Exploring the Wound Healing Potential of a Cuscuta chinensis Extract-Loaded Nanoemulsion-Based Gel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Plant Material
2.1.2. Microorganisms
2.1.3. Cell Line and Cytokines
2.1.4. Chemicals
2.2. Methods
2.2.1. Plant Extraction
2.2.2. Chemical Marker Analysis Using High-Performance Liquid Chromatography (HPLC)
2.2.3. Antioxidant Activity Determination
DPPH Radical Scavenging Assay
Ferric Reducing Antioxidant Power (FRAP) Assay
Lipid Peroxidation Inhibition Assay
2.2.4. Antibacterial Activity Determination
Agar Disc Diffusion Method
Minimum Inhibitory Concentration (MIC) Determination
2.2.5. Anti-Inflammatory Activity Determination
Cell Culture
Cytotoxicity Test
IL-1β, IL-6, and TNF-α Expression
2.2.6. In Vitro Irritation Test Using Hen’s Egg Chorioallantoic Membrane (HET-CAM) Assay
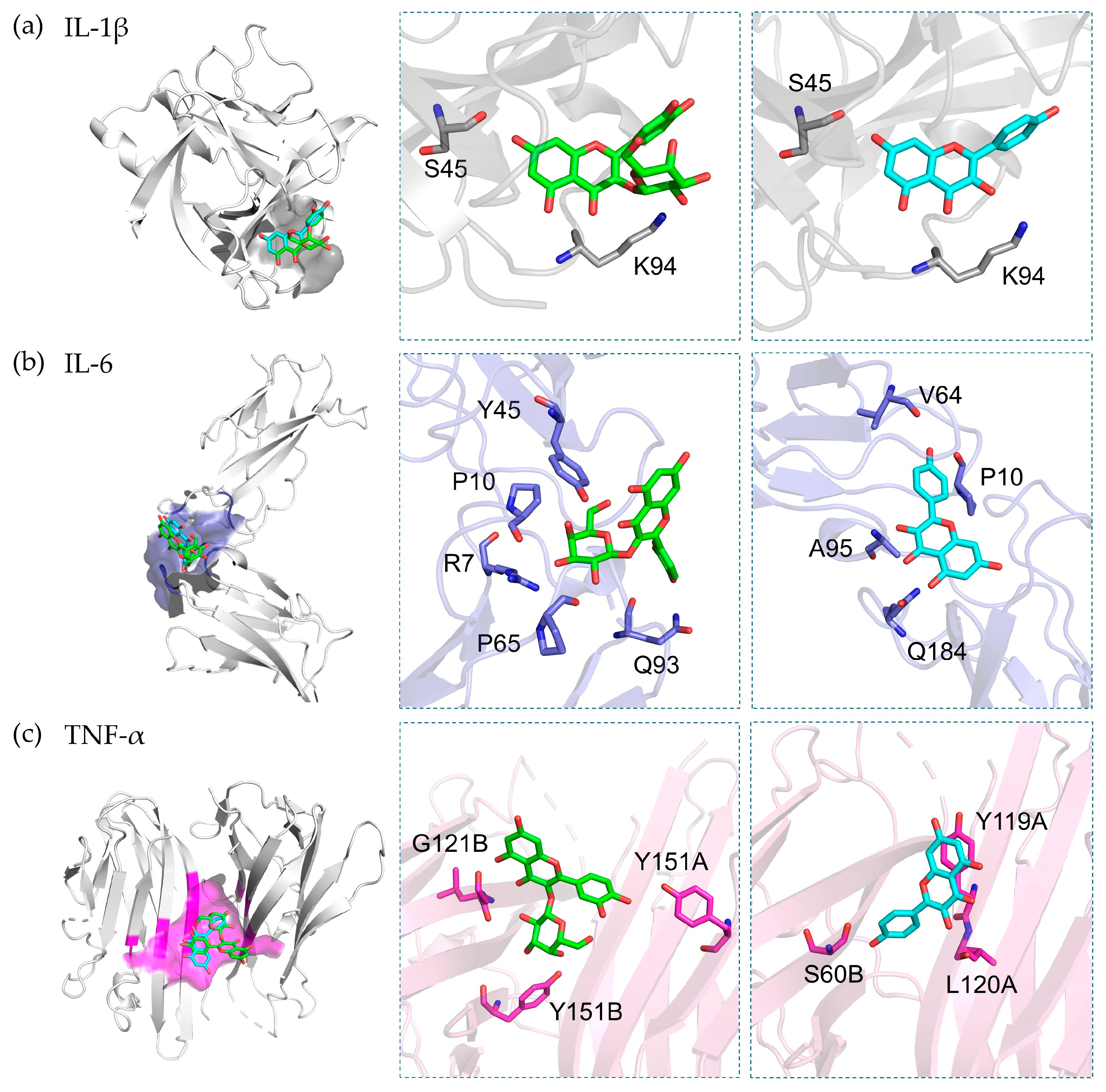
2.2.7. Molecular Docking
2.2.8. Development of C. chinensis Seed-Extract-Loaded Nanoemulsions
Solubility Study
Preparation of C. chinensis Seed-Extract-Loaded Nanoemulsions
Characterization of C. chinensis Seed Extract-Loaded Nanoemulsions
Morphology of C. chinensis Seed Extract-Loaded Nanoemulsions Determined Using a Transmission Electron Microscope (TEM)
Entrapment Efficiency of C. chinensis Seed Extract-Loaded Nanoemulsions
Stability Study of C. chinensis Seed Extract-Loaded Nanoemulsions
2.2.9. Preparation of C. chinensis Seed Extract-Loaded Nanoemulsion-Based Gel
2.2.10. In Vivo Wound Care Using C. chinensis Seed Extract-Loaded Nanoemulsion-Based Gel
Experimental Animals
Wound Care Model
2.2.11. Statistical Analysis
3. Results and Discussion
3.1. C. chinensis Seed Extraction
3.2. Chemical Markers Analysis Using High-Performance Liquid Chromatography (HPLC)
3.3. Antioxidant Activity of C. chinensis Seed Extracts
3.4. Antibacterial Activity of C. chinensis Seed Extracts
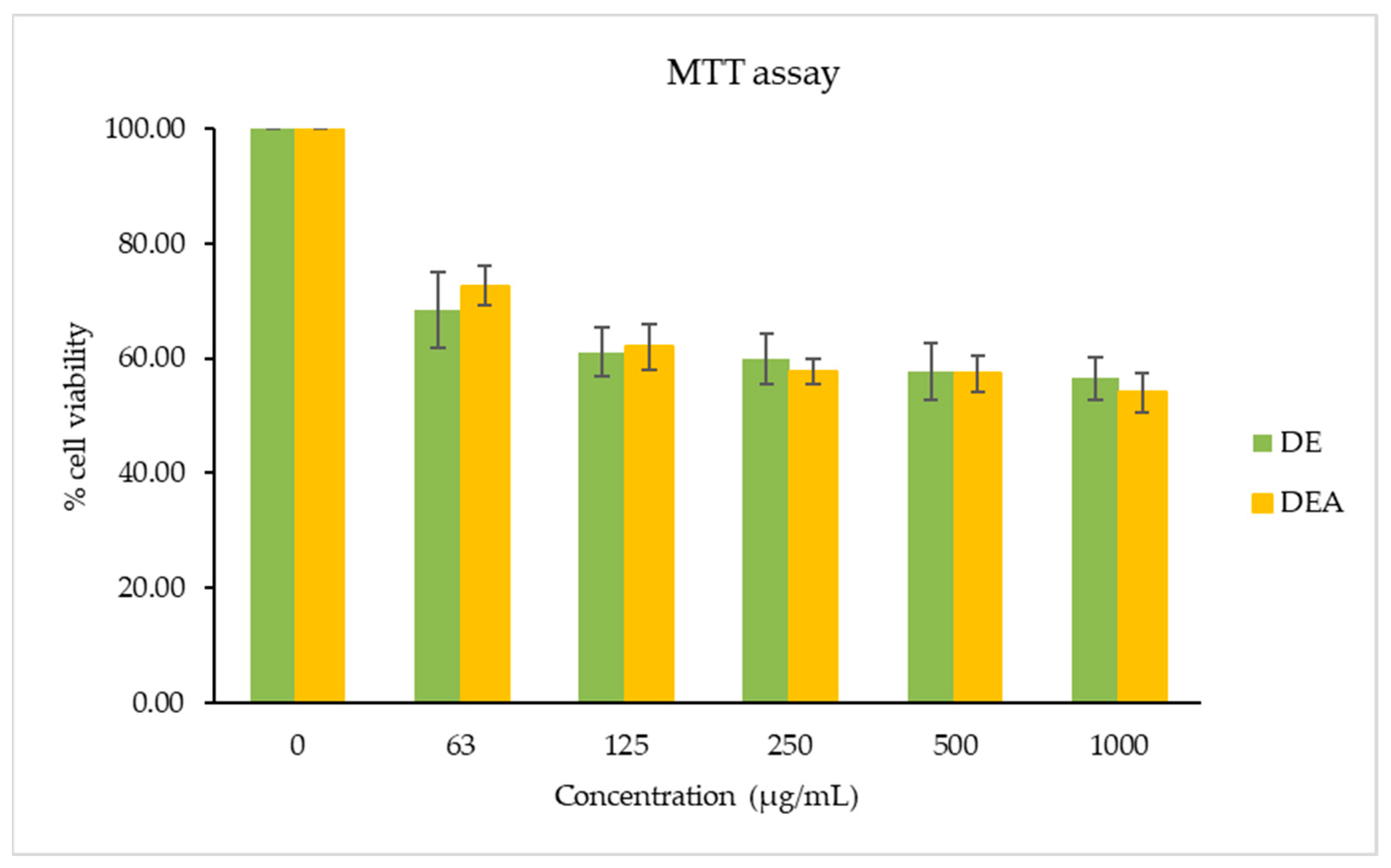
3.5. Cytotoxicity Test
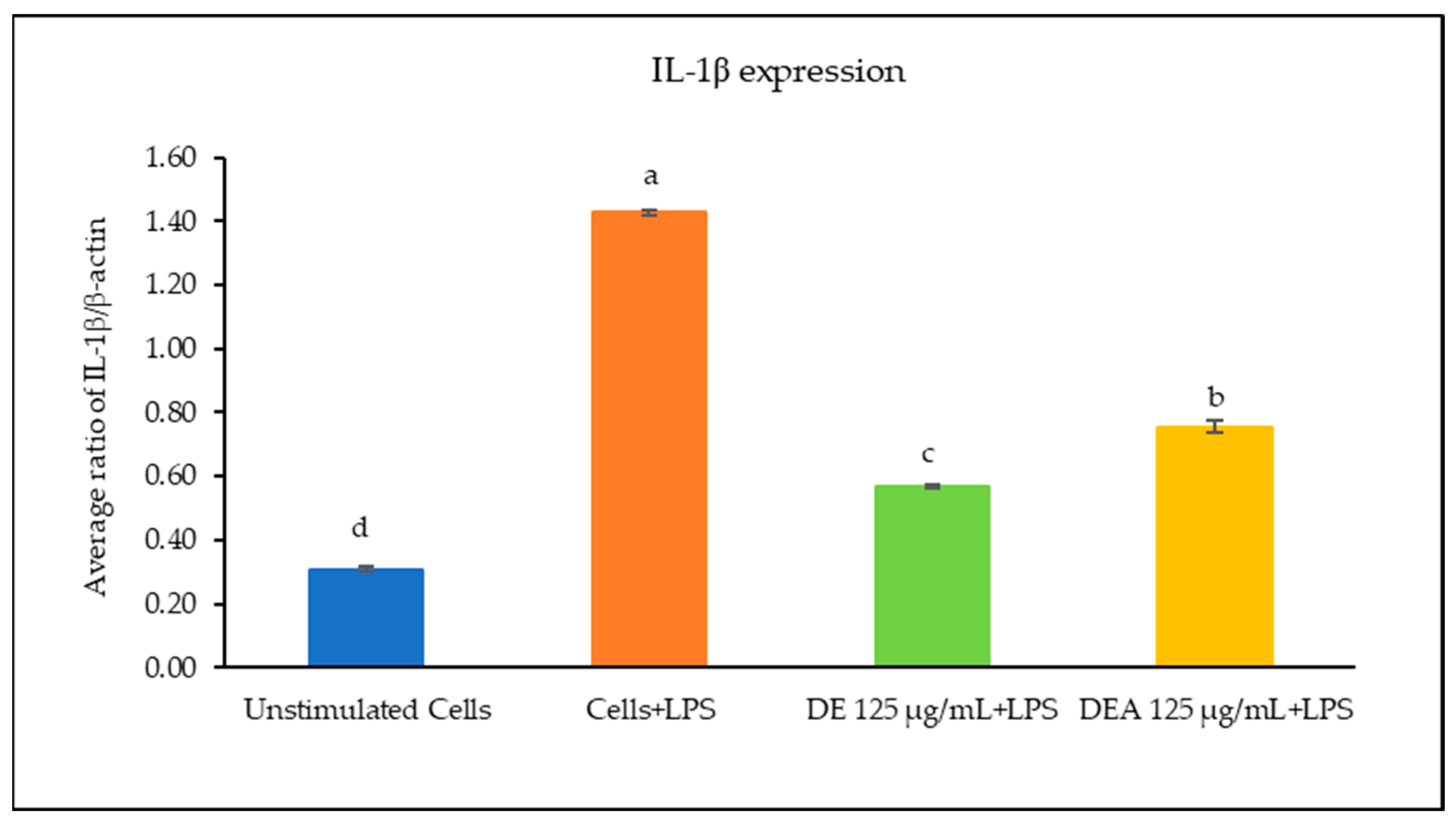
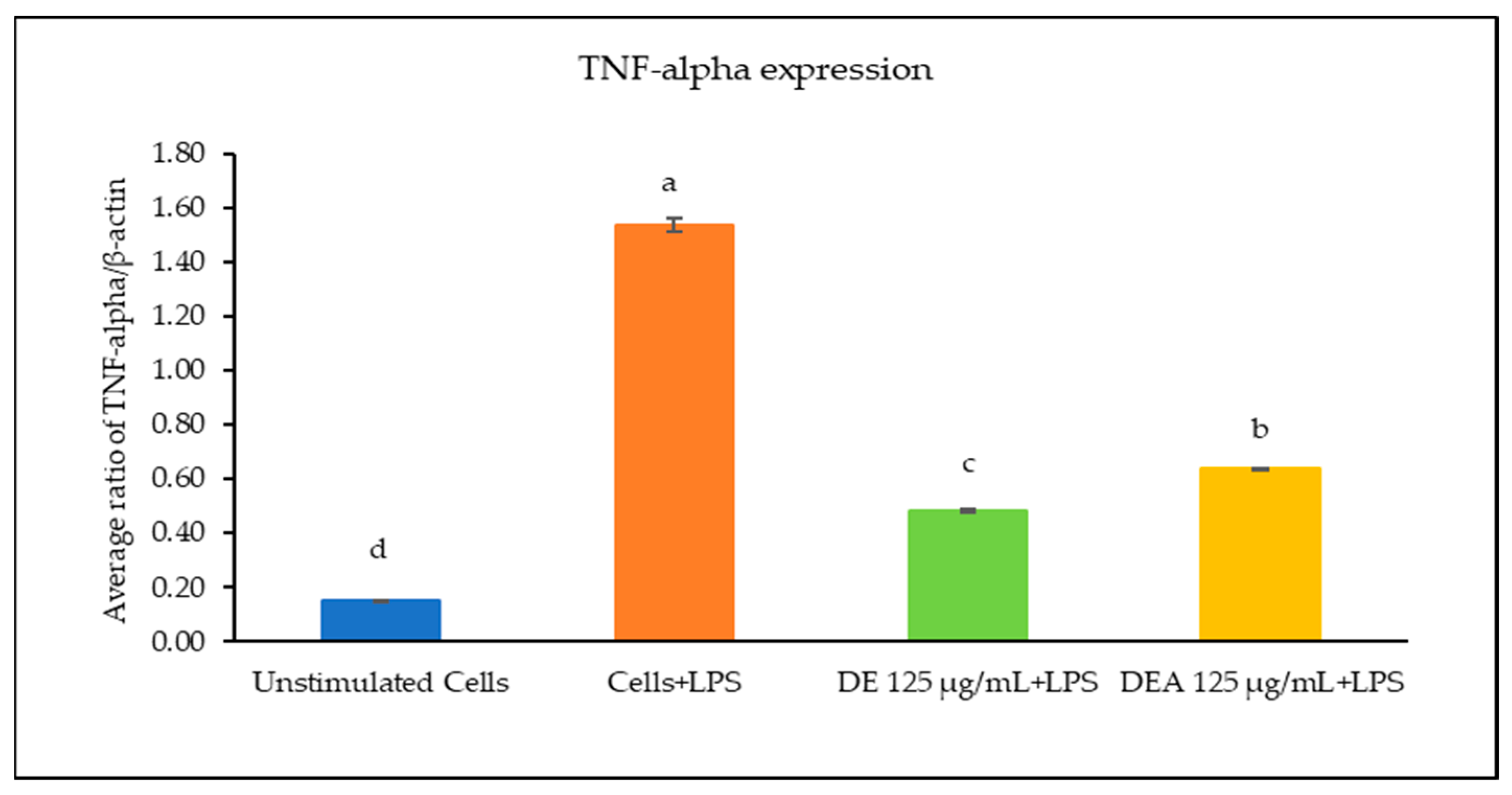
3.6. The Effects of C. chinensis Seed Extracts on Inhibiting Gene Expression of Inflammatory Cytokines
3.7. Molecular Docking
3.8. Irritation Assessment Using Hen’s Egg Test on the Chorioallantoic Membrane (HET-CAM) Assay
3.9. Development and Characterization of Unloaded Nanoemulsions and C. chinensis Extract-Loaded Nanoemulsions
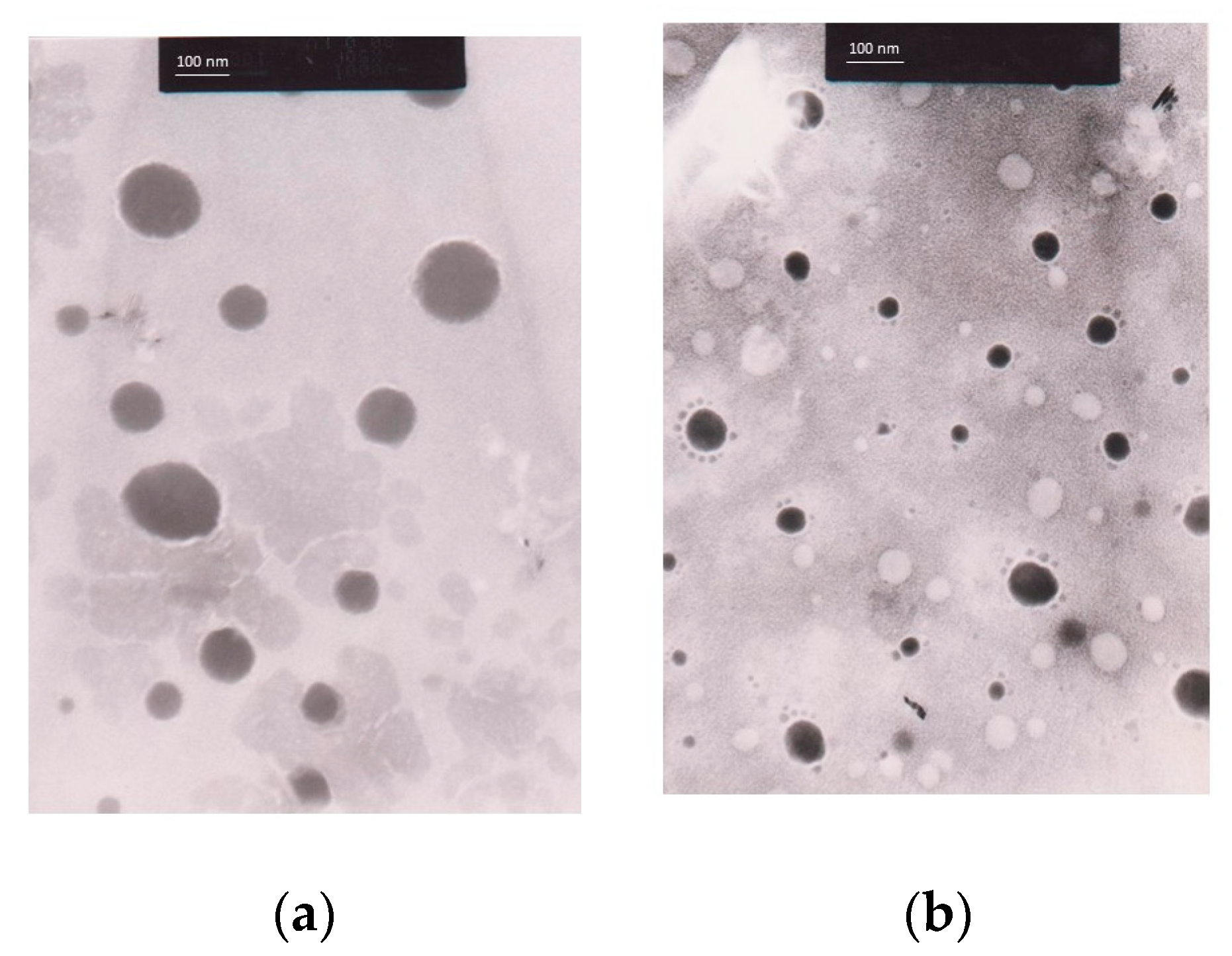
3.9.1. Morphology of Nanoemulsions and C. chinensis Extract-Loaded Nanoemulsions Determined Using Transmission Electron Microscope (TEM) Images
3.9.2. Stability Study of Unloaded Nanoemulsions and C. chinensis Extract-Loaded Nanoemulsions
3.9.3. Entrapment Efficacy of C. chinensis Extract-Loaded Nanoemulsions
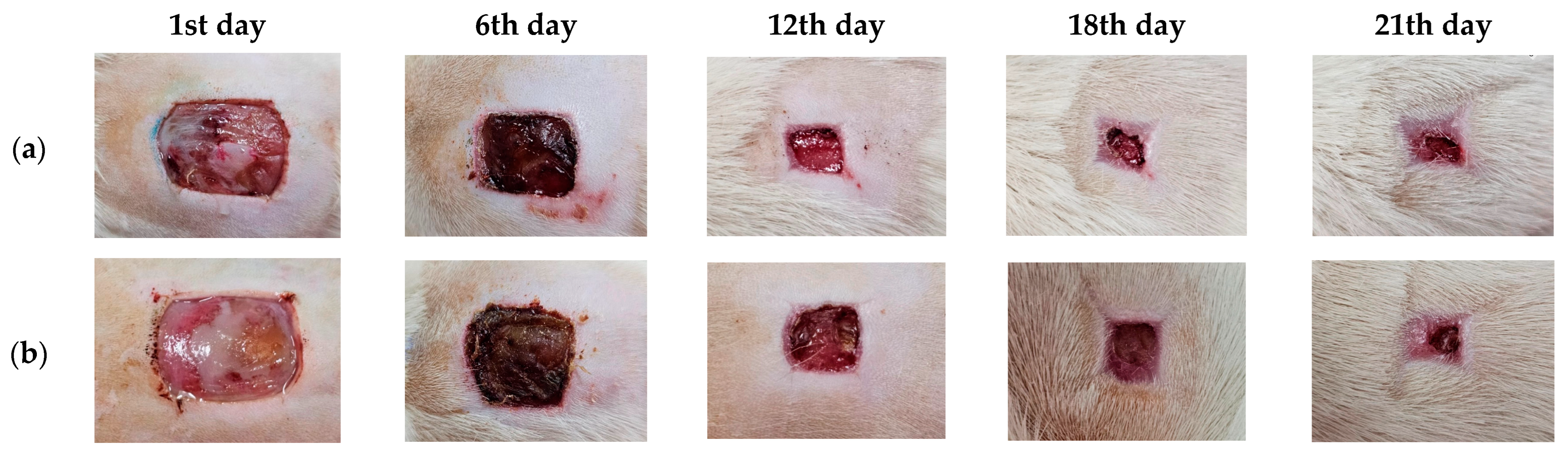
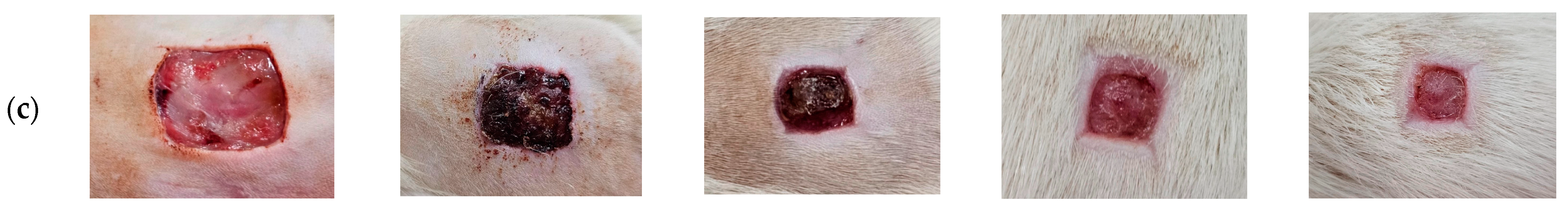
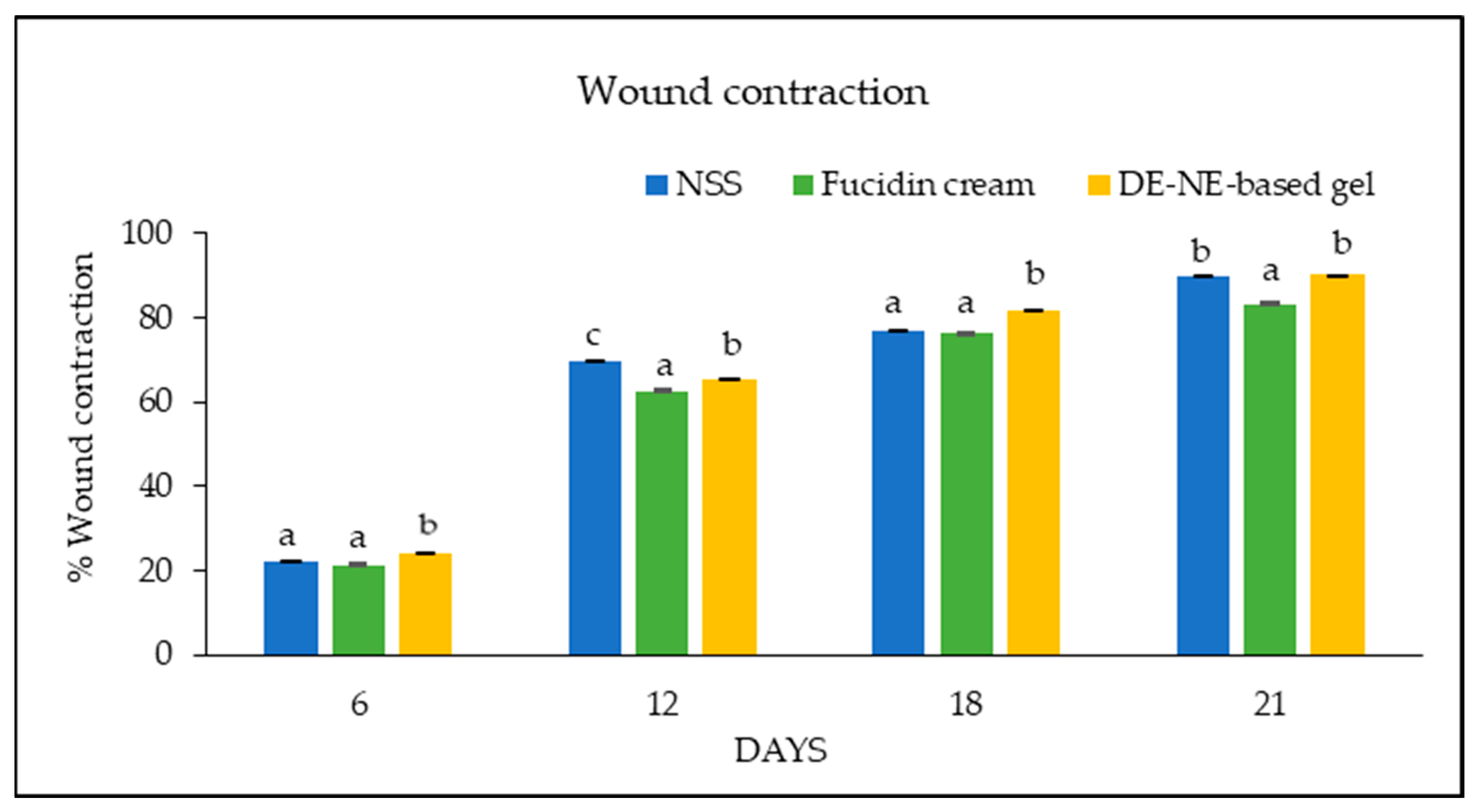
3.10. Wound Care Effect of C. chinensis Extract-Loaded Nanoemulsion-Based Gel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Kodzius, R. Management and prevention strategies for non-communicable diseases (NCDs) and their risk factors. Public Health Front. 2020, 8, 574111. [Google Scholar] [CrossRef]
- Aranaz, P.; Navarro-Herrera, D.; Zabala, M.; Miguéliz, I.; Romo-Hualde, A.; López-Yoldi, M. American Diabetes Association 2010. Diagnosis and classification of diabetes mellitus. Diabetes Care 2023, 33, 189. [Google Scholar]
- Sothornwit, J.; Srisawasdi, G.; Suwannakin, A.; Sriwijitkamol, A. Decreased health-related quality of life in patients with diabetic foot problems. Diabetes Metab. Syndr. Obes. 2018, 11, 35–43. [Google Scholar] [CrossRef]
- Donnapee, S.; Li, J.; Yang, X.; Ge, A.; Donkor, P.O.; Gao, X.; Chang, Y. Cuscuta chinensis Lam.: A systematic review on ethnopharmacology, phytochemistry and pharmacology of an important traditional herbal medicine. J. Ethnopharmacol. 2014, 157, 292–308. [Google Scholar] [CrossRef]
- Mo, H.; Zhang, N.; Li, H.; Li, F.; Pu, R. Beneficial effects of Cuscuta chinensis extract on glucocorticoid-induced osteoporosis through modulation of RANKL/OPG signals. Braz. J. Med. Biol. Res. 2019, 52, 87–94. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Q.; Wang, F.; Zhang, G. Antiosteoporotic compounds from seeds of Cuscuta chinensis. J. Ethnopharmacol. 2011, 135, 553–560. [Google Scholar] [CrossRef]
- Yang, H.M.; Shin, H.-K.; Kang, Y.-H.; Kim, J.-K. Cuscuta chinensis extract promotes osteoblast differentiation and mineralization in human osteoblast-like MG-63 cells. J. Med. Food 2009, 12, 85–92. [Google Scholar] [CrossRef]
- Dermani, F.K.; Saidijam, M.; Najafi, R.; Moradkhani, S.; Mohammadzaheri, Z.; Beiranvand, N.; Mohammadi, S.; Shabab, N.; Kalvandi, R.; Zeraati, F. Cytotoxic effects of hydroalcoholic extract of Cuscuta chinensis on PC3 and MCF7 cancer cell lines. Avicenna J. Phytomed. 2021, 11, 258. [Google Scholar]
- Lee, M.S.; Chen, C.J.; Wan, L.; Koizumi, A.; Chang, W.T.; Yang, M.J.; Lin, W.H.; Tsai, F.J.; Lin, M.K. Quercetin is increased in heat-processed Cuscuta campestris seeds, which enhances the seed’s anti-inflammatory and anti-proliferative activities. Process Biochem. 2011, 46, 2248–2254. [Google Scholar] [CrossRef]
- Gao, J.; Li, R.; Zhang, L.; Jia, L.; Ying, X.; Dou, D.; Li, J.; Li, H.-B. Cuscuta chinensis seeds water extraction protecting murine osteoblastic MC3T3-E1 cells against tertiary butyl hydroperoxide induced injury. J. Ethnopharmacol. 2013, 148, 587–595. [Google Scholar] [CrossRef]
- Liao, J.C.; Chang, W.T.; Lee, M.S.; Chiu, Y.J.; Chao, W.K.; Lin, Y.C.; Lin, M.K.; Peng, W.H. Antinociceptive and anti-inflammatory activities of Cuscuta chinensis seeds in mice. Am. J. Chin. Med. 2014, 42, 223–242. [Google Scholar] [CrossRef]
- Kang, S.Y.; Jung, H.W.; Lee, M.Y.; Lee, H.W.; Chae, S.W.; Park, Y.K. Effect of the semen extract of Cuscuta chinensis on inflammatory responses in LPS-stimulated BV-2 microglia. Chin. J. Integr. Med. 2014, 12, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yang, H.; Jung, D.H.; Hwang, J.T.; Ko, B.S. Ameliorating effects of Cuscuta chinensis Lamak extract on hind-limb ischemia, and angiogenic or inflammatory associated factors in ovariectomized mice. Mol. Med. Rep. 2019, 19, 3321–3329. [Google Scholar] [CrossRef]
- Thakur, N.; Garg, G.; Sharma, P.K.; Kumar, N. Nanoemulsions: A review on various pharmaceutical application. Glob. J. Pharm. 2012, 6, 222–225. [Google Scholar]
- Zain, M.S.C.; Edirisinghe, S.L.; Kim, C.-H.; De Zoysa, M.; Shaari, K. Nanoemulsion of flavonoid-enriched oil palm (Elaeis guineensis Jacq.) leaf extract enhances wound healing in zebrafish. Phytomed. Plus 2021, 1, 100–124. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Shaikh, I.A.; Abdel-Wahab, B.A.; Nourein, I.H.; Ahmad, J. Thymoquinone loaded topical nanoemulgel for wound healing: Formulation design and in-vivo evaluation. Molecules 2021, 26, 3863. [Google Scholar] [CrossRef]
- Choi, Y.; Jang, J.; Koo, H.J.; Tanaka, M.; Lee, K.H.; Choi, J. Alginate-chitosan hydrogel patch with beta-glucan nanoemulsion for antibacterial applications. Biotechnol. Bioprocess Eng. 2021, 26, 71–77. [Google Scholar] [CrossRef]
- Pathan, I.B.; Munde, S.J.; Shelke, S.; Ambekar, W.; Setty, C.M. Curcumin loaded fish scale collagen-HPMC nanogel for wound healing application: Ex-vivo and in-vivo evaluation. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 165–174. [Google Scholar] [CrossRef]
- Lo, S.; Fauzi, M.B. Current update of collagen nanomaterials fabrication, characterization, and its applications: A review. Pharmaceutics 2021, 13, 316. [Google Scholar] [CrossRef]
- Kiattisin, K.; Intasai, N.; Nitthikan, N.; Nantarat, T.; Lee, K.H.; Lin, W.C.; Lue, S.C.; Leelapornpisid, P. Antioxidant, anti-tyrosinase, anti-aging potentials and safety of arabica coffee cherry extract. Chiang Mai J. Sci. 2019, 46, 930–945. [Google Scholar]
- Liu, J.; Zou, S.; Liu, W.; Li, J.; Wang, H.; Hao, J.; He, J.; Gao, X.; Liu, E.; Chang, Y. An established HPLC-MS/MS method for evaluation of the influence of salt processing on pharmacokinetics of six compounds in cuscutae semen. Molecules 2019, 24, 2502. [Google Scholar] [CrossRef]
- Phumat, P.; Chaichit, S.; Potprommanee, S.; Preedalikit, W.; Sainakham, M.; Poomanee, W.; Chaiyana, W.; Kiattisin, K. Influence of Benincasa hispida peel extracts on antioxidant and anti-aging activities, including molecular docking simulation. Foods 2023, 12, 3555. [Google Scholar] [CrossRef]
- Wayne, P. CLSI Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100 (ISBN 978-1-68440-066-9 [Print]; ISBN 978-1-68440-067-6 [Electronic]); Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Romulo, A.; Zuhud, E.A.; Rondevaldova, J.; Kokoska, L. Screening of in vitro antimicrobial activity of plants used in traditional Indonesian medicine. Pharm. Biol. 2018, 56, 287–293. [Google Scholar] [CrossRef]
- Vajrabhaya, L.; Korsuwannawong, S. Cytotoxicity evaluation of a thai herb using tetrazolium (MTT) and sulforhodamine B (SRB) assays. J. Anal. Sci. Technol. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Chaiyana, W.; Punyoyai, C.; Somwongin, S.; Leelapornpisid, P.; Ingkaninan, K.; Waranuch, N.; Srivilai, J.; Thitipramote, N.; Wisuitiprot, W.; Schuster, R. Inhibition of 5α-reductase, IL-6 secretion, and oxidation process of Equisetum debile Roxb. ex vaucher extract as functional food and nutraceuticals ingredients. Nutrients 2017, 9, 1105. [Google Scholar] [CrossRef]
- Hommel, U.; Hurth, K.; Rondeau, J.-M.; Vulpetti, A.; Ostermeier, D.; Boettcher, A.; Brady, J.P.; Hediger, M.; Lehmann, S.; Koch, K. Discovery of a selective and biologically active low-molecular weight antagonist of human IL-1b. Nat. Commun. 2023, 14, 5497–5509. [Google Scholar] [CrossRef]
- Adams, R.; Burnley, R.J.; Valenzano, C.R.; Qureshi, O.; Doyle, C.; Lumb, S.; del Carmen Lopez, M.; Griffin, R.; McMillan, D.; Taylor, R.D. Discovery of a junctional epitope antibody that stabilizes IL-6 and gp80 protein: Protein interaction and modulates its downstream signaling. Sci. Rep. 2017, 7, 377–386. [Google Scholar] [CrossRef]
- He, M.M.; Smith, A.S.; Oslob, J.D.; Flanagan, W.M.; Braisted, A.C.; Whitty, A.; Cancilla, M.T.; Wang, J.; Lugovskoy, A.A.; Yoburn, J.C. Small-molecule inhibition of TNF-α. Science 2005, 310, 1022–1025. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Schrodinger, L. The PyMOL molecular graphics system. Version 2015, 1, 8. [Google Scholar]
- Nitthikan, N.; Leelapornpisid, P.; Natakankitkul, S.; Chaiyana, W.; Mueller, M.; Viernstein, H.; Kiattisin, K. Improvement of stability and transdermal delivery of bioactive compounds in green robusta coffee beans extract loaded nanostructured lipid carriers. J. Nanotechnol. 2018, 12, 47–51. [Google Scholar] [CrossRef]
- Subramanian, S.; Duraipandian, C.; Alsayari, A.; Ramachawolran, G.; Wong, L.S.; Sekar, M.; Gan, S.H.; Subramaniyan, V.; Seethalakshmi, S.; Jeyabalan, S. Wound healing properties of a new formulated flavonoid-rich fraction from Dodonaea viscosa Jacq. leaves extract. Front. Pharmacol. 2023, 14, 1096–1905. [Google Scholar] [CrossRef]
- Dhar, G.; Akther, S.; Sultana, A.; May, U.; Islam, M.M.; Dhali, M.; Sikdar, D. Effect of extraction solvents on phenolic contents and antioxidant capacities of Artocarpus chaplasha and Carissa carandas fruits from Bangladesh. J. Appl. Biol. 2017, 5, 39–44. [Google Scholar]
- Wang, H.; Hou, X.; Li, B.; Yang, Y.; Li, Q.; Si, Y. Study on active components of cuscuta chinensis promoting neural stem cells proliferation: Bioassay-guided fractionation. Molecules 2021, 26, 6634. [Google Scholar] [CrossRef]
- Williamson, G.; Barron, D.; Shimoi, K.; Terao, J. In vitro biological properties of flavonoid conjugates found in vivo. Free Radic. Res. 2005, 39, 457–469. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial properties, sources, clinical, and traditional applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9580–9604. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.C.; Zhou, S.J.; Li, Y.; Zheng, T.T.; Zhou, C.Z.; Wan, X.H. Hyperoside: A review on its sources, biological activities, and molecular mechanisms. Phytother. Res. 2022, 36, 2779–2802. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K. Abnormal solvent effects on hydrogen atom abstractions. 1. The reactions of phenols with 2, 2-diphenyl-1-picrylhydrazyl (DPPH) in alcohols. J. Org. Chem. 2003, 68, 3433–3438. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Gülcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Han, R.-M.; Zhang, J.-P.; Skibsted, L.H. Reaction dynamics of flavonoids and carotenoids as antioxidants. Molecules 2012, 17, 2140–2160. [Google Scholar] [CrossRef] [PubMed]
- Egbuna, C.; Awuchi, C.G.; Kushwaha, G.; Rudrapal, M.; Patrick-Iwuanyanwu, K.C.; Singh, O.; Odoh, U.E.; Khan, J.; Jeevanandam, J.; Kumarasamy, S. Bioactive compounds effective against type 2 diabetes mellitus: A systematic review. Curr. Top. Med. Chem. 2021, 21, 1067–1095. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Twinomuhwezi, H. The medical, pharmaceutical, and nutritional biochemistry and uses of some common medicinal plants. In Medicinal and Aromatic Plants of the World; Encyclopedia of Life Support Systems: Paris, France, 2021; pp. 1–32. [Google Scholar]
- Shahbaz, M.; Imran, M.; Alsagaby, S.A.; Naeem, H.; Al Abdulmonem, W.; Hussain, M.; Abdelgawad, M.A.; El-Ghorab, A.H.; Ghoneim, M.M.; El-Sherbiny, M. Anticancer, antioxidant, ameliorative and therapeutic properties of kaempferol. Int. J. Food Prop. 2023, 26, 1140–1166. [Google Scholar] [CrossRef]
- Xu, S.; Chen, S.; Xia, W.; Sui, H.; Fu, X. Hyperoside: A review of its structure, synthesis, pharmacology, pharmacokinetics, and toxicity. Molecules 2022, 27, 3009. [Google Scholar] [CrossRef] [PubMed]
- Jang, E. Hyperoside as a potential natural product targeting oxidative stress in liver diseases. Antioxidants 2022, 11, 1437. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, L.; Wang, X.; Lan, R.; Wang, M.; Du, G.; Guan, W.; Liu, J.; Brennan, M.; Guo, H. Antioxidant activity evaluation of dietary flavonoid hyperoside using saccharomyces cerevisiae as a model. Molecules 2019, 24, 788. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Fu, R.; Cai, Y. Hyperoside protected against oxidative stress-induced liver injury via the PHLPP2-AKT-GSK-3β signaling pathway in vivo and in vitro. Front. Pharmacol. 2020, 11, 550–579. [Google Scholar] [CrossRef]
- Salih, S.; Alkhazraji, T.; Ali, A. Alkaloid content and effectiveness of anti-bacterial and oxidation extracts of the species Cuscuta chinensis Lam. (convolvulaceae). J. Plant Prod. 2016, 7, 117–122. [Google Scholar] [CrossRef]
- Ahmad, A.; Tandon, S.; Xuan, T.D.; Nooreen, Z. A review on phytoconstituents and biological activities of Cuscuta species. Biomed. Pharmacother. 2017, 92, 772–795. [Google Scholar] [CrossRef]
- Noureen, S.; Noreen, S.; Ghumman, S.A.; Batool, F.; Bukhari, S.N.A. The genus Cuscuta (Convolvolaceac): An updated review on indigenous uses, phytochemistry, and pharmacology. Iran. J. Basic Med. Sci. 2019, 22, 12–25. [Google Scholar]
- Azad, A.K.; Laboni, F.R.; Rashid, H.; Ferdous, S.; Rashid, S.S.; Kamal, N.; Labu, Z.K.; Islam, M.; Islam Sarker, Z. In vitro evaluation of Cuscuta reflexa Roxb. for thrombolytic, antioxidant, membrane stabilizing and antimicrobial activities. Nat. Prod. Res. 2020, 34, 2394–2397. [Google Scholar] [CrossRef] [PubMed]
- Manirujjaman, M.; Suchana, S.; Collet, T.; Nawshin, L.; Chowdhury, M. Antimicrobial effects of ethanolic extracts from Cuscuta reflexa Roxb.(Convolvulaceae). Int. J. Pharmacogn. Pharm. Res. 2016, 8, 930–932. [Google Scholar]
- Biswas, S.; Chowdhury, A.; Das, J.; Karamkar, U.; Raihan, S.; Das, A. Phytochemical investigation and chromatographic evaluation with antimicrobial and cytotoxic potential of Cuscuta epithymum. Int. J. Pharm. 2012, 8, 422–427. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial effects of flavonoids and their structure-activity relationship study: A comparative interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Thebti, A.; Meddeb, A.; Ben Salem, I.; Bakary, C.; Ayari, S.; Rezgui, F.; Essafi-Benkhadir, K.; Boudabous, A.; Ouzari, H.-I. Antimicrobial activities and mode of flavonoid actions. Antibiotics 2023, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Brem, H.; Stojadinovic, O.; Diegelmann, R.F.; Entero, H.; Lee, B.; Pastar, I.; Golinko, M.; Rosenberg, H.; Tomic-Canic, M. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol. Med. 2007, 13, 30–39. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, F.; Xu, G.; Wang, D. Inflammatory microenvironment of skin wounds. Front. Immunol. 2022, 13, 789274. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hamad, K.; Al Shibitini, A.; Juma, S.; Sharifi, S.; Gould, L.; Mahmoudi, M. Investigating inflammatory markers in wound healing: Understanding implications and identifying artifacts. ACS Pharmacol. Transl. Sci. 2024, 7, 18–27. [Google Scholar] [CrossRef]
- Bagher, Z.; Ehterami, A.; Safdel, M.H.; Khastar, H.; Semiari, H.; Asefnejad, A.; Davachi, S.M.; Mirzaii, M.; Salehi, M. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. J. Drug Deliv. Sci. Technol. 2020, 55, 101–109. [Google Scholar] [CrossRef]
- Özay, Y.; Güzel, S.; Yumrutaş, Ö.; Pehlivanoğlu, B.; Erdoğdu, İ.H.; Yildirim, Z.; Türk, B.A.; Darcan, S. Wound healing effect of kaempferol in diabetic and nondiabetic rats. J. Surg. Res. 2019, 233, 284–296. [Google Scholar] [CrossRef]
- Hu, W.-H.; Wang, H.-Y.; Xia, Y.-T.; Dai, D.K.; Xiong, Q.-P.; Dong, T.T.-X.; Duan, R.; Chan, G.K.-L.; Qin, Q.-W.; Tsim, K.W.-K. Kaempferol, a major flavonoid in ginkgo folium, potentiates angiogenic functions in cultured endothelial cells by binding to vascular endothelial growth factor. Front. Pharmacol. 2020, 11, 521988. [Google Scholar] [CrossRef]
- Kurt-Celep, İ.; Celep, E.; Akyüz, S.; İnan, Y.; Barak, T.H.; Akaydın, G.; Telci, D.; Yesilada, E. Hypericum olympicum L. recovers DNA damage and prevents MMP–9 activation induced by UVB in human dermal fibroblasts. J. Ethnopharmacol. 2020, 246, 112202. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Li, G.; Yang, L. Hyperoside protects against cerebral ischemia-reperfusion injury by alleviating oxidative stress, inflammation, and apoptosis in rats. Biotechnol. Biotechnol. Equip. 2019, 33, 798–806. [Google Scholar] [CrossRef]
- Chen, X.; Famurewa, A.C.; Tang, J.; Olatunde, O.O.; Olatunji, O.J. Hyperoside attenuates neuroinflammation, cognitive impairment and oxidative stress via suppressing TNF-α/NF-κB/caspase-3 signaling in type 2 diabetes rats. Nutr. Neurosci. 2022, 25, 1774–1784. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-H.; Zhu, L.-B.; Li, T.; Zhu, H.; Wang, Y.-N.; Ren, X.-L.; Hu, B.-L.; Huang, C.-P.; Zhu, J.-H.; Zhang, X. Hyperoside inhibits lipopolysaccharide-induced inflammatory responses in microglial cells via p38 and NFκB pathways. Int. Immunopharmacol. 2017, 50, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Luo, J.; Jing, X.; Xiang, W.; Guo, J.; Yao, X.; Liang, S.; Guo, F.; Xu, T. Hyperoside ameliorates the progression of osteoarthritis: An in vitro and in vivo study. Phytomedicine 2021, 80, 153387. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, J.; Dong, W.; Li, P.; Li, L.; Hou, J.; Zheng, Y.; Lin, C.; Ren, J. Protective effect of kaempferol on LPS plus ATP-induced inflammatory response in cardiac fibroblasts. Inflammation 2015, 38, 94–101. [Google Scholar] [CrossRef]
- Lee, W.; Lee, E.; Sung, M.; Yoo, W. Kaempferol inhibits IL-1β-stimulated, RANKL-mediated osteoclastogenesis via downregulation of MAPKs, c-Fos, and NFATc1. Inflammation 2014, 37, 1221–1230. [Google Scholar] [CrossRef]
- Modi, J.D.; Patel, J.K. Nanoemulsion-based gel formulation of aceclofenac for topical delivery. Int. J. Pharm. Pharm. Sci. 2011, 1, 6–12. [Google Scholar]
- Arora, R.; Aggarwal, G.; Harikumar, S.; Kaur, K. Nanoemulsion based hydrogel for enhanced transdermal delivery of ketoprofen. Adv. Pharm. J. 2014, 2014, 468456. [Google Scholar] [CrossRef]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for formulation of nanoemulsion drug delivery system: A review. Prev. Nutr. Food Sci. 2019, 24, 225. [Google Scholar] [CrossRef] [PubMed]
- Solè, I.; Pey, C.M.; Maestro, A.; González, C.; Porras, M.; Solans, C.; Gutiérrez, J.M. Nano-emulsions prepared by the phase inversion composition method: Preparation variables and scale up. J. Colloid Interface Sci. 2010, 344, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Nikmaram, N.; Roohinejad, S.; Estevinho, B.N.; Rocha, F.; Greiner, R.; McClements, D.J. Production, properties, and applications of solid self-emulsifying delivery systems (S-SEDS) in the food and pharmaceutical industries. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 108–126. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic emulsification of food-grade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrason. Sonochem. 2013, 20, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Smruthi, M.; Nallamuthu, I.; Anand, T. A comparative study of optimized naringenin nanoformulations using nano-carriers (PLA/PVA and zein/pectin) for improvement of bioavailability. Food Chem. 2022, 369, 130–134. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Chunhavacharatorn, P.; Khanashyam, A.C.; Li, L.; Al-Asmari, F. Cinnamon bark oil in water nanoemulsion formulation, characterization, and antimicrobial activities. LWT 2023, 179, 114–121. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Pandya, N.S.; Said, Z.; Öztop, H.F.; Abu-Hamdeh, N. 4S consideration (synthesis, sonication, surfactant, stability) for the thermal conductivity of CeO2 with MWCNT and water-based hybrid nanofluid: An experimental assessment. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125–128. [Google Scholar] [CrossRef]
- Sharma, N.; Kaur, G.; Khatkar, S.K. Optimization of emulsification conditions for designing ultrasound assisted curcumin loaded nanoemulsion: Characterization, antioxidant assay and release kinetics. LWT 2021, 141, 112–116. [Google Scholar] [CrossRef]
- Salami, A.A.; Imosemi, I.O.; Owoeye, O.O. A comparison of the effect of chlorhexidine, tap water and normal saline on healing wounds. Int. J. Morphol. 2006, 24, 673–676. [Google Scholar] [CrossRef]
- Mirza, R.E.; Fang, M.M.; Ennis, W.J.; Koh, T.J. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013, 62, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U. Flavonoids as potential wound-healing molecules: Emphasis on pathways perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, R.D.D.G.; Perini, J.A.; Machado, D.E.; Angeli-Gamba, T.; dos Santos Esteves, R.; Santos, M.G.; Oliveira, A.P.; Rocha, L. Wound healing activity and chemical standardization of Eugenia pruniformis cambess. Pharmacogn. Mag. 2016, 12, 288. [Google Scholar] [PubMed]
- Kang, Y.; Xu, L.; Dong, J.; Yuan, X.; Ye, J.; Fan, Y.; Liu, B.; Xie, J.; Ji, X. Programmed microalgae-gel promotes chronic wound healing in diabetes. Nat. Commun. 2024, 15, 1042. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer | Sequence: (5′-3′) |
|---|---|---|
| β–actin | Forward Reverse | TCATGAAGTGTGACGTTGACATCCGT CCTAGAAGCATTTGCGGTGCACGATG |
| IL-1β | Forward Reverse | CAGGATGAGGACATGAGCACC CTCTGCAGACTCAAACTCCAC |
| IL-6 | Forward Reverse | CATCCAGTTGCCTTCTTGGGA GCATTGGAAATTGGGGTAGGAAG |
| TNF-α | Forward Reverse | ATGAGCACAGAAAGCATGATC TACAGGCTTGTCACTCGAATT |
| Compositions | NE1 | NE2 | NE3 | NE4 | NE5 | NE6 |
|---|---|---|---|---|---|---|
| Avocado oil | 10 | 10 | 10 | 10 | 10 | 10 |
| Smix ratio | 1:1 | 1:1 | 2:1 | 2:1 | 3:1 | 3:1 |
| Smix (%) | 10 | 15 | 10 | 15 | 10 | 15 |
| Water | 80 | 75 | 80 | 75 | 80 | 75 |
| Samples | DPPH IC50 (µg/mL) | FRAP Value (mg FeSO4/g Extract) | Lipid Peroxidation Inhibition IC50 (µg/mL) |
|---|---|---|---|
| DEA | 4.85 ± 0.18 a | 0.37 ± 0.01 b | 4.73 ± 0.18 b |
| DE | 1.57 ± 0.01 b | 0.20 ± 0.02 c | 6.29 ± 0.54 a |
| Trolox | 1.05 ± 0.06 c | 1.44 ± 0.04 a | 0.21 ± 0.01 c |
| Concentration (mg/mL) | Inhibition Zone (mm.) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | Tetracycline (30 µg/mL) | DMSO | |||||
| Extracts/ Microbial | DE | DEA | DE | DEA | DE | DEA | DE | DEA | ||
| E. faecalis | - | 11 ± 1.0 | - | 10 ± 0.6 | - | - | - | - | 15 ± 1.7 | - |
| E. coli | - | 7 ± 0 | - | - | - | - | - | - | 30 ± 0 | - |
| P. aeruginosa | - | 9 ± 4.0 | - | 8 ± 3.2 | - | - | - | - | 24.3 ± 0.6 | - |
| Extracts /Microbial | MIC (mg/mL) | ||
|---|---|---|---|
| E. faecalis | E. coli | P. aeruginosa | |
| DEA | 6.25 ± 0.0 | 6.25 ± 0.0 | 8.3 ± 3.6 |
| Samples | IS | Irritation Assessment |
|---|---|---|
| Positive control (1% w/v SLS) | 11.9 ± 0.6 | Severe |
| Negative control (0.9% w/v NaCl) | 0.0 ± 0.0 | No irritation |
| DE (5 mg/mL) | 0.0 ± 0.0 | No irritation |
| DEA (5 mg/mL) | 0.0 ± 0.0 | No irritation |
| Formulation | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| Unloaded NE F1 | 174.90 ± 2.34 d | 0.28 ± 0.04 c | −18.03 ± 0.40 b |
| Unloaded NE F2 | 211.27 ± 2.04 e | 0.37 ± 0.01 c | −19.70 ± 0.44 b |
| Unloaded NE F3 | 152.90 ± 1.21 c | 0.20 ± 0.03 a | −0.25 ± 0.49 a |
| Unloaded NE F4 | 124.13 ± 1.15 b | 0.25 ± 0.02 b | −20.20 ± 0.26 b |
| Unloaded NE F5 | 88.42 ± 1.10 a | 0.27 ± 0.01 b | −20.90 ± 0.56 b |
| Unloaded NE F6 | 119.37 ± 0.45 a,b | 0.26 ± 0.01 b | −19.20 ± 0.78 b |
| Conditions | Particle Size (nm) | PDI | Zeta Potential (mV) | |
|---|---|---|---|---|
| Unloaded NE F5 | Initial | 132.04 ± 1.41 a | 0.27 ± 0.02 a | −20.90 ± 0.56 a |
| HC | 142.60 ± 1.20 b | 0.28 ± 0.00 a | −26.60 ± 0.82 b | |
| 30 °C | 134.27 ± 1.67 a | 0.26 ± 0.00 a | −23.33 ± 0.85 a,b | |
| DE-loaded NE | Initial | 136.80 ± 1.95 a | 0.25 ± 0.01 a | −24.07 ± 0.25 b |
| HC | 206.83 ± 2.62 b | 0.27 ± 0.01 b | −21.20 ± 0.82 a | |
| 30 °C | 131.07 ± 1.02 a | 0.26 ± 0.01 a | −23.33 ± 0.85 a,b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitthikan, N.; Preedalikit, W.; Supadej, K.; Chaichit, S.; Leelapornpisid, P.; Kiattisin, K. Exploring the Wound Healing Potential of a Cuscuta chinensis Extract-Loaded Nanoemulsion-Based Gel. Pharmaceutics 2024, 16, 573. https://doi.org/10.3390/pharmaceutics16050573
Nitthikan N, Preedalikit W, Supadej K, Chaichit S, Leelapornpisid P, Kiattisin K. Exploring the Wound Healing Potential of a Cuscuta chinensis Extract-Loaded Nanoemulsion-Based Gel. Pharmaceutics. 2024; 16(5):573. https://doi.org/10.3390/pharmaceutics16050573
Chicago/Turabian StyleNitthikan, Nichcha, Weeraya Preedalikit, Kanittapon Supadej, Siripat Chaichit, Pimporn Leelapornpisid, and Kanokwan Kiattisin. 2024. "Exploring the Wound Healing Potential of a Cuscuta chinensis Extract-Loaded Nanoemulsion-Based Gel" Pharmaceutics 16, no. 5: 573. https://doi.org/10.3390/pharmaceutics16050573