Five Novel Polymorphs of Cardarine/GW501516 and Their Characterization by X-ray Diffraction, Computational Methods, Thermal Analysis and a Pharmaceutical Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Crystallization Experiments
- (i)
- GW-1 (starting polymorph) from acetonitrile;
- (ii)
- GW-2 from mixture of pentane and acetone;
- (iii)
- GW-3 in isopropyl alcohol;
- (iv)
- GW-5 in methyl isobutyl ketone;
- (v)
- GW-4 was found in the bulk of the starting sample as single crystals but was not further reproduced.
2.2. X-ray Single-Crystal Diffraction and Structure Refinement
2.3. X-ray Powder Diffraction
2.4. Differential Scanning Calorimetry
2.5. Evaluation of Intermolecular Interaction Energies
2.6. Evaluation of Stability and Preliminary Solubility Check
3. Results
3.1. Crystal Structures Descriptions
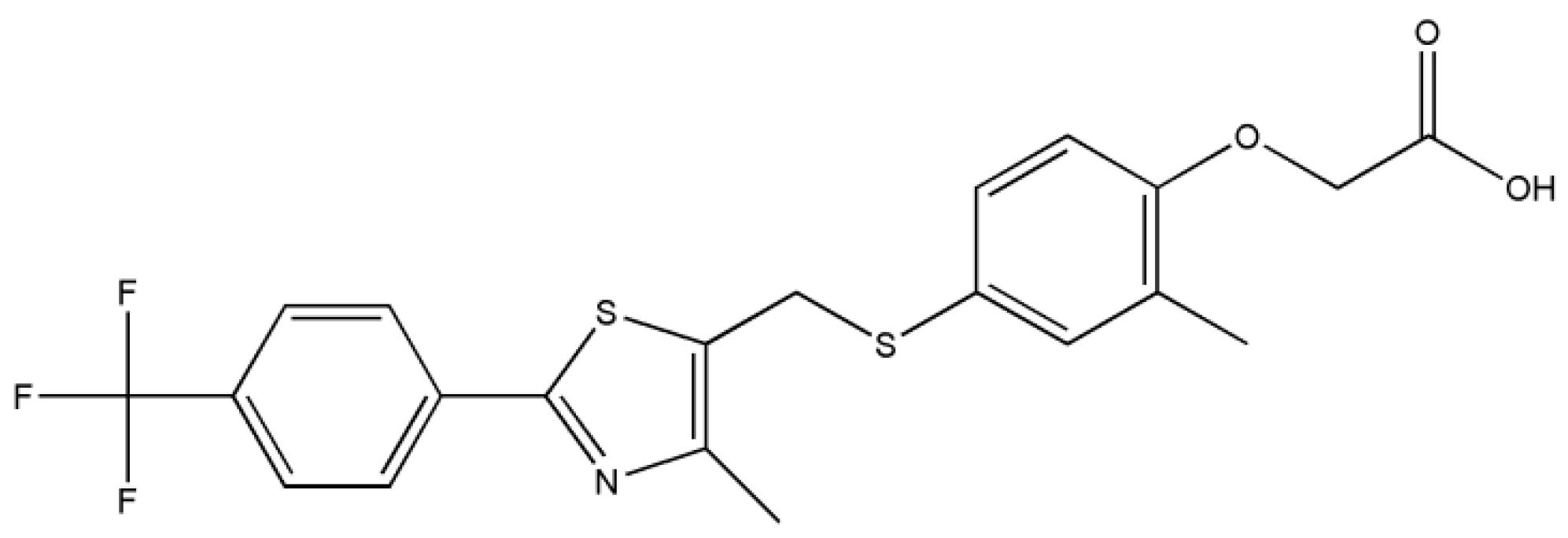
3.1.1. GW-1
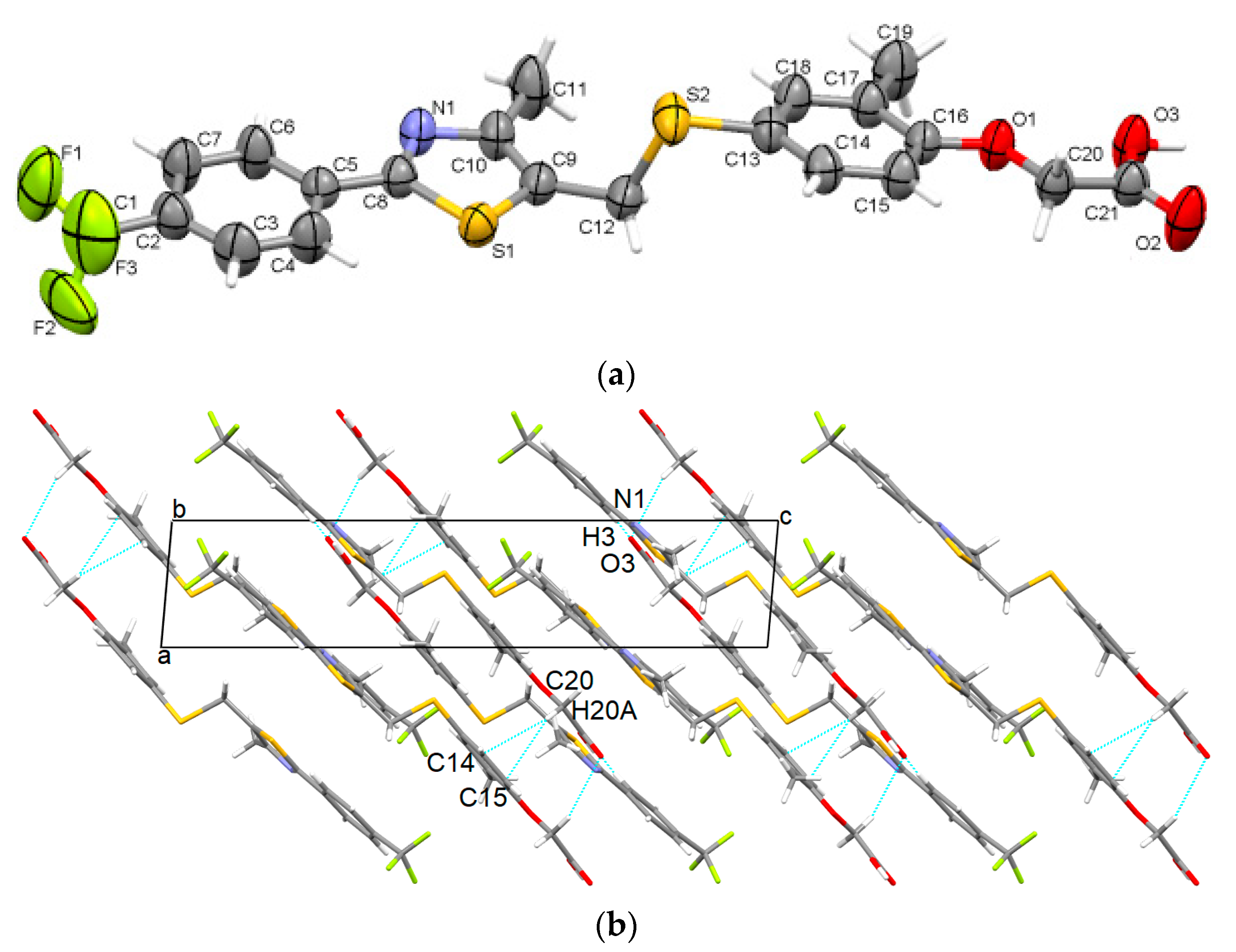
3.1.2. GW-2
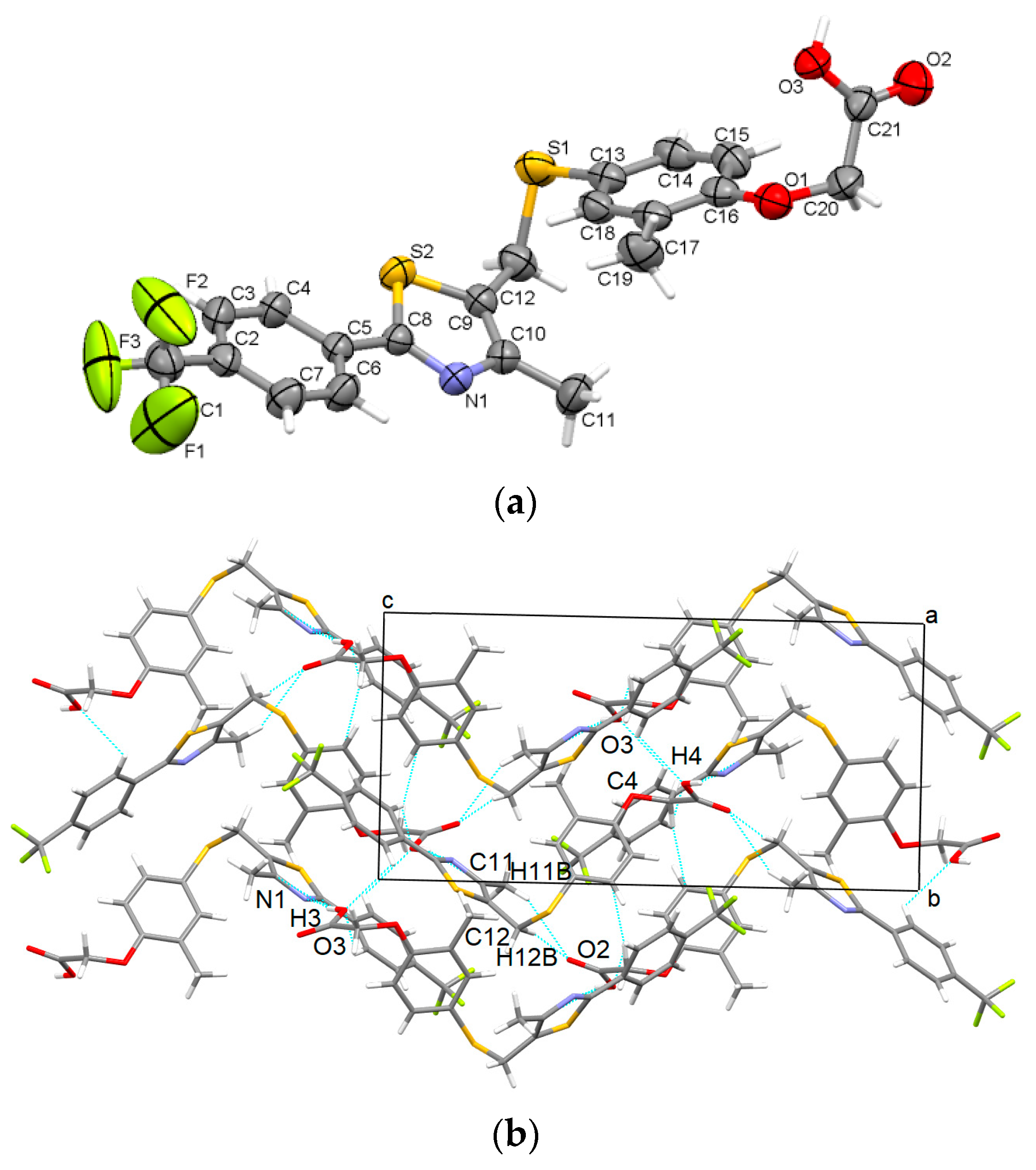
3.1.3. GW-3
3.1.4. GW-4
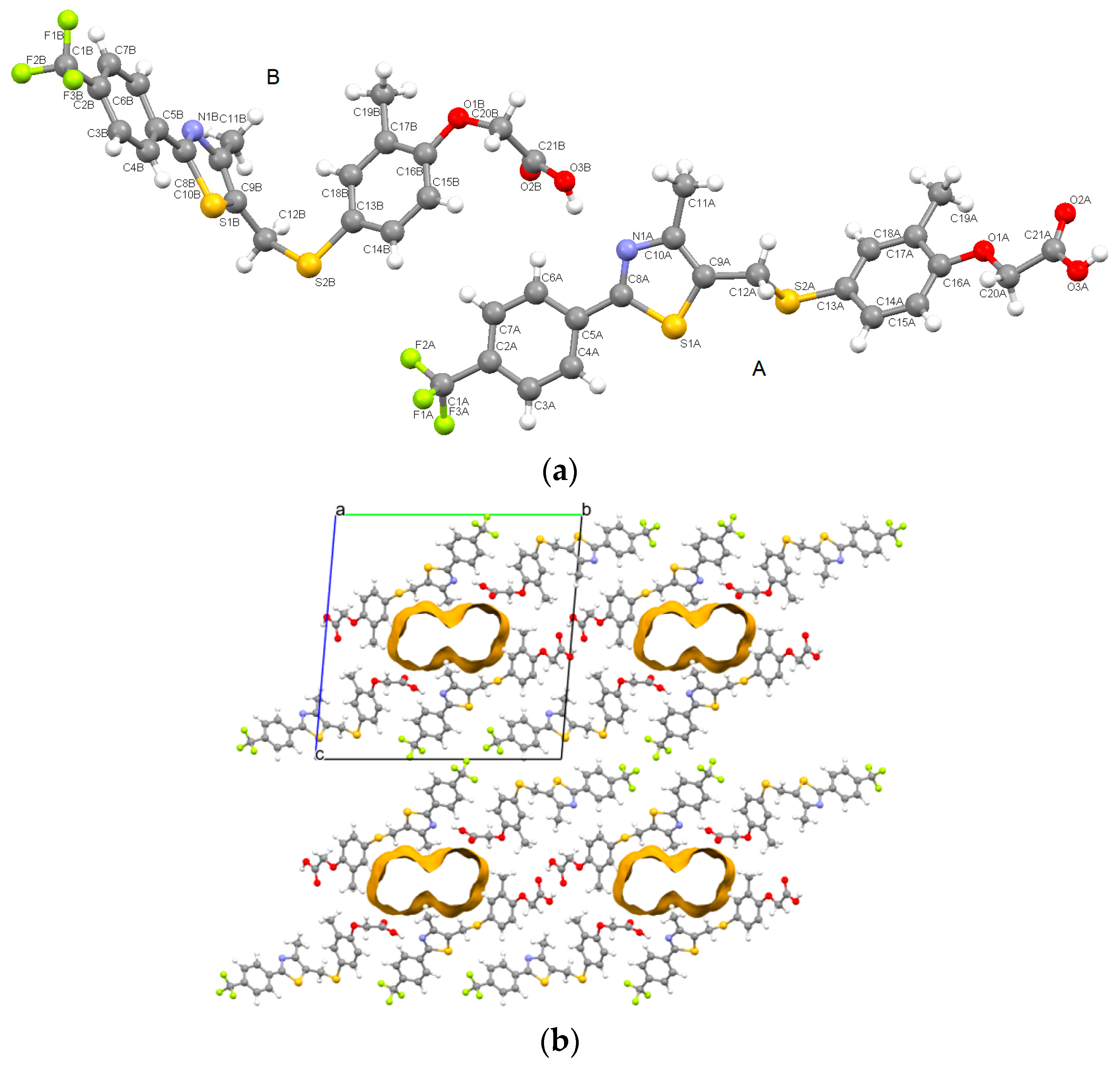
3.1.5. Crystal Structure Solved by Powder X-ray Diffraction for GW-5
- (i)
- GW501516 shows a high ability to be packed in various distinct supramolecular arrangements (polymorphs); the ease of crystallizing in new polymorphs is mainly due to the high flexibility of the carbon-sulfur (C12)-(S2) bond at the methylsulfanyl group and the C20-O1 bond between the acetate group and phenoxy moiety.
- (ii)
- GW-1, GW-2, and GW-3 are centrosymmetric, crystallized in the monoclinic system and having the following space groups: (GW-1: P21/c, GW-2: P21/n and GW-3: C2/c). GW-4 is non-centrosymmetric orthorhombic and has space group Pca21 and GW-5 has triclinic space group P-1.
- (iii)
- GW-4, found in the start probe, has always recrystallized centrosymmetrically upon dissolution and slow evaporation in various solvents. This is due to the lower overall lattice energy compared with the other polymorphs.
- (iv)
- The stability and cohesion in the crystal is mainly ensured as follows: strong O-H···N hydrogen bonds between the carboxyl group and thiazole ring for GW-1, GW-2, and GW-3; strong carboxyl···carboxyl O-H···O hydrogen bonds are found in GW-4 while GW-5 contains both O-H···N and O-H···O interactions. Other weaker interactions that are present in all polymorphs are C-H···π, C-H···F, and C-H···O.
3.2. X-ray Powder Diffraction Analysis
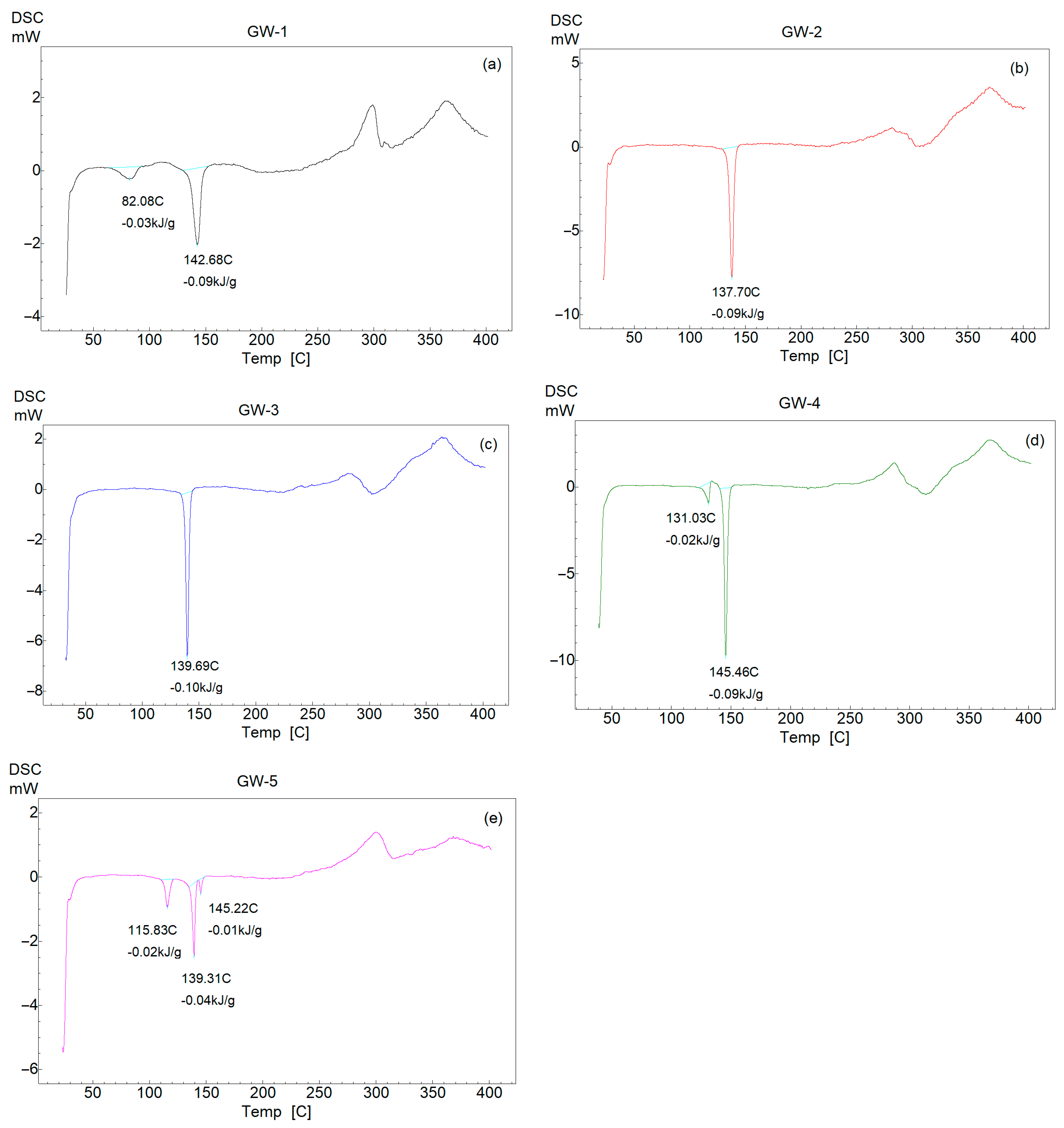
3.3. DSC Thermal Analysis
3.4. Pairwise Intermolecular and Lattice Energies Evaluation
- (i)
- Polymorphs GW-1, GW-2, GW-3, and GW-5 show hydroxyl···thiazole O-H···N hydrogen bonds which are characterized by high interaction energies mostly dominated by an electrostatic component (Eele).
- (ii)
- Polymorphs GW-4 and GW-5 are characterized by an unused nitrogen atom of thiazole rings in the formation of O-H···N hydrogen bonds but form strong mutual carboxyl···carboxyl O-H···O hydrogen bonds with very high binding energy (Etot = −148 kJ/mol) which is overwhelmed by the electrostatic energy (Eele = −114 kJ/mol) in GW-4 and Etot = −140 kJ/mol and with Eele = −106 kJ/mol in GW-5.
- (iii)
- Rather low polarization energies (Epol) suggest that the molecules are little polarized.
- (iv)
- In all five crystals, the stacking by C-H···π contacts are involved mostly in stability and cohesion, an aspect that is observed from the dominant values of dispersion energies (Edis) where these interactions are involved; it is observed that in the crystals containing C-H···π interactions, the dispersion energies are much higher.
3.5. Hirshfeld Diagrams and Fingerprint Plots Analysis
- (i)
- The fingerprint plots of GW-1, GW-2, and GW-3 are symmetric and specific to the crystals with one molecule in asymmetric units; the fingerprint diagrams of GW-4 and GW-5 are asymmetric, suggesting that the molecular environment is different for each individual molecule in the asymmetric unit (Figure S3, Supporting information).
- (ii)
- The presence of sharp and protruding H···N/N···H spikes in GW-1, GW-2, GW-3, and GW-5 is attributable to the strong hydroxyl···thiazole N-H···O interactions which play an important role in packing.
- (iii)
- The sharp protruding H···O/O···H spikes in GW-4 and molecule A of GW-5 are representative of the existence of strong carbonyl···carbonyl O-H···O bonds and have major role in overall stability.
- (iv)
- The above both pair of spikes (H···N/N···H and H···O/O···H) present in all crystals corroborated with the high absolute values of interaction energies (Table S1, Supporting Information) denote the overall importance in cohesion.
- (v)
- A breakdown of fingerprint diagrams (Table S3, Supporting Information) shows the greatest percentage comprises H···H contacts, followed by F···H/H···F, O···H/H···O, C···H/H···C, S···H/H···S contacts. This points to the fact that crystals are governed by hydrogen bonds and combinations of van der Waals interactions.
- (vi)
- From the fingerprint plots of GW-5 it can be observed that di and de pairs extend to distances up to 3.0 Å and do not fit into the usual two-dimensional geometry of the fingerprint plots [31]. This could be explained by the presence of the solvent in the lattice that was not located by X-ray diffraction calculations on powders. This represents a possibly incomplete crystal structure solving of GW-5. However, the two cardarine molecules are well highlighted.
3.6. Stability of Polymorphic Samples and Solubility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terada, M.; Araki, M.; Ashibe, B.; Motojima, K. GW501516 acts as an efficient PPARα activator in the mouse liver. Drug Discov. Ther. 2011, 5, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Brunmair, B.; Staniek, K.; Dörig, J.; Szöcs, Z.; Stadlbauer, K.; Marian, V.; Gras, F.; Anderwald, C.; Nohl, H.; Waldhäusl, W.; et al. Activation of PPAR-delta in isolated rat skeletal muscle switches fuel preference from glucose to fatty acids. Diabetologia 2006, 49, 2713–2722. [Google Scholar] [CrossRef]
- Dressel, U.; Allen, T.L.; Pippal, J.B.; Rohde, P.R.; Lau, P.; Muscat, G.E. The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol. Endocrinol. 2003, 17, 2477–2493. [Google Scholar] [CrossRef]
- Kintz, P.; Gheddar, L.; Paradis, C.; Chinellato, M.; Ameline, A.; Raul, J.S.; Oliva-Labadie, M. Peroxisome Proliferator-Activated Receptor Delta Agonist (PPAR-δ) and Selective Androgen Receptor Modulator (SARM) Abuse: Clinical, Analytical and Biological Data in a Case Involving a Poisonous Combination of GW1516 (Cardarine) and MK2866 (Ostarine). Toxics 2021, 9, 251. [Google Scholar] [CrossRef]
- Barish, G.D.; Narkar, V.A.; Evans, R.M. PPAR delta: A dagger in the heart of the metabolic syndrome. J. Clin. Investig. 2006, 116, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Coll, T.; Alvarez-Guardia, D.; Barroso, E.; Gómez-Foix, A.M.; Palomer, X.; Laguna, J.C.; Vázquez-Carrera, M. Activation of peroxisome proliferator-activated receptor-δ by GW501516 prevents fatty acid-induced nuclear factor-κB activation and insulin resistance in skeletal muscle cells. Endocrinology 2010, 151, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Paw, M.; Wnuk, D.; Madeja, Z.; Michalik, M. PPARδ Agonist GW501516 Suppresses the TGF-β-Induced Profibrotic Response of Human Bronchial Fibroblasts from Asthmatic Patients. Int. J. Mol. Sci. 2023, 24, 7721. [Google Scholar] [CrossRef] [PubMed]
- Kintz, P.; Ameline, A.; Gheddar, L.; Raul, J.S. Testing for G W501516 (cardarine) in human hair using LC/MS- MS and confirmation by LC/HRMS. Drug Test Anal. 2020, 12, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Weihrauch, M.; Handschin, C. Pharmacological targeting of exercise adaptations in skeletal muscle: Benefits and pitfalls. Biochem. Pharmacol. 2018, 147, 211–220. [Google Scholar] [CrossRef]
- Trevisiol, S.; Moulard, Y.; Delcourt, V.; Jaubert, M.; Boyer, S.; Tendon, S.; Haryouli, H.; Taleb, W.; Caroff, M.; Chabot, B.; et al. Comprehensive characterization of peroxisome proliferator activated receptor-δ agonist GW501516 for horse doping control analysis. Drug Test. Anal. 2021, 13, 1191–1202. [Google Scholar] [CrossRef]
- Bernstein, J. Polymorphism in Molecular Crystals; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Vippagunta, S.R.; Brittain, H.G.; Grant, D.J.W. Crystalline solids. Adv. Drug Deliv. Rev. 2001, 48, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, D.; Sergeev, G. The Polymorphism of Drugs: New Approaches to the Synthesis of Nanostructured Polymorphs. Pharmaceutics 2020, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- CrysAlis PRO. Rigaku Oxford Diffraction; CrysAlis PRO: Yarnton, UK, 2015. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer model energies and energy frameworks: Extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef]
- Allen, F.H.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. International Tables for Crystallography, 3rd ed.; Prince, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 790–811. [Google Scholar]
- Meden, A. Crystal structure solution from powder diffraction data—State of the art and perspectives. Croat. Chem. Acta 1998, 71, 615–633. [Google Scholar]
- Neumann, M.A. Crystal X-Cell: A novel indexing algorithm for routine tasks and difficult cases. J. Appl. Cryst. 2003, 36, 356–365. [Google Scholar] [CrossRef]
- Materials Studio, v8.0.0.843; Dassault Systèmes BIOVIA: San Diego, CA, USA, 2014.
- Favre-Nicolin, V.; Cerny, R. FOX, free objects for crystallography’: A modular approach to ab initio structure determination from powder diffraction. Biol. Mass. Spectrom. 2002, 35, 734–743. [Google Scholar] [CrossRef]
- Caglioti, G.; Paoletti, A.; Ricci, F.P. Choice of collimators for a crystal spectrometer for neutron diffraction. Nucl. Instrum. 1958, 3, 223–228. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Drebushchak, V.A.; McGregor, L.; Rychkov, D.A. Cooling rate “window” in the crystallization of metacetamol form II. J. Therm. Anal. Calorim. 2017, 127, 1807–1814. [Google Scholar] [CrossRef]
- Dubok, A.S.; Rychkov, D.A. Relative Stability of Pyrazinamide polymorphs Revisited: A Computational Study of Bending and Brittle Forms Phase Transitions in a Broad Temperature Range. Crystals 2023, 13, 617. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]



| Identification Code | GW-1 | GW-2 | GW-3 | GW-4 |
|---|---|---|---|---|
| Empirical formula | C21H18F3NO3S2 | C21H18F3NO3S2 | C21H18F3NO3S2 | C21H18F3NO3S2 |
| Formula weight | 453.48 | 453.48 | 453.48 | 453.48 |
| Temperature/K | 293(2) | 293(2) | 293(2) | 293(2) |
| Crystal system | monoclinic | monoclinic | monoclinic | orthorhombic |
| Space group | P21/c | P21/n | C2/c | Pca21 |
| a/Å | 4.75852(13) | 10.92669(10) | 33.1318(18) | 35.1542(3) |
| b/Å | 19.4117(6) | 9.68349(11) | 4.5002(2) | 4.906 |
| c/Å | 22.6359(6) | 19.7719(2) | 29.4838(11) | 24.1208(3) |
| α/° | 90 | 90 | 90 | 90 |
| β/° | 94.909(3) | 98.2167(10) | 107.691(5) | 90 |
| γ/° | 90 | 90 | 90 | 90 |
| Volume/Å3 | 2083.23(11) | 2070.56(4) | 4188.1(4) | 4159.78(6) |
| Z | 4 | 4 | 8 | 8 |
| Z’ | 1 | 1 | 1 | 2 |
| ρcalc g/cm3 | 1.446 | 1.455 | 1.438 | 1.447 |
| μ/mm−1 | 2.769 | 2.785 | 2.754 | 2.773 |
| F(000) | 936.0 | 936.0 | 1872.0 | 1872.0 |
| Crystal size/mm3 | 0.2 × 0.1 × 0.1 | 0.25 × 0.2 × 0.15 | 0.3 × 0.05 × 0.04 | 0.25 × 0.15 × 0.10 |
| Radiation | CuKα (λ = 1.54184) | CuKα (λ = 1.54184) | CuKα (λ = 1.54184) | CuKα (λ = 1.54184) |
| 2Θ range/° | 6.008 to 141.96 | 8.758 to 141.298 | 6.294 to 149.676 | 6.222 to 141.31 |
| Index ranges | −5 ≤ h ≤ 5, −23 ≤ k ≤ 22, −27 ≤ l ≤ 27 | −13 ≤ h ≤ 13, −11 ≤ k ≤ 11, −22 ≤ l ≤ 24 | −38 ≤ h ≤ 40, −5 ≤ k ≤ 5, −36 ≤ l ≤ 26 | −42 ≤ h ≤ 42, −5 ≤ k ≤ 5, −27 ≤ l ≤ 29 |
| Reflections collected | 12,876 | 29,259 | 7093 | 59,180 |
| Independent reflections | 3951 [Rint = 0.0281, Rsigma = 0.0261] | 3936 [Rint = 0.0247, Rsigma = 0.0130] | 3938 [Rint = 0.0224, Rsigma = 0.0365] | 7692 [Rint = 0.0498, Rsigma = 0.0239] |
| Data/restraints/parameters | 3951/0/277 | 3936/1/274 | 3938/1/277 | 7692/3/553 |
| Goodness-of-fit on F2 | 1.067 | 1.071 | 1.188 | 1.035 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0481, wR2 = 0.1241 | R1 = 0.0556, wR2 = 0. 1968 | R1 = 0.0538, wR2 = 0.1620 | R1 = 0.0514, wR2 = 0.1377 |
| Final R indexes [all data] | R1 = 0.0641, wR2 = 0.1360 | R1 = 0.0625, wR2 = 0.2041 | R1 = 0.0845, wR2 = 0.1892 | R1 = 0.0553, wR2 = 0.1435 |
| Largest diff. peak/hole/e Å−3 | 0.35/−0.24 | 1.04/−0.59 | 0.54/−0.34 | 0.98/−0.25 |
| Flack parameter | 0.54(2) |
| GW-1 | GW-2 | GW-3 | GW-4 | GW-5 | |
|---|---|---|---|---|---|
| Plane P1 and RMSD (Å) | 0.039 Å | 0.087 Å | 0.148 Å | 0.046 Å/ 0.052 Å | 0.091 Å/ 0.053 Å |
| Plane P2 and RMSD (Å) | 0.016 Å | 0.018 Å | 0.01 Å | 0.07 Å/ 0.011 Å | 0.016 Å/ 0.025 Å |
| Angle between planes P1 and P2 | 12.0° | 40.6° | 4.15° | 9.2°/ 8.6° | 41.6°/ 85.2° |
| Torsion angle [C9-C12-S2-C13] (°) | −164.5° | 72.4° | −163.0° | 154.9°/ −151.3° | 174.6°/ −75.0° |
| Polymorph | GW-5 |
|---|---|
| Chemical formula | C21H18F3NO3S2 |
| Formula weight (g/mol) | 453.48 |
| Crystal system | triclinic |
| Space group | P-1 |
| Z | 4 |
| Z’ | 2 |
| a (Å) | 4.9088(7) |
| b (Å) | 21.269(3) |
| c (Å) | 21.312(4) |
| A (°) | 94.476(13) |
| Β (°) | 96.387(13) |
| γ (°) | 92.795(12) |
| V (Å3) | 2200.82 |
| Rwp (%) | 0.0751 |
| Rp (%) | 0.0547 |
| ρcalc (g/cm3) | 1.369 |
| Polymorph | Temperature (°C) | Enthalpy (kJ/mol) |
|---|---|---|
| GW-1 | 82 | −13.60 |
| 142 | −40.81 | |
| GW-2 | 137 | −40.81 |
| GW-3 | 139 | −45.34 |
| GW-4 | 131 | −9.06 |
| GW-5 | 115 | −9.06 |
| 139 | −18.13 | |
| 145 | −4.53 |
| Crystal | Eele (kJ/mol) | Epol (kJ/mol) | Edisp (kJ/mol) | Erep (kJ/mol) | Elatt (kJ/mol) | Melting Point (°C) |
|---|---|---|---|---|---|---|
| GW-1 | −77.1 | −21.9 | −189.4 | 57.4 | −231.0 | 142 |
| GW-2 | −114.1 | −18.2 | −183.5 | 92.3 | −223.5 | 137 |
| GW-3 | −91.9 | −19.9 | −176.8 | 51.8 | −236.8 | 139 |
| GW-4 | −63.9 | −14.6 | −171.7 | 54.5 | −195.7 | 131 |
| GW-5 | −84.4 | −19.7 | −151.6 | 45.6 | −210.1 | 139 and 145 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turza, A.; Pascuta, P.; Muresan-Pop, M.; Mare, L.; Borodi, G.; Popescu, V. Five Novel Polymorphs of Cardarine/GW501516 and Their Characterization by X-ray Diffraction, Computational Methods, Thermal Analysis and a Pharmaceutical Perspective. Pharmaceutics 2024, 16, 623. https://doi.org/10.3390/pharmaceutics16050623
Turza A, Pascuta P, Muresan-Pop M, Mare L, Borodi G, Popescu V. Five Novel Polymorphs of Cardarine/GW501516 and Their Characterization by X-ray Diffraction, Computational Methods, Thermal Analysis and a Pharmaceutical Perspective. Pharmaceutics. 2024; 16(5):623. https://doi.org/10.3390/pharmaceutics16050623
Chicago/Turabian StyleTurza, Alexandru, Petru Pascuta, Marieta Muresan-Pop, Liviu Mare, Gheorghe Borodi, and Violeta Popescu. 2024. "Five Novel Polymorphs of Cardarine/GW501516 and Their Characterization by X-ray Diffraction, Computational Methods, Thermal Analysis and a Pharmaceutical Perspective" Pharmaceutics 16, no. 5: 623. https://doi.org/10.3390/pharmaceutics16050623
APA StyleTurza, A., Pascuta, P., Muresan-Pop, M., Mare, L., Borodi, G., & Popescu, V. (2024). Five Novel Polymorphs of Cardarine/GW501516 and Their Characterization by X-ray Diffraction, Computational Methods, Thermal Analysis and a Pharmaceutical Perspective. Pharmaceutics, 16(5), 623. https://doi.org/10.3390/pharmaceutics16050623










