Effect of Tight Junction-Modulating FCIGRL-Modified Peptides on the Intestinal Absorption of Doxorubicin in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Preparation of Doxorubicin Solutions for the Animal Study
2.4. Intraduodenal Administration of Doxorubicin Solutions to Rats
2.5. HPLC-Fluorescence Conditions
2.6. Data Analysis
3. Results
3.1. Preparation of FCIGRL-Modified Peptides
3.2. Intraduodenal Administration of Doxorubicin with FCIGRL-Modified Peptides to Rats
3.3. Effect of Pep3 and Pep4 with Levan on the Permeability of Doxorubicin
3.4. Effect of Pep2 with Levan and BC on the Permeability of Doxorubicin
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Slingerland, M.; Guchelaar, H.J.; Gelderblom, H. Liposomal drug formulations in cancer therapy: 15 years along the road. Drug Discov. Today 2012, 17, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, D.R.; Sharma, G.; Beniwal, V.; Ravi Kumar, M.N. Design of biodegradable nanoparticles for oral delivery of doxorubicin: In vivo pharmacokinetics and toxicity studies in rats. Pharm. Res. 2009, 26, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Pathomthongtaweechai, N.; Muanprasat, C. Potential Applications of Chitosan-Based Nanomaterials to Surpass the Gastrointestinal Physiological Obstacles and Enhance the Intestinal Drug Absorption. Pharmaceutics 2021, 13, 887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benival, D.M.; Devarajan, P.V. Lipomer of doxorubicin hydrochloride for enhanced oral bioavailability. Int. J. Pharm. 2012, 423, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Patil, S.R.; Swarnakar, N.K.; Agrawal, A.K. Oral delivery of doxorubicin using novel polyelectrolyte-stabilized liposomes (layersomes). Mol. Pharm. 2012, 9, 2626–2635. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Cho, H.J.; Kim, J.S.; Shim, C.K.; Chung, S.J.; Oak, M.H.; Yoon, I.S.; Kim, D.D. The limited intestinal absorption via paracellular pathway is responsible for the low oral bioavailability of doxorubicin. Xenobiotica 2013, 43, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, M.K.; Song, I.S. Recent Advances in Doxorubicin Formulation to Enhance Pharmacokinetics and Tumor Targeting. Pharmaceuticals 2023, 16, 802. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenthal, R.; Heydt, M.S.; Amasheh, M.; Stein, C.; Fromm, M.; Amasheh, S. Analysis of absorption enhancers in epithelial cell models. Ann. N. Y. Acad. Sci. 2012, 1258, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Deli, M.A. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim. Biophys. Acta 2009, 1788, 892–910. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Baudry, B.; Pumplin, D.W.; Wasserman, S.S.; Tall, B.D.; Ketley, J.M.; Kaper, J.B. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc. Natl. Acad. Sci. USA 1991, 88, 5242–5246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fasano, A.; Fiorentini, C.; Donelli, G.; Uzzau, S.; Kaper, J.B.; Margaretten, K.; Ding, X.; Guandalini, S.; Comstock, L.; Goldblum, S.E. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J. Clin. Investig. 1995, 96, 710–720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fasano, A.; Uzzau, S. Modulation of intestinal tight junctions by Zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J. Clin. Investig. 1997, 99, 1158–1164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fasano, A. Modulation of intestinal permeability: An innovative method of oral drug delivery for the treatment of inherited and acquired human diseases. Mol. Genet. Metab. 1998, 64, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.S.; Gao, H.; Raje, S.; Scott, K.R.; Eddington, N.D. Enhancing the permeation of marker compounds and enaminone anticonvulsants across Caco-2 monolayers by modulating tight junctions using zonula occludens toxin. Eur. J. Pharm. Biopharm. 2001, 52, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, M.; Lu, R.; Uzzau, S.; Wang, W.; Margaretten, K.; Pazzani, C.; Maimone, F.; Fasano, A. Zonula occludens toxin structure-function analysis. Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J. Biol. Chem. 2001, 276, 19160–19165. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.S.; Raje, S.; Gao, H.; Salama, N.N.; Eddington, N.D. Enhanced permeability of molecular weight markers and poorly bioavailable compounds across Caco-2 cell monolayers using the absorption enhancer, zonula occludens toxin. Pharm. Res. 2002, 19, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Karyekar, C.S.; Fasano, A.; Raje, S.; Lu, R.; Dowling, T.C.; Eddington, N.D. Zonula occludens toxin increases the permeability of molecular weight markers and chemotherapeutic agents across the bovine brain microvessel endothelial cells. J. Pharm. Sci. 2003, 92, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Fasano, A.; Eddington, N.D. Effect of the six-mer synthetic peptide (AT1002) fragment of zonula occludens toxin on the intestinal absorption of cyclosporin A. Int. J. Pharm. 2008, 351, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Fasano, A.; Eddington, N.D. Enhanced nasal absorption of hydrophilic markers after dosing with AT1002, a tight junction modulator. Eur. J. Pharm. Biopharm. 2008, 69, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Kim, S.B.; Shim, C.K.; Chung, S.J.; Kim, D.D.; Rhee, S.K.; Choi, G.J.; Kim, C.H.; Kim, K. Paracellular permeation-enhancing effect of AT1002 C-terminal amidation in nasal delivery. Drug Des. Dev. Ther. 2015, 9, 1815–1823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, K.H. Effect of C-terminal amidated tight junction modulator and fructose polymer on the nasal and intestinal permeation of atenolol. Yakhak Hoeji 2019, 63, 37–42. [Google Scholar] [CrossRef]
- Li, M.; Oliver, E.; Kitchens, K.M.; Vere, J.; Alkan, S.S.; Tamiz, A.P. Structure-activity relationship studies of permeability modulating peptide AT-1002. Bioorg. Med. Chem. Lett. 2008, 18, 4584–4586. [Google Scholar] [CrossRef] [PubMed]
- Öner, E.T.; Hernández, L.; Combie, J. Review of Levan polysaccharide: From a century of past experiences to future prospects. Biotechnol. Adv. 2016, 34, 827–844. [Google Scholar] [CrossRef] [PubMed]
- Combie, J.; Öner, E.T. From healing wounds to resorbable electronics, levan can fill bioadhesive roles in scores of markets. Bioinspir. Biomim. 2018, 14, 011001. [Google Scholar] [CrossRef] [PubMed]
- Goldblum, S.E.; Rai, U.; Tripathi, A.; Thakar, M.; De Leo, L.; Di Toro, N.; Not, T.; Ramachandran, R.; Puche, A.C.; Hollenberg, M.D.; et al. The active Zot domain (aa 288–293) increases ZO-1 and myosin 1C serine/threonine phosphorylation, alters interaction between ZO-1 and its binding partners, and induces tight junction disassembly through proteinase activated receptor 2 activation. FASEB J. 2011, 25, 144–158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Kang, X.; Huang, Z.Q.; Shen, L.; Luo, Q.; Li, M.Y.; Luo, L.P.; Tu, J.H.; Han, M.; Ye, J. Protease-Activated Receptor-2 Decreased Zonula Occlidens-1 and Claudin-1 Expression and Induced Epithelial Barrier Dysfunction in Allergic Rhinitis. Am. J. Rhinol. Allergy 2021, 35, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Enjoji, S.; Ohama, T.; Sato, K. Regulation of epithelial cell tight junctions by protease-activated receptor 2. J. Vet. Med. Sci. 2014, 76, 1225–1229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
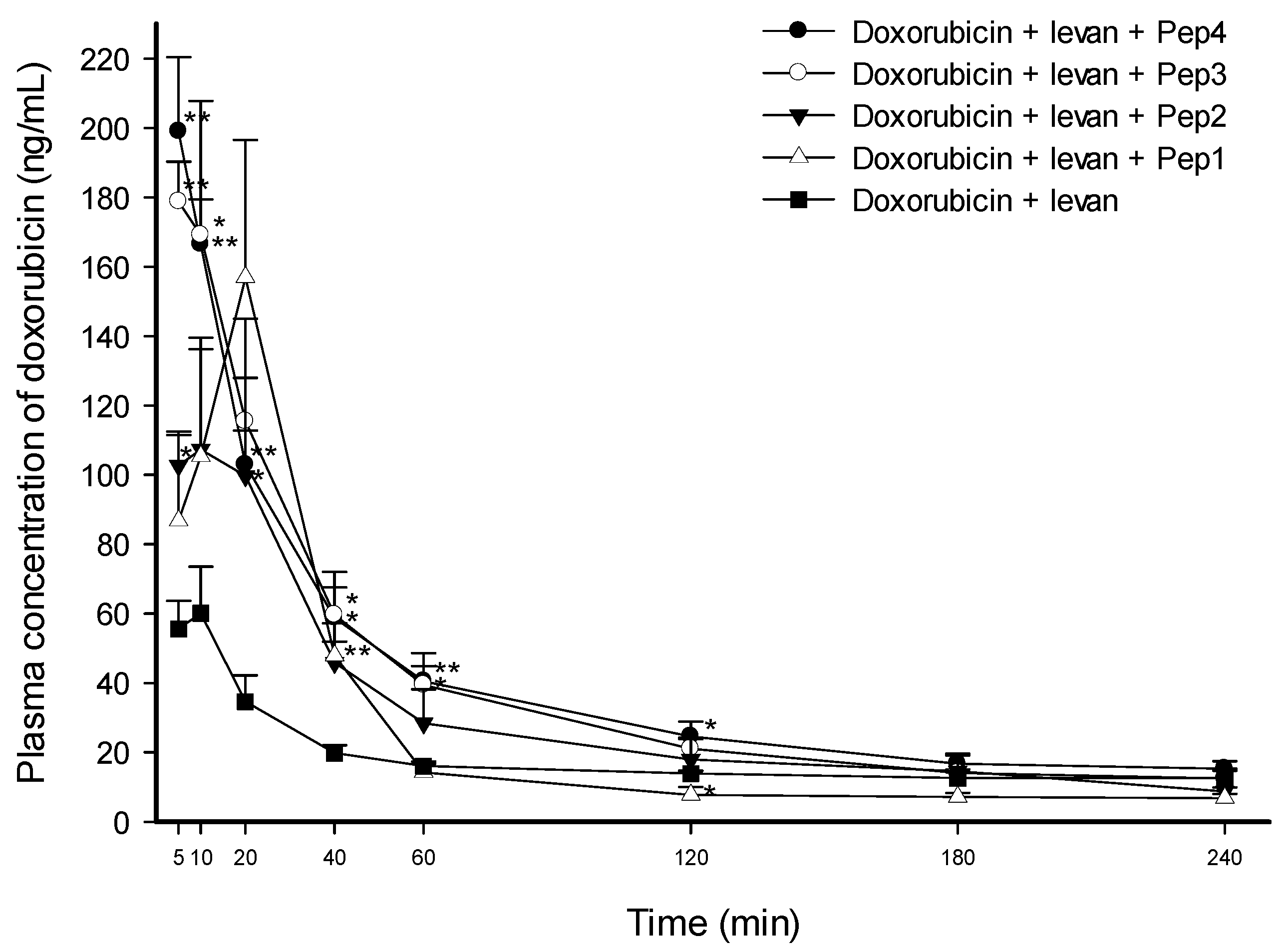
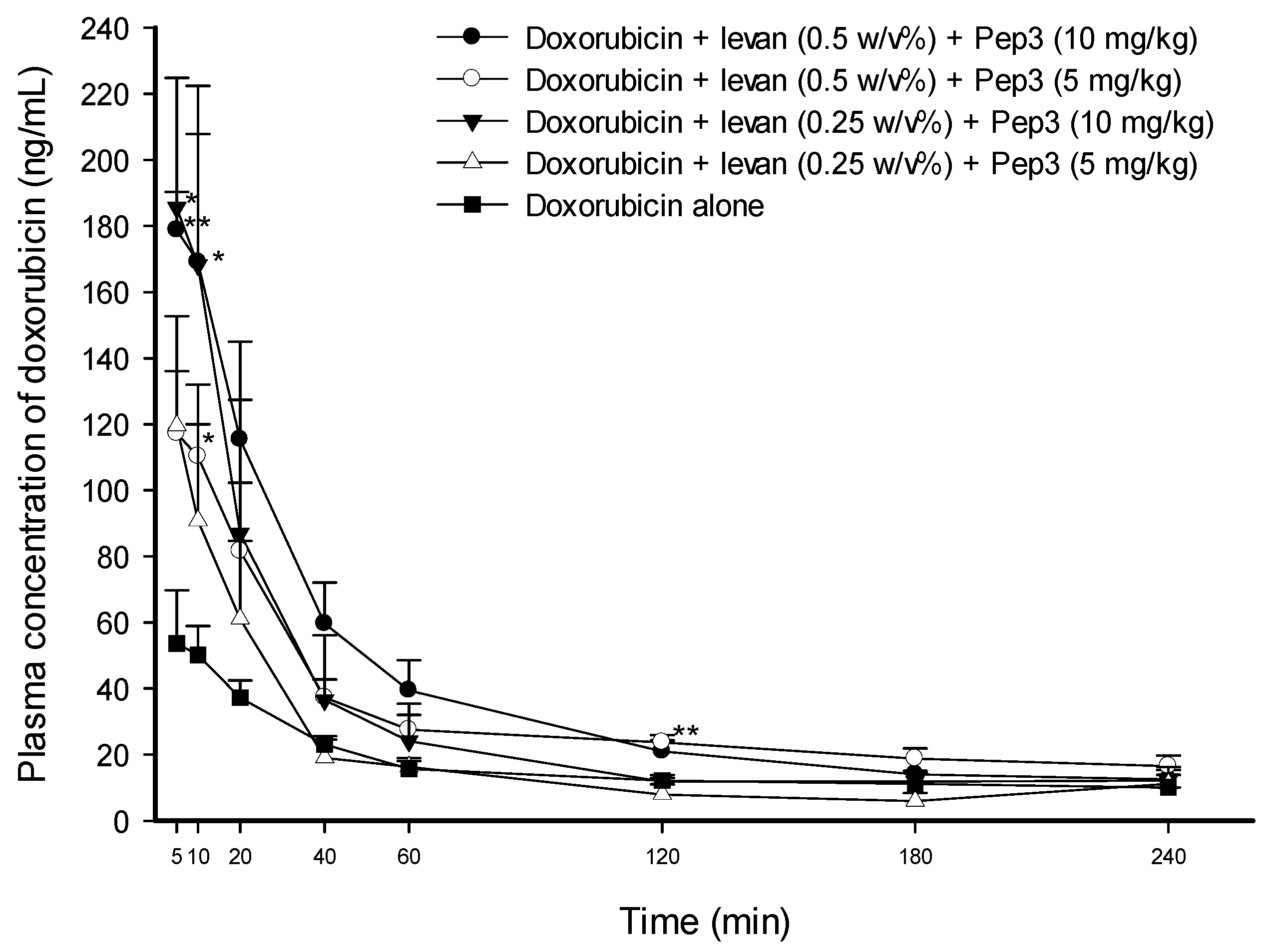

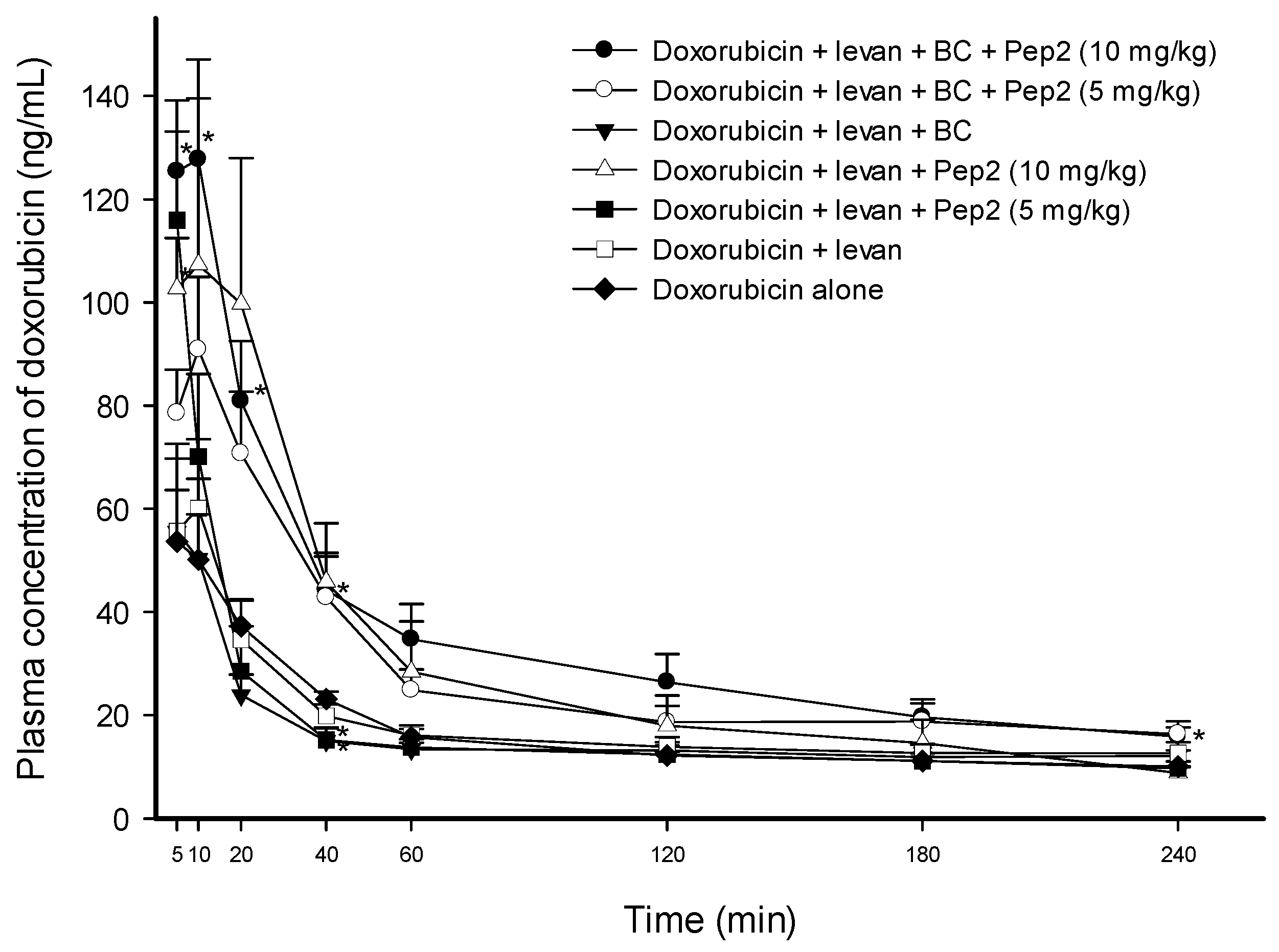
| AUC0–240min (min ng·mL−1) | Cmax (ng·mL−1) | Tmax (min) | T1/2 (min) | Vd/F (L·kg−1) | Cl/F (mL kg·min−1) | |
|---|---|---|---|---|---|---|
| Doxorubicin + levan + Pep4 | 9519.18 ± 953.95 * (2.24-fold) | 198.99 ± 21.38 ** (2.97-fold) | 5.00 ± 0.00 (0.71-fold) | 226.57 ± 29.13 (0.89-fold) | 112.25 ± 3.43 * (0.54-fold) | 359.77 ± 63.88 (0.61-fold) |
| Doxorubicin + levan + Pep3 | 9144.32 ± 1343.45 ** (2.15-fold) | 205.68 ± 25.84 ** (3.07-fold) | 7.00 ± 1.22 (1.00-fold) | 236.63 ± 22.15 (0.93-fold) | 133.35 ± 17.61 * (0.64-fold) | 390.52 ± 37.94 * (0.66-fold) |
| Doxorubicin + levan + Pep2 | 7084.95 ± 1459.19 (1.66-fold) | 132.09 ± 19.61 * (1.97-fold) | 11.67 ± 4.41 (1.67-fold) | 243.63 ± 52.78 (0.96-fold) | 184.98 ± 49.10 (0.89-fold) | 514.63 ± 73.03 (0.87-fold) |
| Doxorubicin + levan + Pep1 | 6206.53 ± 1119.41 (1.46-fold) | 156.94 ± 39.57 * (2.34-fold) | 20.00 ± 0.00 ** (2.86-fold) | 210.64 ± 10.91 (0.83-fold) | 196.37 ± 39.05 (0.94-fold) | 651.39 ± 137.14 (1.10-fold) |
| Doxorubicin + levan (control) | 4258.49 ± 194.97 | 67.10 ± 12.03 | 7.00 ± 1.22 | 253.74 ± 30.70 | 208.20 ± 19.13 | 590.29 ± 64.15 |
| AUC0–240min (min ng·mL−1) | Cmax (ng·mL−1) | Tmax (min) | T1/2 (min) | Vd/F (L·kg−1) | Cl/F (mL kg·min−1) | |
|---|---|---|---|---|---|---|
| Doxo + levan (0.5 w/v%) + Pep4 (10 mg·kg−1) | 9519.18 ± 953.95 ** (2.38-fold) | 198.99 ± 21.38 ** (3.30-fold) | 5.00 ± 0.00 (0.75-fold) | 226.57 ± 29.13 (1.06-fold) | 112.25 ± 3.43 * (0.52-fold) | 359.77 ± 63.88 * (0.51-fold) |
| Doxo + levan (0.5 w/v%) + Pep4 (5 mg·kg−1) | 8443.43 ± 1567.91 (2.11-fold) | 147.34 ± 31.64 (2.44-fold) | 10.00 ± 0.00 (1.50-fold) | 232.67 ± 36.44 (1.09-fold) | 152.42 ± 54.19 (0.70-fold) | 442.77 ± 106.21 (0.63-fold) |
| Doxo + levan (0.25 w/v%) + Pep4 (10 mg·kg−1) | 7225.12 ± 238.62 ** (1.81-fold) | 104.06 ± 15.55 (1.72-fold) | 10.00 ± 2.74 (1.50-fold) | 266.83 ± 37.85 (1.25-fold) | 130.94 ± 6.81 ** (0.60-fold) | 359.30 ± 35.23 ** (0.51-fold) |
| Doxo + levan (0.5 w/v%) + Pep3 (10 mg·kg−1) | 9144.32 ± 1343.45 * (2.29-fold) | 205.68 ± 25.84 ** (3.41-fold) | 7.00 ± 1.22 (1.05-fold) | 236.63 ± 22.15 (1.11-fold) | 133.35 ± 17.61 * (0.61-fold) | 390.52 ± 37.94 ** (0.55-fold) |
| Doxo + levan (0.5 w/v%) + Pep3 (5 mg·kg−1) | 7534.28 ± 1005.76 * (1.89-fold) | 125.09 ± 17.40 * (2.07-fold) | 8.00 ± 1.22 (1.20-fold) | 251.33 ± 55.61 (1.17-fold) | 127.69 ± 16.28 * (0.59-fold) | 422.07 ± 103.89 (0.60-fold) |
| Doxo + levan (0.25 w/v%) + Pep3 (10 mg·kg−1) | 6970.39 ± 2061.62 (1.75-fold) | 190.03 ± 43.57 (3.15-fold) | 6.67 ± 1.67 (1.00-fold) | 185.51 ± 14.15 (0.87-fold) | 139.90 ± 23.66 (0.64-fold) | 536.14 ± 122.06 (0.76-fold) |
| Doxo + levan (0.25 w/v%) + Pep3 (5 mg·kg−1) | 4400.23 ± 744.27 (1.10-fold) | 119.66 ± 33.04 (1.98-fold) | 5.00 ± 0.00 (0.75-fold) | 269.95 ± 16.03 (1.26-fold) | 254.94 ± 64.39 (1.17-fold) | 668.18 ± 196.89 (0.95-fold) |
| Doxo + levan (0.5 w/v%) + BC (0.5 w/v%) + Pep2 (10 mg·kg−1) | 8308.38 ± 979.26 * (2.08-fold) | 134.20 ± 16.64 * (2.22-fold) | 7.50 ± 1.44 (1.13-fold) | 214.57 ± 28.83 (1.00-fold) | 116.69 ± 12.28 ** (0.54-fold) | 392.27 ± 53.17 ** (0.56-fold) |
| Doxo + levan (0.5 w/v%) + BC (0.5 w/v%) + Pep2 (5 mg·kg−1) | 6723.07 ± 503.45 ** (1.68-fold) | 92.85 ± 13.93 (1.54-fold) | 9.00 ± 1.00 (1.35-fold) | 231.27 ± 19.68 (1.08-fold) | 137.78 ± 10.72 ** (0.63-fold) | 422.87 ± 46.98 ** (0.60-fold) |
| Doxo + levan (0.5 w/v%) + Pep2 (10 mg·kg−1) | 7084.95 ± 1459.19 (1.77-fold) | 132.09 ± 19.61 * (2.19-fold) | 11.67 ± 4.41 (1.75-fold) | 243.63 ± 52.78 (1.14-fold) | 184.98 ± 49.10 (0.85-fold) | 514.63 ± 73.03 (0.73-fold) |
| Doxo + levan (0.5 w/v%) + Pep2 (5 mg·kg−1) | 4090.05 ± 539.47 (1.02-fold) | 115.95 ± 17.14 * (1.92-fold) | 5.00 ± 0.00 (0.75-fold) | 238.79 ± 5.21 (1.12-fold) | 238.97 ± 29.87 (1.10-fold) | 697.92 ± 103.17 (0.99-fold) |
| Doxo + levan (0.5 w/v%) + BC (0.5 w/v%) | 3719.75 ± 554.33 (0.93-fold) | 57.37 ± 15.70 (0.95-fold) | 8.33 ± 1.67 (1.25-fold) | 218.04 ± 38.81 (1.02-fold) | 204.55 ± 12.93 (0.94-fold) | 688.98 ± 120.39 (0.98-fold) |
| Doxo + levan (0.5 w/v%) | 4258.49 ± 194.97 (1.07-fold) | 67.10 ± 12.03 (1.11-fold) | 7.00 ± 1.22 (1.05-fold) | 253.74 ± 30.70 (1.19-fold) | 208.20 ± 19.13 (0.96-fold) | 590.29 ± 64.15 (0.84-fold) |
| Doxo alone (control) | 3994.46 ± 326.83 | 60.33 ± 9.53 | 6.67 ± 1.67 | 214.02 ± 20.47 | 217.51 ± 20.98 | 704.36 ± 1.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.-H. Effect of Tight Junction-Modulating FCIGRL-Modified Peptides on the Intestinal Absorption of Doxorubicin in Rats. Pharmaceutics 2024, 16, 650. https://doi.org/10.3390/pharmaceutics16050650
Song K-H. Effect of Tight Junction-Modulating FCIGRL-Modified Peptides on the Intestinal Absorption of Doxorubicin in Rats. Pharmaceutics. 2024; 16(5):650. https://doi.org/10.3390/pharmaceutics16050650
Chicago/Turabian StyleSong, Keon-Hyoung. 2024. "Effect of Tight Junction-Modulating FCIGRL-Modified Peptides on the Intestinal Absorption of Doxorubicin in Rats" Pharmaceutics 16, no. 5: 650. https://doi.org/10.3390/pharmaceutics16050650
APA StyleSong, K.-H. (2024). Effect of Tight Junction-Modulating FCIGRL-Modified Peptides on the Intestinal Absorption of Doxorubicin in Rats. Pharmaceutics, 16(5), 650. https://doi.org/10.3390/pharmaceutics16050650





