Films for Wound Healing Fabricated Using a Solvent Casting Technique
Abstract
:1. Introduction
2. Wound Healing
2.1. Hemostasis
2.2. Inflammation
2.3. Proliferation
2.4. Remodeling
3. Polymeric Films for Wound Healing
3.1. Natural Polymeric Films
3.2. Synthetic Polymeric Films
3.3. Blended Polymeric Films
4. The Solvent Casting Method
4.1. Advantages
4.2. The Importance of the Polymer
4.3. The Influence of the Plasticizing Agent
4.4. Blend of Polymers
4.5. Polymer–Polymer, Drug–Polymer Incompatibility Phenomena
4.6. Growth of Microorganisms
5. Fabrication
5.1. The Solvent Process
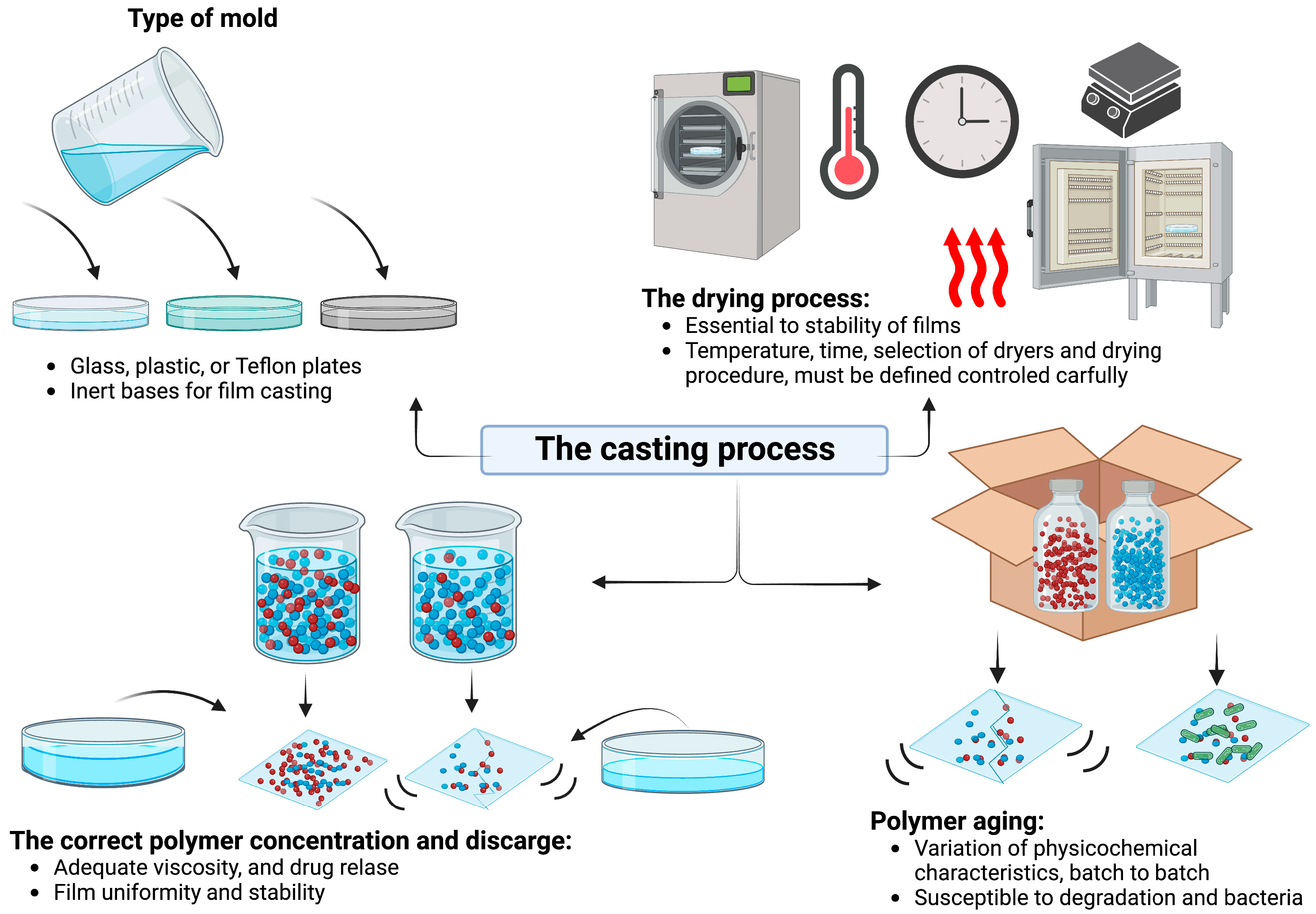
5.2. The Casting Process
6. Reproducibility, Establishing Quality Control Parameters
| PF | Properties | Dimensions | Organoleptic Properties | Mechanical Properties | Ref. |
|---|---|---|---|---|---|
| -Chitosan 1% (w/v) -Mansoa hirsuta fraction (MHF) 1.5% (w/v) | -Advanced healing -Re-epithelization -Cell proliferation -Collagen formation | -Film samples: 5 × 15 cm strips -Thickness: 26.57 ± 2.052 μm | -Smooth and continuous surface | -TS: 22.60 ± 2.79 MPa -EB: 68.75% | [116] |
| -Soy protein isolate 5% (w/w) -Glycerol 35% (w/w) -Glyoxal 1% (w/w) -Drug 3% (w/w) | -Analgesic: bupivacaine -Antibiotics: gentamicin, clindamycin | NA | -Soft film (drug incorporation had a softening effect | -YM: 342.5 ± 95.9 MPa -EB: 73.1 ± 35.7% -TS: 17.2 ± 2.8 MPa | [119] |
| -Chitosan 1% (w/v) -Carbopol 0.5% (w/v) -Glycerin 5% (w/v) | -Antibiotic: mupirocin | -Thickness: 0.504 ± 0.018 mm | -Swelling index: till 900% after 24 h -Moisture loss: 1.120 ± 2.067% | -TS: 0.695 ± 0.11 N/cm2 -EB: 211.763 ± 27.119 N | [38] |
| -Chitosan 1% (w/v) -Tween 80 0.1% (v/w) -H. perforatum oil 0.25–1.5% (v/v) | -H. perforatum oil: anti-inflammatory, antimicrobial, antioxidant agent, wound healing, and pain relief effect | -Thickness: 0.033–0.066 mm (0.066 ± 0.0029 the one of 1.5% oil v/v) | -Smooth surface -Transparent and colorless | -TS (without oil): 44.6 Mpa -TS (with 1.5% (v/v) of oil): 14.8 Mpa -EB (without oil): 7% -EB: 8–21% | [115] |
| -Chitosan 1% (w/v) -Bentonite 0.5% (w/v) Ratio CS:BN: 1:1–6:1 | -Bentonite: antimicrobial activity | -Thickness: 17.50 ± 5–42.50 ± 9.75 μm. -WVTR: 1093 ± 20.5–1954 ± 51 g/m2/day | -Porosity: increased from 78% to 88% with BN | -Folding strength: 145.25 ± 2.21–289.50 ± 0.57 | [117] |
| -Keratin:Fibrin: Gelatin 1:1:3 ratio (K:F:G) | -Mupirocin: topical antibiotic | NA | -Smooth surface | -EB: 3.61% -TS: 9.48 Mpa | [113] |
| -Chitosan 1% (w/v) -PVA 5% (w/v) -Glycerol 10% (v/v) -Glyoxal 5% (v/v) | -For biomedical application | -Thickness: 0.784 mm -Swelling: continuous swelling for 1st and 2nd h (4 h: 1.8 g) | -Smooth surface -Porous, rough surface in a non-crosslinked film | -Hardness value: 53.8 -TS: 0.74 MPa -Maximum load: 0.92 N -EB: 34% | [126] |
| -Poly(3-HO)/n-BG nanocomposite system -n-BG: 17% for 5 wt% or 9% for 10 wt% polymer solutions | -Matrix support for skin tissue | NA | -Smooth surface | -YM: 3 ± 1–4 ± 1 MPa -TS: 3.3 MPa. -EB: 222 ± 6%–236 ± 10% | [127] |
| -Sodium alginate (SG): Pectin (PC) solution (5%) (1:1 w/w) -Glycerol 7% (v/v) -TP: 5–25 mg/mL | -Tridax Procumbenns (TP): biodegradable, biocompatible, and antibacterial hemostatic agent | -Swelling: up to 251% within 15 min in PBS. -Thickness: 0.193 ± 0.002 mm–0.278 ± 0.002 mm | -Pale yellowish to green-brown color | -TS: 5–6 MPa. -EB: 70% -WVTR: 1500–2000 g/m2/24 h | [121] |
| -Soy protein isolate (SPI) 8.5% (wt) -PVA 10% (wt) -Glycerol 5% (wt) | -Potential wound healing application | -Thickness: 50 μm. -Swelling: 69.243 ± 22.7% -WVTR: 266.7 g/m2 day−1 | -Smooth surface | -TS: 0.85 MPa -EB: 2.0825% | [120] |
| -Gellan gum (GG): 16 mg/mL -Glycerol: 66 mg/mL -Silibinin nanocapsules: 10 mL | -Silibinin (SB): hepato-protector, antioxidant, and anti-inflammatory | -Thickness: 19 ± 2 μm. -Swelling: 5.11 ± 2.40% | -Transparent -Smooth and continuous surface | -TS: 3.70 ± 0.26 MPa -EB: 4.71 ± 0.37% | [128] |
7. Principal Applications of Films for Wound Healing
7.1. Current Medical Applications
7.2. Future Perspectives and Areas of Improvement
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Romero-Montero, A.; Labra-Vázquez, P.; del Valle, L.J.; Puiggalí, J.; García-Arrazola, R.; Montiel, C.; Gimeno, M. Development of an Antimicrobial and Antioxidant Hydrogel/Nano-Electrospun Wound Dressing. RSC Adv. 2020, 10, 30508–30518. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Guo, B. Smart Wound Dressings for Wound Healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Qiao, B.; Pang, Q.; Yuan, P.; Luo, Y.; Ma, L. Smart Wound Dressing for Infection Monitoring and NIR-Triggered Antibacterial Treatment. Biomater. Sci. 2020, 8, 1649–1657. [Google Scholar] [CrossRef]
- Yoshimura, M.; Ohura, N.; Tanaka, J.; Ichimura, S.; Kasuya, Y.; Hotta, O.; Kagaya, Y.; Sekiyama, T.; Tannba, M.; Suzuki, N. Soft Silicone Foam Dressing Is More Effective than Polyurethane Film Dressing for Preventing Intraoperatively Acquired Pressure Ulcers in Spinal Surgery Patients: The Border Operating Room Spinal Surgery (BOSS) Trial in Japan. Int. Wound J. 2018, 15, 188–197. [Google Scholar] [CrossRef]
- Chin, C.-Y.; Jalil, J.; Ng, P.Y.; Ng, S.-F. Development and Formulation of Moringa Oleifera Standardised Leaf Extract Film Dressing for Wound Healing Application. J. Ethnopharmacol. 2018, 212, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.; Labbaf, S.; Mirhaj, M.; Salehi, S.; Seifalian, A.M.; Firuzeh, M. Natural Polymers in Wound Healing: From Academic Studies to Commercial Products. J. Appl. Polym. Sci. 2023, 140, e53910. [Google Scholar] [CrossRef]
- Mwiiri, F.K.; Brandner, J.M.; Daniels, R. Electrospun Bioactive Wound Dressing Containing Colloidal Dispersions of Birch Bark Dry Extract. Pharmaceutics 2020, 12, 770. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for Wound Dressings: An Up-to-Date Overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef]
- Yasuda, K.; Ogushi, M.; Nakashima, A.; Nakano, Y.; Suzuki, K. Accelerated Wound Healing on the Skin Using a Film Dressing with β-Glucan Paramylon. In Vivo 2018, 32, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Weller, C.D.; Team, V.; Sussman, G. First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front. Pharmacol. 2020, 11, 155. [Google Scholar] [CrossRef] [Green Version]
- Rao, K.M.; Kim, E.; Kim, H.J.; Uthappa, U.T.; Han, S.S. Hyaluronic Acid-Quercetin Pendant Drug Conjugate for Wound Healing Applications. Int. J. Biol. Macromol. 2023, 240, 124336. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Deng, Z.; Guo, Y.; Xu, P. Engineering Functional Natural Polymer-Based Nanocomposite Hydrogels for Wound Healing. Nanoscale Adv. 2023, 5, 27–45. [Google Scholar] [CrossRef]
- Khalil, H.; Cullen, M.; Chambers, H.; Carroll, M.; Walker, J. Elements Affecting Wound Healing Time: An Evidence Based Analysis. Wound Repair Regen. 2015, 23, 550–556. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Critical Review in Oral Biology & Medicine: Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Derm. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falabella, A.F.; Kirsner, R.S. Wound Healing (Basic and Clinical Dermatology); Fallabela, A.F., Kirsner Robert, S., Eds.; Taylor & Francis Group: Abingdon, UK, 2005; Volume 33, ISBN 9780824754587. [Google Scholar]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Reinke, J.M.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Balaji, S.; Watson, C.L.; Ranjan, R.; King, A.; Bollyky, P.L.; Keswani, S.G. Chemokine Involvement in Fetal and Adult Wound Healing. Adv. Wound Care 2015, 4, 660–672. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound Repair and Regeneration: Mechanisms, Signaling, and Translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [Green Version]
- Broughton, G.; Janis, J.E.; Attinger, C.E. The Basic Science of Wound Healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef] [PubMed]
- Nalliappan, G.; Siva Subramaniyan, V.; Rajkumar, D.; Ramraj Jayabalan, A.; Kumarakrishnan, V.B. Molecular Biology of Wound Healing. J. Pharm. Bioallied. Sci. 2012, 4, S334. [Google Scholar]
- Singh, S.; Young, A.; McNaught, C.E. The Physiology of Wound Healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Schultz, G.; Clark, W.; Rotatori, D.S. EGF and TGF-α in Wound Healing and Repair. J. Cell. Biochem. 1991, 45, 346–352. [Google Scholar] [CrossRef]
- Gonzalez, A.C.D.O.; Andrade, Z.D.A.; Costa, T.F.; Medrado, A.R.A.P. Wound Healing—A Literature Review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Yannas, I.V.; Tzeranis, D.S.; So, P.T.C. Regeneration of Injured Skin and Peripheral Nerves Requires Control of Wound Contraction, Not Scar Formation. Wound Repair Regen. 2017, 25, 177–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Prunotto, M.; Desmoulière, A.; Varga, J.; De Wever, O.; Mareel, M.; Gabbiani, G. Recent Developments in Myofibroblast Biology. Am. J. Pathol. 2012, 180, 1340–1355. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases; StatPearls: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Carla Daunton, S.D.; Kothari, S.; Smith, L.; Steele, D. A History of Materials and Practices for Wound Management. Wound Pract. Res. J. Aust. Wound Manag. Assoc. 2012, 20, 174–176, 178–180, 182–186. [Google Scholar]
- Bloom, H. ‘Cellophane’ Dressing for Second-Degree Burns. Lancet 1945, 3, 559. [Google Scholar] [CrossRef]
- Wang, B.; Li, W.; Harrison, J. An Evaluation of Wound Healing Efficacy of a Film Dressing Made from Polymer-Integrated Amnion Membrane. Organogenesis 2020, 16, 126–136. [Google Scholar] [CrossRef]
- Rani Raju, N.; Silina, E.; Stupin, V.; Manturova, N.; Chidambaram, S.B.; Achar, R.R. Multifunctional and Smart Wound Dressings—A Review on Recent Research Advancements in Skin Regenerative Medicine. Pharmaceutics 2022, 14, 1574. [Google Scholar] [CrossRef]
- Zasadziński, K.; Spałek, M.J.; Rutkowski, P. Modern Dressings in Prevention and Therapy of Acute and Chronic Radiation Dermatitis—A Literature Review. Pharmaceutics 2022, 14, 1204. [Google Scholar] [CrossRef] [PubMed]
- Savencu, I.; Iurian, S.; Porfire, A.; Bogdan, C.; Tomuță, I. Review of Advances in Polymeric Wound Dressing Films. React. Funct. Polym. 2021, 168, 105059. [Google Scholar] [CrossRef]
- Naseri-Nosar, M.; Ziora, Z.M. Wound Dressings from Naturally-Occurring Polymers: A Review on Homopolysaccharide-Based Composites. Carbohydr. Polym. 2018, 189, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Üstündağ Okur, N.; Hökenek, N.; Okur, M.E.; Ayla, Ş.; Yoltaş, A.; Siafaka, P.I.; Cevher, E. An Alternative Approach to Wound Healing Field; New Composite Films from Natural Polymers for Mupirocin Dermal Delivery. Saudi Pharm. J. 2019, 27, 738–752. [Google Scholar] [CrossRef]
- Wu, D.; Wei, W.; Li, H.; Wang, X.; Wang, T.; Tang, S.; Li, Q.; Yao, Y.; Pan, Y.; Wei, J. Blended Films Containing Polybutyrolactam and Chitosan for Potential Wound Dressing Applications. J. Appl. Polym. Sci. 2018, 135, 46511. [Google Scholar] [CrossRef]
- Riccio, B.V.F.; Silvestre, A.L.P.; Meneguin, A.B.; de Cassia Ribeiro, T.; Klosowski, A.B.; Ferrari, P.C.; Chorilli, M. Exploiting Polymeric Films as a Multipurpose Drug Delivery System: A Review. AAPS PharmSciTech 2022, 23, 269. [Google Scholar] [CrossRef]
- Bunge, K.E.; Dezzutti, C.S.; Hendrix, C.W.; Marzinke, M.A.; Spiegel, H.M.L.; Moncla, B.J.; Schwartz, J.L.; Meyn, L.A.; Richardson-Harman, N.; Rohan, L.C.; et al. FAME-04: A Phase 1 Trial to Assess the Safety, Acceptability, Pharmacokinetics and Pharmacodynamics of Film and Gel Formulations of Tenofovir. J. Int. AIDS Soc. 2018, 21, e25156. [Google Scholar] [CrossRef] [Green Version]
- Gayathri, D.; Jayakumari, L.S. Evaluation of Commercial Arrowroot Starch/CMC Film for Buccal Drug Delivery of Glipizide. Polimeros 2019, 29, e2019047. [Google Scholar] [CrossRef]
- Marie Arockianathan, P.; Sekar, S.; Sankar, S.; Kumaran, B.; Sastry, T.P. Evaluation of Biocomposite Films Containing Alginate and Sago Starch Impregnated with Silver Nano Particles. Carbohydr. Polym. 2012, 90, 717–724. [Google Scholar] [CrossRef]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic Polymeric Biomaterials for Wound Healing: A Review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Alven, S.; Nqoro, X.; Aderibigbe, B.A. Polymer-Based Materials Loaded with Curcumin for Wound Healing Applications. Polymers 2020, 12, 2286. [Google Scholar] [CrossRef] [PubMed]
- Kong, I.; Tshai, K.Y.; Hoque, M.E. Manufacturing of Natural Fibre-Reinforced Polymer Composites by Solvent Casting Method. In Manufacturing of Natural Fibre Reinforced Polymer Composites; Springer International Publishing: Cham, Switzerland, 2015; pp. 331–349. [Google Scholar]
- Prasad, A.; Sankar, M.R.; Katiyar, V. State of Art on Solvent Casting Particulate Leaching Method for Orthopedic ScaffoldsFabrication. Mater. Today Proc. 2017, 4, 898–907. [Google Scholar] [CrossRef]
- Zena, Y.; Periyasamy, S.; Tesfaye, M.; Tumsa, Z.; Jayakumar, M.; Mohamed, B.A.; Asaithambi, P.; Aminabhavi, T.M. Essential Characteristics Improvement of Metallic Nanoparticles Loaded Carbohydrate Polymeric Films—A Review. Int. J. Biol. Macromol. 2023, 242, 124803. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Shagdarova, B.; Sinitsyna, O.; Sinitsyn, A.; Varlamov, V. Evaluation of Antibacterial and Antifungal Properties of Low Molecular Weight Chitosan Extracted from Hermetia Illucens Relative to Crab Chitosan. Molecules 2022, 27, 577. [Google Scholar] [CrossRef]
- Aramwit, P.; Ratanavaraporn, J.; Ekgasit, S.; Tongsakul, D.; Bang, N. A Green Salt-Leaching Technique to Produce Sericin/PVA/Glycerin Scaffolds with Distinguished Characteristics for Wound-Dressing Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 915–924. [Google Scholar] [CrossRef]
- Orava, J.; Kohoutek, T.; Wagner, T. Deposition Techniques for Chalcogenide Thin Films. In Chalcogenide Glasses; Elsevier: Amsterdam, The Netherlands, 2014; pp. 265–309. [Google Scholar]
- Sahu, N.; Parija, B.; Panigrahi, S. Fundamental Understanding and Modeling of Spin Coating Process: A Review. Indian J. Phys. 2009, 83, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Jun, Y.; Qin, J.; Lee, S.-H. Electrospinning versus Microfluidic Spinning of Functional Fibers for Biomedical Applications. Biomaterials 2017, 114, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Kim, H.; Na, S.J.; Shin, D.; Jo, K.; Lee, J. Thin Films as an Emerging Platform for Drug Delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Kang, X.; Liu, Y.; Feng, F.; Zhang, H. Characterization of Gelatin/Zein Films Fabricated by Electrospinning vs Solvent Casting. Food Hydrocoll. 2018, 74, 324–332. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Gain, S.; Verma, S.; Singh, B.; Vyas, M.; Mehta, M.; Haque, A. Polymers in Designing the Mucoadhesive Films: A Comprehensive Review. Int. J. Green Pharm. 2018, 12, S330–S344. [Google Scholar]
- Anbukarasu, P.; Sauvageau, D.; Elias, A. Tuning the Properties of Polyhydroxybutyrate Films Using Acetic Acid via Solvent Casting. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Tenorová, K.; Masteiková, R.; Pavloková, S.; Kostelanská, K.; Bernatonienė, J.; Vetchý, D. Formulation and Evaluation of Novel Film Wound Dressing Based on Collagen/Microfibrillated Carboxymethylcellulose Blend. Pharmaceutics 2022, 14, 782. [Google Scholar] [CrossRef]
- Leyva-Gómez, G.; González-Torres, M.; Alcalá-Alcalá, S.; Bernal-Chávez, S.A.; Morales-Morfin, J.C.; González-Del Carmen, M.; Sharifi-Rad, J.; Figueroa-González, G.; Reyes-Hernández, O.D.; del Prado-Audelo, M.L.; et al. Development of Films from Natural Sources for Infections during Wound Healing. Cell. Mol. Biol. 2021, 67, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Sussman, G. Understanding Film Dressings. Wounds Int. 2010, 1, 23–25. [Google Scholar]
- Palo, M.; Rönkönharju, S.; Tiirik, K.; Viidik, L.; Sandler, N.; Kogermann, K. Bi-Layered Polymer Carriers with Surface Modification by Electrospinning for Potential Wound Care Applications. Pharmaceutics 2019, 11, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and Synthetic Polymers for Wounds and Burns Dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef]
- Li, X.; Kong, X.; Zhang, Z.; Nan, K.; Li, L.L.; Wang, X.H.; Chen, H. Cytotoxicity and Biocompatibility Evaluation of N,O-Carboxymethyl Chitosan/Oxidized Alginate Hydrogel for Drug Delivery Application. Int. J. Biol. Macromol. 2012, 50, 1299–1305. [Google Scholar] [CrossRef]
- Robles-Kanafany, C.M.; del Prado-Audelo, M.L.; González-Torres, M.; Giraldo-Gomez, D.M.; Caballero-Florán, I.H.; González-Del Carmen, M.; Sharifi-Rad, J.; Figueroa-González, G.; Reyes-Hernández, O.D.; Cortés, H.; et al. Development of a Guar Gum Film with Lysine Clonixinate for Periodontal Treatments. Cell. Mol. Biol. 2021, 67, 89–95. [Google Scholar] [CrossRef]
- Mishra, R.K.; Ramasamy, K.; Lim, S.M.; Ismail, M.F.; Majeed, A.B.A. Antimicrobial and in Vitro Wound Healing Properties of Novel Clay Based Bionanocomposite Films. J. Mater. Sci. Mater. Med. 2014, 25, 1925–1939. [Google Scholar] [CrossRef] [PubMed]
- Guadarrama-Acevedo, M.C.; Mendoza-Flores, R.A.; del Prado-Audelo, M.L.; Urbán-Morlán, Z.; Giraldo-Gomez, D.M.; Magaña, J.J.; González-Torres, M.; Reyes-Hernández, O.D.; Figueroa-González, G.; Caballero-Florán, I.H.; et al. Development and Evaluation of Alginate Membranes with Curcumin-Loaded Nanoparticles for Potential Wound-Healing Applications. Pharmaceutics 2019, 11, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villar-Padilla, A.; del Prado-Audelo, M.L.; González-Torres, M.; Giraldo-Gomez, D.M.; Caballero-Florán, I.H.; del Carmen, M.G.; Sharifi-Rad, J.; Figueroa-González, G.; Reyes-Hernández, O.D.; Cortés, H.; et al. Development of a Xanthan Gum Film for the Possible Treatment of Vaginal Infections. Cell. Mol. Biol. 2021, 67, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-Based Plasticizers and Biopolymer Films: A Review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Bergo, P.; Sobral, P.J.A. Effects of Plasticizer on Physical Properties of Pigskin Gelatin Films. Food Hydrocoll. 2007, 21, 1285–1289. [Google Scholar] [CrossRef]
- de Souza, R.F.B.; de Souza, F.C.B.; Bierhalz, A.C.K.; Pires, A.L.R.; Moraes, Â.M. Biopolymer-Based Films and Membranes as Wound Dressings. In Biopolymer Membranes and Films; Elsevier: Amsterdam, The Netherlands, 2020; pp. 165–194. [Google Scholar]
- Sharma, B.; Malik, P.; Jain, P. Biopolymer Reinforced Nanocomposites: A Comprehensive Review. Mater. Today Commun. 2018, 16, 353–363. [Google Scholar] [CrossRef]
- Sarath, C.C.; Shanks, R.A.; Thomas, S. Polymer Blends. In Nanostructured Polymer Blends; William Andrew: Norwich, NY, USA, 2013; pp. 1–14. [Google Scholar] [CrossRef]
- Pereda, M.; Ponce, A.G.; Marcovich, N.E.; Ruseckaite, R.A.; Martucci, J.F. Chitosan-Gelatin Composites and Bi-Layer Films with Potential Antimicrobial Activity. Food Hydrocoll. 2011, 25, 1372–1381. [Google Scholar] [CrossRef]
- Meng, F.; Dave, V.; Chauhan, H. Qualitative and Quantitative Methods to Determine Miscibility in Amorphous Drug-Polymer Systems. Eur. J. Pharm. Sci. 2015, 77, 106–111. [Google Scholar] [CrossRef]
- Ricarte, R.G.; van Zee, N.J.; Li, Z.; Johnson, L.M.; Lodge, T.P.; Hillmyer, M.A. Recent Advances in Understanding the Micro- and Nanoscale Phenomena of Amorphous Solid Dispersions. Mol. Pharm. 2019, 16, 4089–4103. [Google Scholar] [CrossRef]
- Donelli, G. Biofilm-Based Healthcare-Associated Infections: Volume II. Adv. Exp. Med. Biol. 2015, 831, 93–117. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric Materials with Antimicrobial Activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, J.H.; Li, T.S.; Wang, S.H.; Yao, C.H.; Chung, W.Y.; Ko, T.H. Dressing with Epigallocatechin Gallate Nanoparticles for Wound Regeneration. Wound Repair Regen. 2016, 24, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.W.; Lv, Y.L.; Zhong, Y.F.; Xue, Y.N.; Wang, Y.; Zhang, L.Y.; Hu, X.; Tan, W.Q. Beneficial Effects of Green Tea EGCG on Skin Wound Healing: A Comprehensive Review. Molecules 2021, 26, 6123. [Google Scholar] [CrossRef] [PubMed]
- Salawi, A. An Insight into Preparatory Methods and Characterization of Orodispersible Film—A Review. Pharmaceuticals 2022, 15, 844. [Google Scholar] [CrossRef] [PubMed]
- Dangre, P.V.; Phad, R.D.; Surana, S.J.; Chalikwar, S.S. Quality by Design (QbD) Assisted Fabrication of Fast Dissolving Buccal Film for Clonidine Hydrochloride: Exploring the Quality Attributes. Adv. Polym. Technol. 2019, 2019, 3682402. [Google Scholar] [CrossRef]
- Naseri, E.; Ahmadi, A. A Review on Wound Dressings: Antimicrobial Agents, Biomaterials, Fabrication Techniques, and Stimuli-Responsive Drug Release. Eur. Polym. J. 2022, 173, 111293. [Google Scholar] [CrossRef]
- Boateng, J.S.; Stevens, H.N.E.; Eccleston, G.M.; Auffret, A.D.; Humphrey, M.J.; Matthews, K.H. Development and Mechanical Characterization of Solvent-Cast Polymeric Films as Potential Drug Delivery Systems to Mucosal Surfaces. Drug Dev. Ind. Pharm. 2009, 35, 986–996. [Google Scholar] [CrossRef]
- Hafezi, F.; Scoutaris, N.; Douroumis, D.; Boateng, J. 3D Printed Chitosan Dressing Crosslinked with Genipin for Potential Healing of Chronic Wounds. Int. J. Pharm. 2019, 560, 406–415. [Google Scholar] [CrossRef]
- Croll, S.G. The Origin of Residual Internal Stress in Solvent-Cast Thermoplastic Coatings. J. Appl. Polym. Sci. 1979, 23, 847–858. [Google Scholar] [CrossRef] [Green Version]
- Eulálio, H.Y.C.; Vieira, M.; Fideles, T.B.; Tomás, H.; Silva, S.M.L.; Peniche, C.A.; Fook, M.V.L. Physicochemical Properties and Cell Viability of Shrimp Chitosan Films as Affected by Film Casting Solvents. I-Potential Use as Wound Dressing. Materials 2020, 13, 5005. [Google Scholar] [CrossRef] [PubMed]
- Bedell, M.L.; Guo, J.L.; Xie, V.Y.; Navara, A.M.; Mikos, A.G. Polymer Scaffold Fabrication. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 295–315. [Google Scholar]
- Eskandarinia, A.; Kefayat, A.; Rafienia, M.; Agheb, M.; Navid, S.; Ebrahimpour, K. Cornstarch-Based Wound Dressing Incorporated with Hyaluronic Acid and Propolis: In Vitro and in Vivo Studies. Carbohydr. Polym. 2019, 216, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kim, S.G.; Hyun, Y.H.; Jhon, M.S. Preparation and Rheological Characteristics of Solvent-Cast Poly (Ethylene Oxide)/Montmorillonite Nanocomposites. Macromol. Rapid Commun. 2001, 22, 320–325. [Google Scholar] [CrossRef]
- Silvestro, I.; Lopreiato, M.; Scotto d’Abusco, A.; di Lisio, V.; Martinelli, A.; Piozzi, A.; Francolini, I. Hyaluronic Acid Reduces Bacterial Fouling and Promotes Fibroblasts’ Adhesion onto Chitosan 2D-Wound Dressings. Int. J. Mol. Sci. 2020, 21, 2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixit, R.P.; Puthli, S.P. Oral Strip Technology: Overview and Future Potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef]
- Kim, J.O.; Noh, J.-K.; Thapa, R.K.; Hasan, N.; Choi, M.; Kim, J.H.; Lee, J.-H.; Ku, S.K.; Yoo, J.-W. Nitric Oxide-Releasing Chitosan Film for Enhanced Antibacterial and in Vivo Wound-Healing Efficacy. Int. J. Biol. Macromol. 2015, 79, 217–225. [Google Scholar] [CrossRef]
- Cupone, I.E.; Sansone, A.; Marra, F.; Giori, A.M.; Jannini, E.A. Orodispersible Film (ODF) Platform Based on Maltodextrin for Therapeutical Applications. Pharmaceutics 2022, 14, 2011. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Kefayat, A.; Agheb, M.; Rafienia, M.; Amini Baghbadorani, M.; Navid, S.; Ebrahimpour, K.; Khodabakhshi, D.; Ghahremani, F. A Novel Bilayer Wound Dressing Composed of a Dense Polyurethane/Propolis Membrane and a Biodegradable Polycaprolactone/Gelatin Nanofibrous Scaffold. Sci. Rep. 2020, 10, 3063. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, F.S.; Sharifianjazi, F.; Esmaeilkhanian, A.; Salehi, E. Chitosan Films and Scaffolds for Regenerative Medicine Applications: A Review. Carbohydr. Polym. 2021, 273, 118631. [Google Scholar] [CrossRef]
- Germini, G.; Peltonen, L. 3D Printing of Drug Nanocrystals for Film Formulations. Molecules 2021, 26, 3941. [Google Scholar] [CrossRef]
- Prest, W.M.; Luca, D.J. The Origin of the Optical Anisotropy of Solvent Cast Polymeric Films. J. Appl. Phys. 1979, 50, 6067–6071. [Google Scholar] [CrossRef]
- Ghosal, K.; Chandra, A.; Praveen, G.; Snigdha, S.; Roy, S.; Agatemor, C.; Thomas, S.; Provaznik, I. Electrospinning over Solvent Casting: Tuning of Mechanical Properties of Membranes. Sci. Rep. 2018, 8, 5058. [Google Scholar] [CrossRef] [Green Version]
- Cilurzo, F.; Cupone, I.E.; Minghetti, P.; Selmin, F.; Montanari, L. Fast Dissolving Films Made of Maltodextrins. Eur. J. Pharm. Biopharm. 2008, 70, 895–900. [Google Scholar] [CrossRef]
- Kanai, T.C.G. Film Processing Advances; Hanser: München, Germany, 1999. [Google Scholar]
- Kunte, S.; Tandale, P. Fast Dissolving Strips: A Novel Approach for the Delivery of Verapamil. J. Pharm. Bioallied. Sci. 2010, 2, 325. [Google Scholar] [CrossRef]
- Krampe, R.; Visser, J.C.; Frijlink, H.W.; Breitkreutz, J.; Woerdenbag, H.J.; Preis, M. Oromucosal Film Preparations: Points to Consider for Patient Centricity and Manufacturing Processes. Expert Opin. Drug Deliv. 2016, 13, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.D.; Chung, Y.G.; Gil, E.S.; Seth, A.; Franck, D.; Cristofaro, V.; Sullivan, M.P.; di Vizio, D.; Gomez, P.; Adam, R.M.; et al. Bladder Tissue Regeneration Using Acellular Bi-Layer Silk Scaffolds in a Large Animal Model of Augmentation Cystoplasty. Biomaterials 2013, 34, 8681–8689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandeep, K.; Rana, A.C.; Nimrata, S. Fast Dissolving Films: An Innovative Drug Delivery System. Int. J. Pharm. Res. Allied Sci. 2013, 2, 14–24. [Google Scholar]
- Siemann, U. Solvent Cast Technology—A Versatile Tool for Thin Film Production. In Scattering Methods and the Properties of Polymer Materials; Stribeck, N., Smarsly, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–14. [Google Scholar]
- Ayensu, I.; Mitchell, J.C.; Boateng, J.S. Development and Physico-Mechanical Characterisation of Lyophilised Chitosan Wafers as Potential Protein Drug Delivery Systems via the Buccal Mucosa. Colloids Surf. B Biointerfaces 2012, 91, 258–265. [Google Scholar] [CrossRef]
- Raju, P.N.; Kumar, M.S.; Reddy, C.M.; Ravishankar, K. Formulation and Evaluation of Fast Dissolving Films of Loratidine by Solvent Casting Method. Pharma. Innov. 2013, 2, 31–35. [Google Scholar]
- Haq, A.; Kumar, S.; Mao, Y.; Berthiaume, F.; Michniak-Kohn, B. Thymoquinone-Loaded Polymeric Films and Hydrogels for Bacterial Disinfection and Wound Healing. Biomedicines 2020, 8, 386. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Romero-Guzmán, D.; Fernández-Grajera, M.; González-Martín, M.L.; Gallardo-Moreno, A.M. Aging of Solvent-Casting PLA-Mg Hydrophobic Films: Impact on Bacterial Adhesion and Viability. Coatings 2019, 9, 814. [Google Scholar] [CrossRef] [Green Version]
- Anbukarasu, P.; Sauvageau, D.; Elias, A. The Effects of Solvent Casting Temperature and Physical Aging on polyhydroxybutyrate-graphene Nanoplatelet Composites. Polym. Compos. 2021, 42, 1451–1461. [Google Scholar] [CrossRef]
- Kakkar, P.; Madhan, B.; Shanmugam, G. Extraction and Characterization of Keratin from Bovine Hoof: A Potential Material for Biomedical Applications. J. Korean Phys. Soc. 2014, 3, 596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellappan, L.K.; Anandhavelu, S.; Doble, M.; Perumal, G.; Jeon, J.H.; Vikraman, D.; Kim, H.S. Biopolymer Film Fabrication for Skin Mimetic Tissue Regenerative Wound Dressing Applications. Int. J. Polym. Mater. Polym. Biomater. 2020, 71, 196–207. [Google Scholar] [CrossRef]
- Neamtu, B.M.; Barbu, A. Trends in Alginate-Based Films and Membranes for Wound Healing. Rom. Biotechnol. Lett. 2020, 25, 1683–1689. [Google Scholar] [CrossRef]
- Güneş, S.; Tıhmınlıoğlu, F. Hypericum Perforatum Incorporated Chitosan Films as Potential Bioactive Wound Dressing Material. Int. J. Biol. Macromol. 2017, 102, 933–943. [Google Scholar] [CrossRef]
- Pereira, J.R.; Bezerra, G.S.; Furtado, A.A.; de Carvalho, T.G.; da Silva, V.C.; Monteiro, A.L.B.; Guerra, G.C.B.; de Araújo Júnior, R.F.; Sant’ana, A.E.G.; de Freitas Fernandes-Pedrosa, M.; et al. Chitosan Film Containing Mansoa Hirsuta Fraction for Wound Healing. Pharmaceutics 2020, 12, 484. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Dutta, J. Preparation and Characterization of Chitosan-Bentonite Nanocomposite Films for Wound Healing Application. Int. J. Biol. Macromol. 2017, 104, 1897–1904. [Google Scholar] [CrossRef]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Physically Crosslinked-Sacran Hydrogel Films for Wound Dressing Application. Int. J. Biol. Macromol. 2016, 89, 465–470. [Google Scholar] [CrossRef]
- Baranes-Zeevi, M.; Goder, D.; Zilberman, M. Novel Drug-Eluting Soy-Protein Structures for Wound Healing Applications. Polym. Adv. Technol. 2019, 30, 2523–2538. [Google Scholar] [CrossRef]
- Khabbaz, B.; Solouk, A.; Mirzadeh, H. Polyvinyl Alcohol/Soy Protein Isolate Nanofibrous Patch for Wound-Healing Applications. Prog. Biomater. 2019, 8, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Sutar, T.; Bangde, P.; Dandekar, P.; Adivarekar, R. Fabrication of Herbal Hemostat Films Loaded with Medicinal Tridax Procumbenns Extracts. Fibers Polym. 2021, 22, 2135–2144. [Google Scholar] [CrossRef]
- Byun, Y.; Whiteside, S.; Thomas, R.; Dharman, M.; Hughes, J.; Kim, Y.T. The Effect of Solvent Mixture on the Properties of Solvent Cast Polylactic Acid (PLA) Film. J. Appl. Polym. Sci. 2012, 124, 3577–3582. [Google Scholar] [CrossRef]
- Figoli, A.; Marino, T.; Simone, S.; Di Nicolò, E.; Li, X.M.; He, T.; Tornaghi, S.; Drioli, E. Towards Non-Toxic Solvents for Membrane Preparation: A Review. Green Chem. 2014, 16, 4034–4059. [Google Scholar] [CrossRef]
- Dong, X.; Lu, D.; Harris, T.A.L.; Escobar, I.C. Polymers and Solvents Used in Membrane Fabrication: A Review Focusing on Sustainable Membrane Development. Membranes 2021, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Parın, F.N.; El-Ghazali, S.; Yeşilyurt, A.; Parın, U.; Ullah, A.; Khatri, M.; Kim, I.S. PVA/Inulin-Based Sustainable Films Reinforced with Pickering Emulsion of Niaouli Essential Oil for Potential Wound Healing Applications. Polymers 2023, 15, 1002. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A. Development and Characterization of Tripolymeric and Bipolymeric Composite Films Using Glyoxal as a Potent Crosslinker for Biomedical Application. Mater. Sci. Eng. C 2017, 73, 333–339. [Google Scholar] [CrossRef]
- Rai, R.; Roether, J.A.; Knowles, J.C.; Mordan, N.; Salih, V.; Locke, I.C.; Gordge, M.P.; Mccormick, A.; Mohn, D.; Stark, W.J.; et al. Highly Elastomeric Poly(3-Hydroxyoctanoate) Based Natural Polymer Composite for Enhanced Keratinocyte Regeneration. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 326–335. [Google Scholar] [CrossRef]
- Gehrcke, M.; de Bastos Brum, T.; da Rosa, L.S.; Ilha, B.D.; Soares, F.Z.M.; Cruz, L. Incorporation of Nanocapsules into Gellan Gum Films: A Strategy to Improve the Stability and Prolong the Cutaneous Release of Silibinin. Mater. Sci. Eng. C 2021, 119, 111624. [Google Scholar] [CrossRef]
- Gonçalves, M.M.; Carneiro, J.; Justus, B.; Espinoza, J.T.; Budel, J.M.; Farago, P.V.; de Paula, J.P. Preparation and Characterization of a Novel Antimicrobial Film Dressing for Wound Healing Application. Braz. J. Pharm. Sci. 2020, 56, e18784. [Google Scholar] [CrossRef]
- Shi, R.; Geng, H.; Gong, M.; Ye, J.; Wu, C.; Hu, X.; Zhang, L. Long-Acting and Broad-Spectrum Antimicrobial Electrospun Poly (ε-Caprolactone)/Gelatin Micro/Nanofibers for Wound Dressing. J. Colloid Interface Sci. 2018, 509, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, R.M.; Abdel-Mohsen, A.M.; Hrdina, R.; Burgert, L.; Fohlerova, Z.; Pavliňák, D.; Sayed, O.N.; Jancar, J. Wound Dressing Based on Chitosan/Hyaluronan/Nonwoven Fabrics: Preparation, Characterization and Medical Applications. Int. J. Biol. Macromol. 2016, 89, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Gianino, E.; Miller, C.; Gilmore, J. Smart Wound Dressings for Diabetic Chronic Wounds. Bioengineering 2018, 5, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; McAnulty, J.F.; Schurr, M.J.; Murphy, C.J.; Abbott, N.L. Polymeric Materials for Chronic Wound and Burn Dressings. In Advanced Wound Repair Therapies; Elsevier: Amsterdam, The Netherlands, 2011; pp. 186–208. [Google Scholar]
- Broussard, K.C.; Powers, J.G. Wound Dressings: Selecting the Most Appropriate Type. Am. J. Clin. Dermatol. 2013, 14, 449–459. [Google Scholar] [CrossRef]
- Meuleneire, F. A Vapour-Permeable Film Dressing Used on Superficial Wounds. Br. J. Nurs. 2014, 23, S36–S43. [Google Scholar] [CrossRef]
- Souto, E.B.; Yoshida, C.M.P.; Leonardi, G.R.; Cano, A.; Sanchez-Lopez, E.; Zielinska, A.; Viseras, C.; Severino, P.; da Silva, C.F.; de Melo Barbosa, R. Lipid-Polymeric Films: Composition, Production and Applications in Wound Healing and Skin Repair. Pharmaceutics 2021, 13, 1199. [Google Scholar] [CrossRef]
- Costa, N.N.; de Faria Lopes, L.; Ferreira, D.F.; de Prado, E.M.L.; Severi, J.A.; Resende, J.A.; de Paula Careta, F.; Ferreira, M.C.P.; Carreira, L.G.; de Souza, S.O.L.; et al. Polymeric Films Containing Pomegranate Peel Extract Based on PVA/Starch/PAA Blends for Use as Wound Dressing: In Vitro Analysis and Physicochemical Evaluation. Mater. Sci. Eng. C 2020, 109, 110643. [Google Scholar] [CrossRef]
- Ni, Y.; Lin, W.; Mu, R.; Wu, C.; Lin, Z.; Chen, S.; Pang, J. Facile Fabrication of Novel Konjac Glucomannan Films with Antibacterial Properties via Microfluidic Spinning Strategy. Carbohydr. Polym. 2019, 208, 469–476. [Google Scholar] [CrossRef]
- Duan, Y.; Li, K.; Wang, H.; Wu, T.; Zhao, Y.; Li, H.; Tang, H.; Yang, W. Preparation and Evaluation of Curcumin Grafted Hyaluronic Acid Modified Pullulan Polymers as a Functional Wound Dressing Material. Carbohydr. Polym. 2020, 238, 116195. [Google Scholar] [CrossRef]
- Ahmad, N.; Tayyeb, D.; Ali, I.; K. Alruwaili, N.; Ahmad, W.; ur Rehman, A.; Khan, A.H.; Iqbal, M.S. Development and Characterization of Hemicellulose-Based Films for Antibacterial Wound-Dressing Application. Polymers 2020, 12, 548. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Ahmad, M.M.; Alruwaili, N.K.; Alrowaili, Z.A.; Alomar, F.A.; Akhtar, S.; Alsaidan, O.A.; Alhakamy, N.A.; Zafar, A.; Elmowafy, M.; et al. Antibiotic-Loaded Psyllium Husk Hemicellulose and Gelatin-Based Polymeric Films for Wound Dressing Application. Pharmaceutics 2021, 13, 236. [Google Scholar] [CrossRef] [PubMed]
- Bardania, H.; Mahmoudi, R.; Bagheri, H.; Salehpour, Z.; Fouani, M.H.; Darabian, B.; Khoramrooz, S.S.; Mousavizadeh, A.; Kowsari, M.; Moosavifard, S.E.; et al. Facile Preparation of a Novel Biogenic Silver-Loaded Nanofilm with Intrinsic Anti-Bacterial and Oxidant Scavenging Activities for Wound Healing. Sci. Rep. 2020, 10, 6129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.Gov. Available online: https://beta.clinicaltrials.gov/ (accessed on 2 June 2023).
- EktoTherixTM Regenerative Tissue Scaffold for Repair of Surgical Excision Wounds. Available online: https://beta.clinicaltrials.gov/study/NCT02409628 (accessed on 2 June 2023).
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial Anti-Oxidant Electroactive Injectable Hydrogel as Self-Healing Wound Dressing with Hemostasis and Adhesiveness for Cutaneous Wound Healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- de Tayrac, R.; Devoldere, G.; Renaudie, J.; Villard, P.; Guilbaud, O.; Eglin, G. Prolapse Repair by Vaginal Route Using a New Protected Low-Weight Polypropylene Mesh: 1-Year Functional and Anatomical Outcome in a Prospective Multicentre Study. Int. Urogynecol. J. 2007, 18, 251–256. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent Advances on Antimicrobial Wound Dressing: A Review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial Biomaterials for Skin Wound Dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef]
- Hawthorne, B.; Simmons, J.K.; Stuart, B.; Tung, R.; Zamierowski, D.S.; Mellott, A.J. Enhancing Wound Healing Dressing Development through Interdisciplinary Collaboration. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1967–1985. [Google Scholar] [CrossRef]
- Eleroui, M.; Feki, A.; Hamzaoui, A.; Kammoun, I.; Bouhamed, M.; Boudawara, O.; Ben Ayed, I.; Ben Amara, I. Preparation and Characterization of a Novel Hamada Scoparia Polysaccharide Composite Films and Evaluation of Their Effect on Cutaneous Wound Healing in Rat. Int. J. Pharm. 2021, 608, 121056. [Google Scholar] [CrossRef]
- Gomes Neto, R.J.; Genevro, G.M.; de Almeida Paulo, L.; Lopes, P.S.; de Moraes, M.A.; Beppu, M.M. Characterization and in Vitro Evaluation of Chitosan/Konjac Glucomannan Bilayer Film as a Wound Dressing. Carbohydr. Polym. 2019, 212, 59–66. [Google Scholar] [CrossRef]
- Alzarea, A.I.; Alruwaili, N.K.; Ahmad, M.M.; Munir, M.U.; Butt, A.M.; Alrowaili, Z.A.; Bin Shahari, M.S.; Almalki, Z.S.; Alqahtani, S.S.; Dolzhenko, A.V.; et al. Development and Characterization of Gentamicin-Loaded Arabinoxylan-Sodium Alginate Films as Antibacterial Wound Dressing. Int. J. Mol. Sci. 2022, 23, 2899. [Google Scholar] [CrossRef] [PubMed]
- Pawar, H.V.; Tetteh, J.; Boateng, J.S. Preparation, Optimisation and Characterisation of Novel Wound Healing Film Dressings Loaded with Streptomycin and Diclofenac. Colloids Surf. B Biointerfaces 2013, 102, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.P.; Chen, X.G.; Li, Y.; Hui, Y.Z. Characterization and Ornidazole Release in Vitro of a Novel Composite Film Prepared with Chitosan/Poly(Vinyl Alcohol)/Alginate. J. Biomed. Mater. Res. A 2008, 85, 566–572. [Google Scholar] [CrossRef]
- Pereira, G.G.; Guterres, S.S.; Balducci, A.G.; Colombo, P.; Sonvico, F. Polymeric Films Loaded with Vitamin e and Aloe Vera for Topical Application in the Treatment of Burn Wounds. Biomed. Res. Int. 2014, 2014, 641590. [Google Scholar] [CrossRef] [Green Version]




| Type of Polymer | Examples | Properties | Ref. |
|---|---|---|---|
| Natural | Chitosan, hyaluronic acid, starch, silk fibroin, sericin, keratin, sodium alginate, gelatin, collagen, zein, cellulose, and konjac glucomannan | Biocompatibility, biodegradability, high disponibility, healing properties, permeability, inertness, and bioadhesiveness | [36,37] |
| Synthetic | Polyvinyl alcohol, polyacrylic acid, polycaprolactone, polyethylene glycol, polyvinylpyrrolidone, polylactic acid, and polydimethylsiloxane. | Resistance, flexibility, structure, high degree of polymerization, thermo-responsiveness, hydrophilicity, and occlusivity | [36] |
| Blended | Chitosan + pectin | Fluconazole drug administration, antifungal, vaginal film, fast-dissolving | [41] |
| Arrow root starch + carboxy methyl cellulose (CMC) | Glipizide in vitro drug administration, buccal film, thin, mucoadhesive, increased tensile strength, clear, biodegradable, edible, smooth | [42] | |
| Chitosan + PVP | White coloration, reduced mechanical strength (brittle and easier to tear than chitosan film), increased water vapor permeability, reduced antimicrobial properties (>50% PVP) | [40] | |
| Alginate + Sago Starch + silver nanoparticles | Reduction in inflammation, faster healing, greater tensile strength, porous (good absorption and oxygen exchange) | [43] |
| Polymer | Formulation (% w/v) | Application | Outcomes | Ref. |
|---|---|---|---|---|
| Chitosan (Ch) | -4% Ch stock -6% oxidized SA stock -1:2 Ch/SA solution | -Drug delivery | -Non-cytotoxic -No cutaneous reaction after 72 h of in vivo subcutaneous injection -Cytocompatibility in vitro | [63] |
| Guar gum (GG) | -1% GG -0.5% PVP/0.5 to 5% propylene glycol (PG) as copolymers -200 to 1000 mg Lysine Clonixinate (LC) | -Periodontal treatment | -Highly homogeneous structure -Prolonged API release -Suitable swelling behavior | [64] |
| Methyl cellulose (MC) | -3% MC -2% SA as copolymer -1 to 5% Montmorillonite (MMT) | -Wound healing and antimicrobial properties | -Delayed thermal drug degradation -Increase in film tensile strength -Bacteriostatic properties | [65] |
| Sodium alginate (SA) | -4% SA -2% PVA/PVP as copolymers -10% Glycerol/12% PG as plasticizers -Curcumin-loaded PCL nanoparticles | -Wound healing | -High absorbency capacities for exudate removal -Gradual release of the drug -High adherence -Pores with controlled dimensions | [66] |
| Xanthan gum (XG) | -10% XG -1% Glycerol as a plasticizer -300 mg metronidazole | -Treatment of vaginal infections | -Less frequency of administration compared to conventional treatments. -High adherence -Adequate pH | [67] |
| Characteristic | Advantages | Ref. |
|---|---|---|
| Transparency | Easy assessment of the wound | [33,36,58,59,60] |
| Impermeability | Effective barrier to water and bacteria | |
| Porosity | Transmit water vapor from beneath the dressing to the external environment | |
| Structure |
|
| Formulation | Characteristics | Application | Ref. |
|---|---|---|---|
| Collagen 1% + modified microfibrillar carboxymethylcellulose 1% (CMC) | -Good adherence -Flexibility and cohesiveness -Acidic pH and low degree of swelling -Durablility | Swelling and mechanical tests were performed using an artificial wound model (Petri dish and sponge soaked with BSS). | [58] |
| Polysaccharides extracted from Hammada scoparia leaves (PSP) + PVA | -Thickness: 0.0556 mm -In vitro antioxidant activity -Increase the rate of hydroxyproline in the wound site -Accelerate wound closure and re-epithelialization | Tested in a male adult Wistar rat with a circular wound on the dorsal region by excising the skin. | [151] |
| Chitosan (CS) + konjac glucomannan (KGM) bilayer film | -High biocompatibility -Low cytotoxicity -Transparent -Inhibits growth and penetration of microorganisms -Good thermostability and miscibility -Resist natural deformation of human skin | -Cytotoxicity-viability, and genotoxicity tests were measured using CHO cell line -Proliferation, cytotoxicity-viability, immunofluorescence, and fibroblasts adhesion tests were performed using Human dermal primary fibroblasts | [152] |
| Chitosan (CS) + hyaluronic acid (HA) at different percentages (1–35%)—2D matrices | -Lower transparency and homogeneity than CS pure -Improved water uptake and surface wettability (HA ≥ 10%) -Hampered water vapor permeability (HA > 5%) -HA affected mechanical properties but provided more flexible matrices (HA = 1–5%) -Fibroblast adhesion and high proliferation (HA = 5%) -Intrinsic antibacterial fouling properties (HA ≥ 5%) | -Proliferation, cytotoxicity-viability, immunofluorescence, and fibroblasts adhesion tests were performed using human dermal primary fibroblasts | [91] |
| Arabinoxylan (AX) (1.5%, 2%, 2.5%, 3%) and glycerol (2.5%) + sodium alginate (SA) (2%, 2.5%, 3%, 3.5%) loaded with gentamicin sulfate (0.1%) | -Thermally stable -Transparency -Uniform thickness -Smooth surface morphology -Similar tensile strength as human skin -Water transmission rate suitable -Mild water/exudate uptake capacity -Excellent cytocompatibility ->80% GS released in two phases in 24 h (Fickian diffusion mechanism) -Antibacterial effect against E. coli, S. aureus, and P. aeruginosa. | -Cytotoxicity-viability tests were performed using human lung fibroblasts: MRC-5 cells (ATCC CCL-171) | [153] |
| Polybutyrolactam (PBA) + chitosan (CS) 50%-50% composite film | -Non-toxic -Biodegradable in phosphate buffer saline -Cytocompatibility -Non-allergenic -Higher flexibility -Natural skin-like mechanical properties -Higher water vapor transmission rates -Strength -Promotes cell proliferation | -Cell attachment and proliferation tests were performed using a culture of L929 cells | [39] |
| Chitosan (CS) + Carbopol + Glycerine loaded with mupirocin | -Bioadhesive -Accelerates the regeneration of the epidermal layer -Improved swelling ability -Promotes epithelialization and angiogenesis | -Ex vivo permeation studies were performed using vertical Franz diffusion cells -Ex vivo bioadhesion and permeation studies were performed using Balb-c mice | [38] |
| Polyox® (POL) 1% (w/w) + hydroxypropylmethylcellulose (HPMC), carrageenan (CAR), sodium alginate (SA) or chitosan (CS) (75/25 ratio) and glycerol (GLY) as a plasticizer, loaded with streptomycin (STP) and diclofenac (DLF) | -Homogenous -High swelling -Reduced bacterial infection -Reduced inflammation -Flexible -Transparent | -In vitro, drug dissolution studies were performed using Franz diffusion cells | [154] |
| Chitosan 2% (w/w) + PVA 3% (w/w) + sodium alginate 2% (w/w) loaded with ornidazole (OD) 1.0 mg/cm2 | -Excellent light transmittance -Control of water vapor transmission rate -Fluid drainage ability promotion | -Antibacterial studies were performed using Staphylococcus aureus and Escherichia coli | [155] |
| Sodium alginate (SA 14.25% w/w) + PVA 30–25% (w/w) loaded with vitamin E 3.60% (w/w) and Aloe vera 1% (w/w) | -High elasticity and strength -Considerable thickness -Biphasic controlled release of vitamin E acetate for more than 12 h -Deep accumulation of vitamin E acetate in the stratum corneum | -In vitro, Vitamin E acetate release studies were performed using modified Franz permeation cells with a synthetic membrane of regenerated cellulose -Tape stripping test with five volunteers (two males and three females) | [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borbolla-Jiménez, F.V.; Peña-Corona, S.I.; Farah, S.J.; Jiménez-Valdés, M.T.; Pineda-Pérez, E.; Romero-Montero, A.; Del Prado-Audelo, M.L.; Bernal-Chávez, S.A.; Magaña, J.J.; Leyva-Gómez, G. Films for Wound Healing Fabricated Using a Solvent Casting Technique. Pharmaceutics 2023, 15, 1914. https://doi.org/10.3390/pharmaceutics15071914
Borbolla-Jiménez FV, Peña-Corona SI, Farah SJ, Jiménez-Valdés MT, Pineda-Pérez E, Romero-Montero A, Del Prado-Audelo ML, Bernal-Chávez SA, Magaña JJ, Leyva-Gómez G. Films for Wound Healing Fabricated Using a Solvent Casting Technique. Pharmaceutics. 2023; 15(7):1914. https://doi.org/10.3390/pharmaceutics15071914
Chicago/Turabian StyleBorbolla-Jiménez, Fabiola V., Sheila I. Peña-Corona, Sonia J. Farah, María Teresa Jiménez-Valdés, Emiliano Pineda-Pérez, Alejandra Romero-Montero, María Luisa Del Prado-Audelo, Sergio Alberto Bernal-Chávez, Jonathan J. Magaña, and Gerardo Leyva-Gómez. 2023. "Films for Wound Healing Fabricated Using a Solvent Casting Technique" Pharmaceutics 15, no. 7: 1914. https://doi.org/10.3390/pharmaceutics15071914
APA StyleBorbolla-Jiménez, F. V., Peña-Corona, S. I., Farah, S. J., Jiménez-Valdés, M. T., Pineda-Pérez, E., Romero-Montero, A., Del Prado-Audelo, M. L., Bernal-Chávez, S. A., Magaña, J. J., & Leyva-Gómez, G. (2023). Films for Wound Healing Fabricated Using a Solvent Casting Technique. Pharmaceutics, 15(7), 1914. https://doi.org/10.3390/pharmaceutics15071914









