Early Detection of Myeloid-Derived Suppressor Cells in the Lung Pre-Metastatic Niche by Shortwave Infrared Nanoprobes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rare-Earth Albumin Nanocomposite Synthesis
2.2. Nanocomposite Functionalization
2.3. Nanocomposite Characterization
2.4. Cell Culture
2.5. Animals
2.6. MDSC Isolation
2.7. Nanocomposite Targeting Assessment by Flow Cytometry
2.8. Tumor Models
2.9. ReANC Administration and SWIR Imaging
2.10. Image Analysis
2.11. Metastasis Counting
2.12. Immunohistochemistry
2.13. Statistical Analysis
3. Results
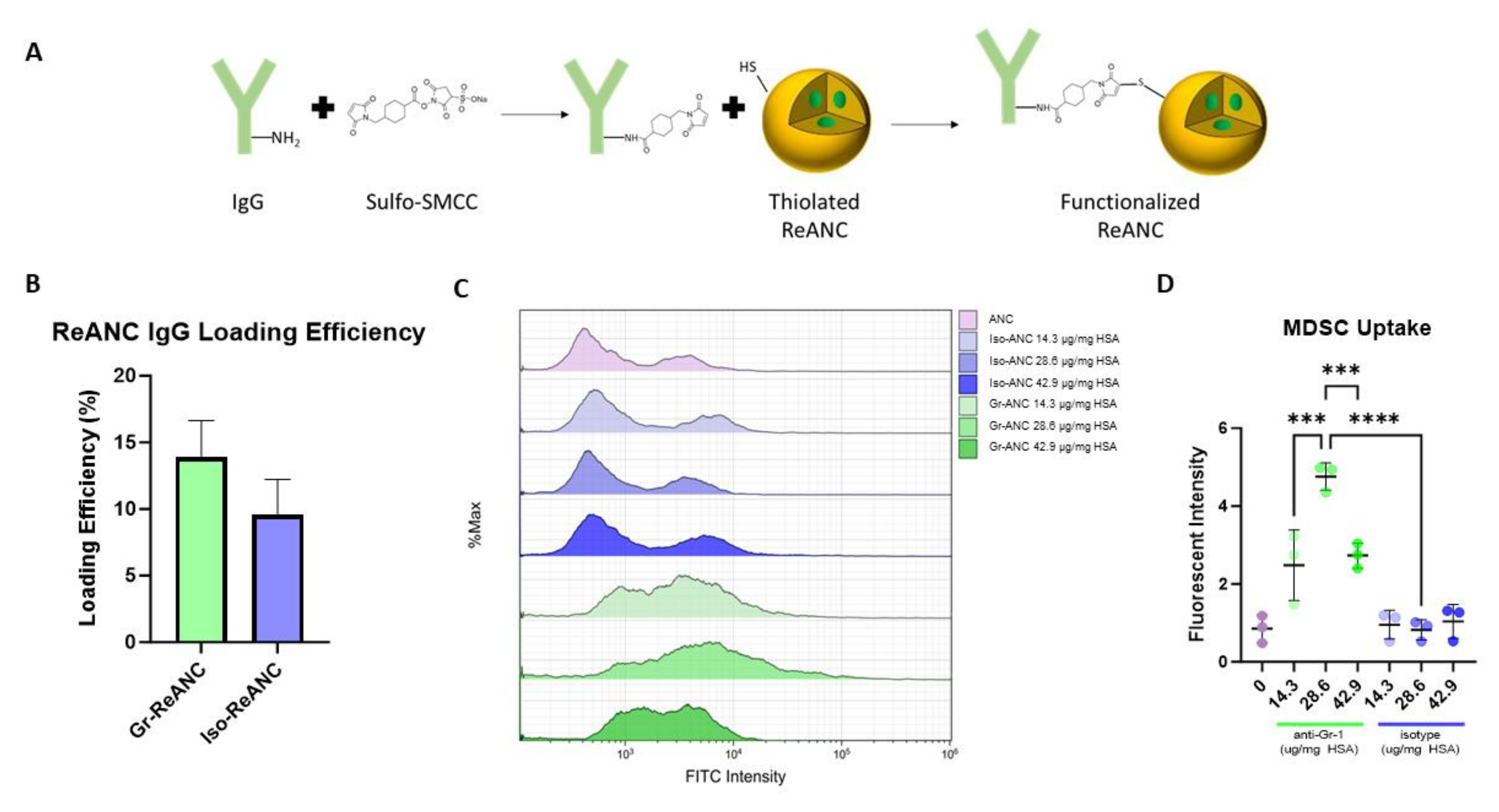
3.1. Characterization of MDSC-Targeted Nanocomposites
3.2. Validation of MDSC Targeting
3.3. Balb/c Breast Cancer Tumor Models
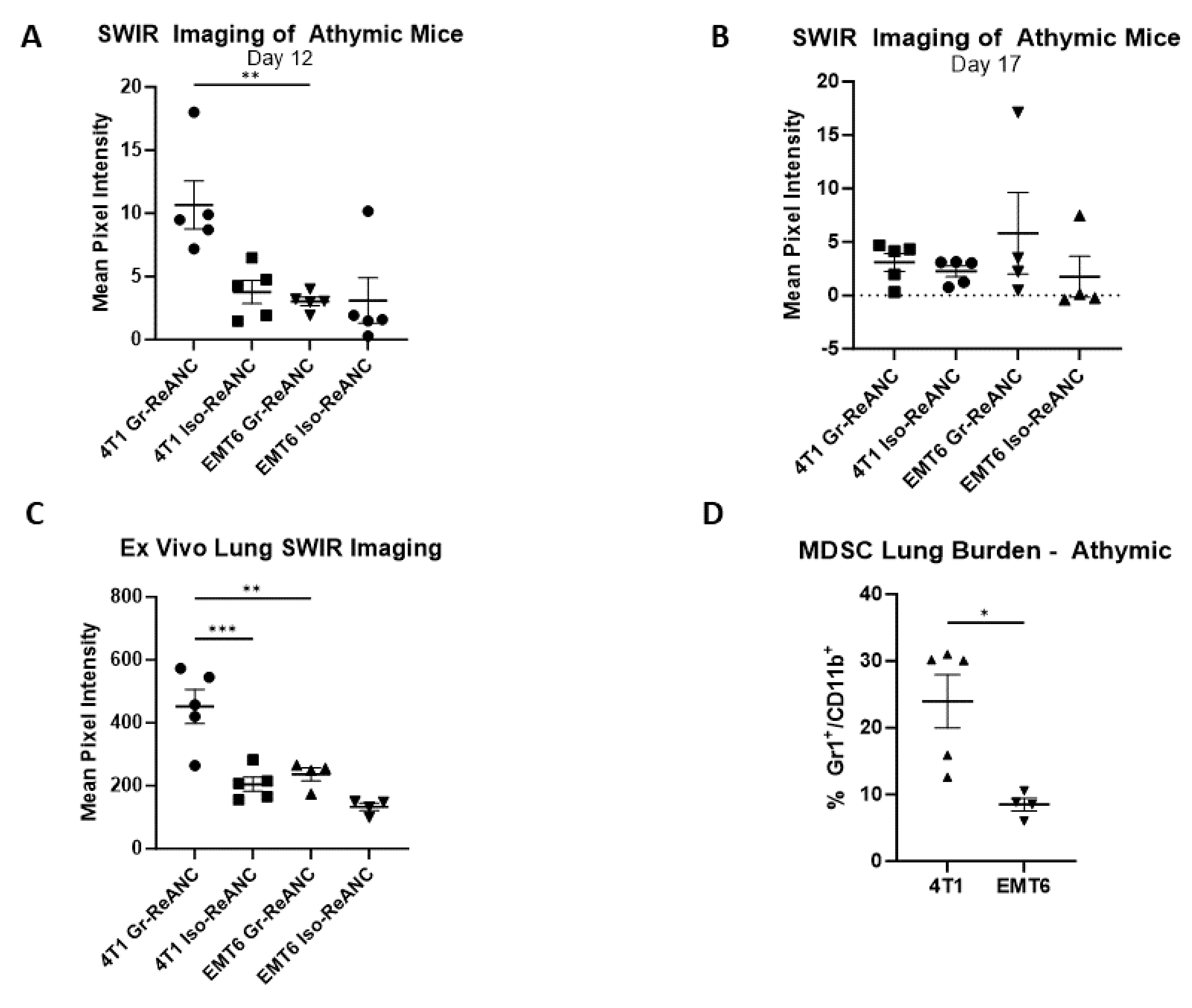
3.4. Proof-of-Concept Imaging in Athymic Mice
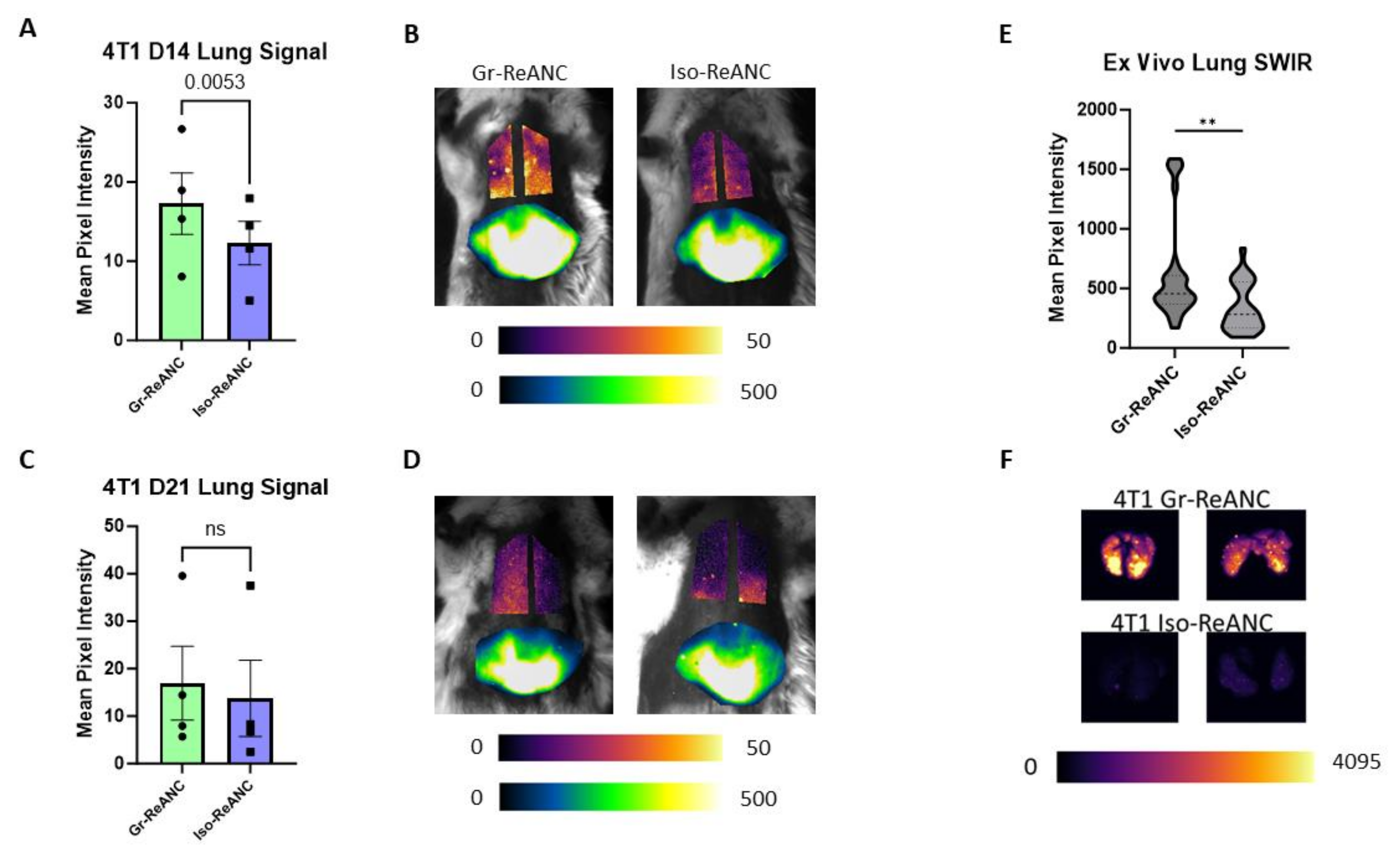
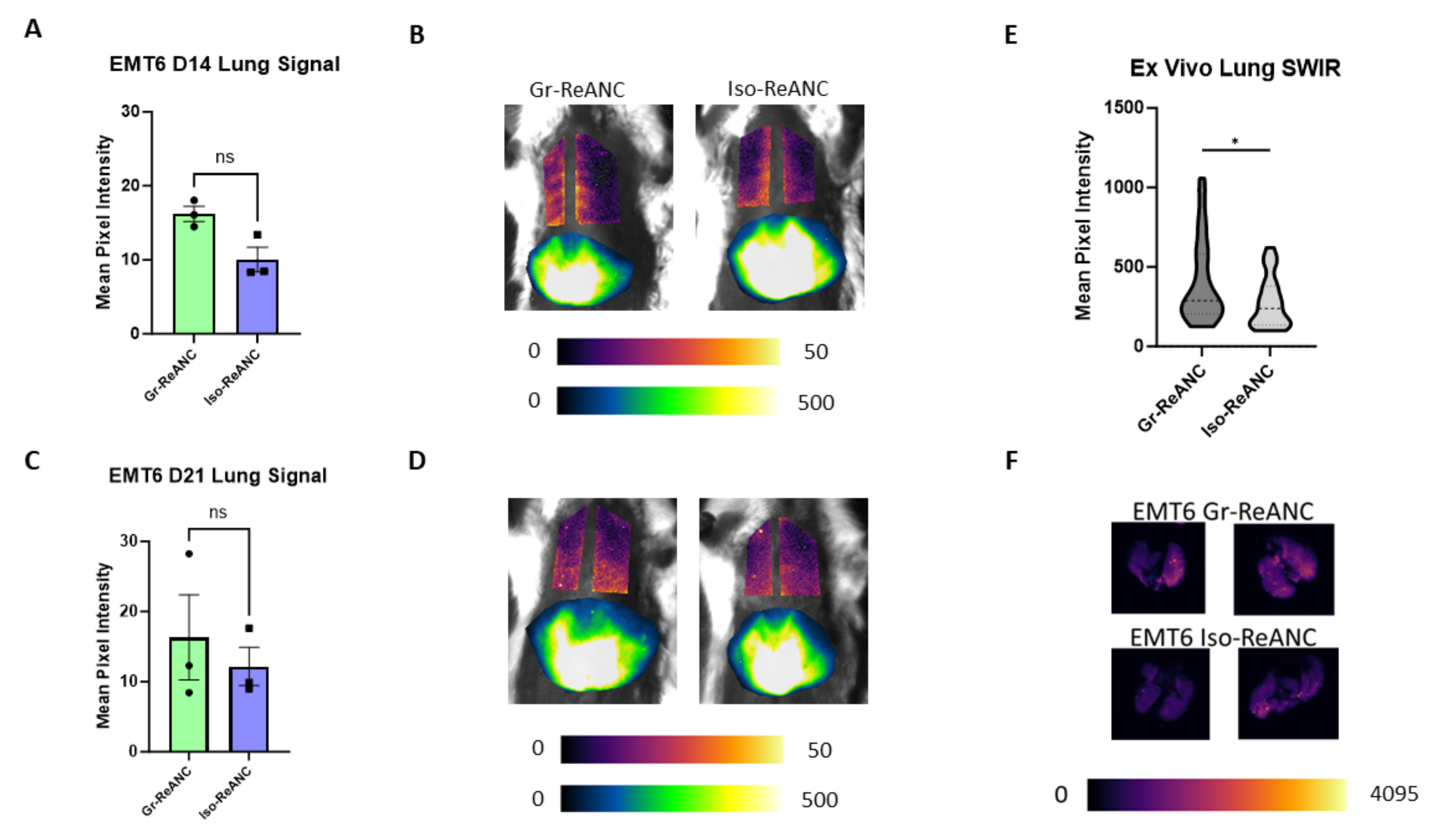
3.5. In Vivo Imaging of the Pre-Metastatic Niche
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Ren, Z.; Hameed, O.; Chanda, D.; Morgan, C.J.; Siegal, G.P.; Wei, S. Breast Cancer Subtypes Predispose the Site of Distant Metastases. Am. J. Clin. Pathol. 2015, 143, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Han, B.; Siegel, E.; Cui, Y.; Giuliano, A.; Cui, X. Breast Cancer Lung Metastasis: Molecular Biology and Therapeutic Implications. Cancer Biol. Ther. 2018, 19, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, S.; Corrigan, M.; O’Reilly, S. The Clinicomolecular Landscape of de Novo versus Relapsed Stage IV Metastatic Breast Cancer. Exp. Mol. Pathol. 2020, 114, 104404. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ahn, S.G.; Lee, H.M.; Park, J.T.; Han, K.; Lee, S.A.; Jeong, J. Metastasis-Free Interval Is Closely Related to Tumor Characteristics and Has Prognostic Value in Breast Cancer Patients with Distant Relapse. J. Breast Cancer 2015, 18, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W. Curing Metastatic Breast Cancer. J. Oncol. Pract. 2016, 12, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, M.G.; Naghavi-Behzad, M.; Vogsen, M. A Role of FDG-PET/CT for Response Evaluation in Metastatic Breast Cancer? Semin. Nucl. Med. 2022, 52, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Ichiba, T.; Sakuyama, T.; Arakawa, Y.; Nagasaki, E.; Aiba, K.; Nogi, H.; Kawase, K.; Takeyama, H.; Toriumi, Y.; et al. Possible Clinical Cure of Metastatic Breast Cancer: Lessons from Our 30-Year Experience with Oligometastatic Breast Cancer Patients and Literature Review. Breast Cancer 2012, 19, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Ueno, N.T. Improvement of Survival and Prospect of Cure in Patients with Metastatic Breast Cancer. Breast Cancer 2012, 19, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Brewster, A.M.; Hortobagyi, G.N.; Broglio, K.R.; Kau, S.-W.; Santa-Maria, C.A.; Arun, B.; Buzdar, A.U.; Booser, D.J.; Valero, V.; Bondy, M.; et al. Residual Risk of Breast Cancer Recurrence 5 Years After Adjuvant Therapy. J. Natl. Cancer Inst. 2008, 100, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, M.; Sun, Z.; Price, K.N.; Karlsson, P.; Forbes, J.F.; Thürlimann, B.; Gianni, L.; Castiglione, M.; Gelber, R.D.; Coates, A.S.; et al. Annual Hazard Rates of Recurrence for Breast Cancer during 24 Years of Follow-Up: Results From the International Breast Cancer Study Group Trials I to V. J. Clin. Oncol. 2016, 34, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Del Turco, M.R.; Palli, D.; Cariddi, A.; Ciatto, S.; Pacini, P.; Distante, V. Intensive Diagnostic Follow-up after Treatment of Primary Breast Cancer: A Randomized Trial. JAMA 1994, 271, 1593–1597. [Google Scholar] [CrossRef]
- Ghezzi, P.; Magnanini, S.; Rinaldini, M.; Berardi, F.; Di Biagio, G.; Testare, F.; Tavoni, N.; Schittulli, F.; D’Amico, C.; Pedicini, T.; et al. Impact of Follow-up Testing on Survival and Health-Related Quality of Life in Breast Cancer Patients: A Multicenter Randomized Controlled Trial. JAMA 1994, 271, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Aebi, S.; Gelber, S.; Anderson, S.J.; Láng, I.; Robidoux, A.; Martín, M.; Nortier, J.W.R.; Paterson, A.H.G.; Rimawi, M.F.; Cañada, J.M.B.; et al. Chemotherapy for Isolated Locoregional Recurrence of Breast Cancer: The CALOR Randomised Trial. Lancet Oncol. 2014, 15, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X. Characteristics and Significance of the Pre-Metastatic Niche. Cancer Cell 2016, 30, 668–681. [Google Scholar] [CrossRef]
- Patras, L.; Shaashua, L.; Matei, I.; Lyden, D. Immune Determinants of the Pre-Metastatic Niche. Cancer Cell 2023, 41, 546–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, Y.; Guo, N.; Wang, S. MDSCs: Key Criminals of Tumor Pre-Metastatic Niche Formation. Front. Immunol. 2019, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Wang, Z.; Lv, J.; Qin, Y.; Shi, H. MDSCs: The Key Players in the Formation of Pre-Metastatic Niche. Front. Biosci. Landmark 2023, 28, 58. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Lee, O.-Y.; Shon, S.Y.; Nam, O.; Ryu, P.M.; Seo, M.W.; Lee, D.-S. A Mutual Activation Loop between Breast Cancer Cells and Myeloid-Derived Suppressor Cells Facilitates Spontaneous Metastasis through IL-6 Trans-Signaling in a Murine Model. Breast Cancer Res. 2013, 15, R79. [Google Scholar] [CrossRef] [PubMed]
- Eisenblaetter, M.; Flores-Borja, F.; Lee, J.J.; Wefers, C.; Smith, H.; Hueting, R.; Cooper, M.S.; Blower, P.J.; Patel, D.; Rodriguez-Justo, M.; et al. Visualization of Tumor-Immune Interaction—Target-Specific Imaging of S100A8/A9 Reveals Pre-Metastatic Niche Establishment. Theranostics 2017, 7, 2392–2401. [Google Scholar] [CrossRef]
- James, M.L.; Gambhir, S.S. A Molecular Imaging Primer: Modalities, Imaging Agents, and Applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yuan, C.; Cai, J.; Pu, W.; Wu, P.; Li, C.; Li, G.; Zhang, Y.; Zhang, J.; Guo, J.; et al. Early Diagnosis of Breast Cancer Lung Metastasis by Nanoprobe-Based Luminescence Imaging of the Pre-Metastatic Niche. J. Nanobiotechnol. 2022, 20, 134. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Gleysteen, J.; Teraphongphom, N.T.; Li, Y.; Rosenthal, E. In-Vivo Optical Imaging in Head and Neck Oncology: Basic Principles, Clinical Applications and Future Directions. Int. J. Oral Sci. 2018, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Naczynski, D.J.; Tan, M.C.; Riman, R.E.; Moghe, P.V. Rare Earth Nanoprobes for Functional Biomolecular Imaging and Theranostics. J. Mater. Chem. B Mater. Biol. Med. 2014, 2, 2958–2973. [Google Scholar] [CrossRef] [PubMed]
- Sandell, J.L.; Zhu, T.C. A Review of In-Vivo Optical Properties of Human Tissues and Its Impact on PDT. J. Biophotonics 2011, 4, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Tian, R.; Antaris, A.L.; Chen, X.; Dai, H. Near-Infrared-II Molecular Dyes for Cancer Imaging and Surgery. Adv. Mater. 2019, 31, e1900321. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhu, B.; Zheng, K.; He, S.; Meng, L.; Song, J.; Yang, H. Recent Progress in NIR-II Contrast Agent for Biological Imaging. Front. Bioeng. Biotechnol. 2020, 7, 487. [Google Scholar] [CrossRef] [PubMed]
- Diao, S.; Hong, G.; Antaris, A.L.; Blackburn, J.L.; Cheng, K.; Cheng, Z.; Dai, H. Biological Imaging without Autofluorescence in the Second Near-Infrared Region. Nano Res. 2015, 8, 3027–3034. [Google Scholar] [CrossRef]
- Hilderbrand, S.A.; Weissleder, R. Near-Infrared Fluorescence: Application to in Vivo Molecular Imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Naczynski, D.J.; Tan, M.C.; Zevon, M.; Wall, B.; Kohl, J.; Kulesa, A.; Chen, S.; Roth, C.M.; Riman, R.E.; Moghe, P.V. Rare-Earth-Doped Biological Composites as in Vivo Shortwave Infrared Reporters. Nat. Commun. 2013, 4, 2199. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Ma, Z.; Wang, F.; Wang, X.; Yang, Y.; Liu, Y.; Zhao, X.; Li, J.; Du, H.; Zhang, M.; et al. In Vivo Molecular Imaging for Immunotherapy Using Ultra-Bright near-Infrared-IIb Rare-Earth Nanoparticles. Nat. Biotechnol. 2019, 37, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Mendez, C.B.; Gonda, A.; Shah, J.V.; Siebert, J.N.; Zhao, X.; He, S.; Riman, R.E.; Tan, M.C.; Moghe, P.V.; Ganapathy, V.; et al. Short-Wave Infrared Emitting Nanocomposites for Fluorescence-Guided Surgery. IEEE J. Sel. Top. Quantum Electron. 2021, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.V.; Gonda, A.; Pemmaraju, R.; Subash, A.; Mendez, C.B.; Berger, M.; Zhao, X.; He, S.; Riman, R.E.; Tan, M.C.; et al. Shortwave Infrared-Emitting Theranostics for Breast Cancer Therapy Response Monitoring. Front. Mol. Biosci. 2020, 7, 569415. [Google Scholar] [CrossRef] [PubMed]
- Kantamneni, H.; Zevon, M.; Donzanti, M.J.; Zhao, X.; Sheng, Y.; Barkund, S.R.; McCabe, L.H.; Banach-Petrosky, W.; Higgins, L.M.; Ganesan, S.; et al. Surveillance Nanotechnology for Multi-Organ Cancer Metastases. Nat. Biomed. Eng. 2017, 1, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Zevon, M.; Ganapathy, V.; Kantamneni, H.; Mingozzi, M.; Kim, P.; Adler, D.; Sheng, Y.; Tan, M.C.; Pierce, M.; Riman, R.E.; et al. CXCR-4 Targeted, Short Wave Infrared (SWIR) Emitting Nanoprobes for Enhanced Deep Tissue Imaging and Micrometastatic Lesion Detection. Small 2015, 11, 6347–6357. [Google Scholar] [CrossRef] [PubMed]
- Kantamneni, H.; Barkund, S.; Donzanti, M.; Martin, D.; Zhao, X.; He, S.; Riman, R.E.; Tan, M.C.; Pierce, M.C.; Roth, C.M.; et al. Shortwave Infrared Emitting Multicolored Nanoprobes for Biomarker-Specific Cancer Imaging in Vivo. BMC Cancer 2020, 20, 1082. [Google Scholar] [CrossRef] [PubMed]
- Gonda, A.; Zhao, N.; Shah, J.V.; Calvelli, H.R.; Kantamneni, H.; Francis, N.L.; Ganapathy, V. Engineering Tumor-Targeting Nanoparticles as Vehicles for Precision Nanomedicine. Med. One 2019, 4, e190021. [Google Scholar] [CrossRef] [PubMed]
- Naczynski, D.J.; Andelman, T.; Pal, D.; Chen, S.; Riman, R.E.; Roth, C.M.; Moghe, P.V. Albumin Nanoshell Encapsulation of Near-Infrared-Excitable Rare-Earth Nanoparticles Enhances Biocompatibility and Enables Targeted Cell Imaging. Small 2010, 6, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.V.; Siebert, J.N.; Zhao, X.; He, S.; Riman, R.E.; Tan, M.C.; Pierce, M.C.; Lattime, E.C.; Ganapathy, V.; Moghe, P.V. Shortwave-Infrared-Emitting Nanoprobes for CD8 Targeting and In Vivo Imaging of Cytotoxic T Cells in Breast Cancer. Adv. NanoBiomed Res. 2023, 4, 2300092. [Google Scholar] [CrossRef]
- Zhao, X.; He, S.; Tan, M.C. Design of Infrared-Emitting Rare Earth Doped Nanoparticles and Nanostructured Composites. J. Mater. Chem. C 2016, 4, 8349–8372. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques; Elsevier Science & Technology: San Diego, CA, USA, 2013; ISBN 978-0-12-382240-6. [Google Scholar]
- Paschall, A.V.; Liu, K. An Orthotopic Mouse Model of Spontaneous Breast Cancer Metastasis. J. Vis. Exp. 2016, 114, 54040. [Google Scholar] [CrossRef]
- Ouzounova, M.; Lee, E.; Piranlioglu, R.; El Andaloussi, A.; Kolhe, R.; Demirci, M.F.; Marasco, D.; Asm, I.; Chadli, A.; Hassan, K.A.; et al. Monocytic and Granulocytic Myeloid Derived Suppressor Cells Differentially Regulate Spatiotemporal Tumour Plasticity during Metastatic Cascade. Nat. Commun. 2017, 8, 14979. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, Y.; Mizuno, S.; Kato, K.; Mizuno-Iijima, S.; Tanimoto, Y.; Ishida, M.; Kajiwara, N.; Sakasai, T.; Miwa, Y.; Takahashi, S.; et al. Simple Generation of Hairless Mice for in Vivo Imaging. Exp. Anim. 2017, 66, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, L.N.; Wilkinson, K.H.; Kong, A. Triple-Negative Breast Cancer: Who Should Receive Neoadjuvant Chemotherapy? Surg. Oncol. Clin. N. Am. 2018, 27, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S. Triple-Negative Breast Cancer Molecular Subtyping and Treatment Progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- So, J.Y.; Ohm, J.; Lipkowitz, S.; Yang, L. Triple Negative Breast Cancer (TNBC): Non-Genetic Tumor Heterogeneity and Immune Microenvironment: Emerging Treatment Options. Pharmacol. Ther. 2022, 237, 108253. [Google Scholar] [CrossRef] [PubMed]
- Groth, C.; Hu, X.; Weber, R.; Fleming, V.; Altevogt, P.; Utikal, J.; Umansky, V. Immunosuppression Mediated by Myeloid-Derived Suppressor Cells (MDSCs) during Tumour Progression. Br. J. Cancer 2019, 120, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Renz, B.W.; Ilmer, M.; Koch, D.; Yang, Y.; Werner, J.; Bazhin, A.V. Myeloid-Derived Suppressor Cells in Solid Tumors. Cells 2022, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.R.; Revi, N.; Murugappan, S.; Singh, S.P.; Rengan, A.K. Enhanced Permeability and Retention Effect: A Key Facilitator for Solid Tumor Targeting by Nanoparticles. Photodiagnosis Photodyn. Ther. 2022, 39, 102915. [Google Scholar] [CrossRef] [PubMed]
- Ya, G.; Ren, W.; Qin, R.; He, J.; Zhao, S. Role of Myeloid-Derived Suppressor Cells in the Formation of Pre-Metastatic Niche. Front. Oncol. 2022, 12, 975261. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for Myeloid-Derived Suppressor Cell Nomenclature and Characterization Standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yi, M.; Niu, M.; Mei, Q.; Wu, K. Myeloid-Derived Suppressor Cells: An Emerging Target for Anticancer Immunotherapy. Mol. Cancer 2022, 21, 184. [Google Scholar] [CrossRef] [PubMed]
- Loeuillard, E.; Yang, J.; Buckarma, E.; Wang, J.; Liu, Y.; Conboy, C.; Pavelko, K.D.; Li, Y.; O’Brien, D.; Wang, C.; et al. Targeting Tumor-Associated Macrophages and Granulocytic Myeloid-Derived Suppressor Cells Augments PD-1 Blockade in Cholangiocarcinoma. J. Clin. Investig. 2020, 130, 5380–5396. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, X.; Fang, D.; Wang, X.; Guan, J.; Shi, Z.-W.; Chen, X. Gemcitabine-Facilitated Modulation of the Tumor Microenvironment and PD-1/PD-L1 Blockade Generate a Synergistic Antitumor Effect in a Murine Hepatocellular Carcinoma Model. Clin. Res. Hepatol. Gastroenterol. 2021, 46, 101853. [Google Scholar] [CrossRef] [PubMed]
- Dawod, B.; Liu, J.; Gebremeskel, S.; Yan, C.; Sappong, A.; Johnston, B.; Hoskin, D.W.; Marshall, J.S.; Wang, J. Myeloid-Derived Suppressor Cell Depletion Therapy Targets IL-17A-Expressing Mammary Carcinomas. Sci. Rep. 2020, 10, 13343. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, P.C.; Ochoa, A.C. Arginine Regulation by Myeloid Derived Suppressor Cells and Tolerance in Cancer: Mechanisms and Therapeutic Perspectives. Immunol. Rev. 2008, 222, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Fultang, L.; Panetti, S.; Ng, M.; Collins, P.; Graef, S.; Rizkalla, N.; Booth, S.; Lenton, R.; Noyvert, B.; Shannon-Lowe, C.; et al. MDSC Targeting with Gemtuzumab Ozogamicin Restores T Cell Immunity and Immunotherapy against Cancers. eBioMedicine 2019, 47, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced Targeted Therapies in Cancer: Drug Nanocarriers, the Future of Chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siebert, J.N.; Shah, J.V.; Tan, M.C.; Riman, R.E.; Pierce, M.C.; Lattime, E.C.; Ganapathy, V.; Moghe, P.V. Early Detection of Myeloid-Derived Suppressor Cells in the Lung Pre-Metastatic Niche by Shortwave Infrared Nanoprobes. Pharmaceutics 2024, 16, 549. https://doi.org/10.3390/pharmaceutics16040549
Siebert JN, Shah JV, Tan MC, Riman RE, Pierce MC, Lattime EC, Ganapathy V, Moghe PV. Early Detection of Myeloid-Derived Suppressor Cells in the Lung Pre-Metastatic Niche by Shortwave Infrared Nanoprobes. Pharmaceutics. 2024; 16(4):549. https://doi.org/10.3390/pharmaceutics16040549
Chicago/Turabian StyleSiebert, Jake N., Jay V. Shah, Mei Chee Tan, Richard E. Riman, Mark C. Pierce, Edmund C. Lattime, Vidya Ganapathy, and Prabhas V. Moghe. 2024. "Early Detection of Myeloid-Derived Suppressor Cells in the Lung Pre-Metastatic Niche by Shortwave Infrared Nanoprobes" Pharmaceutics 16, no. 4: 549. https://doi.org/10.3390/pharmaceutics16040549
APA StyleSiebert, J. N., Shah, J. V., Tan, M. C., Riman, R. E., Pierce, M. C., Lattime, E. C., Ganapathy, V., & Moghe, P. V. (2024). Early Detection of Myeloid-Derived Suppressor Cells in the Lung Pre-Metastatic Niche by Shortwave Infrared Nanoprobes. Pharmaceutics, 16(4), 549. https://doi.org/10.3390/pharmaceutics16040549





