Development of Clarstatin, a Novel Drug Lead for the Therapy of Autoimmune Uveitis
Abstract
:1. Introduction
2. Experimental Method
2.1. Fluorescent Ca2+ Imaging
2.2. Experimental Autoimmune Uveitis Model
2.2.1. Experimental Autoimmune Uveitis Induction
2.2.2. Treatment with Clarstatin
2.2.3. Histological Evaluation of Eye Slices
2.3. Cell Death Assay
2.4. Acute Toxicity Evaluation of Clarstatin in Mice
2.5. Statistics
3. Results
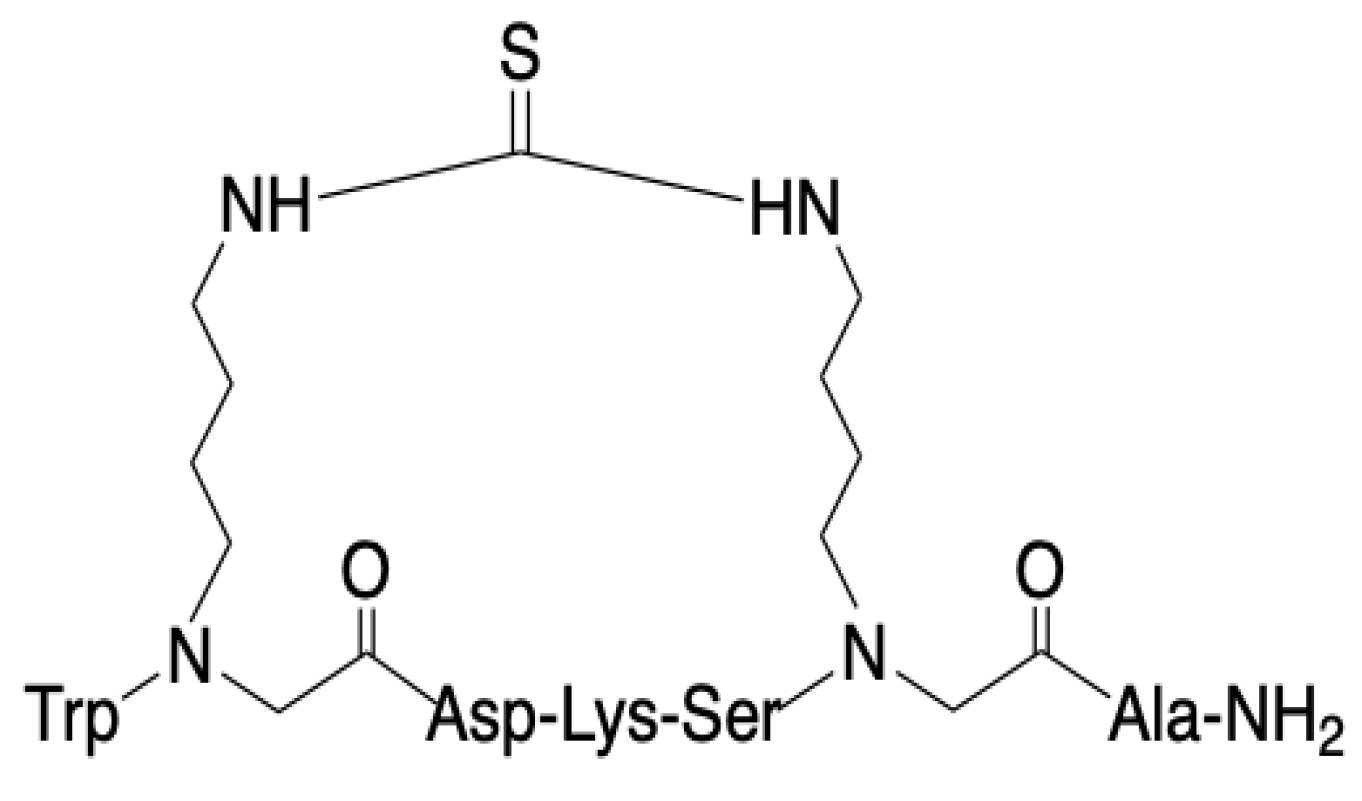
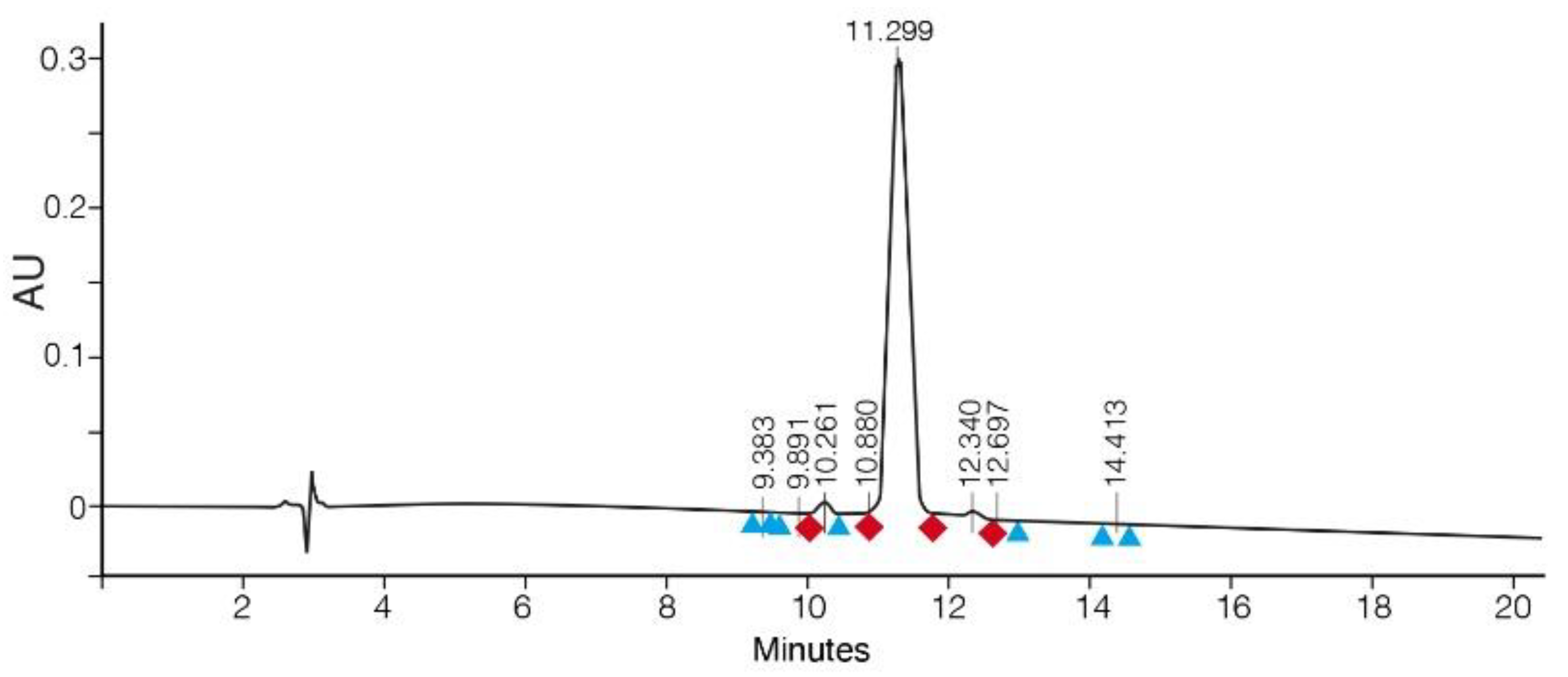
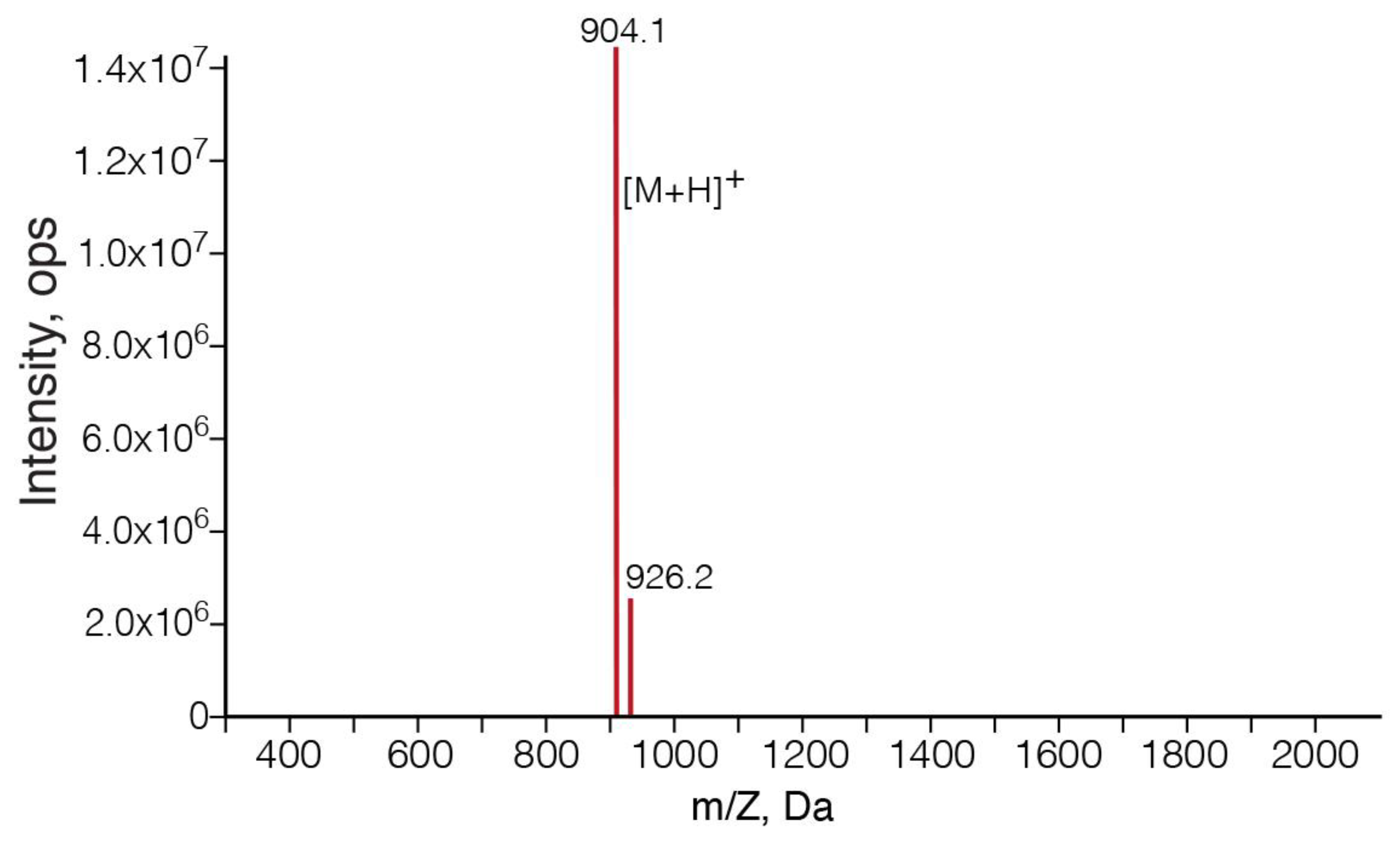
3.1. SPPS of Clarstatin
3.2. Ca2+ Signaling Was Reduced in Jurkat Cells upon Clarstatin Treatment
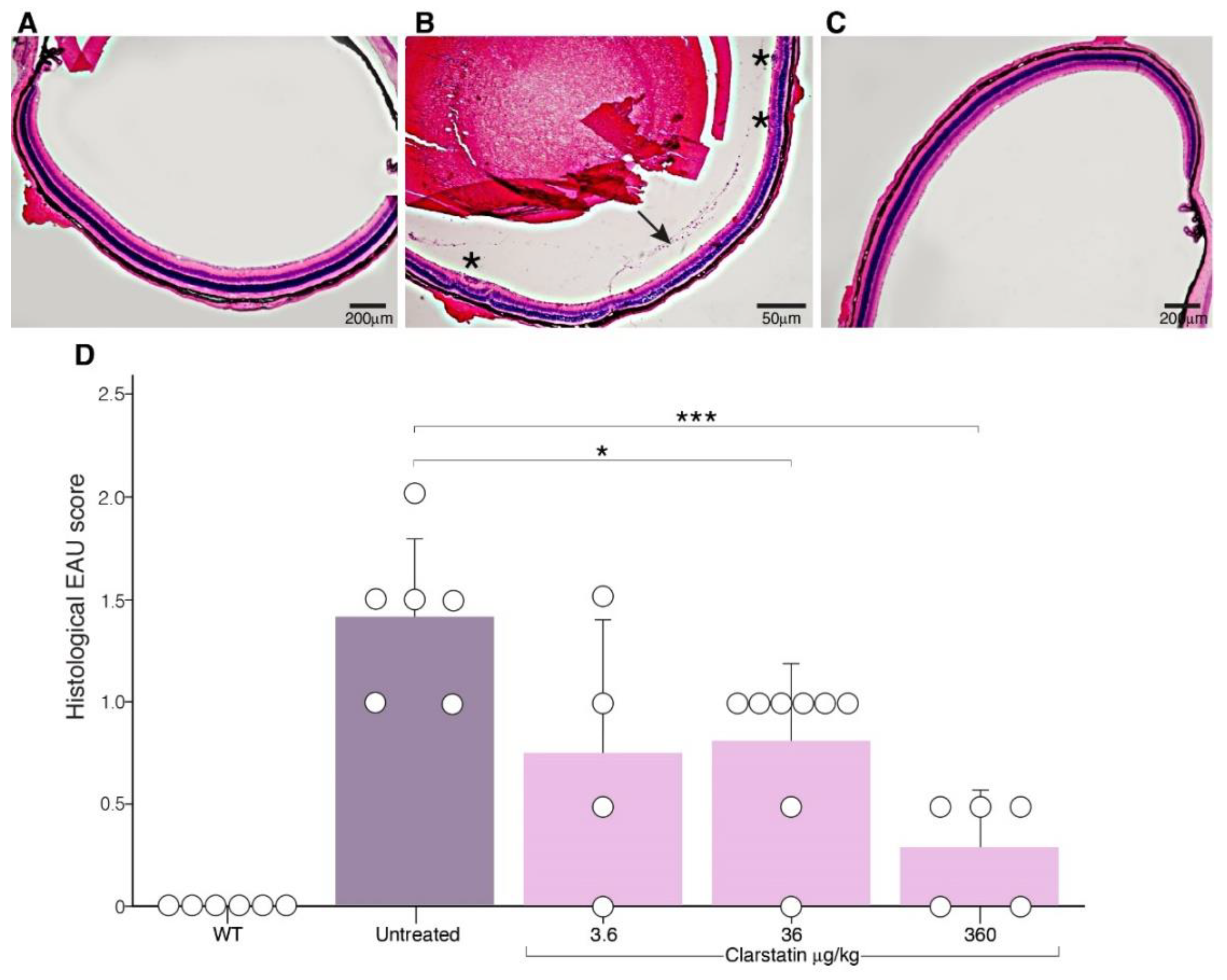
3.3. The Severity of EAU Was Reduced in Mice upon Clarstatin Treatment
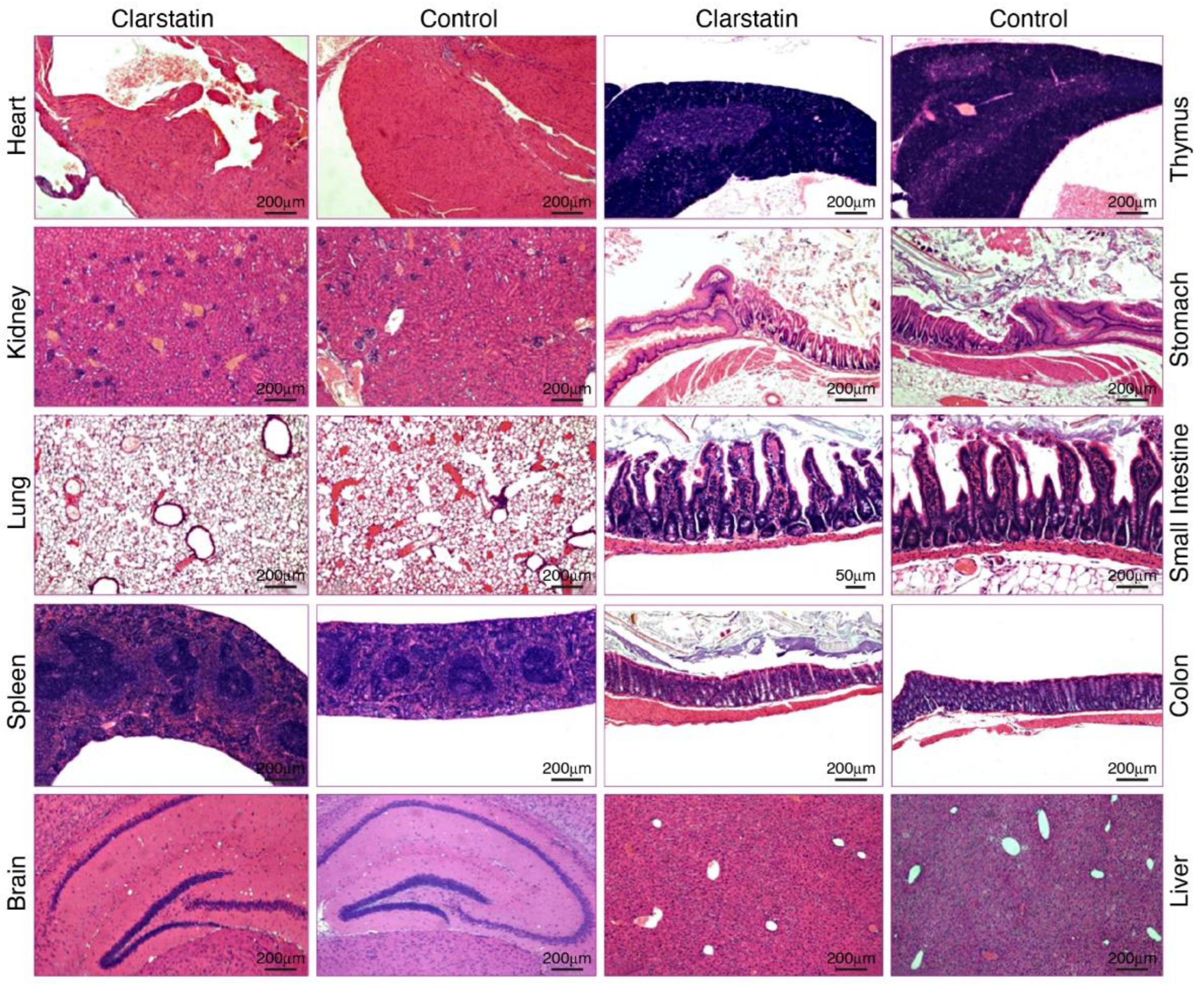
3.4. Acute Tolerability of Clarstatin in Mice without Short-Term Adverse Pathological Effects on Major Organs
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barisani-Asenbauer, T.; MacA, S.M.; Mejdoubi, L.; Emminger, W.; MacHold, K.; Auer, H. Uveitis—A Rare Disease Often Associated with Systemic Diseases and Infections—A Systematic Review of 2619 Patients. Orphanet. J. Rare Dis. 2012, 7, 57. [Google Scholar] [CrossRef]
- Valdes, L.M.; Sobrin, L. Uveitis Therapy: The Corticosteroid Options. Drugs 2020, 80, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.T.; Bodaghi, B.; Couto, C.; Zierhut, M.; Acharya, N.; Pavesio, C.; Tay-Kearney, M.L.; Neri, P.; Douglas, K.; Pathai, S.; et al. New Observations and Emerging Ideas in Diagnosis and Management of Non-Infectious Uveitis: A Review. Semin. Arthritis Rheum. 2019, 49, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.Y.; Fugger, L.; Strominger, J.L.; Siebold, C. MHC Class II Proteins and Disease: A Structural Perspective. Nat. Rev. Immunol. 2006, 6, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Mangalam, A.K.; Rajagopalan, G.; Taneja, V.; David, C.S. HLA Class II Transgenic Mice Mimic Human Inflammatory Diseases. Adv. Immunol. 2008, 97, 65–147. [Google Scholar] [PubMed]
- Li, S.; Yan, L.; Li, M.; Fan, N. Analysis of HLA Profiles in 390 Uveitis Cases: Insights into Clinical Presentations. Altern. Ther. Health Med. 2023, 29, 717–721. [Google Scholar] [PubMed]
- Hou, S.; Li, N.; Liao, X.; Kijlstra, A.; Yang, P. Uveitis Genetics. Exp. Eye Res. 2020, 190, 107853. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, P.K.; Silver, J.; Winchester, R.J. The Shared Epitope Hypothesis. An Approach to Understanding the Molecular Genetics of Susceptibility to Rheumatoid Arthritis. Arthritis Rheum. 1987, 30, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Guarene, M.; Capittini, C.; De Silvestri, A.; Pasi, A.; Badulli, C.; Sbarsi, I.; Cremaschi, A.L.; Garlaschelli, F.; Pizzochero, C.; Monti, M.C.; et al. Targeting the Immunogenetic Diseases with the Appropriate HLA Molecular Typing: Critical Appraisal on 2666 Patients Typed in One Single Centre. Biomed. Res. Int. 2013, 2013, 904247. [Google Scholar] [CrossRef]
- Naveh, S.; Tal-Gan, Y.; Ling, S.; Hoffman, A.; Holoshitz, J.; Gilon, C. Developing Potent Backbone Cyclic Peptides Bearing the Shared Epitope Sequence as Rheumatoid Arthritis Drug-Leads. Bioorganic Med. Chem. Lett. 2012, 22, 493–496. [Google Scholar] [CrossRef]
- Fu, J.; Ling, S.; Liu, Y.; Yang, J.; Naveh, S.; Hannah, M.; Gilon, C.; Zhang, Y.; Holoshitz, J. A Small Shared Epitope-Mimetic Compound Potently Accelerates Osteoclast-Mediated Bone Damage in Autoimmune Arthritis. J. Immunol. 2013, 191, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Liu, Y.; Fu, J.; Colletta, A.; Gilon, C.; Holoshitz, J. Shared Epitope-Antagonistic Ligands: A New Therapeutic Strategy in Mice with Erosive Arthritis. Arthritis Rheumatol. 2015, 67, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, V.R.; Michalak, M.; Abdullah, T.M.; Bremer, E.; Eggleton, P. Mechanisms of Translocation of ER Chaperones to the Cell Surface and Immunomodulatory Roles in Cancer and Autoimmunity. Front. Oncol. 2015, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Solheim, J.C. Class I MHC Molecules: Assembly and Antigen Presentation. Immunol. Rev. 1999, 172, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Adibzadeh, M.; Friccius, H.; Bornhak, S.; Max, H.; Hambrecht, A.; Sansom, D.; Kalbacher, H.; Schenk, A.; Pohla, H.; Pawelec, G. Role of Three Quantitatively Dominant Endogenous Peptides from HLA-DRB1*0401 Molecules in Class II Specific Alloreactivity. Transpl. Immunol. 1994, 2, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Cheng, A.; Pumpens, P.; Michalak, M.; Holoshitz, J. Identification of the Rheumatoid Arthritis Shared Epitope Binding Site on Calreticulin. PLoS ONE 2010, 5, e11703. [Google Scholar] [CrossRef] [PubMed]
- Onuora, S. Pharmacotherapy: Going Upstream: Peptidomimetics Block Shared-Epitope Signalling. Nat. Rev. Rheumatol. 2015, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, P.; Ackerman, A.L.; Giodini, A.; Peaper, D.R.; Wearsch, P.A. Mechanisms of MHC Class I-Restricted Antigen Processing and Cross-Presentation. Immunol. Rev. 2005, 207, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Ohkuro, M.; Kim, J.D.; Kuboi, Y.; Hayashi, Y.; Mizukami, H.; Kobayashi-Kuramochi, H.; Muramoto, K.; Shirato, M.; Michikawa-Tanaka, F.; Moriya, J.; et al. Calreticulin and Integrin Alpha Dissociation Induces Anti-Inflammatory Programming in Animal Models of Inflammatory Bowel Disease. Nat. Commun. 2018, 9, 1982. [Google Scholar] [CrossRef]
- Liu, C.-C.; Leclair, P.; Yap, S.Q.; Lim, C.J. The Membrane-Proximal KXGFFKR Motif of α-Integrin Mediates Chemoresistance. Mol. Cell. Biol. 2013, 33, 4334–4345. [Google Scholar] [CrossRef]
- Gold, L.I.; Eggleton, P.; Sweetwyne, M.T.; Van Duyn, L.B.; Greives, M.R.; Naylor, S.; Michalak, M.; Murphy-Ullrich, J.E. Calreticulin: Non-Endoplasmic Reticulum Functions in Physiology and Disease. FASEB J. 2010, 24, 665–683. [Google Scholar] [CrossRef] [PubMed]
- Touati-Jallabe, Y.; Bojnik, E.; Legrand, B.; Mauchauffée, E.; Chung, N.N.; Schiller, P.W.; Benyhe, S.; Averlant-Petit, M.C.; Martinez, J.; Hernandez, J.F. Cyclic Enkephalins with a Diversely Substituted Guanidine Bridge or a Thiourea Bridge: Synthesis, Biological and Structural Evaluations. J. Med. Chem. 2013, 56, 5964–5973. [Google Scholar] [CrossRef] [PubMed]
- Verbeken, M.; Wynendaele, E.; Mauchauffée, E.; Bracke, N.; Stalmans, S.; Bojnik, E.; Benyhe, S.; Peremans, K.; Polis, I.; Burvenich, C.; et al. Blood-Brain Transfer and Antinociception of Linear and Cyclic N-Methyl-Guanidine and Thiourea-Enkephalins. Peptides 2015, 63, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Geron, M.; Kumar, R.; Matzner, H.; Lahiani, A.; Gincberg, G.; Cohen, G.; Lazarovici, P.; Priel, A. Protein Toxins of the Echis Coloratus Viper Venom Directly Activate TRPV1. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Thurau, S.R.; Chan, C.C.; Nussenblatt, R.B.; Caspi, R.R. Oral Tolerance in a Murine Model of Relapsing Experimental Autoimmune Uveoretinitis (EAU): Induction of Protective Tolerance in Primed Animals. Clin. Exp. Immunol. 1997, 109, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Lahiani, A.; Hidmi, A.; Katzhendler, J.; Yavin, E.; Lazarovici, P. Novel Synthetic PEGylated Conjugate of α-Lipoic Acid and Tempol Reduces Cell Death in a Neuronal PC12 Clonal Line Subjected to Ischemia. ACS Chem. Neurosci. 2016, 7, 1452–1462. [Google Scholar] [CrossRef]
- Naveh, S. Development of Chemical Tools for Studying Protein-Protein Interactions; The Hebrew University of Jerusalem: Jerusalem, Israel, 2012. [Google Scholar]
- Boas, U.; Gertz, H.; Christensen, B.; Heegaard, P.M.H. Facile Synthesis of Aliphatic Isothiocyanates and Thioureas on Solid Phase Using Peptide Coupling Reagents Q. Tetrahedron Lett. 2004, 45, 269–272. [Google Scholar] [CrossRef]
- Weinstein, J.E.; Pepple, K.L. Cytokines in Uveitis. Curr. Opin. Ophthalmol. 2018, 29, 267–274. [Google Scholar] [CrossRef]
- Gupta, S.; Shyamsundar, K.; Agrawal, M.; Vichare, N.; Biswas, J. Current Knowledge of Biologics in Treatment of Noninfectious Uveitis. J. Ocul. Pharmacol. Ther. 2022, 38, 203–222. [Google Scholar] [CrossRef]
- Michalak, M.; Corbett, E.F.; Mesaeli, N.; Nakamura, K.; Opas, M. Calreticulin: One Protein, One Gene, Many Functions. Biochem. J. 1999, 344 Pt 2, 281–292. [Google Scholar] [CrossRef]
- Liu, N.; Fine, R.E.; Simons, E.; Johnson, R.J. Decreasing Calreticulin Expression Lowers the Ca2+ Response to Bradykinin and Increases Sensitivity to Ionomycin in NG-108-15 Cells. J. Biol. Chem. 1994, 269, 28635–28639. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.R.; Raghavan, M. Endoplasmic Reticulum Calcium Depletion Impacts Chaperone Secretion, Innate Immunity, and Phagocytic Uptake of Cells. J. Immunol. 2011, 187, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Klaska, I.; Forrester, J. Mouse Models of Autoimmune Uveitis. Curr. Pharm. Des. 2015, 21, 2453–2467. [Google Scholar] [CrossRef] [PubMed]
- Gardner, P. Calcium and T Lymphocyte Activation. Cell 1989, 59, 15–20. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merzbach, S.; Hoffman, A.; Lazarovici, P.; Gilon, C.; Amer, R. Development of Clarstatin, a Novel Drug Lead for the Therapy of Autoimmune Uveitis. Pharmaceutics 2024, 16, 723. https://doi.org/10.3390/pharmaceutics16060723
Merzbach S, Hoffman A, Lazarovici P, Gilon C, Amer R. Development of Clarstatin, a Novel Drug Lead for the Therapy of Autoimmune Uveitis. Pharmaceutics. 2024; 16(6):723. https://doi.org/10.3390/pharmaceutics16060723
Chicago/Turabian StyleMerzbach, Shira, Amnon Hoffman, Philip Lazarovici, Chaim Gilon, and Radgonde Amer. 2024. "Development of Clarstatin, a Novel Drug Lead for the Therapy of Autoimmune Uveitis" Pharmaceutics 16, no. 6: 723. https://doi.org/10.3390/pharmaceutics16060723
APA StyleMerzbach, S., Hoffman, A., Lazarovici, P., Gilon, C., & Amer, R. (2024). Development of Clarstatin, a Novel Drug Lead for the Therapy of Autoimmune Uveitis. Pharmaceutics, 16(6), 723. https://doi.org/10.3390/pharmaceutics16060723







