Metformin-Mediated Improvement in Solubility, Stability, and Permeability of Nonsteroidal Anti-Inflammatory Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Salt Synthesis
2.3. Characterization
2.3.1. Single Crystal X-ray Diffraction (SCXRD) Analysis
2.3.2. Powder X-ray Diffraction (PXRD) Analysis
2.3.3. Differential Scanning Calorimetry (DSC) Analysis
2.3.4. Infrared Spectroscopy (IR) Analysis
2.4. Solubility Experiments
2.5. Intrinsic Dissolution Rate (IDR) Experiments
2.6. Permeability Experiments
2.7. Dynamic Vapor Sorption (DVS) Experiment
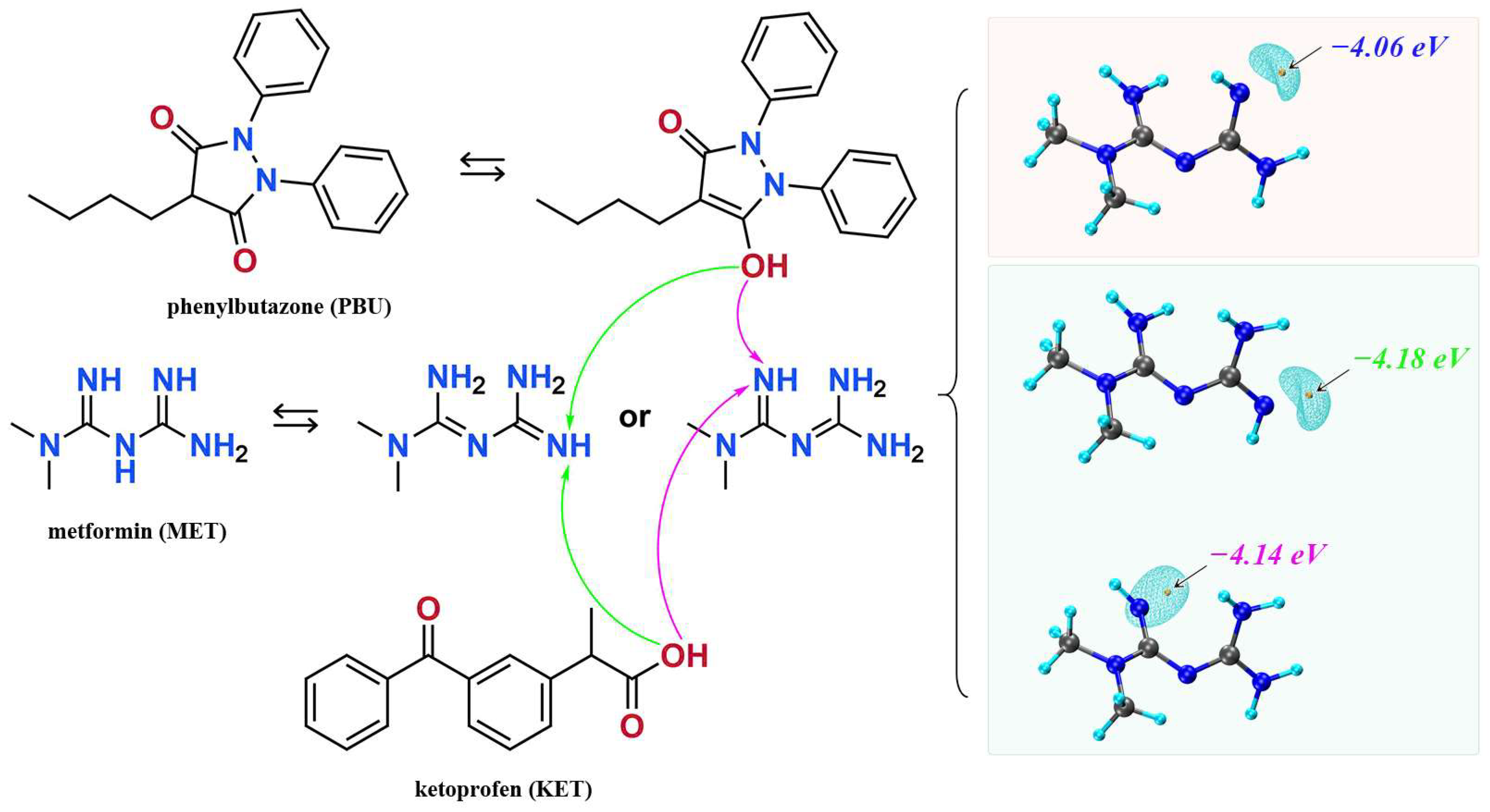
2.8. Theoretical Calculation
2.9. In Vivo Pharmacokinetic Study
3. Results and Discussions
3.1. Characterization Analysis
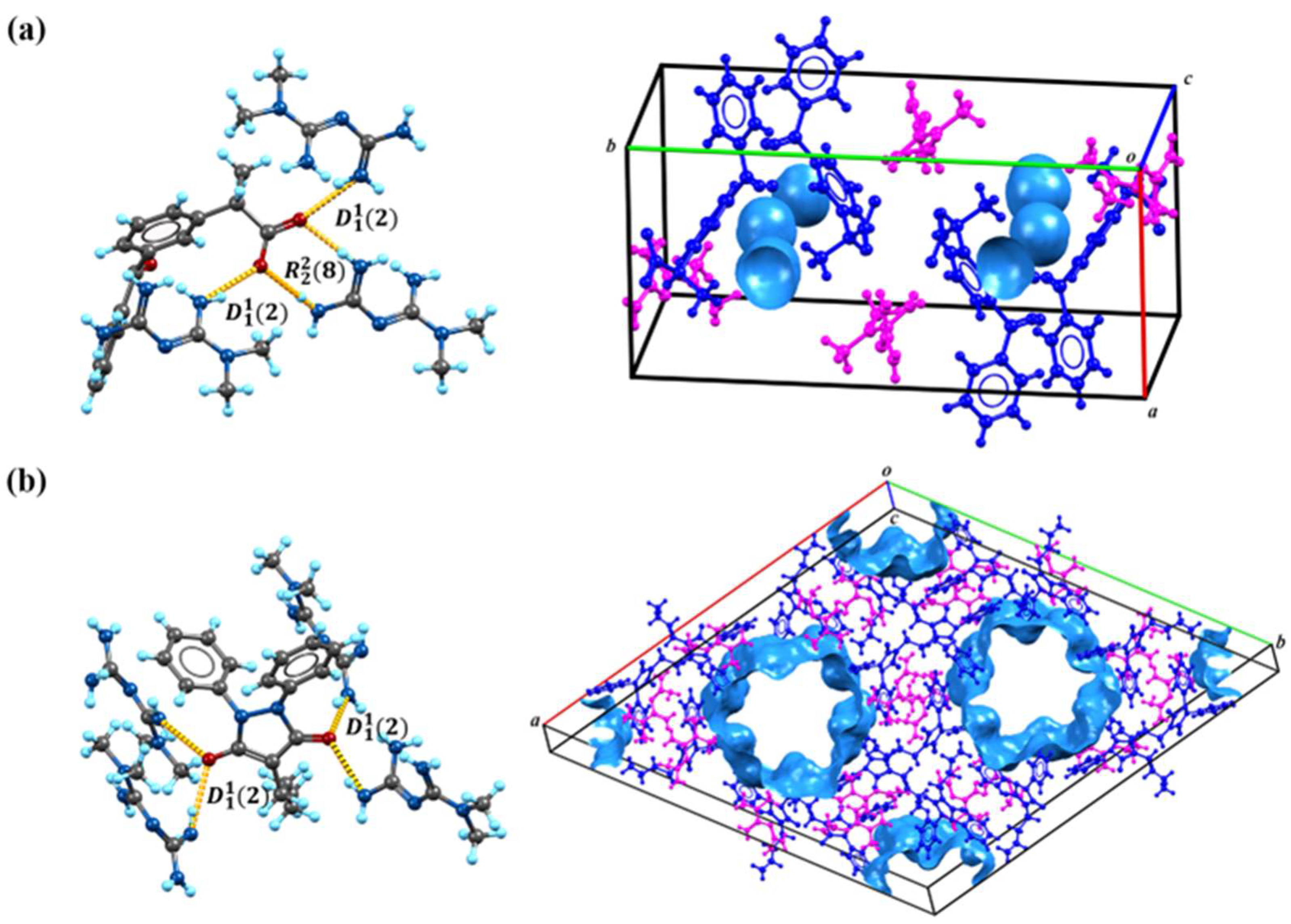
3.1.1. SCXRD Analysis
3.1.2. PXRD Analysis
3.1.3. DSC Analysis
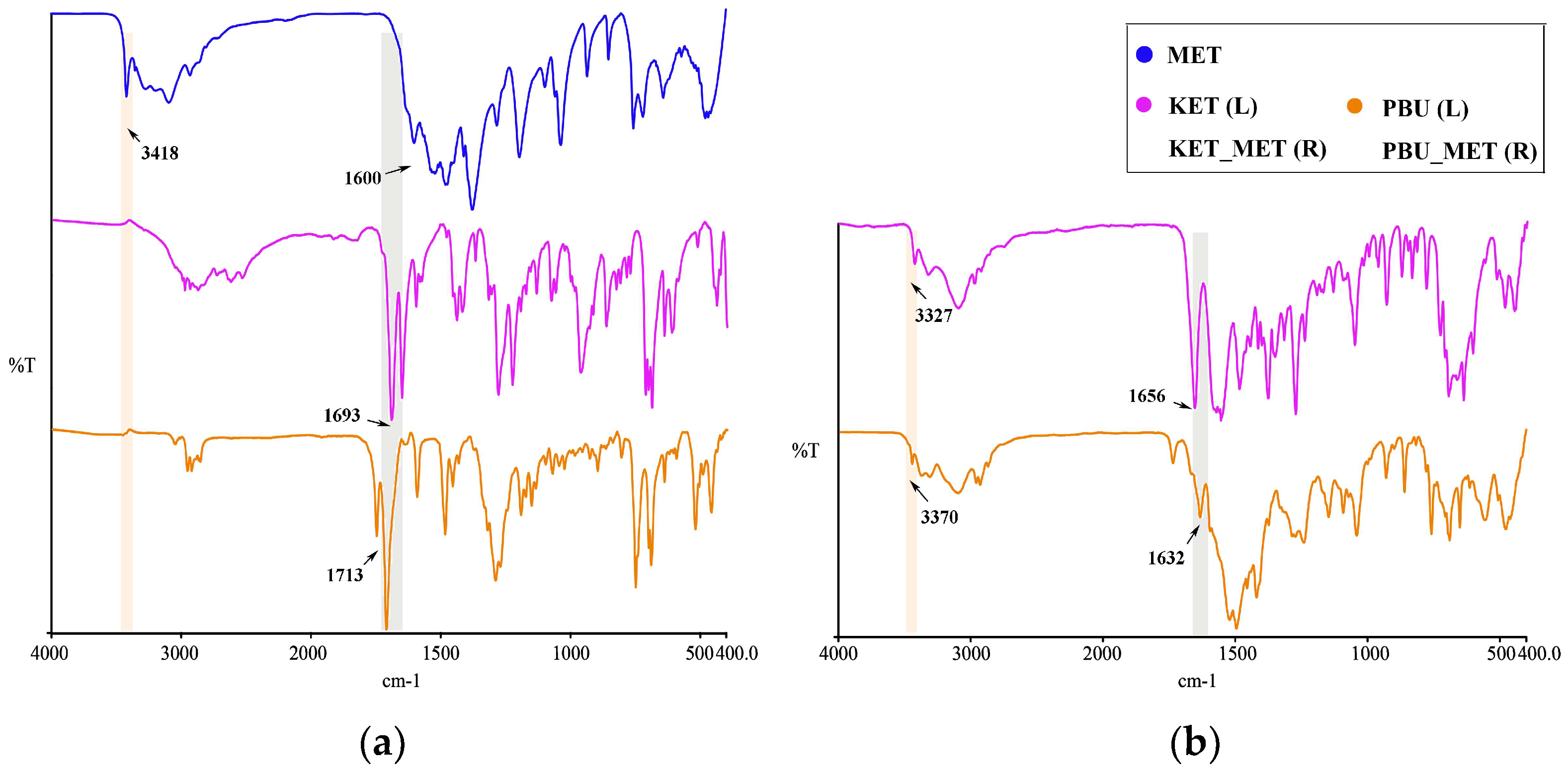
3.1.4. IR Analysis
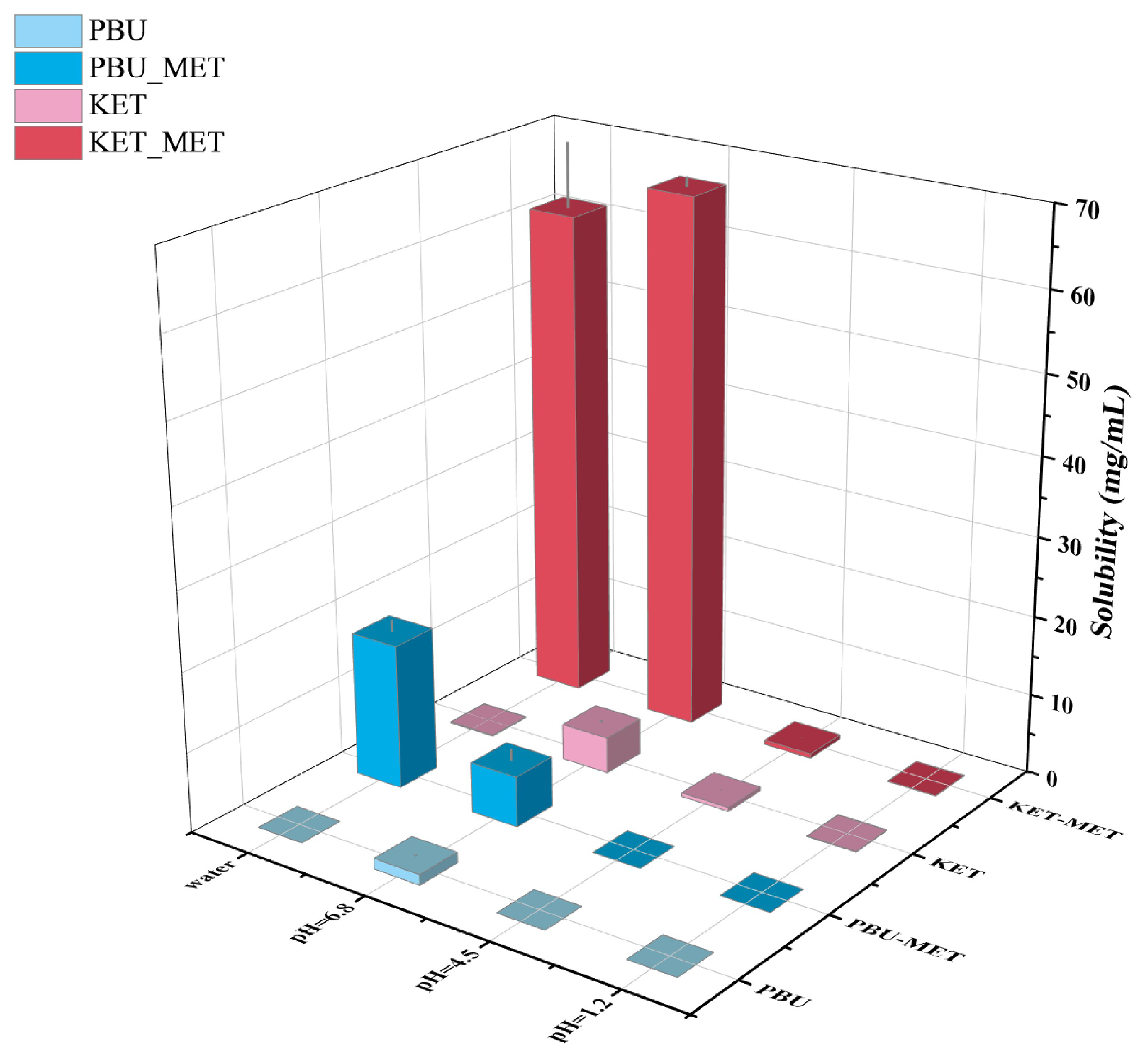
3.2. Solubility Studies
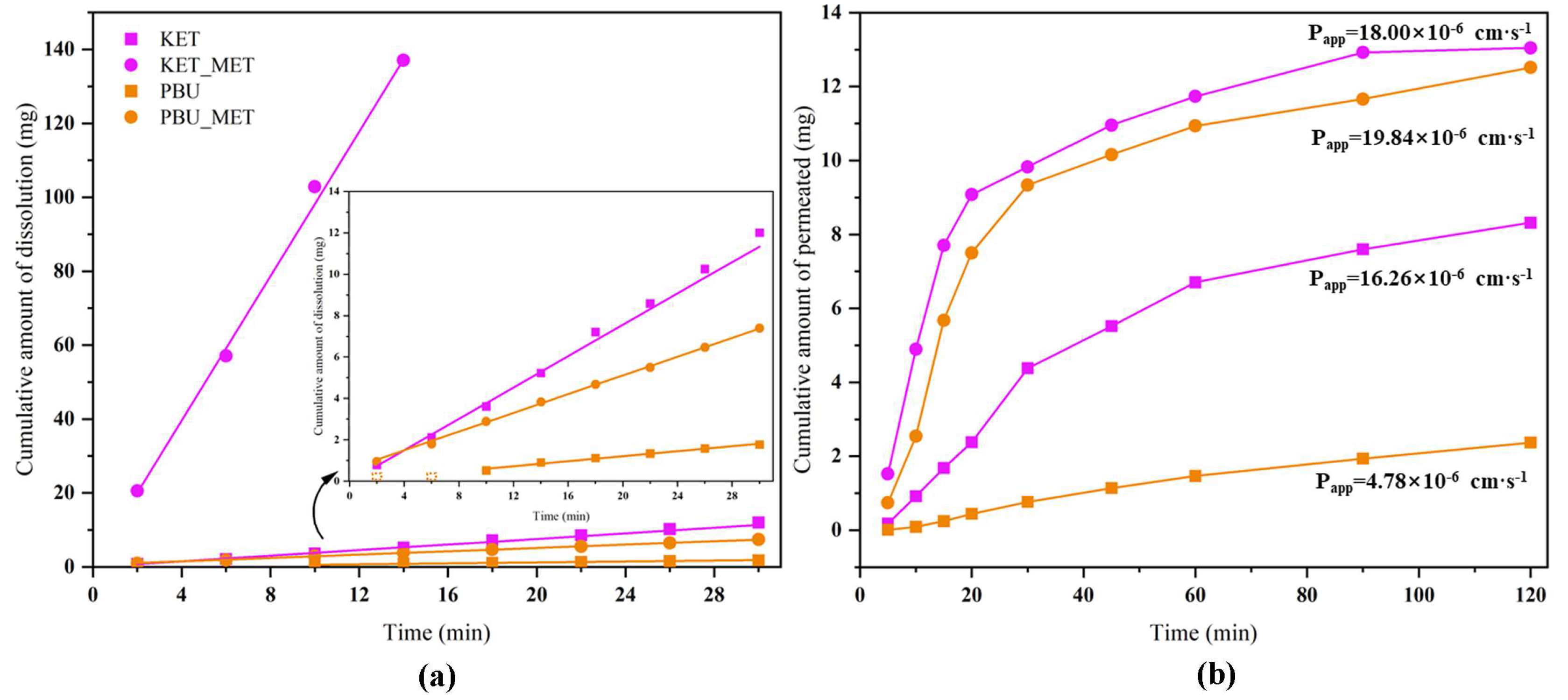
3.3. Intrinsic Dissolution Rate (IDR) Studies
3.4. Permeability Studies
3.5. DVS Studies
3.6. In Vivo Pharmacokinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schultheiss, N.; Newman, A. Pharmaceutical cocrystals and their physicochemical properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Tiţa, D.; Fuliaş, A.; Tiţa, B. Thermal stability of ketoprofen—Active substance and tablets: Part 1. Kinetic study of the active substance under non-isothermal conditions. J. Therm. Anal. Calorim. 2011, 105, 501–508. [Google Scholar] [CrossRef]
- Akbari, J.; Saeedi, M.; Morteza-Semnani, K.; Rostamkalaei, S.S.; Asadi, M.; Asare-Addo, K.; Nokhodchi, A. The Design of Ketoprofen Solid Lipid Nanoparticles to Target Skin Layers. Colloids Surf. B 2016, 145, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chávez, J.J.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. In Vivo Skin Permeation of Sodium Ketoprofen Formulated in Pluronic F-127 Gels: Effect of Azone and Transcutol. Drug Dev. Ind. Pharm. 2005, 31, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Patel, N.; Lin, S. Solubility and dissolution enhancement strategies: Current understanding and recent trends. Drug Dev. Ind. Pharm. 2015, 41, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Fahr, A.; Liu, X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin. Drug Deliv. 2007, 4, 403–416. [Google Scholar] [CrossRef]
- Göke, K.; Lorenz, T.; Repanas, A.; Schneider, F.; Steiner, D.; Baumann, K.; Bunjes, H.; Andreas, D.; Finke, J.H.; Glasmacher, B.; et al. Novel strategies for the formulation and processing of poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2018, 126, 40–56. [Google Scholar] [CrossRef]
- Nascimento, A.L.; Fernandes, R.P.; Charpentier, M.D.; ter Horst, J.H.; Caires, F.J.; Chorilli, M. Co-crystals of non-steroidal anti-inflammatory drugs (NSAIDs): Insight toward formation, methods, and drug enhancement. Particuology 2021, 58, 227–241. [Google Scholar] [CrossRef]
- Lanas, A. Nonsteroidal antiinflammatory drugs and cyclooxygenase inhibition in the gastrointestinal tract: A trip from peptic ulcer to colon cancer. Am. J. Med. Sci. 2009, 338, 96–106. [Google Scholar] [CrossRef]
- Wong, R.S. Role of nonsteroidal anti-inflammatory drugs (NSAIDs) in cancer prevention and cancer promotion. Adv. Pharmacol. Pharm. Sci. 2019, 2019, 3418975. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Chen, T.; Wang, L.; An, Q.; Jin, Y.; Yang, D.Z.; Zhang, L.; Chen, T.; Du, G.H.; Lu, Y. Two novel co-crystals of Ketoprofen: Comparison of stability, solubility and intermolecular interaction. Pharmaceuticals 2022, 15, 807. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.; Azim, Y. Challenges and opportunities of pharmaceutical cocrystals: A focused review on non-steroidal anti-inflammatory drugs. RSC Med. Chem. 2021, 12, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Tiţa, B.; Fuliaş, A.; Bandur, G.; Rusu, G.; Tiţa, D. Thermal stability of ibuprofen. Kinetic study under non-isothermal conditions. Rev. Roum. Chim. 2010, 55, 553–558. [Google Scholar]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Acebedo-Martínez, F.J.; Domínguez-Martín, A.; Alarcón-Payer, C.; Garcés-Bastida, C.; Verdugo-Escamilla, C.; Gómez-Morales, J.; Choquesillo-Lazarte, D. Metformin-NSAIDs Molecular Salts: A Path towards Enhanced Oral Bioavailability and Stability. Pharmaceutics 2023, 15, 449. [Google Scholar] [CrossRef] [PubMed]
- Flory, J.; Lipska, K. Metformin in 2019. JAMA 2019, 321, 1926–1927. [Google Scholar] [CrossRef]
- Liu, H.J.; Nie, J.J.; Chan, H.S.; Zhang, H.L.; Li, L.; Lin, H.Q.; Tong, H.H.Y.; Ma, A.D.; Zhou, Z.Z. Phase solubility diagrams and energy surface calculations support the solubility enhancement with low hygroscopicity of bergenin: 4-aminobenzamide (1:1) cocrystal. Int. J. Pharm. 2021, 60, 120537. [Google Scholar] [CrossRef]
- Mohamed, S.; Tocher, D.A.; Vickers, M.; Karamertzanis, P.G.; Price, S.L. Salt or cocrystal? A new series of crystal structures formed from simple pyridines and carboxylic acids. Cryst. Growth Des. 2009, 9, 2881–2889. [Google Scholar] [CrossRef]
- Wu, Y.H.; Hao, X.J.; Li, J.T.; Guan, A.Y.; Zhou, Z.Z.; Guo, F. New insight into improving the solubility of poorly soluble drugs by preventing the formation of their hydrogen-bonds: A case of dapsone salts with camphorsulfonic and 5-sulfosalicylic acid. CrystEngComm 2021, 23, 6191–6198. [Google Scholar] [CrossRef]
- Yang, D.Z.; Wang, L.; Yuan, P.H.; An, Q.; Su, B.; Yu, M.C.; Chen, T.; Hu, K.; Zhang, L.; Lu, Y.; et al. Cocrystal virtual screening based on the XGBoost machine learning model. Chin. Chem. Lett. 2022, 34, 107964. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J. Acid–base crystalline complexes and the pKa rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Sathisaran, I.; Dalvi, S.V. Engineering cocrystals of poorly water-soluble drugs to enhance dissolution in aqueous medium. Pharmaceutics 2018, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Esam, Z. A proposed mechanism for the possible therapeutic potential of Metformin in COVID-19. Diabetes Res. Clin. Pract. 2020, 167, 108282. [Google Scholar] [CrossRef]
- Feng, W.Q.; Wang, L.Y.; Gao, J.; Zhao, M.Y.; Li, Y.T.; Wu, Z.Y.; Yan, C.W. Solid state and solubility study of a potential anticancer drug-drug molecular salt of diclofenac and metformin. J. Mol. Struct. 2021, 1234, 130166. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Glomme, A.; März, J.; Dressman, J.B. Comparison of a Miniaturized Shake-Flask Solubility Method with Automated Potentiometric Acid/Base Titrations and Calculated Solubilities. J. Pharm. Sci. 2005, 94, 1–16. [Google Scholar] [CrossRef]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 75, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian; Version 16; Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Yang, D.Z.; Wang, H.J.; Liu, Q.W.; Yuan, P.H.; Chen, T.; Zhang, L.; Yang, S.Y.; Zhou, Z.Z.; Lu, Y.; Du, G.H. Structural landscape on a series of rhein: Berberine cocrystal salt solvates: The formation, dissolution elucidation from experimental and theoretical investigations. Chin. Chem. Lett. 2022, 33, 3207–3211. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Mercury, version 2023. 1 Windows; Cambridge Crystallographic Database Centre: Cambridge, UK, 2023. [Google Scholar]
- Singh, T.P.; Vijayan, M. Structural studies of analgesics and their interactions. Part 4. Crystal structures of phenylbutazone and a 2:1 complex between phenylbutazone and piperazine. J. Chem. Soc. Perkin Trans. 2 1977, 5, 693–699. [Google Scholar] [CrossRef]
- Tiwari, M.; Chawla, G.; Bansal, A.K. Quantification of olanzapine polymorphs using powder X-ray diffraction technique. J. Pharm. Biomed. Anal. 2007, 43, 865–872. [Google Scholar] [CrossRef]
- Qiao, N.; Li, M.; Schlindwein, W.; Malek, N.; Davies, A.; Trappitt, G. Pharmaceutical cocrystals: An overview. Int. J. Pharm. 2011, 419, 1–11. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring nature and predicting strength of hydrogen bonds: A correlation analysis between atoms-in-molecules descriptors, binding energies, and energy components of symmetry-adapted perturbation theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef]
- Maeda, H.; Ogawa, Y.; Nakayama, H. Inclusion complexes of melatonin with modified cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 217–224. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Natarajan, R.K.; Renganayaki, V.; Natarajan, S. Vibrational spectra and thermodynamic analysis of metformin. Indian J. Pure Appl. Phys. 2006, 44, 495–500. [Google Scholar]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Babu, N.J.; Nangia, A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.Y.; Liu, F.; Li, Y.T.; Wu, Z.Y.; Yan, C.W. Simultaneously enhancing the in vitro/in vivo performances of acetazolamide using proline as a zwitterionic coformer for cocrystallization. CrystEngComm 2019, 21, 3064–3073. [Google Scholar] [CrossRef]
- An, Q.; Li, N.; Zhao, Z.H.; Wang, N.Q.; Wang, X.Y.; Yang, X.Y.; Yang, D.Z.; Zhang, L.; Lu, Y.; Du, G.H.; et al. Two Novel Metformin Carboxylate Salts and the Accidental Discovery of Two 1, 3, 5-Triazine Antihyperglycemic Agent. ACS Omega 2023, 8, 48028–48041. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Miller, J.M. The solubility–permeability interplay and its implications in formulation design and development for poorly soluble drugs. AAPS J. 2012, 14, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.M.; Liu, L.; Bu, F.Z.; Li, Y.T.; Yan, C.W.; Wu, Z.Y. A novice cocrystal nanomicelle formulation of 5-fluorouracil with proline: The design, self-assembly and in vitro/vivo biopharmaceutical characteristics. Int. J. Pharm. 2022, 617, 121635. [Google Scholar] [CrossRef]
- Ji, X.; Wu, D.; Li, C.; Li, J.; Sun, Q.; Chang, D.; Yin, Q.; Zhou, L.; Xie, C.; Gong, J.; et al. Enhanced solubility, dissolution, and permeability of abacavir by salt and cocrystal formation. Cryst. Growth Des. 2021, 22, 428–440. [Google Scholar] [CrossRef]
- Jung, S.J.; Choi, S.O.; Um, S.Y.; Kim, J.I.; Choo, H.Y.P.; Choi, S.Y.; Chung, S.Y. Prediction of the permeability of drugs through study on quantitative structure–permeability relationship. J. Pharm. Biomed. Anal. 2006, 41, 469–475. [Google Scholar] [CrossRef]
- Emami, S.; Siahi-Shadbad, M.; Adibkia, K.; Barzegar-Jalali, M. Recent advances in improving oral drug bioavailability by cocrystals. BioImpacts BI 2018, 8, 305. [Google Scholar] [CrossRef]
- Yang, Y.H.; Yu, M.C.; Ren, L.W.; An, Q.; Li, W.; Yang, H.; Zhang, Y.Z.; Zhang, S.; Hao, Y.; Du, G.H.; et al. Design, synthesis and characterization of a novel multicomponent salt of bexarotene with metformin and application in ameliorating psoriasis with T2DM. Int. J. Pharm. 2023, 646, 123501. [Google Scholar] [CrossRef]
- Zhou, W.X.; Zhao, H.W.; Chen, H.H.; Zhang, Z.Y.; Chen, D.Y. Characterization of drug-drug salt forms of metformin and aspirin with improved physicochemical properties. CrystEngComm 2019, 21, 3770–3773. [Google Scholar] [CrossRef]
- Yu, M.C.; Liang, M.D.; An, Q.; Wang, W.W.; Zhang, B.X.; Yang, S.Y.; Zhou, J.; Yang, X.Y.; Yang, D.Z.; Zhang, L.; et al. Versatile Solid Modifications of Multicomponent Pharmaceutical Salts: Novel Metformin–Rhein Salts Based on Advantage Complementary Strategy Design. Pharmaceutics 2023, 15, 1196. [Google Scholar] [CrossRef] [PubMed]




| KET-MET | PBU-MET | |
|---|---|---|
| Formula | C16H13O3·C4H12N5 | C19H19N2O2·C4H12N5 |
| Crystal size (mm) | 0.20 × 0.20 × 0.20 | 0.20 × 0.20 × 0.20 |
| Molecular weight | 383.45 | 436.74 |
| Temperature (K) | 293 (2) | 293 (2) |
| Crystal system | monoclinic | trigonal |
| Space group | ||
| α (Å) | 10.513 (1) | 40.224 (1) |
| b (Å) | 22.394 (2) | 40.224 (1) |
| c (Å) | 8.807 (1) | 9.958 (1) |
| a (deg) | 90 | 90 |
| β (deg) | 93.628 (5) | 90 |
| γ (deg) | 90 | 120 |
| Volume (Å3) | 2069.45 (19) | 13,953.5 (7) |
| Z | 4 | 18 |
| Density (g/cm3) | 1.231 | 0.936 |
| R1 (I 2σ(I)) | 0.0656 | 0.0624 |
| wR2 (I 3σ(I)) | 0.1798 | 0.1770 |
| Goodness-of-fit on F2 | 1.039 | 1.058 |
| Completeness (%) | 99.9% | 98.9% |
| CCDC deposition no. | 2,324,339 | 2,324,340 |
| Solubility (mg/mL) | ||||
|---|---|---|---|---|
| Compound | pH 1.2 Buffer | pH 4.5 Buffer | pH 6.8 Buffer | Water |
| KET KET-MET | 0.076 ± 0.0358 | 0.494 ± 0.0394 | 4.555 ± 0.1932 | 0.118 ± 0.0529 |
| 0.049 ± 0.0433 | 0.567 ± 0.0278 | 66.987 ± 1.2080 | 61.668 ± 8.0857 | |
| PBU PBU-MET | 0.006 ± 0.0022 | 0.001 ± 0.0006 | 1.326 ± 0.0849 | 0.005 ± 0.0041 |
| 0.007 ± 0.0007 | 0.010 ± 0.0075 | 6.327 ± 1.3960 | 18.152 ± 1.4396 | |
| Parameter | KET | KET-MET | PBU | PBU-MET |
|---|---|---|---|---|
| AUC0-t (mg/L*h) | 1150.30 ± 133.78 | 1214.72 ± 305.84 | 1593.36 ± 457.03 | 1532.80 ± 501.89 |
| AUC0-∞ (mg/L*h) | 1995.02 ± 1011.62 | 2273.14 ± 155.47 | 1631.16 ± 435.64 | 1570.51 ± 504.48 |
| MRT0-t (h) | 12.87 ± 0.90 | 11.27 ± 1.22 | 9.75 ± 1.68 | 9.78 ± 1.74 |
| MRT0-∞ (h) | 26.16 ± 10.27 | 25.01 ± 2.14 | 10.25 ± 2.07 | 10.30 ± 2.41 |
| t1/2z (h) | 13.04 ± 8.75 | 15.90 ± 1.65 | 3.07 ± 120 | 3.59 ± 1.38 |
| Tmax (h) | 8.00 ± 2.00 | 0.67 ± 0.29 | 8.00 ± 2.00 | 9.60 ± 1.67 |
| CLz/F (L/h/kg) | 0.06 ± 0.02 | 0.07 ± 0.01 | 0.10 ± 0.04 | 0.07 ± 0.02 |
| Vz/F (L/kg) | 0.90 ± 0.34 | 1.519 ± 0.05 | 0.30 ± 0.20 | 0.49 ± 0.15 |
| Cmax (mg/L) | 112.00 ± 17.05 | 125.77 ± 39.89 | 164.76 ± 43.81 | 149.04 ± 28.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Q.; Xing, C.; Wang, Z.; Li, S.; Wang, W.; Yang, S.; Kong, L.; Yang, D.; Zhang, L.; Du, G.; et al. Metformin-Mediated Improvement in Solubility, Stability, and Permeability of Nonsteroidal Anti-Inflammatory Drugs. Pharmaceutics 2024, 16, 382. https://doi.org/10.3390/pharmaceutics16030382
An Q, Xing C, Wang Z, Li S, Wang W, Yang S, Kong L, Yang D, Zhang L, Du G, et al. Metformin-Mediated Improvement in Solubility, Stability, and Permeability of Nonsteroidal Anti-Inflammatory Drugs. Pharmaceutics. 2024; 16(3):382. https://doi.org/10.3390/pharmaceutics16030382
Chicago/Turabian StyleAn, Qi, Cheng Xing, Zhipeng Wang, Shuang Li, Wenwen Wang, Shiying Yang, Linglei Kong, Dezhi Yang, Li Zhang, Guanhua Du, and et al. 2024. "Metformin-Mediated Improvement in Solubility, Stability, and Permeability of Nonsteroidal Anti-Inflammatory Drugs" Pharmaceutics 16, no. 3: 382. https://doi.org/10.3390/pharmaceutics16030382
APA StyleAn, Q., Xing, C., Wang, Z., Li, S., Wang, W., Yang, S., Kong, L., Yang, D., Zhang, L., Du, G., & Lu, Y. (2024). Metformin-Mediated Improvement in Solubility, Stability, and Permeability of Nonsteroidal Anti-Inflammatory Drugs. Pharmaceutics, 16(3), 382. https://doi.org/10.3390/pharmaceutics16030382








