Chitosan Alginate Nanoparticles of Protein Hydrolysate from Acheta domesticus with Enhanced Stability for Skin Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Fabrication of CANPs
2.3. Optimization of Process Parameters for CANPs Production
2.4. Preparation of PH Using Enzymatic Hydrolysis
2.5. Fabrication of PH-Loaded CANPs
2.6. Determination of Total Protein Content of PH
2.7. Determination of Encapsulation Efficiency and Loading Capacity of PH-Loaded CANPs
2.8. Stability Study of CANPs and PH-Loaded CANPs
2.9. Physicochemical Characterization of CANPs
2.9.1. Particle Size, Particle Size Distribution, and Zeta Potential Analysis
2.9.2. External Appearance and Morphological Analysis
2.9.3. Fourier Transform Infrared (FT-IR) Spectroscopy
2.9.4. Differential Scanning Calorimetry (DSC)
2.10. Irritation Test by Hen’s Egg Test on the Chorioallantoic Membrane (HET-CAM) Assay
2.11. In Vitro Release Study of PH-Loaded CANPs
2.12. Skin Permeation and Skin Retention Study of PH-Loaded CANPs
2.12.1. Skin Permeation Determination
2.12.2. Skin Retention Determination
2.13. Statistical Analysis
3. Results and Discussions
3.1. Fabrication of CANPs
3.2. Fabrication of PH-Loaded CANPs
3.2.1. Effect of Incorporating PH on CANPs Characteristics
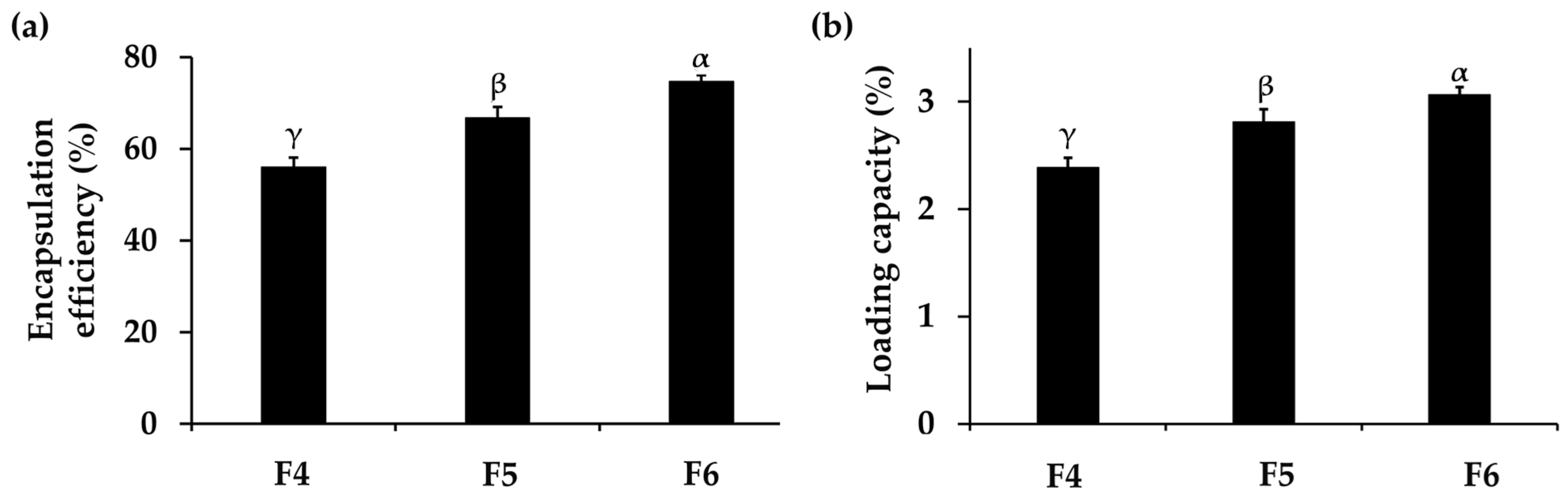
3.2.2. Encapsulation Efficiency and Loading Capacity of PH-Loaded CANPs
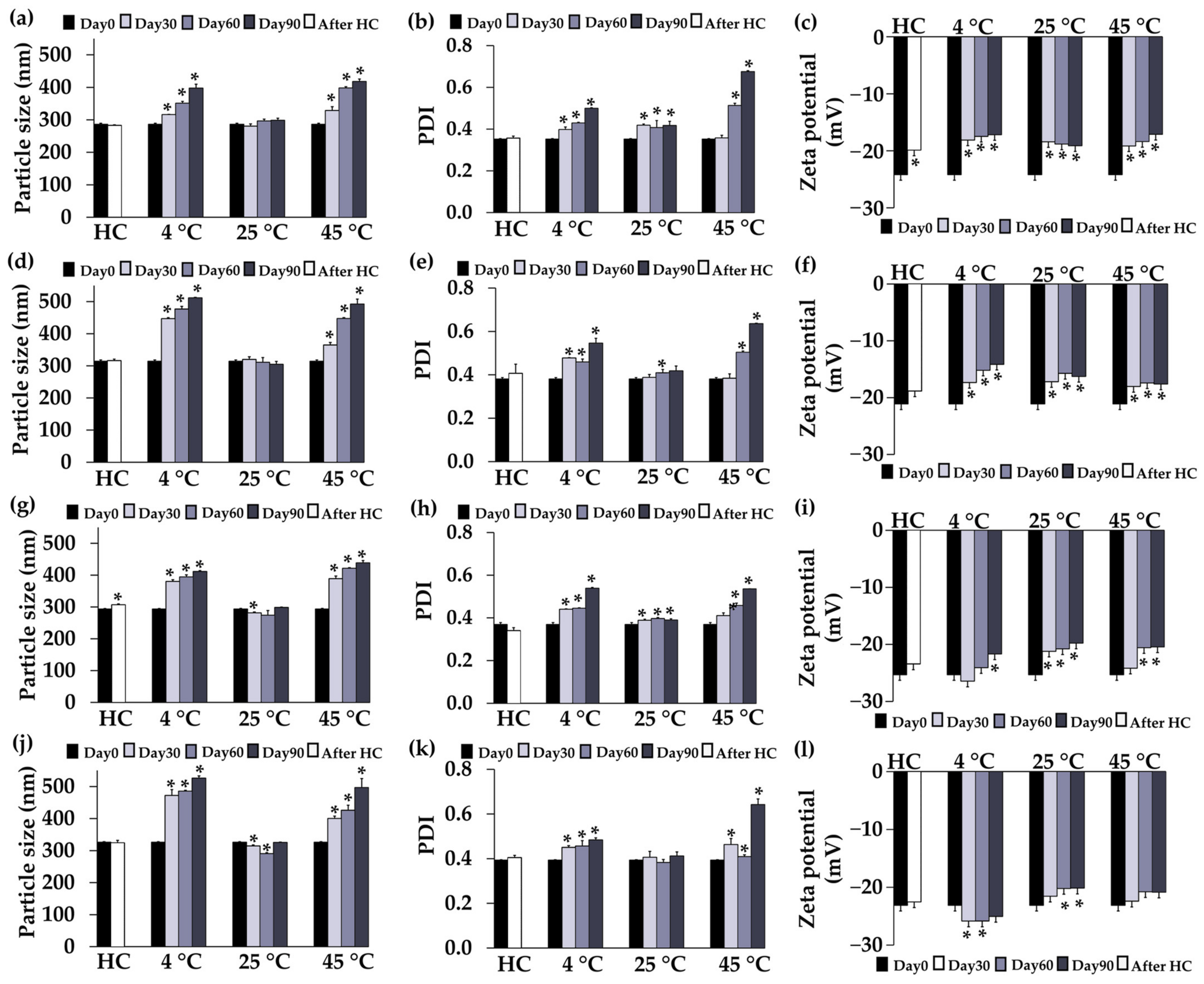
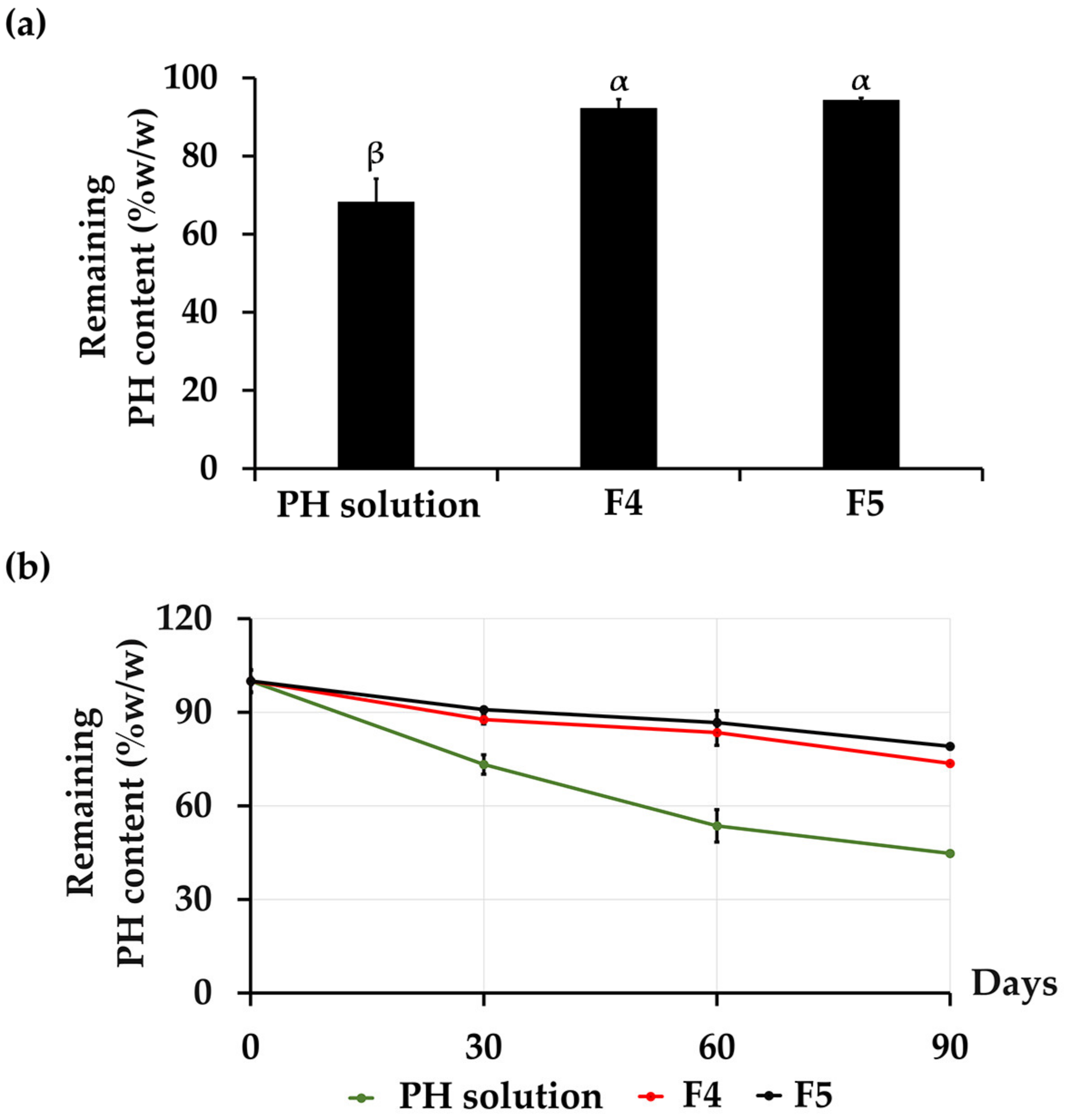
3.3. Stability Profiles of CANPs and PH-Loaded CANPs
3.3.1. Physical Stability
3.3.2. Chemical Stability
3.4. Characterization of CANPs and PH-Loaded CANPs
3.4.1. External Appearance and Morphology
3.4.2. FT-IR Results
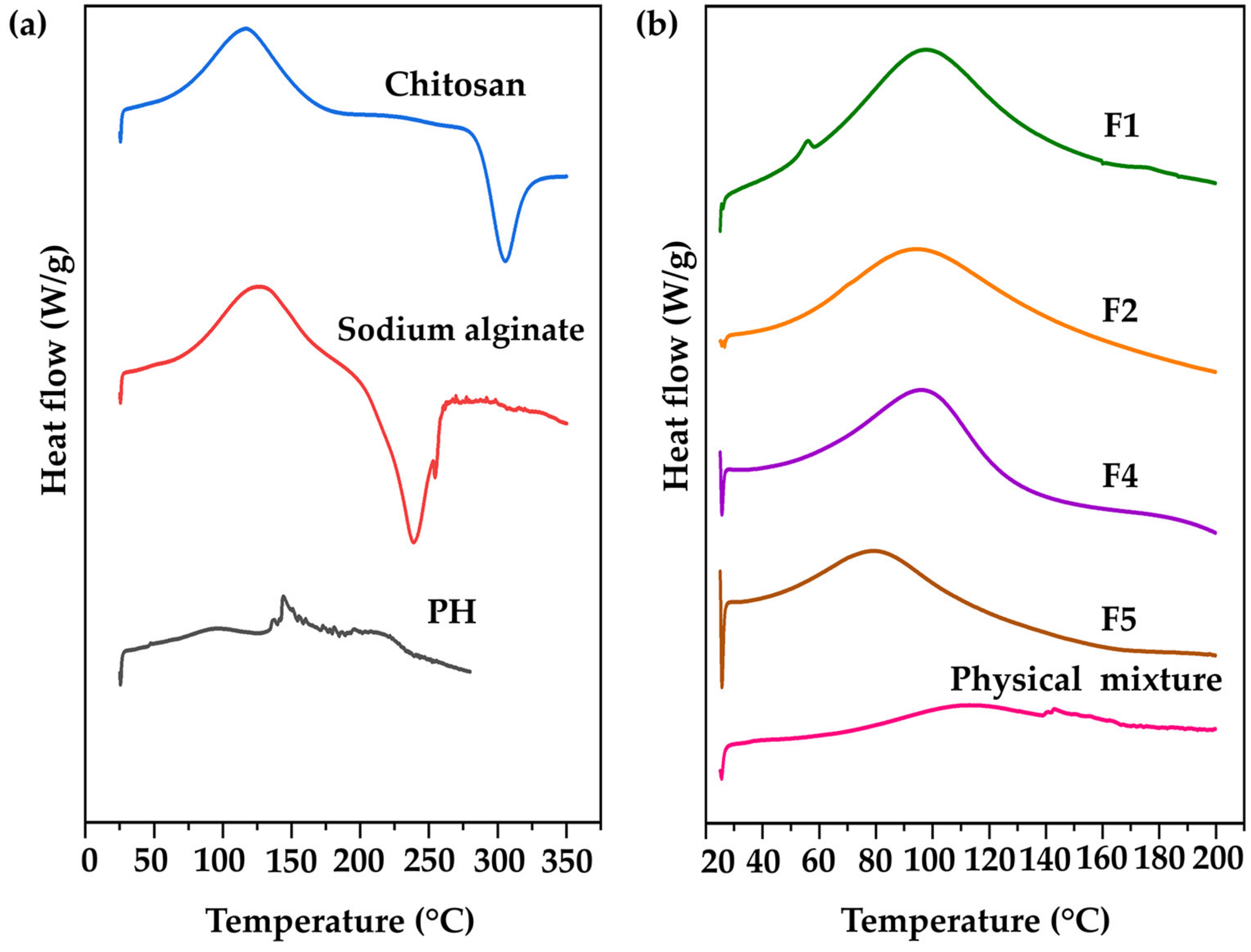
3.4.3. DSC Results
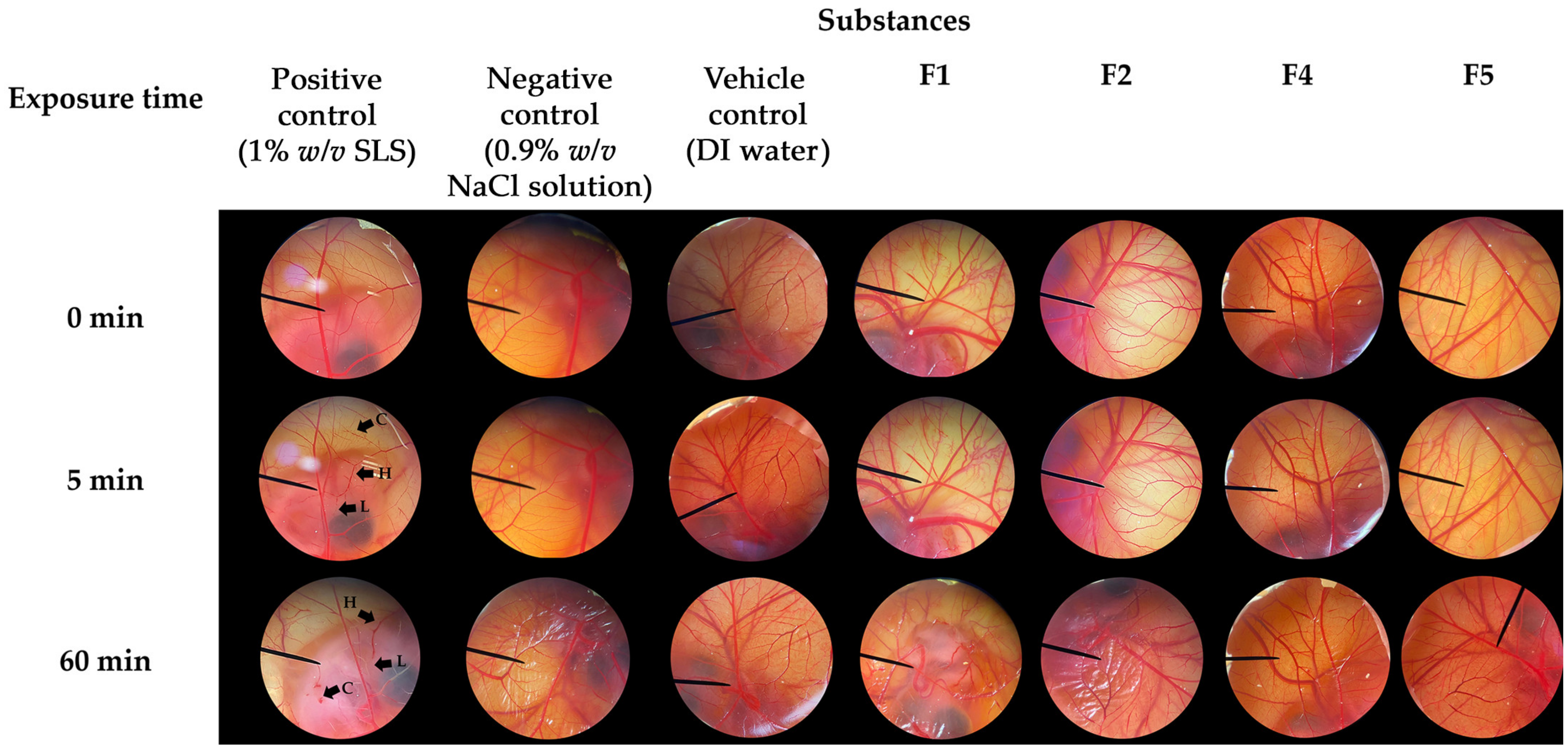
3.5. Irritative Potential of CANPs and PH-Loaded CANPs
3.6. Release Profiles of PH-Loaded CANPs
3.7. Skin Permeation and Skin Retention Profiles of PH-Loaded CANPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngoc, L.T.N.; Moon, J.; Lee, Y.C. Insights into Bioactive Peptides in Cosmetics. Cosmetics 2023, 10, 111. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive Peptides Derived from Seaweed Protein and Their Health Benefits: Antihypertensive, Antioxidant, and Antidiabetic Properties. J. Food Sci. 2017, 83, 6–16. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lin, L.; Zheng, H.; Mo, Y.; Zhou, C.; Sun, S.; Hong, P.; Qian, Z. Potential Anti-Skin Aging Effect of a Peptide AYAPE Isolated from Isochrysis zhanjiangensis on UVB-Induced HaCaT Cells and H2O2-Induced BJ Cells. J. Photochem. Photobiol. B Biol. 2022, 233, 112481. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, I.; Nam, T. Bioactive Peptide from Pyropia yezoensis and Its Anti-Inflammatory Activities. Int. J. Mol. Med. 2015, 36, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Al-Sahlany, S.T.G.; Altemimi, A.B.; Al-Manhel, A.J.A.; Niamah, A.K.; Lakhssassi, N.; Ibrahim, S.A. Purification of Bioactive Peptide with Antimicrobial Properties Produced by Saccharomyces cerevisiae. Foods 2020, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Mancusi, A. Food Peptides for the Nutricosmetic Industry. Antioxidants 2023, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Yeerong, K.; Sriyab, S.; Somwongin, S.; Punyoyai, C.; Chantawannakul, P.; Anuchapreeda, S.; Prommaban, A.; Chaiyana, W. Skin Irritation and Potential Antioxidant, Anti-Collagenase, and Anti-Elastase Activities of Edible Insect Extracts. Sci. Rep. 2021, 11, 22954. [Google Scholar] [CrossRef]
- Yeerong, K.; Chantawannakul, P.; Anuchapreeda, S.; Wangtueai, S.; Chaiyana, W. Optimization of Hydrolysis Conditions, Isolation, and Identification of Biologically Active Peptides Derived from Acheta domesticus for Antioxidant and Collagenase Inhibition. Antioxidants 2024, 13, 367. [Google Scholar] [CrossRef] [PubMed]
- David-Birman, T.; Raften, G.; Lesmes, U. Effects of Thermal Treatments on the Colloidal Properties, Antioxidant Capacity and In-Vitro Proteolytic Degradation of Cricket Flour. Food Hydrocoll. 2018, 79, 48–54. [Google Scholar] [CrossRef]
- Ndiritu, A.K.; Kinyuru, J.; Gichuhi, P.N.; Kenji, G.M. Effects of NaCl and pH on the Functional Properties of Edible Crickets (Acheta domesticus) Protein Concentrate. J. Food Meas. Charact. 2019, 13, 1788–1796. [Google Scholar] [CrossRef]
- Edward, E.; Wongprasert, T.; Bunyakanchana, T.; Siripitakpong, P.; Supabowornsathit, K.; Vilaivan, T.; Suppavorasatit, I. Cricket Protein Isolate Extraction: Effect of Ammonium Sulfate on Physicochemical and Functional Properties of Proteins. Foods 2023, 12, 4032. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K. Functional coatings and microencapsulation: A general perspective. In Functional Coatings: By Polymer Microencapsulation, 1st ed.; Ghosh, S.K., Ed.; Wiley-VCH, Verlag GmbH & Co. KgaA: Weinheim, Germany, 2006; pp. 1–28. [Google Scholar]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric Nanoparticles: The Future of Nanomedicine. WIREs Nanomed. Nanobiotechnol. 2015, 8, 271–299. [Google Scholar] [CrossRef] [PubMed]
- Jawahar, N.; Meyyanathan, S.N. Polymeric Nanoparticles for Drug Delivery and Targeting: A Comprehensive Review. Int. J. Health Sci. 2012, 1, 217. [Google Scholar] [CrossRef]
- Anwunobi, A.P.; Emeje, M.O. Recent Applications of Natural Polymers in Nanodrug Delivery. J. Nanomedic. Nanotechnol. 2011, S4, 002. [Google Scholar] [CrossRef]
- Fatima, S.; Quadri, S.N.; Parveen, S.; Beg, S.; Rahman, M.; Ahmad, F.J.; Abdin, M.Z. Polymeric Nanoparticles for Potential Drug Delivery Applications in Cancer. In Nanoformulation Strategies for Cancer Treatment, 1st ed.; Beg, S., Rahman, M., Choudhry, H., Souto, E.B., Ahmad, F.J., Eds.; Elsevier Publishing Company Ltd.: Amsterdamn, The Netherlands, 2021; pp. 65–88. [Google Scholar]
- Aluani, D.; Tzankova, V.; Kondeva-Burdina, M.; Yordanov, Y.; Nikolova, E.; Odzhakov, F.; Apostolov, A.; Markova, T.; Yoncheva, K. Evaluation of Biocompatibility and Antioxidant Efficiency of Chitosan-Alginate Nanoparticles Loaded with Quercetin. Int. J. Biol. Macromol. 2017, 103, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Ammala, A. Biodegradable Polymers as Encapsulation Materials for Cosmetics and Personal Care Markets. Int. J. Cosmet. Sci. 2013, 35, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.N.; Raghava, S. Smart Systems Based on Polysaccharides. In Natural-Based Polymers for Biomedical Applications; Woodhead Publishing: Sawston, UK, 2008; pp. 129–161. [Google Scholar]
- Esposito, E.; Cortesi, R.; Nastruzzi, C. Gelatin Microspheres: Influence of Preparation Parameters and Thermal Treatment on Chemico-Physical and Biopharmaceutical Properties. Biomaterials 1996, 17, 2009–2020. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Abraham, T.E. Polyionic Hydrocolloids for the Intestinal Delivery of Protein Drugs: Alginate and Chitosan—A Review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Luo, X.; Huang, Q.; Chen, W. Calcium-Crosslinked Alginate-Encapsulated Bacteria for Remediating of Cadmium-Polluted Water and Production of CdS Nanoparticles. Appl. Microbiol. Biotechnol. 2021, 105, 2171–2179. [Google Scholar] [CrossRef]
- El-Houssiny, A.S.; Ward, A.A.; Mostafa, D.M.; Abd-El-Messieh, S.L.; Abd-El-Nour, K.N.; Darwish, M.; Khalil, W.A. Sodium Alginate Nanoparticles as a New Transdermal Vehicle of Glucosamine Sulfate for Treatment of Osteoarthritis. Eur. J. Nanomed. 2017, 9, 105–114. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Setty, C.M.; Sahoo, S.S.; Sa, B. Alginate-Coated Alginate-Polyethyleneimine Beads for Prolonged Release of Furosemide in Simulated Intestinal Fluid. Drug Dev. Ind. Pharm. 2005, 31, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Balaji, R.A.; Sathya, R.; Revathy, R. Levofloxacin: Formulation and In-Vitro Evaluation of Alginate and Chitosan Nanospheres. Egypt. Pharm. J. 2015, 14, 30. [Google Scholar] [CrossRef]
- Picart, C. Natural-Based Multilayer Films for Biomedical Applications. In Natural-Based Polymers for Biomedical Applications, 1st ed.; Reis, R.L., Neves, N.M., Mano, F.J., Gomes, E.M., Marques, P.A., Azevedo, S.H., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 231–259. [Google Scholar]
- Panonnummal, R.; Kumar, V.S.; Jayakumar, R.; Sabitha, M. Application of Chitosan and Its Derivatives in Transdermal Drug Delivery. In Chitosan for Biomaterials IV, 1st ed.; Jayakumar, R., Prabaharan, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 411–446. [Google Scholar]
- Ngo, D.; Kim, S. Antioxidant Effects of Chitin, Chitosan, and Their Derivatives. In Advances in Food and Nutrition Research, 1st ed.; Kim, S.W., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 15–31. [Google Scholar]
- El-Hack, M.E.A.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd-Elhakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal Activity of Chitosan Nanoparticles and Correlation with Their Physical Properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Fu, E.; Shen, E. Chitosan Inhibits Prostaglandin E2 Formation and Cyclooxygenase-2 Induction in Lipopolysaccharide-Treated RAW 264.7 Macrophages. Biochem. Biophys. Res. Commun. 2003, 308, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Tchemtchoua, V.T.; Atanasova, G.; Aqil, A.; Filée, P.; Garbacki, N.; Vanhooteghem, O.; Deroanne, C.; Noël, A.; Jérôme, C.; Nusgens, B.; et al. Development of a Chitosan Nanofibrillar Scaffold for Skin Repair and Regeneration. Biomacromolecules 2011, 12, 3194–3204. [Google Scholar] [CrossRef] [PubMed]
- Ta, Q.; Ting, J.; Harwood, S.; Browning, N.; Simm, A.; Ross, K.; Olier, I.; Al-Kassas, R. Chitosan Nanoparticles for Enhancing Drugs and Cosmetic Components Penetration through the Skin. Eur. J. Pharm. Sci. 2021, 160, 105765. [Google Scholar] [CrossRef]
- Wani, T.U.; Raza, S.N.; Khan, N.A. Rosmarinic Acid Loaded Chitosan Nanoparticles for Wound Healing in Rats. Int. J. Pharm. Sci. Res. 2019, 10, 1138–1147. [Google Scholar] [CrossRef]
- Patil, J.S.; Kamalapur, M.V.; Marapur, S.C.; Kadam, D.V. Ionotropic Gelation and Polyelectrolyte Complexation: The Novel Techniques to Design Hydrogel Particulate Sustained, Modulated Drug Delivery System: A Review. Dig. J. Nanomater. Biostructures 2010, 5, 241–248. [Google Scholar]
- De, S.; Robinson, D.H. Polymer Relationships during Preparation of Chitosan–Alginate and Poly-l-Lysine–Alginate Nanospheres. J. Control. Release 2003, 89, 101–112. [Google Scholar] [CrossRef]
- Loquercio, A.; Castell-Perez, M.E.; Gomes, C.; Moreira, R.G. Preparation of Chitosan-Alginate Nanoparticles for Trans-cinnamaldehyde Entrapment. J. Food Sci. 2015, 8, N2305–N2315. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Chakraborty, S.; Bhattacharya, S.; Mishra, R.; Kundu, P.P. pH-Sensitive Chitosan/Alginate Core-Shell Nanoparticles for Efficient and Safe Oral Insulin Delivery. Int. J. Biol. Macromol. 2015, 72, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Chattopadhyay, M.; Sen, K.K.; Saha, M.K. Development and Characterization of Alginate Coated Low Molecular Weight Chitosan Nanoparticles as New Carriers for Oral Vaccine Delivery in Mice. Carbohydr. Polym. 2015, 121, 403–410. [Google Scholar] [CrossRef]
- Zohri, M.; Nomani, A.; Gazori, T.; Haririan, I.; Mirdamadi, S.; Sadjadi, S.K.; Ehsani, M.R. Characterization of Chitosan/Alginate Self-Assembled Nanoparticles as a Protein Carrier. J. Dispers. Sci. Technol. 2011, 32, 576–582. [Google Scholar] [CrossRef]
- Azevedo, M.A.; Bourbon, A.I.; Vicente, A.A.; Cerqueira, M.A. Alginate/Chitosan Nanoparticles for Encapsulation and Controlled Release of Vitamin B2. Int. J. Biol. Macromol. 2014, 71, 141–146. [Google Scholar] [CrossRef]
- Chen, G.L.; Cai, H.; Chen, J.; Li, R.; Zhong, S.; Jia, X.; Liu, X.; Song, B. Chitosan/Alginate Nanoparticles for the Enhanced Oral Antithrombotic Activity of Clam Heparinoid from the Clam Coelomactra antiquata. Mar. Drugs 2022, 20, 136. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-Alginate Nanoparticles as Effective Oral Carriers to Improve the Stability, Bioavailability, and Cytotoxicity of Curcumin Diethyl Disuccinate. Carbohydr. Polym. 2021, 256, 117426. [Google Scholar] [CrossRef] [PubMed]
- Jiamphun, S.; Chaiyana, W. Enhancing Skin Delivery and Stability of Vanillic and Ferulic Acids in Aqueous Enzymatically Extracted Glutinous Rice Husk by Nanostructured Lipid Carriers. Pharmaceutics 2023, 15, 1961. [Google Scholar] [CrossRef]
- Hong-In, P.; Chaiyana, W. Potential Cosmeceutical Lamellar Liquid Crystals Containing Black Longan (Dimocarpus longan Lour.) Seed Extract for MMP-1 and Hyaluronidase Inhibition. Sci. Rep. 2022, 12, 7683. [Google Scholar] [CrossRef]
- Lertsutthiwong, P.; Rojsitthisak, P.; Nimmannit, U. Preparation of Turmeric Oil-Loaded Chitosan-Alginate Biopolymeric Nanocapsules. Mater. Sci. Eng. C 2009, 29, 856–860. [Google Scholar] [CrossRef]
- Thewanjutiwong, S.; Phokasem, P.; Disayathanoowat, T.; Juntrapirom, S.; Kanjanakawinkul, W.; Chaiyana, W. Development of Film-Forming Gel Formulations Containing Royal Jelly and Honey Aromatic Water for Cosmetic Applications. Gels 2023, 9, 816. [Google Scholar] [CrossRef]
- Ribeiro, A.J.; Silva, C.; Ferreira, D.; Veiga, F. Chitosan-Reinforced Alginate Microspheres Obtained through the Emulsification/Internal Gelation Technique. Eur. J. Pharm. Sci. 2005, 25, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Tammasorn, P.; Charoensup, W.; Bunrod, A.; Kanjanakawinkul, W.; Chaiyana, W. Promising Anti-Wrinkle Applications of Aromatic Extracts of Hedychium coronarium J. Koenig via Antioxidation and Collagenase Inhibition. Pharmaceuticals 2023, 16, 1738. [Google Scholar] [CrossRef] [PubMed]
- Neimkhum, W.; Anuchapreeda, S.; Lin, W.C.; Lue, S.C.; Lee, K.H.; Chaiyana, W. Enhancement of Stability and Dermal Delivery of Carissa carandas Linn. Leaf Extract by Liquid Crystals. J. Drug Deliv. Sci. Technol. 2023, 82, 104258. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef]
- Marsup, P.; Yeerong, K.; Neimkhum, W.; Sirithunyalug, J.; Anuchapreeda, S.; To-Anun, C.; Chaiyana, W. Enhancement of Chemical Stability and Dermal Delivery of Cordyceps militaris Extracts by Nanoemulsion. Nanomaterials 2020, 10, 1565. [Google Scholar] [CrossRef]
- Khong, T.T.; Aarstad, O.A.; Skjåk-Bræk, G.; Draget, K.I.; Vårum, K.M. Gelling Concept Combining Chitosan and Alginate—Proof of Principle. Biomacromolecules 2013, 14, 2765–2771. [Google Scholar] [CrossRef]
- Sarmento, B.; Ribeiro, A.J.; Veiga, F.; Ferreira, D.; Neufeld, R.J. Insulin-Loaded Nanoparticles are Prepared by Alginate Ionotropic Pre-Gelation Followed by Chitosan Polyelectrolyte Complexation. J. Nanosci. Nanotechnol. 2007, 7, 2833–2841. [Google Scholar] [CrossRef]
- Natrajan, D.; Srinivasan, S.; Sundar, K.; Ravindran, A. Formulation of Essential Oil-Loaded Chitosan–Alginate Nanocapsules. J. Food Drug Anal. 2015, 23, 560–568. [Google Scholar] [CrossRef]
- Rizg, W.Y.; Naveen, N.R.; Kurakula, M.; Bukhary, H.A.; Safhi, A.Y.; Alfayez, E.; Sindi, A.M.; Ali, S.; Murshid, S.S.; Hosny, K.M. QbD Supported Optimization of the Alginate-Chitosan Nanoparticles of Simvastatin in Enhancing the Anti-Proliferative Activity against Tongue Carcinoma. Gels 2022, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; El-Say, K.M. Development of Alginate-Reinforced Chitosan Nanoparticles Utilizing W/O Nanoemulsification/Internal Crosslinking Technique for Transdermal Delivery of Rabeprazole. Life Sci. 2014, 110, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Phan, J.; Schairer, D.; Champer, J.; Qin, M.; Pirouz, A.; Blecher-Paz, K.; Oren, A.; Liu, P.T.; Modlin, R.L.; et al. Antimicrobial and Anti-Inflammatory Activity of Chitosan–Alginate Nanoparticles: A Targeted Therapy for Cutaneous Pathogens. J. Investig. Dermatol. 2013, 133, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Rahaiee, S.; Shojaosadati, S.A.; Hashemi, M.; Moini, S.; Razavi, S.H. Improvement of Crocin Stability by Biodegradable Nanoparticles of Chitosan-Alginate. Int. J. Biol. Macromol. 2015, 79, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Ribeiro, A.J.; Veiga, F.; Sampaio, P.; Neufeld, R.J.; Ferreira, D. Alginate/Chitosan Nanoparticles are Effective for Oral Insulin Delivery. Pharm. Res. 2007, 24, 2198–2206. [Google Scholar] [CrossRef]
- Ong, S.G.M.; Ming, L.C.; Lee, K.S.; Yuen, K.H. Influence of the Encapsulation Efficiency and Size of Liposome on the Oral Bioavailability of Griseofulvin-Loaded Liposomes. Pharmaceutics 2016, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Kyzioł, A.; Mazgała, A.; Michna, J.; Regiel-Futyra, A.; Sebastián, V. Preparation and Characterization of Alginate/Chitosan Formulations for Ciprofloxacin-Controlled Delivery. J. Biomater. Appl. 2017, 32, 162–174. [Google Scholar] [CrossRef]
- Cegnar, M.; Kerč, J. Self-Assembled Polyelectrolyte Nanocomplexes of Alginate, Chitosan and Ovalbumin. Acta Chim. Slov. 2010, 57, 431–441. [Google Scholar]
- Yoncheva, K.; Merino, M.; Shenol, A.; Daskalov, N.T.; St Petkov, P.; Vayssilov, G.N.; Garrido, M.J. Optimization and In-Vitro/In-Vivo Evaluation of Doxorubicin-Loaded Chitosan-Alginate Nanoparticles using a Melanoma Mouse Model. Int. J. Pharm. 2019, 556, 1–8. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Bhuket, P.R.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/Alginate Nanoparticles as a Promising Carrier of Novel Curcumin Diethyl Diglutarate. Int. J. Biol. Macromol. 2019, 131, 1125–1136. [Google Scholar] [CrossRef]
- Koç, M.L.; Özdemir, Ü.; İmren, D. Prediction of the pH and the Temperature-Dependent Swelling Behavior of Ca2+-Alginate Hydrogels by Artificial Neural Networks. Chem. Eng. Sci. 2009, 64, 1908. [Google Scholar] [CrossRef]
- Abnoos, M.; Mohseni, M.; Mousavi, S.A.; Ashtari, K.; Ilka, R.; Mehravi, B. Chitosan-Alginate Nano-Carrier for Transdermal Delivery of Pirfenidone in Idiopathic Pulmonary Fibrosis. Int. J. Biol. Macromol. 2018, 118, 1319–1325. [Google Scholar] [CrossRef]
- Bajpai, S.; Tankhiwale, R. Investigation of Dynamic Release of Vitamin B2 from Calcium Alginate/Chitosan Multilayered Beads: Part II. React. Funct. Polym. 2006, 66, 1565–1574. [Google Scholar] [CrossRef]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sutariya, V.B. Reversed Chitosan–Alginate Polyelectrolyte Complex for Stability Improvement of Alpha-Amylase: Optimization and Physicochemical Characterization. Eur. J. Pharm. Biopharm. 2007, 65, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Bhuket, P.R.N.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/Alginate Nanoparticles as a Promising Approach for Oral Delivery of Curcumin Diglutaric Acid for Cancer Treatment. Mater. Sci. Eng. C 2018, 93, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Haldar, K.; Chakraborty, S. Role of Chemical Reaction and Drag Force during Drop Impact Gelation Process. Colloids Surf. A Physicochem. Eng. 2018, 559, 401–409. [Google Scholar] [CrossRef]
- Drabczyk, A.; Kudłacik–Kramarczyk, S.; Głąb, M.; Kędzierska, M.; Jaromin, A.; Mierzwiński, D.; Tyliszczak, B. Physicochemical Investigations of Chitosan-Based Hydrogels Containing Aloe vera Designed for Biomedical Use. Materials 2020, 13, 3073. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.J.; Siddique, A. Ionic Studies of Sodium Alginate Isolated from Sargassum terrarium (Brown Algea) Karachi Coast with 2,1-Electrolyte. J. Saudi Chem. Soc. 2010, 14, 117–123. [Google Scholar] [CrossRef]
- Sahu, S.K.; Prusty, A. Design and Evaluation of a Nanoparticulate System Prepared by Biodegradable Polymers for Oral Administration of Protein Drugs. Die Pharm. 2010, 65, 824–829. [Google Scholar]
- Bolat, B.; Ugur, A.E.; Öztop, M.H.; Alpas, H. Effects of High Hydrostatic Pressure Assisted Degreasing on the Technological Properties of Insect Powders Obtained from Acheta domesticus & Tenebrio molitor. J. Food Eng. 2021, 292, 110359. [Google Scholar] [CrossRef]
- Ebrahimi, S.E.; Koocheki, A.; Milani, E.; Mohebbi, M. Interactions between Lepidium perfoliatum Seed Gum—Grass Pea (Lathyrus sativus) Protein Isolate in Composite Biodegradable Film. Food Hydrocoll. 2016, 54, 302–314. [Google Scholar] [CrossRef]
- Lozano-Vazquez, G.; Lobato-Calleros, C.; Escalona-Buendía, H.B.; Chávez, G.A.G.; Álvarez-Ramírez, J.; Vernon-Carter, E.J. Effect of the Weight Ratio of Alginate-Modified Tapioca Starch on the Physicochemical Properties and Release Kinetics of Chlorogenic Acid Containing Beads. Food Hydrocoll. 2015, 48, 301–311. [Google Scholar] [CrossRef]
- Leonardi, M.; Caruso, G.; Carroccio, S.; Boninelli, S.; Curcuruto, G.; Zimbone, M.; Allegra, M.; Torrisi, B.; Ferlito, F.; Miritello, M. Smart Nanocomposites of Chitosan/Alginate Nanoparticles Loaded with Copper Oxide as Alternative Nanofertilizers. Environ. Sci. Nano 2021, 8, 174–187. [Google Scholar] [CrossRef]
- Kumar, S.; Chauhan, N.; Gopal, M.; Kumar, R.; Dilbaghi, N. Development and Evaluation of Alginate–Chitosan Nanocapsules for Controlled Release of Acetamiprid. Int. J. Biol. Macromol. 2015, 81, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Tummino, M.L.; Magnacca, G.; Cimino, D.; Laurenti, E.; Nisticò, R. The Innovation Comes from the Sea: Chitosan and Alginate Hybrid Gels and Films as Sustainable Materials for Wastewater Remediation. Int. J. Mol. Sci. 2020, 21, 550. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Christie, G. Measurement of Starch Thermal Transitions Using Differential Scanning Calorimetry. Carbohydr. Polym. 2001, 46, 179–184. [Google Scholar] [CrossRef]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® Synthetic Membrane: Permeability Comparison to Human Cadaver Skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
- Leong, M.Y.; Kong, Y.L.; Burgess, K.; Wong, W.F.; Sethi, G.; Looi, C.Y. Recent Development of Nanomaterials for Transdermal Drug Delivery. Biomedicines 2023, 11, 1124. [Google Scholar] [CrossRef]





| Sodium Alginate Concentration (mg/mL) | Calcium Chloride Concentration (mg/mL) | Chitosan Concentration (mg/mL) | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|
| 0.1 | 1.3 | 0.2 | 240 ± 7 a | 0.61 ± 0.02 c | −16.0 ±0.2 c |
| 0.2 | 1.3 | 0.2 | 258 ± 11 b | 0.45 ± 0.01 b | −18.8 ±0.6 b |
| 0.4 | 1.3 | 0.2 | 283 ± 4 c | 0.39 ± 0.01 a | −20.0 ±0.1 b |
| 0.6 | 1.3 | 0.2 | 288 ± 5 c | 0.40 ± 0.01 a | −27.8 ±2.3 a |
| 0.8 | 1.3 | 0.2 | 319 ± 5 d | 0.40 ± 0.01 a | −29.5 ±1.0 a |
| 1.0 | 1.3 | 0.2 | 330 ± 2 d | 0.41 ± 0.01 a | −30.2 ± 1.2 a |
| 0.6 | 1.3 | 0.2 | 302 ± 2 b | 0.41 ± 0.01 b | −25.3 ± 0.3 c |
| 0.6 | 1.6 | 0.2 | 301 ± 8 b | 0.40 ± 0.00 b | −24.7 ± 0.3 c,d |
| 0.6 | 1.8 | 0.2 | 269 ± 5 a | 0.29 ± 0.00 a | −27.8 ± 0.1 a |
| 0.6 | 2.0 | 0.2 | 290 ± 3 b | 0.42 ± 0.01 b | −26.7 ± 0.1 b |
| 0.6 | 2.2 | 0.2 | 320 ± 8 c | 0.41 ± 0.02 b | −25.5 ± 0.4 c |
| 0.6 | 2.4 | 0.2 | 334 ± 3 c | 0.45 ± 0.01 c | −24.3 ± 0.6 d |
| 0.6 | 1.8 | 0.1 | 275 ± 5 a | 0.35 ± 0.00 a | −24.9 ± 0.8 a |
| 0.6 | 1.8 | 0.2 | 284 ± 4 a,b | 0.36 ± 0.01 a | −23.6 ± 0.3 a |
| 0.6 | 1.8 | 0.4 | 295 ± 7 b | 0.36 ± 0.01 a | −19.6 ± 0.6 b |
| 0.6 | 1.8 | 0.6 | 330 ± 1 c | 0.44 ± 0.00 b | −18.9 ± 0.4 b |
| 0.6 | 1.8 | 0.8 | 356 ± 8 d | 0.47 ± 0.01 c | −17.5 ± 0.3 c |
| 0.6 | 1.8 | 1.0 | 393 ± 4 e | 0.50 ± 0.01 d | −17.2 ± 0.1 c |
| Formulation | PH Concentration (mg/mL) | Sodium Alginate Concentration (mg/mL) | Calcium Chloride Concentration (mg/mL) | Chitosan Concentration (mg/mL) | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|---|---|
| F1 | - | 0.6 | 1.8 | 0.1 | 275 ± 5 a | 0.35 ± 0.00 a | −24.9 ± 0.8 a |
| F2 | - | 0.6 | 1.8 | 0.2 | 284 ± 4 a,b | 0.36 ± 0.01 a | −23.6 ± 0.3 a |
| F3 | - | 0.6 | 1.8 | 0.4 | 295 ± 7 b | 0.36 ± 0.01 a | −19.6 ± 0.6 b |
| F4 | 1.25 | 0.6 | 1.8 | 0.1 | 309 ± 0 c | 0.39 ± 0.01 b | −26.0 ± 0.9 a |
| F5 | 1.25 | 0.6 | 1.8 | 0.2 | 319 ± 1 d | 0.40 ± 0.01 b | −24.3 ± 0.1 a |
| F6 | 1.25 | 0.6 | 1.8 | 0.4 | 352 ± 5 e | 0.40 ± 0.00 b | −22.6 ± 0.3 a |
| Sample | IS | Irritative Level |
|---|---|---|
| Positive control (1% w/v SLS) | 18 ± 1 a | Severe irritation |
| Negative control (0.9% w/v NaCl) | 0 ± 0 b | No irritation |
| Vehicle (DI water) | 0 ± 0 b | No irritation |
| F1 | 0 ± 0 b | No irritation |
| F2 | 0 ± 0 b | No irritation |
| F4 | 0 ± 0 b | No irritation |
| F5 | 0 ± 0 b | No irritation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeerong, K.; Chantawannakul, P.; Anuchapreeda, S.; Juntrapirom, S.; Kanjanakawinkul, W.; Müllertz, A.; Rades, T.; Chaiyana, W. Chitosan Alginate Nanoparticles of Protein Hydrolysate from Acheta domesticus with Enhanced Stability for Skin Delivery. Pharmaceutics 2024, 16, 724. https://doi.org/10.3390/pharmaceutics16060724
Yeerong K, Chantawannakul P, Anuchapreeda S, Juntrapirom S, Kanjanakawinkul W, Müllertz A, Rades T, Chaiyana W. Chitosan Alginate Nanoparticles of Protein Hydrolysate from Acheta domesticus with Enhanced Stability for Skin Delivery. Pharmaceutics. 2024; 16(6):724. https://doi.org/10.3390/pharmaceutics16060724
Chicago/Turabian StyleYeerong, Kankanit, Panuwan Chantawannakul, Songyot Anuchapreeda, Saranya Juntrapirom, Watchara Kanjanakawinkul, Anette Müllertz, Thomas Rades, and Wantida Chaiyana. 2024. "Chitosan Alginate Nanoparticles of Protein Hydrolysate from Acheta domesticus with Enhanced Stability for Skin Delivery" Pharmaceutics 16, no. 6: 724. https://doi.org/10.3390/pharmaceutics16060724











