Nanodrug Delivery Systems for Myasthenia Gravis: Advances and Perspectives
Abstract
:1. Introduction
2. Pathogenesis of MG
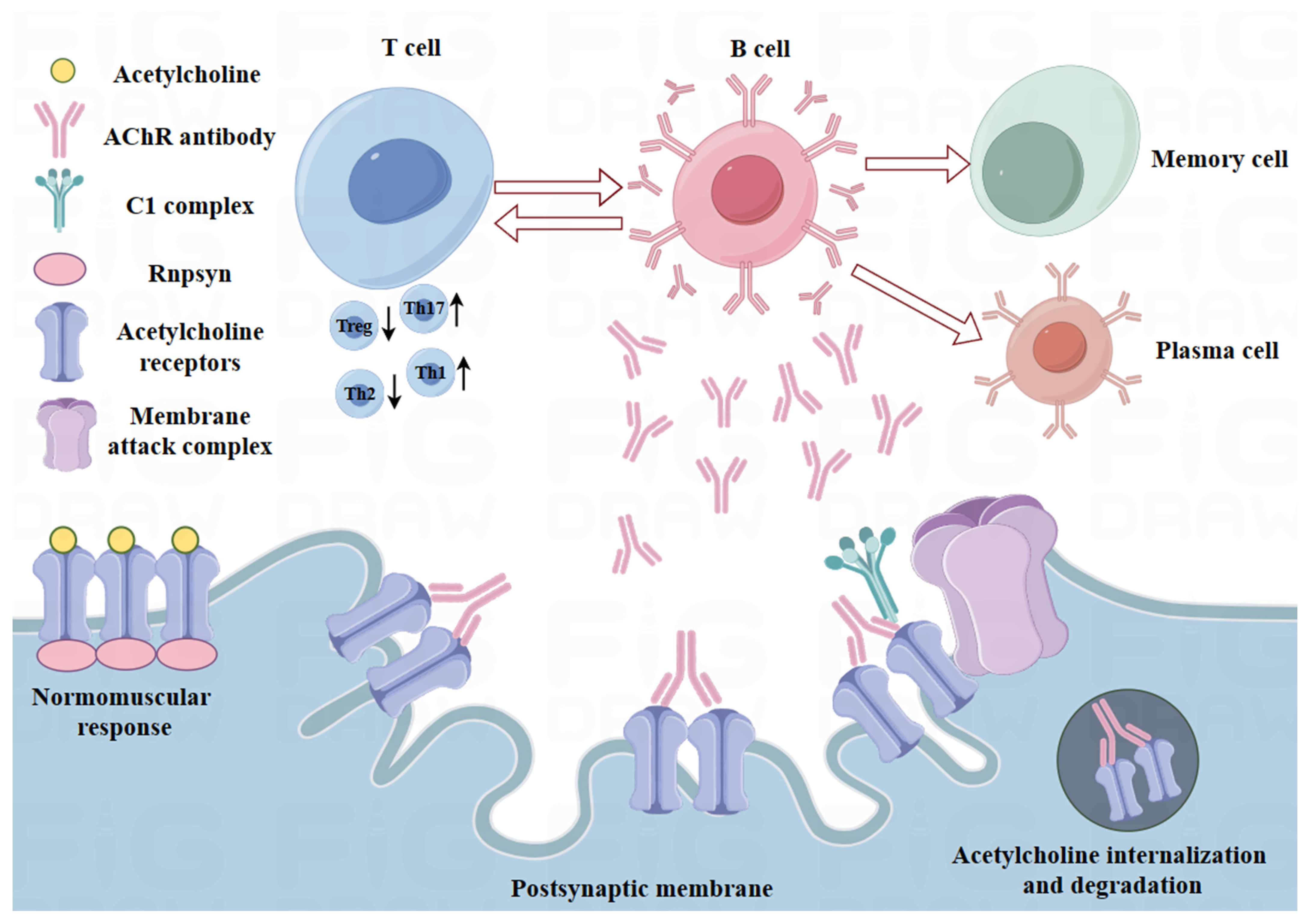
2.1. Immunoregulatory Defects in MG
2.2. Pathological Role of Mitochondria in MG
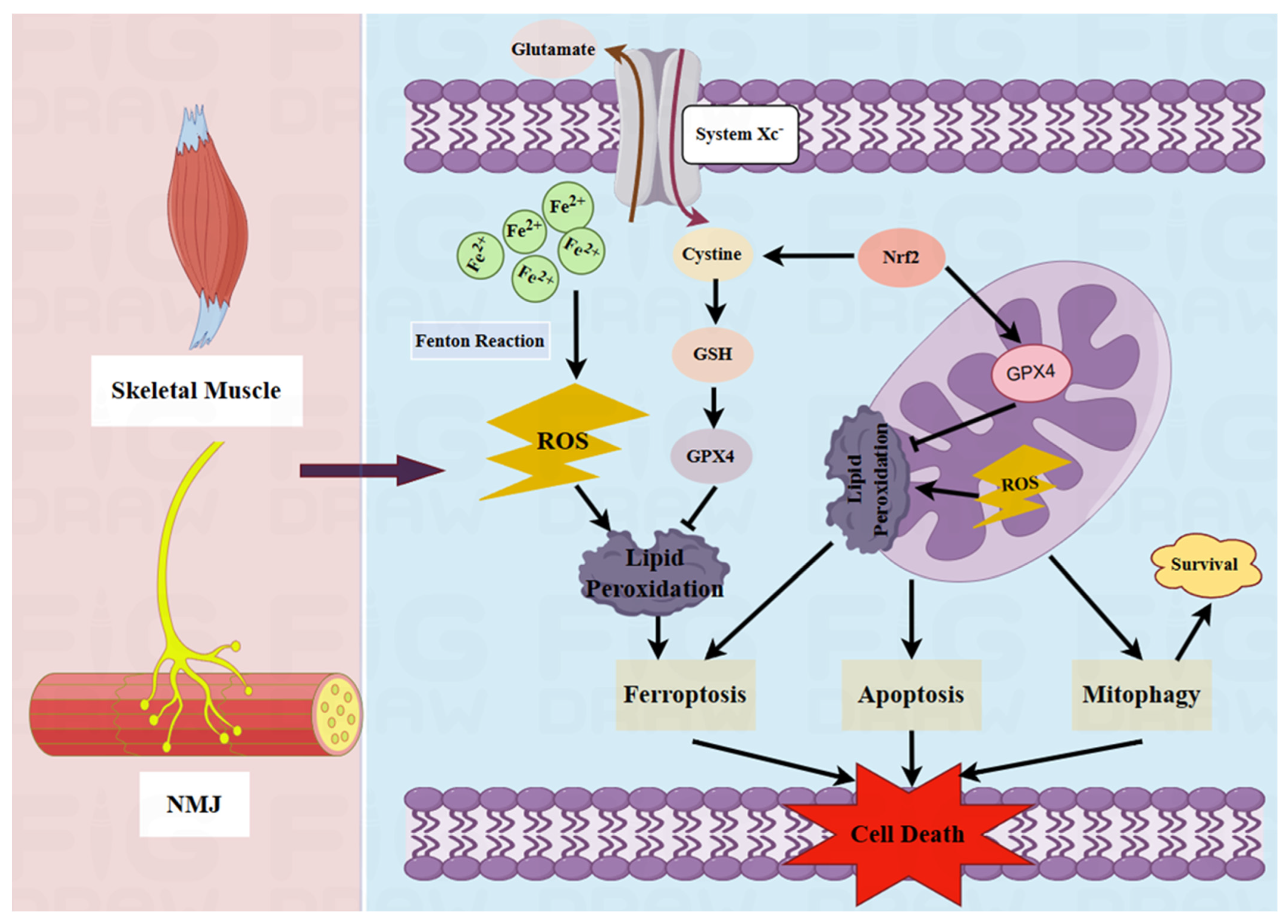
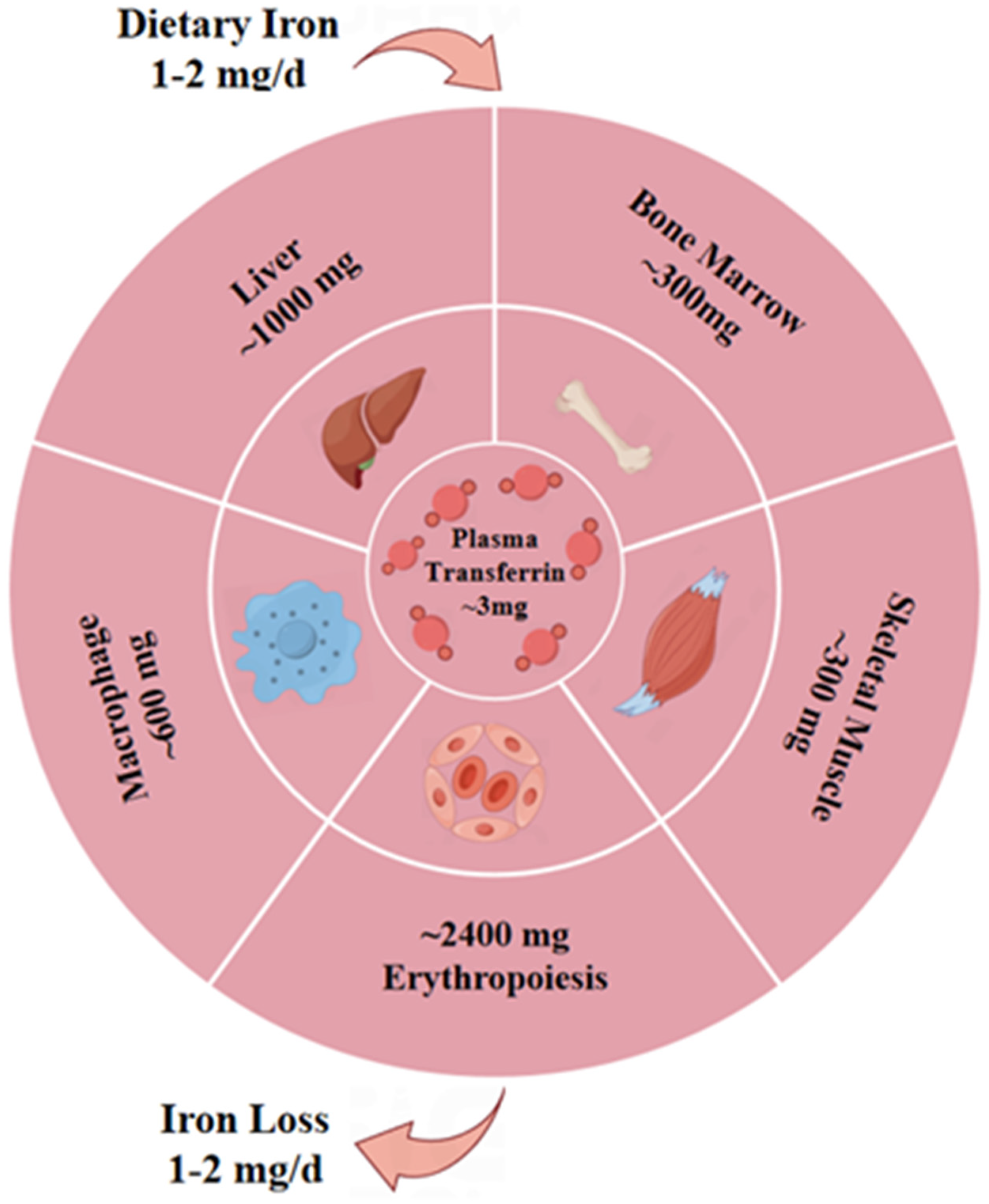
2.3. Potential Pathogenic Effects of Ferroptosis on MG
3. Therapeutic Potential of Nano-Biomedicines in MG
3.1. Potential Role of Nano-Biomedicines in Immunomodulation
3.2. Potential Role of Nano-Biomedicines in Targeted Mitochondrial Therapy
3.3. Potential Effects of Nano-Biomedicines on Ferroptosis
| Target | Delivery System | Active Drug/Agent | Treatment Outcomes | References |
|---|---|---|---|---|
| Immune system | Extracellular vesicles | Caspase-1 inhibitor | Targeted macrophages to inhibit the Th17 response and GC response and thereby improve EAMG | [115] |
| AuNPs | IL-4 or IL-10 | Shifted the immune response in chronically inflamed dystrophic muscle | [146] | |
| PLA and nano-HAP | Doxycycline | Decreased salivary MMP-8 and plasma IL-1 and TNF-α concentrations | [147] | |
| Nano-liposomes | MPS | Decreased serum TGF-β levels and reduced macrophage infiltration in the diaphragm | [119] | |
| PLGA composites | Polydeoxyribonucleotide | Regulated the M1-to-M2 polarization of macrophages and caused immune modulation | [148] | |
| LNPs and polyplex nanomicelles | mRNA | Supported rapid mRNA expression and a potent immune response | [149] | |
| Liposomes | Alendronate | Regulated the M1-to-M2 polarization of macrophages and T-cell functionality | [150] | |
| Flexible liposome hydrogel | DEX | Reduced joint swelling by increasing macrophage uptake | [151] | |
| GO nanosheets | GO | Reversed the dynamic changes to CKs and reduced the activity of Ca2+ | [152] | |
| Erythrocyte membrane-camouflaged NPs | CD22-shRNA, Aβ aptamers | Ameliorated a pro-inflammatory immune environment and could be used to visualize Aβ plaques | [153] | |
| AuNPs | IL-4 | Directed M2 macrophage polarization and promoted regeneration | [154] | |
| Mitochondria | PLGA NPs | Sonosensitizer IR780 and ferroptosis activator RSL-3 | Inhibited the activity of GPX4 and induced ROS generation | [155] |
| Lipid-polymer hybrid nano-system | Calycosin and tanshinone | Increased drug accumulation in cardiac tissue and enabled better infarct size reduction | [156] | |
| Lipid nanocarriers | siRNA-loaded magnesium phosphate core | Reversed mitochondrial dysfunction and alleviated AD neuropathology | [157] | |
| Ceria NPs | Atorvastatin | Eliminated excessive ROS and protected mitochondrial structure | [158] | |
| Polydopamine-coated NPs | PDA and α-TOS | Enabled nanomedicine accumulation in mitochondria to destroy tumor cells | [159] | |
| Molecularly imprinted polymer NPs | Molecularly imprinted polymer | Blocked the catalytic activity of DHFR to inhibit DNA synthesis | [160] | |
| Porous silicon NPs | Bovine serum albumin | Disrupted the mitochondrial respiratory chain | [161] | |
| PLGA-b-PEG NPs | CoQ10 | Effectively increased the tricarboxylic acid cycle rate | [128] | |
| Lipidosomes | Quercetin | Decreased ROS generation, increased ATP levels, and enhanced lactate dehydrogenase activity | [130] | |
| Biomimetic nanocrystals | Curcumin | Reversed mitochondrial dysfunction, TH+ neuron injury, and abnormal α-syn aggregation | [162] | |
| ZIF-8-coated Prussian blue nanocomposite | Quercetin | Restored mitochondrial function, restored energy metabolism, and reduced ROS | [163] | |
| BPNSs | Matrine | Improved neurotransmitter delivery, removed excess ROS, and decreased neuroinflammation | [164] | |
| BPNSs-based hydrogel | Methylene blue | Improved mitochondrial function, and suppressed tau neuropathology | [165] | |
| Platelet membranes-ICG-SS31-PLGA | Indocyanine green and elamipretide | Reduced mitochondrial oxidative stress, inflammation, and apoptosis | [166] | |
| Ferroptosis | Polydopamine NPs | Polydopamine | Depleted ROS, chelated iron, and inhibited the ubiquitination of GPX4 | [144] |
| Ceria-based NPs | Cerium oxide | Alleviated oxidative stress and lipid peroxidation and increased GPX4 activity | [143] | |
| DSPE-PEG 2000 NPs | Iron oxide | Regulated the Beclin1/ATG5-dependent autophagy pathway | [167] | |
| Melanin NPs | Melanin | Inhibited ROS-related ferroptosis to reduce myocardial injury | [168] | |
| Poly-PLGA co-polymers | Alpha lipoic acid | Reduced ROS-induced damage and restored heart function. | [169] | |
| Metal-phenolic nanocomplexes | Quercetin | Attenuated the free radical burst induced by iron overload and restored iron metabolism homeostasis | [170] | |
| PDN@AGL | AGL | Decreased lipid peroxidation, reduced ROS levels, and attenuated ferroptosis | [171] | |
| MPEG-PTMC NPs | Curcumin | Enhanced the delivery of Cur to inhibit ferroptosis | [141] | |
| Polymer NPs | Resveratrol | Inhibited ROS generation and excessive accumulation to attenuate ferroptosis | [172] | |
| PAA@Mn3O4 NPs | Mn3O4 | Resisted lipid peroxidation and detoxified ROS to suppress ferroptosis | [173] |
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Payet, C.A.; You, A.; Fayet, O.M.; Dragin, N.; Berrih-Aknin, S.; Le Panse, R. Myasthenia Gravis: An Acquired Interferonopathy? Cells 2022, 11, 1218. [Google Scholar] [CrossRef] [PubMed]
- Albazli, K.; Kaminski, H.J.; Howard, J.F., Jr. Complement Inhibitor Therapy for Myasthenia Gravis. Front. Immunol. 2020, 11, 917. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.R.; Shah, S.B.; Lovering, R.M. The Neuromuscular Junction: Roles in Aging and Neuromuscular Disease. Int. J. Mol. Sci. 2021, 22, 8058. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.S.; Cardwell, C.R.; McCarron, P.O.; McConville, J. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol. 2010, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tian, D.C.; Zhang, C.; Li, Z.; Zhai, Y.; Xiu, Y.; Gu, H.; Li, H.; Wang, Y.; Shi, F.D. Incidence, mortality, and economic burden of myasthenia gravis in China: A nationwide population-based study. Lancet Reg. Health West. Pac. 2020, 5, 100063. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Su, Y.; Chang, T. Knowledge mapping of global trends for myasthenia gravis development: A bibliometrics analysis. Front. Immunol. 2023, 14, 1132201. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Vicente, E.; Álvarez-Velasco, R.; Segovia, S.; Paradas, C.; Casasnovas, C.; Guerrero-Sola, A.; Pardo, J.; Ramos-Fransi, A.; Sevilla, T.; López de Munain, A.; et al. Clinical and therapeutic features of myasthenia gravis in adults based on age at onset. Neurology 2020, 94, e1171–e1180. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, K.; Tzartos, S.J. Myasthenia Gravis: Autoantibody Specificities and Their Role in MG Management. Front. Neurol. 2020, 11, 596981. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, A.M.; Verschuuren, J.; Tannemaat, M.R. Prevalence and associated factors of fatigue in autoimmune myasthenia gravis. Neuromuscul. Disord. 2021, 31, 612–621. [Google Scholar] [CrossRef]
- Huijbers, M.G.; Marx, A.; Plomp, J.J.; Le Panse, R.; Phillips, W.D. Advances in the understanding of disease mechanisms of autoimmune neuromuscular junction disorders. Lancet Neurol. 2022, 21, 163–175. [Google Scholar] [CrossRef]
- Dresser, L.; Wlodarski, R.; Rezania, K.; Soliven, B. Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations. J. Clin. Med. 2021, 10, 2235. [Google Scholar] [CrossRef]
- Lehnerer, S.; Jacobi, J.; Schilling, R.; Grittner, U.; Marbin, D.; Gerischer, L.; Stascheit, F.; Krause, M.; Hoffmann, S.; Meisel, A. Burden of disease in myasthenia gravis: Taking the patient’s perspective. J. Neurol. 2022, 269, 3050–3063. [Google Scholar] [CrossRef] [PubMed]
- Son, J.M.; Lee, C. Aging: All roads lead to mitochondria. Semin. Cell Dev. Biol. 2021, 116, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Makinde, E.; Ma, L.; Mellick, G.D.; Feng, Y. Mitochondrial Modulators: The Defender. Biomolecules 2023, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Shirihai, O.S. Mitochondrial signal transduction. Cell Metab. 2022, 34, 1620–1653. [Google Scholar] [CrossRef] [PubMed]
- Valenti, D.; Atlante, A. Mitochondrial Bioenergetics in Different Pathophysiological Conditions 2.0. Int. J. Mol. Sci. 2022, 23, 5552. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Hossain, T.; Eckmann, D.M. Mitochondrial dynamics involves molecular and mechanical events in motility, fusion and fission. Front. Cell Dev. Biol. 2022, 10, 1010232. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Liang, K.; Zhu, H.; Zhao, C.; Hu, H.; Yin, S. Ferroptosis and Its Role in Chronic Diseases. Cells 2022, 11, 2040. [Google Scholar] [CrossRef]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2022, 24, 449. [Google Scholar] [CrossRef]
- Li, J.; Jia, B.; Cheng, Y.; Song, Y.; Li, Q.; Luo, C. Targeting Molecular Mediators of Ferroptosis and Oxidative Stress for Neurological Disorders. Oxid. Med. Cell Longev. 2022, 2022, 3999083. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.; Gupta, R.; Sahu, M.; Srivastava, D.; Das, A.; Ambasta, R.K.; Kumar, P. Free radical biology in neurological manifestations: Mechanisms to therapeutics interventions. Environ. Sci. Pollut. Res. Int. 2022, 29, 62160–62207. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Chen, X.; Zhong, H.; Wang, Y. Ferroptosis in life: To be or not to be. Biomed. Pharmacother. 2023, 159, 114241. [Google Scholar] [CrossRef] [PubMed]
- Villalón-García, I.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Talaverón-Rey, M.; Suárez-Rivero, J.M.; Suárez-Carrillo, A.; Munuera-Cabeza, M.; Reche-López, D.; Cilleros-Holgado, P.; Piñero-Pérez, R.; et al. Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration. Neural Regen. Res. 2023, 18, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target. Ther. 2020, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xin, L.; Xiang, M.; Shang, C.; Wang, Y.; Wang, Y.; Cui, X.; Lu, Y. The molecular mechanisms of ferroptosis and its role in cardiovascular disease. Biomed. Pharmacother. 2022, 145, 112423. [Google Scholar] [CrossRef]
- Lai, B.; Wu, C.H.; Wu, C.Y.; Luo, S.F.; Lai, J.H. Ferroptosis and Autoimmune Diseases. Front. Immunol. 2022, 13, 916664. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P.; et al. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021, 7, 193. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef]
- Ren, J.X.; Li, C.; Yan, X.L.; Qu, Y.; Yang, Y.; Guo, Z.N. Crosstalk between Oxidative Stress and Ferroptosis/Oxytosis in Ischemic Stroke: Possible Targets and Molecular Mechanisms. Oxid. Med. Cell. Longev. 2021, 2021, 6643382. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Dewi, M.K.; Chaerunisaa, A.Y.; Muhaimin, M.; Joni, I.M. Improved Activity of Herbal Medicines through Nanotechnology. Nanomaterials 2022, 12, 4073. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Pande, S.; Sagathia, V.; Ranch, K.; Beladiya, J.; Boddu, S.H.S.; Jacob, S.; Al-Tabakha, M.M.; Hassan, N.; Shahwan, M. Nanocarriers for the Delivery of Neuroprotective Agents in the Treatment of Ocular Neurodegenerative Diseases. Pharmaceutics 2023, 15, 837. [Google Scholar] [CrossRef] [PubMed]
- Makhathini, S.S.; Mdanda, S.; Kondiah, P.J.; Kharodia, M.E.; Rumbold, K.; Alagidede, I.; Pathak, Y.; Bulbulia, Z.; Rants’o, T.A.; Kondiah, P.P.D. Biomedicine Innovations and Its Nanohydrogel Classifications. Pharmaceutics 2022, 14, 2839. [Google Scholar] [CrossRef] [PubMed]
- Vodyashkin, A.A.; Kezimana, P.; Vetcher, A.A.; Stanishevskiy, Y.M. Biopolymeric Nanoparticles-Multifunctional Materials of the Future. Polymers 2022, 14, 2287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Poon, K.; Masonsong, G.S.P.; Ramaswamy, Y.; Singh, G. Sustainable Nanomaterials for Biomedical Applications. Pharmaceutics 2023, 15, 922. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Yu, Z.; Xu, T.; Wang, L.; Meng, N.; Jin, H.; Xu, B. Novel Nano-Drug Delivery System for Brain Tumor Treatment. Cells 2022, 11, 3761. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.A.; Kim, J.H.; Ryu, K.; Kaushik, N. Current Nanomedicine for Targeted Vascular Disease Treatment: Trends and Perspectives. Int. J. Mol. Sci. 2022, 23, 12397. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.H.; Khattak, S.; Rauf, M.A.; Ansari, M.A.; Alomary, M.N.; Razak, S.; Yang, C.Y.; Wu, D.D.; Ji, X.Y. Role of Nanomedicine-Based Therapeutics in the Treatment of CNS Disorders. Molecules 2023, 28, 1283. [Google Scholar] [CrossRef]
- Ko, C.N.; Zang, S.; Zhou, Y.; Zhong, Z.; Yang, C. Nanocarriers for effective delivery: Modulation of innate immunity for the management of infections and the associated complications. J. Nanobiotechnol. 2022, 20, 380. [Google Scholar] [CrossRef]
- Waheed, S.; Li, Z.; Zhang, F.; Chiarini, A.; Armato, U.; Wu, J. Engineering nano-drug biointerface to overcome biological barriers toward precision drug delivery. J. Nanobiotechnol. 2022, 20, 395. [Google Scholar] [CrossRef]
- Kumari, S.; Goyal, A.; Sönmez Gürer, E.; Algın Yapar, E.; Garg, M.; Sood, M.; Sindhu, R.K. Bioactive Loaded Novel Nano-Formulations for Targeted Drug Delivery and Their Therapeutic Potential. Pharmaceutics 2022, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Alharbi, F.D.; Alhibs, A.S.; Alanazi, N.B.; Alshehri, B.Y.; Saleh, M.A.; Alshehri, F.S.; Algarni, M.A.; Almugaiteeb, T.; Uddin, M.N.; et al. PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases. Pharmaceutics 2022, 14, 2728. [Google Scholar] [CrossRef] [PubMed]
- Pozharov, V.P.; Minko, T. Nanotechnology-Based RNA Vaccines: Fundamentals, Advantages and Challenges. Pharmaceutics 2023, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Z.; Lei, H.; Zhang, D. Recent progress in nanotechnology-based drug carriers for resveratrol delivery. Drug Deliv. 2023, 30, 2174206. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, M.L.; Jiang, R.; Bourke, A.; Nowak, R.J.; O’Connor, K.C. Autoimmune Pathology in Myasthenia Gravis Disease Subtypes Is Governed by Divergent Mechanisms of Immunopathology. Front. Immunol. 2020, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Berrih-Aknin, S.; Le Panse, R. Myasthenia gravis: A comprehensive review of immune dysregulation and etiological mechanisms. J. Autoimmun. 2014, 52, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Le Panse, R.; Cizeron-Clairac, G.; Cuvelier, M.; Truffault, F.; Bismuth, J.; Nancy, P.; De Rosbo, N.K.; Berrih-Aknin, S. Regulatory and pathogenic mechanisms in human autoimmune myasthenia gravis. Ann. N. Y. Acad. Sci. 2008, 1132, 135–142. [Google Scholar] [CrossRef]
- Castañeda, J.; Hidalgo, Y.; Sauma, D.; Rosemblatt, M.; Bono, M.R.; Núñez, S. The Multifaceted Roles of B Cells in the Thymus: From Immune Tolerance to Autoimmunity. Front. Immunol. 2021, 12, 766698. [Google Scholar] [CrossRef]
- Berrih-Aknin, S.; Morel, E.; Raimond, F.; Safar, D.; Gaud, C.; Binet, J.P.; Levasseur, P.; Bach, J.F. The role of the thymus in myasthenia gravis: Immunohistological and immunological studies in 115 cases. Ann. N. Y. Acad. Sci. 1987, 505, 50–70. [Google Scholar] [CrossRef]
- DuPage, M.; Bluestone, J.A. Harnessing the plasticity of CD4+ T cells to treat immune-mediated disease. Nat. Rev. Immunol. 2016, 16, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Gertel-Lapter, S.; Mizrachi, K.; Berrih-Aknin, S.; Fuchs, S.; Souroujon, M.C. Impairment of regulatory T cells in myasthenia gravis: Studies in an experimental model. Autoimmun. Rev. 2013, 12, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Akamine, H.; Uzawa, A.; Kojima, Y.; Ozawa, Y.; Yasuda, M.; Onishi, Y.; Kuwabara, S. Role of soluble forms of follicular helper T-cell membrane molecules in the pathogenesis of myasthenia gravis. J. Neuroimmunol. 2023, 375, 578014. [Google Scholar] [CrossRef] [PubMed]
- Danikowski, K.M.; Jayaraman, S.; Prabhakar, B.S. Regulatory T cells in multiple sclerosis and myasthenia gravis. J. Neuroinflammation 2017, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, X.S.; Wang, Y.G.; Lu, C.; Li, J.; Zhang, P. Imbalance of Th17 and Tregs in thymoma may be a pathological mechanism of myasthenia gravis. Mol. Immunol. 2021, 133, 67–76. [Google Scholar] [CrossRef]
- Villegas, J.A.; Van Wassenhove, J.; Le Panse, R.; Berrih-Aknin, S.; Dragin, N. An imbalance between regulatory T cells and T helper 17 cells in acetylcholine receptor-positive myasthenia gravis patients. Ann. N. Y. Acad. Sci. 2018, 1413, 154–162. [Google Scholar] [CrossRef]
- Shibui, A.; Shimura, E.; Nambu, A.; Yamaguchi, S.; Leonard, W.J.; Okumura, K.; Sugano, S.; Sudo, K.; Nakae, S. Th17 cell-derived IL-17 is dispensable for B cell antibody production. Cytokine 2012, 59, 108–114. [Google Scholar] [CrossRef]
- Uzawa, A.; Kuwabara, S.; Suzuki, S.; Imai, T.; Murai, H.; Ozawa, Y.; Yasuda, M.; Nagane, Y.; Utsugisawa, K. Roles of cytokines and T cells in the pathogenesis of myasthenia gravis. Clin. Exp. Immunol. 2021, 203, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Gu, W.; He, L.; Sun, B. Th1/Th2 cell’s function in immune system. Adv. Exp. Med. Biol. 2014, 841, 45–65. [Google Scholar] [CrossRef]
- Alahgholi-Hajibehzad, M.; Kasapoglu, P.; Jafari, R.; Rezaei, N. The role of T regulatory cells in immunopathogenesis of myasthenia gravis: Implications for therapeutics. Expert Rev. Clin. Immunol. 2015, 11, 859–870. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Ono, M.; Setoguchi, R.; Yagi, H.; Hori, S.; Fehervari, Z.; Shimizu, J.; Takahashi, T.; Nomura, T. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006, 212, 8–27. [Google Scholar] [CrossRef]
- Dominguez-Villar, M.; Hafler, D.A. Regulatory T cells in autoimmune disease. Nat. Immunol. 2018, 19, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Thiruppathi, M.; Rowin, J.; Ganesh, B.; Sheng, J.R.; Prabhakar, B.S.; Meriggioli, M.N. Impaired regulatory function in circulating CD4+CD25highCD127low/− T cells in patients with myasthenia gravis. Clin. Immunol. 2012, 145, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ma, Q.; Yu, L.; Huang, H.; Liu, X.; Chen, P.; Ran, H.; Liu, W. JAK2 inhibitor ameliorates the progression of experimental autoimmune myasthenia gravis and balances Th17/Treg cells via regulating the JAK2/STAT3-AKT/mTOR signaling pathway. Int. Immunopharmacol. 2023, 115, 109693. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Tang, X. Gut Microbiota as Regulators of Th17/Treg Balance in Patients With Myasthenia Gravis. Front. Immunol. 2021, 12, 803101. [Google Scholar] [CrossRef] [PubMed]
- Cenacchi, G.; Papa, V.; Fanin, M.; Pegoraro, E.; Angelini, C. Comparison of muscle ultrastructure in myasthenia gravis with anti-MuSK and anti-AChR antibodies. J. Neurol. 2011, 258, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Pelz, A.; Trautmann, G.; Block, K.; Furlan, S.; Gutsmann, M.; Kohler, S.; Volpe, P.; Blottner, D.; Meisel, A.; et al. Opposite Regulation of Homer Signal at the NMJ Postsynaptic Micro Domain between Slow- and Fast-Twitch Muscles in an Experimentally Induced Autoimmune Myasthenia Gravis (EAMG) Mouse Model. Int. J. Mol. Sci. 2022, 23, 15052. [Google Scholar] [CrossRef] [PubMed]
- Lisowski, P.; Kannan, P.; Mlody, B.; Prigione, A. Mitochondria and the dynamic control of stem cell homeostasis. EMBO Rep. 2018, 19, e45432. [Google Scholar] [CrossRef] [PubMed]
- Reggiani, C.; Marcucci, L. A controversial issue: Can mitochondria modulate cytosolic calcium and contraction of skeletal muscle fibers? J. Gen. Physiol. 2022, 154, e202213167. [Google Scholar] [CrossRef]
- Bolaños, P.; Calderón, J.C. Excitation-contraction coupling in mammalian skeletal muscle: Blending old and last-decade research. Front. Physiol. 2022, 13, 989796. [Google Scholar] [CrossRef]
- Ke, L.; Li, Q.; Song, J.; Jiao, W.; Ji, A.; Chen, T.; Pan, H.; Song, Y. The mitochondrial biogenesis signaling pathway is a potential therapeutic target for myasthenia gravis via energy metabolism (Review). Exp. Ther. Med. 2021, 22, 702. [Google Scholar] [CrossRef]
- Slavin, M.B.; Memme, J.M.; Oliveira, A.N.; Moradi, N.; Hood, D.A. Regulatory networks coordinating mitochondrial quality control in skeletal muscle. Am. J. Physiol. Cell Physiol. 2022, 322, C913–C926. [Google Scholar] [CrossRef]
- Oudbier, S.J.; Goh, J.; Looijaard, S.; Reijnierse, E.M.; Meskers, C.G.M.; Maier, A.B. Pathophysiological Mechanisms Explaining the Association Between Low Skeletal Muscle Mass and Cognitive Function. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1959–1968. [Google Scholar] [CrossRef]
- Alizadeh Pahlavani, H.; Laher, I.; Knechtle, B.; Zouhal, H. Exercise and mitochondrial mechanisms in patients with sarcopenia. Front. Physiol. 2022, 13, 1040381. [Google Scholar] [CrossRef]
- Saoji, M.; Petersen, C.E.; Sen, A.; Tripoli, B.A.; Smyth, J.T.; Cox, R.T. Reduction of Drosophila Mitochondrial RNase P in Skeletal and Heart Muscle Causes Muscle Degeneration, Cardiomyopathy, and Heart Arrhythmia. Front. Cell Dev. Biol. 2022, 10, 788516. [Google Scholar] [CrossRef]
- Xu, H.; Ranjit, R.; Richardson, A.; Van Remmen, H. Muscle mitochondrial catalase expression prevents neuromuscular junction disruption, atrophy, and weakness in a mouse model of accelerated sarcopenia. J. Cachexia Sarcopenia Muscle 2021, 12, 1582–1596. [Google Scholar] [CrossRef]
- Jiao, W.; Hu, F.; Li, J.; Song, J.; Liang, J.; Li, L.; Song, Y.; Chen, Z.; Li, Q.; Ke, L. Qiangji Jianli Decoction promotes mitochondrial biogenesis in skeletal muscle of myasthenia gravis rats via AMPK/PGC-1α signaling pathway. Biomed. Pharmacother. 2020, 129, 110482. [Google Scholar] [CrossRef]
- Song, J.; Lei, X.; Jiao, W.; Song, Y.; Chen, W.; Li, J.; Chen, Z. Effect of Qiangji Jianli decoction on mitochondrial respiratory chain activity and expression of mitochondrial fusion and fission proteins in myasthenia gravis rats. Sci. Rep. 2018, 8, 8623. [Google Scholar] [CrossRef]
- Li, L.; Cai, D.; Zhong, H.; Liu, F.; Jiang, Q.; Liang, J.; Li, P.; Song, Y.; Ji, A.; Jiao, W.; et al. Mitochondrial dynamics and biogenesis indicators may serve as potential biomarkers for diagnosis of myasthenia gravis. Exp. Ther. Med. 2022, 23, 307. [Google Scholar] [CrossRef]
- Li, L.; Huang, T.; Yang, J.; Yang, P.; Lan, H.; Liang, J.; Cai, D.; Zhong, H.; Jiao, W.; Song, Y. PINK1/Parkin pathway-mediated mitophagy by AS-IV to explore the molecular mechanism of muscle cell damage. Biomed. Pharmacother. 2023, 161, 114533. [Google Scholar] [CrossRef]
- Pereyra, A.S.; Lin, C.T.; Sanchez, D.M.; Laskin, J.; Spangenburg, E.E.; Neufer, P.D.; Fisher-Wellman, K.; Ellis, J.M. Skeletal muscle undergoes fiber type metabolic switch without myosin heavy chain switch in response to defective fatty acid oxidation. Mol. Metab. 2022, 59, 101456. [Google Scholar] [CrossRef]
- Salles, J.; Chanet, A.; Guillet, C.; Vaes, A.M.; Brouwer-Brolsma, E.M.; Rocher, C.; Giraudet, C.; Patrac, V.; Meugnier, E.; Montaurier, C.; et al. Vitamin D status modulates mitochondrial oxidative capacities in skeletal muscle: Role in sarcopenia. Commun. Biol. 2022, 5, 1288. [Google Scholar] [CrossRef]
- Chatzinikita, E.; Maridaki, M.; Palikaras, K.; Koutsilieris, M.; Philippou, A. The Role of Mitophagy in Skeletal Muscle Damage and Regeneration. Cells 2023, 12, 716. [Google Scholar] [CrossRef]
- Gambarotto, L.; Metti, S.; Chrisam, M.; Cerqua, C.; Sabatelli, P.; Armani, A.; Zanon, C.; Spizzotin, M.; Castagnaro, S.; Strappazzon, F.; et al. Ambra1 deficiency impairs mitophagy in skeletal muscle. J. Cachexia Sarcopenia Muscle 2022, 13, 2211–2224. [Google Scholar] [CrossRef]
- Huot, J.R.; Pin, F.; Chatterjee, R.; Bonetto, A. PGC1α overexpression preserves muscle mass and function in cisplatin-induced cachexia. J. Cachexia Sarcopenia Muscle 2022, 13, 2480–2491. [Google Scholar] [CrossRef]
- Europa, T.A.; Nel, M.; Lebeko, M.R.; Heckmann, J.M. Mitochondrial bioenergetics in ocular fibroblasts of two myasthenia gravis cases. IBRO Neurosci. Rep. 2022, 12, 297–302. [Google Scholar] [CrossRef]
- López-Bellón, S.; Rodríguez-López, S.; González-Reyes, J.A.; Burón, M.I.; de Cabo, R.; Villalba, J.M. CYB5R3 overexpression preserves skeletal muscle mitochondria and autophagic signaling in aged transgenic mice. GeroScience 2022, 44, 2223–2241. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, D.M.; Yu, R.R.; Zhang, L.L.; Liu, Y.Z.; Chen, J.X.; Chen, H.C.; Liu, Y.P. The Effect of Aerobic Exercise on the Oxidative Capacity of Skeletal Muscle Mitochondria in Mice with Impaired Glucose Tolerance. J. Diabetes Res. 2022, 2022, 3780156. [Google Scholar] [CrossRef]
- Qualls, A.E.; Southern, W.M.; Call, J.A. Mitochondria-cytokine crosstalk following skeletal muscle injury and disuse: A mini-review. Am. J. Physiol. Cell Physiol. 2021, 320, C681–C688. [Google Scholar] [CrossRef]
- Wesolowski, L.T.; Semanchik, P.L.; White-Springer, S.H. Beyond antioxidants: Selenium and skeletal muscle mitochondria. Front. Vet. Sci. 2022, 9, 1011159. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Isern, J.; Campanario, S.; Perdiguero, E.; Ramírez-Pardo, I.; Segalés, J.; Hernansanz-Agustín, P.; Curtabbi, A.; Deryagin, O.; Pollán, A.; et al. Mitochondrial dynamics maintain muscle stem cell regenerative competence throughout adult life by regulating metabolism and mitophagy. Cell Stem Cell 2022, 29, 1298–1314.e1210. [Google Scholar] [CrossRef]
- Yan, Y.; Li, M.; Lin, J.; Ji, Y.; Wang, K.; Yan, D.; Shen, Y.; Wang, W.; Huang, Z.; Jiang, H.; et al. Adenosine monophosphate activated protein kinase contributes to skeletal muscle health through the control of mitochondrial function. Front. Pharmacol. 2022, 13, 947387. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; Paez, H.G.; Pitzer, C.R.; Ferrandi, P.J.; Khan, M.M.; Mohamed, J.S.; Carson, J.A.; Deschenes, M.R. Mitochondria transplant therapy improves regeneration and restoration of injured skeletal muscle. J. Cachexia Sarcopenia Muscle 2023, 14, 493–507. [Google Scholar] [CrossRef]
- Zhang, R.F.; Zeng, M.; Lv, N.; Wang, L.M.; Yang, Q.Y.; Gan, J.L.; Li, H.H.; Yu, B.; Jiang, X.J.; Yang, L. Ferroptosis in neurodegenerative diseases: Inhibitors as promising candidate mitigators. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 46–65. [Google Scholar] [CrossRef]
- Tong, X.; Tang, R.; Xiao, M.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Yu, X.; Shi, S. Targeting cell death pathways for cancer therapy: Recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J. Hematol. Oncol. 2022, 15, 174. [Google Scholar] [CrossRef]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 7–23. [Google Scholar] [CrossRef]
- Galaris, D.; Pantopoulos, K. Oxidative stress and iron homeostasis: Mechanistic and health aspects. Crit. Rev. Clin. Lab. Sci. 2008, 45, 1–23. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, B.; Shen, D.; Chen, J.; Yu, Z.; Chen, C. Ferroptosis in a sarcopenia model of senescence accelerated mouse prone 8 (SAMP8). Int. J. Biol. Sci. 2021, 17, 151–162. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, R.; Peng, Y.; Wang, Z.; Huang, J.; Meng, H.; Min, J.; Wang, F.; Ma, Q. ACSL4 contributes to ferroptosis-mediated rhabdomyolysis in exertional heat stroke. J. Cachexia Sarcopenia Muscle 2022, 13, 1717–1730. [Google Scholar] [CrossRef]
- Wang, D.; Liang, W.; Huo, D.; Wang, H.; Wang, Y.; Cong, C.; Zhang, C.; Yan, S.; Gao, M.; Su, X.; et al. SPY1 inhibits neuronal ferroptosis in amyotrophic lateral sclerosis by reducing lipid peroxidation through regulation of GCH1 and TFR1. Cell Death Differ. 2023, 30, 369–382. [Google Scholar] [CrossRef]
- La Rosa, P.; Petrillo, S.; Turchi, R.; Berardinelli, F.; Schirinzi, T.; Vasco, G.; Lettieri-Barbato, D.; Fiorenza, M.T.; Bertini, E.S.; Aquilano, K.; et al. The Nrf2 induction prevents ferroptosis in Friedreich’s Ataxia. Redox Biol. 2021, 38, 101791. [Google Scholar] [CrossRef]
- Huang, P. The relationship between serum iron levels and AChR-Ab and IL-6 in patients with myasthenia gravis. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 98–102. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Song, C.; Sun, J.; Liu, W. Association Between Serum Iron Status and Muscle Mass in Adults: Results From NHANES 2015-2018. Front. Nutr. 2022, 9, 941093. [Google Scholar] [CrossRef]
- Li, K.; Hou, L.; Tan, Y.; Huang, Y.; Shi, J.; Han, J.; Yan, J.; Guan, Y.; Cui, L. Iron metabolism in non-anemic myasthenia gravis patients: A cohort study. J. Neuroimmunol. 2023, 375, 578015. [Google Scholar] [CrossRef]
- Scaramellini, N.; Fischer, D.; Agarvas, A.R.; Motta, I.; Muckenthaler, M.U.; Mertens, C. Interpreting Iron Homeostasis in Congenital and Acquired Disorders. Pharmaceuticals 2023, 16, 329. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, Q.; Wu, D.; Chen, L. Mitochondrial iron metabolism and its role in diseases. Clin. Chim. Acta 2021, 513, 6–12. [Google Scholar] [CrossRef]
- Duan, G.; Li, J.; Duan, Y.; Zheng, C.; Guo, Q.; Li, F.; Zheng, J.; Yu, J.; Zhang, P.; Wan, M.; et al. Mitochondrial Iron Metabolism: The Crucial Actors in Diseases. Molecules 2022, 28, 29. [Google Scholar] [CrossRef]
- Rouault, T.A. Mitochondrial iron overload: Causes and consequences. Curr. Opin. Genet. Dev. 2016, 38, 31–37. [Google Scholar] [CrossRef]
- Sung, H.K.; Murugathasan, M.; Abdul-Sater, A.A.; Sweeney, G. Autophagy deficiency exacerbates iron overload induced reactive oxygen species production and apoptotic cell death in skeletal muscle cells. Cell Death Dis. 2023, 14, 252. [Google Scholar] [CrossRef]
- Zhang, Q.; Qu, H.; Chen, Y.; Luo, X.; Chen, C.; Xiao, B.; Ding, X.; Zhao, P.; Lu, Y.; Chen, A.F.; et al. Atorvastatin Induces Mitochondria-Dependent Ferroptosis via the Modulation of Nrf2-xCT/GPx4 Axis. Front. Cell Dev. Biol. 2022, 10, 806081. [Google Scholar] [CrossRef]
- Ding, H.; Chen, S.; Pan, X.; Dai, X.; Pan, G.; Li, Z.; Mai, X.; Tian, Y.; Zhang, S.; Liu, B.; et al. Transferrin receptor 1 ablation in satellite cells impedes skeletal muscle regeneration through activation of ferroptosis. J. Cachexia Sarcopenia Muscle 2021, 12, 746–768. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.M.; Kysenius, K.; Caldow, M.K.; Hardee, J.P.; Crouch, P.J.; Ayton, S.; Bush, A.I.; Lynch, G.S.; Koopman, R. Iron accumulation in skeletal muscles of old mice is associated with impaired regeneration after ischaemia-reperfusion damage. J. Cachexia Sarcopenia Muscle 2021, 12, 476–492. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.; Barnett, C.; Bril, V. Novel Treatments in Myasthenia Gravis. Front. Neurol. 2020, 11, 538. [Google Scholar] [CrossRef]
- Al-Lawati, H.; Aliabadi, H.M.; Makhmalzadeh, B.S.; Lavasanifar, A. Nanomedicine for immunosuppressive therapy: Achievements in pre-clinical and clinical research. Expert Opin. Drug Deliv. 2018, 15, 397–418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Du, T.; Yang, C.L.; Li, T.; Li, X.L.; Liu, W.; Zhang, P.; Dong, J.; Si, W.Y.; Duan, R.S.; et al. Extracellular vesicles encapsulated with caspase-1 inhibitor ameliorate experimental autoimmune myasthenia gravis through targeting macrophages. J. Control. Release 2023, 364, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Azharuddin, M.; Zhu, G.H.; Sengupta, A.; Hinkula, J.; Slater, N.K.H.; Patra, H.K. Nano toolbox in immune modulation and nanovaccines. Trends Biotechnol. 2022, 40, 1195–1212. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Fadeel, B. Understanding the bidirectional interactions between two-dimensional materials, microorganisms, and the immune system. Adv. Drug Deliv. Rev. 2022, 188, 114422. [Google Scholar] [CrossRef]
- Gomes, G.S.; Frank, L.A.; Contri, R.V.; Longhi, M.S.; Pohlmann, A.R.; Guterres, S.S. Nanotechnology-based alternatives for the topical delivery of immunosuppressive agents in psoriasis. Int. J. Pharm. 2023, 631, 122535. [Google Scholar] [CrossRef] [PubMed]
- Turjeman, K.; Yanay, N.; Elbaz, M.; Bavli, Y.; Gross, M.; Rabie, M.; Barenholz, Y.; Nevo, Y. Liposomal steroid nano-drug is superior to steroids as-is in mdx mouse model of Duchenne muscular dystrophy. Nanomedicine 2019, 16, 34–44. [Google Scholar] [CrossRef]
- Ahmed, Z.; Qaisar, R. Nanomedicine for Treating Muscle Dystrophies: Opportunities, Challenges, and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 12039. [Google Scholar] [CrossRef]
- Chen, B.Q.; Zhao, Y.; Zhang, Y.; Pan, Y.J.; Xia, H.Y.; Kankala, R.K.; Wang, S.B.; Liu, G.; Chen, A.Z. Immune-regulating camouflaged nanoplatforms: A promising strategy to improve cancer nano-immunotherapy. Bioact. Mater. 2023, 21, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Steffens, R.C.; Wagner, E. Directing the Way-Receptor and Chemical Targeting Strategies for Nucleic Acid Delivery. Pharm. Res. 2023, 40, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Lo Buglio, A.; Vendemiale, G. Muscle Delivery of Mitochondria-Targeted Drugs for the Treatment of Sarcopenia: Rationale and Perspectives. Pharmaceutics 2022, 14, 2588. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Huot, J.R.; Bonetto, A. The Mitochondria-Targeting Agent MitoQ Improves Muscle Atrophy, Weakness and Oxidative Metabolism in C26 Tumor-Bearing Mice. Front. Cell Dev. Biol. 2022, 10, 861622. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Perumal, E.; Bi, X.; Wang, Y.; Ding, W. Potential mechanisms of uremic muscle wasting and the protective role of the mitochondria-targeted antioxidant Mito-TEMPO. Int. Urol. Nephrol. 2020, 52, 1551–1561. [Google Scholar] [CrossRef]
- van de Weijer, T.; Phielix, E.; Bilet, L.; Williams, E.G.; Ropelle, E.R.; Bierwagen, A.; Livingstone, R.; Nowotny, P.; Sparks, L.M.; Paglialunga, S.; et al. Evidence for a direct effect of the NAD+ precursor acipimox on muscle mitochondrial function in humans. Diabetes 2015, 64, 1193–1201. [Google Scholar] [CrossRef]
- Pirinen, E.; Cantó, C.; Jo, Y.S.; Morato, L.; Zhang, H.; Menzies, K.J.; Williams, E.G.; Mouchiroud, L.; Moullan, N.; Hagberg, C.; et al. Pharmacological Inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 2014, 19, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Sena Ozbay, H.; Yabanoglu-Ciftci, S.; Baysal, I.; Gultekinoglu, M.; Can Eylem, C.; Ulubayram, K.; Nemutlu, E.; Topaloglu, R.; Ozaltin, F. Mitochondria-targeted CoQ10 loaded PLGA-b-PEG-TPP nanoparticles: Their effects on mitochondrial functions of COQ8B-/- HK-2 cells. Eur. J. Pharm. Biopharm. 2022, 173, 22–33. [Google Scholar] [CrossRef]
- Faria, R.; Boisguérin, P.; Sousa, Â.; Costa, D. Delivery Systems for Mitochondrial Gene Therapy: A Review. Pharmaceutics 2023, 15, 572. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ling, L.; Lu, J.; Jiang, F.; Sun, J.; Zhang, Z.; Huang, Y.; Liu, X.; Zhu, Y.; Fu, X.; et al. Reactive oxygen species-responsive mitochondria-targeted liposomal quercetin attenuates retinal ischemia-reperfusion injury via regulating SIRT1/FOXO3A and p38 MAPK signaling pathways. Bioeng. Transl. Med. 2023, 8, e10460. [Google Scholar] [CrossRef]
- Liu, X.; Gao, J.; Yan, Y.; Georgiou, E.A.; Lou, J.; Feng, M.; Zhang, X.; Gao, F.; Liu, J.; Kostakis, I.K.; et al. Mitochondria-Targeted Triphenylphosphonium-Hydroxytyrosol Prevents Lipotoxicity-Induced Endothelial Injury by Enhancing Mitochondrial Function and Redox Balance via Promoting FoxO1 and Nrf2 Nuclear Translocation and Suppressing Inflammation via Inhibiting p38/NF-кB Pathway. Antioxidants 2023, 12, 175. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yao, Z.; Zhang, X.; Li, J.; Huang, C.; Ouyang, Y.; Qian, Y.; Fan, C. Energy-Supporting Enzyme-Mimic Nanoscaffold Facilitates Tendon Regeneration Based on a Mitochondrial Protection and Microenvironment Remodeling Strategy. Adv. Sci. 2022, 9, e2202542. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, V.; Carton, F.; Vattemi, G.; Arpicco, S.; Stella, B.; Berlier, G.; Marengo, A.; Boschi, F.; Malatesta, M. Uptake and intracellular distribution of different types of nanoparticles in primary human myoblasts and myotubes. Int. J. Pharm. 2019, 560, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Van Steenbergen, V.; Lavoie-Cardinal, F.; Kazwiny, Y.; Decet, M.; Martens, T.; Verstreken, P.; Boesmans, W.; De Koninck, P.; Vanden Berghe, P. Nano-positioning and tubulin conformation contribute to axonal transport regulation of mitochondria along microtubules. Proc. Natl. Acad. Sci. USA 2022, 119, e2203499119. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Qin, X.; Yang, Z.; Song, S.; Liu, X.; Wu, C.; Qian, J.; Huang, X.; Zhang, Y.; He, W. Engineered a dual-targeting HA-TPP/A nanoparticle for combination therapy against KRAS-TP53 co-mutation in gastrointestinal cancers. Bioact. Mater. 2024, 32, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, F.; Liu, K.; Liu, S.; Xiao, T.; Zhu, Y.; Xu, L. Mitochondria-Targeting Polymer Micelles in Stepwise Response Releasing Gemcitabine and Destroying the Mitochondria and Nucleus for Combined Antitumor Chemotherapy. Int. J. Mol. Sci. 2022, 23, 12624. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, G.H.; Yoo, K.H.; Khang, D. Overcoming multidrug-resistant lung cancer by mitochondrial-associated ATP inhibition using nanodrugs. J. Nanobiotechnol. 2023, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C.; Chang, H.S.; Yeh, C.Y.; Chang, H.J.; Cheng, W.L.; Lin, T.T.; Liu, C.S.; Chen, S.T. Regulation of mitochondrial fusion and mitophagy by intra-tumoral delivery of membrane-fused mitochondria or Midiv-1 enhances sensitivity to doxorubicin in triple-negative breast cancer. Biomed. Pharmacother. 2022, 153, 113484. [Google Scholar] [CrossRef] [PubMed]
- Jaganjac, M.; Borovic Sunjic, S.; Zarkovic, N. Utilizing Iron for Targeted Lipid Peroxidation as Anticancer Option of Integrative Biomedicine: A Short Review of Nanosystems Containing Iron. Antioxidants 2020, 9, 191. [Google Scholar] [CrossRef]
- Sang, M.; Luo, R.; Bai, Y.; Dou, J.; Zhang, Z.; Liu, F.; Feng, F.; Xu, J.; Liu, W. Mitochondrial membrane anchored photosensitive nano-device for lipid hydroperoxides burst and inducing ferroptosis to surmount therapy-resistant cancer. Theranostics 2019, 9, 6209–6223. [Google Scholar] [CrossRef]
- Yang, C.; Han, M.; Li, R.; Zhou, L.; Zhang, Y.; Duan, L.; Su, S.; Li, M.; Wang, Q.; Chen, T.; et al. Curcumin Nanoparticles Inhibiting Ferroptosis for the Enhanced Treatment of Intracerebral Hemorrhage. Int. J. Nanomed. 2021, 16, 8049–8065. [Google Scholar] [CrossRef] [PubMed]
- Mavridi-Printezi, A.; Menichetti, A.; Mordini, D.; Amorati, R.; Montalti, M. Recent Applications of Melanin-like Nanoparticles as Antioxidant Agents. Antioxidants 2023, 12, 863. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Peng, J.; Cheng, S.; Zhang, Q.; Zhang, N.; Zhou, Z.; Zhang, Y.; Zhao, Y.; Liu, T. Biomimetic Nanozymes Suppressed Ferroptosis to Ameliorate Doxorubicin-Induced Cardiotoxicity via Synergetic Effect of Antioxidant Stress and GPX4 Restoration. Nutrients 2023, 15, 1090. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, Y.; Guo, J.; Li, J.; Zhang, P.; Yang, H.; Rong, K.; Zhou, T.; Fu, J.; Zhao, J. Polydopamine Nanoparticles Targeting Ferroptosis Mitigate Intervertebral Disc Degeneration Via Reactive Oxygen Species Depletion, Iron Ions Chelation, and GPX4 Ubiquitination Suppression. Adv. Sci. 2023, 10, e2207216. [Google Scholar] [CrossRef] [PubMed]
- She, H.; Tan, L.; Du, Y.; Zhou, Y.; Guo, N.; Zhang, J.; Du, Y.; Wang, Y.; Wu, Z.; Ma, C.; et al. VDAC2 malonylation participates in sepsis-induced myocardial dysfunction via mitochondrial-related ferroptosis. Int. J. Biol. Sci. 2023, 19, 3143–3158. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, T.M.; Mooney, D.J. Anti-inflammatory nanoparticles significantly improve muscle function in a murine model of advanced muscular dystrophy. Sci. Adv. 2021, 7, eabh3693. [Google Scholar] [CrossRef] [PubMed]
- Andrei, V.; Andrei, S.; Gal, A.F.; Rus, V.; Gherman, L.M.; Boșca, B.A.; Niculae, M.; Barabas, R.; Cadar, O.; Dinte, E.; et al. Immunomodulatory Effect of Novel Electrospun Nanofibers Loaded with Doxycycline as an Adjuvant Treatment in Periodontitis. Pharmaceutics 2023, 15, 707. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, D.S.; Hwang, G.Y.; Lee, J.K.; Lee, H.L.; Jung, J.W.; Hwang, S.Y.; Baek, S.W.; Yoon, S.L.; Ha, Y.; et al. Multi-modulation of immune-inflammatory response using bioactive molecule-integrated PLGA composite for spinal fusion. Mater. Today Bio. 2023, 19, 100611. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yada, E.; Terai, Y.; Takahashi, T.; Nakanishi, H.; Tanaka, H.; Akita, H.; Itaka, K. Comprehensive Evaluation of Lipid Nanoparticles and Polyplex Nanomicelles for Muscle-Targeted mRNA Delivery. Pharmaceutics 2023, 15, 2291. [Google Scholar] [CrossRef]
- Islam, M.R.; Patel, J.; Back, P.I.; Shmeeda, H.; Kallem, R.R.; Shudde, C.; Markiewski, M.; Putnam, W.C.; Gabizon, A.A.; La-Beck, N.M. Pegylated Liposomal Alendronate Biodistribution, Immune Modulation, and Tumor Growth Inhibition in a Murine Melanoma Model. Biomolecules 2023, 13, 1309. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Han, J.F.; Zhang, F.Y.; Liao, T.T.; Na, R.; Yuan, X.F.; He, G.B.; Ye, W. Flexible nano-liposomes-based transdermal hydrogel for targeted delivery of dexamethasone for rheumatoid arthritis therapy. Drug Deliv. 2022, 29, 2269–2282. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, G.; Amoriello, R.; Lozano, N.; Carnasciali, A.; Guasti, D.; Becucci, M.; Cellot, G.; Kostarelos, K.; Ballerini, C.; Ballerini, L. Graphene Oxide Nanosheets Reduce Astrocyte Reactivity to Inflammation and Ameliorate Experimental Autoimmune Encephalomyelitis. ACS Nano 2023, 17, 1965–1978. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Huang, Y.; Kou, Q.; Lu, L.; Jiang, H.; Li, X.; Gui, R.; Huang, R.; Huang, X.; Ma, J.; et al. Study on the Role of an Erythrocyte Membrane-Coated Nanotheranostic System in Targeted Immune Regulation of Alzheimer’s Disease. Adv. Sci. 2023, 10, e2301361. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, T.M.; Mooney, D.J. Functional muscle recovery with nanoparticle-directed M2 macrophage polarization in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 10648–10653. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Z.; Liu, Y.; Cao, X.; Li, F.; Ran, H.; Cao, Y.; Wu, C. ‘Mito-Bomb’: A novel mitochondria-targeting nanosystem for ferroptosis-boosted sonodynamic antitumor therapy. Drug Deliv. 2022, 29, 3111–3122. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guo, J.; Wang, Y.; Xing, X.; Zhang, X.; Zhang, G.; Dong, Z. Acute myocardial infarction therapy using calycosin and tanshinone co-loaded mitochondria targeted lipid-polymer hybrid nano-system: Preparation, characterization, and anti myocardial infarction activity assessment. Biomed. Pharmacother. 2022, 155, 113650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Song, Q.; Yu, R.; Wang, A.; Jiang, G.; Huang, Y.; Chen, J.; Xu, J.; Wang, D.; Chen, H.; et al. Nano-Brake Halts Mitochondrial Dysfunction Cascade to Alleviate Neuropathology and Rescue Alzheimer’s Cognitive Deficits. Adv. Sci. 2023, 10, e2204596. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jin, F.; Liu, D.; Shu, G.; Wang, X.; Qi, J.; Sun, M.; Yang, P.; Jiang, S.; Ying, X.; et al. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics 2020, 10, 2342–2357. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Wang, B.; Liu, Y.; Wan, Y.; Liu, Q.; Xu, H.; Liang, R.; Shi, Y.; Tu, P.; Wu, H.; et al. Mitochondria-targeting polydopamine-coated nanodrugs for effective photothermal- and chemo-synergistic therapies against lung cancer. Regen. Biomater. 2022, 9, rbac051. [Google Scholar] [CrossRef]
- Qin, Y.T.; Ma, Y.J.; Feng, Y.S.; He, X.W.; Li, W.Y.; Zhang, Y.K. Targeted Mitochondrial Fluorescence Imaging-Guided Tumor Antimetabolic Therapy with the Imprinted Polymer Nanomedicine Capable of Specifically Recognizing Dihydrofolate Reductase. ACS Appl. Mater. Interfaces 2021, 13, 40332–40341. [Google Scholar] [CrossRef]
- Li, J.; Fan, J.; Gao, Y.; Huang, S.; Huang, D.; Li, J.; Wang, X.; Santos, H.A.; Shen, P.; Xia, B. Porous Silicon Nanocarriers Boost the Immunomodulation of Mitochondria-Targeted Bovine Serum Albumins on Macrophage Polarization. ACS Nano 2023, 17, 1036–1053. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Liu, Y.; Liu, W.; Yu, G.; Huang, Y.; Yang, Y.; Chen, X.; Chen, T. Brain-Targeted Biomimetic Nanodecoys with Neuroprotective Effects for Precise Therapy of Parkinson’s Disease. ACS Cent. Sci. 2022, 8, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hong, H.; Xue, J.; Luo, J.; Liu, Q.; Chen, X.; Pan, Y.; Zhou, J.; Liu, Z.; Chen, T. Near-Infrared Radiation-Assisted Drug Delivery Nanoplatform to Realize Blood-Brain Barrier Crossing and Protection for Parkinsonian Therapy. ACS Appl. Mater. Interfaces 2021, 13, 37746–37760. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Li, Z.; Liu, Y.; Ma, R.; Chen, X.; Liu, W.; Song, Y.; Zhang, Y.; Yu, G.; Wu, Z.; et al. “Swiss Army Knife” black phosphorus-based nanodelivery platform for synergistic antiparkinsonian therapy via remodeling the brain microenvironment. J. Control. Release 2023, 353, 752–766. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, Y.; Cheng, G.; Ni, Y.; Xie, A.; Zhu, X.; Yin, C.; Zhang, Y.; Chen, T. Customized Intranasal Hydrogel Delivering Methylene Blue Ameliorates Cognitive Dysfunction against Alzheimer’s Disease. Adv. Mater. 2024, e2307081. [Google Scholar] [CrossRef]
- Yao, S.; Wu, D.; Hu, X.; Chen, Y.; Fan, W.; Mou, X.; Cai, Y.; Yang, X. Platelet membrane-coated bio-nanoparticles of indocyanine green/elamipretide for NIR diagnosis and antioxidant therapy in acute kidney injury. Acta Biomater. 2023, 173, 482–497. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Chen, H.; Ren, Z.; Zhang, P.; Chen, J.; Jiang, S. Ultrasmall iron oxide nanoparticles induced ferroptosis via Beclin1/ATG5-dependent autophagy pathway. Nano Converg. 2021, 8, 10. [Google Scholar] [CrossRef]
- Liu, C.; Zou, Q.; Tang, H.; Liu, J.; Zhang, S.; Fan, C.; Zhang, J.; Liu, R.; Liu, Y.; Liu, R.; et al. Melanin nanoparticles alleviate sepsis-induced myocardial injury by suppressing ferroptosis and inflammation. Bioact. Mater. 2023, 24, 313–321. [Google Scholar] [CrossRef]
- Xie, D.M.; Zhong, Q.; Xu, X.; Li, Y.; Chen, S.; Li, M.; Peng, C. Alpha lipoic acid-loaded electrospun fibrous patch films protect heart in acute myocardial infarction mice by inhibiting oxidative stress. Int. J. Pharm. 2023, 632, 122581. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, D.; Yang, F.; Ye, C.; Chen, Z.; Chen, Y.; Yu, X.; Xie, J.; Dou, Y.; Chang, J. In Situ Self-Assembled Phytopolyphenol-Coordinated Intelligent Nanotherapeutics for Multipronged Management of Ferroptosis-Driven Alzheimer’s Disease. ACS Nano 2024, 18, 7890–7906. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Z.; Wu, G.; Yin, L.; Xu, L.; Wang, N.; Peng, J. Apigenin-7-glucoside-loaded nanoparticle alleviates intestinal ischemia-reperfusion by ATF3/SLC7A11-mediated ferroptosis. J. Control. Release 2024, 366, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Duan, L.; Yang, Y.; Liu, W.; Zhang, Y.; Zhou, L.; Su, S.; Lo, P.C.; Cai, J.; Gao, L.; et al. Nanoparticles improved resveratrol brain delivery and its therapeutic efficacy against intracerebral hemorrhage. Nanoscale 2021, 13, 3827–3840. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Li, J.; Liu, J.; Feng, B.; Zhang, T.; Liu, Q.; Ma, H.; Wu, H.; Wu, H. Targeting ferroptosis by poly(acrylic) acid coated Mn3O4 nanoparticles alleviates acute liver injury. Nat. Commun. 2023, 14, 7598. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.S.; Qin, C.; Dong, M.H.; Heming, M.; Zhou, L.Q.; Wang, W.; Cai, S.B.; You, Y.F.; Shang, K.; Xiao, J.; et al. B cell lineage reconstitution underlies CAR-T cell therapeutic efficacy in patients with refractory myasthenia gravis. EMBO Mol. Med. 2024, 16, 966–987. [Google Scholar] [CrossRef]
- Dalakas, M.C. Progress in the therapy of myasthenia gravis: Getting closer to effective targeted immunotherapies. Curr. Opin. Neurol. 2020, 33, 545–552. [Google Scholar] [CrossRef]
| Treatment Strategy | NCT Number | Drug | Actual Enrollment | Age | Phase | Status |
|---|---|---|---|---|---|---|
| Antagonize neonatal Fc receptor | NCT05681715 | Rozanolixizumab | 62 | ≥18 | Phase 3 | On going |
| NCT04951622 | Nipocalimab | 198 | ≥18 | Phase 3 | Recruiting | |
| NCT05265273 | Nipocalimab | 12 | 2~17 | Phase 2 Phase 3 | Recruiting | |
| NCT05403541 | Batoclimab | 240 | ≥18 | Phase 3 | Recruiting | |
| NCT04980495 | Efgartigimod | 69 | ≥18 | Phase 3 | On going | |
| NCT05374590 | Efgartigimod | 12 | 2~18 | Phase 2 Phase 3 | Recruiting | |
| NCT04833894 | Efgartigimod | 12 | 2~18 | Phase 2 Phase 3 | Recruiting | |
| NCT04818671 | Efgartigimod | 183 | ≥18 | Phase 3 | On going | |
| Inhibit complement | NCT06055959 | Zilucoplan | 8 | 12~17 | Phase 2 Phase 3 | Recruiting |
| NCT04225871 | Zilucoplan | 200 | ≥18 | Phase 3 | On going | |
| NCT05514873 | Zilucoplan | 26 | 18~85 | Phase 3 | On going | |
| NCT05644561 | Ravulizumab | 12 | Not limited | Phase 3 | Recruiting | |
| NCT05070858 | Pozelimab and Cemdisiran | 235 | ≥18 | Phase 3 | Recruiting | |
| NCT06282159 | DNTH103 | 60 | 18~75 | Phase 2 | Recruiting | |
| Target IL-6R | NCT05067348 | Tocilizumab | 64 | 18~80 | Phase 2 | Recruiting |
| NCT05716035 | Tocilizumab | 64 | 18~80 | Phase 2 Phase 3 | Recruiting | |
| NCT04963270 | Satralizumab | 185 | ≥12 | Phase 3 | Recruiting | |
| CAR-T cells | NCT05828225 | CD19 CAR-T cells | 9 | ≥18 | Phase 1 | Recruiting |
| NCT04146051 | Descartes-08 | 30 | ≥18 | Phase 2 | Recruiting | |
| Target B cells | NCT04524273 | Inebilizumab | 238 | ≥18 | Phase 3 | On going |
| NCT05737160 | Telitacicept | 100 | 18~80 | Phase 3 | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Yan, Z.; Song, Y.; Chen, T. Nanodrug Delivery Systems for Myasthenia Gravis: Advances and Perspectives. Pharmaceutics 2024, 16, 651. https://doi.org/10.3390/pharmaceutics16050651
Huang J, Yan Z, Song Y, Chen T. Nanodrug Delivery Systems for Myasthenia Gravis: Advances and Perspectives. Pharmaceutics. 2024; 16(5):651. https://doi.org/10.3390/pharmaceutics16050651
Chicago/Turabian StyleHuang, Jiayan, Zhao Yan, Yafang Song, and Tongkai Chen. 2024. "Nanodrug Delivery Systems for Myasthenia Gravis: Advances and Perspectives" Pharmaceutics 16, no. 5: 651. https://doi.org/10.3390/pharmaceutics16050651
APA StyleHuang, J., Yan, Z., Song, Y., & Chen, T. (2024). Nanodrug Delivery Systems for Myasthenia Gravis: Advances and Perspectives. Pharmaceutics, 16(5), 651. https://doi.org/10.3390/pharmaceutics16050651







