Formulation of Ascorbic Acid and Betaine-based Therapeutic Deep Eutectic System for Enhanced Transdermal Delivery of Ascorbic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AA:Bet:H2O THEDES
2.3. Characterization of AA:Bet:H2O THEDES
2.3.1. Polarized Optical Microscopy Analysis
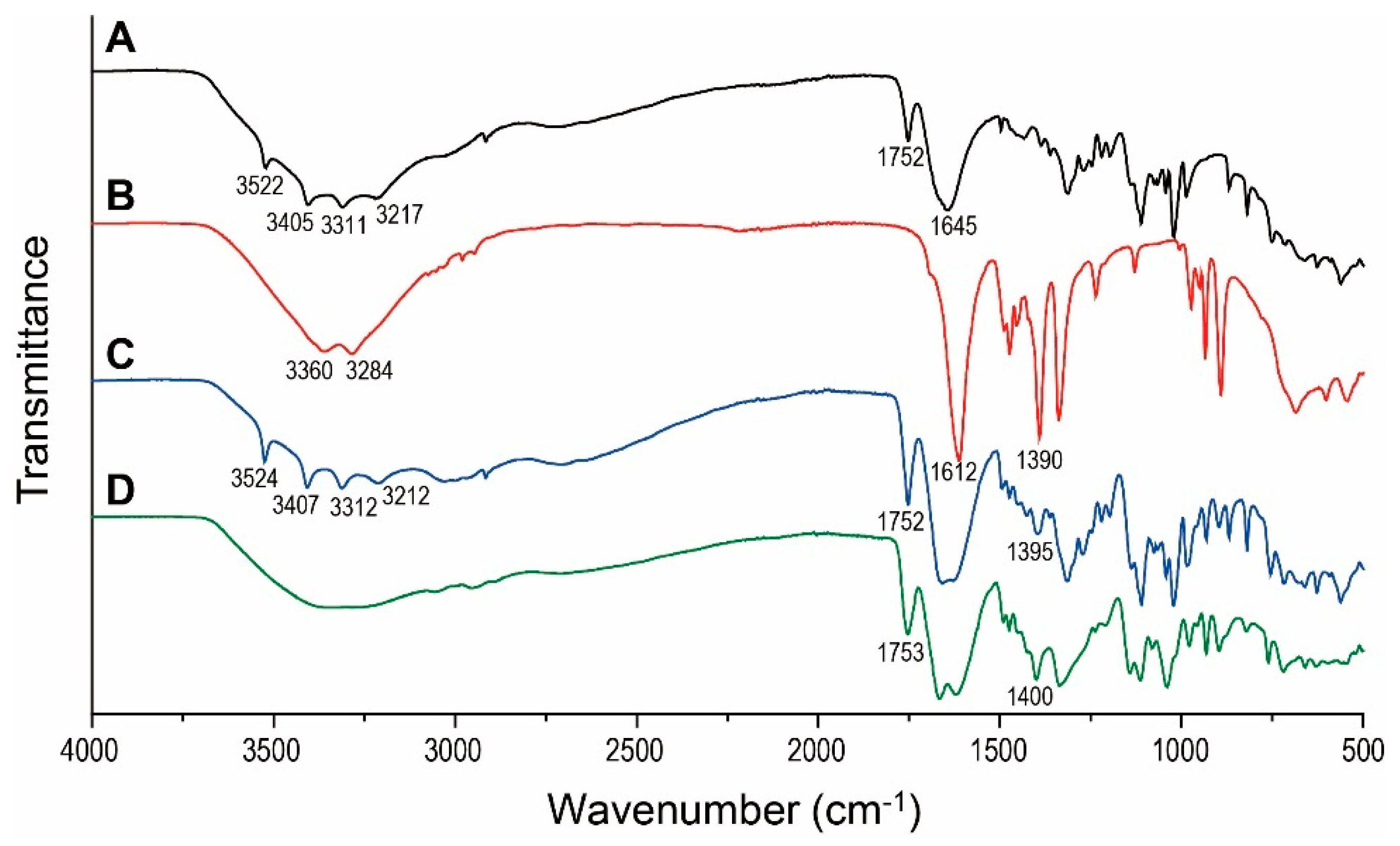
2.3.2. Fourier Transform Infrared Spectroscopy Analysis
2.3.3. Differential Scanning Calorimetry Analysis
2.3.4. Solubility Measurement in Polyols
2.4. In Vitro Skin Permeation Studies
2.5. In Vivo Efficacy Test
3. Results and Discussion
3.1. Polarized Optical Microscopy Analysis
3.2. Fourier Transform Infrared Spectroscopy Analysis
3.3. Differential Scanning Calorimetry and Solubility Studies
3.4. In Vitro Skin Permeation Studies
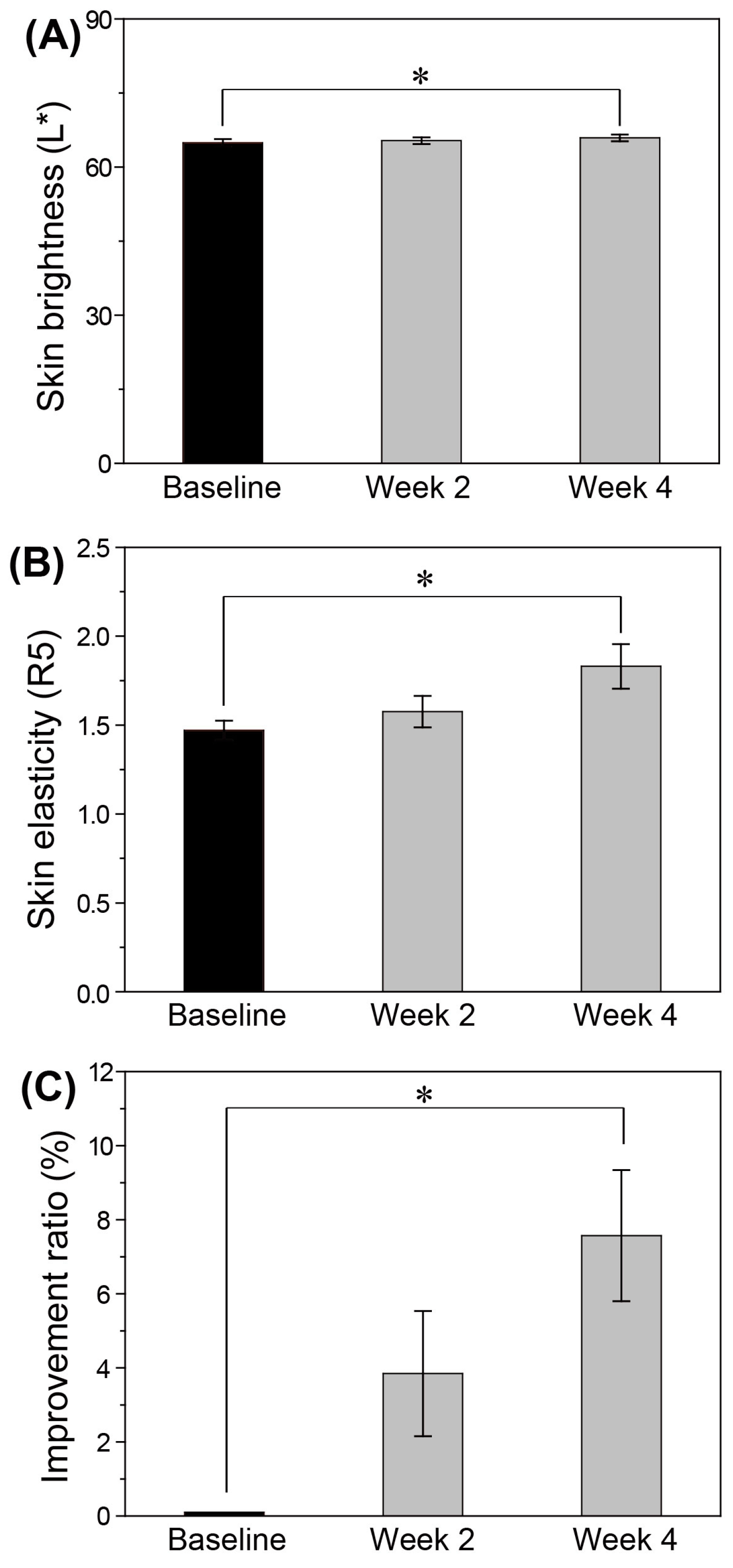
3.5. In Vivo Skin Changes after THEDES Serum Use
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zhao, J.; Duan, H.; Guan, Y.; Zhao, L. Green and efficient extraction of four bioactive flavonoids from Pollen Typhae by ultrasound-assisted deep eutectic solvents extraction. J. Pharm. Biomed. Anal. 2018, 161, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Carriazo, D.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Deep-eutectic solvents playing multiple roles in the synthesis of polymers and related materials. Chem. Soc. Rev. 2012, 41, 4996–5014. [Google Scholar] [CrossRef] [PubMed]
- Pollet, P.; Davey, E.A.; Ureña-Benavides, E.E.; Eckert, C.A.; Liotta, C.L. Solvents for sustainable chemical processes. Green Chem. 2014, 16, 1034–1055. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Ionic liquids and deep eutectic solvents in natural products research: Mixtures of solids as extraction solvents. J. Nat. Prod. 2013, 76, 2162–2173. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Hammond, O.S.; Mudring, A.-V. Ionic liquids and deep eutectics as a transformative platform for the synthesis of nanomaterials. Chem. Commun. 2022, 58, 3865–3892. [Google Scholar] [CrossRef]
- Rojas, O.G.; Nakayama, T.; Hall, S.R. Green and cost-effective synthesis of the superconductor BSCCO (Bi-2212), using a natural deep eutectic solvent. Ceram. Int. 2019, 45, 8546–8552. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Ali, S.; Li, P.; Zhang, W.; Rauch, M.C.R.; Willot, S.J.; Ribitsch, D.; Choi, Y.H.; Alcalde, M. Natural deep eutectic solvents as performance additives for peroxygenase catalysis. ChemCatChem 2020, 12, 989–994. [Google Scholar] [CrossRef]
- Benoit, C.; Virginie, C.; Boris, V. The use of NADES to support innovation in the cosmetic industry. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 97, pp. 309–332. ISBN 0065-2296. [Google Scholar]
- Punzo, A.; Porru, E.; Silla, A.; Simoni, P.; Galletti, P.; Roda, A.; Tagliavini, E.; Samorì, C.; Caliceti, C. Grape Pomace for Topical Application: Green NaDES Sustainable Extraction, Skin Permeation Studies, Antioxidant and Anti-Inflammatory Activities Characterization in 3D Human Keratinocytes. Biomolecules 2021, 11, 1181. [Google Scholar] [CrossRef] [PubMed]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural deep eutectic solvents (Nades): Phytochemical extraction performance enhancer for pharmaceutical and nutraceutical product development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, N.R.; Spelbos, V.S.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Solubility and stability of some pharmaceuticals in natural deep eutectic solvents-based formulations. Molecules 2021, 26, 2645. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Ahmed, E.I.; Prasad, K.; Qader, I.B.; Ryder, K.S. Liquid pharmaceuticals formulation by eutectic formation. Fluid Phase Equilibria 2017, 448, 2–8. [Google Scholar] [CrossRef]
- Stott, P.W.; Williams, A.C.; Barry, B.W. Transdermal delivery from eutectic systems: Enhanced permeation of a model drug, ibuprofen. J. Control. Release 1998, 50, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gurau, G.; Shamshina, J.; Cojocaru, O.A.; Janikowski, J.; MacFarlane, D.R.; Davis, J.H.; Rogers, R.D. Simultaneous membrane transport of two active pharmaceutical ingredients by charge assisted hydrogen bond complex formation. Chem. Sci. 2014, 5, 3449–3456. [Google Scholar] [CrossRef]
- Yin, T.; Wu, J.; Yuan, J.; Wang, X. Therapeutic deep eutectic solvent based on osthole and paeonol: Preparation, characterization, and permeation behavior. J. Mol. Liq. 2022, 346, 117133. [Google Scholar] [CrossRef]
- Farris, P.K. Topical vitamin C: A useful agent for treating photoaging and other dermatologic conditions. Dermatol. Surg. 2005, 31, 814–818. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Honeywell-Nguyen, P.L.; Gooris, G.S.; Ponec, M. Structure of the skin barrier and its modulation by vesicular formulations. Prog. Lipid Res. 2003, 42, 1–36. [Google Scholar] [CrossRef]
- Kim, M.; Yang, H.; Kim, H.; Jung, H.; Jung, H. Novel cosmetic patches for wrinkle improvement: Retinyl retinoate-and ascorbic acid-loaded dissolving microneedles. Int. J. Cosmet. Sci. 2014, 36, 207–212. [Google Scholar] [CrossRef]
- Starr, N.J.; Hamid, K.A.; Wibawa, J.; Marlow, I.; Bell, M.; Pérez-García, L.; Barrett, D.A.; Scurr, D.J. Enhanced vitamin C skin permeation from supramolecular hydrogels, illustrated using in situ ToF-SIMS 3D chemical profiling. Int. J. Pharm. 2019, 563, 21–29. [Google Scholar] [CrossRef]
- Ebihara, M.; Akiyama, M.; Ohnishi, Y.; Tajima, S.; Komata, K.; Mitsui, Y. Iontophoresis promotes percutaneous absorption of L-ascorbic acid in rat skin. J. Dermatol. Sci. 2003, 32, 217–222. [Google Scholar] [CrossRef]
- Raschke, T.; Koop, U.; Düsing, H.-J.; Filbry, A.; Sauermann, K.; Jaspers, S.; Wenck, H.; Wittern, K.-P. Topical activity of ascorbic acid: From in vitro optimization to in vivo efficacy. Skin Pharmacol. Physiol. 2004, 17, 200–206. [Google Scholar] [CrossRef]
- Gallarate, M.; Carlotti, M.E.; Trotta, M.; Bovo, S. On the stability of ascorbic acid in emulsified systems for topical and cosmetic use. Int. J. Pharm. 1999, 188, 233–241. [Google Scholar] [CrossRef]
- Austria, R.; Semenzato, A.; Bettero, A. Stability of vitamin C derivatives in solution and topical formulations. J. Pharm. Biomed. Anal. 1997, 15, 795–801. [Google Scholar] [CrossRef]
- Ahmad, I.; Sheraz, M.A.; Ahmed, S.; Shaikh, R.H.; Vaid, F.H.M.; Ansari, S.A. Photostability and interaction of ascorbic acid in cream formulations. Aaps Pharmscitech 2011, 12, 917–923. [Google Scholar] [CrossRef]
- Maione-Silva, L.; de Castro, E.G.; Nascimento, T.L.; Cintra, E.R.; Moreira, L.C.; Cintra, B.A.S.; Valadares, M.C.; Lima, E.M. Ascorbic acid encapsulated into negatively charged liposomes exhibits increased skin permeation, retention and enhances collagen synthesis by fibroblasts. Sci. Rep. 2019, 9, 522. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, W.; Zou, L.; Liu, W.; Liu, C.; Liang, R.; Chen, J. Storage stability and skin permeation of vitamin C liposomes improved by pectin coating. Colloids Surf. B: Biointerfaces 2014, 117, 330–337. [Google Scholar] [CrossRef]
- Heber, G.K.; Markovic, B.; Hayes, A. An immunohistological study of anhydrous topical ascorbic acid compositions on ex vivo human skin. J. Cosmet. Dermatol. 2006, 5, 150–156. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Stamford, N.P.J. Stability, transdermal penetration, and cutaneous effects of ascorbic acid and its derivatives. J. Cosmet. Dermatol. 2012, 11, 310–317. [Google Scholar] [CrossRef]
- Humbert, P.G.; Haftek, M.; Creidi, P.; Lapière, C.; Nusgens, B.; Richard, A.; Schmitt, D.; Rougier, A.; Zahouani, H. Topical ascorbic acid on photoaged skin. Clinical, topographical and ultrastructural evaluation: Double-blind study vs. placebo. Exp. Dermatol. 2003, 12, 237–244. [Google Scholar] [CrossRef]
- Espinal-Perez, L.E.; Moncada, B.; Castanedo-Cazares, J.P. A double-blind randomized trial of 5% ascorbic acid vs. 4% hydroquinone in melasma. Int. J. Dermatol. 2004, 43, 604–607. [Google Scholar] [CrossRef]
- Kameyama, K.; Sakai, C.; Kondoh, S.; Yonemoto, K.; Nishiyama, S.; Tagawa, M.; Murata, T.; Ohnuma, T.; Quigley, J.; Dorsky, A. Inhibitory effect of magnesium L-ascorbyl-2-phosphate (VC-PMG) on melanogenesis in vitro and in vivo. J. Am. Acad. Dermatol. 1996, 34, 29–33. [Google Scholar] [CrossRef]
- Matsuda, S.; Shibayama, H.; Hisama, M.; Ohtsuki, M.; Iwaki, M. Inhibitory effects of a novel ascorbic derivative, disodium isostearyl 2-O-L-ascorbyl phosphate on melanogenesis. Chem. Pharm. Bull. 2008, 56, 292–297. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Saito, N.; Kurita, K.; Shimokado, K.; Maruyama, N.; Ishigami, A. Ascorbic acid enhances the expression of type 1 and type 4 collagen and SVCT2 in cultured human skin fibroblasts. Biochem. Biophys. Res. Commun. 2013, 430, 579–584. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z. Transport and intracellular accumulation of vitamin C in endothelial cells: Relevance to collagen synthesis. Arch. Biochem. Biophys. 2005, 434, 178–186. [Google Scholar] [CrossRef]
- Ghosh, N.; VK, P.; Sharma, J.D.; Vajarekar, A.S. Estimation of dermal absorption parameters of cleaning chemical ingredients. J. Chem. Heal. Saf. 2019, 26, 9–14. [Google Scholar] [CrossRef]
- Abdelmalek, M.F.; Angulo, P.; Jorgensen, R.A.; Sylvestre, P.B.; Lindor, K.D. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: Results of a pilot study. Am. J. Gastroenterol. 2001, 96, 2711–2717. [Google Scholar] [CrossRef]
- Hayes, K.C.; Pronczuk, A.; Cook, M.W.; Robbins, M.C. Betaine in sub-acute and sub-chronic rat studies. Food Chem. Toxicol. 2003, 41, 1685–1700. [Google Scholar] [CrossRef]
- Chen, A.; An, Y.; Huang, W.; Xuan, T.; Zhang, Q.; Ye, M.; Luo, S.; Xuan, X.; He, H.; Zheng, J. Highly Water-Preserving Zwitterionic Betaine-Incorporated Collagen Sponges with Anti-oxidation and Anti-inflammation for Wound Regeneration. Front. Cell Dev. Biol. 2020, 8, 491. [Google Scholar] [CrossRef]
- Wattanaploy, S.; Chinaroonchai, K.; Namviriyachote, N.; Muangman, P. Randomized controlled trial of polyhexanide/betaine gel versus silver sulfadiazine for partial-thickness burn treatment. Int. J. Low. Extrem. Wounds 2017, 16, 45–50. [Google Scholar] [CrossRef]
- Rantanen, I.; Nicander, I.; Jutila, K.; Ollmar, S.; Tenovuo, J.; Söderling, E. Betaine reduces the irritating effect of sodium lauryl sulfate on human oral mucosa in vivo. Acta Odontol. Scand. 2002, 60, 306–310. [Google Scholar] [CrossRef]
- Aroso, I.M.; Silva, J.C.; Mano, F.; Ferreira, A.S.D.; Dionísio, M.; Sá-Nogueira, I.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur. J. Pharm. Biopharm. 2016, 98, 57–66. [Google Scholar] [CrossRef]
- Panicker, C.Y.; Varghese, H.T.; Philip, D. FT-IR, FT-raman and SERS spectra of vitamin C. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 65, 802–804. [Google Scholar] [CrossRef]
- Yadav, R.A.; Rani, P.; Kumar, M.; Singh, R.; Singh, P.; Singh, N.P. Experimental IR and Raman spectra and quantum chemical studies of molecular structures, conformers and vibrational characteristics of L-ascorbic acid and its anion and cation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 84, 6–21. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, B.; Sun, S.; Wu, P. Skin-like mechanoresponsive self-healing ionic elastomer from supramolecular zwitterionic network. Nat. Commun. 2021, 12, 4082. [Google Scholar] [CrossRef]
- Contreras, R.; Lodeiro, L.; Rozas-Castro, N.; Ormazábal-Toledo, R. On the role of water in the hydrogen bond network in DESs: An ab initio molecular dynamics and quantum mechanical study on the urea–betaine system. Phys. Chem. Chem. Phys. 2021, 23, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Vanda, H.; Dai, Y.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. Comptes Rendus Chim. 2018, 21, 628–638. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Zhao, Y.; Wang, J.; Yu, Z. Insights into the hydrogen bond interactions in deep eutectic solvents composed of choline chloride and polyols. ACS Sustain. Chem. Eng. 2019, 7, 7760–7767. [Google Scholar] [CrossRef]
- Maréchal, Y. Infrared and related spectroscopies of H-bonded systems: Experimental point of view. Hydrog. Bond Water Mol. 2007, 77–113. [Google Scholar] [CrossRef]
- Pinnell, S.R.; Yang, H.; Omar, M.; Riviere, N.M.; Debuys, H.V.; Walker, L.C.; Wang, Y.; Levine, M. Topical L-ascorbic acid: Percutaneous absorption studies. Dermatologic Surg. 2001, 27, 137–142. [Google Scholar] [CrossRef]
- Chaudhari, S.P.; Patil, P.S. Pharmaceutical excipients: A review. Int. J. Adv. Pharm. Biol. Chem. 2012, 1, 21–34. [Google Scholar]
- Iliopoulos, F.; Sil, B.C.; Al Hossain, A.S.M.M.; Moore, D.J.; Lucas, R.A.; Lane, M.E. Topical delivery of niacinamide: Influence of neat solvents. Int. J. Pharm. 2020, 579, 119137. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.R.; Monteiro e Silva, S.A.; Leonardi, G.R. Effects of 1, 3-propanediol associated, or not, with butylene glycol and/or glycerol on skin hydration and skin barrier function. Int. J. Cosmet. Sci. 2024, 46, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Stricker-Krongrad, A.; Shoemake, C.R.; Liu, J.; Brocksmith, D.; Bouchard, G. The importance of minipigs in dermal safety assessment: An overview. Cutan. Ocul. Toxicol. 2017, 36, 105–113. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chormale, J.H.; Bansal, A.K. Deep eutectic systems: An overview of fundamental aspects, current understanding and drug delivery applications. Int. J. Pharm. 2021, 610, 121203. [Google Scholar] [CrossRef]
- Leveque, N.; Muret, P.; Makki, S.; Mac-Mary, S.; Kantelip, J.P.; Humbert, P. Ex vivo cutaneous absorption assessment of a stabilized ascorbic acid formulation using a microdialysis system. Skin Pharmacol. Physiol. 2004, 17, 298–303. [Google Scholar] [CrossRef]
- Green, B.A.; Ruey, J.Y.; Van Scott, E.J. Clinical and cosmeceutical uses of hydroxyacids. Clin. Dermatol. 2009, 27, 495–501. [Google Scholar] [CrossRef]
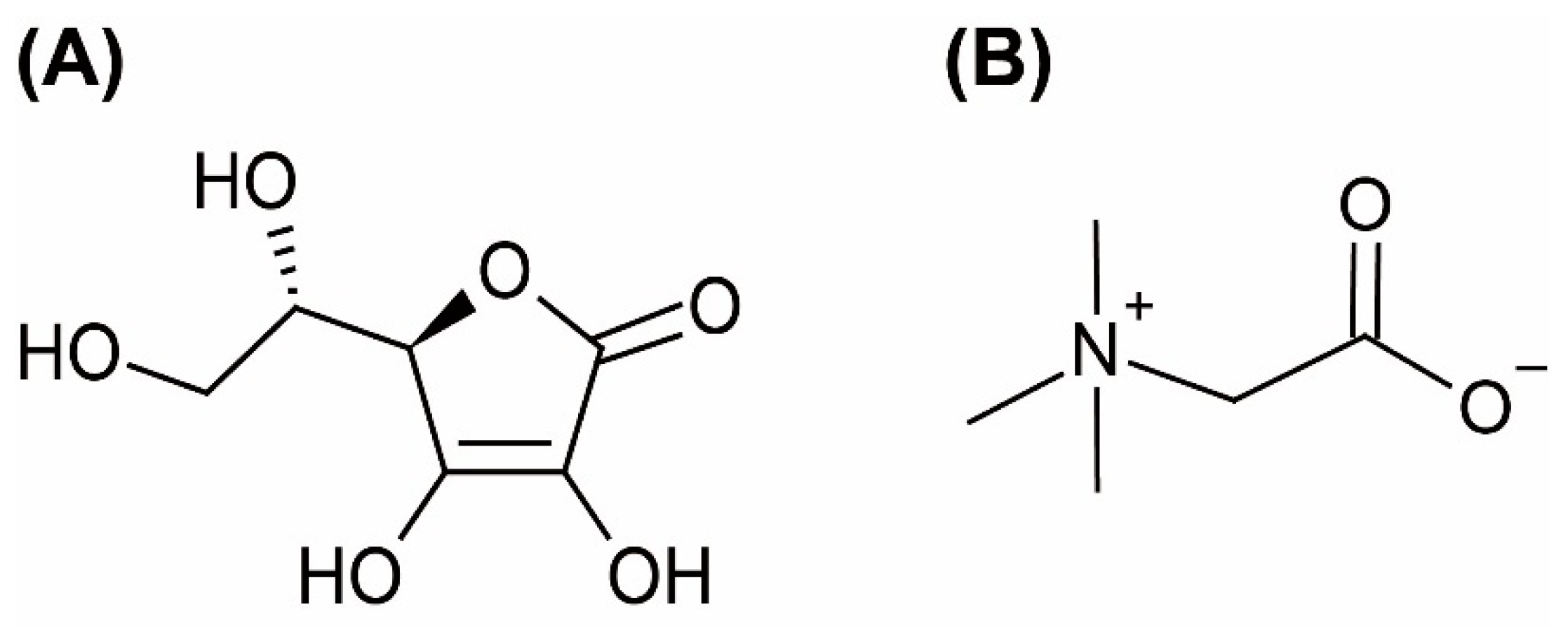
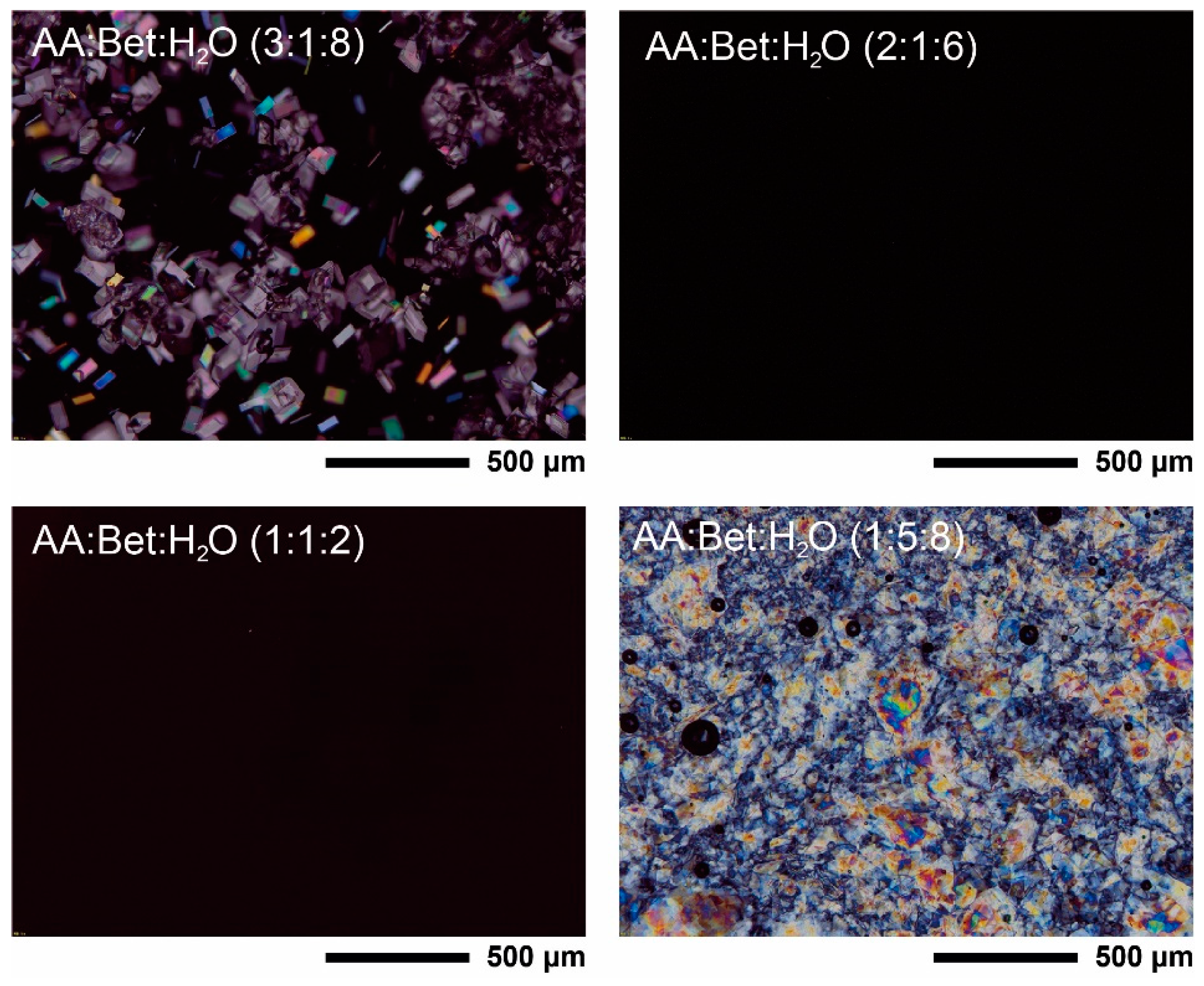


| Component | Molar Ratio | Abbreviation | Appearance | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Ascorbic acid | Betaine | Water | 3:1:8 a | - | Solid paste |
| 2:1:6 b | AA:Bet:H2O (2:1:6) | Clear liquid at room temperature | |||
| 1:1:2 | AA:Bet:H2O (1:1:2) | ||||
| 1:5:8 | - | Solid paste | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.-E.; Jun, S.-H.; Ryoo, J.-Y.; Kang, N.-G. Formulation of Ascorbic Acid and Betaine-based Therapeutic Deep Eutectic System for Enhanced Transdermal Delivery of Ascorbic Acid. Pharmaceutics 2024, 16, 687. https://doi.org/10.3390/pharmaceutics16050687
Song J-E, Jun S-H, Ryoo J-Y, Kang N-G. Formulation of Ascorbic Acid and Betaine-based Therapeutic Deep Eutectic System for Enhanced Transdermal Delivery of Ascorbic Acid. Pharmaceutics. 2024; 16(5):687. https://doi.org/10.3390/pharmaceutics16050687
Chicago/Turabian StyleSong, Ji-Eun, Seung-Hyun Jun, Joo-Yeon Ryoo, and Nae-Gyu Kang. 2024. "Formulation of Ascorbic Acid and Betaine-based Therapeutic Deep Eutectic System for Enhanced Transdermal Delivery of Ascorbic Acid" Pharmaceutics 16, no. 5: 687. https://doi.org/10.3390/pharmaceutics16050687
APA StyleSong, J.-E., Jun, S.-H., Ryoo, J.-Y., & Kang, N.-G. (2024). Formulation of Ascorbic Acid and Betaine-based Therapeutic Deep Eutectic System for Enhanced Transdermal Delivery of Ascorbic Acid. Pharmaceutics, 16(5), 687. https://doi.org/10.3390/pharmaceutics16050687






