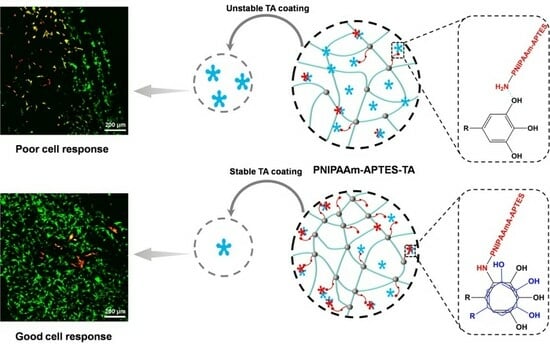
In Situ Preparation of Tannic Acid-Modified Poly(N-isopropylacrylamide) Hydrogel Coatings for Boosting Cell Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Amino-Terminated PNIPAAm-Modified Surface
2.3. Preparation of TA-Modified PNIPAAm-APTES Hydrogel Coatings
2.4. Thermo-Sensitive Behaviors of Different Coatings
2.5. Protein Behaviors on Various Coatings
2.6. Biological Investigation
2.6.1. Cell Culture
2.6.2. Cell Response on Various Coatings
2.7. Statistical Analysis
3. Results and Discussion
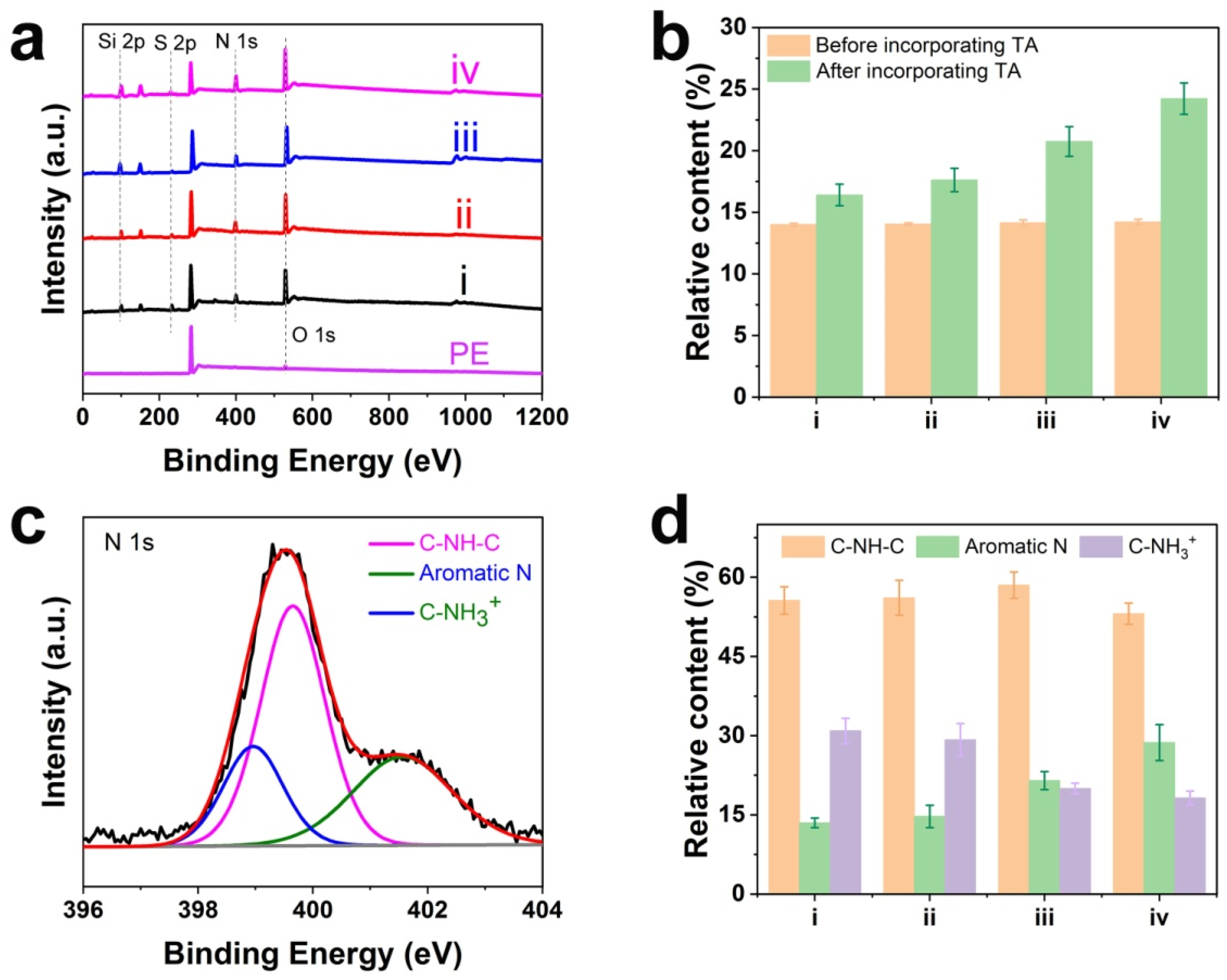
3.1. Chemical Components and Content of Various Coatings
3.2. Morphologies of Various Coatings
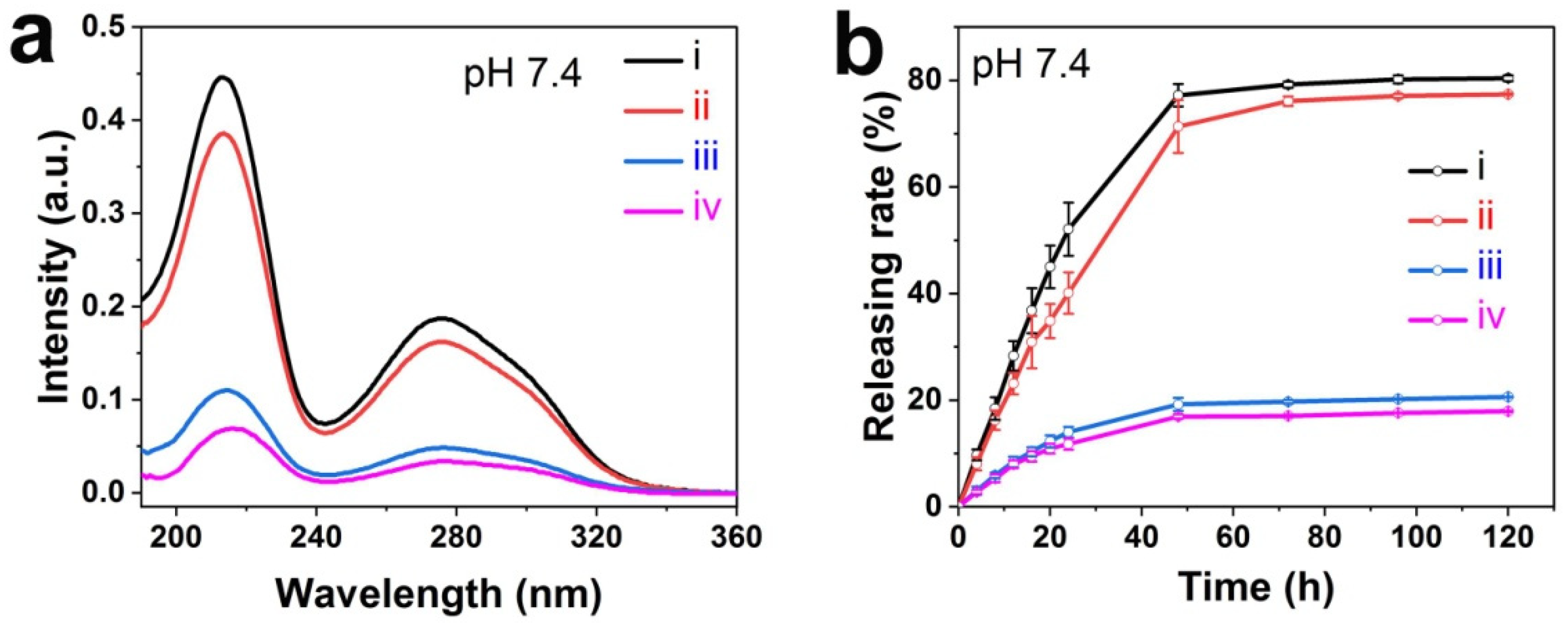
3.3. Stability of TA-Modified Hydrogel Coatings
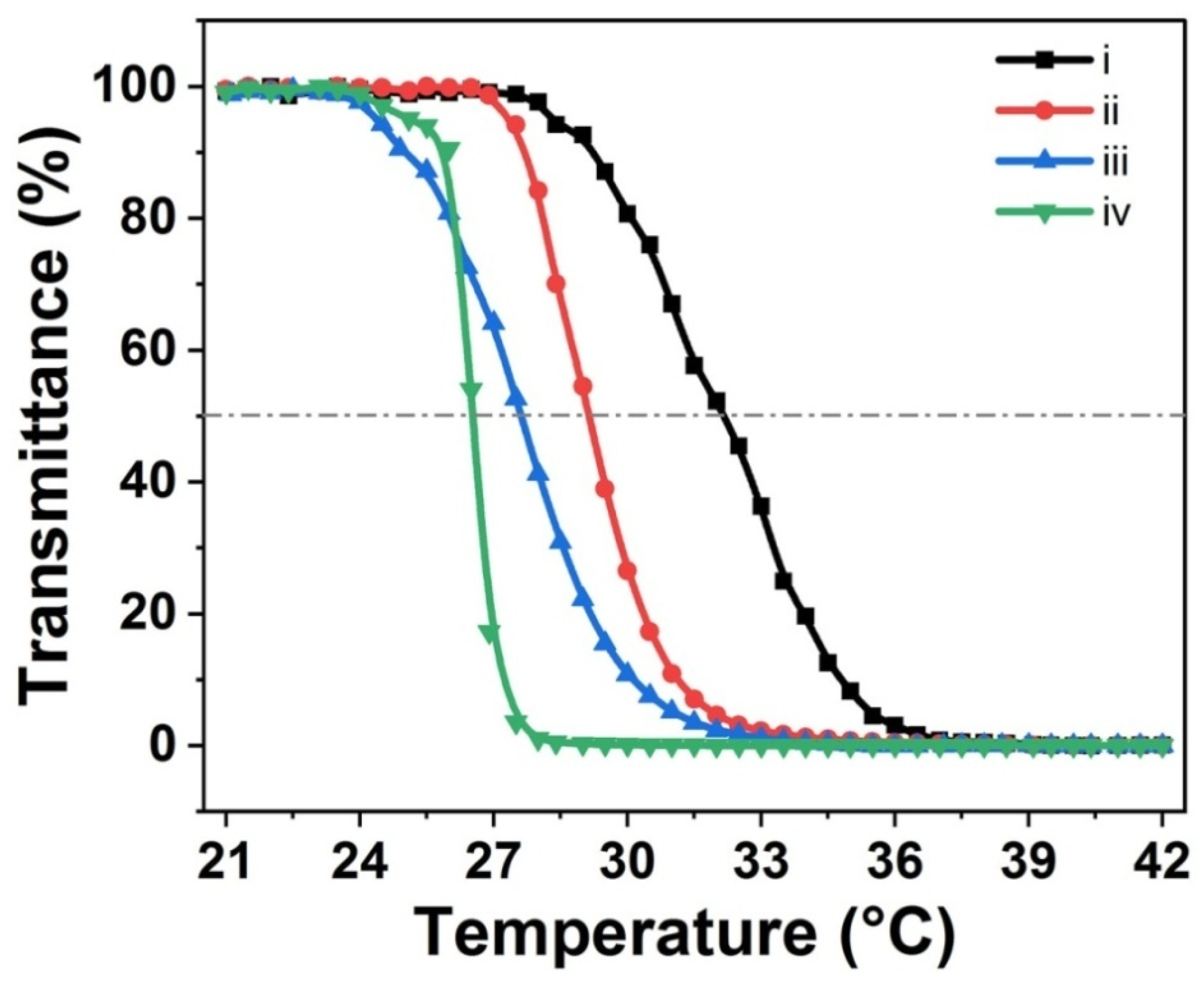
3.4. Thermo-Responsiveness of Various Hydrogel Coatings
3.5. Wetting of Diverse Hydrogel Coatings
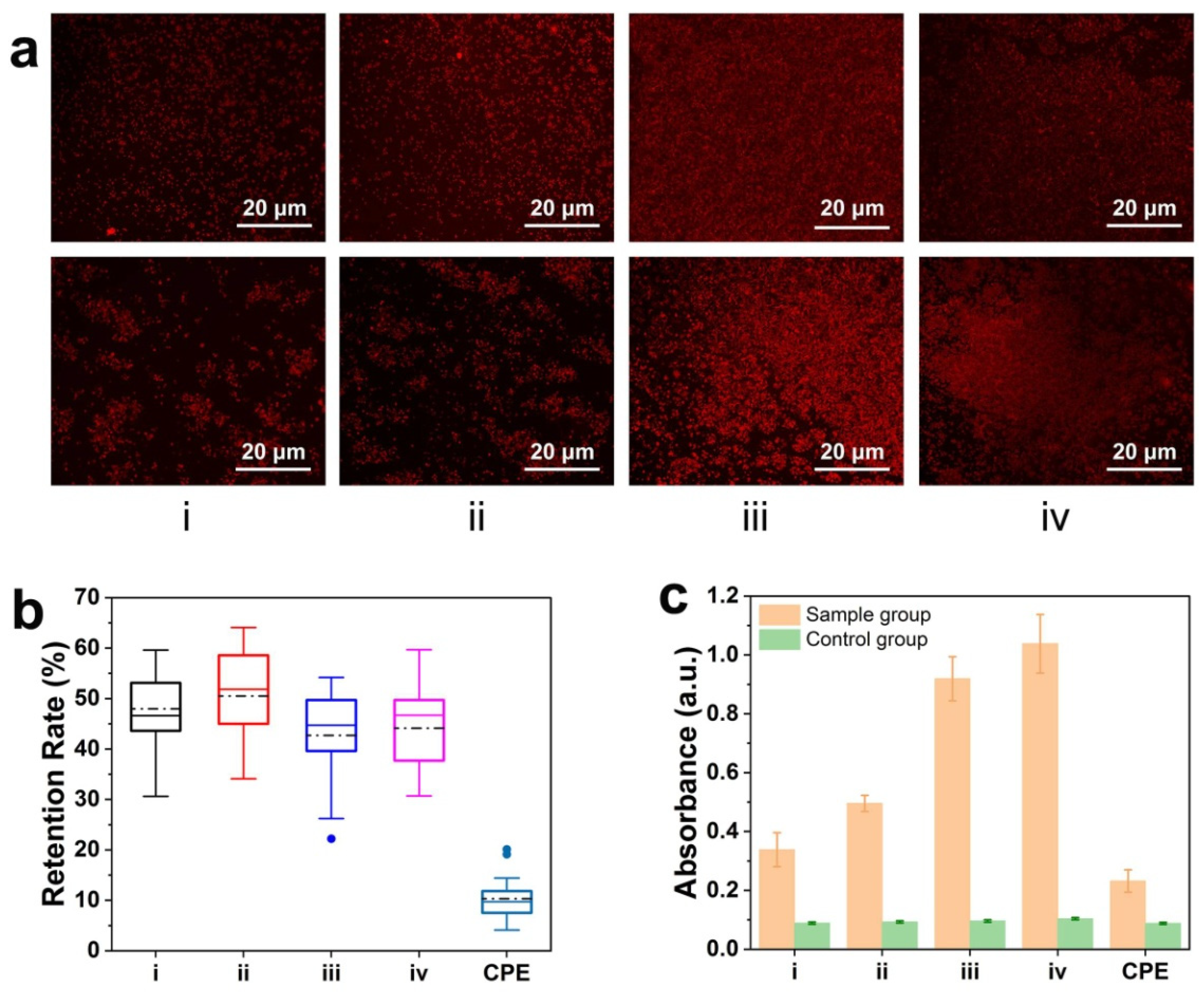
3.6. Protein Behaviors on Hydrogel Coatings
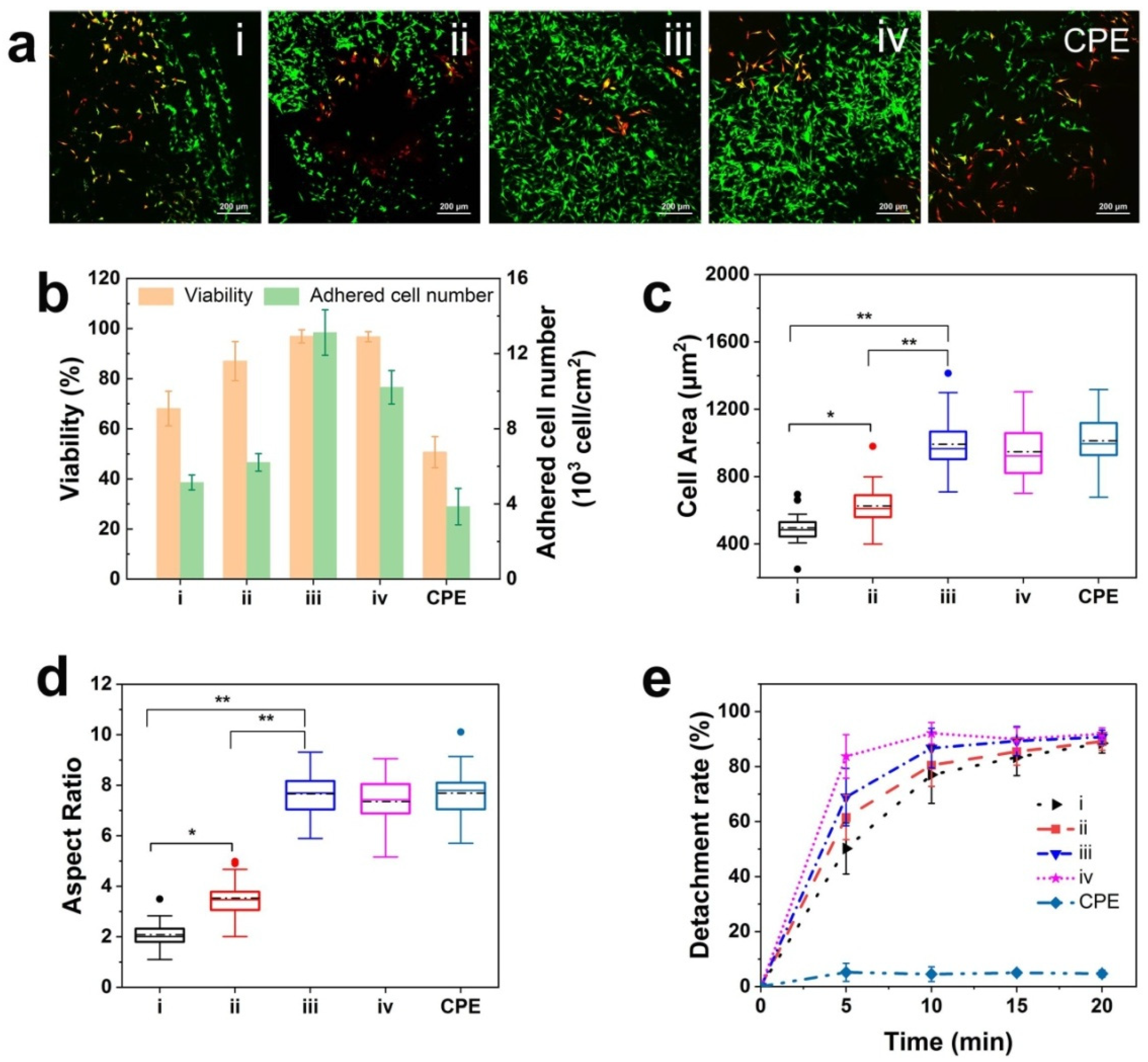
3.7. Cell Response on Diverse Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gepstein, L. Derivation and potential applications of human embryonic stem cells. Circ. Res. 2002, 91, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Stojkoic, M.; Lako, M.; Strachan, T.; Murdoch, A. Derivation, growth and applications of human embryonic stem cells. Reproduction 2004, 128, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Silva, E.A.; Reseland, J.E.; Heyward, C.A.; Haugen, H.J. Biological responses to physicochemical properties of biomaterial surface. Chem. Soc. Rev. 2020, 49, 5178–5224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, F.W.; Wang, Z.T.; Liu, Y.J.; Fu, X.; Jin, A.; Yung, B.C.; Chen, W.; Fan, J.; Yang, X.Y.; et al. Hierarchical assembly of bioactive amphiphilic molecule pairs into supramolecular nanofibril self-supportive scaffolds for stem cell differentiation. J. Am. Chem. Soc. 2016, 138, 15027–15034. [Google Scholar] [CrossRef] [PubMed]
- Kanchanawong, P.; Shtengel, G.; Pasapera, A.M.; Ramko, E.B.; Davidson, M.W.; Hess, H.F.; Waterman, C.M. Nanoscale architecture of integrin-based cell adhesions. Nature 2010, 468, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Cheng, Z.G.; Cheng, K.; Weng, W.J. SiO2/TiO2 nanocomposite films on polystyrene for light-induced cell detachment application. ACS Appl. Mater. Interfaces 2017, 9, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.L.; Akiyama, Y.; Okano, T. Temperature-responsive cell culture surfaces for cell sheet tissue engineering. In Biomaterials in Asia: In Commemoration of the 1st Asian Biomaterials Congress, Tsukuba, Japan, 6–8 December 2007; World Scientific: Singapore, 2008; pp. 55–70. [Google Scholar] [CrossRef]
- Yang, J.; Yamato, M.; Kohno, C.; Nishimoto, A.; Sekine, H.; Fukai, F.; Okano, T. Cell sheet engineering: Recreating tissues without biodegradable scaffolds. Biomaterials 2005, 26, 6415–6422. [Google Scholar] [CrossRef] [PubMed]
- Muscolino, E.; Costa, M.A.; Sabatino, M.A.; Alessi, S.; Bulone, D.; SanBiagio, P.L.; Passantino, R.; Giacomazza, D.; Dispenza, C. Recombinant mussel protein Pvfp5β enhances cell adhesion of poly(vinylalcohol)/k-carrageenan hydrogel scaffolds. Int. J. Biol. Macromol. 2022, 211, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Cushing, M.C.; Anseth, K.S. Hydrogel cell cultures. Science 2007, 316, 1133–1134. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell line age specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Grim, J.C.; Brown, T.E.; Aguado, B.A.; Chapnick, D.A.; Viert, A.L.; Liu, X.D.; Anseth, K.S. A reversible and repeatable thiol-ene bioconjugation for dynamic patterning of signaling proteins in hydrogels. ACS Cent. Sci. 2018, 4, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Gu, L.; Darnell, M.; Klumpers, D.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Mooney, D.J. Substrate stress relaxation regulates cell spreading. Nat. Commun. 2015, 6, 6365. [Google Scholar] [CrossRef]
- Nagase, K.; Yamato, M.; Kanazawa, H.; Okano, T. Poly(N-isopropylacrylamide)-based thermoresponsive surfaces provide new types of biomedical applications. Biomaterials 2018, 153, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Ultrathin poly(N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir 2004, 20, 5506–5511. [Google Scholar] [CrossRef] [PubMed]
- Fukumori, K.; Akiyama, Y.; Kumashiro, Y.; Kobayashi, J.; Yamato, M.; Sakai, K.; Okano, T. Characterization of ultra-thin temperature-responsive polymer layer and its polymer thickness dependency on cell attachment/detachment properties. Macromol. Biosci. 2010, 10, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Shymborska, Y.; Budkowski, A.; Raczkowska, J.; Donchak, V.; Melnyk, Y.; Vasiichuk, V.; Stetsyshyn, Y. Switching it up: The promise of stimuli-responsive polymer systems in biomedical science. Chem. Rec. 2024, 24, e202300217. [Google Scholar] [CrossRef] [PubMed]
- Erel-Unal, I.; Sukhishvili, S.A. Hydrogen-bonded multilayers of a neutral polymer and a polyphenol. Macromolecules 2008, 41, 3962–3970. [Google Scholar] [CrossRef]
- Xu, L.Q.; Neoh, K.G.; Kang, E.T. Natural polyphenols as versatile platforms for material engineering and surface functionalization. Prog. Polym. Sci. 2018, 87, 165–196. [Google Scholar] [CrossRef]
- Jafari, H.; Ghaffari-Bohlouli, P.; Niknezhad, S.V.; Abedi, A.; Izadifar, Z.; Mohammadinejad, R.; Varma, R.S.; Shavandi, A. Tannic acid: A versatile polyphenol for design of biomedical hydrogels. J. Mater. Chem. B 2022, 10, 5873–5912. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, Z.; Correia, A.; Hasany, M.; Figueiredo, P.; Dobakhti, F.; Eskandari, M.R.; Hosseini, S.H.; Abiri, R.; Khorshid, S.; Hirvonen, J.; et al. Ahydrogen-bonded extracellular matrix-mimicking bactericidal hydrogel with radical scavenging and hemostatic function for pH-responsive wound healing acceleration. Adv. Healthc. Mater. 2021, 10, 2001122. [Google Scholar] [CrossRef]
- Wang, Q.; He, M.; Zhao, W.F.; Zhao, C.S. A unidirectional drug-release Janus membrane based on hydrogen bonding barrier effect for preventing post operative adhesion and promoting tissue repair. View 2023, 4, 20230026. [Google Scholar] [CrossRef]
- Choi, D.; Gwon, K.; Hong, H.J.; Baskaran, H.; Calvo-Lozano, O.; Gonzalez-Suarez, A.M.; Park, K.; DeHoyos-Vega, J.M.; Lechuga, L.M.; Hong, J.; et al. Coating bioactive microcapsules with tannic acid enhances the phenotype of the encapsulated pluripotents tem cells. ACS Appl. Mater. Interfaces 2022, 14, 27274–27286. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Kobayashi, J.; Yamato, M.; Okano, T. Temperature-responsive poly(N-isopropylacrylamide)-grafted microcarriers for large-scale non-invasive harvest of achorage-dependent cells. Biomaterials 2012, 33, 3803–3812. [Google Scholar] [CrossRef] [PubMed]
- Wallart, X.; de Villeneuve, C.H.; Allongue, P. Truly quantitative XPS characterization of organic monolayers on silicon: Study of alkyl and alkoxy monolayers on H-Si(111). J. Am. Chem. Soc. 2005, 127, 7871–7878. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Wang, R.G.; Hu, H.L.; Liu, W.B. Surface modification of self-healing poly(urea-formaldehyde) microcapsules using silane-coupling agent. Appl. Surf. Sci. 2008, 255, 1894–1900. [Google Scholar] [CrossRef]
- Zhou, J.; Shen, H.L.; Li, Z.H.; Zhang, S.; Zhao, Y.T.; Bi, X.; Wang, Y.S.; Cui, H.Y.; Zhuo, S.P. Porous carbon materials with dual N,S-doping and uniform ultra-microporosity for high performance super capacitors. Electrochim. Acta 2016, 209, 557–564. [Google Scholar] [CrossRef]
- Ding, Y.; Weng, L.T.; Yang, M.; Yang, Z.; Lu, X.; Huang, N.; Leng, Y. Insights into the aggregation/deposition and structure of a polydopamine film. Langmuir 2014, 30, 12258–12269. [Google Scholar] [CrossRef]
- Choi, C.; Chae, S.Y.; Nah, J.W. Thermosensitive poly(N-isopropylacrylamide)-b-poly(ε-caprolactone) nanoparticles for efficient drug delivery system. Polymer 2006, 47, 4571–4580. [Google Scholar] [CrossRef]
- Tian, Y.; Su, B.; Jiang, L. Interfacial material system exhibiting superwettability. Adv. Mater. 2014, 26, 6872–6897. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Feng, J.; Gao, H.; Feng, X.; Jiang, L. Superwettability-based interfacial chemical reactions. Adv. Mater. 2019, 31, 1800718. [Google Scholar] [CrossRef] [PubMed]
- Ishtikhar, M.; Ahmad, E.; Siddiqui, Z.; Ahmad, S.; Khan, M.V.; Zaman, M.; Siddiqi, M.K.; Nusrat, S.; Chandel, T.I.; Ajmal, M.R.; et al. Biophysical insight into the interaction mechanism of plant derived polyphenolic compound tannic acid with homologous mammalian serum albumins. Int. J. Biol. Macromol. 2018, 107, 2450–2464. [Google Scholar] [CrossRef] [PubMed]
- Altankov, G.; Grinnell, F.; Groth, T. Studies on the biocompatibility of materials: Fibroblast reorganization of substratum-bound fibronectin on surfaces varying in wettability. J. Biomed. Mater. Res. 1996, 30, 385–391. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces: Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive polymers and their biomedical application in tissue engineering—A review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Ji, S.Q.; He, F.; Cao, M.Y.; Peng, S.Q.; Li, Y.X. One-step transformation of highly hydrophobic membranes into superhydrophilic and underwater superoleophobic ones for high-efficiency separation of oil-in-water emulsions. J. Mater. Chem. A 2018, 6, 3391–3396. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, J.; He, F.; Zhao, J.G.; Wang, Z.X. (TA-APTES)Plus: Arapid, green and universal coating for membrane modification toward oil-in-water emulsion separation. J. Membr. Sci. 2023, 680, 121741. [Google Scholar] [CrossRef]
- Lu, Y.T.; Zeng, K.; Fuhrmann, B.; Woelk, C.; Zhang, K.; Groth, T. Engineering of stable cross-linked multilayers based on thermo-responsive PNIPAM-grafted-chitosan/heparin to tailor their physiochemical properties and biocompatibility. ACS Appl. Mater. Interfaces 2022, 14, 29550–29562. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.Q.; Zhang, D.; Feng, C.L. Wettability of supramolecular nanofibers for controlled cell adhesion and proliferation. Langmuir 2013, 29, 15359–15366. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, Y.T.; Zeng, K.; Heinze, T.; Groth, T.; Zhang, K. Recent progress on cellulose-based ionic compounds for biomaterials. Adv. Mater. 2021, 33, 202000717. [Google Scholar] [CrossRef] [PubMed]
- Jonckheere, D.; Steele, J.A.; Claes, B.; Bueken, B.; Claes, L.; Lagrain, B.; Roeffaers, M.B.J.; DeVos, D.E. Adsorption and separation of aromatic amino acids from aqueous solutions using metal organic frame works. ACS Appl. Mater. Interfaces 2017, 9, 30064–30073. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Tanaka, S.; Tsuruoka, T.; Nagahama, K. Two-dimensional metal-organic framework-based cellular scaffolds with high protein adsorption, retention, and replenishment capabilities. ACS Appl. Mater. Interfaces 2022, 14, 34443–34454. [Google Scholar] [CrossRef] [PubMed]
- Secundo, F. Conformational changes of enzymes upon immobilisation. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J.; et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 2015, 6, 7240. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Alizadeh, E. Cell form and function: Interpreting and controlling the shape of adherent cells. Trends Biotechnol. 2019, 37, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Tymetska, S.; Shymborska, Y.; Stetsyshyn, Y.; Budkowski, A.; Bernasik, A.; Awsiuk, K.; Donchak, V.; Raczkowska, J. Thermoresponsive smart copolymer coatings based on P(NIPAM-co-HEMA) and P(OEGMA-co-HEMA) brushes for regenerative medicine. ACS Biomater. Sci. Eng. 2023, 9, 6256–6272. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.Z.; Mooney, D.J. Chemical strategies to engineer hydrogels for cell culture. Nat. Rev. Chem. 2022, 6, 726–744. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.H.; Kikuchi, A.; Yamato, M.; Sakurai, Y.; Okano, T. Rapid cell sheet detachment from poly(N-isopropylacrylamide)-grafted porous cell culture membranes. J. Biomed. Mater. Res. 2000, 50, 82–89. [Google Scholar] [CrossRef]
- Tamura, A.; Uchida, K.; Yajima, H. Reversible temperature-dependent dispersion-aggregation transition of poly(N-isopropylacrylamide)-60 Fullerene conjugates. Chem. Lett. 2006, 35, 282–283. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; Koeverden, M.P.; Such, G.K.; Cui, J.W.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef] [PubMed]






| Sample Code | Abbreviation | Coating Composition | ||
|---|---|---|---|---|
| TA-Free Nanohydrogel Coatings | TA (mg) | |||
| Type | Mass (g) | |||
| I | PNIPAAm2000-APTES-TA | PNIPAAm2000-APTES | 1.0 | 12.5 |
| II | PNIPAAm1300-APTES-TA | PNIPAAm1300-APTES | 1.0 | 12.5 |
| III | PNIPAAm860-APTES-TA | PNIPAAm860-APTES | 1.0 | 12.5 |
| IV | PNIPAAm500-APTES-TA | PNIPAAm500-APTES | 1.0 | 12.5 |
| V | PNIPAAm2000-APTES | PNIPAAm2000-APTES | 1.0 | 0 |
| VI | PNIPAAm1300-APTES | PNIPAAm1300-APTES | 1.0 | 0 |
| VII | PNIPAAm860-APTES | PNIPAAm860-APTES | 1.0 | 0 |
| VIII | PNIPAAm500-APTES | PNIPAAm500-APTES | 1.0 | 0 |
| PE | – | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Liu, X.; Liang, P.; Yuan, H.; Yang, T. In Situ Preparation of Tannic Acid-Modified Poly(N-isopropylacrylamide) Hydrogel Coatings for Boosting Cell Response. Pharmaceutics 2024, 16, 538. https://doi.org/10.3390/pharmaceutics16040538
Xu J, Liu X, Liang P, Yuan H, Yang T. In Situ Preparation of Tannic Acid-Modified Poly(N-isopropylacrylamide) Hydrogel Coatings for Boosting Cell Response. Pharmaceutics. 2024; 16(4):538. https://doi.org/10.3390/pharmaceutics16040538
Chicago/Turabian StyleXu, Jufei, Xiangzhe Liu, Pengpeng Liang, Hailong Yuan, and Tianyou Yang. 2024. "In Situ Preparation of Tannic Acid-Modified Poly(N-isopropylacrylamide) Hydrogel Coatings for Boosting Cell Response" Pharmaceutics 16, no. 4: 538. https://doi.org/10.3390/pharmaceutics16040538
APA StyleXu, J., Liu, X., Liang, P., Yuan, H., & Yang, T. (2024). In Situ Preparation of Tannic Acid-Modified Poly(N-isopropylacrylamide) Hydrogel Coatings for Boosting Cell Response. Pharmaceutics, 16(4), 538. https://doi.org/10.3390/pharmaceutics16040538