Li, Y.-Y.; Zhao, B.; Yang, Y.; Weerasooriya, R.; Chen, X. Sensitive detection of SARS-CoV-2 spike protein based on electrochemical impedance spectroscopy of Fe3O4@SiO2–Au/GCE biosensor. Adv. Sens. Energy Mater. 2023, 2, 100067. [Google Scholar] [CrossRef]
- Bouali, W.; Erk, N.; Kholafazadehastamal, G.; Naser, M.; Tiris, G. Low-cost voltammetric sensor based on reduced graphene oxide anchored on platinum nanoparticles for robust determination of Favipiravir in real samples. Diam. Relat. Mater. 2023, 131, 109609. [Google Scholar] [CrossRef]
- Rupar, J.; Aleksić, M.M.; Dobričić, V.; Brborić, J.; Čudina, O. An electrochemical study of 9-chloroacridine redox behavior and its interaction with double-stranded DNA. Bioelectrochemistry 2020, 135, 107579. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Brett, A.M. Electrochemical DNA Assays. In Bioelectrochemistry: Fundamentals, Experimental Techniques and Applications; Bartlett, P.N., Ed.; John Wiley & Sons: New York, NY, USA, 2008; pp. 411–442. [Google Scholar]
- Galal, A.; Ahmed, Y.M.; Ahmed, M.H.M.; Atta, N.F. Electrochemistry and determination of an antiviral drug at ionic liquids crystals-carbon nanotubes modified glassy carbon electrode. J. Electrochem. Soc. 2021, 168, 116512. [Google Scholar] [CrossRef]
- Nafisi, S.; Saboury, A.A.; Keramat, N.; Neault, J.F.; Tajmir-Riahi, H.A. Stability and structural features of DNA intercalation with ethidium bromide, acridine orange and methylene blue. J. Mol. Struct. 2007, 827, 35–43. [Google Scholar] [CrossRef]
- Agafonova, L.; Tikhonova, E.; Sanzhakov, M.; Kostryukova, L.; Shumyantseva, V. Electrochemical studies of the interaction of phospholipid nanoparticles with dsDNA. Processes 2022, 10, 2324. [Google Scholar] [CrossRef]
- Campos-Carrillo, A.; Weitzel, J.N.; Sahoo, P.; Rockne, R.; Mokhnatkin, J.V.; Murtaza, M.; Gray, S.W.; Goetz, L.; Goel, A.; Schorka, N.; et al. Circulating tumor DNA as an early cancer detection tool. Pharmacol. Ther. 2020, 207, 107458. [Google Scholar] [CrossRef] [PubMed]
- Chiorcea-Paquim, A.-M.; Oliveira-Brett, A.M. Electrochemistry of chemotherapeutic alkylating agents and their interaction with DNA. J. Pharm. Biomed. Anal. 2023, 222, 115036. [Google Scholar] [CrossRef] [PubMed]
- Bafna, V.; Mischel, P.S. Extrachromosomal DNA in Cancer. Annu. Rev. Genom. Hum. Genet. 2022, 23, 29–52. [Google Scholar] [CrossRef]
- Salma, U.; Siciliano, G.; Primiceri, E.; Turco, A.; Tarantini, I.; Ferrara, F.; Chiriaco, M.S. Electrochemical sensors for liquid biopsy and their integration into lab-on-chip platforms: Revolutionizing the approach to diseases. Chemosensors 2023, 11, 517. [Google Scholar] [CrossRef]
- Talapa, J.; Zhaob, J.; Shena, M.; Songa, Z.; Zhoua, H.; Kanga, Y.; Suna, L.; Yua, L.; Zenga, S.; Caia, S. Recent advances in therapeutic nucleic acids and their analytical methods. J. Pharm. Biomed. Anal. 2021, 206, 114368. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Shah, M.R.; Barek, J.; Malik, M.I. Cancer biomarkers and their biosensors: A comprehensive review. Tr. Anal. Chem. 2023, 158, 116813. [Google Scholar] [CrossRef]
- Madelain, V.; Nguyen, T.H.T.; Olivo, A.; De Lamballerie, X.; Guedj, J.; Taburet, A.-M.; Mentré, F. Ebola virus infection: Review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin. Pharmacokinet. 2016, 55, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Morawska, K.; Popławski, T.; Ciesielski, W.; Smarzewska, S. Electrochemical and spectroscopic studies of the interaction of antiviral drug Tenofovir with single and double stranded DNA. Bioelectrochemistry 2018, 123, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Kostryukova, L.V.; Tereshkina, Y.A.; Tikhonova, E.G.; Khudoklinova, Y.Y.; Bobrova, D.V.; Gisina, A.M.; Morozevich, G.E.; Pronina, V.V.; Bulko, T.V.; Shumyantseva, V.V. Effect of an NGR Peptide on the Efficacy of the Doxorubicin Phospholipid Delivery System. Nanomaterials 2023, 13, 2229. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.E.; Velea, L.M. Intercalative Binding of Small Molecules to Nucleic Acids. Curr. Org. Chem. 2000, 4, 915–929. [Google Scholar] [CrossRef]
- Paleček, E.; Bartošík, M. Electrochemistry of Nucleic Acids. Chem. Rev. 2012, 112, 3427–3481. [Google Scholar] [CrossRef]
- Bolat, G. Investigation of poly(CTAB-MWCNTs) composite based electrochemical DNA biosensor and interaction study with anticancer drug Irinotecan. Microchem. J. 2020, 159, 105426. [Google Scholar] [CrossRef]
- Muti, M.; Muti, M. Electrochemical monitoring of the interaction between anticancer drug and DNA in the presence of antioxidant. Talanta 2018, 178, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
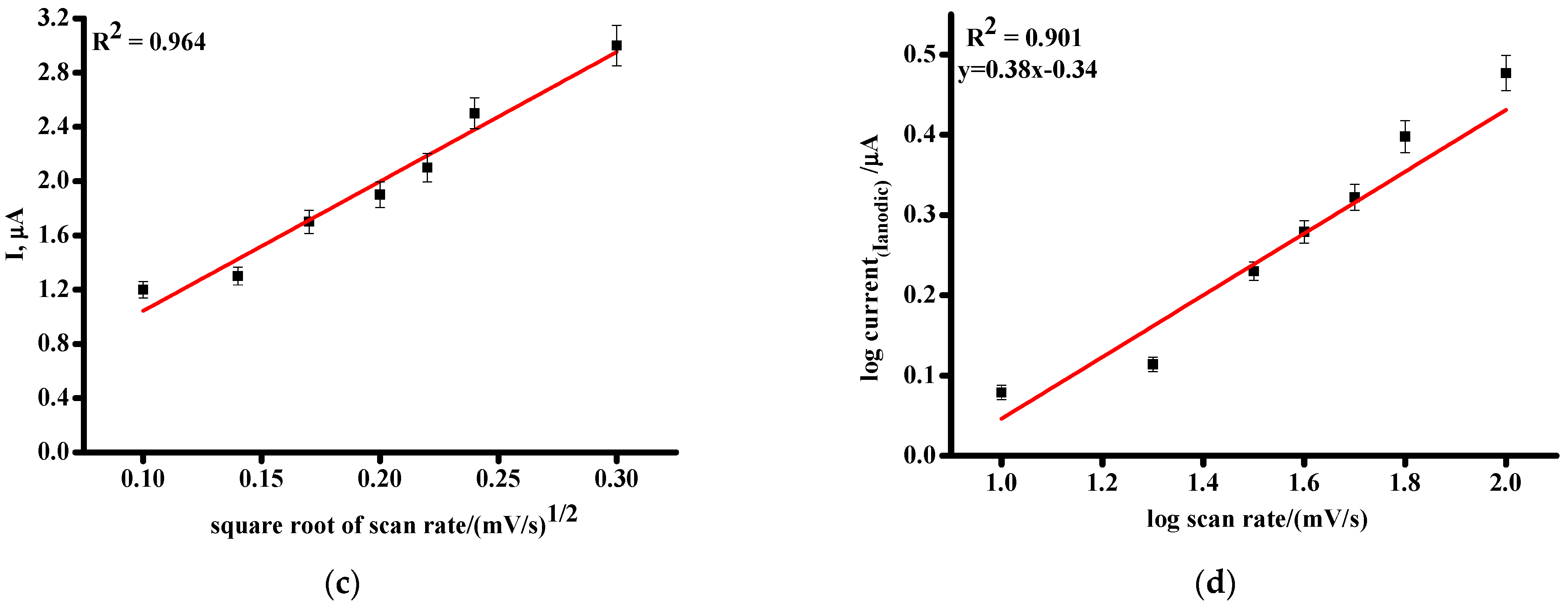

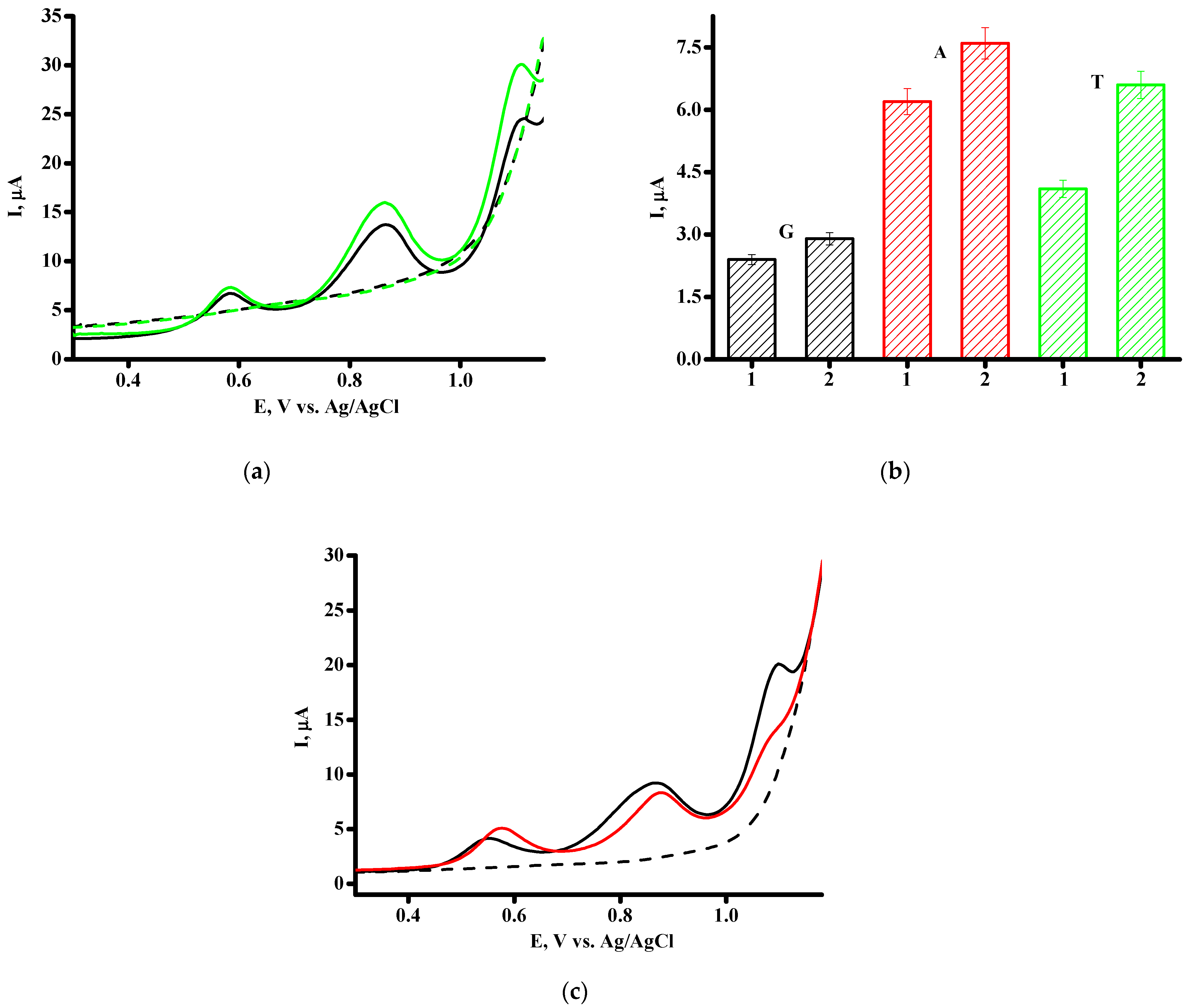

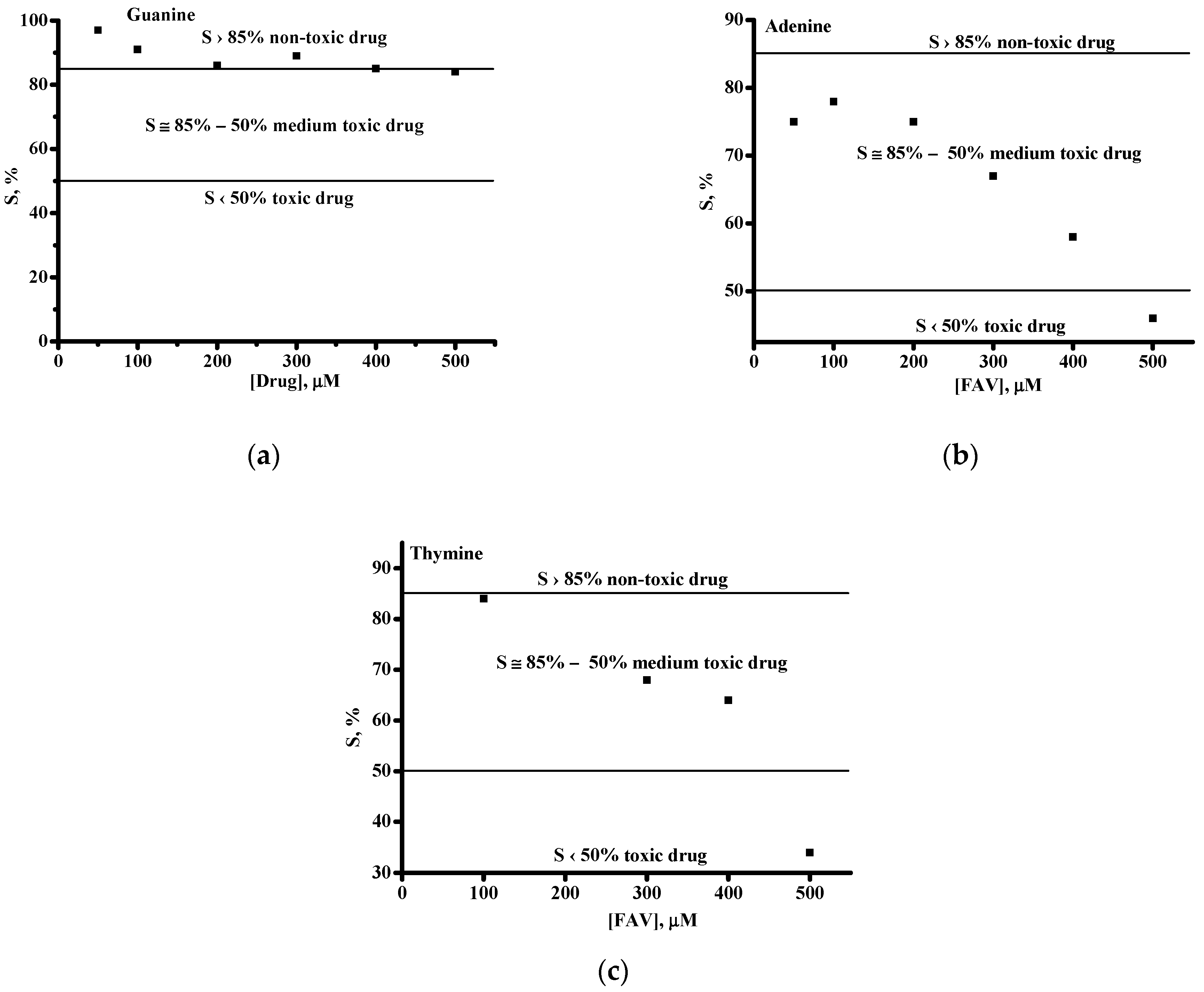


| Parameters | SPE/CNT | SPE/CNT/TiO2 |
|---|---|---|
| Eox, V | 0.972 ± 0.003 | 0.967 ± 0.003 |
| Sensitivity, µA/µM (Slope) | 0.0049 | 0.0082 |
| Linear range, µM | 50–500 | 50–500 |
| LOD, µM | 60 | 37 |
| Equation for linear regression 1 | Iox = (0.0049 ± 0.0005) [FAV] − 0.38 ± 0.10 | Iox = (0.0082 ± 0.0005) [FAV] + 0.52 ± 0.10 |
| Correlation coefficient, R2 | 0.958 | 0.980 |
| FAV/dsDNA | Kb, M−1 | ΔG = −RTln Kb, kJ/mol |
|---|---|---|
| Based on G oxidation signals | 0.24 × 104 | −18.96 |
| Based on A oxidation signals | 1.03 × 104 | −22.51 |
| Based on T oxidation signals | 0.20 × 104 | −18.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumyantseva, V.V.; Bulko, T.V.; Chistov, A.A.; Kolesanova, E.F.; Agafonova, L.E. Pharmacogenomic Studies of Antiviral Drug Favipiravir. Pharmaceutics 2024, 16, 503. https://doi.org/10.3390/pharmaceutics16040503
Shumyantseva VV, Bulko TV, Chistov AA, Kolesanova EF, Agafonova LE. Pharmacogenomic Studies of Antiviral Drug Favipiravir. Pharmaceutics. 2024; 16(4):503. https://doi.org/10.3390/pharmaceutics16040503
Chicago/Turabian StyleShumyantseva, Victoria V., Tatiana V. Bulko, Alexey A. Chistov, Ekaterina F. Kolesanova, and Lyubov E. Agafonova. 2024. "Pharmacogenomic Studies of Antiviral Drug Favipiravir" Pharmaceutics 16, no. 4: 503. https://doi.org/10.3390/pharmaceutics16040503
APA StyleShumyantseva, V. V., Bulko, T. V., Chistov, A. A., Kolesanova, E. F., & Agafonova, L. E. (2024). Pharmacogenomic Studies of Antiviral Drug Favipiravir. Pharmaceutics, 16(4), 503. https://doi.org/10.3390/pharmaceutics16040503







