Abstract
Cutaneous leishmaniasis (CL) is a neglected tropical disease. The treatment is restricted to drugs, such as meglumine antimoniate and amphotericin B, that exhibit toxic effects, high cost, long-term treatment, and limited efficacy. The development of new alternative therapies, including the identification of effective drugs for the topical and oral treatment of CL, is of great interest. In this sense, a combination of topical photodynamic therapy (PDT) with chloroaluminum phthalocyanine liposomes (Lip-ClAlPc) and the oral administration of a self-emulsifying drug delivery system containing fexinidazole (SEDDS-FEX) emerges as a new strategy. The aim of the present study was to prepare, characterize, and evaluate the efficacy of combined therapy with Lip-ClAlPc and SEDDS-FEX in the experimental treatment of Leishmania (Leishmania) major. Lip-ClAlPc and SEDDS-FEX were prepared, and the antileishmanial efficacy study was conducted with the following groups: 1. Lip-ClAlPc (0.05 mL); 2. SEDDS-FEX (50 mg/kg/day); 3. Lip-ClAlPc (0.05 mL)+SEDDS-FEX (50 mg/kg/day) combination; 4. FEX suspension (50 mg/kg/day); and 5. control (untreated). BALB/c mice received 10 sessions of topical Lip-ClAlPc on alternate days and 20 consecutive days of SEDDS-FEX or FEX oral suspension. Therapeutical efficacy was evaluated via the parasite burden (limiting-dilution assay), lesion size (mm), healing of the lesion, and histological analyses. Lip-ClAlPc and SEDDS-FEX presented physicochemical characteristics that are compatible with the administration routes used in the treatments. Lip-ClAlPc+SEDDS-FEX led to a significant reduction in the parasitic burden in the lesion and spleen when compared to the control group (p < 0.05) and the complete healing of the lesion in 43% of animals. The Lip-ClAlPc+SEDDS-FEX combination may be promising for the treatment of CL caused by L. major.
1. Introduction
Leishmaniasis is considered a serious public health problem and is distributed worldwide. The main forms of the disease are cutaneous leishmaniasis (CL), visceral leishmaniasis, and mucocutaneous leishmaniasis. CL is endemic in about 87 countries around the world, and it is generally limited to an ulcer that self-heals over about a year; however, in some cases, it can cause scarring and disfigurement [1,2]. Geographically, CL can be divided into ‘Old World’ and ‘New World’ leishmaniasis. In Old World CL is predominantly caused by Leishmania (Leishmania) major [3].
The chemotherapeutic arsenal for CL consists of drugs, such as meglumine antimoniate (SbV), pentamidine, amphotericin B, and miltefosine. These drugs have limitations such as low efficacy, the potential development of resistance, parenteral administration, and the need for hospitalization, making adherence difficult and increasing related costs. In addition, these treatments can cause serious side effects, such as hematological, pancreatic, and cardiac toxicity and teratogenicity [4]. The World Health Organization (WHO) recommends the investigation of alternative drugs and topical (local) and systemic (oral) formulations.
Fexinidazole (FEX) exhibited activity in vitro and in vivo, demonstrating its potential in the oral therapy of leishmaniasis [5,6,7]. However, FEX is sparingly soluble in water, which can be a limiting factor for its oral absorption. A self-emulsifying drug delivery system (SEDDS) is considered a potential carrier for oral administration, and it is a technology based on an isotropic mixture of lipids, surfactants, and co-surfactants, which forms a fine oil-in-water emulsion, in an aqueous medium after slight agitation. The SEDDS has gained interest in recent years for overcoming the challenges of the poor and irregular oral bioavailability of hydrophobic drugs [8,9,10]. This improvement can be explained by factors such as the following: no step being required for dissolution, greater interfacial surface area due to the fine dispersion of globules, and increased membrane fluidity [9,11,12].
Photodynamic therapy (PDT) is based on the photoactivation of photosensitizer molecules (PSs), which can generate cytotoxic reactive oxygen species (ROS), such as singlet oxygen (1O2) and free radicals when irradiated by visible light at a specific wavelength. The species can damage cellular organelles causing cell death and tissue destruction irreversibly [13]. Chloroaluminum phthalocyanine (ClAlPc) is a great PS due to its chemical stability, strong absorption spectrum in the red-light range (670–780 nm), high efficiency in generating ROS, and excellent activity against different Leishmania species after PDT [13,14,15]. The performance of a PS as an antileishmanial drug for topical application can be improved if an appropriate carrier is used for transdermal delivery. Liposomes are a vesicular nanosystem, constructed by the self-assembly of amphipathic molecules in a bilayer in which the hydrophilic head groups face the outer aqueous environment and the hydrocarbon chains assemble within the hydrophobic interior. The incorporation of hydrophilic and lipophilic drugs into liposomes makes them suitable nanocarriers for drug delivery through different administration routes [16]. Liposomes are used as carriers of some antileishmanial drugs, such as AmB, SbV, and miltefosine. The incorporation of ClAlPc into liposomes can facilitate its access to Leishmania-infected cells, increase its photoactivity by conserving its monomeric structure, and reduce toxicity [17].
Thus, this work reports the evaluation of the therapeutical efficacy of chloroaluminum phthalocyanine-loaded liposomes (Lip-ClAlPc) combined with the oral treatment of FEX-loaded SEDDS (SEDDS-FEX) as a new therapy for the treatment of CL caused by L. (L.) major.
2. Materials and Methods
2.1. Preparation of Formulations
Lip-ClAlPcs composed of egg phosphatidylcholine (Lipoid GmbH, Ludwigshafen, Germany) were obtained as previously published by Lopes and collaborators [15], with the following modifications: after preparation, the vesicles were submitted to extrusion using polycarbonate membranes (0.4 μm, 10 times) and purified by ultracentrifugation at 150,000× g at 4 °C for 190 min (Optima® L-80XP; Beckman Coulter, Indianapolis, IN, USA). Finally, the obtained pellet was resuspended to a final volume three times lower than the original.
To prepare SEDDS-FEX, surfactants and co-surfactants were weighed (Tween 80®, Span 80®, and Kolliphor RH 40®) (Sigma Chemical Company, Saint Louis, MO, USA) and homogenized for 2 min on a magnetic stirrer. Then, FEX (Centipharm, Grasse, France) (10 mg/g) was added, and the mixture was stirred for 2 min. After homogenization, the medium-chain triglyceride (MTC) (Lipoid GmbH, Ludwigshafen, Germany) was added slowly and stirred for another 2 min. As the last step of the process, the formulation was placed in an ultrasound bath for 30 min at 40 °C to obtain an isotropic mixture and then kept under magnetic stirring for 24 ± 2 h at room temperature [7]. The selection of the components used in the preparation of SEDDS-FEX was carried out considering the toxicity, solvent capacity, miscibility, physical state at room temperature, and stability.
FEX was weighed in a centrifuge tube (15 mL) containing glass spheres, along with Tween 80®, to improve the homogenization process during the preparation of the FEX suspension. Then, this mixture was homogenized in a vortex until the FEX was completely covered in the Tween 80®. Purified water was added, and the mixture was homogenized again by vortex until a suspension was obtained. The formulation was protected from light and stored at 4 °C for 7 days [6].
2.2. Physicochemical and Chemical Characterization of Formulations
The average diameter and polydispersity index (Pdi) of the systems were investigated via photon correlation spectroscopy, and the zeta potential (ZP) was determined via dynamic light scattering associated with electrophoretic mobility [7,15]. The measurements were performed using Zetasizer Nano ZS90 equipment (Malvern Instruments Ltd., Worcestershire, UK). The Lip-ClAlPc and SEDDS-FEX samples were diluted with a sodium chloride 0.9% (w/v) solution and Milli-Q water, respectively [7,15]. Measurements are performed in triplicate, and values are represented as mean ± standard deviation.
The identification of FEX crystals that are insoluble in SEDDS-FEX and FEX suspension samples was carried out using polarized light microscopy analysis and Microscope Optical Zeiss® (San Francisco, CA, USA). The analyses were carried out at 100× magnification.
The encapsulation percentage (EP) of chloroaluminum phthalocyanine (ClAlPc) in liposomes was determined via the spectrophotometric method with detection at 674 nm (UV mini-1240, Shimadzu, Kyoto, Japan). Initially, the vesicles were opened with ethyl alcohol (95%) at a volume of 1 mL Lip-ClAlPc:2.3 mL ethyl alcohol 95%. The preparations were diluted in ethyl alcohol (95%). The concentration of ClAlPc in the liposome was determined using a regression equation obtained from the ClAlPc standard curve y = 0.467x − 0.0191 [15].
The EP of the ClAlPc was calculated according to the following equation:
The FEX content in SEDDS was measured via the reversed-phase high-performance liquid chromatography (HPLC) method, as previously published by Damasio and collaborators [7]. The concentration of FEX in SEDDS was determined via the regression equation y = 34965x − 1093 obtained from the FEX standard curve [7].
2.3. In Vivo Antileishmanial Efficacy
For the in vitro propagation of L. (L.) major (MHOM/IL/80/Friendlin) promastigotes, complete Schneider’s medium (Merck, Darmstadt, Germany) was used, enriched with 20% fetal bovine serum (Gibco, Eggenstein, Germany). The promastigotes were maintained in culture until they reached the stationary phase of growth.
BALB/c female mice (age: 7 weeks) were shaved and inoculated subcutaneously with 0.02 mL of a dispersion containing 1 × 107 promastigotes of L. (L.) major at the base of the tail [18]. The study was approved by the Ethics Committee for Animal Experimentation of the Universidade Federal de Minas Gerais (CEUA/UFMG—18/2020).
After the development of homogeneous lesions (average diameter of about 8 mm), the animals were divided into 5 groups of treatment containing 7 animals per group: group 1—Lip-ClAlPc (0.05 mL); group 2—FEX suspension (50 mg/kg/day); group 3—SEDDS-FEX (50 mg/kg/day); group 4—Lip-ClAlPc (0.05 mL)+ SEDDS-FEX (50 mg/kg/day) combined; group 5—infected untreated animals (control).
The Lip-ClAlPc formulation was applied topically (10 doses) in the lesion and protected from light. After 15 min, the lesion was exposed to visible light irradiation for 20 min at a wavelength of 660 nm, releasing 0–95 J/cm2 at an intensity of 81 mW/cm2. The distance between the light source and the lesion was 5 cm. After the session, the residues of the formulation were removed using 0.9% (w/v) sterile saline [15]. The FEX suspension and SEDDS-FEX were administered daily (0.2 mL) for 20 consecutive days by oral gavage, with SEDDS-FEX being previously diluted in water (1:1) before administration [7]. For the control group, the animals did not receive treatment.
Lesion size was measured weekly using a digital caliper (Mitutoyo, São Paulo, Brazil). The size of the lesion was determined by the average value obtained between the longest line that could be traced from one border of the lesion to another and the line that bisected this distance at a 90° angle [18]. The lesions were photographed at the beginning and end of the treatments.
The body weight of animals was evaluated at the beginning of the treatment (0), day 7, day 14, and end of the treatment (21 days). Other signs such as piloerection were used as indicators of the toxicity of the treatment. Additional evaluations included checking the appearance of nodules and metastasis in other locations on the animal skin as signs of disease progression.
The parasite burden in the lesions, as well as in the spleen, was evaluated via the limiting dilution assay, as described by Aguiar and collaborators [19]. The results were expressed as the mean obtained from the triplicate.
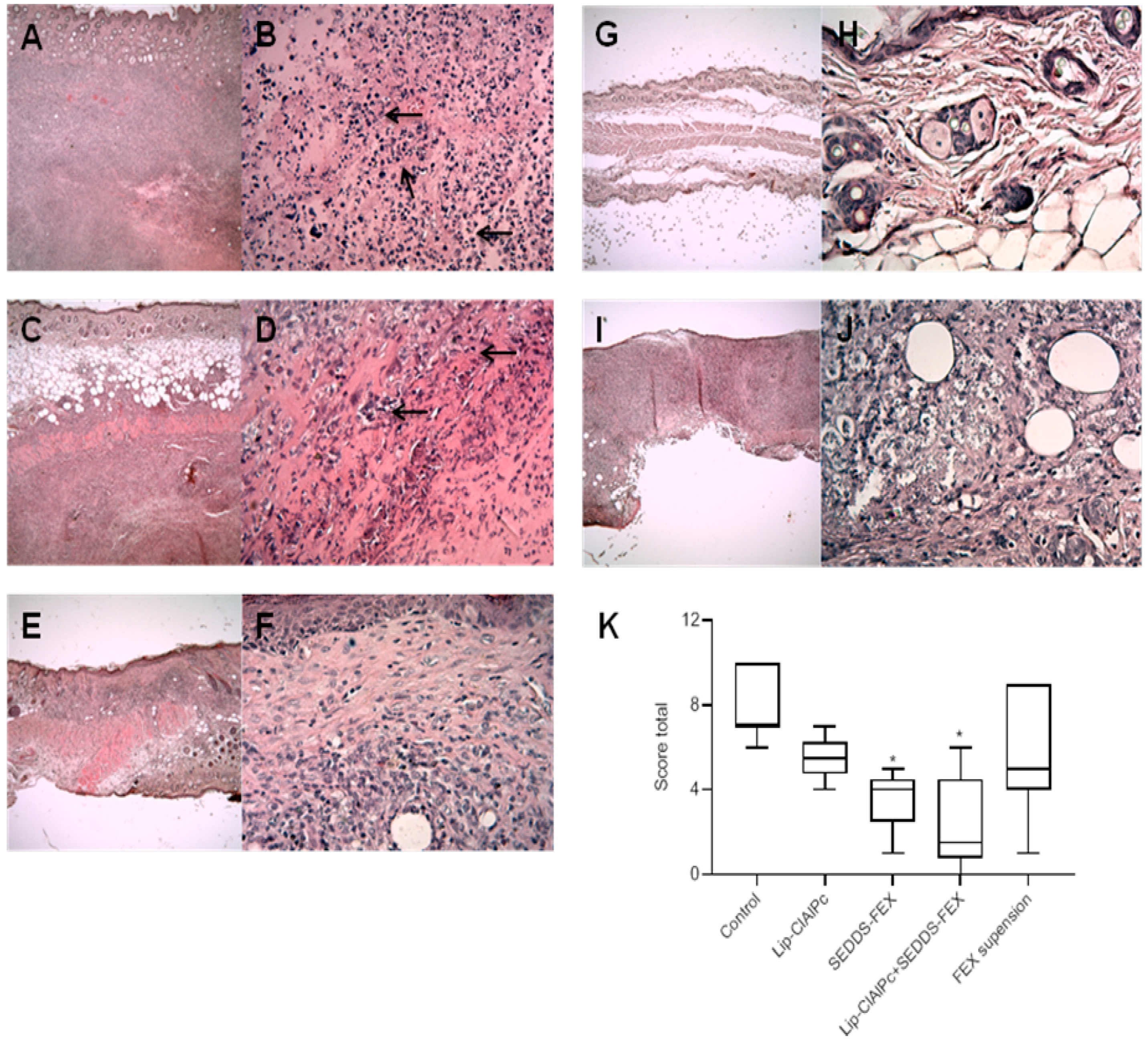
After the euthanasia procedure, the skin samples of the lesions of mice were collected and fixed in 10% neutral buffered formalin (pH 7.2) for a minimum period of 48 h. After processing, the fragments of the lesions were embedded in paraffin, submitted to microtomy with 5 μm, and stained with hematoxylin and eosin (H&E). The images were obtained using a microcamera (Q-Color5, Olympus, Tokyo, Japan) coupled to a BX53 microscope (Olympus), and the Q-Capture Pro 7.0 software was used [20]. The inflammatory reaction was evaluated using a semiquantitative procedure assessing the presence of leukocytes in the dermis and hypodermis [21,22]. The inflammatory infiltrate score system was adapted as follows: 0 = absent, no mononuclear cell exudate (apparently normal dermis); 1 = slight, diffuse mononuclear exudate in the upper dermis (1–9 cells per field/20 fields); 2 = moderate, a diffuse or focal mononuclear exudate around the vessels, glands, and hair follicles in the deep dermis or hypodermis (10–30 cells per field/20 fields); 3 = intense, a severe diffuse or focal mononuclear exudate around the vessels, glands, and hair follicles in deep dermis or hypodermis; 4 = intense, severe, and widespread inflammatory infiltrate in all dermis (>30 cells per field/20 fields). The analyzed morphological parameters of the epidermis were acanthosis, dyskeratosis, papillomatosis, and exocytosis: 0 = absent; 1 = present [23]. The total score was calculated by the sum of all parameters evaluated in the epidermis, dermis, and hypodermis. Histopathological analysis was performed using a single-blinded model [22].
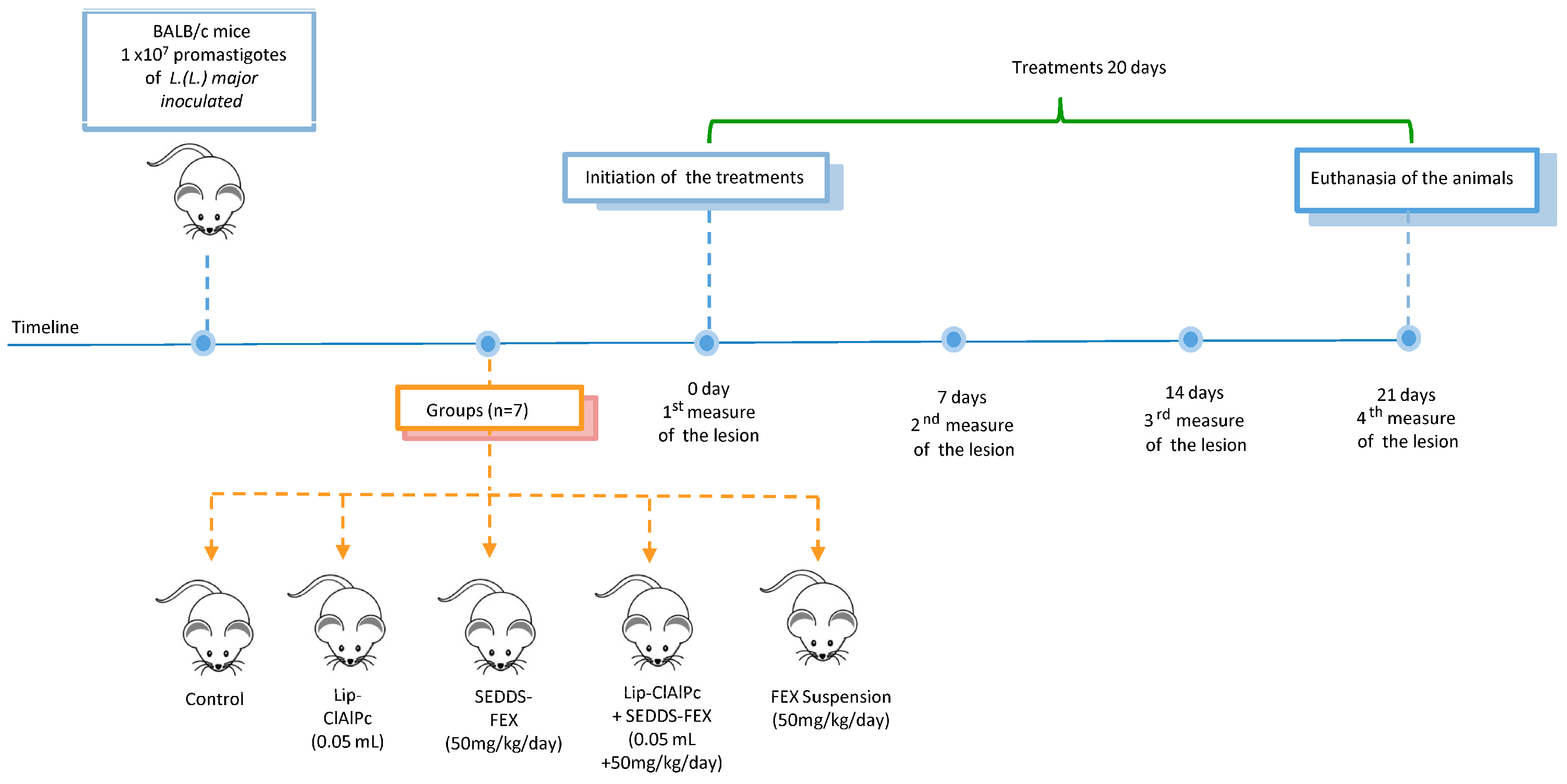
The course of the timeline and treatment regimen is schematized in Figure 1.
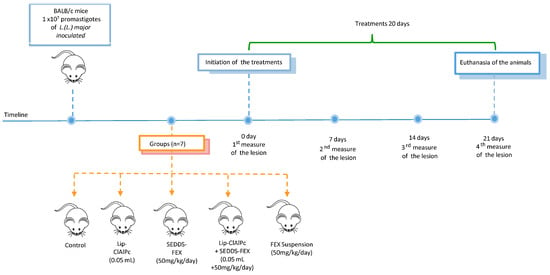
Figure 1.
Timeline course and treatment regimen. Female BALB/c mice were infected with L. (L.) major promastigotes and received 10 sessions of topical Lip-ClAlPc on alternate days and 20 consecutive days of SEDDS-FEX or FEX suspension oral in isolated or combined treatments. Therapeutical efficacy was evaluated by measuring the lesion size (0, 7, 14, and 21 days), the parasite burden, and histological analysis after euthanasia.
2.4. Statistical Analysis
The data were processed using GraphPad Prism software. Normality and homogeneity of variance were assessed using the Kolmogorov–Smirnov and Bartlett’s tests, respectively. Then, the comparison of parasite loads, weights of animals, and lesion sizes among the groups was evaluated via ANOVA, and in the case of statistical differences, the results were confirmed via Tukey’s test. For histopathological analysis, a comparison among groups was performed using the Kruskal–Wallis test. The difference was considered significant when the p-value was <0.05.
3. Results
3.1. Formulations Characterization
The diameter, polydispersion index, zeta potential, and concentration measured for the two formulations (Lip-ClAlPc and SEDDS-FEX) prepared for the in vivo study are shown in Table 1.

Table 1.
Physicochemical characteristics of Lip-ClAlPc and SEDDS-FEX formulations.
Lip-ClAlPc and SEDDS-FEX formulations presented suitable characteristics for topical and oral administration, respectively.
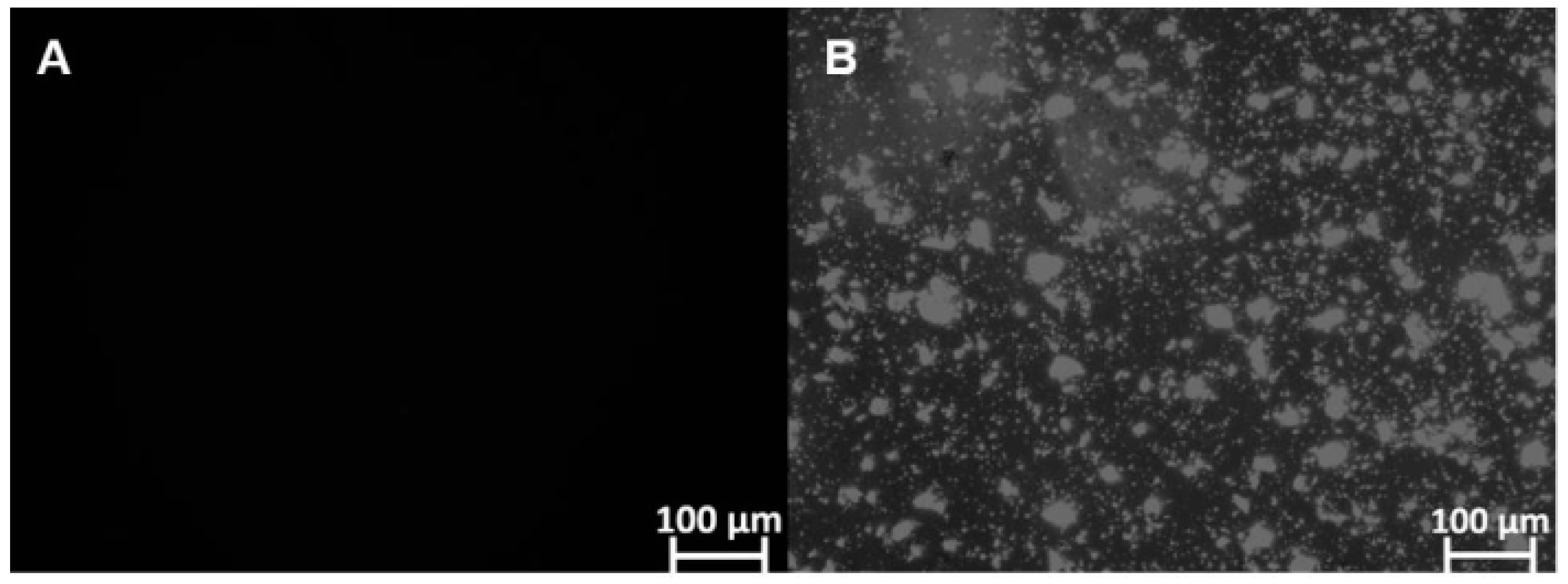
Furthermore, microscopic analysis showed that FEX is soluble in SEDDS-FEX (Figure 2A), while in the suspension, it is possible to verify the presence of FEX crystals (Figure 2B). FEX has low solubility in water, which may be a limiting factor for its absorption when administered orally. In fact, SEDDS-FEX was able to increase the solubility of FEX by keeping it in the dissolved state as a colloidal dispersion.
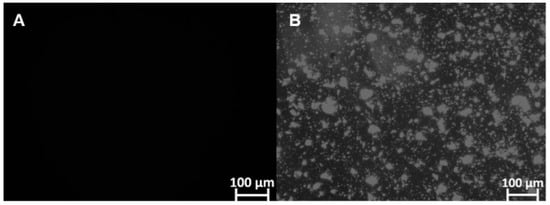
Figure 2.
The identification of FEX crystals that are insoluble in SEDDS-FEX and FEX suspensions (undiluted samples) was carried out using polarized light microscopy analysis and the Optical Zeiss® microscope. The analyses were carried out at 100× magnification: (A) SEDDS-Fex and (B) Fex suspension.
3.2. In Vivo Antileishmanial Efficacy
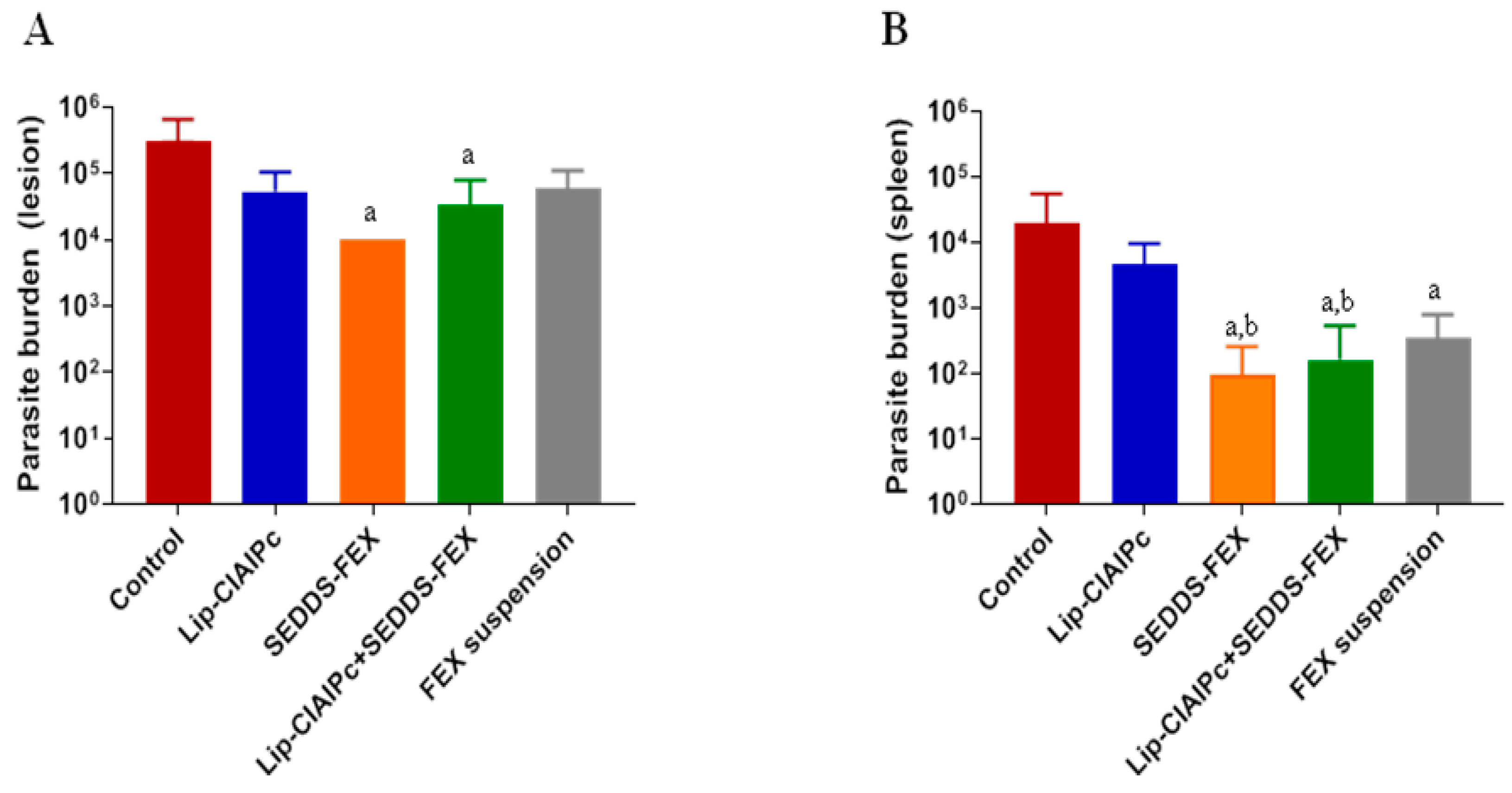
Initially, the animals were experimentally infected with L. (L.) major and the development of lesions followed the prognosis described in the literature [18,24]. It is important to highlight that when evaluating the parasite burden in the lesion, only the SEDDS-FEX (1 × 104), and Lip-ClAlPc+SEDDS-FEX (3 × 104) groups showed a significant reduction (p < 0.05) in relation to the control group (3 × 105) (Figure 3A). However, when comparing all treatments, there is no significant difference between them (p > 0.05) (Figure 3A). The groups treated with the Lip-ClAlPc and FEX suspensions exhibited parasite burdens of 5 × 104 and 6 × 104, respectively.
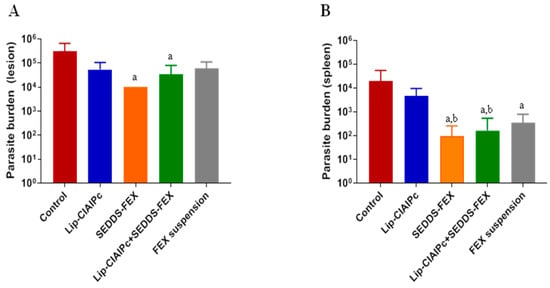
Figure 3.
In vivo efficacy of different treatments in L. (L.) major. Female BALB/c mice were infected with L (L.) major pomastigotes in the base of the tail. The treatments used were Lip-ClAlPc, SEDDS-FEX, Lip-ClAlPc+SEDDS-FEX, FEX suspension, and control group (untreated). The Lip-ClAlPc and Lip-ClAlPc+SEDDS-FEX groups received 10 sessions of topical Lip-ClAlPc on alternate days and 20 consecutive days of SEDDS-FEX or FEX oral suspension in isolated or combined treatments. One day after the end of the treatments, the parasite burden was determined via the limiting dilution method. (A) Parasite burden in lesions. The letter “a” indicates a statistically significant difference in relation to the control group (p < 0.05). (B) Parasite burden in the spleen. The letters “a” and “b” indicate statistically significant differences in relation to the control and Lip-ClAlPc groups, respectively (p < 0.05). The bars represent the averages and standard deviations (n = 7).
The parasite burden in the spleen is presented in Figure 3B. In fact, all formulations containing fexinidazole that were administered at doses of 50 mg/kg/day (Lip-ClAlPc+SEDDS-FEX (6 × 102), SEDDS-FEX (1 × 102), and FEX suspensions (8 × 102)) were able to significantly reduce the parasite load in the spleen (Figure 3B) when compared with the control group (2 × 104) (p < 0.05). It was also possible to observe a statistical difference in the Lip-ClAlPc group (6 × 103) when compared with the SEDDS-FEX and Lip-ClAlPc+SEDDS-FEX groups (p < 0.05) (Figure 3B). However, the Lip-ClAlPc group does not differ statistically from the FEX suspension group (p > 0.05). It is interesting to note that the SEDDS-FEX treatment led to a greater reduction in parasite loads in the lesion and the spleen than the FEX suspension treatment. This finding is probably related to increased bioavailability, which may be related to the complete solubilization of FEX when administered in the form of SEDDS-FEX.
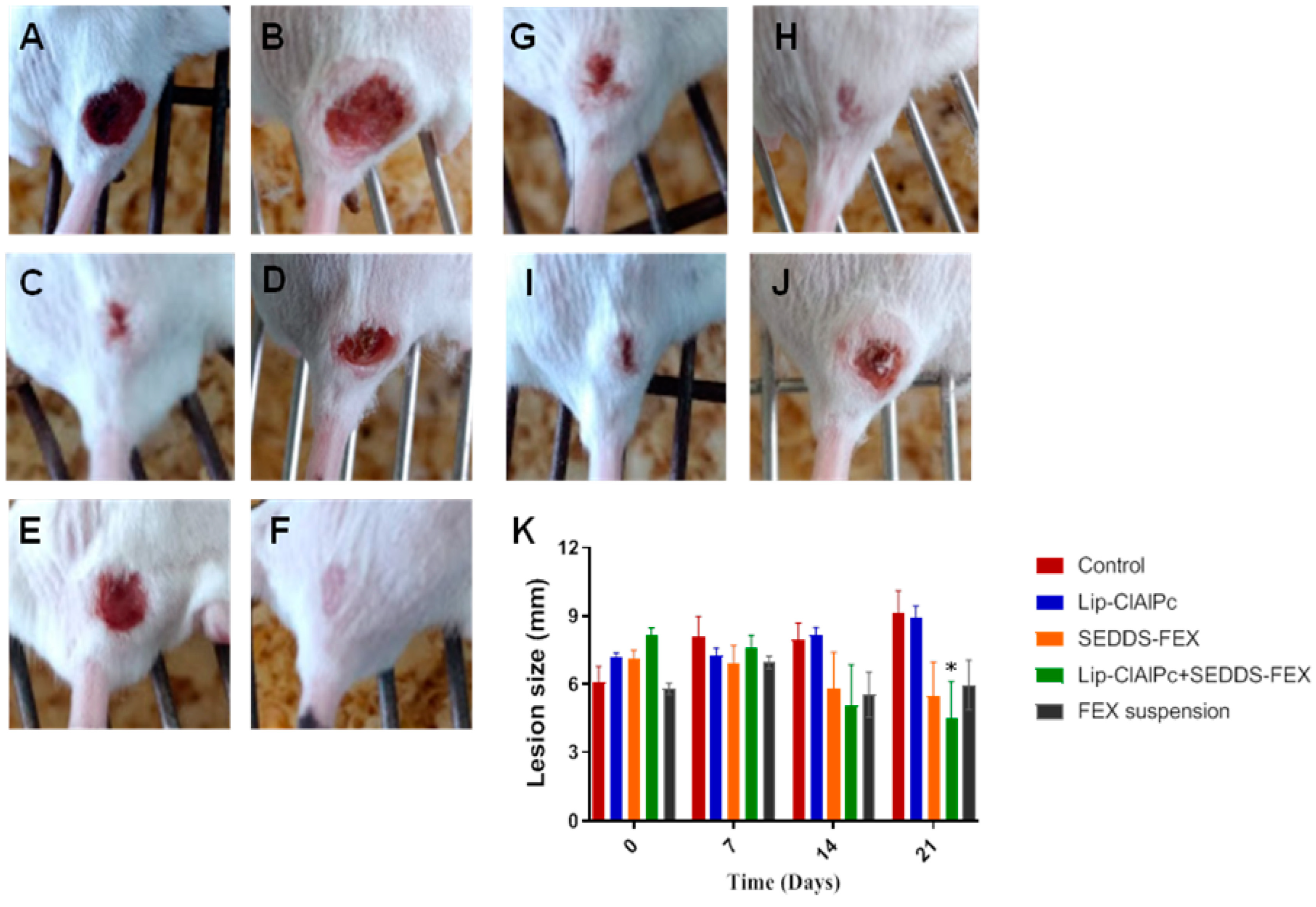
At the beginning of the treatment, the animals presented lesions with an average diameter equal to the following: control group (6.1 ± 1.9 mm), Lip-ClAlPc (7.2 ± 0.6 mm), SEDDS-FEX (7.1 ± 1.0 mm), Lip-ClAlPc+SEDDS-FEX (8.1 ± 0.9 mm), and FEX suspension (5.8 ± 0.7 mm); there were no significant differences between all groups (p > 0.05) (Figure 4K). It is possible to verify that the Lip-ClAlPc+SEDDS-FEX group was the only one that significantly reduced the size of the lesion in relation to the control group on the 21st day (p < 0.05) (Figure 4K). The images of the animals lesions at the beginning of treatment (day 0) and at the end (21 days) are presented in Figure 4: control group (Figure 4A,B), Lip-ClAlPc (Figure 4C,D), SEDDS-FEX (Figure 4E,F), Lip-ClAlPc+SEDDS-FEX (Figure 4G,H), and FEX suspension (Figure 4I,J).
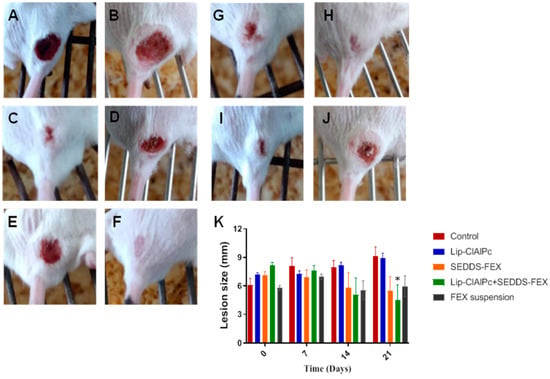
Figure 4.
In vivo efficacy of different treatments in L. (L.) major. Female BALB/c mice were infected with L (L.) major pomastigotes in the base of the tail. The treatments used were Lip-ClAlPc, SEDDS-FEX, Lip-ClAlPc+SEDDS-FEX, FEX suspension, and control group (untreated). The Lip-ClAlPc and Lip-ClAlPc+SEDDS-FEX groups received 10 sessions of topical Lip-ClAlPc on alternate days and 20 consecutive days of SEDDS-FEX or FEX suspension in isolated or combined treatments. Images of the macroscopic aspect of the lesions (A–J). Control group: infected and untreated animals at 0 days (A) and 21 days (B). Animal treated with Lip-ClAlPc at 0 days (C) and 21 days (D). Animal treated with SEDDS-FEX at 0 days (E) and 21 days (F). Animal treated with Lip-ClAlPc+SEDDS-FEX at 0 days (G) and 21 days (H). Animal treated with the FEX suspension at 0 days (I) and 21 days (J). (K) Monitoring of average lesion size in response to different treatments. Lesion size is shown as the average and standard error of the mean. * Indicates statistically significant difference in relation to the control group (p < 0.05) on the 21st day (n = 7).
The histopathological analysis of skin lesions of the mice in the control group revealed extensive inflammatory and necrotic lesions (Figure 5A,B). The lesion was characterized by large areas of necrosis and ulceration, as well as a mixed inflammatory infiltrate, with a predominance of mononuclear macrophages in deeper regions of the dermis. Vacuolated and parasitized macrophages were also detected, as well as the points of dystrophic calcification. In the group that received Lip-ClAlPc treatment (Figure 5C,D), the changes detected were less intense, and no changes were observed in the epidermis, including the absence of ulcerated areas in almost all samples. The inflammatory infiltrate and areas of necrosis ranged from moderate. Similar characteristics were seen in the group that received the treatment with SEDDS-FEX (Figure 5E,F).
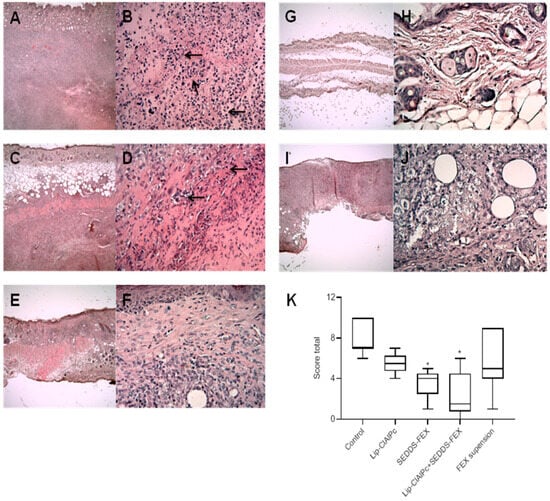
Figure 5.
Histopathological analysis (H&E) was performed in the control group (A,B), Lip-ClAlPc (C,D), SEDDS-FEX (E,F), Lip-ClAlPc+SEDDS-FEX (G,H), and FEX suspension (I,J). The first image on the right presents 4× magnification, and the second image on the left the image presents 40× magnification. Arrows indicate the regions with inflammatory processes. (K) Total score of the histopathological analysis. Data presented as median, maximum, and minimum. The groups treated with SEDDS-FEX and Lip-ClAlPc+SEDDS-FEX exhibited statistical differences when compared to the control group (* p < 0.05) (n = 7).
The treatment with Lip-ClAlPc+SEDDS-FEX (Figure 5G,H) attenuated the lesion phenotype with no epidermal lesions, and the absence of necrosis was observed in most samples. Finally, in the FEX suspension-treated group (Figure 5I,J), a variation in the lesion was observed among the animals, with some presenting intense alterations as described in the control group while others presented mild lesions in limited areas. The total score is shown in Figure 5K, where we can see that the SEDDS-FEX and Lip-ClAlPc+SEDDS-FEX-treated groups exhibited a significant difference when compared to the control group (p < 0.05).
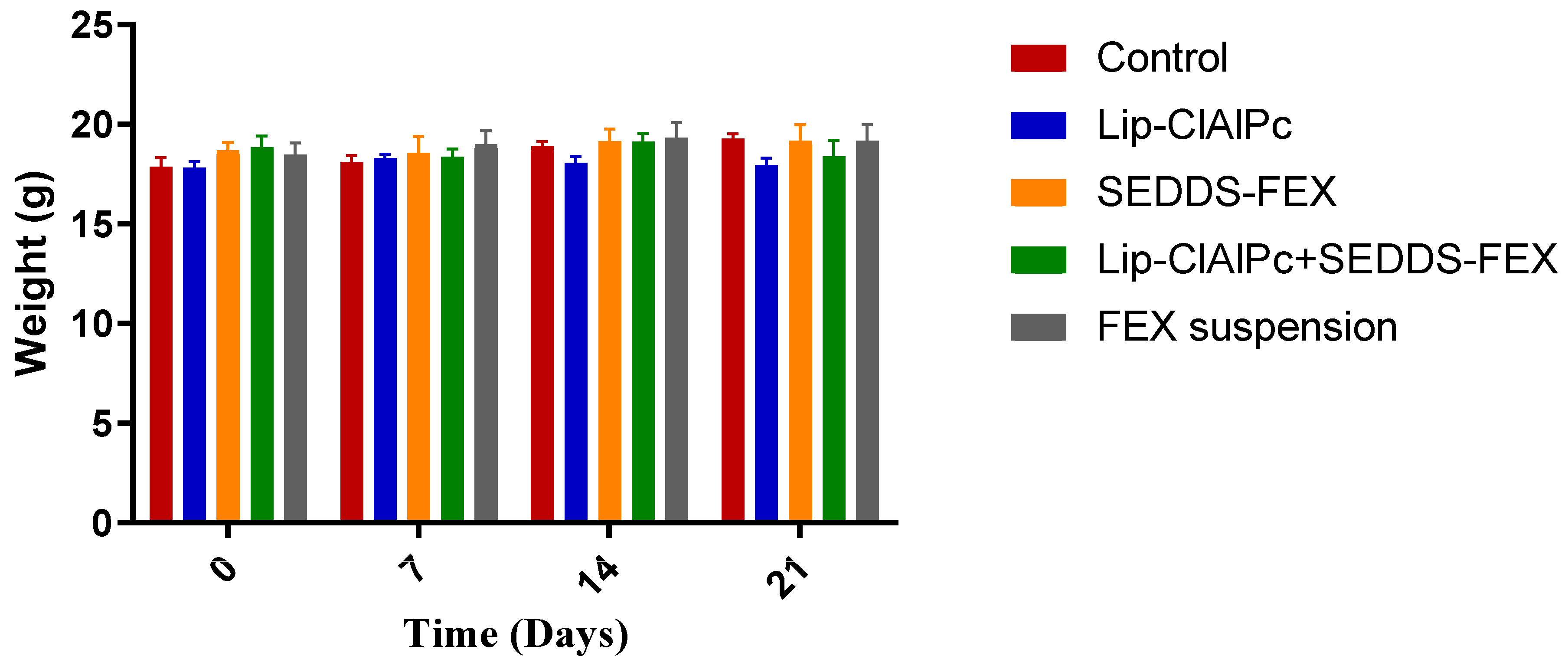
The comparisons of the average body weight of the animals of each group on days 0, 7, 14, and 21 were not statistically different (p > 0.05) (Figure 6). Other signs of toxicity evaluated, such as piloerection and death, were not observed.
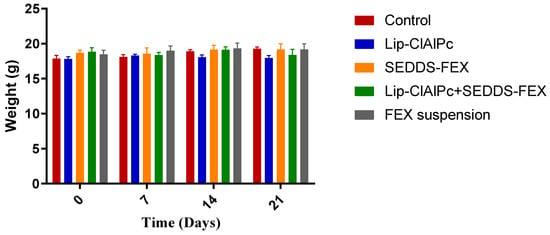
Figure 6.
Evaluation of the weight of BALB/c mice infected with L. (L.) major and submitted to different treatments. Female BALB/c mice were infected with L (L.) major promastigotes at the base of the tail. After the development of ulcerated lesions, the animals were treated with the Lip-ClAlPc, SEDDS-FEX, Lip-ClAlPc+SEDDS-FEX, FEX suspension, and Control (untreated). Animals were weighed at baseline (0 days) and on days 7, 14, and 21 after starting treatment. The bars represent means and standard deviations. Comparisons between the weights at all times were not statistically significant (p > 0.05).
4. Discussion
L. (L.) major is one of the main species causing CL in the Mediterranean basin, Middle East, and Africa. Co-infection with HIV has led to atypical manifestations of CL, as was observed in an outbreak related to this species in Burkina Faso [2]. Therefore, these factors show the importance of searching for new therapeutic alternatives for the treatment of CL caused by this species. It is important to highlight that each species of Leishmania presents specific biochemical and molecular characteristics, and their sensitivity to drugs is different [25]. Published studies reported the in vivo efficacy of treatments with FEX and ClAlPc against different species that cause leishmaniasis, such as L. donovani, L. infantum, L. amazonensis, and L. braziliensis, showing promising results [5,6,7,13,15,26]. However, until now, no study has been found that evaluated the effectiveness of these treatments against L. (L.) major. On another point, combination therapy is a very promising alternative to improve the efficacy of the treatments against leishmaniasis. Through this strategy, it is possible to test drugs with synergistic or additive activity, which can reduce the duration of therapy or decrease the effective doses, allowing treatment with less toxicity than therapies with isolated drugs [27,28].
The physicochemical and chemical properties of nanostructures, such as size and composition, are factors that can influence their interaction with the organism, potentially impacting the stability, effectiveness, and toxicity of these systems [29,30]. When evaluating the size obtained for the Lip-ClAlPc (348 nm), the size obtained is compatible with the opening of the membrane (0.4 µM) used for their extrusion. It is important to consider that liposomes with sizes greater than 500 nm may compromise their use as a drug-release vehicle [26]. SEDDS-FEX, after dilution, showed globules with an average size of around 100 nm (Table 1), which indicates a compact arrangement of the surfactant at the oil–water interface [31], a relevant parameter in improving drug absorption after oral administrations, as discussed by Chatterjee and coworkers (2016) [8]. The Pdi value provides information about the homogeneity of the size distribution of nanosystems. The values found for Lip-ClAlPc and SEDDS-FEX formulations were at most equal to 0.3, which indicates the obtainment of monodisperse systems, further contributing to an adequate therapeutic response, and these systems are appropriate for topical and oral administration [26]. High values of ZP induce electrostatic repulsion, preventing coalescence and improving stability [29]. The ZP values obtained were slightly negative. It is important to highlight that the Lip-ClAlPc and SEDDS-FEX formulations were stable for 2 months at 4 °C and 6 months at 25 °C, respectively [7,15].
As observed through microscopic analysis, no FEX crystals were present in the SEDDS-FEX formulation. This finding is desirable to ensure that the emulsion formed after diluting the SEDDS does not present the precipitation of the active pharmaceutical ingredient, as the presence of insoluble crystals in the medium can lead to a delay or decrease in its absorption [32,33]. Damasio and collaborators (2023) [7] also observed that for this formulation, at dilutions of 1:200 in water and simulated gastric or intestinal fluids, FEX remained soluble.
Skin lesions are easily accessible for light sources and provide an opportunity for the introduction of PDT, which is interesting for the topical treatment of CL [34]. In this study, an improvement was observed in all parameters evaluated after the Lip-ClAlPc+SEDDS-FEX treatment compared with the control group. In vivo studies are an effective method for screening new treatments for leishmaniasis. It is important to note that BALB/c mice infected with L. major have been widely used for decades to test the efficacy of potential anti-leishmanial drugs, and they are considered a rigorous non-cure model in which only the most active drugs are effective. Thus, any improvement in the disease can be attributed to the effects of chemotherapy. The lesion cure model in BALB/c mice is highly reproducible and consistent, and it has some clinical similarities with human CL [35]. However, this criterion is not always correlated with a reduction in parasite burden, since part of the lesion is composed of inflammatory cells with amastigotes restricted to the layer dermal [36]. Interestingly, our results showed that treatment with the Lip-ClAlPc+SEDDS-FEX was able to reduce lesions, as well as parasite burden.
It is also interesting to note that the SEDDS-FEX treatment was able to lead to a greater reduction in the parasite load in the lesion than the FEX suspension treatment. This is probably because FEX is completely solubilized when administered in the form of SEDDS-FEX. According to the Biopharmaceutical Classification System, FEX is a class II drug and has low solubility and high permeability [37]; therefore, its low solubility is the limiting factor for absorption. When SEDDS-FEX is released into the lumen of the intestine, it can disperse to form a nanometer-sized emulsion, thereby improving the dissolution rate of the drug. Tarral and collaborators (2014) [38] showed that the bioavailability of FEX administered in tablet form in humans is approximately 25% lower than when administered in suspension form. The reduced absorption of FEX in tablet form is explained by the time required for disintegration and dissolution, as the rate of dissolution of the drug is the determining factor for the degree of absorption within a given period of gastric transit.
In the present study, the combined treatment of Lip-ClAlPc+SEDDS-FEX was able to significantly reduce the parasite burden in the lesion and spleen, in addition to the complete healing of the lesions in 43% of the animals. On the other hand, in previous studies conducted on species causing CL in the New World, a high dose was required to reduce the parasite burden in the organs evaluated, as well as for the healing of the lesions [6]. Despite the difference between the species, our results show a clinical improvement correlated with anti-leishmanial activity, which can be attributed to the combined effects of the drugs, as well as the form of drug delivery. Conventional drug delivery systems are not capable of targeted delivery, while solutions based on nanotechnology are the innovative approach to therapeutic advancement in the fight against leishmaniasis [11]. The industrial preparation of SEDDS is economical and simple, as it is almost like preparing the solution, using basic production equipment [10], while the proposed method for preparing liposomes was the most common (hydration of the lipid film) [16].
Drug discovery and development are expensive, time-consuming, and risky endeavors. Pharmaceutical companies show low interest in the development of anti-leishmanial drugs, most likely due to low financial returns. Therefore, the development of new formulations has become an interesting strategy for infectious and neglected diseases, especially in emerging countries [39,40].
5. Conclusions
In the present study, we observed both a reduction in parasitic burden in the regions evaluated (lesion and spleen) and a clinical improvement in lesions, with a reduction in size and healing when the animals were treated with the combination of Lip-ClAlPc+SEDDS-FEX. This finding indicates that this combination may be promising for the treatment of CL caused by L. (L.) major.
Author Contributions
Conceptualization, R.A.S., L.A.F., M.C.O. and M.G.A.; methodology, R.A.S., D.S.D., L.D.C., E.d.M.-T., C.M.Q.-J., L.A.F., P.E.S., R.B.A., A.T., M.C.O. and M.G.A.; validation, R.A.S., D.S.D., L.D.C., C.M.Q.-J. and. P.E.S.; formal analysis, R.A.S., D.S.D., L.D.C., E.d.M.-T., C.M.Q.-J., L.A.F., P.E.S., R.B.A., A.T., M.C.O. and M.G.A.; investigation, R.A.S., D.S.D., L.D.C., E.d.M.-T., C.M.Q.-J., L.A.F., P.E.S., R.B.A., A.T., M.C.O. and M.G.A.; resources, L.A.F., M.C.O. and M.G.A.; data curation, R.A.S. and M.G.A.; writing—original draft preparation, R.A.S.; writing—review and editing, R.A.S., L.A.F., M.C.O. and M.G.A.; visualization, R.A.S. and M.G.A.; supervision, M.G.A. and M.C.O.; project administration, M.G.A. and M.C.O.; funding acquisition, M.G.A. and M.C.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado de Minas Gerais—FAPEMIG (CDS-RED-00007-14-Rede Mineira de Pesquisas em Nanobiotecnologia); Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (307098/2018-4); and INCT em Nanobiotecnologia—CNPq (573880/2008-5).
Institutional Review Board Statement
This study was approved by the Animal Ethics Committee for Experimentation of the Universidade Federal de Minas Gerais (CEUA/UFMG) under protocol no 18/2020.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in a publicly accessible repository that does not issue DOIs. Publicly available datasets were analyzed in this study. These data can be found here: http://hdl.handle.net/1843/41158, accessed on 4 April 2024.
Acknowledgments
The authors also thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES for supporting Raphaela Ariany dos Santos Silva with a scholarship.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leishmaniasis. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 7 April 2023).
- Burza, S.; Croft, S.L. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Van Der Snoek, E.M.; Robinson, D.J. A review of photodynamic therapy in cutaneous leishmaniasis. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 918–922. [Google Scholar] [CrossRef] [PubMed]
- de SOUZA, M.L.; Dos Santos, W.M. Cutaneous leishmaniasis: New oral therapeutic approaches under development. Int. J. Dermatol. 2022, 61, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, S.; Patterson, S. The anti-trypanosome drug fexinidazole shows potential for treating visceral leishmaniasis. Sci. Transl. Med. 2012, 4, 119re1. [Google Scholar] [CrossRef] [PubMed]
- de Morais-Teixeira, E.; Rabello, A. In vitro activity and in vivo efficacy of fexinidazole against New World Leishmania species. J. Antimicrob. Chemother. 2019, 74, 2318–2325. [Google Scholar] [CrossRef] [PubMed]
- Damasio, D.S.N.; Antunes, P.A. A new oral self-emulsifying drug delivery system improves the antileishmania efficacy of fexinidazole in vivo. Int. J. Pharm. 2023, 631, 122505. [Google Scholar] [CrossRef] [PubMed]
- Chartterjee, B.; Hamed, A.S. Controversies with self-emulsifying drug delivery system from pharmacokinetic point of view. Drug Deliv. 2016, 23, 3639–3652. [Google Scholar] [CrossRef]
- Rani, E.R.; Radha, G.V. Insights into Novel Excipients of Self-Emulsifying Drug Delivery Systems and Their Significance: An Updated Review. Crit. Rev. Ther. Drug Carrier Syst. 2021, 38, 27–74. [Google Scholar] [CrossRef] [PubMed]
- Salawi, A. Self-emulsifying drug delivery systems: A novel approach to deliver drugs. Drug Deliv. 2022, 29, 1811–1823. [Google Scholar] [CrossRef]
- Jamshaid, H.; Din, F.U. Nanotechnology based solutions for anti-leishmanial impediments: A detailed insight. J. Nanobiotechnol. 2021, 19, 106. [Google Scholar] [CrossRef]
- Gupta, S.; Kesarla, R. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. ISRN Pharm. 2013, 26, 848043. [Google Scholar] [CrossRef] [PubMed]
- Leal Pinto, S.M.; Muehlmann, L.A. Nanoemulsions with Chloroaluminium Phthalocyanine and Paromomycin for Combined Photodynamic and Antibiotic Therapy for Cutaneous Leishmaniasis. Infect. Chemother. 2021, 53, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.P.; Mittamnn, J. Photodynamic effects of zinc phthalocyanines on intracellular amastigotes of Leishmania amazonensis and Leishmania braziliensis. Lasers Med. Sci. 2014, 30, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.C.; Silva, R.A. Topical photodynamic therapy with chloroaluminum phthalocyanine liposomes is as effective as systemic pentavalent antimony in the treatment of experimental cutaneous leishmaniasis. Photodiagnosis Photodyn. Ther. 2019, 28, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Large, D.E.; Abdelmessih, R.G. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef] [PubMed]
- Hernández, I.P.; Montanari, J. In vitro phototoxicity of ultradeformable liposomes containing chloroaluminum phthalocyanine against New World Leishmania species. J. Photochem. Photobiol. B 2012, 117, 157–163. [Google Scholar] [CrossRef]
- Aguiar, M.G.; Silva, D.L. Combined topical paromomycin and oral miltefosine treatment of mice experimentally infected with Leishmania (Leishmania) major leads to reduction in both lesion size and systemic parasite burdens. J. Antimicrob. Chemother. 2009, 64, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, M.G.; Pereira, A.M.M. Reductions in skin and systemic parasite burdens as a combined effect of topical paromomycin and oral miltefosine treatment of mice experimentally infected with Leishmania (Leishmania) amazonensis. Antimicrob. Agents Chemother. 2010, 54, 4699–4704. [Google Scholar] [CrossRef]
- Kawakami, M.Y.M.; Zamora, L.O. Efficacy of nanoemulsion with Pterodon emarginatus Vogel oleoresin for topical treatment of cutaneous leishmaniasis. Biomed. Pharmacother. 2021, 134, 111109. [Google Scholar] [CrossRef]
- Oliveira, L.G.; Souza-Testasicca, M.C. Annexin A1 Is Involved in the Resolution of Inflammatory Responses during Leishmania braziliensis Infection. J. Immunol. 2017, 198, 3227–3236. [Google Scholar] [CrossRef]
- Alves, C.F.; Alves, C.F. American tegumentary leishmaniasis: Effectiveness of an immunohistochemical protocol for the detection of leishmania in skin. PLoS ONE 2013, 8, e63343. [Google Scholar] [CrossRef]
- Viana, A.G.; Mayrink, W. Histopathological and immunohistochemical aspects of American cutaneous leishmaniasis before and after different treatments. An. Bras. Dermatol. 2013, 88, 32–40. [Google Scholar] [CrossRef]
- Mansur-Alves, I.; Lima, B.L.F. Cholesterol improves stability of amphotericin B nanoemulsion: Promising use in the treatment of cutaneous leishmaniasis. Nanomedicine 2022, 18, 1237–1251. [Google Scholar] [CrossRef]
- Escobar, P.; Matu, S. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH(3) (edelfosine) and amphotericin B. Acta Trop. 2002, 81, 151–157. [Google Scholar] [CrossRef]
- Ribeiro, J.B.P.; Miranda-Vilela, A.L. Study of the efficacy of N-methyl glucamine antimoniate (SbV) associated with photodynamic therapy using liposomal chloroaluminium phthalocyanine in the treatment of cutaneous leishmaniasis caused by Leishmania (L.) amazonensis in C57BL6 mice. Photodiagnosis Photody Ther. 2019, 26, 261–269. [Google Scholar]
- De Souza, M.L.; Gonzaga da Costa, L.A. Recent strategies for the development of oral medicines for the treatment of visceral leishmaniasis. Drug Dev. Res. 2020, 81, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Uliana, S.R.B.; Trinconi, C.T. Chemotherapy of leishmaniasis: Present challenges. Parasitology 2018, 145, 464–480. [Google Scholar] [CrossRef] [PubMed]
- Mazzeti, A.L.; Oliveira, L.T. Benznidazole self-emulsifying delivery system: A novel alternative dosage form for Chagas disease treatment. Eur. J. Pharm. Sci. 2020, 30, 105234. [Google Scholar] [CrossRef]
- Ganta, S.; Talekar, M. Nanoemulsions in translational research-opportunities and challenges in targeted cancer therapy. AAPS PharmSciTech 2014, 15, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Kommuru, T.R.; Gurley, B. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: Formulation development and bioavailability assessment. Int. J. Pharm. 2001, 212, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Cerpnjak, K.; Zvonar, A. Lipid-based systems as a promising approach for enhancing the bioavailability of poorly water-soluble drugs. Acta Pharm. 2013, 63, 427–445. [Google Scholar] [CrossRef]
- Gupta, S.; Chavhan, S. Self-nanoemulsifying drug delivery system for adefovirdipivoxil: Design, characterization, in vitro and ex vivo evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 145–155. [Google Scholar] [CrossRef]
- Silva, M.L.F.; Alves, P.M. Analysis of Macrophage Activation Markers in an Experimental Model of Cutaneous Leishmaniasis Treated with Photodynamic Therapy Mediated by 5-Aminolevulinic Acid. Photobiomodul Photomed. Laser Surg. 2019, 37, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Caridha, D.; Vesely, B. Route map for the discovery and pre-clinical development of new drugs and treatments for cutaneous leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 2019, 11, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Sundar, S. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Assessment Report: Fexinidazole Winthrop. 2018. Available online: https://www.ema.europa.eu/en/documents/outside-eu-assessment-report/fexinidazole-winthrop-assessment-report_en.pdf (accessed on 15 August 2022).
- Tarral, A.; Blesson, S. Determination of an optimal dosing regimen for fexinidazole, a novel oral drug for the treatment of human African trypanosomiasis: First-in-human studies. Clin. Pharmacokinet. 2014, 53, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Van Bocxlaer, K.; Croft, S.L. Pharmacokinetics and pharmacodynamics in the treatment of cutaneous leishmaniasis—Challenges and opportunities. RSC Med. Chem. 2021, 12, 472–482. [Google Scholar] [CrossRef]
- Padhy, B.M.; Gupta, Y.K. Drug repositioning: Re-investigating existing drugs for new therapeutic indications. J. Postgrad. Med. 2011, 57, 153–160. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).