Progress in the Use of Hydrogels for Antioxidant Delivery in Skin Wounds
Abstract
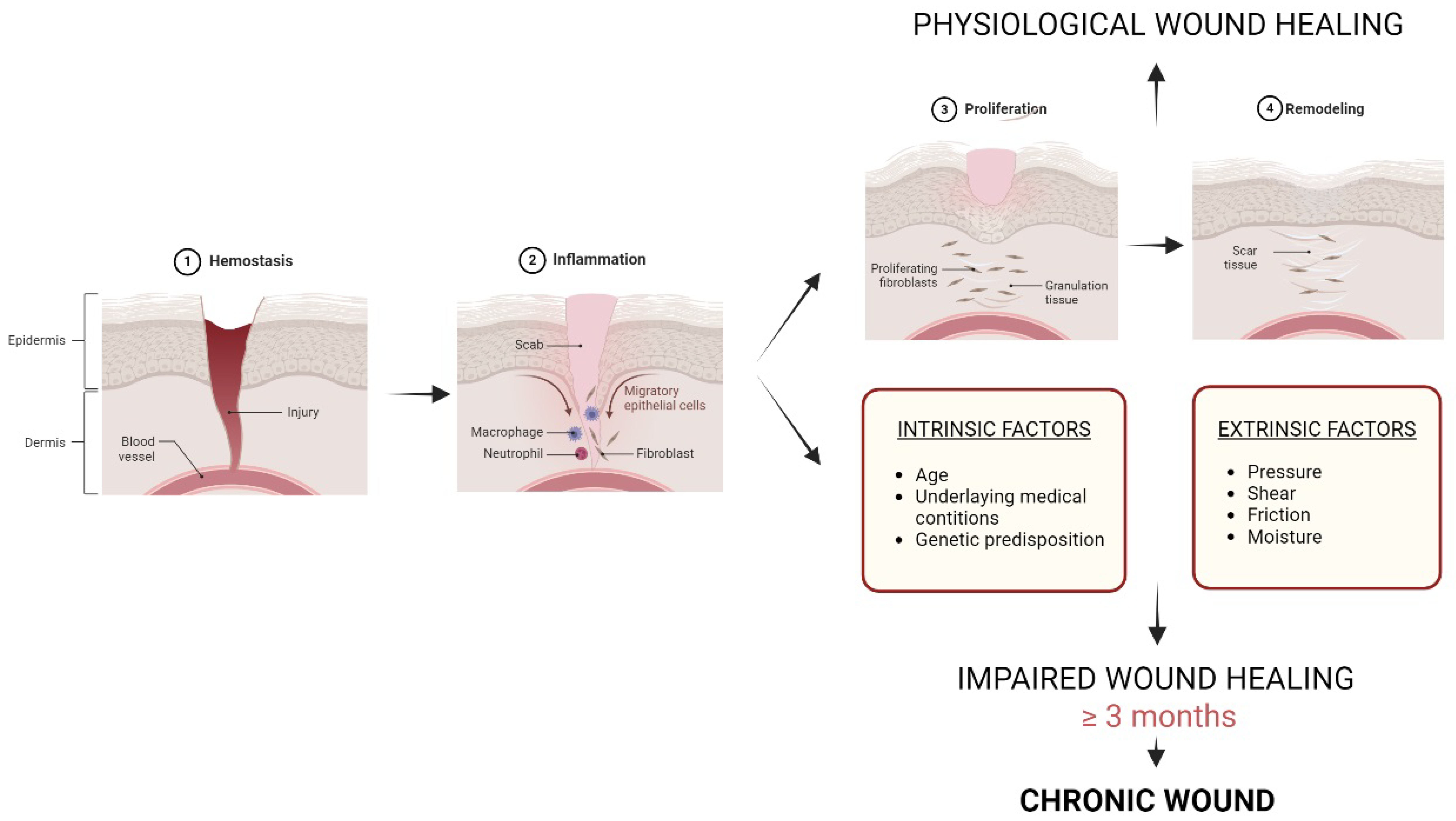
:1. Introduction
- Skin and chronic wounds
- b.
- Oxidative stress and its role in chronic wounds
2. Antioxidants, a Skin Ally
- Hydrophilic antioxidants
| Antioxidant | Solubility | Classification | Mechanism | Source | Refs. |
|---|---|---|---|---|---|
| Ascorbic acid (Vitamin C) | Water-soluble | Endogenous and exogenous | Free radical scavenger, regenerates vitamin E | Fruits and vegetables, especially citrus fruits, strawberries, kiwi, mango, papaya, pineapple, bell peppers, broccoli, Brussels sprouts, and tomatoes | [46,47,48,49] |
| Melatonin | Water-soluble | Endogenous | Free radical scavenger, induces the activity of some antioxidant enzymes | Produced endogenously in the body, dietary sources include eggs, fish, nuts, milk, grapes, mushrooms, oats, corn, tart cherries, and tomatoes | [46,50] |
| Glutathione (GSH) | Water-soluble | Endogenous | Free radical scavenger, regenerates other antioxidants, detoxification | Produced endogenously in the body, dietary sources include fruits and vegetables, especially avocados, asparagus, spinach, and okra | [46,51] |
| Alpha-Tocopherol (Vitamin E) | Lipid-soluble | Exogenous | Free radical scavenger, regenerates vitamin C | Found in nuts, seeds, vegetable oils, and leafy green vegetables | [46,52] |
| Ubiquinone (Coenzyme Q10) | Lipid-soluble | Endogenous and exogenous | Free radical scavenger, protects cell membranes | Found in small amounts in meats, fish, and whole grains, and also available as a dietary supplement | [46,53] |
| Carotenoids | Lipid-soluble | Exogenous | Free radical scavenger, singlet oxygen quenching, protection against exposure to UV radiation, regenerates vitamin E and vitamin C | Found in fruits and vegetables, especially those that are red, orange, and yellow in color, and also available as a dietary supplement | [46,54] |
| alpha-lipoic acid (ALA) | Water and lipid-soluble | Endogenous and exogenous | Free radical scavenger, regenerates other antioxidants, metal chelation, effect on gene expression | Found in small amounts in foods such as spinach, broccoli, and potatoes, and also available as a dietary supplement | [46,55] |
| Flavonoids and other polyphenols | Water and lipid-soluble | Exogenous | Peroxyl radical scavenger, metal chelation | Found in fruits, vegetables, tea, wine, and chocolate | [56] |
- Indirect antioxidant effects: Melatonin also stimulates the activity of antioxidant enzymes, such as superoxide dismutase (SOD) and glutathione peroxidase (GPx), which further enhance the body’s antioxidant defense systems [50].
- b.
- Hydrophobic antioxidants
- c.
- Amphiphilic antioxidants
- Free radical scavenging: ALA can directly neutralize a wide range of free radicals and reactive oxygen species by donating electrons. This is helpful when preventing oxidative damage to lipids, proteins, and also DNA.
- Regeneration of other antioxidants: ALA is able to regenerate and enhance the activity of other antioxidants, such as vitamin C and E, glutathione, and coenzyme Q10.
- Metal chelation: ALA can bind to certain metal ions, such as iron and copper. In this manner, it reduces their ability to promote the formation of harmful ROS.
- Effects on gene expression: ALA can induce the expression of antioxidant enzymes through nuclear factor E2-related factor 2 (Nrf2).
3. Hydrogels for Chronic Wound Repair
- Natural polymer hydrogels
- b.
- Synthetic polymer hydrogels
| Classification | Material | Properties | Refs. |
|---|---|---|---|
| Natural polymer hydrogel | Collagen | It presents exceptional biodegradability, low antigenicity, and biocompatibility. It can self-assemble and crosslink, forming robust and stable fibers making it a promising candidate for the development of scaffolds. | [127,128,129] |
| Alginate | It presents structural similarities to the extracellular matrix, biocompatibility, lack of toxicity, biodegradability, cost-effectiveness in extraction, and ease of gelation. Due to its poor stability and relatively soft mechanical properties, it is used to combine alginate with other polymers to improve its properties. | [135,138,139,140] | |
| Hyaluronic acid (HA) | It presents good biocompatibility, biodegradability, bioresorbability, high viscosity, and mechanical stability, leading to an ideal biomaterial for the design and development of non-adhesive, non-thrombogenic, and non-immunogenic scaffolds. | [148,149] | |
| Chitosan | It presents biodegradability, non-toxicity, non-antigenicity, inertness, bioadhesiveness, and hemostatic effects. It can be modified to create multifunctional constructs that closely resemble the natural matrix. | [151,152,153,154,155,156] | |
| Gelatin | It presents good biocompatibility, biodegradability, degradation mediated by matrix metalloproteinases, preservation of natural cell adhesion motifs, low antigenicity, and minimal inflammatory response when introduced in vivo due to its degradation process. The functional groups allow for modifications with crosslinkers or therapeutic agents, expanding its versatility as a material applicable to wound healing and tissue regeneration. | [161,162,163,164] | |
| Synthetic polymer hydrogels | Poly(ethylene glycol) (PEG) | It is non-ionic, biocompatible with optimal physicochemical and biological attributes; it typically does not generate an immune response. Chemical modification of PEG scaffolds can further enhance its biological properties. | [173,176] |
| Poly(vinyl alcohol) (PVA) | It has reduced protein-binding tendencies, relatively higher elasticity and water content. It has good biocompatibility, non-carcinogenic properties, non-toxicity, swelling characteristics, and bioadhesive features. | [179,180,181] | |
| Poly(lactic acid) (PLA) | Since lactides lack functionality, they are copolymerized with hydrophilic monomers or conjugated with hydrophilic moieties to create hydrogels. Given that PLA presents high hydrophobicity, modifications to the PLA attributes can be made by copolymerizing or crosslinking with other polymers such as polyethylene oxide, polyethylene glycol, polysaccharides, and polypeptides. | [186] |
4. Applications of Antioxidant-Incorporated Hydrogels in Chronic Wound Repair
| Hydrogel | Antioxidant | Loading Strategy | Release Method | Healing Mechanism | Efficacy | Refs. |
|---|---|---|---|---|---|---|
| Gellan-g-poly(ethylene glycol) methacrylate matrix (GPMA) | Ascorbic acid (AA) | Diffusion filling method. | Release controlled by swelling behavior and mechanical properties of the hydrogel. GPMA hydrogel showed sustained release of ascorbic acid at pH 5.4 and 7.4. It has pH sensitivity, and the release at lower pH was decreased. | Collagen synthesis, anti-oxidant, and anti-inflammatory activity. | 55% wound closure within 24 h (HaCaT cells). | [189] |
| Graphene oxide-collagen scaffold (GO-COL) | N-acetylcysteine (NAC) | NAC is mixed with the precursor polymer solution, and gelation occurs with the drug within the matrix. | NAC loaded in the scaffolds could be persistently released for at least 18 days, GO-COL hydrogel showed sustained release of NAC. | Resist oxidative stress, promote angiogenesis, accelerate ECM-synthesis, and facilitate epithelization. | 58.176% wound closure within 24 h (NIH 3T3 fibroblasts). | [191] |
| Hyaluronic acid-g-lipoic acid (HA-LA) | Lipoic acid (LA) | Grafted LA forms part of the granular gel, being mixed in the precursor polymer solution. | Controlled release of LA for at least 3 days (dressings changed every 3 days). | Eliminate excessive ROS at the wound site and up-regulate the secretion of reparative growth factor. | Significant promotion of wound healing in both normal and diabetic mice. | [195] |
| Oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system (TOCN-PVA) | Curcumin (Cur) | Cur solubilization by ethanol (100%) and pluronic® (4%) to be mixed in the precursor solution. | In contact with fluid, Cur is released from the hydrogel system, along with Cur/Plu cargo/misceli. Curcumin release increased dramatically after 1 day of incubation. | Scavenge ROS and lipid peroxidation. | Significant wound contraction after 2 weeks of application in rat skin excisional wound model. | [196] |
| Silk Fibroin and Polyvinyl Alcohol Composite Hydrogel Film | Curcumin (Cur) | Cur nanoparticles were fabricated and loaded into hydrogel films. | Nanoparticles mediated release of curcumin. | Antibacterial activity, increase in fibroblasts and angiogenesis, inhibit inflammation, and accelerate deposition of collagen. | After 7 days, skin wound remodeling and rebirth were higher on rats’ whole skin injury model. | [197] |
| Chitosan (3D printed) | α-tocopherol | Dispersion of α-tocopherol in chitosan solution. | Controlled drug release by geometrically complex devices designed for 3D printing. | Radical scavenging activity and antibacterial activity. | High biocompatibility was shown on human fibroblast cultures. | [198] |
5. Concluding Remarks and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A.A.C.C. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2014, 357, 8–42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Arda, O.; Göksügür, N.; Tüzün, Y. Basic histological structure and functions of facial skin. Clin. Dermatol. 2014, 32, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The role of chemokines in wound healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [PubMed]
- Nunan, R.; Harding, K.G.; Martin, P. Clinical challenges of chronic wounds: Searching for an optimal animal model to recapitulate their complexity. DMM Dis. Models Mech. 2014, 7, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Järbrink, K.K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair. Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Chronic Wounds: A Growing Problem; Mission Regional Medical Center: Mission, TX, USA, 2020.
- Díaz, F.J.G.; Conde, M.M.; Jaime, R.C. Comparación entre el coste y el cierre de heridas en una unidad de gestión clínica que incluye una enfermera de práctica avanzada en heridas crónicas complejas. Gerokomos 2021, 32, 193–198. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair. Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Fortune Business Insights. Chronic Wound Care Market Size [2022–2029] Worth USD 19.52 Billion | Exhibiting a CAGR of 6.7% [Internet]. 2022. Available online: https://www.fortunebusinessinsights.com/enquiry/request-sample-pdf/chronic-wound-care-market-100222 (accessed on 15 February 2024).
- Saoudi, M.; Badraoui, R.; Chira, A.; Saeed, M.; Bouali, N.; Elkahoui, S.; Alam, J.M.; Kallel, C.; El Feki, A. The Role of Allium subhirsutum L. in the Attenuation of Dermal Wounds by Modulating Oxidative Stress and Inflammation in Wistar Albino Rats. Molecules 2021, 26, 4875. [Google Scholar] [CrossRef]
- Shi, X.M.; Xu, G.M.; Zhang, G.J.; Liu, J.R.; Wu, Y.M.; Gao, L.G.; Yang, Y.; Chang, Z.S.; Yao, C.W. Low-temperature Plasma Promotes Fibroblast Proliferation in Wound Healing by ROS-activated NF-κB Signaling Pathway. Curr. Med. Sci. 2018, 38, 107–114. [Google Scholar] [CrossRef]
- Lindley, L.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and Biomarkers for Wound Healing. Plast. Reconstr. Surg. 2016, 138 (Suppl. S3), 18S–28S. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and Impact of Antioxidant Hydrogel in Chronic Wound Healing. Adv. Healthc. Mater. 2020, 9, e1901502. [Google Scholar] [CrossRef]
- Koo, M.A.; Hong, S.H.; Lee, M.H.; Kwon, B.J.; Seon, G.M.; Kim, M.S.; Kim, D.; Chang Nam, K.; Park, J.C. Effective stacking and transplantation of stem cell sheets using exogenous ROS-producing film for accelerated wound healing. Acta Biomater. 2019, 95, 418–426. [Google Scholar] [CrossRef]
- Li, Y.; Shen, C.; Zhou, X.; Zhang, J.; Lai, X.; Zhang, Y. Local Treatment of Hydrogen-Rich Saline Promotes Wound Healing in vivo by Inhibiting Oxidative Stress via Nrf-2/HO-1 Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 2949824. [Google Scholar] [CrossRef]
- Zhou, X.; Ruan, Q.; Ye, Z.; Chu, Z.; Xi, M.; Li, M.; Hu, W.; Guo, X.; Yao, P.; Xie, W. Resveratrol accelerates wound healing by attenuating oxidative stress-induced impairment of cell proliferation and migration. Burns 2021, 47, 133–139. [Google Scholar] [CrossRef]
- Ibrahim, N.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.Y.; Ima-Nirwana, S.; Shuid, A.N. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef]
- Fitzmaurice, S.D.; Sivamani, R.K.; Isseroff, R.R. Antioxidant Therapies for Wound Healing: A Clinical Guide to Currently Commercially Available Products. Skin Pharmacol. Physiol. 2011, 24, 113–126. [Google Scholar] [CrossRef]
- Dreiss, C.A. Hydrogel design strategies for drug delivery. Curr. Opin. Colloid Interface Sci. 2020, 48, 1–17. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.V.; Shukla, P.; Kikkeri, N.N. Antioxidants in dermatology. An. Bras. Dermatol. 2017, 92, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Lee, M.G. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016, 21, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermato-endocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The Role of Antioxidants on Wound Healing: A Review of the Current Evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef]
- Viaña-Mendieta, P.; Sánchez, M.L.; Benavides, J. Rational selection of bioactive principles for wound healing applications: Growth factors and antioxidants. Int. Wound J. 2022, 19, 100–113. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, A.; Rocha-Uribe, A. Antioxidant Hydrophobicity and Emulsifier Type Influences the Partitioning of Antioxidants in the Interface Improving Oxidative Stability in O/W Emulsions Rich in n-3 Fatty Acids. Eur. J. Lipid Sci. Technol. 2018, 120, 1700277. [Google Scholar] [CrossRef]
- Polutchko, S.K.; Glime, G.N.E.; Demmig-Adams, B. Synergistic Action of Membrane-Bound and Water-Soluble Antioxidants in Neuroprotection. Molecules 2021, 26, 5385. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Sousa, M.J.; Ferreira, I.C.F.R. Comparative Study of Lipophilic and Hydrophilic Antioxidants from in vivo and in vitro Grown Coriandrum sativum. Plant Foods Hum. Nutr. 2011, 66, 181–186. [Google Scholar] [CrossRef]
- Bechara, N.; Flood, V.M.; Gunton, J.E. A Systematic Review on the Role of Vitamin C in Tissue Healing. Antioxidants 2022, 11, 1605. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Ward, S.; Wayne, J.S.; Brophy, D.F.; Fowler, A.A., 3rd; Yager, D.R.; Natarajan, R. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int. Wound J. 2016, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- DePhillipo, N.N.; Aman, Z.S.; Kennedy, M.I.; Begley, J.P.; Moatshe, G.; LaPrade, R.F. Efficacy of Vitamin C Supplementation on Collagen Synthesis and Oxidative Stress After Musculoskeletal Injuries: A Systematic Review. Orthop. J. Sports Med. 2018, 6, 2325967118804544. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Fisher, B.J.; Huynh, Q.K.; Wijesinghe, D.S.; Chalfant, C.E.; Brophy, D.F.; Fowler, A.A., 3rd; Natarajan, R. Resolution of Sterile Inflammation: Role for Vitamin C. Mediat. Inflamm. 2014, 2014, 173403. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Chakraborty, R. The Role of Antioxidants in Human Health. ACS Symp. Ser. 2011, 1083, 1–37. [Google Scholar] [CrossRef]
- Zehiroglu, C.; Sarikaya, S.B.O. The importance of antioxidants and place in today’s scientific and technological studies. J. Food Sci. Technol. 2019, 56, 4757–4774. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.; Madduma-Liyanage, K.; Mobley, J.A.; Assimos, D.G.; Holmes, R.P. Ascorbic acid intake and oxalate synthesis. Urolithiasis 2016, 44, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, N.; Andolina, G.; Cannata, A.; Costanzo, M.G.; Rizzo, V.; Currò, M.; Ientile, R.; Caccamo, D. Is Melatonin the Cornucopia of the 21st Century? Antioxidants 2020, 9, 1088. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Tucker, J.M.; Townsend, D.M. Alpha-tocopherol: Roles in prevention and therapy of human disease. Biomed. Pharmacother. 2005, 59, 380–387. [Google Scholar] [CrossRef]
- Knott, A.; Achterberg, V.; Smuda, C.; Mielke, H.; Sperling, G.; Dunckelmann, K.; Vogelsang, A.; Krüger, A.; Schwengler, H.; Behtash, M.; et al. Topical treatment with coenzyme Q 10-containing formulas improves skin’s Q 10 level and provides antioxidative effects. Biofactors 2015, 41, 383–390. [Google Scholar] [CrossRef]
- Baswan, S.M.; Klosner, A.E.; Weir, C.; Salter-Venzon, D.; Gellenbeck, K.W.; Leverett, J.; Krutmann, J. Role of ingestible carotenoids in skin protection: A review of clinical evidence. Photodermatol. Photoimmunol. Photomed. 2021, 37, 490–504. [Google Scholar] [CrossRef]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 2009, 1790, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil ABin Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. The physiological function of melatonin in plants. Plant Signal. Behav. 2006, 1, 89–95. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; González-Menéndez, P.; Hevia, D.; Cernuda-Cernuda, R. Melatonin transport into mitochondria. Cell. Mol. Life Sci. 2017, 74, 3927–3940. [Google Scholar] [CrossRef] [PubMed]
- Sharafati-Chaleshtori, R.; Shirzad, H.; Rafieian-Kopaei, M.; Soltani, A. Melatonin and human mitochondrial diseases. J. Res. Med. Sci. 2017, 22, 2. [Google Scholar] [CrossRef]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.H.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef]
- Pugazhenthi, K.; Kapoor, M.; Clarkson, A.N.; Hall, I.; Appleton, I. Melatonin accelerates the process of wound repair in full-thickness incisional wounds. J. Pineal Res. 2008, 44, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Ren, L.; Ma, H.; Hu, R.; Gao, H.; Wang, L.; Chen, X.; Zhao, Z.; Liu, J. Melatonin promotes diabetic wound healing in vitro by regulating keratinocyte activity. Am. J. Transl. Res. 2016, 8, 4682–4693. [Google Scholar] [PubMed]
- Winterbourn, C.C. Regulation of intracellular glutathione. Redox Biol. 2019, 22, 101086. [Google Scholar] [CrossRef] [PubMed]
- Averill-Bates, D.A. The antioxidant glutathione. Vitam. Horm. 2023, 121, 109–141. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Tamer, T.M.; Hassan, M.A.; Valachová, K.; Omer, A.M.; El-Shafeey, M.E.A.; Eldin, M.S.M.; Šoltés, L. Enhancement of wound healing by chitosan/hyaluronan polyelectrolyte membrane loaded with glutathione: In vitro and in vivo evaluations. J. Biotechnol. 2020, 310, 103–113. [Google Scholar] [CrossRef]
- Pham-Huy, C.A.; Huy, B.P. Free Radicals and Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Hobson, R. Vitamin E and wound healing: An evidence-based review. Int. Wound J. 2016, 13, 331–335. [Google Scholar] [CrossRef]
- Chong, W.T.; Tan, C.P.; Cheah, Y.K.; Lai, O.M. In-vitro and in-vivo evaluations of tocotrienol-rich nanoemulsified system on skin wound healing. PLoS ONE 2022, 17, e0267381. [Google Scholar] [CrossRef]
- Saini, R. Coenzyme Q10: The essential nutrient. J. Pharm. Bioallied Sci. 2011, 3, 466–467. [Google Scholar] [CrossRef]
- Rodick, T.; Seibels, D.; Babu, J.R.; Huggins, K.; Ren, G.; Mathews, S. Potential role of coenzyme Q10 in health and disease conditions. Nutr. Diet. Suppl. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Manzar, H.; Abdulhussein, D.; Yap, T.E.; Cordeiro, M.F. Cellular Consequences of Coenzyme Q10 Deficiency in Neurodegeneration of the Retina and Brain. Int. J. Mol. Sci. 2020, 21, 9299. [Google Scholar] [CrossRef]
- Sifuentes-Franco, S.; Sánchez-Macías, D.C.; Carrillo-Ibarra, S.; Rivera-Valdés, J.J.; Zuñiga, L.Y.; Sánchez-López, V.A. Antioxidant and Anti-Inflammatory Effects of Coenzyme Q10 Supplementation on Infectious Diseases. Healthcare 2022, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Sangsefidi, Z.S.; Yaghoubi, F.; Hajiahmadi, S.; Hosseinzadeh, M. The effect of coenzyme Q10 supplementation on oxidative stress: A systematic review and meta-analysis of randomized controlled clinical trials. Food Sci. Nutr. 2020, 8, 1766–1776. [Google Scholar] [CrossRef] [PubMed]
- Hormozi, M.; Mirzaei, R.; Nakhaee, A.; Payandeh, A.; Izadi, S.; Haghighi, J.D. Effects of coenzyme Q10 supplementation on oxidative stress and antioxidant enzyme activity in glazers with occupational cadmium exposure: A randomized, double-blind, placebo-controlled crossover clinical trial. Toxicol. Ind. Health 2019, 35, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Terao, J. Revisiting carotenoids as dietary antioxidants for human health and disease prevention. Food Funct. 2023, 14, 7799–7824. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Yuan, J.; Du, Y.; Zeng, J.; Sun, Y.; Wu, Y.; Gao, X.H.; Chen, H.D. Effects of Oral Carotenoids on Oxidative Stress: A Systematic Review and Meta-Analysis of Studies in the Recent 20 Years. Front. Nutr. 2022, 9, 754707. [Google Scholar] [CrossRef] [PubMed]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Kulawik, A.; Cielecka-Piontek, J.; Zalewski, P. The Importance of Antioxidant Activity for the Health-Promoting Effect of Lycopene. Nutrients 2023, 15, 3821. [Google Scholar] [CrossRef]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ren, B.; Guo, R.; Zhang, W.; Ma, S.; Yao, Y.; Yuan, T.; Liu, Z.; Liu, X. Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-κB transcriptional pathway. Food Chem. Toxicol. 2017, 109 Pt 1, 505–516. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Khedr, N.F.; El-Bahrawy, H.A.; Hamada, O.B. Suppression of inducible nitric oxide synthase and tumor necrosis factor-alpha level by lycopene is comparable to methylprednisolone in acute pancreatitis. Dig. Liver Dis. 2018, 50, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, L.; Wang, Z.; Cui, Y.; Tan, X.; Yuan, T.; Liu, Q.; Liu, Z.; Liu, X. Supplementation of lycopene attenuates lipopolysaccha-ride-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J. Nutr. Biochem. 2018, 56, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, H.; Wang, J.; Liu, P.; Tan, X.; Ren, B.; Liu, Z.; Liu, X. Lycopene Supplementation Attenuates Oxidative Stress, Neuroinflammation, and Cognitive Impairment in Aged CD 1 Mice. J. Agric. Food Chem. 2018, 66, 3127–3136. [Google Scholar] [CrossRef] [PubMed]
- Palombo, P.; Fabrizi, G.; Ruocco, V.; Ruocco, E.; Fluhr, J.; Roberts, R.; Morganti, P. Beneficial Long-Term Effects of Combined Oral/Topical Antioxidant Treatment with the Carotenoids Lutein and Zeaxanthin on Human Skin: A Dou-ble-Blind, Placebo-Controlled Study. Skin Pharmacol. Physiol. 2007, 20, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Tibullo, D.; Volti, G.L.; Giallongo, C.; Grasso, S.; Tomassoni, D.; Anfuso, C.D.; Lupo, G.; Amenta, F.; Avola, R.; Bramanti, V. Biochemical and clinical relevance of alpha lipoic acid: Antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm. Res. 2017, 66, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Ghibu, S.; Richard, C.; Zeller, M.; Cottin, Y.; Vergely, C. Direct and indirect antioxidant properties of α-lipoic acid and therapeutic potential. Mol. Nutr. Food Res. 2013, 57, 114–125. [Google Scholar] [CrossRef]
- Zonooz, S.R.; Hasani, M.; Morvaridzadeh, M.; Pizarro, A.B.; Heydari, H.; Yosaee, S.; Rezamand, G.; Heshmati, J. Effect of alpha-lipoic acid on oxidative stress parameters: A systematic review and meta-analysis. J. Funct. Foods. 2021, 87, 104774. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Alwasel, S.H. Metal Ions, Metal Chelators and Metal Chelating Assay as Antioxidant Method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and An-ti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef] [PubMed]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P.; et al. Iron Complexes of Flavonoids-Antioxidant Capacity and Beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Zhu, Q.Q.; Yang, X.Y.; Xu, H.H.; Sun, B.; Wang, X.J.; Sheng, J. Wound healing can be improved by (-)-epigallocatechin gallate through targeting Notch in streptozotocin-induced diabetic mice. FASEB J. 2019, 33, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kawazoe, T.; Han, D.W.; Matsumara, K.; Suzuki, S.; Tsutsumi, S.; Hyon, S.H. Enhanced wound healing by an ep-igallocatechin gallate-incorporated collagen sponge in diabetic mice. Wound Repair Regen. 2008, 16, 714–720. [Google Scholar] [CrossRef]
- Li, S.; Yan, J.; Zhu, Q.; Liu, X.; Li, S.; Wang, S.; Wang, X.; Sheng, J. Biological Effects of EGCG@MOF Zn(BTC)4 System Improves Wound Healing in Diabetes. Molecules 2022, 27, 5427. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, R.; Liu, M.; Wang, L.; Chen, W.; Sun, Y.; Wei, W.; Ye, S. A novel wound dressing based on epigallocatechin-3-gallate self-assemble hydrogels promotes effects on wound healing. Mater. Today Sustain. 2022, 18, 100125. [Google Scholar] [CrossRef]
- Zawani, M.; Fauzi, M.B. Epigallocatechin Gallate: The Emerging Wound Healing Potential of Multifunctional Biomaterials for Future Precision Medicine Treatment Strategies. Polymers 2021, 13, 3656. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Ram, M.; Kumawat, S.; Tandan, S.; Kumar, D. Quercetin accelerated cutaneous wound healing in rats by increasing levels of VEGF and TGF-β1. Indian J. Exp. Biol. 2016, 54, 187–195. [Google Scholar] [PubMed]
- Nunes, S.; Danesi, F.; Rio DDel Silva, P. Resveratrol and inflammatory bowel disease: The evidence so far. Nutr. Res. Rev. 2018, 31, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Yoksa, D.T.; Abba, Y.; Shamaki, B.U.; Satumari, N.A. Effects of resveratrol topical ointment on wound healing of full-thickness cutaneous burns in albino rats. J. Wound Care 2022, 31, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Tejada, S.; Manayi, A.; Daglia, M.; Nabavi, S.F.; Sureda, A.; Hajheydari, Z.; Gortzi, O.; Pazoki-Toroudi, H.; Nabavi, S.M. Wound Healing Effects of Curcumin: A Short Review. Curr. Pharm. Biotechnol. 2016, 17, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Maugeri, A.; Favara, G.; Lio, R.M.S.; Evola, G.; Agodi, A.; Basile, G. Nutrition and Wound Healing: An Overview focusing on the Beneficial Effects of Curcumin. Int. J. Mol. Sci. 2019, 20, 1119. [Google Scholar] [CrossRef]
- Kant, V.; Kumar, D.; Prasad, R.; Gopal, A.; Pathak, N.N.; Kuma, P.; Tandan, S.K. Combined effect of substance P and curcumin on cutaneous wound healing in diabetic rats. J. Surg. Res. 2017, 212, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.; Pu, C.; Liu, C.; Chen, Y.; Chen, Y.; Liang, C.; Hsieh, J.H.; Huang, H.F.; Chen, Y.L. Curcumin accelerates cutaneous wound healing via multiple biological actions: The involvement of TNF-α, MMP-9, α-SMA, and collagen. Int. Wound J. 2018, 15, 605–617. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Kumar, D.; Pathak, N.N.; Ram, M.; Jangir, B.L.; Tandan, S.K.; Kumar, D. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J. Surg. Res. 2015, 193, 978–988. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Pathak, N.N.; Kumar, P.; Tandan, S.K.; Kumar, D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int. Immunopharmacol. 2014, 20, 322–330. [Google Scholar] [CrossRef]
- Lev, R.; Seliktar, D. Hydrogel biomaterials and their therapeutic potential for muscle injuries and muscular dystrophies. J. R. Soc. Interface 2018, 15, 20170380. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Hydrogel-Based Strategies to Advance Therapies for Chronic Skin Wounds. Annu. Rev. Biomed. Eng. 2019, 21, 145–169. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Qin, D.; Sun, M.; Wang, T.; Chen, X. Research status of self-healing hydrogel for wound management: A review. Int. J. Biol. Macromol. 2020, 164, 2108–2123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef]
- Karami, P.; Wyss, C.S.; Khoushabi, A.; Schmocker, A.; Broome, M.; Moser, C.; Bourban, P.E.; Pioletti, D.P. Composite Double-Network Hydrogels to Improve Adhesion on Biological Surfaces. ACS Appl. Mater. Interfaces 2018, 10, 38692–38699. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.P.; Espiga, A.; Silva, D.; Baptista, P.; Henriques, J.; Ferreira, C.; Silva, J.C.; Borges, J.P.; Pires, E.; Chaves, P.; et al. Development of a new chitosan hydrogel for wound dressing. Wound Repair Regen. 2009, 17, 817–824. [Google Scholar] [CrossRef]
- Xiang, J.; Shen, L.; Hong, Y. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Pol. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Ming, Z.; Han, L.; Bao, M.; Zhu, H.; Qiang, S.; Xue, S.; Liu, W. Living Bacterial Hydrogels for Accelerated Infected Wound Healing. Adv. Sci. 2021, 8, e2102545. [Google Scholar] [CrossRef]
- Hu, B.; Gao, M.; Boakye-Yiadom, K.O.; Ho, W.; Yu, W.; Xu, X.; Zhang, X.Q. An intrinsically bioactive hydrogel with on-demand drug release behaviors for diabetic wound healing. Bioact. Mater. 2021, 6, 4592–4606. [Google Scholar] [CrossRef]
- Liu, D.; Wang, T.; Lu, Y. Untethered Microrobots for Active Drug Delivery: From Rational Design to Clinical Settings. Adv. Healthc. Mater. 2022, 11, e2102253. [Google Scholar] [CrossRef]
- David, G.; Bargan, A.I.; Drobota, M.; Bele, A.; Rosca, I. Comparative Investigation of Collagen-Based Hybrid 3D Structures for Potential Biomedical Applications. Materials 2021, 14, 3313. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Coradin, T.; Desimone, M.F. Bio-inspired silica–collagen materials: Applications and perspectives in the medical field. Biomater. Sci. 2013, 1, 688–702. [Google Scholar] [CrossRef] [PubMed]
- David, G. Collagen-based 3D structures—Versatile, efficient materials for biomedical applications. In Biopolymer-Based Formulations: Biomedical and Food Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 881–906. [Google Scholar]
- Desimone, M.F.; Hélary, C.; Rietveld, I.B.; Bataille, I.; Mosser, G.; Giraud-Guille, M.M.; Livage, J.; Coradin, T. Silica–collagen bionanocomposites as three-dimensional scaffolds for fibroblast immobilization. Acta Biomater. 2010, 6, 3998–4004. [Google Scholar] [CrossRef]
- Desimone, M.F.; Hélary, C.; Quignard, S.; Rietveld, I.B.; Bataille, I.; Copello, G.J.; Mosser, G.; Giraud-Guille, M.M.; Livage, J.; Meddahi-Pellé, A.; et al. In vitro studies and preliminary in vivo evaluation of silicified concentrated collagen hydrogels. ACS Appl. Mater. Interfaces 2011, 3, 3831–3838. [Google Scholar] [CrossRef] [PubMed]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Horak, W.; Nowakowska, M. Collagen/chitosan/hyaluronic acid-based injectable hydrogels for tissue engineering applications—Design, physicochemical and biological characterization. Colloids Surf. B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Zhang, C.; Gan, B.; Liu, W.; Liang, J.; Fan, Z.; Wen, Y.; Yang, Y.; Peng, X.; Zhou, Y. Collagen-tussah silk fibroin hybrid scaffolds loaded with bone mesenchymal stem cells promote skin wound repair in rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110611. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Rastogi, K.; Tyagi, P.; Rawat, H.; Mittal, G.; Jamini, A.; Singh, H.; Tyagi, A. Comparative Analysis of Collagen and Chitosan-based Dressing for Haemostatic and Wound Healing Application. AAPS PharmSciTech 2021, 22, 76. [Google Scholar] [CrossRef] [PubMed]
- Ågren, M. Wound Healing Biomaterials—Volume 2: Functional Biomaterials; Elsevier Science: San Diego, CA, USA, 2016; 542p. [Google Scholar]
- Antezana, P.E.; Municoy, S.; Perez, C.J.; Desimone, M.F. Collagen Hydrogels Loaded with Silver Nanoparticles and Cannabis Sativa Oil. Antibiotics 2021, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.; Williams, P. Handbook of Hydrocolloids; Woodhead Publishing: Sawston, UK, 2009. [Google Scholar]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Fan, W.; Liu, Y.; Wang, Q.; Weng, L. Fabrication, Property and Application of Calcium Alginate Fiber: A Review. Polymers 2022, 14, 3227. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Datta, S.; Barua, R.; Das, J. Importance of Alginate Bioink for 3D Bioprinting in Tissue Engineering and Regenerative Medicine. Alginates—Recent Uses of This Natural Polymer; Intechopen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Antezana, P.E.; Municoy, S.; Orive, G.; Desimone, M.F. Design of a New 3D Gelatin-Alginate Scaffold Loaded with Cannabis sativa Oil. Polymers 2022, 14, 4506. [Google Scholar] [CrossRef]
- Stubbe, B.; Mignon, A.; Declercq, H.; Van Vlierberghe, S.; Dubruel, P. Development of Gelatin-Alginate Hydrogels for Burn Wound Treatment. Macromol. Biosci. 2019, 19, e1900123. [Google Scholar] [CrossRef]
- Ducheyne, P. Comprehensive Biomaterials; Elsevier: Amsterdam, The Netherlands, 2015; 3672p. [Google Scholar]
- Garantziotis, S.; Savani, R.C. Hyaluronan biology: A complex balancing act of structure, function, location and context. Matrix Biol. 2019, 78–79, 1–10. [Google Scholar] [CrossRef]
- Slobounov, S.; Cao, C.; Jaiswal, N.; Newell, K.M. Neural basis of postural instability identified by VTC and EEG. Exp. Brain Res. 2009, 199, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Agarwal, A.; Agrawal, H.; Agrawal, G.P. Double-Liposome – Based Dual-Drug Delivery System as Vectors for Effective Management of Peptic Ulcer. J. Liposome Res. 2012, 22, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Khabarov, V.; Boykov, P.; Selyanin, M. Hyaluronic Acid: Production, Properties, Application in Biology and Medicine; Willey: Hoboken, NJ, USA, 2015; 224p. [Google Scholar]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Sierra-Sánchez Fernández-González, A.; Lizana-Moreno, A.; Espinosa-Ibáñez, O.; Martinez-Lopez, A.; Guerrero-Calvo, J.; Fernández-Porcel, N.; Ruiz-García, A.; Ordóñez-Luque, A.; Carriel, V. Hyaluronic acid biomaterial for human tissue-engineered skin substitutes: Preclinical comparative in vivo study of wound healing. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2414–2427. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Zhang, X.; Zhang, R.; Hu, Y.; Boyer, C.; Xu, F.J. Photo-responsive supramolecular hyaluronic acid hydrogels for accelerated wound healing. J. Control. Release 2020, 323, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.A.; Rahman, S.S.; Qureshi, A.; Ullah, A. Biopolymers: Opportunities and Challenges for 3D Printing. In Chapter 12—Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 281–303. [Google Scholar]
- Alvarez Echazú, M.I.; Olivetti, C.E.; Peralta, I.; Alonso, M.R.; Anesini, C.; Perez, C.J.; Alvarez, G.S.; Desimone, M.F. Development of pH-responsive biopolymer-silica composites loaded with Larrea divaricata Cav. extract with antioxidant activity. Colloids Surf. B Biointerfaces 2018, 169, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Echazú, M.I.; Olivetti, C.E.; Anesini, C.; Perez, C.J.; Alvarez, G.S.; Desimone, M.F. Development and evaluation of thymol-chitosan hydrogels with antimicrobial-antioxidant activity for oral local delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. Molecules 2021, 26, 2683. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti-Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef]
- Pérez, R.A.; Won, J.E.; Knowles, J.C.; Kim, H.W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Wang, X.; Chen, Y.; Yan, Y.; Zhang, L.; Zhang, L. Peptide-modified chitosan hydrogels promote skin wound healing by enhancing wound angiogenesis and inhibiting inflammation. Am. J. Transl. Res. 2017, 9, 2352–2362. [Google Scholar]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel Trends in Hydrogel Development for Biomedical Applications : A Review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef]
- Leucht, A.; Volz, A.C.; Rogal, J.; Borchers, K.; Kluger, P.J. Advanced gelatin-based vascularization bioinks for extrusion-based bioprinting of vascularized bone equivalents. Sci. Rep. 2020, 10, 5330. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, S.; Zhang, C.; Ikoma, T.; Guo, H.; Zhang, X.; Li, X.; Chen, W. Novel microsphere-packing synthesis, microstructure, formation mechanism and in vitro biocompatibility of porous gelatin/hydroxyapatite microsphere scaffolds. Ceram. Int. 2021, 47, 32187–32194. [Google Scholar] [CrossRef]
- Acevedo, C.A.; Olguín, Y.; Briceño, M.; Forero, J.C.; Osses, N.; Díaz-Calderón, P.; Jaques, A.; Ortiz, R. Design of a biodegradable UV-irradiated gelatin-chitosan/nanocomposed membrane with osteogenic ability for application in bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control. Release 2014, 190, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Campiglio, C.E.; Negrini, N.C.; Farè, S.; Draghi, L. Cross-Linking Strategies for Electrospun Gelatin Scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef] [PubMed]
- Besser, R.R.; Bowles, A.C.; Alassaf, A.; Carbonero, D.; Claure, I.; Jones, E.; Reda, J.; Wubker, L.; Batchelor, W.; Ziebarth, N.; et al. Enzymatically crosslinked gelatin–laminin hydrogels for applications in neuromuscular tissue engineering. Biomater. Sci. 2020, 8, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Zheng, X.; Zhang, A.; Zhang, X.; Tang, K. Pullulan dialdehyde crosslinked gelatin hydrogels with high strength for biomedical applications. Carbohydr. Polym. 2019, 216, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, M.; Peng, Y.; He, S.; Zhu, X.; Hu, C.; Xia, G.; Zuo, T.; Zhang, X.; Yun, Y.; et al. A physicochemical double-cross-linked gelatin hydrogel with enhanced antibacterial and anti-inflammatory capabilities for improving wound healing. J. Nanobiotechnology 2022, 20, 426. [Google Scholar] [CrossRef]
- Dong, Y.; Sigen, A.; Rodrigues, M.; Li, X.; Kwon, S.H.; Kosaric, N.; Khong, S.; Gao, Y.; Wang, W.; Gurtner, G.C. Injectable and Tunable Gelatin Hydrogels Enhance Stem Cell Retention and Improve Cutaneous Wound Healing. Adv. Funct. Mater. 2017, 27, 1606619. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Rawn, J.D. Synthetic Polymer. In Principles of Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 397–419. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X. Synthetic polymers for organ 3D printing. Polymers 2020, 12, 1765. [Google Scholar] [CrossRef]
- French, A.C.; Thompson, A.L.; Davis, B.G. High-Purity Discrete PEG-Oligomer Crystals Allow Structural Insight. Angew. Chem. Int. Ed. Engl. 2009, 48, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Zalipsky, S.; Harris, J.M. Introduction to Chemistry and Biological Applications of Poly(ethylene glycol). ACS Symp. Ser. 1997, 680, 1–13. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels—A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Hassanajili, S.; Azarpira, N.; Bagher Karimi, M.; Geramizadeh, B. Development of thermal-crosslinkable chitosan/maleic terminated polyethylene glycol hydrogels for full thickness wound healing: In vitro and in vivo evaluation. Eur. Polym. J. 2019, 118, 113–127. [Google Scholar] [CrossRef]
- Schmedlen, R.H.; Masters, K.S.; West, J.L. Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials 2002, 23, 4325–4332. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Design and optimization of process parameters of polyvinyl (alcohol)/chitosan/nano zinc oxide hydrogels as wound healing materials. Carbohydr. Polym. 2019, 207, 542–554. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic acid (PLA) synthesis and modifications: A review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, B.; Yang, G.; Gauthier, M. Poly(Lactic Acid)-Based Biomaterials: Synthesis, Modification and Applications. In Biomedical Science, Engineering and Technology; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Fukushima, K.; Kimura, Y. An efficient solid-state polycondensation method for synthesizing stereocomplexed poly(lactic acid)s with high molecular weight. J. Polym. Sci. A Polym. Chem. 2008, 46, 3714–3722. [Google Scholar] [CrossRef]
- Sun, M.; Chen, S.; Ling, P.; Ma, J.; Wu, S. Electrospun methacrylated gelatin/poly(L-lactic acid) nanofibrous hydrogel scaffolds for potential wound dressing application. Nanomaterials 2021, 12, 6. [Google Scholar] [CrossRef]
- Ravi, D.; Rajalekshmy, G.P.; Rekha, M.R.; Joseph, R. Ascorbic acid-loaded gellan-g-poly(ethylene glycol) methacrylate matrix as a wound-healing material. Int. J. Biol. Macromol. 2023, 251, 126243. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Mahmood, R. Carnosine and N-acetyl cysteine protect against sodium nitrite-induced oxidative stress in rat blood. Cell Biol. Int. 2018, 42, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Li, J.; Guo, K.; Guo, N.; Zhong, A.; Yang, J.; Wang, J.; Xiao, P.; Sun, J.; Xiong, L. Antioxidant biocompatible composite collagen dressing for diabetic wound healing in rat model. Regen. Biomater. 2021, 8, rbab003. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Belaid, H.; Nagarajan, S.; Teyssier, C.; Barou, C.; Barés, J.; Balme, S.; Garay, H.; Huon, V.; Cornu, D.; Cavaillès, V.; et al. Development of new biocompatible 3D printed graphene oxide-based scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110595. [Google Scholar] [CrossRef] [PubMed]
- Moini, H.; Packer, L.; Saris, N.E.L. Antioxidant and Prooxidant Activities of α-Lipoic Acid and Dihydrolipoic Acid. Toxicol. Appl. Pharmacol. 2002, 182, 84–90. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, Y.; Mao, Z.; Zhang, J.; Zhang, K.; Yin, J.; Wang, C. Hyaluronic acid-g-lipoic acid granular gel for promoting diabetic wound healing. Bioeng. Transl. Med. 2022, 8, e10402. [Google Scholar] [CrossRef] [PubMed]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.G.; Lee, B.T. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Wang, R.; Ruan, L.; Jiang, G.; Li, P.; Aharodnikau, U.E.; Yunusov, K.E.; Gao, X.; Solomevich, S.O. Fabrication of Curcumin-Loaded Silk Fibroin and Polyvinyl Alcohol Composite Hydrogel Films for Skin Wound Healing. ACS Appl. Bio Mater. 2022, 5, 4400–4412. [Google Scholar] [CrossRef] [PubMed]
- Bergonzi, C.; Bianchera, A.; Remaggi, G.; Ossiprandi, M.C.; Zimetti, F.; Marchi, C.; Bernini, F.; Bettini, R.; Elviri, L. Biocompatible 3D Printed Chitosan-Based Scaffolds Containing α-Tocopherol Showing Antioxidant and Antimicrobial Activity. Appl. Sci. 2021, 11, 7253. [Google Scholar] [CrossRef]
- Huang, C.; Dong, L.; Zhao, B.; Lu, Y.; Huang, S.; Yuan, Z.; Luo, G.; Xu, Y.; Qian, W. Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 2022, 12, e1094. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, W.; He, G.; Yang, J.; Li, J.; Ma, H.; Wang, S. Hydrogel-Transformable Antioxidant Poly-γ-Glutamic Acid/Polyethyleneimine Hemostatic Powder for Efficient Wound Hemostasis. Gels 2024, 10, 68. [Google Scholar] [CrossRef]
- Zhao, N.; Yuan, W. Self-healing and shape-adaptive nanocomposite hydrogels with anti-inflammatory, antioxidant, antibacterial activities and hemostasis for real-time visual regeneration of diabetic wounds. Compos. B Eng. 2023, 262, 110819. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, J.; Li, M.; Liu, Z.; Wang, X.; Zhang, L.; Wang, Z. Hydrogel-Based Biomaterials Engineered from Natural-Derived Polysaccharides and Proteins for Hemostasis and Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 780187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeso, L.; Antezana, P.E.; Hvozda Arana, A.G.; Evelson, P.A.; Orive, G.; Desimone, M.F. Progress in the Use of Hydrogels for Antioxidant Delivery in Skin Wounds. Pharmaceutics 2024, 16, 524. https://doi.org/10.3390/pharmaceutics16040524
Maeso L, Antezana PE, Hvozda Arana AG, Evelson PA, Orive G, Desimone MF. Progress in the Use of Hydrogels for Antioxidant Delivery in Skin Wounds. Pharmaceutics. 2024; 16(4):524. https://doi.org/10.3390/pharmaceutics16040524
Chicago/Turabian StyleMaeso, Lidia, Pablo Edmundo Antezana, Ailen Gala Hvozda Arana, Pablo Andrés Evelson, Gorka Orive, and Martín Federico Desimone. 2024. "Progress in the Use of Hydrogels for Antioxidant Delivery in Skin Wounds" Pharmaceutics 16, no. 4: 524. https://doi.org/10.3390/pharmaceutics16040524





