Tiny Green Army: Fighting Malaria with Plants and Nanotechnology
Abstract
:1. Introduction
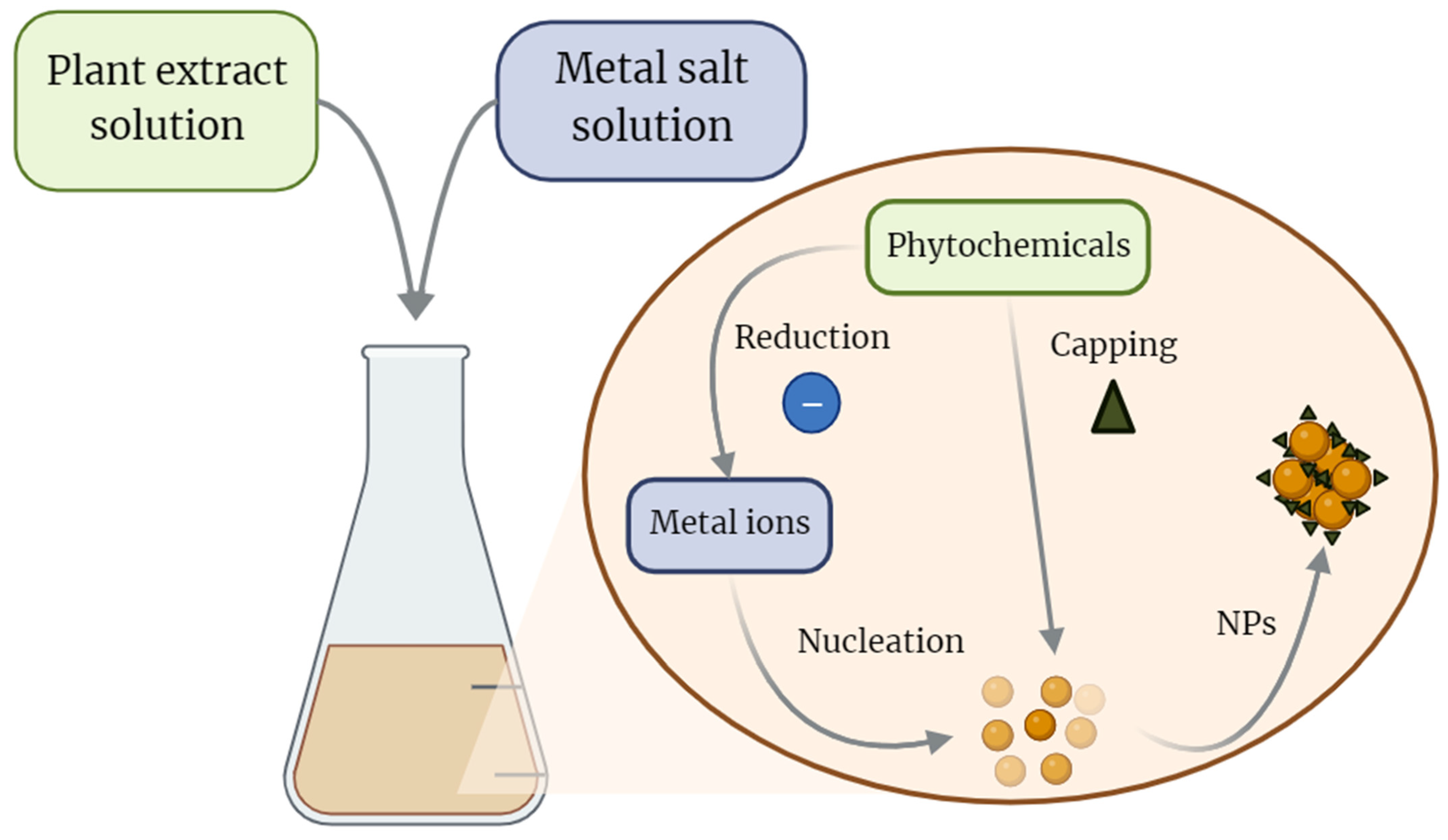
2. Green-Nanotechnology
3. Plants and Nano Formulations
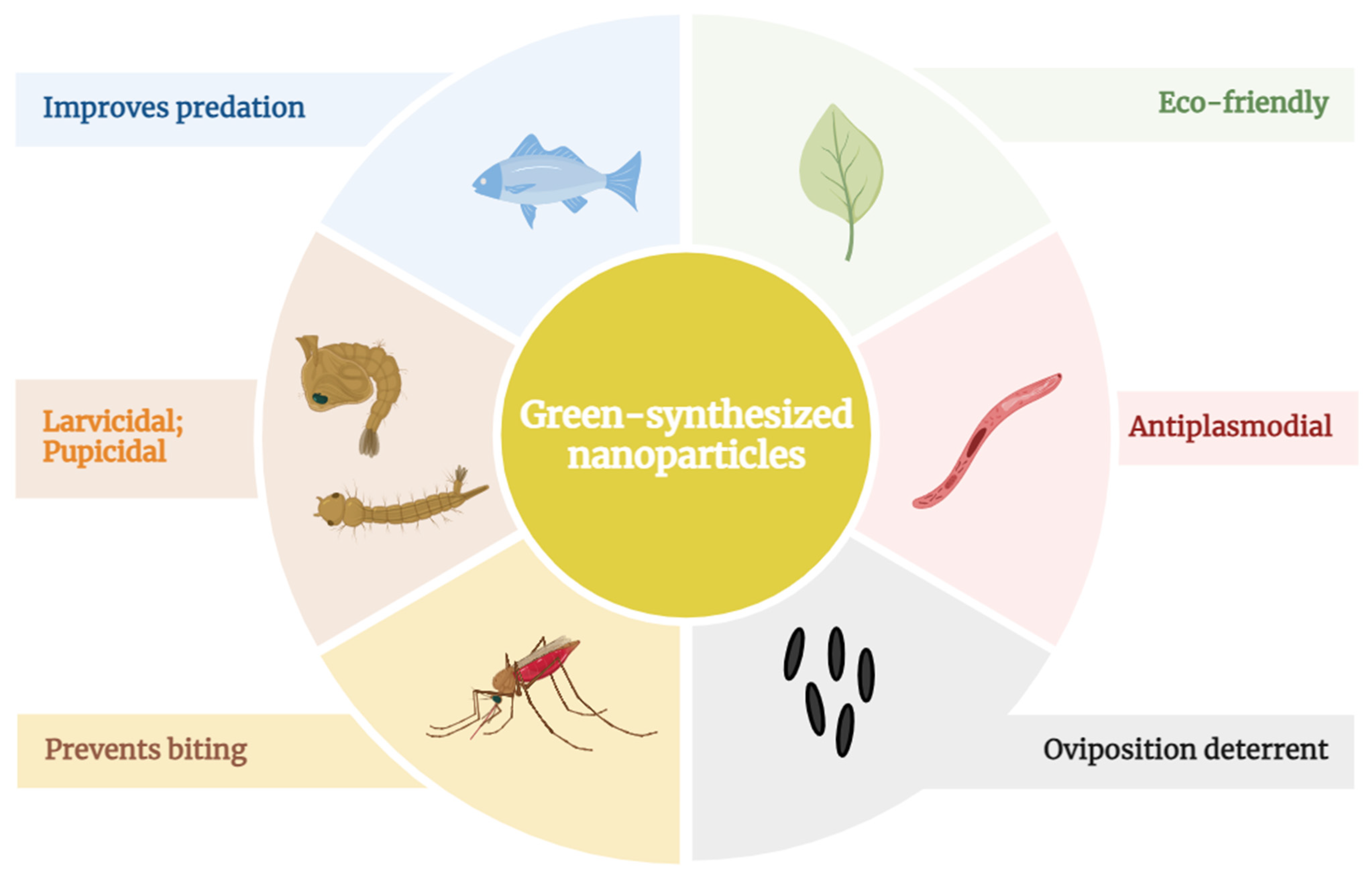
4. Malaria Prevention Strategies
| Plant Extract | Plant Use | Type of Nanotechnology | Size of Nanoparticles | Effects | References |
|---|---|---|---|---|---|
| Alchornea cordifolia leaf | Infections, inflammation, rheumatism, analgesia, and arthritis | Ag NPs | 5–25 nm (TEM) | Larvicidal, impact on larvae behavior and morphology, antiplasmodial and hemolytic | [109] |
| Aristolochia indica leaf | Post–partum infections, snakebites, fever, malaria, skin diseases, helminths, edema, intestinal disorders, and antibacterial | Ag NPs | 30–55 nm (SEM) | Larvicidal and pupicidal in laboratory and field, predation booster | [110] |
| Azadirachta indica leaf and bark | Antimalarial, intestinal disorders, and diabetes | Ag NPs | 4–28 nm (TEM) | Antiplasmodial and hemolytic | [41] |
| Cedrus deodara oil | Insecticidal | Pectin nanocapsules | 40–80 nm (TEM) | Larvicidal and impact on larvae behavior and morphology | [111] |
| Cocos nucifera mesocarp | Food | Ag NPs | mean 23 nm (TEM) | Larvicidal | [112] |
| Couroupita guianensis flower | Antibiotic, antifungal, antidepressant, antiseptic, analgesia, stomach ache, and skin diseases | Au NPs | 29.2–43.8 nm (TEM) | Larvicidal and pupicidal in laboratory and field, adulticidal, predation booster, and antiplasmodial | [113] |
| Codium tomentosum | Antioxidant | Ag NPs | 20–40 nm (SEM) | Larvicidal, pupicidal, antiplasmodial, antioxidant, antibacterial, and predation booster | [114] |
| Eclipta prostrata leaf | Lipidemia, atherosclerosis, hepatic diseases, and snake-venom poisoning | Pd NPs | 18–64 nm (TEM) | Antiplasmodial and cytotoxic | [84] |
| Mimusops elengi leaf | Cardiotonic, stomach ache, anti-helminthic, dysentery, antimicrobial, anti-ulcer, antianxiety, anti-oxidant, antihyperglycemic, anti-hyperlipidemic, anti-inflammatory, and fever | Ag NPs | 25–40 nm (TEM) | Larvicidal, pupicidal, adulticidal, and predation booster in laboratory and field | [115] |
| Arachis hypogaea peel | Cattle food | Ag NPs | 20–50 nm (TEM) | Larvicidal and impact on larvae morphology | [116] |
| Vitex negundo leaf | Bactericidal, diabetes, inflammation, and asthma | ZnO NPs | 28–42 nm (TEM) | Larvicidal, pupicidal, antioxidant, cytotoxic, and photocatalytic | [117] |
| Pteridium aquilinum leaf | Analgesia and food | Ag NPs | 35–65 nm (SEM) | Larvicidal and pupicidal in laboratory and field, adulticidal, ovicidal, antiplasmodial, and repellent smoke | [96] |
| Ulva lactuca | Antioxidant, antibacterial, and antiviral | Ag NPs | 20–35 nm (SEM) | Larvicidal, pupicidal, antiplasmodial, and repellent smoke | [97] |
| Citrus limon leaf | Natural pesticide, insect repellent, and antimicrobial | Au-Pd NPs | 1.5–18.5 nm (TEM) | Larvicidal and predation booster | [98] |
| Lagenaria siceraria peel | Anti-swelling, diuretic, antibacterial, and cytotoxic | ZnO NPs | - | Larvicidal, impact on larvae behavior and morphology, antiplasmodial, predation booster, and cytotoxic | [99] |
| Cymbopogon citratus leaf | - | Au NPs | 20–50 nm (TEM) | Larvicidal, pupicidal, and predation booster | [100] |
| Ichnocarpus frutescens leaf | Anti-diabetes, anti-tumor, anti-inflammatory, and analgesia | Ag NPs | 5–47 nm (TEM) | Larvicidal and biocompatible with non-target organisms | [101] |
| Rubus ellipticus leaf | Diabetes, diarrhea, gastralgia, wound healing, dysentery, antifertility, antimicrobial, analgesia, and epilepsy | Ag NPs | 1–25 nm (TEM) | Larvicidal, ovicidal, and adulticidal, oviposition deterrent, and biocompatible with non-target organisms | [102] |
| Naregamia alata leaf | Wound healing, ulcers, halitosis, cough, asthma, bronchitis, splenomegaly, scabies, pruritus, dysentery, dyspepsia, catarrh, anemia, and malarial fevers | Ag NPs | 0–5.5 nm (TEM) | Larvicidal, ovicidal, adulticidal, and biocompatible with non-target organisms | [103] |
| Hugonia mystax leaf | Anthelmintic and rheumatism | Ag NPs | 40–90 nm (SEM) | Larvicidal and biocompatible with non-target organisms | [104] |
| Solanum xanthocarpum leaf | Anti-cancer, antioxidant, anti-HIV, antibacterial, and insecticidal | Ag NPs | 10–20 nm (TEM) | Larvicidal and biocompatible with non-target organisms | [105] |
| Eucalyptus globulus oil | Natural pesticide | Nanoemulsion | 22–40 nm (TEM) | Larvicidal in laboratory and semi-field | [118] |
| Mangifera indica leaf | Antioxidant and antibacterial | TiO2 NPs | 30 nm (TEM) | Larvicidal and acaricidal | [84] |
| Barleria cristata leaf | Antioxidant, cytotoxic, and antimicrobial | Ag NPs | 38–41 nm (TEM) | Larvicidal, biocompatible with non-target organisms | [119] |
| Malva sylvestris leaf | Antioxidant, anti–inflammatory, and antimicrobial | Ag NPs | 18–25 nm (TEM) | Larvicidal and biocompatible with non-target organisms | [108] |
| Ammania baccifera aerial | Analgesia, antifertility, hypothermic, hypertensive, antiurolithiasis, antisteroidogenic, antimicrobial, antiurolithic, anti–inflammatory, and antioxidant | Ag NPs | 10–30 nm (TEM) | Larvicidal | [120] |
| Artemisia nilagirica leaf | Antimicrobial, anthelmintic, antiseptic, and larvicidal | Ag NPs | <30 nm (SEM) | Larvicidal and pupicidal | [121] |
| Valoniopsis pachynema | - | CdS NPs | <100 nm (SEM) | Larvicidal, pupicidal, antiplasmodial, and toxic to non–target organisms | [107] |
| Vitex negundo leaf | Antimicrobial, anti–inflammatory, diabetes, asthma, cytotoxic, and larvicidal | ZnO NPs | 28.48–42.14 nm (TEM) | Larvicidal and antioxidant | [117] |
| Annona squamosa leaf | Pesticidal, cytotoxic, and antioxidant | Ag NPs | 200–500 nm (SEM) | Larvicidal, pupicidal, ovicidal, and adulticidal | [122] |
| Momordica charantia leaf | Antidiabetic, antiviral, antitumor, antileukemic, antibacterial, anthelmintic, antimutagenic, antimycobacterial, antioxidant, antiulcer, anti-inflammatory, hypocholesterolemic, hypotriglyceridemic, hypotensive, immunostimulant, and insecticidal | ZnO NPs | 21.32 nm (SEM) | Larvicidal, acaricidal, and pediculicidal | [123] |
| TiO2 NPs | 70 nm (TEM) | Larvicidal, pupicidal, antiplasmodial, and biocompatible with non–target organisms | [106] | ||
| Euphorbia hirta leaf | Natural insecticide | Ag NPs | 30–60 nm (SEM) | Larvicidal and pupicidal | [124] |
| Nerium oleander leaf | Anticancer, antimicrobial, anxiolytic, and antipsychotic | Ag NPs | 20–35 nm (SEM) | Larvicidal and pupicidal | [125] |
| Heliotropium indicum leaf | Fever, throat infection, ulcer, gonorrhea, localized inflammation, rheumatism, ring worm, wounds, aphrodisiac, astringent, and expectorant | Ag NPs | 18–45 nm (TEM) | Adulticidal | [126] |
| Zornia diphylla leaf | Dysentery, venereal diseases, and sleep induction | Ag NPs | 30–60 nm (SEM) | Larvicidal and biocompatible with non-target organisms | [127] |
| Mussaenda glabra leaf | - | Ag NPs | 15–25 nm (TEM) | Larvicidal and biocompatible with non-target organisms | [128] |
| Anisomeles indica leaf | Inflammatory skin diseases, liver protection, intestinal infections, abdominal pain and immune system deficiencies, expectorant, diaphoretic, rheumatism, and psoriasis | Ag NPs | 18–35 nm (TEM) | Larvicidal | [129] |
| Holostemma adakodien leaf | - | Ag NPs | - | Larvicidal, antibacterial, and biocompatible with non-target organisms | [81] |
| Quisqualis indica leaf | Antimicrobial and anticoccidial | Ag NPs | <30 nm (SEM) | Larvicidal and biocompatible with non-target organisms | [130] |
| Nicandra physalode leaf | Antidiuretic, mydriasis, analgesia, antibacterial, anti-inflammatory, and insecticidal | Ag NPs | 5–35 nm (SEM) | Larvicidal and biocompatible with non-target organisms | [131] |
| Gmelina asiatica leaf | Hepatic diseases | Ag NPs | 20–64 nm (TEM) | Larvicidal | [132] |
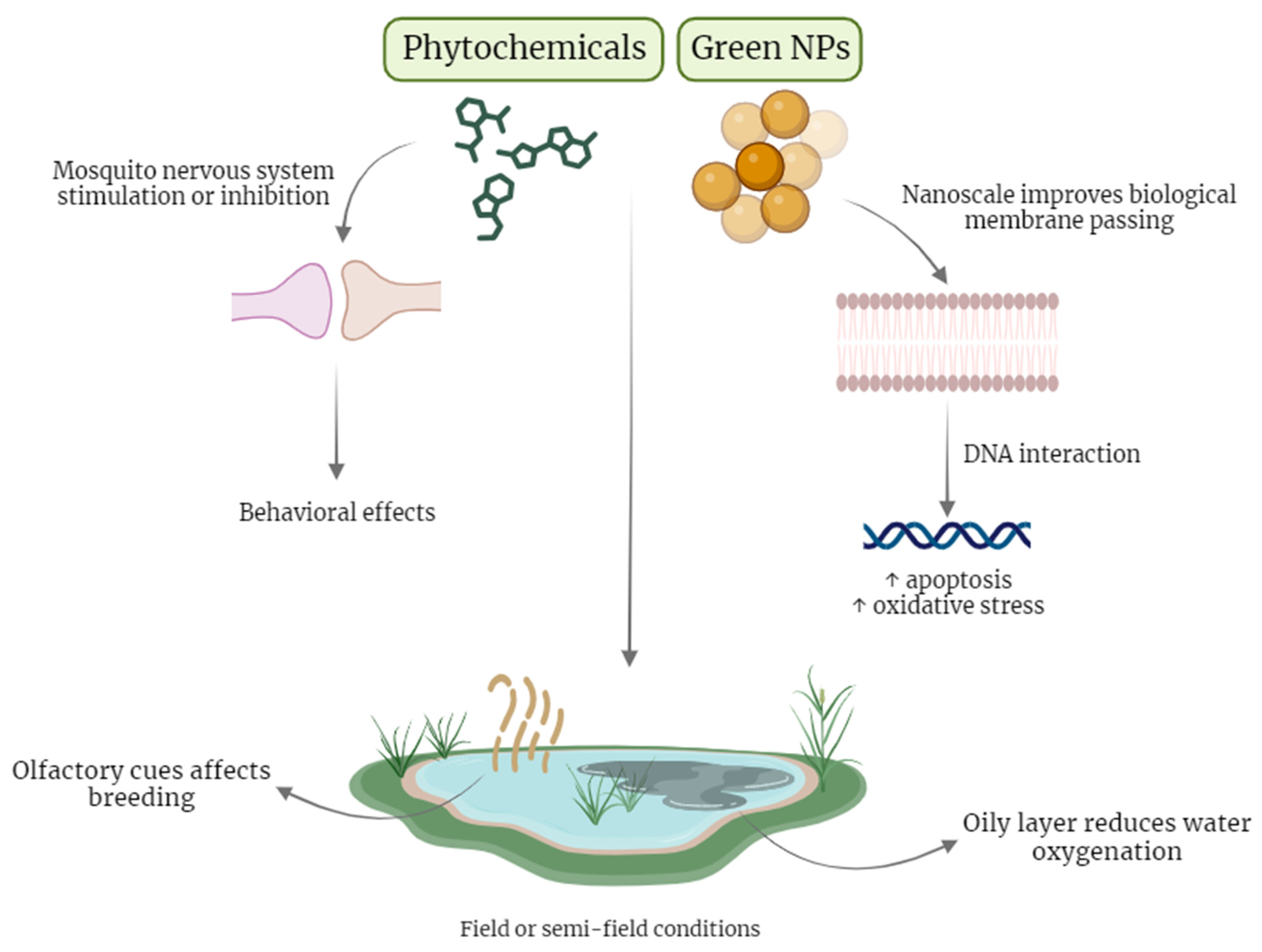
5. Plant Extracts and Green NPs Action Mechanisms
6. Antimalarial Effects of Green NPs
7. Conclusions
8. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- WHO. World Malaria Report; WHO: Geneva, Switzerland, 2022; p. 293.
- Gonzalez-Sanz, M.; Berzosa, P.; Norman, F.F. Updates on Malaria Epidemiology and Prevention Strategies. Curr. Infect. Dis. Rep. 2023, 25, 131–139. [Google Scholar] [CrossRef]
- Lu, H.Z.; Sui, Y.; Lobo, N.F.; Fouque, F.; Gao, C.; Lu, S.; Lv, S.; Deng, S.Q.; Wang, D.Q. Challenge and opportunity for vector control strategies on key mosquito-borne diseases during the COVID-19 pandemic. Front. Public Health 2023, 11, 1207293. [Google Scholar] [CrossRef] [PubMed]
- Loonen, J.; Dery, D.B.; Musaka, B.Z.; Bandibabone, J.B.; Bousema, T.; van Lenthe, M.; Pop-Stefanija, B.; Fesselet, J.F.; Koenraadt, C.J.M. Identification of main malaria vectors and their insecticide resistance profile in internally displaced and indigenous communities in Eastern Democratic Republic of the Congo (DRC). Malar. J. 2020, 19, 425. [Google Scholar] [CrossRef] [PubMed]
- Yokoly, F.N.; Zahouli, J.B.Z.; Small, G.; Ouattara, A.F.; Opoku, M.; de Souza, D.K.; Koudou, B.G. Assessing Anopheles vector species diversity and transmission of malaria in four health districts along the borders of Cote d’Ivoire. Malar. J. 2021, 20, 409. [Google Scholar] [CrossRef] [PubMed]
- Okuneye, K.; Eikenberry, S.E.; Gumel, A.B. Weather-driven malaria transmission model with gonotrophic and sporogonic cycles. J. Biol. Dyn. 2019, 13, 288–324. [Google Scholar] [CrossRef] [PubMed]
- Gallichotte, E.N.; Dobos, K.M.; Ebel, G.D.; Hagedorn, M.; Rasgon, J.L.; Richardson, J.H.; Stedman, T.T.; Barfield, J.P. Towards a method for cryopreservation of mosquito vectors of human pathogens. Cryobiology 2021, 99, 1–10. [Google Scholar] [CrossRef]
- Tripathi, H.; Bhalerao, P.; Singh, S.; Arya, H.; Alotaibi, B.S.; Rashid, S.; Hasan, M.R.; Bhatt, T.K. Malaria therapeutics: Are we close enough? Parasit. Vectors 2023, 16, 130. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines for Malaria; WHO: Geneva, Switzerland, 2023; p. 447.
- Azmi, W.A.; Rizki, A.F.M.; Djuardi, Y.; Artika, I.M.; Siregar, J.E. Molecular insights into artemisinin resistance in Plasmodium falciparum: An updated review. Infect. Genet. Evol. 2023, 112, 105460. [Google Scholar] [CrossRef]
- Wang, S.; Huang, F.; Yan, H.; Yin, J.; Xia, Z. A review of malaria molecular markers for drug resistance in Plasmodium falciparum and Plasmodium vivax in China. Front. Cell. Infect. Microbiol. 2023, 13, 1167220. [Google Scholar] [CrossRef]
- Chaves, J.B.; Portugal Tavares de Moraes, B.; Regina Ferrarini, S.; Noe da Fonseca, F.; Silva, A.R.; Goncalves-de-Albuquerque, C.F. Potential of nanoformulations in malaria treatment. Front. Pharmacol. 2022, 13, 999300. [Google Scholar] [CrossRef]
- Haldar, K.; Bhattacharjee, S.; Safeukui, I. Drug resistance in Plasmodium. Nat. Rev. Microbiol. 2018, 16, 156–170. [Google Scholar] [CrossRef]
- Wicht, K.J.; Mok, S.; Fidock, D.A. Molecular Mechanisms of Drug Resistance in Plasmodium falciparum Malaria. Annu. Rev. Microbiol. 2020, 74, 431–454. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Mishra, V.K.; Kashaw, V.; Iyer, A.K.; Kashaw, S.K. Comprehensive review on various strategies for antimalarial drug discovery. Eur. J. Med. Chem. 2017, 125, 1300–1320. [Google Scholar] [CrossRef] [PubMed]
- Sougoufara, S.; Diedhiou, S.M.; Doucoure, S.; Diagne, N.; Sembene, P.M.; Harry, M.; Trape, J.F.; Sokhna, C.; Ndiath, M.O. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: A new challenge to malaria elimination. Malar. J. 2014, 13, 125. [Google Scholar] [CrossRef]
- Matowo, N.S.; Martin, J.; Kulkarni, M.A.; Mosha, J.F.; Lukole, E.; Isaya, G.; Shirima, B.; Kaaya, R.; Moyes, C.; Hancock, P.A.; et al. An increasing role of pyrethroid-resistant Anopheles funestus in malaria transmission in the Lake Zone, Tanzania. Sci. Rep. 2021, 11, 13457. [Google Scholar] [CrossRef] [PubMed]
- Soma, D.D.; Zogo, B.M.; Some, A.; Tchiekoi, B.N.; Hien, D.F.S.; Pooda, H.S.; Coulibaly, S.; Gnambani, J.E.; Ouari, A.; Mouline, K.; et al. Anopheles bionomics, insecticide resistance and malaria transmission in southwest Burkina Faso: A pre-intervention study. PLoS ONE 2020, 15, e0236920. [Google Scholar] [CrossRef] [PubMed]
- Riveron, J.M.; Chiumia, M.; Menze, B.D.; Barnes, K.G.; Irving, H.; Ibrahim, S.S.; Weedall, G.D.; Mzilahowa, T.; Wondji, C.S. Rise of multiple insecticide resistance in Anopheles funestus in Malawi: A major concern for malaria vector control. Malar. J. 2015, 14, 344. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Zahoor, M.; Nazir, N.; Iftikhar, M.; Naz, S.; Zekker, I.; Burlakovs, J.; Uddin, F.; Kamran, A.W.; Kallistova, A.; Pimenov, N.; et al. A Review on Silver Nanoparticles: Classification, Various Methods of Synthesis, and Their Potential Roles in Biomedical Applications and Water Treatment. Water 2021, 13, 2216. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Aldakheel, F.M.; Sayed, M.M.E.; Mohsen, D.; Fagir, M.H.; El Dein, D.K. Green Synthesis of Silver Nanoparticles Loaded Hydrogel for Wound Healing; Systematic Review. Gels 2023, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Yue, G.; Guo, L.; Hu, Y.; Cui, Q.; Wang, J.; Tang, J.; Liu, H. Nanodrugs systems for therapy and diagnosis of esophageal cancer. Front. Bioeng. Biotechnol. 2023, 11, 1233476. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ji, Z.; Zhou, H.; Wu, D.; Gu, Z.; Wang, D.; Ten Dijke, P. Lipid-based nanoparticles as drug delivery systems for cancer immunotherapy. MedComm 2023, 4, e339. [Google Scholar] [CrossRef] [PubMed]
- Shoeb, E.; Badar, U.; Venkataraman, S.; Hefferon, K. Frontiers in Bioengineering and Biotechnology: Plant Nanoparticles for Anti-Cancer Therapy. Vaccines 2021, 9, 830. [Google Scholar] [CrossRef]
- Yazdanian, M.; Rostamzadeh, P.; Rahbar, M.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Yazdanian, A. The Potential Application of Green-Synthesized Metal Nanoparticles in Dentistry: A Comprehensive Review. Bioinorg. Chem. Appl. 2022, 2022, 2311910. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals-A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Elkodous, M.A.; Shabaka, S.H.; Younis, S.A.; Alshangiti, D.M.; Madani, M.; Al-Gahtany, S.A.; Elkhatib, W.F.; Noreddin, A.M.; Nady, N.; et al. An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment. Nanotechnol. Rev. 2021, 10, 954–977. [Google Scholar] [CrossRef]
- Noga, M.; Milan, J.; Frydrych, A.; Jurowski, K. Toxicological Aspects, Safety Assessment, and Green Toxicology of Silver Nanoparticles (AgNPs)— Critical Review: State of the Art. Int. J. Mol. Sci. 2023, 24, 5133. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.U.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Borges, D.F.; Lopes, E.A.; Fialho Moraes, A.R.; Soares, M.S.; Visôtto, L.E.; Oliveira, C.R.; Moreira Valente, V.M. Formulation of botanicals for the control of plant-pathogens: A review. Crop. Protect. 2018, 110, 135–140. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K.; Prasad, K.; Kulkarni, A.R. Plant system: Nature’s nanofactory. Colloids Surf. B Biointerfaces 2009, 73, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Miu, B.A.; Dinischiotu, A. New Green Approaches in Nanoparticles Synthesis: An Overview. Molecules 2022, 27, 6472. [Google Scholar] [CrossRef] [PubMed]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Tehrani, A.; Omranpoor, M.M.; Vatanara, A.; Seyedabadi, M.; Ramezani, V. Formation of nanosuspensions in bottom-up approach: Theories and optimization. Daru 2019, 27, 451–473. [Google Scholar] [CrossRef]
- Chugh, D.; Viswamalya, V.S.; Das, B. Green synthesis of silver nanoparticles with algae and the importance of capping agents in the process. J. Genet. Eng. Biotechnol. 2021, 19, 126. [Google Scholar] [CrossRef]
- Ahmed, S.; Alhareth, K.; Mignet, N. Advancement in nanogel formulations provides controlled drug release. Int. J. Pharm. 2020, 584, 119435. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Hawadak, J.; Kojom Foko, L.P.; Pande, V.; Singh, V. In vitro antiplasmodial activity, hemocompatibility and temporal stability of Azadirachta indica silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2022, 50, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Santos-Magalhaes, N.S.; Mosqueira, V.C. Nanotechnology applied to the treatment of malaria. Adv. Drug Deliv. Rev. 2010, 62, 560–575. [Google Scholar] [CrossRef]
- Joshi, M.; Pathak, S.; Sharma, S.; Patravale, V. Solid microemulsion preconcentrate (NanOsorb) of artemether for effective treatment of malaria. Int. J. Pharm. 2008, 362, 172–178. [Google Scholar] [CrossRef]
- Kumar, R.; Ray, P.C.; Datta, D.; Bansal, G.P.; Angov, E.; Kumar, N. Nanovaccines for malaria using Plasmodium falciparum antigen Pfs25 attached gold nanoparticles. Vaccine 2015, 33, 5064–5071. [Google Scholar] [CrossRef] [PubMed]
- Zaker, M. Natural Plant Products as Eco-friendly Fungicides for Plant Diseases Control—A Review. Agriculturists 2016, 14, 134–141. [Google Scholar] [CrossRef]
- Benelli, G.; Maggi, F.; Pavela, R.; Murugan, K.; Govindarajan, M.; Vaseeharan, B.; Petrelli, R.; Cappellacci, L.; Kumar, S.; Hofer, A.; et al. Mosquito control with green nanopesticides: Towards the One Health approach? A review of non-target effects. Environ. Sci. Pollut. Res. Int. 2018, 25, 10184–10206. [Google Scholar] [CrossRef]
- Ali, M.Y.S.; Ravikumar, S.; Beula, J.M. Mosquito larvicidal activity of seaweeds extracts against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Asian Pac. J. Trop. Dis. 2013, 3, 196–201. [Google Scholar] [CrossRef]
- Rajeswary, M.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Zingiber cernuum (Zingiberaceae) essential oil as effective larvicide and oviposition deterrent on six mosquito vectors, with little non-target toxicity on four aquatic mosquito predators. Environ. Sci. Pollut. Res. Int. 2018, 25, 10307–10316. [Google Scholar] [CrossRef]
- Govindarajan, M.; Benelli, G. Eco-friendly larvicides from Indian plants: Effectiveness of lavandulyl acetate and bicyclogermacrene on malaria, dengue and Japanese encephalitis mosquito vectors. Ecotoxicol. Environ. Saf. 2016, 133, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, G.D.J.G.; Rei Yan, S.L.; Palmisano, G.; Wrenger, C. Plant Extracts as a Source of Natural Products with Potential Antimalarial Effects: An Update from 2018 to 2022. Pharmaceutics 2023, 15, 1638. [Google Scholar] [CrossRef]
- Bagavan, A.; Rahuman, A.A.; Kaushik, N.K.; Sahal, D. In vitro antimalarial activity of medicinal plant extracts against Plasmodium falciparum. Parasitol. Res. 2011, 108, 15–22. [Google Scholar] [CrossRef]
- Elango, G.; Rahuman, A.A.; Kamaraj, C.; Bagavan, A.; Zahir, A.A. Efficacy of medicinal plant extracts against malarial vector, Anopheles subpictus Grassi. Parasitol. Res. 2011, 108, 1437–1445. [Google Scholar] [CrossRef]
- Kamaraj, C.; Kaushik, N.K.; Mohanakrishnan, D.; Elango, G.; Bagavan, A.; Zahir, A.A.; Rahuman, A.A.; Sahal, D. Antiplasmodial potential of medicinal plant extracts from Malaiyur and Javadhu hills of South India. Parasitol. Res. 2012, 111, 703–715. [Google Scholar] [CrossRef]
- Kamaraj, C.; Kaushik, N.K.; Rahuman, A.A.; Mohanakrishnan, D.; Bagavan, A.; Elango, G.; Zahir, A.A.; Santhoshkumar, T.; Marimuthu, S.; Jayaseelan, C.; et al. Antimalarial activities of medicinal plants traditionally used in the villages of Dharmapuri regions of South India. J. Ethnopharmacol. 2012, 141, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Bagavan, A.; Rahuman, A.A.; Zahir, A.A.; Kamaraj, C.; Elango, G.; Jayaseelan, C.; Kirthi, A.V.; Santhoshkumar, T.; Marimuthu, S.; et al. Evaluation of antiplasmodial activity of medicinal plants from North Indian Buchpora and South Indian Eastern Ghats. Malar. J. 2015, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Alaithan, H.; Kumar, N.; Islam, M.Z.; Liappis, A.P.; Nava, V.E. Novel Therapeutics for Malaria. Pharmaceutics 2023, 15, 1800. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Zinzuvadia, A.; Prajapati, M.; Tyagi, R.K.; Dalai, S. Swertiamarin-mediated immune modulation/adaptation confers protection against Plasmodium berghei. Future Microbiol. 2022, 17, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, G.; Alfei, S. Development of Phytochemical Delivery Systems by Nano-Suspension and Nano-Emulsion Techniques. Int. J. Mol. Sci. 2023, 24, 9824. [Google Scholar] [CrossRef]
- Ahmed, T.; Hyder, M.Z.; Liaqat, I.; Scholz, M. Climatic Conditions: Conventional and Nanotechnology-Based Methods for the Control of Mosquito Vectors Causing Human Health Issues. Int. J. Environ. Res. Public Health 2019, 16, 3165. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Swathy, K.; Thomas, A.; Kweka, E.J.; Rahman, A.; Pittarate, S.; Krutmuang, P. Insecticidal Efficacy of Microbial-Mediated Synthesized Copper Nano-Pesticide against Insect Pests and Non-Target Organisms. Int. J. Environ. Res. Public Health 2021, 18, 10536. [Google Scholar] [CrossRef]
- Murugan, K.; Subramaniam, J.; Rajaganesh, R.; Panneerselvam, C.; Amuthavalli, P.; Vasanthakumaran, M.; Jayashanthini, S.; Dinesh, D.; Anitha, J.; Wang, L.; et al. Efficacy and side effects of bio-fabricated sardine fish scale silver nanoparticles against malarial vector Anopheles stephensi. Sci. Rep. 2021, 11, 19567. [Google Scholar] [CrossRef]
- Thelma, J.; Balasubramanian, C. Ovicidal, larvicidal and pupicidal efficacy of silver nanoparticles synthesized by Bacillus marisflavi against the chosen mosquito species. PLoS ONE 2021, 16, e0260253. [Google Scholar] [CrossRef]
- Murugan, K.; Anitha, J.; Dinesh, D.; Suresh, U.; Rajaganesh, R.; Chandramohan, B.; Subramaniam, J.; Paulpandi, M.; Vadivalagan, C.; Amuthavalli, P.; et al. Fabrication of nano-mosquitocides using chitosan from crab shells: Impact on non-target organisms in the aquatic environment. Ecotoxicol. Environ. Saf. 2016, 132, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, R.B.; Patil, S.V.; Patil, C.D.; Salunke, B.K. Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti (Linnaeus, 1762) and Anopheles stephensi Liston (Diptera; Culicidae). Parasitol. Res. 2011, 109, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, A.; Murugan, K.; Panneerselvam, C.; Madhiyazhagan, P.; Dinesh, D.; Vadivalagan, C.; Aziz, A.T.; Chandramohan, B.; Suresh, U.; Rajaganesh, R.; et al. Earthworm-mediated synthesis of silver nanoparticles: A potent tool against hepatocellular carcinoma, Plasmodium falciparum parasites and malaria mosquitoes. Parasitol. Int. 2016, 65, 276–284. [Google Scholar] [CrossRef]
- Roux, O.; Robert, V. Larval predation in malaria vectors and its potential implication in malaria transmission: An overlooked ecosystem service? Parasit. Vectors 2019, 12, 217. [Google Scholar] [CrossRef]
- Al Mukhaini, S.K.; Mohammed, O.A.; Gerbers, S.; Al Awaidy, S.T. The Progress Towards National Malaria Elimination: The Experience of Oman. Oman Med. J. 2023, 38, e500. [Google Scholar] [CrossRef]
- Bosly, H.A.E. Evaluation of larvicidal enhanced activity of sandalwood oil via nano-emulsion against Culex pipiens and Ades aegypti. Saudi J. Biol. Sci. 2022, 29, 103455. [Google Scholar] [CrossRef]
- Ferreira, R.; D’Haveloose, N.P.; Cruz, R.A.S.; Araujo, R.S.; Carvalho, J.C.T.; Rocha, L.; Fernandes, L.P.; Da Costa, T.S.; Fernandes, C.P.; Souto, R.N.P. Nano-emulsification Enhances the Larvicidal Potential of the Essential Oil of Siparuna guianensis (Laurales: Siparunaceae) Against Aedes (Stegomyia) aegypti (Diptera: Culicidae). J. Med. Entomol. 2020, 57, 788–796. [Google Scholar] [CrossRef]
- Gnanadesigan, M.; Anand, M.; Ravikumar, S.; Maruthupandy, M.; Vijayakumar, V.; Selvam, S.; Dhineshkumar, M.; Kumaraguru, A.K. Biosynthesis of silver nanoparticles by using mangrove plant extract and their potential mosquito larvicidal property. Asian Pac. J. Trop. Med. 2011, 4, 799–803. [Google Scholar] [CrossRef]
- Hajra, A.; Dutta, S.; Mondal, N.K. Mosquito larvicidal activity of cadmium nanoparticles synthesized from petal extracts of marigold (Tagetes sp.) and rose (Rosa sp.) flower. J. Parasit. Dis. 2016, 40, 1519–1527. [Google Scholar] [CrossRef]
- Lobato Rodrigues, A.B.; Martins, R.L.; Rabelo, E.M.; Tomazi, R.; Santos, L.L.; Brandao, L.B.; Faustino, C.G.; Ferreira Farias, A.L.; Dos Santos, C.B.R.; de Castro Cantuaria, P.; et al. Development of nano-emulsions based on Ayapana triplinervis essential oil for the control of Aedes aegypti larvae. PLoS ONE 2021, 16, e0254225. [Google Scholar] [CrossRef]
- Rajaganesh, R.; Murugan, K.; Panneerselvam, C.; Jayashanthini, S.; Aziz, A.T.; Roni, M.; Suresh, U.; Trivedi, S.; Rehman, H.; Higuchi, A.; et al. Fern-synthesized silver nanocrystals: Towards a new class of mosquito oviposition deterrents? Res. Vet. Sci. 2016, 109, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Suresh, U.; Murugan, K.; Benelli, G.; Nicoletti, M.; Barnard, D.R.; Panneerselvam, C.; Kumar, P.M.; Subramaniam, J.; Dinesh, D.; Chandramohan, B. Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2015, 114, 1551–1562. [Google Scholar] [CrossRef]
- Udappusamy, V.; Mohan, H.; Thinagaran, R. Lantana camara L. essential oil mediated nano-emulsion formulation for biocontrol application: Anti-mosquitocidal, anti-microbial and antioxidant assay. Arch. Microbiol. 2022, 204, 388. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, Y.; Feng, Y.; Ren, X.; Li, Y.; Li, W.; Huang, J.; Kong, L.; Chen, X.; Lin, Z.; et al. Study of the Repellent Activity of 60 Essential Oils and Their Main Constituents against Aedes albopictus, and Nano-Formulation Development. Insects 2022, 13, 1077. [Google Scholar] [CrossRef]
- Abd Elghani, E.M.; El Sayed, A.M.; Abdel-Aziz Emam, M.M.; Al-Mahallawi, A.M.; Tadros, S.H.; Soliman, F.M.; Youssef, F.S. Seasonal metabolic profiling of Valencia orange leaf essential oil using GC coupled with chemometrics, nano-formulation, and insecticidal evaluation: In vivo and in silico. RSC Adv. 2023, 13, 1659–1671. [Google Scholar] [CrossRef]
- Murugan, K.; Dinesh, D.; Nataraj, D.; Subramaniam, J.; Amuthavalli, P.; Madhavan, J.; Rajasekar, A.; Rajan, M.; Thiruppathi, K.P.; Kumar, S.; et al. Iron and iron oxide nanoparticles are highly toxic to Culex quinquefasciatus with little non-target effects on larvivorous fishes. Environ. Sci. Pollut. Res. Int. 2018, 25, 10504–10514. [Google Scholar] [CrossRef] [PubMed]
- Vincent, S.; Kovendan, K.; Chandramohan, B.; Kamalakannan, S.; Kumar, P.M.; Vasugi, C.; Praseeja, C.; Subramaniam, J.; Govindarajan, M.; Murugan, K.; et al. Swift Fabrication of Silver Nanoparticles Using Bougainvillea glabra: Potential Against the Japanese Encephalitis Vector, Culex tritaeniorhynchus Giles (Diptera: Culicidae). J. Clust. Sci. 2017, 28, 37–58. [Google Scholar] [CrossRef]
- Alyahya, S.A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Mothana, R.A.; Al-anbr, M.N.; Vaseeharan, B.; Ishwarya, R.; Yazhiniprabha, M.; et al. Swift fabrication of Ag nanostructures using a colloidal solution of Holostemma ada-kodien (Apocynaceae)—Antibiofilm potential, insecticidal activity against mosquitoes and non-target impact on water bugs. J. Photochem. Photobiol. B Biol. 2018, 181, 70–79. [Google Scholar] [CrossRef]
- Osanloo, M.; Firooziyan, S.; Abdollahi, A.; Hatami, S.; Nematollahi, A.; Elahi, N.; Zarenezhad, E. Nanoemulsion and nanogel containing Artemisia dracunculus essential oil; larvicidal effect and antibacterial activity. BMC Res. Notes 2022, 15, 276. [Google Scholar] [CrossRef]
- Rawani, A.; Ghosh, A.; Chandra, G. Mosquito larvicidal and antimicrobial activity of synthesized nano-crystalline silver particles using leaves and green berry extract of Solanum nigrum L. (Solanaceae: Solanales). Acta Trop. 2013, 128, 613–622. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Roopan, S.M.; Chung, I.M.; Anbarasan, K.; Karthikeyan, V. Efficacy of larvicidal activity of green synthesized titanium dioxide nanoparticles using Mangifera indica extract against blood-feeding parasites. Parasitol. Res. 2015, 114, 571–581. [Google Scholar] [CrossRef]
- Abrantes, D.C.; Rogerio, C.B.; Campos, E.V.R.; Germano-Costa, T.; Vigato, A.A.; Machado, I.P.; Sepulveda, A.F.; Lima, R.; de Araujo, D.R.; Fraceto, L.F. Repellent active ingredients encapsulated in polymeric nanoparticles: Potential alternative formulations to control arboviruses. J. Nanobiotechnol. 2022, 20, 520. [Google Scholar] [CrossRef]
- Chang, M.A.; Impoinvil, D.; Hamre, K.E.S.; Dalexis, P.E.; Merilien, J.B.; Dismer, A.M.; Fouche, B.; Desir, L.; Holmes, K.; Lafortune, W.; et al. Acceptability, Feasibility, Drug Safety, and Effectiveness of a Pilot Mass Drug Administration with a Single Round of Sulfadoxine-Pyrimethamine Plus Primaquine and Indoor Residual Spraying in Communities with Malaria Transmission in Haiti, 2018. Am. J. Trop. Med. Hyg. 2023, 108, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- WHO. Vector Control Products Targeting Outdoor Malaria Transmission: Preferred Product Characteristics; WHO: Geneva, Switzerland, 2023; p. 8.
- Ujihara, K. The history of extensive structural modifications of pyrethroids. J. Pestic. Sci. 2019, 44, 215–224. [Google Scholar] [CrossRef]
- Dong, K.; Du, Y.; Rinkevich, F.; Nomura, Y.; Xu, P.; Wang, L.; Silver, K.; Zhorov, B.S. Molecular biology of insect sodium channels and pyrethroid resistance. Insect. Biochem. Mol. Biol. 2014, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kansal, I.; Kapoor, A.; Solanki, S.; Singh, R. Cypermethrin toxicity in the environment: Analytical insight into detection methods and microbial degradation pathways. J. Appl. Microbiol. 2023, 134, lxad105. [Google Scholar] [CrossRef]
- Miao, W.; Jiang, Y.; Hong, Q.; Sheng, H.; Liu, P.; Huang, Y.; Cheng, J.; Pan, X.; Yu, Q.; Wu, Y.; et al. Systematic evaluation of the toxicological effects of deltamethrin exposure in zebrafish larvae. Environ. Toxicol. Pharmacol. 2023, 100, 104155. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, M.; Bindu, C.F.; Mohanthi, S.; Hema, T.; Poopal, R.K.; Ren, Z.; Li, B. Efficiency of hematological, enzymological and oxidative stress biomarkers of Cyprinus carpio to an emerging organic compound (alphamethrin) toxicity. Environ. Toxicol. Pharmacol. 2023, 101, 104186. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Asensio, M.J.; Hernandez, A.F.; Romero-Molina, D.; Gonzalez-Alzaga, B.; Luzardo, O.P.; Henríquez-Hernández, L.A.; Boada, L.D.; García-Cortés, H.; Lopez-Flores, I.; Sanchez-Piedra, M.D.; et al. Effect of prenatal exposure to organophosphates and pyrethroid pesticides on neonatal anthropometric measures and gestational age. Environ. Res. 2023, 232, 116410. [Google Scholar] [CrossRef]
- Sheikh, I.A.; Beg, M.A.; Hamoda, T.A.; Mandourah, H.M.S.; Memili, E. Androgen receptor signaling and pyrethroids: Potential male infertility consequences. Front. Cell. Dev. Biol. 2023, 11, 1173575. [Google Scholar] [CrossRef]
- Murugan, K.; Aarthi, N.; Kovendan, K.; Panneerselvam, C.; Chandramohan, B.; Kumar, P.M.; Amerasan, D.; Paulpandi, M.; Chandirasekar, R.; Dinesh, D.; et al. Mosquitocidal and antiplasmodial activity of Senna occidentalis (Cassiae) and Ocimum basilicum (Lamiaceae) from Maruthamalai hills against Anopheles stephensi and Plasmodium falciparum. Parasitol. Res. 2015, 114, 3657–3664. [Google Scholar] [CrossRef]
- Panneerselvam, C.; Murugan, K.; Roni, M.; Aziz, A.T.; Suresh, U.; Rajaganesh, R.; Madhiyazhagan, P.; Subramaniam, J.; Dinesh, D.; Nicoletti, M.; et al. Fern-synthesized nanoparticles in the fight against malaria: LC/MS analysis of Pteridium aquilinum leaf extract and biosynthesis of silver nanoparticles with high mosquitocidal and antiplasmodial activity. Parasitol. Res. 2016, 115, 997–1013. [Google Scholar] [CrossRef]
- Murugan, K.; Samidoss, C.M.; Panneerselvam, C.; Higuchi, A.; Roni, M.; Suresh, U.; Chandramohan, B.; Subramaniam, J.; Madhiyazhagan, P.; Dinesh, D.; et al. Seaweed-synthesized silver nanoparticles: An eco-friendly tool in the fight against Plasmodium falciparum and its vector Anopheles stephensi? Parasitol. Res. 2015, 114, 4087–4097. [Google Scholar] [CrossRef]
- Minal, S.P.; Prakash, S. Laboratory analysis of Au–Pd bimetallic nanoparticles synthesized with Citrus limon leaf extract and its efficacy on mosquito larvae and non-target organisms. Sci. Rep. 2020, 10, 21610. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Alarjani, K.M.; Rajeswari, V.D. Enhancing malaria control using Lagenaria siceraria and its mediated zinc oxide nanoparticles against the vector Anopheles stephensi and its parasite Plasmodium falciparum. Sci. Rep. 2020, 10, 21568. [Google Scholar] [CrossRef]
- Murugan, K.; Benelli, G.; Panneerselvam, C.; Subramaniam, J.; Jeyalalitha, T.; Dinesh, D.; Nicoletti, M.; Hwang, J.S.; Suresh, U.; Madhiyazhagan, P. Cymbopogon citratus-synthesized gold nanoparticles boost the predation efficiency of copepod Mesocyclops aspericornis against malaria and dengue mosquitoes. Exp. Parasitol. 2015, 153, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Hoti, S.L.; Benelli, G. Facile fabrication of eco-friendly nano-mosquitocides: Biophysical characterization and effectiveness on neglected tropical mosquito vectors. Enzyme Microb. Technol. 2016, 95, 155–163. [Google Scholar] [CrossRef] [PubMed]
- AlQahtani, F.S.; AlShebly, M.M.; Govindarajan, M.; Senthilmurugan, S.; Vijayan, P.; Benelli, G. Green and facile biosynthesis of silver nanocomposites using the aqueous extract of Rubus ellipticus leaves: Toxicity and oviposition deterrent activity against Zika virus, malaria and filariasis mosquito vectors. J. Asia-Pac. Entomol. 2017, 20, 157–164. [Google Scholar] [CrossRef]
- Azarudeen, R.M.S.T.; Govindarajan, M.; Amsath, A.; Muthukumaran, U.; Benelli, G. Single-Step Biofabrication of Silver Nanocrystals Using Naregamia alata: A Cost Effective and Eco-Friendly Control Tool in the Fight Against Malaria, Zika Virus and St. Louis Encephalitis Mosquito Vectors. J. Clust. Sci. 2017, 28, 179–203. [Google Scholar] [CrossRef]
- Govindarajan, M.; Kadaikunnan, S.; Alharbi, N.S.; Benelli, G. Single-step biological fabrication of colloidal silver nanoparticles using Hugonia mystax: Larvicidal potential against Zika virus, dengue, and malaria vector mosquitoes. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1317–1325. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, D.; Kumar, V.; Chauhan, R.; Singh, H. Mosquito larvicidal potential of Solanum xanthocarpum leaf extract derived silver nanoparticles and its bio-toxicity on non-target aquatic organism. J. Vector Borne Dis. 2022, 59, 216–227. [Google Scholar] [CrossRef]
- Gandhi, P.R.; Jayaseelan, C.; Kamaraj, C.; Rajasree, S.R.R.; Regina Mary, R. In vitro antimalarial activity of synthesized TiO2 nanoparticles using Momordica charantia leaf extract against Plasmodium falciparum. J. Appl. Biomed. 2018, 16, 378–386. [Google Scholar] [CrossRef]
- Sujitha, V.; Murugan, K.; Dinesh, D.; Pandiyan, A.; Aruliah, R.; Hwang, J.S.; Kalimuthu, K.; Panneerselvam, C.; Higuchi, A.; Aziz, A.T.; et al. Green-synthesized CdS nano-pesticides: Toxicity on young instars of malaria vectors and impact on enzymatic activities of the non-target mud crab Scylla serrata. Aquat. Toxicol. 2017, 188, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Hoti, S.L.; Rajeswary, M.; Benelli, G. One-step synthesis of polydispersed silver nanocrystals using Malva sylvestris: An eco-friendly mosquito larvicide with negligible impact on non-target aquatic organisms. Parasitol. Res. 2016, 115, 2685–2695. [Google Scholar] [CrossRef]
- Kojom Foko, L.P.; Hawadak, J.; Verma, V.; Belle Ebanda Kedi, P.; Eboumbou Moukoko, C.E.; Kamaraju, R.; Pande, V.; Singh, V. Phytofabrication and characterization of Alchornea cordifolia silver nanoparticles and evaluation of antiplasmodial, hemocompatibility and larvicidal potential. Front. Bioeng. Biotechnol. 2023, 11, 1109841. [Google Scholar] [CrossRef]
- Murugan, K.; Labeeba, M.A.; Panneerselvam, C.; Dinesh, D.; Suresh, U.; Subramaniam, J.; Madhiyazhagan, P.; Hwang, J.S.; Wang, L.; Nicoletti, M.; et al. Aristolochia indica green-synthesized silver nanoparticles: A sustainable control tool against the malaria vector Anopheles stephensi? Res. Vet. Sci. 2015, 102, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kala, S.; Sogan, N.; Naik, S.N.; Agarwal, A.; Kumar, J. Impregnation of pectin-cedarwood essential oil nanocapsules onto mini cotton bag improves larvicidal performances. Sci. Rep. 2020, 10, 14107. [Google Scholar] [CrossRef]
- Roopan, S.M.; Rohit; Madhumitha, G.; Rahuman, A.A.; Kamaraj, C.; Bharathi, A.; Surendra, T.V. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind. Crops Prod. 2013, 43, 631–635. [Google Scholar] [CrossRef]
- Subramaniam, J.; Murugan, K.; Panneerselvam, C.; Kovendan, K.; Madhiyazhagan, P.; Dinesh, D.; Kumar, P.M.; Chandramohan, B.; Suresh, U.; Rajaganesh, R.; et al. Multipurpose effectiveness of Couroupita guianensis-synthesized gold nanoparticles: High antiplasmodial potential, field efficacy against malaria vectors and synergy with Aplocheilus lineatus predators. Environ. Sci. Pollut. Res. Int. 2016, 23, 7543–7558. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Panneerselvam, C.; Subramaniam, J.; Madhiyazhagan, P.; Hwang, J.S.; Wang, L.; Dinesh, D.; Suresh, U.; Roni, M.; Higuchi, A.; et al. Eco-friendly drugs from the marine environment: Spongeweed-synthesized silver nanoparticles are highly effective on Plasmodium falciparum and its vector Anopheles stephensi, with little non-target effects on predatory copepods. Environ. Sci. Pollut. Res. Int. 2016, 23, 16671–16685. [Google Scholar] [CrossRef]
- Subramaniam, J.; Murugan, K.; Panneerselvam, C.; Kovendan, K.; Madhiyazhagan, P.; Kumar, P.M.; Dinesh, D.; Chandramohan, B.; Suresh, U.; Nicoletti, M.; et al. Eco-friendly control of malaria and arbovirus vectors using the mosquitofish Gambusia affinis and ultra-low dosages of Mimusops elengi-synthesized silver nanoparticles: Towards an integrative approach? Environ. Sci. Pollut. Res. 2015, 22, 20067–20083. [Google Scholar] [CrossRef]
- Velu, K.; Elumalai, D.; Hemalatha, P.; Janaki, A.; Babu, M.; Hemavathi, M.; Kaleena, P.K. Evaluation of silver nanoparticles toxicity of Arachis hypogaea peel extracts and its larvicidal activity against malaria and dengue vectors. Environ. Sci. Pollut. Res. 2015, 22, 17769–17779. [Google Scholar] [CrossRef]
- Kamaraj, C.; Gandhi, P.R.; Ragavendran, C.; Sugumar, V.; Kumar, R.C.S.; Ranjith, R.; Priyadharsan, A.; Cherian, T. Sustainable development through the bio-fabrication of ecofriendly ZnO nanoparticles and its approaches to toxicology and environmental protection. Biomass Convers. Biorefin. 2022, 1–17. [Google Scholar] [CrossRef]
- Sabzalizade, S.; Amani, A.; Vatandoost, H.; Hosseini, F.; Najafi-Taher, R.; Basseri, H.R. Evaluation of Nanoemulsion of Eucalyptus globulus Oil as Potent Botanical Larvicide against Malaria Vector, Anopheles stephensi and West Nile Vector, Culex pipiens Under Laboratory and Semi-Field Conditions. J. Arthropod. Borne Dis. 2021, 15, 380–388. [Google Scholar] [CrossRef]
- Govindarajan, M.; Benelli, G. Facile biosynthesis of silver nanoparticles using Barleria cristata: Mosquitocidal potential and biotoxicity on three non-target aquatic organisms. Parasitol. Res. 2016, 115, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Suman, T.Y.; Elumalai, D.; Kaleena, P.K.; Rajasree, S.R.R. GC–MS analysis of bioactive components and synthesis of silver nanoparticle using Ammannia baccifera aerial extract and its larvicidal activity against malaria and filariasis vectors. Ind. Crops Prod. 2013, 47, 239–245. [Google Scholar] [CrossRef]
- Nalini, M.; Lena, M.; Sumathi, P.; Sundaravadivelan, C. Effect of phyto-synthesized silver nanoparticles on developmental stages of malaria vector, Anopheles stephensi and dengue vector, Aedes aegypti. Egypt. J. Basic Appl. Sci. 2019, 4, 212–218. [Google Scholar] [CrossRef]
- Arjunan, N.K.; Murugan, K.; Rejeeth, C.; Madhiyazhagan, P.; Barnard, D.R. Green synthesis of silver nanoparticles for the control of mosquito vectors of malaria, filariasis, and dengue. Vector Borne Zoonotic Dis. 2012, 12, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, P.R.; Jayaseelan, C.; Mary, R.R.; Mathivanan, D.; Suseem, S.R. Acaricidal, pediculicidal and larvicidal activity of synthesized ZnO nanoparticles using Momordica charantia leaf extract against blood feeding parasites. Exp. Parasitol. 2017, 181, 47–56. [Google Scholar] [CrossRef]
- Priyadarshini, K.A.; Murugan, K.; Panneerselvam, C.; Ponarulselvam, S.; Hwang, J.S.; Nicoletti, M. Biolarvicidal and pupicidal potential of silver nanoparticles synthesized using Euphorbia hirta against Anopheles stephensi Liston (Diptera: Culicidae). Parasitol. Res. 2012, 111, 997–1006. [Google Scholar] [CrossRef]
- Roni, M.; Murugan, K.; Panneerselvam, C.; Subramaniam, J.; Hwang, J.S. Evaluation of leaf aqueous extract and synthesized silver nanoparticles using Nerium oleander against Anopheles stephensi (Diptera: Culicidae). Parasitol. Res. 2013, 112, 981–990. [Google Scholar] [CrossRef]
- Veerakumar, K.; Govindarajan, M.; Hoti, S.L. Evaluation of plant-mediated synthesized silver nanoparticles against vector mosquitoes. Parasitol. Res. 2014, 113, 4567–4577. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Muthukumaran, U.; Hoti, S.L.; Khater, H.F.; Benelli, G. Single-step biosynthesis and characterization of silver nanoparticles using Zornia diphylla leaves: A potent eco-friendly tool against malaria and arbovirus vectors. J. Photochem. Photobiol. B 2016, 161, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.L.; Nicoletti, M.; Benelli, G. Facile synthesis of mosquitocidal silver nanoparticles using Mussaenda glabra leaf extract: Characterisation and impact on non-target aquatic organisms. Nat. Prod. Res. 2016, 30, 2491–2494. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Rajeswary, M.; Veerakumar, K.; Muthukumaran, U.; Hoti, S.L.; Benelli, G. Green synthesis and characterization of silver nanoparticles fabricated using Anisomeles indica: Mosquitocidal potential against malaria, dengue and Japanese encephalitis vectors. Exp. Parasitol. 2016, 161, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Vijayan, P.; Kadaikunnan, S.; Alharbi, N.S.; Benelli, G. One-pot biogenic fabrication of silver nanocrystals using Quisqualis indica: Effectiveness on malaria and Zika virus mosquito vectors, and impact on non-target aquatic organisms. J. Photochem. Photobiol. B 2016, 162, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Khater, H.F.; Panneerselvam, C.; Benelli, G. One-pot fabrication of silver nanocrystals using Nicandra physalodes: A novel route for mosquito vector control with moderate toxicity on non-target water bugs. Res. Vet. Sci. 2016, 107, 95–101. [Google Scholar] [CrossRef]
- Muthukumaran, U.; Govindarajan, M.; Rajeswary, M.; Hoti, S.L. Synthesis and characterization of silver nanoparticles using Gmelina asiatica leaf extract against filariasis, dengue, and malaria vector mosquitoes. Parasitol. Res. 2015, 114, 1817–1827. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, P.; Singh, H.; Agrawal, V. Biocontrol of mosquito vectors through herbal-derived silver nanoparticles: Prospects and challenges. Environ. Sci. Pollut. Res. Int. 2020, 27, 25987–26024. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Jiang, X.; Miclăuş, T.; Chen, C.; Autrup, H.; Beer, C. Silver nanoparticles—wolves in sheep’s clothing? Toxicol. Res. 2015, 4, 563–575. [Google Scholar] [CrossRef]
- Gopinath, P.; Gogoi, S.K.; Sanpui, P.; Paul, A.; Chattopadhyay, A.; Ghosh, S.S. Signaling gene cascade in silver nanoparticle induced apoptosis. Colloids Surf. B Biointerfaces 2010, 77, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef]
- Almeida, J.P.; Chen, A.L.; Foster, A.; Drezek, R. In vivo biodistribution of nanoparticles. Nanomedicine 2011, 6, 815–835. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular Targets for Components of Essential Oils in the Insect Nervous System—A Review. Molecules 2017, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.M.; Choi, J. Identification, characterization and expression profiles of Chironomus riparius glutathione S-transferase (GST) genes in response to cadmium and silver nanoparticles exposure. Aquat. Toxicol. 2011, 101, 550–560. [Google Scholar] [CrossRef]
- Govindarajan, M.; Benelli, G. alpha-Humulene and beta-elemene from Syzygium zeylanicum (Myrtaceae) essential oil: Highly effective and eco-friendly larvicides against Anopheles subpictus, Aedes albopictus, and Culex tritaeniorhynchus (Diptera: Culicidae). Parasitol. Res. 2016, 115, 2771–2778. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.L.; Benelli, G. Larvicidal potential of carvacrol and terpinen-4-ol from the essential oil of Origanum vulgare (Lamiaceae) against Anopheles stephensi, Anopheles subpictus, Culex quinquefasciatus and Culex tritaeniorhynchus (Diptera: Culicidae). Res. Vet. Sci. 2016, 104, 77–82. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.L.; Bhattacharyya, A.; Benelli, G. Eugenol, alpha-pinene and beta-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol. Res. 2016, 115, 807–815. [Google Scholar] [CrossRef]
- AlShebly, M.M.; AlQahtani, F.S.; Govindarajan, M.; Gopinath, K.; Vijayan, P.; Benelli, G. Toxicity of ar-curcumene and epi-beta-bisabolol from Hedychium larsenii (Zingiberaceae) essential oil on malaria, chikungunya and St. Louis encephalitis mosquito vectors. Ecotoxicol. Environ. Saf. 2017, 137, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Bohman, B.; Ignell, R.; Hill, S.R. Odour-mediated oviposition site selection in Aedes aegypti depends on aquatic stage and density. Parasit. Vectors 2023, 16, 264. [Google Scholar] [CrossRef]
- Mwingira, V.S.; Spitzen, J.; Mboera, L.E.G.; Torres-Estrada, J.L.; Takken, W. The Influence of Larval Stage and Density on Oviposition Site-Selection Behavior of the Afrotropical Malaria Mosquito Anopheles coluzzii (Diptera: Culicidae). J. Med. Entomol. 2020, 57, 657–666. [Google Scholar] [CrossRef]
- Mwingira, V.S.; Mboera, L.E.G.; Takken, W. Synergism between nonane and emanations from soil as cues in oviposition-site selection of natural populations of Anopheles gambiae and Culex quinquefasciatus. Malar. J. 2021, 20, 52. [Google Scholar] [CrossRef] [PubMed]
- Bokore, G.E.; Svenberg, L.; Tamre, R.; Onyango, P.; Bukhari, T.; Emmer, A.; Fillinger, U. Grass-like plants release general volatile cues attractive for gravid Anopheles gambiae sensu stricto mosquitoes. Parasit. Vectors 2021, 14, 552. [Google Scholar] [CrossRef] [PubMed]
- Jayaseelan, C.; Rahuman, A.A.; Rajakumar, G.; Santhoshkumar, T.; Kirthi, A.V.; Marimuthu, S.; Bagavan, A.; Kamaraj, C.; Zahir, A.A.; Elango, G.; et al. Efficacy of plant-mediated synthesized silver nanoparticles against hematophagous parasites. Parasitol. Res. 2012, 111, 921–933. [Google Scholar] [CrossRef]
- Haldar, K.M.; Haldar, B.; Chandra, G. Fabrication, characterization and mosquito larvicidal bioassay of silver nanoparticles synthesized from aqueous fruit extract of putranjiva, Drypetes roxburghii (Wall.). Parasitol. Res. 2013, 112, 1451–1459. [Google Scholar] [CrossRef]
- Subarani, S.; Sabhanayakam, S.; Kamaraj, C. Studies on the impact of biosynthesized silver nanoparticles (AgNPs) in relation to malaria and filariasis vector control against Anopheles stephensi Liston and Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol. Res. 2013, 112, 487–499. [Google Scholar] [CrossRef]
- Patil, C.D.; Borase, H.P.; Patil, S.V.; Salunkhe, R.B.; Salunke, B.K. Larvicidal activity of silver nanoparticles synthesized using Pergularia daemia plant latex against Aedes aegypti and Anopheles stephensi and nontarget fish Poecillia reticulata. Parasitol. Res. 2012, 111, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Santhoshkumar, T.; Rahuman, A.A.; Rajakumar, G.; Marimuthu, S.; Bagavan, A.; Jayaseelan, C.; Zahir, A.A.; Elango, G.; Kamaraj, C. Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol. Res. 2011, 108, 693–702. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Rajakumar, G.; Vishnu Kirthi, A.; Santhoshkumar, T.; Marimuthu, S.; Bagavan, A.; Kamaraj, C.; Zahir, A.A.; Elango, G. Synthesis of pediculocidal and larvicidal silver nanoparticles by leaf extract from heartleaf moonseed plant, Tinospora cordifolia Miers. Parasitol. Res. 2011, 109, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, S.; Rahuman, A.A.; Rajakumar, G.; Santhoshkumar, T.; Kirthi, A.V.; Jayaseelan, C.; Bagavan, A.; Zahir, A.A.; Elango, G.; Kamaraj, C. Evaluation of green synthesized silver nanoparticles against parasites. Parasitol. Res. 2011, 108, 1541–1549. [Google Scholar] [CrossRef]
- Sanei-Dehkordi, A.; Ghasemian, A.; Zarenezhad, E.; Qasemi, H.; Nasiri, M.; Osanloo, M. Nanoliposomes containing three essential oils from the Artemisia genus as effective larvicides against Aedes aegypti and Anopheles stephensi. Sci. Rep. 2023, 13, 11002. [Google Scholar] [CrossRef] [PubMed]
- Firooziyan, S.; Osanloo, M.; Moosa-Kazemi, S.H.; Basseri, H.R.; Hajipirloo, H.M.; Sadaghianifar, A.; Amani, A.; Sedaghat, M.M. Preparation of a Nanoemulsion of Essential Oil of Acroptilon repens Plant and Evaluation of Its Larvicidal Activity agianst Malaria Vector, Anopheles stephensi. J. Arthropod. Borne Dis. 2021, 15, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Dinesh, D.; Kumar, P.J.; Panneerselvam, C.; Subramaniam, J.; Madhiyazhagan, P.; Suresh, U.; Nicoletti, M.; Alarfaj, A.A.; Munusamy, M.A.; et al. Datura metel-synthesized silver nanoparticles magnify predation of dragonfly nymphs against the malaria vector Anopheles stephensi. Parasitol. Res. 2015, 114, 4645–4654. [Google Scholar] [CrossRef]
- Dinesh, D.; Murugan, K.; Madhiyazhagan, P.; Panneerselvam, C.; Mahesh Kumar, P.; Nicoletti, M.; Jiang, W.; Benelli, G.; Chandramohan, B.; Suresh, U. Mosquitocidal and antibacterial activity of green-synthesized silver nanoparticles from Aloe vera extracts: Towards an effective tool against the malaria vector Anopheles stephensi? Parasitol. Res. 2015, 114, 1519–1529. [Google Scholar] [CrossRef]
- Santhosh, S.B.; Yuvarajan, R.; Natarajan, D. Annona muricata leaf extract-mediated silver nanoparticles synthesis and its larvicidal potential against dengue, malaria and filariasis vector. Parasitol. Res. 2015, 114, 3087–3096. [Google Scholar] [CrossRef] [PubMed]
- Arokiyaraj, S.; Dinesh Kumar, V.; Elakya, V.; Kamala, T.; Park, S.K.; Ragam, M.; Saravanan, M.; Bououdina, M.; Arasu, M.V.; Kovendan, K.; et al. Biosynthesized silver nanoparticles using floral extract of Chrysanthemum indicum L.—Potential for malaria vector control. Environ. Sci. Pollut. Res. 2015, 22, 9759–9765. [Google Scholar] [CrossRef]
- Elumalai, D.; Hemavathi, M.; Deepaa, C.V.; Kaleena, P.K. Evaluation of phytosynthesised silver nanoparticles from leaf extracts of Leucas aspera and Hyptis suaveolens and their larvicidal activity against malaria, dengue and filariasis vectors. Parasite Epidemiol. Control 2017, 2, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.E.; Gotopo, L.; Gamboa, N.; Rodrigues, J.R.; Henriques, G.C.; Cabrera, G.; Romero, A.H. Antimalarial Activity of Highly Coordinative Fused Heterocycles Targeting β-Hematin Crystallization. ACS Omega 2022, 7, 7499–7514. [Google Scholar] [CrossRef]
| Plant Species | Type of Nanoparticle | Mosquito Species | Mosquito Stage | LC50 of Nanoformulation | LC50 of Bulk Extract | Reference |
|---|---|---|---|---|---|---|
| Eucalyptus globulus | Nanoemulsion | A. stephensi | Larvae | 80.8730 ppm | 122.8343 ppm | [118] |
| Mangifera indica | TiO2 | A. subpictus | Larvae | 7.72 mg/L | 49.45 mg/L | [84] |
| Ichnocarpus frutescens | Ag | A. subpictus | Larvae | 14.22 μg/mL | 185.83 μg/mL | [101] |
| Citrus limon | AuPd | A. stephensi | Larvae | 5.12 mL/L | - | [98] |
| Rubus ellipticus | Ag | A. stephensi | Larvae | 12.50 μg/mL | 167.19 μg/mL | [102] |
| Naregamia alata | Ag | A. stephensi | Larvae | 12.40 μg/mL | 165.15 μg/mL | [103] |
| Alchornea cordifolia | Ag | A. stephensi | Larvae | 10.67 μg/mL | 53.15 μg/mL | [109] |
| Solanum xanthocarpum | Ag | A. stephensi | Larvae | 9.927 ppm | 1764.528 ppm | [105] |
| Barleria cristata | Ag | A. subpictus | Larvae | 12.46 μg/mL | 124.27 μg/mL | [119] |
| Malva sylvestris | Ag | A. stephensi | Larvae | 10.33 μg/mL | 143.61 μg/mL | [108] |
| Ammania baccifera | Ag | A. subpictus | Larvae | 29.54 mg/L | 257.61 mg/L | [120] |
| Artemisia annua | Nanoliposomes | A. stephensi | Larvae | 90 μg/mL | - | [156] |
| Artemisia sieberi | 140 μg/mL | |||||
| Artemisia dracunculus | 23 μg/mL | |||||
| Artemisia nilagirica | Ag | A. stephensi | Larvae | 0.141% | 0.224% | [121] |
| Pupae | 0.050% | 0.066% | ||||
| Valoniopsis pachynema (algae) | CdS | A. stephensi | Larvae | 29.429 μg/mL | 334.084 μg/mL | [107] |
| Pupae | 31.905 μg/mL | 396.868 μg/mL | ||||
| A. sundaicus | Larvae | 22.496 μg/mL | 296.922 μg/mL | |||
| Pupae | 25.009 μg/mL | 311.860 μg/mL | ||||
| Vitex negundo | ZnO | A. subpictus | Larvae | 2.48 mg/L | 36.89 mg/L | [117] |
| Pupae | 3.63 mg/L | 45.76 mg/L | ||||
| Aristolochia indica | Ag | A. stephensi | Larvae | 10.48 ppm | 490.31 ppm | [110] |
| Pupae | 15.65 ppm | 565.02 ppm | ||||
| Annona squamosa | Ag | A. stephensi | Larvae | 2.12 ppm | - | [122] |
| Pupae | 3.74 ppm | |||||
| Ulva lactuca | Ag | A. stephensi | Larvae | 5.261 ppm | 32.692 ppm | [97] |
| Pupae | 6.860 ppm | 37.603 ppm | ||||
| Momordica charantia | ZnO | A. stephensi | Larvae | 5.42 mg/L | 52.86 mg/L | [123] |
| TiO2 | Larvae | 3.43 mg/L | 85.35 mg/L | [106] | ||
| Pupae | 5.04 mg/L | 96.09 mg/L | ||||
| Pteridium aquilinum | Ag | A. stephensi | Larvae | 18.45 ppm | 395.12 ppm | [96] |
| Pupae | 31.51 ppm | 502.20 ppm | ||||
| Euphorbia hirta | Ag | A. stephensi | Larvae | 27.89 ppm | 197.40 ppm | [124] |
| Pupae | 34.52 ppm | 219.15 ppm | ||||
| Nerium oleander | Ag | A. stephensi | Larvae | 33.99 ppm | 369.96 ppm | [125] |
| Pupae | 39.55 ppm | 426.01 ppm | ||||
| Codium tomentosum (algae) | Ag | A. stephensi | Larvae | 29.6 ppm | 410.7 ppm | [114] |
| Pupae | 40.7 ppm | 487.1 ppm | ||||
| Cymbopogon citratus | Au | A. stephensi | Larvae | 31.466 ppm | 362.292 ppm | [100] |
| Pupae | 38.327 ppm | 434.649 ppm | ||||
| Hugonia mystax | Ag | A. stephensi | Larvae | 14.45 μg/mL | 162.66 μg/mL | [104] |
| Lagenaria siceraria | ZnO | A. stephensi | Larvae | 56.46 ppm | 261.67 ppm | [99] |
| Heliotropium indicum | Ag | A. stephensi | Adult | 26.712 μg/mL | 111.680 μg/mL | [126] |
| Zornia diphylla | Ag | A. subpictus | Larvae | 12.53 μg/mL | 61.23 μg/mL | [127] |
| Mussaenda glabra | Ag | A. subpictus | Larvae | 17 μg/mL | 81 μg/mL | [128] |
| Anisomeles indica | Ag | A. subpictus | Larvae | 31.56 μg/mL | 108.98 μg/mL | [129] |
| Holostemma adakodien | Ag | A. stephensi | Larvae | 12.18 μg/mL | 185.79 μg/mL | [81] |
| Quisqualis indica | Ag | A. stephensi | Larvae | 12.52 μg/mL | 185.98 μg/mL | [130] |
| Nicandra physalodes | Ag | A. stephensi | Larvae | 12.39 μg/mL | 202.82 μg/mL | [131] |
| Gmelina asiatica | Ag | A. stephensi | Larvae | 22.44 μg/mL | 113.53 μg/mL | [132] |
| Couroupita guianensis | Au | A. stephensi | Larvae | 24.57 ppm | 307.72 ppm | [113] |
| Pupae | 28.78 ppm | 363.25 ppm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes-de-Souza, I.; de Moraes, B.P.T.; Silva, A.R.; Ferrarini, S.R.; Gonçalves-de-Albuquerque, C.F. Tiny Green Army: Fighting Malaria with Plants and Nanotechnology. Pharmaceutics 2024, 16, 699. https://doi.org/10.3390/pharmaceutics16060699
Moraes-de-Souza I, de Moraes BPT, Silva AR, Ferrarini SR, Gonçalves-de-Albuquerque CF. Tiny Green Army: Fighting Malaria with Plants and Nanotechnology. Pharmaceutics. 2024; 16(6):699. https://doi.org/10.3390/pharmaceutics16060699
Chicago/Turabian StyleMoraes-de-Souza, Isabelle, Bianca P. T. de Moraes, Adriana R. Silva, Stela R. Ferrarini, and Cassiano F. Gonçalves-de-Albuquerque. 2024. "Tiny Green Army: Fighting Malaria with Plants and Nanotechnology" Pharmaceutics 16, no. 6: 699. https://doi.org/10.3390/pharmaceutics16060699







