Unlocking the Potential of Oleanolic Acid: Integrating Pharmacological Insights and Advancements in Delivery Systems
Abstract
1. Introduction
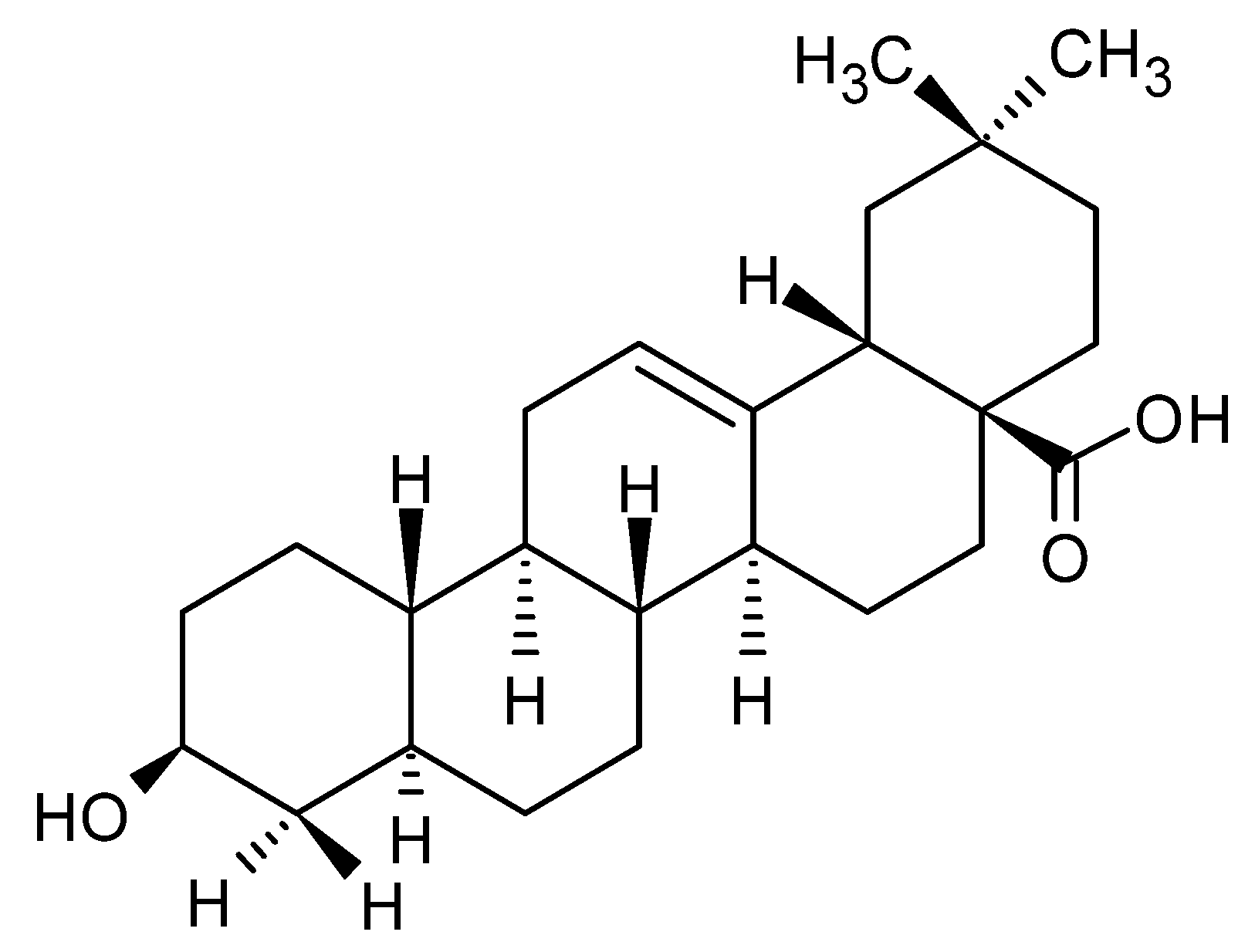
1.1. Chemical Structure and Properties of OA
1.2. Natural Sources of OA and Its Extraction
2. Pharmacological Activities of OA
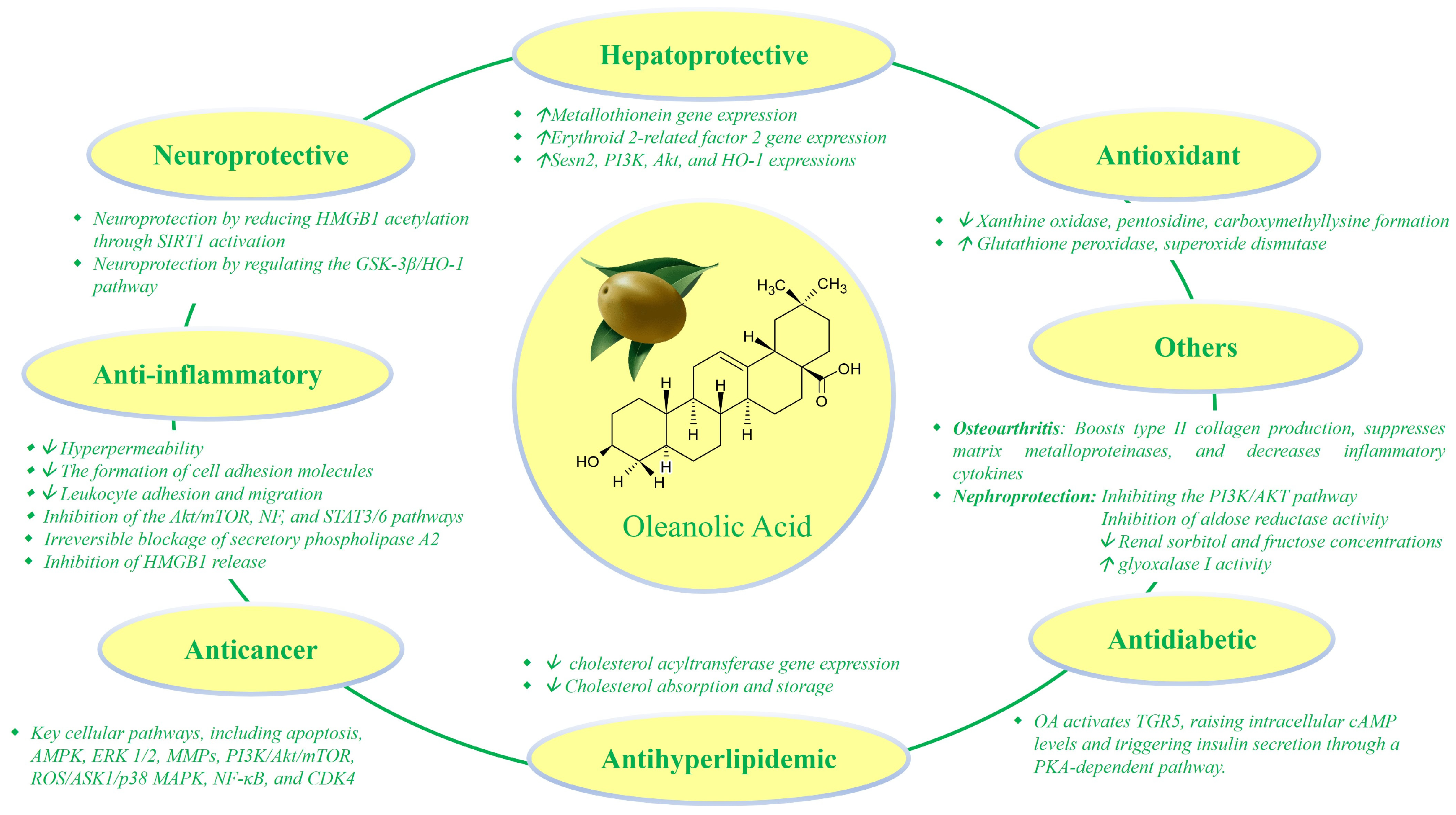
2.1. Anti-Inflammatory Properties
2.2. Antioxidant Effects
2.3. Anticancer Potential
2.4. Hepatoprotective Activity
2.5. Neuroprotective Activity
2.6. Other Therapeutic Effects
3. Combination with Other Natural Compounds or Conventional Drugs
4. Preclinical and Clinical Studies
5. Challenges in OA Delivery
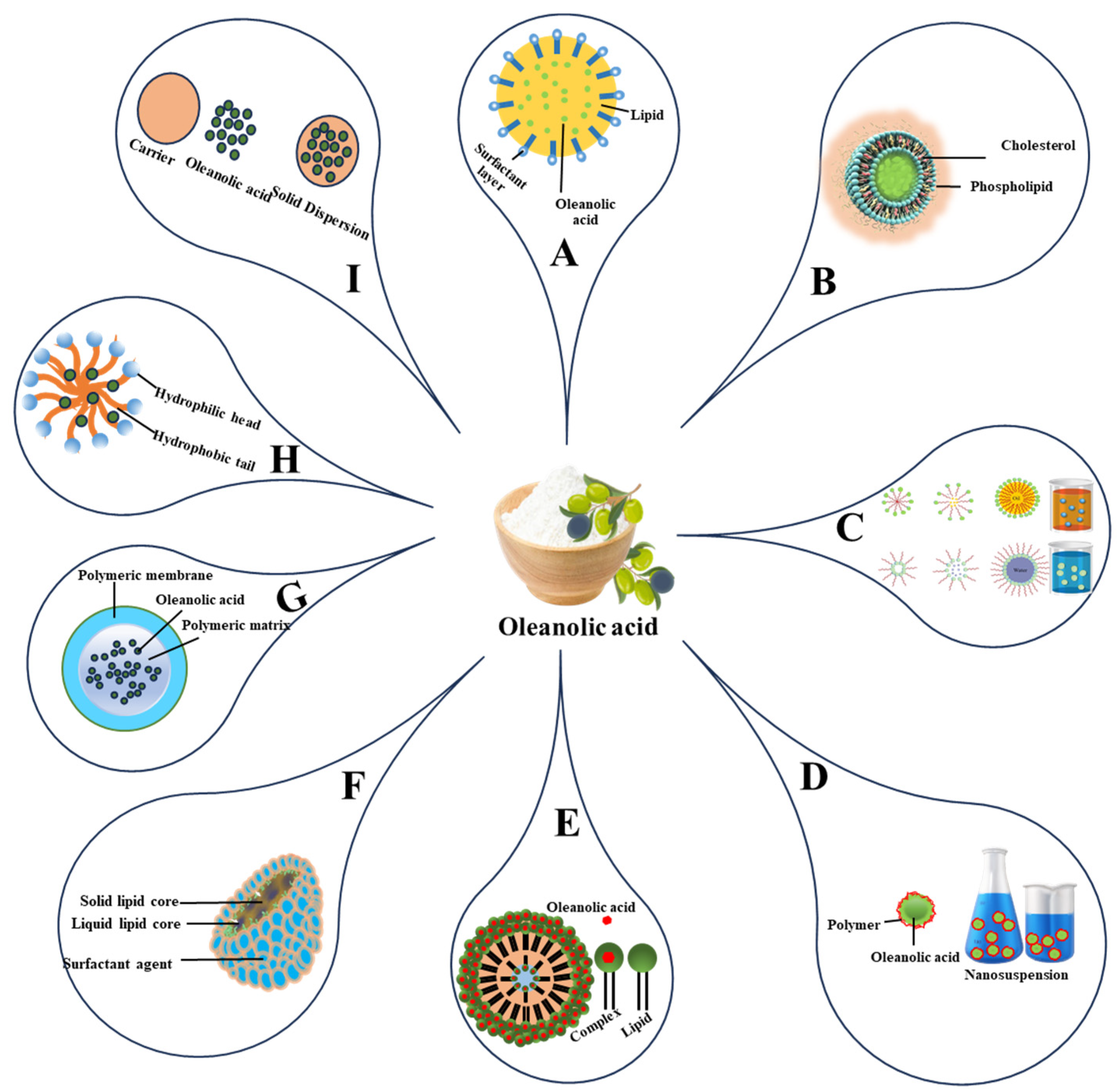
5.1. Lipid-Based Delivery Systems
5.1.1. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
5.1.2. Micro- and Nano-Emulsions
5.1.3. Liposomes
| DDS | Components | Chemical and Physical Parameters | In Vitro Release | Solubility (mg/mL) | Permeability (Pe) | Activity and/or Bioavailability | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EE | DL | Size (nm) | Z-Potential (mV) | PDI | |||||||
| SLN and NLC | OA and lipid | 94.2 ± 3.9% | 4.7 ± 0.2% | 104.5 ± 11.7 | −25.5 ± 1.8 | - | 4.88% per hour | - | - | - | [110] |
| OA and gentiopicrin, glycerin monostearate, oleic acid, Poloxamer 188 | 48.3 ± 2.7% | 8.1 ± 0.4% | 111.0 ± 1.6 | −23.8 ± 0.4 | 0.287 ± 0.01 | 70% after 10 h | - | - | 1520.7 ± 25.3 (μg min/mL) i/v route ↓ ALT ↓ AST | [112] | |
| NLC: Soy lecithin, tristearin, palmitic acid (2:2:1) IPA: sodium dodecyl sulphate, hexadecyltrimethylammonium bromide (1:1) NLCIPA = Lecithin + IPA (2:3, 3:7, 1:4) OA NLCIPA = OA-NLCIPA | NLC: 75% NLCIPA (2:3): 85% NLCIPA (3:7): 90% NLCIPA (1:4): 90% | NLC:8% NLCIPA (2:3): 8.7% NLCIPA (3:7): 9.2% NLCIPA (1:4): 9.2% | NLC (250–258) NLCIPA (139–150) OA-NLCIPA (140–160) OA-NLC (260–265) | NLC (−10 to −12) NLCIPA (−5 to −9) OA-NLCIPA (−4 to −8) OA-NLC (−9 to −9.5) | 0.3–0.5 | NLC = 80% NLCIPA (2:3): 62% NLCIPA (3:7): 42% NLCIPA (1:4): 60% (after 80 h) | - | - | Anticancer activity in HepG2, Huh-7, HCT-116, OA-NLCIPA ˃˃˃OA-NLC | [113] | |
| ME | Smix: Transcutol HP, TPGS (9:1) Oil = Capryol 90 Smix/Oil: (9:1) | - | 2 mg/ mL | 107.8 ± 1.5 | −20.5 ± 0.4 | 0.24 ± 0.02 | 73.2 ± 1.7% after 24 h | 2 | 1.02 × 10−6 ± 1.48 × 10−7 cm/s after 6 h | ↓ HepG2-induced intracellular lipid level | [118] |
| ME-1-OA (Capmul PG-8/NF Transcutol HP, Tween 20, water, 6:17:37:40) ME-2-OA (Nigella oil/ isopropyl myristate (1:1), Transcutol HP, Cremophor EL, water, 4:30:16:50) | - | ME-1-OA (1 mg/mL) ME-2-OA (3 mg/mL) | ME-1-OA (93.0 ± 3.4) ME-2-OA (17.62 ± 0.23) | ME-1-OA (−3.32 ± 0.02) ME-2-OA (−11.63 ± 0.01) | ME-1-OA (0.20 ± 0.04) ME-2-OA (0.20 ± 0.07) | - | ME-1-OA (1000-fold) ME-2-OA (3000-fold) | ME-1-OA 5.70 ± 0.01 × 10−6 cm/s ME-2-OA 4.74 ± 0.04 × 10−5 cm/s | ↑ bioactivity of OA against LPS-induced oxidative stress in RAW 264.7 murine | [44] | |
| Ethyl oleate, Cremophor EL, Ethanol (50:35:15) | - | 13.19 ± 0.33 | 49.7 | - | - | ˃85% in 60 h | 13.19 ± 0.33 mg/g | - | ↑ 1740.1 (ng h/mL) 5.07-fold | [119] | |
| NE | Formulation-I Sefsol 218/Cremophor EL/Labrasol (50:25:25), Formulation-II Sefsol 218/Cremophor EL/Labrasol/Transcutol P (50:22.5:22.5:5), Formulation-III Sefsol 218/Cremophor EL/Labrasol/Transcutol P (50:20:20:10), Formulation-IV Sefsol 218/Cremophor EL/Labrasol/Transcutol P (50:17.5:17.5:15) | - | - | Formulation-I 38.4 ± 0.2 Formulation-II 46.4 ± 0.5 Formulation-III 75.3 ± 0.3 Formulation-IV 110.4 ± 0.6 | - | Formulation-I 0.055 Formulation-II 0.120 Formulation-III 0.238 Formulation-IV 0.258 | ˃75% | - | - | - | [125] |
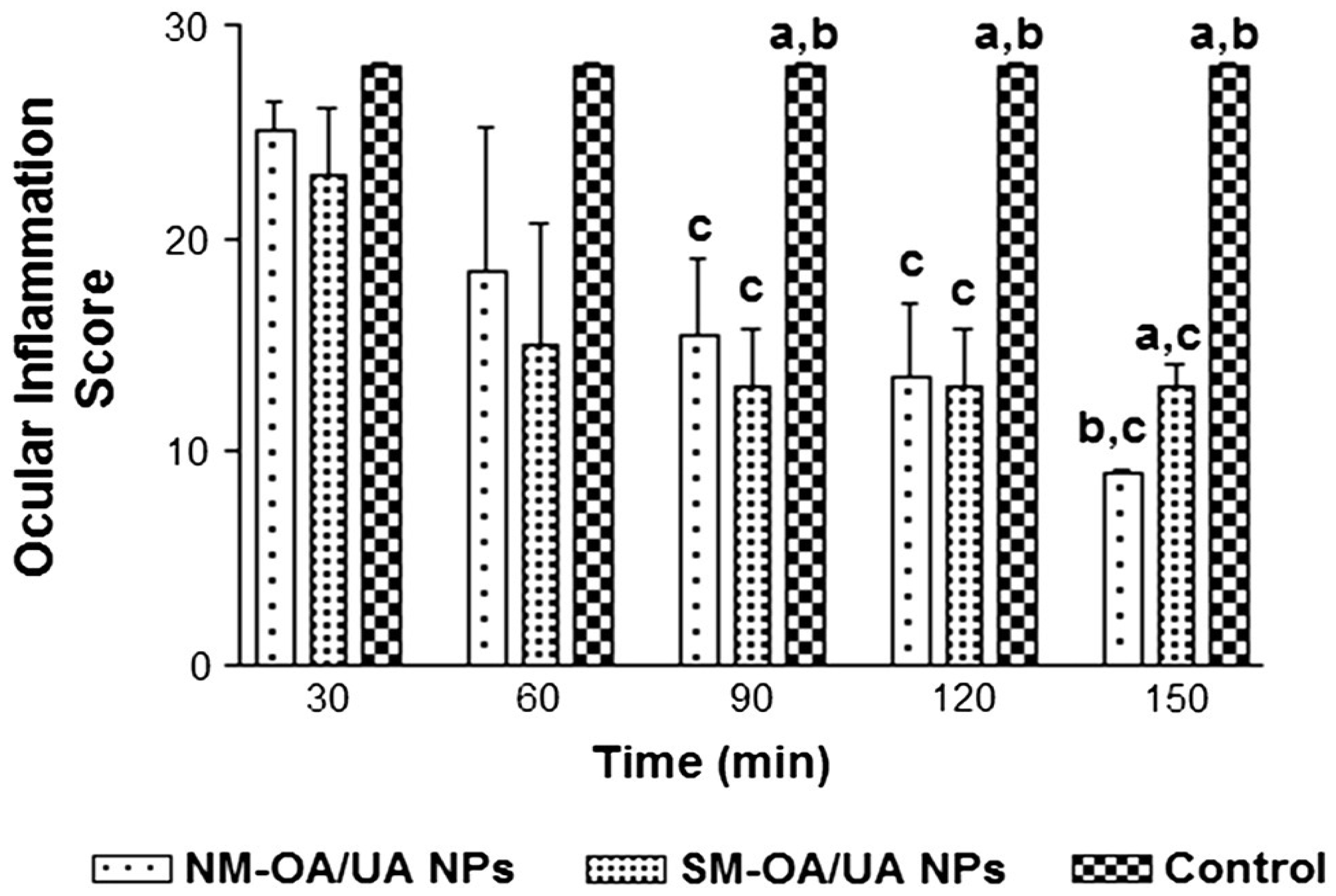
| Natural mixture (NM-OA/UA) and synthetic mixture (SM-OA/UA) castor oil (20%), and propylene glycol (20%) Smix (59.80%): (labrasol, transcutol-P) (4:1) | - | 0.2% (w/w) | NM-OA/UA (200.9–590.5) SM-OA/UA (139.7–450.9) | - | NM-OA/UA (0.25 to 0.73); SM-OA/UA (0.18 to 0.66) | NM-OA/UA DS (56.7 ± 1.2%) SM-OA/UA DS (56.8 ± 2.5%) | - | NM-OA/UA %A48, (6.41–7.39), SM-OA/UA %A48, (4.96–6.24) | ↑ In vivo anti-inflammatory activity | [133] | |
| DDS | Components | Parameters of Formulations | In Vitro Release | Solubility (mg/mL) | Permeability (Pe) | Activity and/or Bioavailability | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EE | DL | Size (nm) | Z-Potential (mV) | PdI | |||||||
| Liposomes | DSPC: 8.7 mg, cholesterol: 2.9 mg OA: 6.85 mg | 79% | - | 353 ± 140 | - | - | 54% release in 24 h | - | - | ↓ OA cytotoxicity ↑ hepatoprotective effect | [129] |
| Soybean phospholipid: 10 mg OA: 1 mg, cholesterol: 1 mg, sodium deoxycholate: 1 mg | 93.9% | 179.4 ± 3.15 | −28.8 ± 1.72 | 0.159 ± 0.061 | - | - | - | Increased bioavailability of 607.9% | [130] | ||
| soybean phosphatidylcholine, cholesterol, PEG-2000 | 63–98% | - | <200 | - | - | slower release | - | - | reduce drug toxicity | [131] | |
| soybean lecithin 80 mg, cholesterol 45.6 mg, triolein 20 mg, stearic acid 1 mg, OA 16 mg, aqueous solution Tween-80, polyvinyl alcohol | 82.3 ± 0.61% | - | Average: 11.57 μm | −13.35 mV | 0.21 | 80% release in 120 h | - | - | suppressed the growth of murine H22 hepatoma AUC0–∞ (h ng mL−1) 26,131.37 | [132] | |
| Micelles | OA: 50 mg Polygalacturonic acid: 50 mg | 76.59 ± 4% | 53.94 ± 3% | 200.0 | −44.7 | - | pH2: 7.5% pH6.8: 13% pH7.4: 18.5% SGF: no release SIF: approx. 12% | - | - | ↑ Increased insulin sensitivity ↓ the blood glucose Cmax: 12.52-fold increased at 6 h | [134] |
| TPGS 120: mg Pluronic P105: 80 mg OA: 7 mg | 93.6 ± 0.05% | 3.5% | 95.7 ± 3.6 | −8.6 ± 0.4 | 0.13 ± 0.03 | ~80% free OA, ~40% OA- micelles at 24 h | - | - | ↑ antitumor effect in A549 and PC-9 cells. | [135] | |
| Polymeric micelles-G w/w% (OA: 0.05; Capryol 90: 2; Poloxamer 407: 6) Polymeric micelles-H w/w (OA: 0.05; Capryol 90: 2; Poloxamer 407: 7) | Polymeric micelles-G: 98.26 ± 0.17% Polymeric micelles-H: 99.18 ± 1.06% | - | Polymeric micelles-G: 80.4 Polymeric micelles-H: 57.0 | - | - | - | - | Polymeric micelles-H: 29.49 ± 4.00% Polymeric micelles-G: 21.39 ± 5.91% at 24 h | alleviated skin wrinkles | [136] | |
| Nanosuspensions | OA and Tween 80 | - | - | 284.9 | −27.6 ± 7.2 | 0.22 | 95% 2 h | 0.026 | - | ↑ hepatoprotective effect | [137] |
| SEL:SEP at 4:1 w/w SE:OA at 2:1 w/w | 45.38 ± 1.81 | - | 96.60 ± 2.30 | - | 0.41 ± 0.03 | 100% 2 h | 1.89 ± 0.08 | - | ↓ proliferation rate of A549 cell lines Bioavailability: 7 times increased | [138] | |
| lyophilized whole whey, lactose monohydrate, OA | - | - | 60.3 ± 7.4 | −14.66 | 0.21 | 97% 2 h | - | - | Improved antidiarrheal effect | [139] | |
5.2. Micelles
5.3. Nanosuspensions
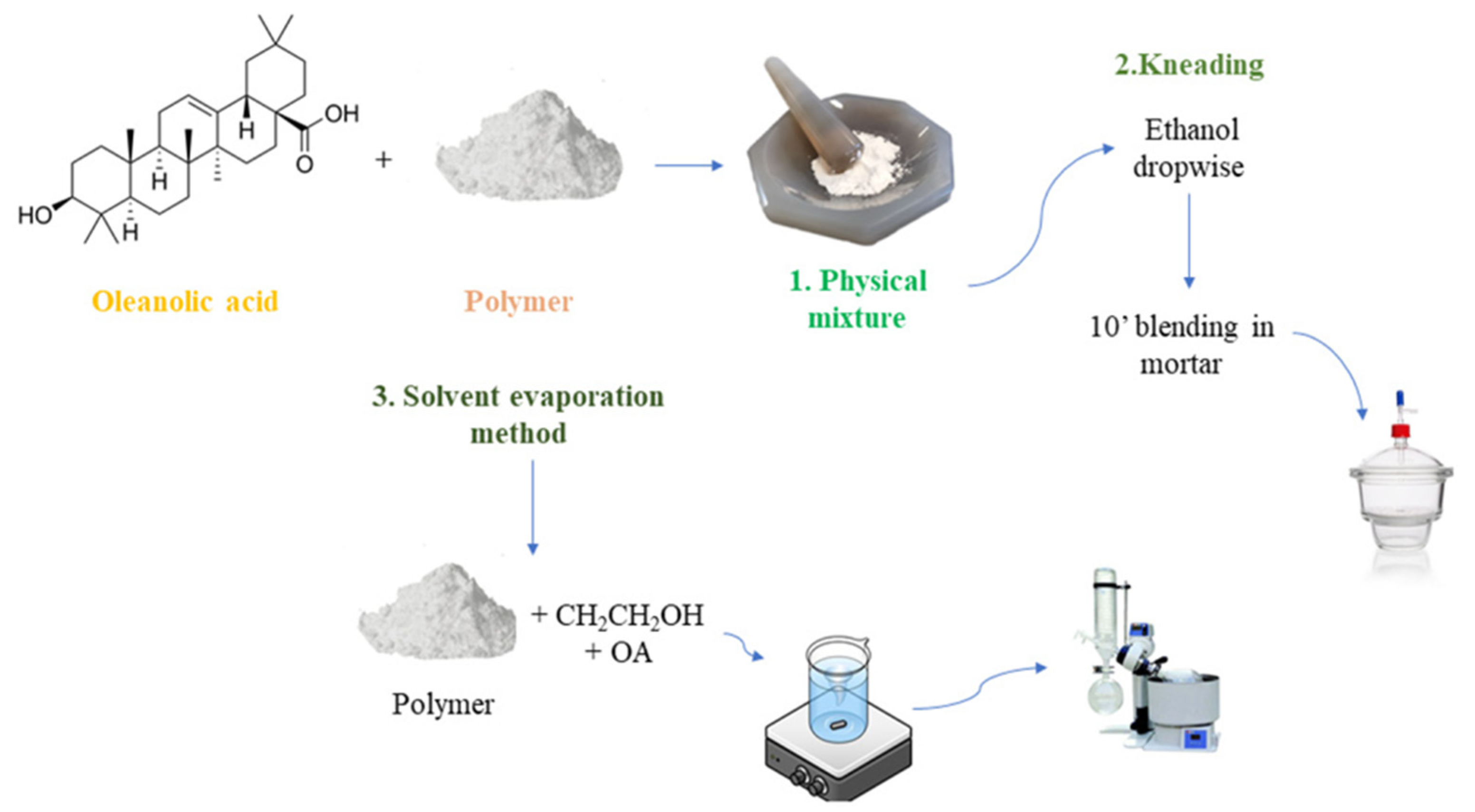
5.4. Nanoparticles
| DDS | Components | Parameters of Formulations | In Vitro Release | Permeability (Pe) | Activity and/or Bioavailability | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| EE | DL | Size (nm) | Z-Potential (mV) | PDI | ||||||
| NP | carboxylated cellulose-graft-Poly(L-lactide) (CC-g-PLLA) copolymers | - | 24.76 ± 0.58 | 196.82 ± 9.14 | −0.23 ± 0.12 | - | 90.5% at pH 6, 80.0% at pH 7.2, over 120 h | - | high efficiency against 4T1 cells and MCF-7 | [147] |
| ALB CTX | OA-ALB-NPs: 82.2 ± 3.9% CTX-OA-ALB-NPs: 78.08 ± 2.3% | - | OA-ALB-NPs: 171 ± 4.8 CTX-OA-ALB-NPs: 180 ± 3.7 | OA-ALB-NPs: −30.5 ± 2.8 CTX-OA-ALB-NPs: −33.3 ± 3.4 | OA-ALB-NPs 0.14 ± 0.07 CTX-OA-ALB-NPs 0.26 ± 0.03 | OA-ALB-NPs pH 5.5: 95% OA-ALB-NPs pH 7.4: 75% CTX-OA-ALB-NPs pH 5.5: 85% CTX-OA-ALB-NPs pH 7.4: 70% | - | ↑ A549 cells’ susceptibility AUC total (ng.h/mL) OA 31,195.8 ± 1127.2 OA-ALB-NPs 68,557.01 ± 1424.36 CTX-OA-ALB-NPs 73,592.7 ± 1148.57 | [148] | |
| ALPs: 50 mg UA: 1 mg OA: 1 mg | 48.98 ± 3.77% | 0.96 ± 0.07 | 199.1 | –7.15 | 0.35–0.40 | - | - | enhanced anti-inflammatory effect | [149] | |
| PLGA, TPGS F1 = 1:5 Drug: polymer F2 = 1:10 Drug: polymer | F1: 66.47 ± 2.12 F2: 40.92 ± 2.37 | F1: 11.08 ± 0.35 F2: 3.72 ± 0.22 | F1 = 377.9 ± 5.7 F2 = 233.9 ± 2.4 | F1: −15.6 ± 1.4 F2: −10.7 ± 1.7 | F1: 0.235 F2: 0.415 | F1: 85.66 ± 0.56% F2: 70.34 ± 1.15% 30 days | - | Enhanced potency against visceral leishmaniasis | [150] | |
| OA: Lactoferrin 1:6 | 92.59 ± 3.24% | 12.44 ± 2.5 | 202.2 ± 8.3 | +27.1 ± 0.32 | 0.15 ± 0.03 | 97.31 ± 2.04% in 20 min | - | ↑ 340.59% AUClast (ng h/mL) 126.53 ± 22.00 | [151] | |
| soybean lecithin, PEG NP-A precipitation method, NP-B: liposomes | NP-A: 86.7% NP-B: 92.6% | - | NP-A: 150 NP-B: 110–140 | - | - | - | - | - | [152] | |
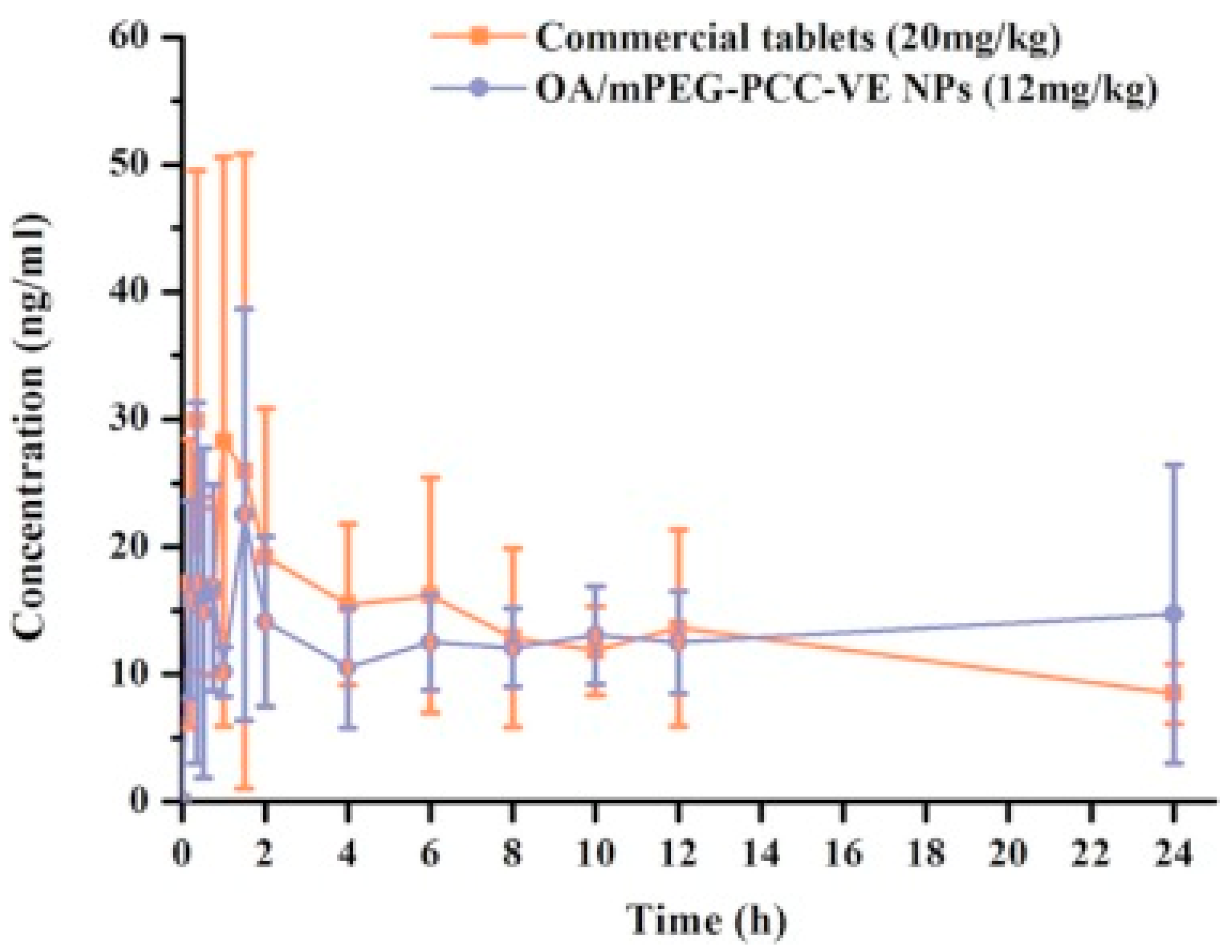
| Vitamin E-modified aliphatic polycarbonate (mPEG-PCC-VE) | 71% | 8.9 | 165.06 ± 1.08 | - | 0.13 ± 0.06 | OA/mPEG-PCC-VE NPs 78% in 30 min | OA/mPEG-PCC-VE NPs Papp Colon: 14 × 103 cm/min Papp Ileum: 4 × 103 cm/min Papp jejunum: 6 × 103 cm/min Papp duodenum: 10 × 103 cm/min | 1.5-fold | [153] | |
| PLGA, polyphenols | OA-PLGA (74.5 ± 3.5) CH-OA-PLGA (71 ± 2.5) OA-B-PLGA (81.1 ± 2.1) CH-OA-B-PLGA (78.6 ± 1.3) | - | PLGA-NP (149.6 ± 3.4) OA-PLGA (184.5 ± 1.5) CH-OA-PLGA (326.8 ± 2.5) OA-B-PLGA (221.5 ± 1.9) CH-OA-B-PLGA (342.2 ± 3.7) | PLGA-NP (−23.1 ± 4.2) OA-PLGA (−23.4 ± 2.2) CH-OA-PLGA (29.8 ± 1.1) OA-B-PLGA (−27.2 ± 2.5) CH-OA-B-PLGA (34.2 ± 3.1) | PLGA-NP (0.30 ± 0.012) OA-PLGA (0.22 ± 0.032) CH-OA-PLGA (0.33 ± 0.043) OA-B-PLGA (0.21 ± 0.021) CH-OA-B-PLGA (0.22 ± 0.011) | OA (~90%) OA-PLGA (~75%) CH-OA-B-PLGA (~40%) CH-OA-PLGA (~50%) OA-B-PLGA (62%) | - | ↑ antitumor effect on breast cancer cells in MDAMB 231 cell line | [154] | |
| PLGA | ∼77% | - | ˂225 | −27 mV | 0.1 | 75% in 40 h | Papp 3.36 ± 0.49 cm s−1 | ↑ anti-inflammatory effect | [133] | |
| OA and Paclitaxel (PTX) | - | - | OA NPs (247.7 ± 0.8) PTX-OA NPs (259.7 ± 3.4) | OA NPs (−8.8 ± 0.2 mV) PTX-OA NPs (−5.8 ± 0.6 mV) | OA NPs (0.035 ± 0.020) PTX-OA NPs (0.020 ± 0.008) | - | - | Synergistically ↑ antitumor activity to 69% from 15% | [155] | |
| OA loaded in liquid crystalline nanoparticle (LCNP)-based gel | 68.31 ± 2.86% to 73.18 ± 3.21% | 12.31 ± 0.41% to 14.12 ± 0.32% | 129 ± 12.11 to 272 ± 21.83 | − 18.3 to − 21.2 | 0.218 to 0.436 | 84.93 to 87.89% at 12 h | Pe (0.98 cm/h to 30.96 cm/h) | effective in a rodent carrageenan-induced hind paw inflammatory model | [156] | |
5.5. Solid Dispersions
| DDS | Components | In Vitro Release | Solubility | Permeability (Pe cm/s) | Activity and/or Bioavailability | References |
|---|---|---|---|---|---|---|
| SD | OA, PVP SD OA, PVP, Polysorbate 80 SD | 70% 90% | - | - | - | [163] |
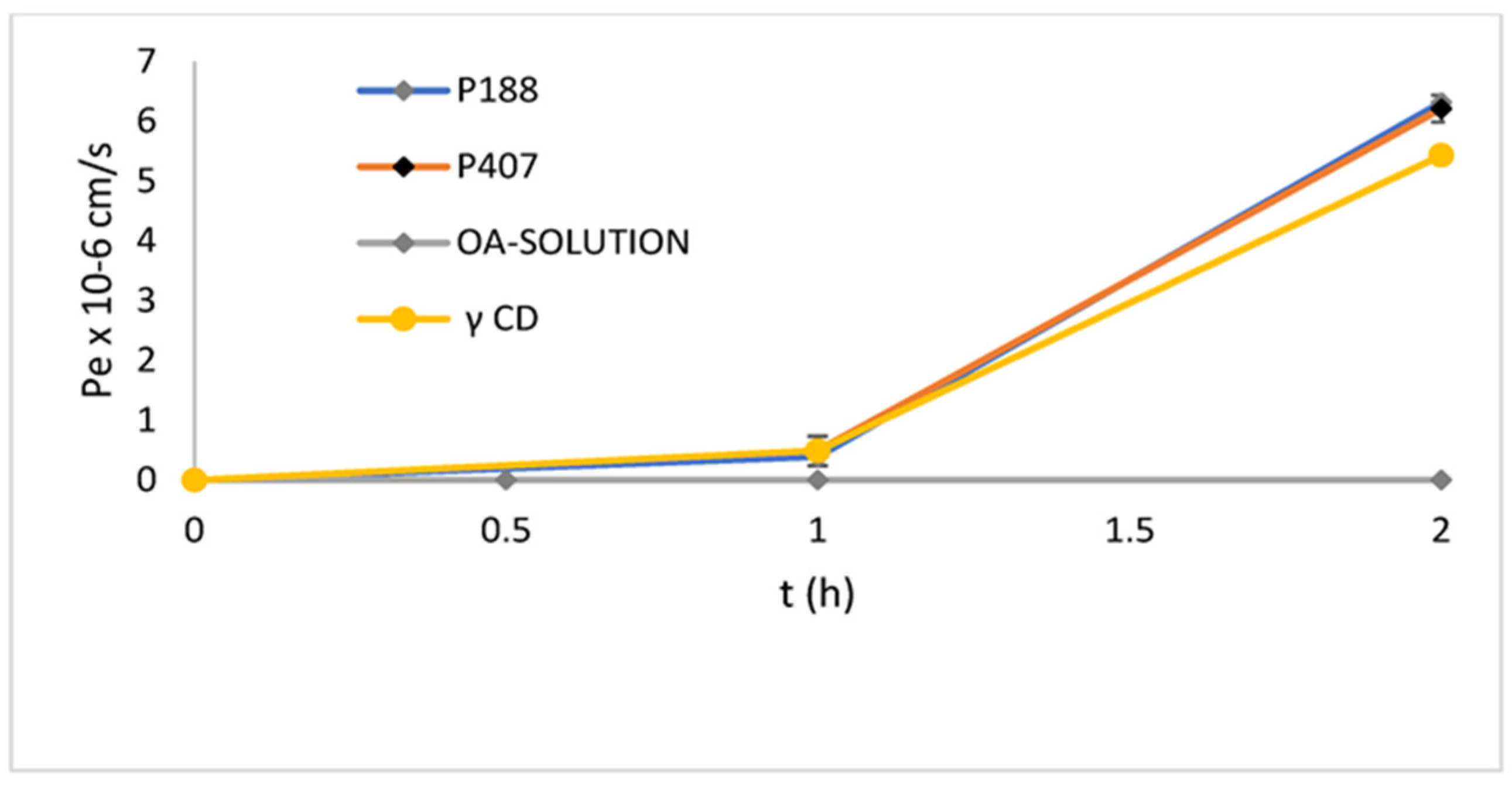
| OA:Poloxamer 188 1:2 OA:Poloxamer 407 1:2 OA:γ-CD 1:2 | 61% 68% 50% | 190 ± 42 μg/mL 170 ± 28 μg/mL 221 ± 17 μg/mL | OA-P407 6.2 ± 0.22 × 10−5 OA-P188 6.3 ± 0.53 × 10−5 OA-γ-CD 5.43 ± 0.12 × 10−5 at 2 h | - | [165] | |
| Formula F (OA:PVP:SC) 1:1:2 Formula G (OA-Na:PVP:SC) (1:1:2) | OA-Na (9.5%) Formula F (59%) Formula G (85%) at 2 h | - | OA 0.138 ± 0.023 0.06% (w/v) SC* 0.381 ± 0.053 at 2 h | Formula F (31,067.44 ± 17,840.92) Formula G (32,657.41 ± 11,832.92) (μg min/mL) | [165] | |
| OA-PVP Physical mixture (PM)-PVP | 90% 45% at 2 h | - | - | AUC0–∞ (ng h/mL) OA-PVP: 2039.5 ± 483.4) PM-PVP: 696.8 ± 151.6 commercial tablet: 875.08 ± 292.1 | [166] | |
| OA:silica 1:7 | 86.4% | - | - | AUC0–24h OA-silica: 228.51 ± 20.35(μg min/mL) 1.9-fold increase | [167] | |
| Phospholipid complexes | OA-phospholipid complex 8:5 | 99.47% in 360 min | 300-fold | - | AUC0–∞ 2.16-fold | [168] |
| OA, phospholipid complex and hydroxyapatite (OPCH) | 90% in 30 min | water 15.3-fold n-octanol 3.19-fold | OPCH increased 1.6–2.6-fold compared to OA. OPCH in the presence of ketoconazole 1.2–2.4-fold compared to OPCH. | AUC0–t (ng h/mL) OPCH: 360.6 ± 19.13 OPCH with ketoconazole: 707.7 ± 30.21 | [19] | |
| OA, Phosphatidylcholine (PC), fumed silica | 0.1% SDS aqueous solution: OA 20%, OA-PC 10%, OA-PC/silica ˃40% 0.3% SDS aqueous solution: OA ˃50%, OA-PC ˃20%, OA-PC/silica 80% 0.5% SDS aqueous solution: OA ˃60%, OA-PC ˃30%, OA-PC/silica ˃80% | OA-PC: 0.12 (30 °C), 0.14 (40 °C), 0.17 (60 °C), 0.15 (80 °C) | - | - | [1] | |
| Other complexes | OA and methyl-β-CD | - | 8.2 | - | ↑ in vitro anticancer activity in HepG2, HT29, and HCT116 cell lines | [169] |
| 2-hydroxypropyl-β-CD and 2-hydroxypropyl-γ-CD | - | - | - | ↑ anti-proliferative A375 (human), B16 4A5 (murine), and SK-Mel 2 (human) cell lines | [170] | |
| hydroxypropyl-β-CD and OA and UA | - | OA (900 times) UA (200 times) | - | - | [171] | |
| amino-appended β-CDs | - | 6.8 × 105 to 2.1 × 106 fold | - | ↑ in vitro anticancer activities in HepG2, HT29, and HCT116 human cancer cell lines | [172] |
5.6. Phospholipid Complexes
5.7. Other Complexes
5.8. Other Formulations
6. Expert Opinion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Jiang, Q.; Du, P.; Zhao, J.; Zhang, T. Preparation and Characterization of Solidified Oleanolic Acid-Phospholipid Complex Aiming to Improve the Dissolution of Oleanolic Acid. Asian J. Pharm. Sci. 2016, 11, 241–247. [Google Scholar] [CrossRef]
- Oprean, C.; Zambori, C.; Borcan, F.; Soica, C.; Zupko, I.; Minorics, R.; Bojin, F.; Ambrus, R.; Muntean, D.; Danciu, C.; et al. Anti-Proliferative and Antibacterial In Vitro Evaluation of the Polyurethane Nanostructures Incorporating Pentacyclic Triterpenes. Pharm. Biol. 2016, 54, 2714–2722. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Ahn, Y.M.; Choi, Y.H.; Yoon, J.J.; Lee, Y.J.; Cho, K.W.; Kang, D.G.; Lee, H.S. Oleanolic Acid Modulates the Renin-Angiotensin System and Cardiac Natriuretic Hormone Concomitantly with Volume and Pressure Balance in Rats. Eur. J. Pharmacol. 2017, 809, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Kim, H.M.; Kang, J.S.; Lee, E.Y.; Yadav, D.; Kwon, M.-H.; Kim, Y.M.; Kim, H.S.; Chung, C.H. Oleanolic Acid and N-Acetylcysteine Ameliorate Diabetic Nephropathy through Reduction of Oxidative Stress and Endoplasmic Reticulum Stress in a Type 2 Diabetic Rat Model. Nephrol. Dial. Transplant. 2016, 31, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Djeziri, F.Z.; Belarbi, M.; Murtaza, B.; Hichami, A.; Benammar, C.; Khan, N.A. Oleanolic Acid Improves Diet-Induced Obesity by Modulating Fat Preference and Inflammation in Mice. Biochimie 2018, 152, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Nieto Callejo, M.L.; Gallardo, I.; Gutierrez, B.; Cabero, M.I.; Ruiz, L.; Alvarez, Y.; Simon, I.; Calvo, H.; Munoz, J.C.; Margolles, A. Oleanolic Acid Protection against Experimental Autoimmune Myocarditis Modulates the Microbiota and the Intestinal Barrier Integrity. Eur. Heart J. 2020, 41, ehaa946-3716. [Google Scholar] [CrossRef]
- Peng, M.; Zhao, X.; Biswas, D. Polyphenols and Tri-Terpenoids from Olea europaea L. in Alleviation of Enteric Pathogen Infections through Limiting Bacterial Virulence and Attenuating Inflammation. J. Funct. Foods 2017, 36, 132–143. [Google Scholar] [CrossRef]
- Du, Y.; Ko, K.M. Oleanolic Acid Protects against Myocardial Ischemia-Reperfusion Injury by Enhancing Mitochondrial Antioxidant Mechanism Mediated by Glutathione and α-Tocopherol in Rats. Planta Med. 2006, 72, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Bachhav, S.S.; Bhutada, M.S.; Patil, S.P.; Sharma, K.S.; Patil, S.D. Oleanolic Acid Prevents Increase in Blood Pressure and Nephrotoxicity in Nitric Oxide Dependent Type of Hypertension in Rats. Pharmacogn. Res. 2015, 7, 385. [Google Scholar]
- Rada, M.; Castellano, J.M.; Perona, J.S.; Guinda, Á. GC-FID Determination and Pharmacokinetic Studies of Oleanolic Acid in Human Serum. Biomed. Chromatogr. 2015, 29, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, R.; Carbó, M.; Pujadas, M.; Biel, S.; Mesa, M.-D.; Covas, M.-I.; Expósito, M.; Espejo, J.-A.; Sanchez-Rodriguez, E.; Díaz-Pellicer, P. Pharmacokinetics of Maslinic and Oleanolic Acids from Olive Oil–Effects on Endothelial Function in Healthy Adults. A Randomized, Controlled, Dose–Response Study. Food Chem. 2020, 322, 126676. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, Y.; Wu, Q.; Xu, S.; Shi, F.; Klaassen, C.D. Oleanolic Acid Reprograms the Liver to Protect against Hepatotoxicants, but Is Hepatotoxic at High Doses. Liver Inter. 2019, 39, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [PubMed]
- Allouche, Y.; Warleta, F.; Campos, M.; Sanchez-Quesada, C.; Uceda, M.; Beltran, G.; Gaforio, J.J. Antioxidant, Antiproliferative, and pro-Apoptotic Capacities of Pentacyclic Triterpenes Found in the Skin of Olives on MCF-7 Human Breast Cancer Cells and Their Effects on DNA Damage. J. Agric. Food Chem. 2011, 59, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, B.; Gallardo, I.; Ruiz, L.; Alvarez, Y.; Cachofeiro, V.; Margolles, A.; Hernandez, M.; Nieto, M.L. Oleanolic Acid Ameliorates Intestinal Alterations Associated with EAE. J. Neuroinflamm. 2020, 17, 363. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Nakane, T.; Masuda, K.; Ishii, H. Ursolic Acid and Oleanolic Acid, Members of Pentacyclic Triterpenoid Acids, Suppress TNF-α-Induced E-Selectin Expression by Cultured Umbilical Vein Endothelial Cells. Phytomedicine 2010, 17, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Dharmappa, K.K.; Kumar, R.V.; Nataraju, A.; Mohamed, R.; Shivaprasad, H.V.; Vishwanath, B.S. Anti-Inflammatory Activity of Oleanolic Acid by Inhibition of Secretory Phospholipase A2. Planta Med. 2009, 75, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yang, X.; Du, P.; Zhang, H.; Zhang, T. Dual Strategies to Improve Oral Bioavailability of Oleanolic Acid: Enhancing Water-Solubility, Permeability and Inhibiting Cytochrome P450 Isozymes. Eur. J. Pharm. Biopharm. 2016, 99, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Kim, Y.H.; Kim, H.H.; Ji, H.Y.; Yoo, S.D.; Choi, W.R.; Lee, S.M.; Han, C.K.; Lee, H.S. Dose-Linear Pharmacokinetics of Oleanolic Acid after Intravenous and Oral Administration in Rats. Biopharm. Drug Dispos. 2007, 28, 51–57. [Google Scholar] [CrossRef]
- Ganjali, M.; Ganjali, M.; Aljabali, A.A.A.; Barhoum, A. Drug Delivery Systems Based on Nano-Herbal Medicine. In Bionanotechnology: Emerging Applications of Bionanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 491–530. [Google Scholar]
- Yang, Y.H.; Dai, S.Y.; Deng, F.H.; Peng, L.H.; Li, C.; Pei, Y.H. Recent Advances in Medicinal Chemistry of Oleanolic Acid Derivatives. Phytochemistry 2022, 203, 113397. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cui, C.; Li, M.; Zhang, Z.; Lv, H. Study of a Novel Disintegrable Oleanolic Acid-Polyvinylpolypyrrolidone Solid Dispersion. Drug Dev. Ind. Pharm. 2017, 43, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, H.L.; Abrego, G.; Souto, E.B.; Garduño-Ramirez, M.L.; Clares, B.; García, M.L.; Calpena, A.C. Nanoemulsions for Dermal Controlled Release of Oleanolic and Ursolic Acids: In Vitro, Ex Vivo and In Vivo Characterization. Colloids Surf. B Biointerfaces 2015, 130, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants–Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Liby, K.T.; Yore, M.M.; Fu, L.; Lopchuk, J.M.; Gribble, G.W. New Synthetic Triterpenoids: Potent Agents for Prevention and Treatment of Tissue Injury Caused by Inflammatory and Oxidative Stress. J. Nat. Prod. 2011, 74, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Ludeña-Huaman, M.A.; Ramos-lnquiltupa, D.A. Determination of the Content of Ursolic and Oleanolic Acid in the Cuticular Wax of Fruits of Different Species of Rosaceae. Rev. Colomb. Quim. 2019, 48, 15–20. [Google Scholar] [CrossRef]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial Activity of Oleanolic and Ursolic Acids: An Update. Evid. Based Complement. Alternat. Med. 2015, 2015, 620472. [Google Scholar] [CrossRef] [PubMed]
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential. Int. J. Mol. Sci. 2021, 22, 4599. [Google Scholar] [CrossRef] [PubMed]
- Tostes, J.B.d.F.; Nakamura, M.J.; de Saboya, C.G.F.; Mazzei, J.L.; Siani, A.C. Efficient and Selective Method to Separate Triterpene Acids by Direct Treatment of Apple Peels with Alkaline Ethanol. Sep. Sci. Technol. 2016, 51, 1986–1993. [Google Scholar] [CrossRef]
- Jin, I.J.; Ko, Y.I.; Kim, Y.M.; Han, S.K. Solubilization of Oleanolic Acid and Ursolic Acid by Cosolvency. Arch. Pharm. Res. 1997, 20, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Hosseiny, S.S.; Szczotka, M.; Jordan, V.; Schlitter, K. Rapid Solubility Determination of the Triterpenes Oleanolic Acid and Ursolic Acid by UV-Spectroscopy in Different Solvents. Phytochem. Lett. 2009, 2, 85–87. [Google Scholar] [CrossRef]
- Schneider, P.; Bischoff, F.; Müller, U.; Bart, H.J.; Schlitter, K.; Jordan, V. Plant Extraction with Aqueous Two-Phase Systems. Chem. Eng. Technol. 2011, 34, 452–458. [Google Scholar] [CrossRef]
- Xia, E.Q.; Yu, Y.Y.; Xu, X.R.; Deng, G.F.; Guo, Y.J.; Li, H.-B. Ultrasound-Assisted Extraction of Oleanolic Acid and Ursolic Acid from Ligustrum lucidum Ait. Ultrason. Sonochem. 2012, 19, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Yang, E.-J.; Ku, S.-K.; Song, K.-S.; Bae, J.-S. Anti-Inflammatory Effects of Oleanolic Acid on LPS-Induced Inflammation In Vitro and In Vivo. Inflammation 2013, 36, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Punia, S.; K Sharma, A. Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with Promising Anti-Inflammatory Activities. Recent. Pat. Inflamm. Allergy Drug Discov. 2016, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Liu, Y.; Niu, K.; Zeng, W.; Wang, R.; Guo, X.; Lin, C.; Hu, L. Oleanolic Acid Alleviate Intestinal Inflammation by Inhibiting Takeda G-Coupled Protein Receptor (TGR) 5 Mediated Cell Apoptosis. Food Funct. 2024, 15, 1963–1976. [Google Scholar] [CrossRef]
- Yang, E.-J.; Lee, W.; Ku, S.-K.; Song, K.-S.; Bae, J.-S. Anti-Inflammatory Activities of Oleanolic Acid on HMGB1 Activated HUVECs. Food Chem. Toxicol. 2012, 50, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, J.; Rodríguez-Rodríguez, R.; González-Díez, M.; Rodríguez, C.; Herrera, M.D.; Ruiz-Gutierrez, V.; Badimon, L. Oleanolic Acid Induces Prostacyclin Release in Human Vascular Smooth Muscle Cells through a Cyclooxygenase-2-Dependent Mechanism. J. Nutr. 2008, 138, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-M.; Lee, M.; An, H.-J. Oleanolic Acid Protects against Mast Cell-Mediated Allergic Responses by Suppressing Akt/NF-ΚB and STAT1 Activation. Phytomedicine 2021, 80, 153340. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, X.L.; Liu, R.; Chen, H.L.; Bai, H.; Liang, X.; Zhang, X.D.; Wang, Z.; Li, W.; Hai, C.X. Antioxidant Activities of Oleanolic Acid In Vitro: Possible Role of Nrf2 and MAP Kinases. Chem. Biol. Interact. 2010, 184, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.C.; Chan, K.C. Nonenzymatic Antioxidative and Antiglycative Effects of Oleanolic Acid and Ursolic Acid. J. Agric. Food Chem. 2007, 55, 7177–7181. [Google Scholar] [CrossRef] [PubMed]
- Somova, L.O.; Nadar, A.; Rammanan, P.; Shode, F.O. Cardiovascular, Antihyperlipidemic and Antioxidant Effects of Oleanolic and Ursolic Acids in Experimental Hypertension. Phytomedicine 2003, 10, 115–121. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, C.; Vasarri, M.; Salvatici, M.C.; Grifoni, L.; Quintela, J.C.; Bilia, A.R.; Degl’Innocenti, D.; Bergonzi, M.C. Microemulsions Enhance the In Vitro Antioxidant Activity of Oleanolic Acid in RAW 264.7 Cells. Pharmaceutics 2022, 14, 2232. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, H.; Liu, M.; Wu, N.; Zhao, J.; Xiao, L.; Han, L.; Chu, E.; Lin, X. Oleanolic Acid Potentiates the Antitumor Activity of 5-Fluorouracil in Pancreatic Cancer Cells. Oncol. Rep. 2012, 28, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Oleanolic Acid and Its Synthetic Derivatives for the Prevention and Therapy of Cancer: Preclinical and Clinical Evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Resende, F.A.; de Andrade Barcala, C.A.M.; da Silva Faria, M.C.; Kato, F.H.; Cunha, W.R.; Tavares, D.C. Antimutagenicity of Ursolic Acid and Oleanolic Acid against Doxorubicin-Induced Clastogenesis in Balb/c Mice. Life Sci. 2006, 79, 1268–1273. [Google Scholar] [CrossRef]
- Pollier, J.; Goossens, A. Oleanolic Acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Žiberna, L.; Šamec, D.; Mocan, A.; Nabavi, S.F.; Bishayee, A.; Farooqi, A.A.; Sureda, A.; Nabavi, S.M. Oleanolic Acid Alters Multiple Cell Signaling Pathways: Implication in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2017, 18, 643. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wen, X.; Sun, H. Oleanolic Acid Derivatives for Pharmaceutical Use: A Patent Review. Expert Opin. Ther. Pat. 2016, 26, 643–655. [Google Scholar] [CrossRef]
- Zheng, Q.; Bo, S.; Zeng, X.; Wei, Y.; Lei, S.; Xiao, X.; Xiao, J.; Wang, Z.; Zheng, X. Advances in Research on Hepatoprotective Activity and Synthesis of Oleanolic Acid Derivatives. J. App. Biopharm. Pharmacokin. 2015, 3, 101551939. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Q.; Lu, Y.-F.; Pi, J. New Insights into Generalized Hepatoprotective Effects of Oleanolic Acid: Key Roles of Metallothionein and Nrf2 Induction. Biochem. Pharmacol. 2008, 76, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.G. Inhibition of Cytochrome P450 2E1 Expression by Oleanolic Acid: Hepatoprotective Effects against Carbon Tetrachloride-Induced Hepatic Injury. Toxicol. Lett. 1999, 105, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rebolledo, G.A.; Siordia-Reyes, A.G.; Meckes-Fischer, M.; Jiménez-Arellanes, A. Hepatoprotective Properties of Oleanolic and Ursolic Acids in Antitubercular Drug-Induced Liver Damage. Asian Pac. J. Trop. Med. 2016, 9, 644–651. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, K. Therapeutic Potential of Oleanolic Acid in Liver Diseases. Naunyn. Schmiedeberg’s Arch. Pharmacol. 2024. [Google Scholar] [CrossRef]
- Hao, B.-B.; Pan, X.-X.; Fan, Y.; Lu, L.; Qian, X.-F.; Wang, X.-H.; Zhang, F.; Rao, J.-H. Oleanolic Acid Attenuates Liver Ischemia Reperfusion Injury by HO-1/Sesn2 Signaling Pathway. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Garcia-Rodriguez, S.; Espinosa, J.M.; Millan-Linares, M.C.; Rada, M.; Perona, J.S. Oleanolic Acid Exerts a Neuroprotective Effect against Microglial Cell Activation by Modulating Cytokine Release and Antioxidant Defense Systems. Biomolecules 2019, 9, 683. [Google Scholar] [CrossRef]
- Caltana, L.; Rutolo, D.; Nieto, M.L.; Brusco, A. Further Evidence for the Neuroprotective Role of Oleanolic Acid in a Model of Focal Brain Hypoxia in Rats. Neurochem. Int. 2014, 79, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, L.; Ji, X.; Shi, R.; Xu, F.; Gu, J. Neuroprotective Effects of Oleanolic Acid against Cerebral Ischemia-Reperfusion Injury in Mice. Exp. Neurol. 2021, 343, 113785. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tong, Z.; Wang, C.; Li, X.; Liang, G. Oleanolic Acid Exerts Neuroprotective Effects in Subarachnoid Hemorrhage Rats through SIRT1-Mediated HMGB1 Deacetylation. Eur. J. Pharmacol. 2021, 893, 173811. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhang, Z.; Zhang, Z.; Zhu, P.; Jiang, X.; Wang, Y.; Deng, Q.; Lam Yung, K.K.; Zhang, S. Oleanolic Acid Alleviates Cerebral Ischemia/Reperfusion Injury via Regulation of the GSK-3β/HO-1 Signaling Pathway. Pharmaceuticals 2021, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Stępnik, K.; Kukula-Koch, W.; Plazinski, W.; Rybicka, M.; Gawel, K. Neuroprotective Properties of Oleanolic Acid—Computational-Driven Molecular Research Combined with In Vitro and In Vivo Experiments. Pharmaceuticals 2023, 16, 1234. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Wu, H.; Liu, J.; Hu, J.; Liao, N.; Peng, J.; Cao, P.; Liang, X.; Hai, C. Antidiabetic Effect of Oleanolic Acid: A Promising Use of a Traditional Pharmacological Agent. Phytother. Res. 2011, 25, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, R.; Zhang, W.; Zhang, X.; Liao, N.; Wang, Z.; Li, W.; Qin, X.; Hai, C. Oleanolic Acid Improves Hepatic Insulin Resistance via Antioxidant, Hypolipidemic and Anti-Inflammatory Effects. Mol. Cell. Endocrinol. 2013, 376, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Genet, C.; Strehle, A.; Thomas, C.; Lobstein, A.; Wagner, A.; Mioskowski, C.; Auwerx, J.; Saladin, R. Anti-Hyperglycemic Activity of a TGR5 Agonist Isolated from Olea europaea. Biochem. Biophys. Res. Commun. 2007, 362, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Maczewsky, J.; Kaiser, J.; Gresch, A.; Gerst, F.; Düfer, M.; Krippeit-Drews, P.; Drews, G. TGR5 Activation Promotes Stimulus-Secretion Coupling of Pancreatic β-Cells via a PKA-Dependent Pathway. Diabetes 2019, 68, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, H.; Wang, X.; Mu, D.; Liao, H.; Zhang, L. Effects of Oleanolic Acid and Maslinic Acid on Hyperlipidemia. Drug Dev. Res. 2007, 68, 261–266. [Google Scholar] [CrossRef]
- Martín, R.; Carvalho-Tavares, J.; Hernández, M.; Arnes, M.; Ruiz-Gutierrez, V.; Nieto, M.L. Beneficial Actions of Oleanolic Acid in an Experimental Model of Multiple Sclerosis: A Potential Therapeutic Role. Biochem. Pharmacol. 2010, 79, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hsu, C.; Huang, C.; Yin, M. Anti-Glycative Effects of Oleanolic Acid and Ursolic Acid in Kidney of Diabetic Mice. Eur. J. Pharmacol. 2010, 628, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zeng, H.; Xu, M.; Huang, C.; Tao, L.; Li, J.; Zhang, T.; Chen, H.; Xia, J.; Li, C. Oleanolic Acid Improves Obesity-Related Inflammation and Insulin Resistance by Regulating Macrophages Activation. Front. Pharmacol. 2021, 12, 697483. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.N.; Mahajan, U.B.; Chandrayan, G.; Kumawat, V.S.; Kamble, S.; Patil, P.; Agrawal, Y.O.; Patil, C.R.; Ojha, S. Protective Effect of Oleanolic Acid on Oxidative Injury and Cellular Abnormalities in Doxorubicin Induced Cardiac Toxicity in Rats. Am. J. Transl. Res. 2016, 8, 60. [Google Scholar] [PubMed]
- Zhang, B.; Zhang, W.; Luo, J.; He, J.; Zheng, X.; Zhu, S.; Rong, B.; Ai, Y.; Zhang, L.; He, T. Effects of Oleanolic Acid on Hair Growth in Mouse Dorsal Skin Mediated via Regulation of Inflammatory Cytokines. J. Appl. Biomed. 2023, 21, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Ruan, H.; Lv, L.; Wei, C.; Yu, Y.; Jia, L.; Song, X.; Zhang, J.; Li, Y. Oleanolic Acid, a Small-Molecule Natural Product, Inhibits ECM Degeneration in Osteoarthritis by Regulating the Hippo/YAP and Wnt/β-Catenin Pathways. Food Funct. 2023, 14, 9999–10013. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, X.; He, J.; Luo, Y.; Zheng, P.; Yu, B.; Chen, D.; Huang, Z. Oleanolic Acid Promotes Skeletal Muscle Fiber Type Transformation by Activating TGR5-Mediated CaN Signaling Pathway. J. Nutr. Biochem. 2024, 123, 109507. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wei, X.; Zhao, T.; Shi, H.; Hao, X.; Wang, Y.; Zhang, H.; Yao, Z.; Zheng, M.; Ma, T. Oleanolic Acid Alleviates Obesity-induced Skeletal Muscle Atrophy via the PI3K/Akt Signaling Pathway. FEBS Open Bio 2024, 14, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Yang, H.; Long, C. Oleanolic Acid Improves the Symptom of Renal Ischemia Reperfusion Injury via the PI3K/AKT Pathway. Urol. Int. 2021, 105, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Ulrich-Merzenich, G. Synergy Research: Approaching a New Generation of Phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between Natural Products and Antibiotics against Infectious Diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57. [Google Scholar] [PubMed]
- Avcı, C.B.; Sogutlu, F.; Ozates, N.P.; Shademan, B.; Gunduz, C. Enhanced Anti-Cancer Potency Using a Combination of Oleanolic Acid and Maslinic Acid to Control Treatment Resistance in Breast Cancer. Adv. Pharm. Bull. 2023, 13, 611. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Debnath, S.K.; Maske, P.; Dighe, V.; Srivastava, R. Targeted Delivery of Ursolic Acid and Oleanolic Acid to Lungs in the Form of an Inhaler for the Management of Tuberculosis: Pharmacokinetic and Toxicity Assessment. PLoS ONE 2022, 17, e0278103. [Google Scholar] [CrossRef] [PubMed]
- Piet, M.; Paduch, R. Ursolic and Oleanolic Acids in Combination Therapy Inhibit Migration of Colon Cancer Cells through Down-Regulation of the UPA/UPAR-Dependent MMPs Pathway. Chem. Biol. Interact. 2022, 368, 110202. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Arellanes, M.A. Hepatoprotective Effect of the Ursolic Acid-Oleanolic Acid Mixture Administered Intragastrically in Mice with Liver Damage Induced by Anti-TB Drugs. Qeios 2023. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Abdelkader, D.; Hassan, W.; Sun, H.; Liu, J. Combination Therapy with Oleanolic Acid and Metformin as a Synergistic Treatment for Diabetes. J. Diabetes Res. 2015, 2015, 973287. [Google Scholar] [CrossRef] [PubMed]
- Kurek, A.; Nadkowska, P.; Pliszka, S.; Wolska, K.I. Modulation of Antibiotic Resistance in Bacterial Pathogens by Oleanolic Acid and Ursolic Acid. Phytomedicine 2012, 19, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Z.; Yang, Y.; Shi, H.; Chen, H.; Gao, Y. A Nanosensitizer Self-Assembled from Oleanolic Acid and Chlorin E6 for Synergistic Chemo/Sono-Photodynamic Cancer Therapy. Phytomedicine 2021, 93, 153788. [Google Scholar] [CrossRef] [PubMed]
- García-González, A.; Espinosa-Cabello, J.M.; Cerrillo, I.; Montero-Romero, E.; Rivas-Melo, J.J.; Romero-Báez, A.; Jiménez-Andreu, M.D.; Ruíz-Trillo, C.A.; Rodríguez-Rodríguez, A.; Martínez-Ortega, A.J. Bioavailability and Systemic Transport of Oleanolic Acid in Humans, Formulated as a Functional Olive Oil. Food Funct. 2023, 14, 9681–9694. [Google Scholar] [CrossRef] [PubMed]
- Minich, D.M.; Bland, J.S.; Katke, J.; Darland, G.; Hall, A.; Lerman, R.H.; Lamb, J.; Carroll, B.; Tripp, M. Clinical Safety and Efficacy of NG440: A Novel Combination of Rho Iso-Alpha Acids from Hops, Rosemary, and Oleanolic Acid for Inflammatory Conditions. Can. J. Physiol. Pharmacol. 2007, 85, 872–883. [Google Scholar] [CrossRef]
- ICH Guideline. M9 Biopharmaceutics Classification System-Based Biowaivers; The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, European Medicines Agency: Amsterdam, The Netherlands, 2019; Available online: https://www.ema.europa.eu/en/ich-m9-biopharmaceutics-classification-system-based-biowaivers-scientific-guideline (accessed on 18 May 2024).
- Moreton, C. Poor Solubility—Where Do We Stand 25 Years after the ‘Rule of Five’? American Pharmaceutical Review, 18 February 2021. [Google Scholar]
- Alizadeh, S.R.; Savadkouhi, N.; Ebrahimzadeh, M.A. Drug Design Strategies That Aim to Improve the Low Solubility and Poor Bioavailability Conundrum in Quercetin Derivatives. Expert Opin. Drug Discov. 2023, 18, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh Chandel, A.K. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef] [PubMed]
- Kesarwani, K.; Gupta, R. Bioavailability Enhancers of Herbal Origin: An Overview. Asian Pac. J. Trop. Biomed. 2013, 3, 253–266. [Google Scholar] [CrossRef]
- Bilia, A.R.; Piazzini, V.; Risaliti, L.; Vanti, G.; Casamonti, M.; Wang, M.; Bergonzi, M.C. Nanocarriers: A Successful Tool to Increase Solubility, Stability and Optimise Bioefficacy of Natural Constituents. Curr. Med. Chem. 2018, 26, 4631–4656. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Biodegradable Nanoparticles for Drug and Gene Delivery to Cells and Tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in Cancer Therapy and Diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) in Cosmetic and Dermatological Preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, H.; Bala, R.; Arora, S. Lipid-Based Drug Delivery Systems. J. Pharm. 2014, 2014, 801820. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Pignatello, R.; Fuochi, V.; Furneri, P.M.; Lauro, M.R.; Santonocito, D.; Cortesi, R.; Esposito, E. Lipid Nanoparticles and Active Natural Compounds: A Perfect Combination for Pharmaceutical Applications. Curr. Med. Chem. 2019, 26, 4681–4696. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; de Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, V.; Cinci, L.; D’Ambrosio, M.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Solid Lipid Nanoparticles and Chitosan-Coated Solid Lipid Nanoparticles as Promising Tool for Silybin Delivery: Formulation, Characterization, and In Vitro Evaluation. Curr. Drug Deliv. 2019, 16, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, V.; Monteforte, E.; Luceri, C.; Bigagli, E.; Bilia, A.R.; Bergonzi, M.C. Nanoemulsion for Improving Solubility and Permeability of Vitex Agnus-Castus Extract: Formulation and in Vitro Evaluation Using PAMPA and Caco-2 Approaches. Drug Deliv. 2017, 24, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Graverini, G.; Piazzini, V.; Landucci, E.; Pantano, D.; Nardiello, P.; Casamenti, F.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Solid Lipid Nanoparticles for Delivery of Andrographolide across the Blood-Brain Barrier: In Vitro and In Vivo Evaluation. Colloids Surf. B Biointerfaces 2018, 161, 302–313. [Google Scholar] [CrossRef]
- Li, W.; Yi, S.; Wang, Z.; Chen, S.; Xin, S.; Xie, J.; Zhao, C. Self-Nanoemulsifying Drug Delivery System of Persimmon Leaf Extract: Optimization and Bioavailability Studies. Int. J. Pharm. 2011, 420, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Rühl, D.; Runge, S.A. Biodegradation of Solid Lipid Nanoparticles as a Function of Lipase Incubation Time. Int. J. Pharm. 1996, 144, 115–121. [Google Scholar] [CrossRef]
- Mei, Z.; Chen, H.; Weng, T.; Yang, Y.; Yang, X. Solid Lipid Nanoparticle and Microemulsion for Topical Delivery of Triptolide. Eur. J. Pharm. Biopharm. 2003, 56, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Doktorovova, S. Solid Lipid Nanoparticle Formulations: Pharmacokinetic and Biopharmaceutical Aspects in Drug Delivery. Methods Enzymol. 2009, 464, 105–129. [Google Scholar] [PubMed]
- Wang, J.W.; Tang, H.F.; Shen, M.; Wang, L.; Fang, K.Q. Preparation and Quality Evaluation of Oleanolic Acid-Loaded Solid Lipid Nanoparticles. J. Fourth Milit. Med. Univ. 2007, 28, 472. [Google Scholar]
- Wang, J.; Zhang, S.; Wen, A. Optimizing for Preparation Technique of Oleanolic Acid Solid Lipid Nanoparticles by Orthogonal Test. Chin. Trad. Herbal Drugs 2007, 38, 683. [Google Scholar]
- Sun, H.; Zhang, X.; Wang, S.; Tu, Y.; Zhao, R.; Xie, Y. Preparation and Characterization of Oleanolic Acid-Loaded Solid Lipid Nanoparticles for Oral Administration. J. Chin. Pharm. Sci. 2011, 20, 259–265. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Md, S.; Sahni, J.K.; Baboota, S.; Dang, S.; Ali, J. Nanostructured Lipid Carriers System: Recent Advances in Drug Delivery. J. Drug Target 2012, 20, 813–830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lv, S.; Li, X.; Feng, Y.; Li, X.; Liu, L.; Li, S.; Li, Y. Preparation, Characterization, and In Vivo Pharmacokinetics of Nanostructured Lipid Carriers Loaded with Oleanolic Acid and Gentiopicrin. Int. J. Nanomed. 2013, 8, 3227–3239. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, G.; Nahak, P.; Roy, B.; Guha, P.; Tsuchiya, K.; Torigoe, K.; Nath, R.K.; Panda, A.K. Use of Ion Pair Amphiphile as an Alternative of Natural Phospholipids in Enhancing the Stability and Anticancer Activity of Oleanolic Acid Loaded Nanostructured Lipid Carriers. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 147–156. [Google Scholar] [CrossRef]
- Nikolaev, B.; Yakovleva, L.; Fedorov, V.; Li, H.; Gao, H.; Shevtsov, M. Nano-and Microemulsions in Biomedicine: From Theory to Practice. Pharmaceutics 2023, 15, 1989. [Google Scholar] [CrossRef]
- Paul, B.K.; Moulik, S.P. Microemulsions: An Overview. J. Dispers. Sci. Technol. 1997, 18, 301–367. [Google Scholar] [CrossRef]
- Madhav, S.; Gupta, D. A Review on Microemulsion Based System. Int. J. Pharm. Sci. Res. 2011, 2, 1888. [Google Scholar]
- Cecchi, L.; Piazzini, V.; D’Ambrosio, M.; Luceri, C.; Rocco, F.; Innocenti, M.; Vanti, G.; Mulinacci, N.; Bergonzi, M.C. Formulation of a Phenol-Rich Extract from Unripe Olives (Olea europaea L.) in Microemulsion to Improve Its Solubility and Intestinal Permeability. Molecules 2020, 25, 3198. [Google Scholar] [CrossRef] [PubMed]
- Vasarri, M.; Degl’Innocenti, D.; Albonetti, L.; Bilia, A.R.; Bergonzi, M.C. Pentacyclic Triterpenes from Olive Leaves Formulated in Microemulsion: Characterization and Role in De Novo Lipogenesis in HepG2 Cells. Int. J. Mol. Sci. 2023, 24, 12113. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Huang, X.; Dou, J.; Zhai, G.; Su, L. Self-Microemulsifying Drug Delivery System for Improved Oral Bioavailability of Oleanolic Acid: Design and Evaluation. Int. J. Nanomed. 2013, 8, 2917–2926. [Google Scholar]
- Anton, N.; Benoit, J.-P.; Saulnier, P. Design and Production of Nanoparticles Formulated from Nano-Emulsion Templates—A Review. J. Control. Release 2008, 128, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Anton, N.; Vandamme, T.F. The Universality of Low-Energy Nano-Emulsification. Int. J. Pharm. 2009, 377, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, P.; Esquena, J.; Tadros, T.F.; Dederen, C.; Garcia, M.J.; Azemar, N.; Solans, C. Formation and Stability of Nano-Emulsions Prepared Using the Phase Inversion Temperature Method. Langmuir 2002, 18, 26–30. [Google Scholar] [CrossRef]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nano-Emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Rathod, N.B.; Meral, R.; Siddiqui, S.A.; Nirmal, N.; Ozogul, F. Nanoemulsion-Based Approach to Preserve Muscle Food: A Review with Current Knowledge. Crit. Rev. Food Sci. Nutr. 2023. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Chang, Q.; Chan, C.K.; Meng, Z.Y.; Wang, G.N.; Sun, J.B.; Wang, Y.T.; Tong, H.H.Y.; Zheng, Y. Formulation Development and Bioavailability Evaluation of a Self-Nanoemulsified Drug Delivery System of Oleanolic Acid. AAPS PharmSciTech 2009, 10, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.; Sultana, Y.; Aqil, M. Liposomal Drug Delivery Systems: An Update Review. Curr. Drug Deliv. 2007, 4, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Wei, X.; Yang, D.; Xing, Z.; Cai, J.; Wang, L.; Zhao, C.; Wei, X.; Jiang, M.; Sun, H.; Zhou, L. Hepatocyte-Targeted Delivery Using Oleanolic Acid-Loaded Liposomes for Enhanced Hepatocellular Carcinoma Therapy. Biomater. Sci. 2023, 11, 3952–3964. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-T.; Wang, Y.-W.; Wu, Y.-C.; Lin, L.-W.; Chen, C.-C.; Chen, C.-Y.; Kuo, S.-M. Reparative Efficacy of Liposome-Encapsulated Oleanolic Acid against Liver Inflammation Induced by Fine Ambient Particulate Matter and Alcohol in Mice. Pharmaceutics 2022, 14, 1108. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, X.; Xu, X.; Gao, N.; Liu, X. Preparation, Characterization and in Vivo Pharmacokinetic Study of PVP-Modified Oleanolic Acid Liposomes. Int. J. Pharm. 2017, 517, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Tang, S.; Tong, Q. Oleanolic Acid Liposomes with Polyethylene Glycol Modification: Promising Antitumor Drug Delivery. Int. J. Nanomedicine 2012, 7, 3517–3526. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, Z.; Zhang, X.; Huang, J.; Yu, X.; Li, J.; Xiong, D.; Sun, X.; Zhong, Z. Effect of a Controlled-Release Drug Delivery System Made of Oleanolic Acid Formulated into Multivesicular Liposomes on Hepatocellular Carcinoma In Vitro and In Vivo. Int. J. Nanomed. 2016, 11, 3111–3129. [Google Scholar]
- Alvarado, H.L.; Abrego, G.; Garduño-Ramirez, M.L.; Clares, B.; Calpena, A.C.; García, M.L. Design and Optimization of Oleanolic/Ursolic Acid-Loaded Nanoplatforms for Ocular Anti-Inflammatory Applications. Nanomedicine 2015, 11, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Wang, Z.; Xu, M.-Z.; Zeng, Z.; Huang, J.; Guan, Y.-Q. Natural Plant-Derived Polygalacturonic Acid-Oleanolic Acid Assemblies as Oral-Delivered Nanomedicine for Insulin Resistance Treatment. Chem. Eng. J. 2020, 390, 124630. [Google Scholar] [CrossRef]
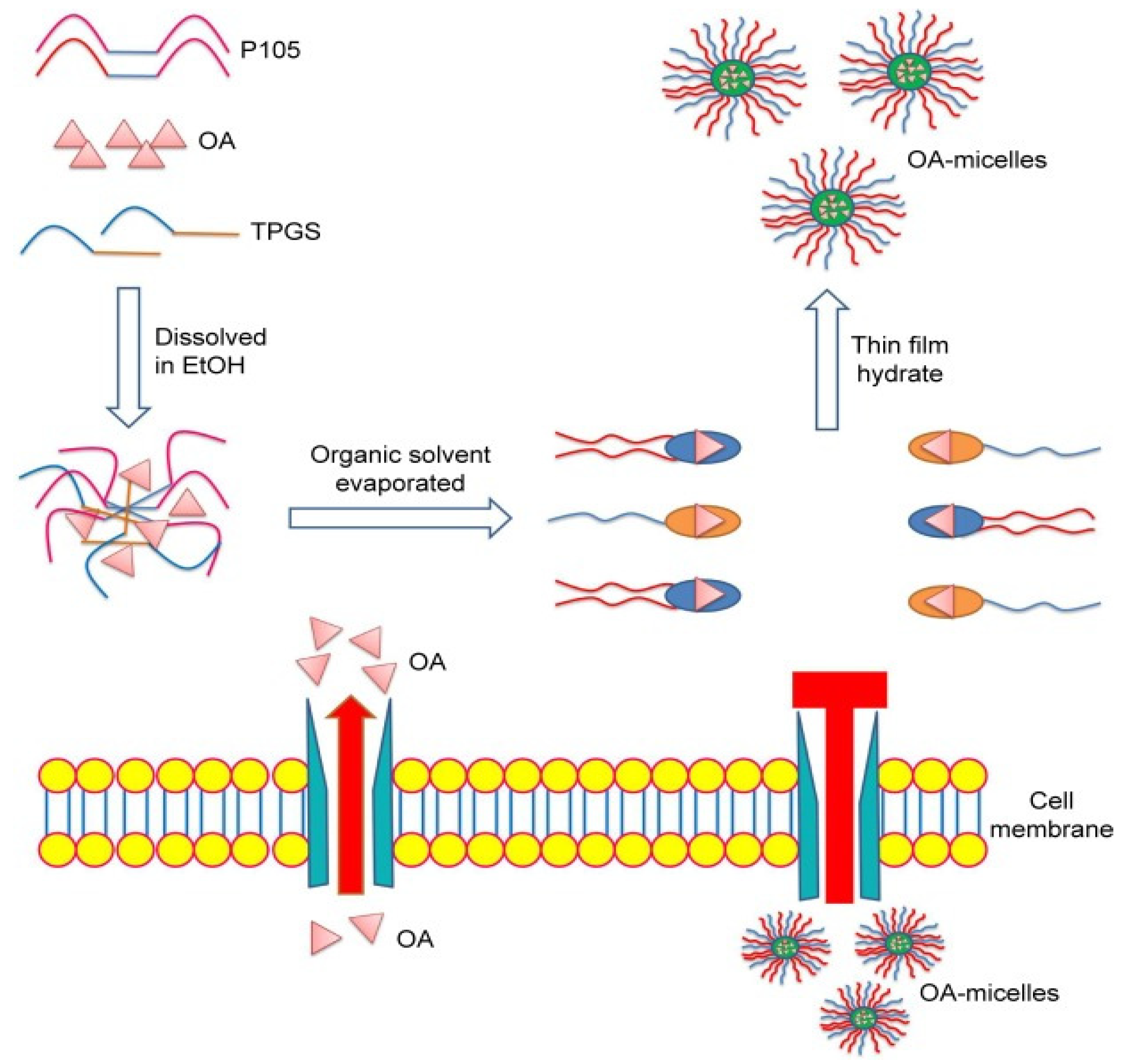
- Wu, H.; Zhong, Q.; Zhong, R.; Huang, H.; Xia, Z.; Ke, Z.; Zhang, Z.; Song, J.; Jia, X. Preparation and Antitumor Evaluation of Self-Assembling Oleanolic Acid-Loaded Pluronic P105/d-α-Tocopheryl Polyethylene Glycol Succinate Mixed Micelles for Non-Small-Cell Lung Cancer Treatment. Int. J. Nanomed. 2016, 11, 6337–6352. [Google Scholar] [CrossRef] [PubMed]
- An, J.Y.; Yang, H.S.; Park, N.R.; Koo, T.; Shin, B.; Lee, E.H.; Cho, S.H. Development of Polymeric Micelles of Oleanolic Acid and Evaluation of Their Clinical Efficacy. Nanoscale Res. Lett. 2020, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, J.; Yang, X.; Zhao, X.; Xu, H. Oleanolic Acid Nanosuspensions: Preparation, in-Vitro Characterization and Enhanced Hepatoprotective Effect. J. Pharm. Pharmacol. 2010, 57, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Das, S.; Ng, K.Y.; Heng, P.W.S. Formulation, Biological and Pharmacokinetic Studies of Sucrose Ester-Stabilized Nanosuspensions of Oleanolic Acid. Pharm. Res. 2011, 28, 2020–2033. [Google Scholar] [CrossRef] [PubMed]
- Banarase, N.B.; Kaur, C.D. Whole Whey Stabilized Oleanolic Acid Nanosuspension: Formulation and Evaluation Study. J. Drug Deliv. Sci. Technol. 2022, 67, 103001. [Google Scholar] [CrossRef]
- Rangel-Yagui, C.d.O.; Pessoa, A., Jr.; Tavares, L.C. Micellar Solubilization of Drugs. J. Pharm. Pharm. Sci. 2005, 8, 147–163. [Google Scholar] [PubMed]
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric Micelles: Basic Research to Clinical Practice. Int. J. Pharm. 2017, 532, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, H.M.; Lavasanifar, A. Polymeric Micelles for Drug Delivery. Expert Opin. Drug Deliv. 2006, 3, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging Role of Nanosuspensions in Drug Delivery Systems. Biomater. Res. 2020, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Rabinow, B.E. Nanosuspensions in Drug Delivery. Nat. Rev. Drug Discov. 2004, 3, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as Drug Delivery Systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, P.; Li, G.; Zhu, S.; Liu, K.; Liu, Y.; He, J.; Lei, J. Amphiphilic Carboxylated Cellulose-g-Poly (L-Lactide) Copolymer Nanoparticles for Oleanolic Acid Delivery. Carbohydr. Polym. 2019, 214, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.N.; Mehata, A.K.; Setia, A.; Kumari, P.; Mahto, S.K.; Muthu, M.S.; Mishra, S.K. EGFR Targeted Albumin Nanoparticles of Oleanolic Acid: In Silico Screening of Nanocarrier, Cytotoxicity and Pharmacokinetics for Lung Cancer Therapy. Int. J. Biol. Macromol. 2023, 246, 125719. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Qiu, Z.; Qiao, X.; Waterhouse, G.I.N.; Zhu, W.; Zhao, W.; He, Q.; Zheng, Z. Creating Burdock Polysaccharide-Oleanolic Acid-Ursolic Acid Nanoparticles to Deliver Enhanced Anti-Inflammatory Effects: Fabrication, Structural Characterization and Property Evaluation. Food Sci. Hum. Wellness 2023, 12, 454–466. [Google Scholar] [CrossRef]
- Ghosh, S.; Kar, N.; Bera, T. Oleanolic Acid Loaded Poly Lactic Co-Glycolic Acid-Vitamin E TPGS Nanoparticles for the Treatment of Leishmania Donovani Infected Visceral Leishmaniasis. Int. J. Biol. Macromol. 2016, 93, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Liu, H.; Lv, H.; Zhang, J.; Zhou, J.; Zhao, Z. Preparation, Characterization, and In Vitro/Vivo Studies of Oleanolic Acid-Loaded Lactoferrin Nanoparticles. Drug Des. Devel. Ther. 2017, 11, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Hao, J.; Gao, D.; Duan, J.; Liu, Z. Preparation and Characterization of Oleanolic Acid Nanoparticles. Curr. Pharm. Anal. 2013, 9, 177–182. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, C.; Liu, H.; Li, Z.; Chen, R.; Zhou, M.; Li, D.; Ye, Q.; Luo, C.; Sun, J. Polymeric Nanoparticles Developed by Vitamin E-Modified Aliphatic Polycarbonate Polymer to Promote Oral Absorption of Oleanolic Acid. Asian J. Pharm. Sci. 2017, 12, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sharma, S.; Sharma, V.; Sharma, K.; Yadav, S.K.; Dwivedi, P.; Agrawal, S.; Paliwal, S.K.; Dwivedi, A.K.; Maikhuri, J.P. Oleanolic–Bioenhancer Coloaded Chitosan Modified Nanocarriers Attenuate Breast Cancer Cells by Multimode Mechanism and Preserve Female Fertility. Int. J. Biol. Macromol. 2017, 104, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhang, S.; Chen, Z.; Chen, A.T.; Ma, J.; Deng, G.; Xu, W.; Zhou, J.; Yu, Z.Q.; Yao, G.; et al. Synergistic Chemotherapy for Breast Cancer and Breast Cancer Brain Metastases via Paclitaxel-Loaded Oleanolic Acid Nanoparticles. Mol. Pharm. 2020, 17, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Pan, S.; Wang, L.; Li, S. Topical Gel Based Nanoparticles for the Controlled Release of Oleanolic Acid: Design and in Vivo Characterization of a Cubic Liquid Crystalline Anti-Inflammatory Drug. BMC Complement Med. Ther. 2021, 21, 224. [Google Scholar] [CrossRef] [PubMed]
- Kumbham, S.; Paul, M.; Itoo, A.; Ghosh, B.; Biswas, S. Oleanolic Acid-Conjugated Human Serum Albumin Nanoparticles Encapsulating Doxorubicin as Synergistic Combination Chemotherapy in Oropharyngeal Carcinoma and Melanoma. Int. J. Pharm. 2022, 614, 121479. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.W.; Zou, C.; Hassan, S.; Din, F.U.; Razak, M.Y.A.; Nawaz, A.; Zeb, A.; Wahab, A.; Bangash, S.A. Cisplatin and Oleanolic Acid Co-Loaded PH-Sensitive CaCO3 Nanoparticles for Synergistic Chemotherapy. RSC Adv. 2022, 12, 14808–14818. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Xu, H.; Bao, X.; Zhang, C.; Guan, X.; Liu, H.; Lv, L.; Deng, S.; Gao, D.; Wang, C. Oleanolic Acid-Loaded PLGA-TPGS Nanoparticles Combined with Heparin Sodium-Loaded PLGA-TPGS Nanoparticles for Enhancing Chemotherapy to Liver Cancer. Life Sci. 2016, 165, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Lee, J.-Y.; Lee, M.-Y.; Song, C.-K.; Choi, J.-H.; Kim, D.-D. Solid Dispersions as a Drug Delivery System. J. Pharm. Investig. 2011, 41, 125–142. [Google Scholar] [CrossRef]
- Nair, A.R.; Lakshman, Y.D.; Anand, V.S.K.; Sree, K.S.N.; Bhat, K.; Dengale, S.J. Overview of Extensively Employed Polymeric Carriers in Solid Dispersion Technology. AAPS PharmSciTech 2020, 21, 309. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, R.J.; Zia, H.; Sandhu, H.K.; Shah, N.H.; Malick, W.A. Improving the Dissolution Rate of Poorly Water Soluble Drug by Solid Dispersion and Solid Solution—Pros and Cons. Drug Deliv. 2007, 14, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, X. Improved Dissolution of Oleanolic Acid with Ternary Solid Dispersions. AAPS PharmSciTech 2007, 8, 267–271. [Google Scholar] [CrossRef]
- De Stefani, C.; Lodovichi, J.; Albonetti, L.; Salvatici, M.C.; Quintela, J.C.; Bilia, A.R.; Bergonzi, M.C. Solubility and Permeability Enhancement of Oleanolic Acid by Solid Dispersion in Poloxamers and γ-CD. Molecules 2022, 27, 3042. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.H.Y.; Du, Z.; Wang, G.N.; Chan, H.M.; Chang, Q.; Lai, L.C.M.; Chow, A.H.L.; Zheng, Y. Spray Freeze Drying with Polyvinylpyrrolidone and Sodium Caprate for Improved Dissolution and Oral Bioavailability of Oleanolic Acid, a BCS Class IV Compound. Int. J. Pharm. 2011, 404, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Guo, M.; Fu, Q.; He, Z. Application of Hot Melt Extrusion to Enhance the Dissolution and Oral Bioavailability of Oleanolic Acid. Asian J. Pharm. Sci. 2017, 12, 66–72. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Guo, B.; Li, Y.; Geng, Y.; Zhao, F.; Zhang, T. Enhancement of Dissolution Rate and Oral Bioavailability in Beagle Dogs of Oleanolic Acid by Adsorbing onto Porous Silica Using Supercritical Carbon Dioxide. J. Drug Deliv. Sci. Technol. 2014, 24, 380–385. [Google Scholar] [CrossRef]
- Cao, F.; Gao, Y.; Yin, Z.; Ping, Q. Enhanced Oral Bioavailability of Oleanolic Acid in Rats with Phospholipid Complex. Lett. Drug Design Discov. 2012, 9, 505–512. [Google Scholar]
- Ren, Y.; Liu, Y.; Niu, R.; Liao, X.; Zhang, J.; Yang, B. Host-Guest Inclusion System of Oleanolic Acid with Methyl-β-Cyclodextrin: Preparation, Characterization and Anticancer Activity. J. Mol. Struct. 2016, 1117, 1–7. [Google Scholar] [CrossRef]
- Oprean, C.; Mioc, M.; Csányi, E.; Ambrus, R.; Bojin, F.; Tatu, C.; Cristea, M.; Ivan, A.; Danciu, C.; Dehelean, C. Improvement of Ursolic and Oleanolic Acids’ Antitumor Activity by Complexation with Hydrophilic Cyclodextrins. Biomed. Pharmacother. 2016, 83, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Quan, P.; Liu, D.F.; Wei, F.D.; Zhang, Q.; Xu, Q.W. The Influence of Cosolvent on the Complexation of HP-β-Cyclodextrins with Oleanolic Acid and Ursolic Acid. AAPS PharmSciTech 2009, 10, 1137–1144. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, Y.; Yang, Z.; Niu, R.; Gao, K.; Yang, B.; Liao, X.; Zhang, J. Solid Inclusion Complexes of Oleanolic Acid with Amino-Appended β-Cyclodextrins (ACDs): Preparation, Characterization, Water Solubility and Anticancer Activity. Mater. Sci. Eng. C 2016, 69, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A Review on Phospholipids and Their Main Applications in Drug Delivery Systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Xin, C.; Liu, S.; Qu, H.; Wang, Z. The Novel Nanocomplexes Containing Deoxycholic Acid-Grafted Chitosan and Oleanolic Acid Displays the Hepatoprotective Effect against CCl4-Induced Liver Injury In Vivo. Int. J. Biol. Macromol. 2021, 185, 338–349. [Google Scholar] [CrossRef]
- Ahmed, S.; Padilla-Gainza, V.M.; Gilkerson, R.; Narula, A.; Lozano, K. Processing-Structure–Property Relationships of Oleanolic Acid Loaded PLGA Fiber Membranes. J. Mater. Sci. 2023, 58, 4240–4255. [Google Scholar] [CrossRef]
- Fan, J.-P.; Zhong, H.; Zhang, X.-H.; Yuan, T.-T.; Chen, H.-P.; Peng, H.-L. Preparation and Characterization of Oleanolic Acid-Based Low-Molecular-Weight Supramolecular Hydrogels Induced by Heating. ACS Appl. Mater. Interfaces 2021, 13, 29130–29136. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Zhou, Z.; Fang, B.; Chen, Z.; Huang, Y.; Hu, Y.; Liu, H. Berberine Oleanolic Acid Complex Salt Grafted Hyaluronic Acid/Silk Fibroin (BOA-g-HA/SF) Composite Scaffold Promotes Cartilage Tissue Regeneration under IL-1β Caused Stress. Int. J. Biol. Macromol. 2023, 250, 126104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Y.; Shi, R.; Kang, T.; Pang, G.; Wang, B.; Zhao, Y.; Zeng, X.; Zou, C.; Wu, P. Synthesis of Hollow Nanocages MOF-5 as Drug Delivery Vehicle to Solve the Load-Bearing Problem of Insoluble Antitumor Drug Oleanolic Acid (OA). Inorg. Chem. Commun. 2018, 96, 20–23. [Google Scholar] [CrossRef]
- Fu, H.; Yen, F.-L.; Huang, P.-H.; Yang, C.-Y.; Yen, C.-H. Oleanolic Acid Nanofibers Attenuated Particulate Matter-Induced Oxidative Stress in Keratinocytes. Antioxidants 2021, 10, 1411. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.G.; Dhiman, S.; Jindal, M.; Sandhu, I.S.; Chitkara, M. Nanobiomaterials: Applications in Biomedicine and Biotechnology, 3rd ed.; Grumezescu, A.M., Ed.; William Andrew Publishing: New York, NY, USA, 2016; Volume 1, pp. 401–429. [Google Scholar]
- Musielak, E.; Feliczak-Guzik, A.; Nowak, I. Synthesis and Potential Applications of Lipid Nanoparticles in Medicine. Materials 2022, 15, 682. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasim, M.; Bergonzi, M.C. Unlocking the Potential of Oleanolic Acid: Integrating Pharmacological Insights and Advancements in Delivery Systems. Pharmaceutics 2024, 16, 692. https://doi.org/10.3390/pharmaceutics16060692
Wasim M, Bergonzi MC. Unlocking the Potential of Oleanolic Acid: Integrating Pharmacological Insights and Advancements in Delivery Systems. Pharmaceutics. 2024; 16(6):692. https://doi.org/10.3390/pharmaceutics16060692
Chicago/Turabian StyleWasim, Muhammad, and Maria Camilla Bergonzi. 2024. "Unlocking the Potential of Oleanolic Acid: Integrating Pharmacological Insights and Advancements in Delivery Systems" Pharmaceutics 16, no. 6: 692. https://doi.org/10.3390/pharmaceutics16060692
APA StyleWasim, M., & Bergonzi, M. C. (2024). Unlocking the Potential of Oleanolic Acid: Integrating Pharmacological Insights and Advancements in Delivery Systems. Pharmaceutics, 16(6), 692. https://doi.org/10.3390/pharmaceutics16060692








