Nivolumab and Ipilimumab Acting as Tormentors of Advanced Tumors by Unleashing Immune Cells and Associated Collateral Damage
Abstract
:1. Introduction
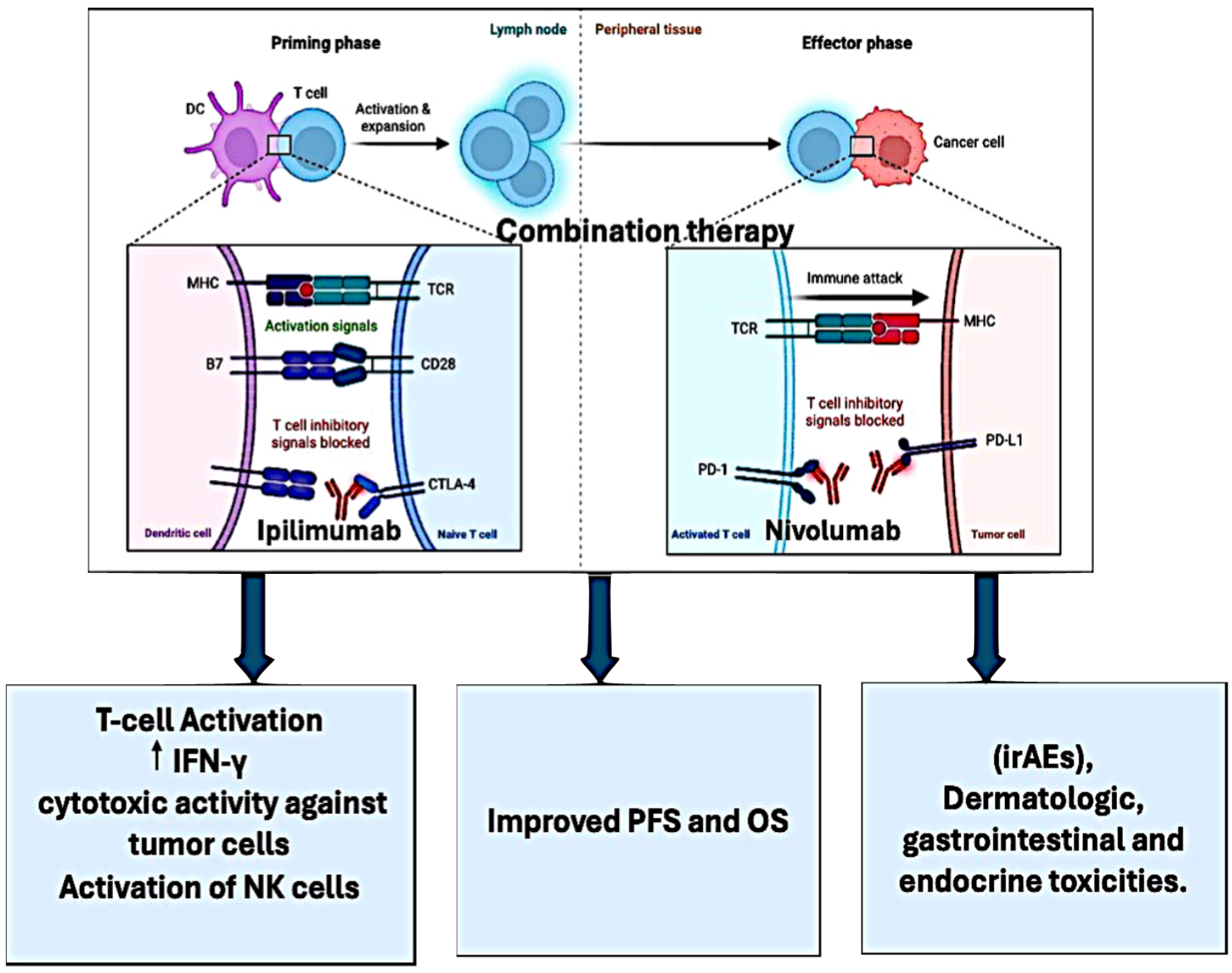
2. Ipilimumab
3. Nivolumab
4. Pharmacokinetics of Ipilimumab in Combination with Nivolumab
5. Clinical Trials of Combination
5.1. Effect on Non-Small Cell Lung Carcinoma
5.2. Effect on Esophageal Carcinoma
| Reference | Trial Phase | Treatment Arms | Primary Endpoints | Results | Clinical Trial Number |
|---|---|---|---|---|---|
| Kato et al., 2022 | Phase 3 | Nivolumab + Ipilimumab (NIVO + IPI), Nivolumab + Chemotherapy (NIVO + Chemo), Chemotherapy (Chemo) | Overall survival (OS), Progression-free survival (PFS) | First-line NIVO + IPI and NIVO + Chemo treatments demonstrated substantial survival advantages over Chemo in Japanese patients with advanced ESCC. Both NIVO + IPI and NIVO + Chemo arms showed acceptable tolerability. | NCT03143153 |
| Meindl-Beinker et al., 2019 | Phase 2 | Nivolumab and Ipilimumab vs. Nivolumab Alone | Overall survival (OS), Time to QoL deterioration, Tumor response, Progression-free survival (PFS), Safety | The RAMONA trial demonstrated a significant survival benefit of nivolumab/ipilimumab in advanced ESCC compared to historical data of standard chemotherapy. Nivolumab/ipilimumab combination therapy showed promising results with acceptable safety profile in elderly patients with ESCC. | NCT03416244 |
| Janjigian, Y. Y., et al. (2018) | Phase 1/2 | Nivolumab, Nivolumab + Ipilimumab | Objective response rate in patients with chemotherapy-refractory esophagogastric cancer | Nivolumab and nivolumab plus ipilimumab showed clinically meaningful antitumor activity, durable responses, encouraging long-term OS, and a manageable safety profile. | NCT01928394 |
| Shitara, K. et al. (2022) | Phase 3 | Nivolumab + Chemotherapy, Nivolumab + Ipilimumab, Chemotherapy | Overall survival in patients with gastroesophageal cancer | Nivolumab + chemotherapy showed improvement in overall survival compared to chemotherapy alone. No significant improvement in overall survival was observed with nivolumab + ipilimumab. | NCT02872116 |
5.3. Effect on Renal Cell Carcinoma
5.4. Effect on Melanoma Cancer
5.5. Effect on Other Cancers
5.6. Resistance to Immune Checkpoint Therapy
5.7. Effect of Immunotherapy on Immunosuppressive MDSCs
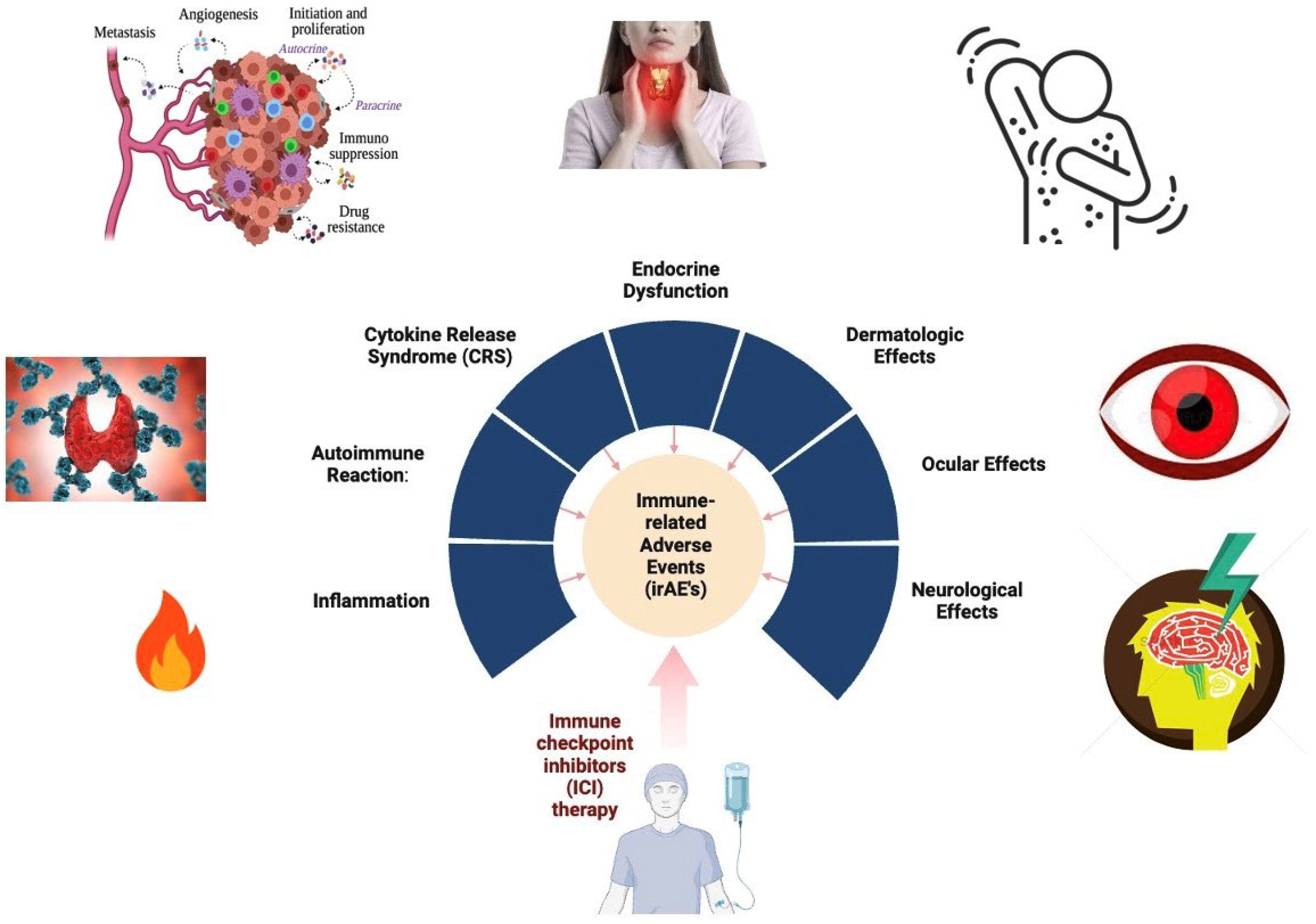
6. Adverse Effects
7. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint In-hibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Albiges, L.; Tannir, N.M.; Burotto, M.; McDermott, D.; Plimack, E.R.; Barthélémy, P.; Porta, C.; Powles, T.; Donskov, F.; George, S.; et al. First-line Nivolumab plus Ipilimumab Versus Sunitinib in Patients Without Nephrectomy and With an Eval-uable Primary Renal Tumor in the CheckMate 214 Trial. Eur. Urol. 2022, 81, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Albiges, L.; Tannir, N.M.; Burotto, M.; McDermott, D.; Plimack, E.R.; Barthélémy, P.; Porta, C.; Powles, T.; Donskov, F.; George, S.; et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020, 5, e001079. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; López-Martin, J.A.; Bendell, J.; Ott, P.A.; Taylor, M.; Eder, J.P.; Jäger, D.; Pietanza, M.C.; Le, D.T.; de Braud, F.; et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016, 17, 883–895. [Google Scholar] [CrossRef]
- Apolo, A.B.; Powles, T.; Escudier, B.; Burotto, M.; Zhang, J.; Simsek, B.; Scheffold, C.; Motzer, R.J.; Choueiri, T.K. Nivolumab plus ipilimumab plus cabozantinib triplet combination for patients with previously untreated advanced renal cell carcinoma: Results from a discontinued arm of the phase III CheckMate 9ER trial. Eur. J. Cancer 2022, 177, 63–71. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Mandalà, M.; Ferrucci, P.F.; Guidoboni, M.; Rutkowski, P.; Ferraresi, V.; Arance, A.; Guida, M.; Maiello, E.; Gogas, H.; et al. Sequencing of Ipilimumab Plus Nivolumab and Encorafenib Plus Binimetinib for Untreated BRAF-Mutated Metastatic Melanoma (SECOMBIT): A Randomized, Three-Arm, Open-Label Phase II Trial. J. Clin. Oncol. 2023, 41, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Jegede, O.A.; Haas, N.B.; McDermott, D.F.; Bilen, M.A.; Stein, M.; Sosman, J.A.; Alter, R.; Plimack, E.R.; Ornstein, M.; et al. Phase II Study of Nivolumab and Salvage Nivolumab/Ipilimumab in Treatment-Naive Patients with Ad-vanced Clear Cell Renal Cell Carcinoma (HCRN GU16-260-Cohort A). J. Clin. Oncol. 2022, 40, 2913–2923. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A mul-ticentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Rozeman, E.A.; Fanchi, L.F.; Sikorska, K.; van de Wiel, B.; Kvistborg, P.; Krijgsman, O.; van den Braber, M.; Philips, D.; Broeks, A.; et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 2018, 24, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Ciuleanu, T.-E.; Lee, J.-S.; Pluzanski, A.; Caro, R.B.; Gutierrez, M.; Ohe, Y.; Nishio, M.; Goldman, J.; Ready, N.; et al. Long-term survival with first-line nivolumab plus ipilimumab in patients with advanced non-small-cell lung cancer: A pooled analysis. Ann. Oncol. 2023, 34, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lee, J.-S.; Ciuleanu, T.-E.; Caro, R.B.; Nishio, M.; Urban, L.; Audigier-Valette, C.; Lupinacci, L.; Sangha, R.; Pluzanski, A.; et al. Five-Year Survival Outcomes with Nivolumab Plus Ipilimumab Versus Chemotherapy as First-Line Treatment for Metastatic Non–Small-Cell Lung Cancer in CheckMate 227. J. Clin. Oncol. 2023, 41, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Cascone, T.; Leung, C.H.; Weissferdt, A.; Pataer, A.; Carter, B.W.; Godoy, M.C.B.; Feldman, H.; William, W.N.; Xi, Y.; Basu, S.; et al. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: The phase 2 platform NEOSTAR trial. Nat. Med. 2023, 29, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Pintova, S.; Sidhu, H.; Friedlander, P.A.; Holcombe, R.F. Sweet’s syndrome in a patient with metastatic melanoma after ipilimumab therapy. Melanoma Res. 2013, 23, 498–501. [Google Scholar] [CrossRef] [PubMed]
- VanderWalde, A.; Bellasea, S.L.; Kendra, K.L.; Khushalani, N.I.; Campbell, K.M.; Scumpia, P.O.; Kuklinski, L.F.; Collichio, F.; Sosman, J.A.; Ikeguchi, A.; et al. Ipilimumab with or without nivolumab in PD-1 or PD-L1 blockade refractory metastatic melanoma: A randomized phase 2 trial. Nat. Med. 2023, 29, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Gibney, G.; Kudchadkar, R.; Yu, B.; Cheng, P.; Martinez, A.J.; Kroeger, J.; Richards, A.; McCormick, L.; Moberg, V.; et al. Phase I/II Study of Metastatic Melanoma Patients Treated with Nivolumab Who Had Progressed after Ipili-mumab. Cancer Immunol. Res. 2016, 4, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Mandalà, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Levinson, B.A.; Laino, A.S.; Pavlick, A.C.; Woods, D.M. Clinical and immune correlate results from a phase 1b study of the histone deacetylase inhibitor mocetinostat with ipilimumab and nivolumab in unresectable stage III/IV melanoma. Melanoma Res. 2022, 32, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Schadendorf, D.; Del Vecchio, M.; Larkin, J.; Atkinson, V.; Schenker, M.; Pigozzo, J.; Gogas, H.; Dalle, S.; Meyer, N.; et al. Adjuvant Therapy of Nivolumab Combined With Ipilimumab Versus Nivolumab Alone in Patients With Resected Stage IIIB-D or Stage IV Melanoma (CheckMate 915). J. Clin. Oncol. 2023, 41, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.-A.; Reed, K.; et al. Nivolumab plus Ipilimumab in Advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef] [PubMed]
- Azarov, I.; Helmlinger, G.; Kosinsky, Y.; Peskov, K. Elaborating on anti CTLA-4 mechanisms of action using an agent-based modeling approach. Front. Appl. Math. Stat. 2022, 8, 993581. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Babamohamadi, M.; Mohammadi, N.; Faryadi, E.; Haddadi, M.; Merati, A.; Ghobadinezhad, F.; Amirian, R.; Izadi, Z.; Hadjati, J. Anti-CTLA-4 nanobody as a promising approach in cancer immunotherapy. Cell Death Dis. 2024, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef]
- Sobhani, N.; Tardiel-Cyril, D.R.; Davtyan, A.; Generali, D.; Roudi, R.; Li, Y. CTLA-4 in Regulatory T Cells for Cancer Immunotherapy. Cancers 2021, 13, 1440. [Google Scholar] [CrossRef] [PubMed]
- Willsmore, Z.N.; Coumbe, B.G.; Crescioli, S.; Reci, S.; Gupta, A.; Harris, R.J.; Chenoweth, A.; Chauhan, J.; Bax, H.J.; McCraw, A.; et al. Combined anti-PD-1 and anti-CTLA-4 checkpoint blockade: Treatment of melanoma and immune mechanisms of action. Eur. J. Immunol. 2021, 51, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F.; Qari, H.A.; Upadhyay, T.K.; Alkhateeb, A.F.; Oves, M. Revolutionization in Cancer Therapeutics via Targeting Major Immune Checkpoints PD-1, PD-L1 and CTLA-4. Pharmaceuticals 2022, 15, 335. [Google Scholar] [CrossRef]
- Davis, K.L.; Fox, E.; Isikwei, E.; Reid, J.M.; Liu, X.; Minard, C.G.; Voss, S.; Berg, S.L.; Weigel, B.J.; Mackall, C.L. A Phase I/II Trial of Nivolumab plus Ipilimumab in Children and Young Adults with Relapsed/Refractory Solid Tumors: A Children’s Oncology Group Study ADVL1412. Clin. Cancer Res. 2022, 28, 5088–5097. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Bai, Y.; Lin, X.; Li, W.; Wang, J.; Zhang, X.; Pan, H.; Bai, C.; Bai, L.; Cheng, Y.; et al. First-line nivolumab plus chemotherapy vs chemotherapy in patients with advanced gastric, gastroesophageal junction and esophageal adenocarcinoma: CheckMate 649 Chinese subgroup analysis. Int. J. Cancer 2023, 152, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, I.J.; Doz, F.; Foreman, N.K.; Hargrave, D.; Lassaletta, A.; André, N.; Hansford, J.R.; Hassall, T.; Eyrich, M.; Gururangan, S.; et al. Nivolumab with or without ipilimumab in pediatric patients with high-grade CNS malignancies: Safety, efficacy, biomarker, and pharmacokinetics—CheckMate908. Neuro-Oncol. 2023, 25, 1530–1545. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.; Luong, G.; Sun, Y. A snapshot of the PD-1/PD-L1 pathway. J. Cancer 2021, 12, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, K.M.; Freeman, G.J.; McDermott, D.F. The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma. Clin. Ther. 2015, 37, 764–782. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Chen, D.S. Immune escape to PD-L1/PD-1 blockade: Seven steps to success (or failure). Ann. Oncol. 2016, 27, 1492–1504. [Google Scholar] [CrossRef] [PubMed]
- Borst, J.; Busselaar, J.; Bosma, D.M.T.; Ossendorp, F. Mechanism of action of PD-1 receptor/ligand targeted cancer immunotherapy. Eur. J. Immunol. 2021, 51, 1911–1920. [Google Scholar] [CrossRef]
- Chen, K.; Cheng, G.; Zhang, F.; Zhu, G.; Xu, Y.; Yu, X.; Huang, Z.; Fan, Y. PD-L1 expression and T cells infiltration in patients with uncommon EGFR-mutant non-small cell lung cancer and the response to immunotherapy. Lung Cancer 2020, 142, 98–105. [Google Scholar] [CrossRef]
- Li, X.; Huntoon, K.; Wang, Y.; Lee, D.; Dong, S.; Antony, A.; Walkey, C.; Kim, B.Y.S.; Jiang, W. Radiation Synergizes with IL-2/IL-15 Stimulation to Enhance Innate Immune Activation and Antitumor Immunity. Mol. Cancer Ther. 2023, 23, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Schoniger, S.; Jasani, B. The PD-1/PD-L1 Pathway: A Perspective on Comparative Immuno-Oncology. Animals 2022, 12, 2661. [Google Scholar] [CrossRef] [PubMed]
- Schoutrop, E.; El-Serafi, I.; Poiret, T.; Zhao, Y.; Gultekin, O.; He, R.; Moyano-Galceran, L.; Carlson, J.W.; Lehti, K.; Hassan, M.; et al. Mesothelin-Specific CAR T Cells Target Ovarian Cancer. Cancer Res 2021, 81, 3022–3035. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, D.; Zhao, F.; Wu, J.; Zhang, B.; Ren, H.; Sun, Q.; Qin, S. Comprehensive analysis of the prognostic value and immune infiltration of FGFR family members in gastric cancer. Front. Oncol. 2022, 12, 936952. [Google Scholar] [CrossRef] [PubMed]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Honjo, T. The PD-1–PD-L pathway in immunological tolerance. Trends Immunol. 2006, 27, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Cheng, Y.; Mok, T.; Chang, J.; Zhang, L.; Feng, J.; Tu, H.Y.; Wu, L.; Zhang, Y.; et al. Nivolumab versus docetaxel in a predominantly Chinese patient pop-ulation with previously treated advanced non-small cell lung cancer: 2-year follow-up from a randomized, open-label, phase 3 study (CheckMate 078). Lung Cancer 2021, 152, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.N.; Horn, L.; Gandhi, L.; Spigel, D.R.; Antonia, S.J.; Rizvi, N.A.; Powderly, J.D.; Heist, R.S.; Carvajal, R.D.; Jackman, D.M.; et al. Overall Survival and Long-Term Safety of Nivolumab (Anti–Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients with Previously Treated Advanced Non–Small-Cell. J. Clin. Oncol. 2015, 33, 2004. [Google Scholar] [CrossRef]
- Reck, M.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open 2021, 6, 100273. [Google Scholar] [CrossRef] [PubMed]
- Cascone, T.; William, W.N., Jr.; Weissferdt, A.; Leung, C.H.; Lin, H.Y.; Pataer, A.; Godoy, M.C.B.; Carter, B.W.; Federico, L.; Reuben, A.; et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: The phase 2 randomized NEOSTAR trial. Nat. Med. 2021, 27, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Ready, N.E.; Audigier-Valette, C.; Goldman, J.W.; Felip, E.; Ciuleanu, T.-E.; Campelo, M.R.G.; Jao, K.; Barlesi, F.; Bordenave, S.; Rijavec, E.; et al. First-line nivolumab plus ipilimumab for metastatic non-small cell lung cancer, including patients with ECOG performance status 2 and other special populations: CheckMate. J. Immunother. Cancer 2023, 11, e006127. [Google Scholar] [CrossRef] [PubMed]
- Diab, A.; Gogas, H.; Sandhu, S.; Long, G.V.; Ascierto, P.A.; Larkin, J.; Sznol, M.; Franke, F.; Ciuleanu, T.E.; Pereira, C.; et al. Bempegaldesleukin Plus Nivolumab in Untreated Advanced Melanoma: The Open-Label, Phase III PIVOT IO 001 Trial Results. J. Clin. Oncol. 2023, 41, 4756–4767. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.; Moreno, V.; Heinhuis, K.M.; Olszanski, A.J.; Spreafico, A.; Ong, M.; Chu, Q.S.; Carvajal, R.D.; Trigo, J.; Ochoa de Olza, M.; et al. OX40 Agonist BMS-986178 Alone or in Combination with Nivolumab and/or Ipilimumab in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 460–472. [Google Scholar] [CrossRef]
- John, T.; Sakai, H.; Ikeda, S.; Cheng, Y.; Kasahara, K.; Sato, Y.; Nakahara, Y.; Takeda, M.; Kaneda, H.; Zhang, H.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in advanced non-small cell lung cancer: A subanalysis of Asian patients in CheckMate 9LA. Int. J. Clin. Oncol. 2022, 27, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Owonikoko, T.K.; Park, K.; Govindan, R.; Ready, N.; Reck, M.; Peters, S.; Dakhil, S.R.; Navarro, A.; Rodríguez-Cid, J.; Schenker, M.; et al. Nivolumab and Ipilimumab as Maintenance Therapy in Extensive-Disease Small-Cell Lung Cancer: CheckMate 451. J. Clin. Oncol. 2021, 39, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; A Rizvi, N.; Goldman, J.W.; Gettinger, S.N.; Borghaei, H.; Brahmer, J.R.; E Ready, N.; E Gerber, D.; Chow, L.Q.; A Juergens, R.; et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): Results of an open-label, phase 1, multicohort study. Lancet Oncol. 2016, 18, 31–41. [Google Scholar] [CrossRef]
- Peters, S.; Pujol, J.-L.; Dafni, U.; Dómine, M.; Popat, S.; Reck, M.; Andrade, J.; Becker, A.; Moro-Sibilot, D.; Curioni-Fontecedro, A.; et al. Consolidation nivolumab and ipilimumab versus observation in limited-disease small-cell lung cancer after chemo-radiotherapy—Results from the randomised phase II ETOP/IFCT 4-12 STIMULI trial. Ann. Oncol. 2021, 33, 67–79. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Bendell, J.; Calvo, E.; Kim, J.W.; Ascierto, P.A.; Sharma, P.; Ott, P.A.; Peltola, K.; Jaeger, D.; Evans, J.; et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J. Clin. Oncol. 2018, 36, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Özgüroğlu, M.; Bang, Y.-J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M.-H.; Fornaro, L.; Olesiński, T.; Caglevic, C.; Muro, K.; et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet 2018, 392, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-K.; Boku, N.; Satoh, T.; Ryu, M.-H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.-S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients with Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Doki, Y.; Ogata, T.; Motoyama, S.; Kawakami, H.; Ueno, M.; Kojima, T.; Shirakawa, Y.; Okada, M.; Ishihara, R.; et al. First-line nivolumab plus ipilimumab or chemotherapy versus chemotherapy alone in advanced esophageal squamous cell carcinoma: A Japanese subgroup analysis of open-label, phase 3 trial (CheckMate 648/ONO-4538-50). Esophagus 2022, 20, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chem-otherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Ajani, J.A.; Moehler, M.; Garrido, M.; Gallardo, C.; Shen, L.; Yamaguchi, K.; Wyrwicz, L.; Skoczylas, T.; Bragagnoli, A.C.; et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 2022, 603, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Meindl-Beinker, N.M.; Betge, J.; Gutting, T.; Burgermeister, E.; Belle, S.; Zhan, T.; Schulte, N.; Maenz, M.; Ebert, M.P.; Haertel, N.; et al. A multicenter open-label phase II trial to evaluate nivolumab and ipilimumab for 2nd line therapy in elderly patients with advanced esophageal squamous cell cancer (RAMONA). BMC Cancer 2019, 19, 231. [Google Scholar] [CrossRef]
- Ebert, M.P.; Meindl-Beinker, N.M.; Gutting, T.; Maenz, M.; Betge, J.; Schulte, N.; Zhan, T.; Weidner, P.; Burgermeister, E.; Hofheinz, R.; et al. Second-line therapy with nivolumab plus ipili-mumab for older patients with oesophageal squamous cell cancer (RAMONA): A multicentre, open-label phase 2 trial. Lancet Healthy Longev. 2022, 3, e417–e427. [Google Scholar] [CrossRef] [PubMed]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined nivolumab and ipilimumab or mono-therapy in untreated melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Tykodi, S.S.; Gordan, L.N.; Alter, R.S.; Arrowsmith, E.; Harrison, M.R.; Percent, I.; Singal, R.; Van Veldhuizen, P.; George, D.J.; Hutson, T.; et al. Safety and efficacy of nivolumab plus ipilimumab in patients with advanced non-clear cell renal cell carcinoma: Results from the phase 3b/4 CheckMate 920 trial. J. Immunother. Cancer 2022, 10, e003844. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Rixe, O.; Oudard, S.; Negrier, S.; Szczylik, C.; Kim, S.T.; et al. Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2007, 356, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Aren Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Rini, B.I.; McDermott, D.F.; Aren Frontera, O.; Hammers, H.J.; Carducci, M.A.; Salman, P.; Escudier, B.; Beuselinck, B.; Amin, A.; et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: Extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019, 20, 1370–1385. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Russo, P.; Grünwald, V.; Tomita, Y.; Zurawski, B.; Parikh, O.; Buti, S.; Barthélémy, P.; Goh, J.C.; Ye, D.; et al. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): A double-blind, randomised, phase 3 trial. Lancet 2023, 401, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Epaillard, N.; Simonaggio, A.; Elaidi, R.; Azzouz, F.; Braychenko, E.; Thibault, C.; Sun, C.-M.; Moreira, M.; Oudard, S.; Vano, Y.-A. BIONIKK: A phase 2 biomarker driven trial with nivolumab and ipilimumab or VEGFR tyrosine kinase inhibitor (TKI) in naïve metastatic kidney cancer. Bull. Cancer 2020, 107, eS22–eS27. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Kluger, H.; George, S.; Tykodi, S.S.; Kuzel, T.M.; Perets, R.; Escudier, B. FRACTION-RCC: Nivolumab plus ipilimumab for ad-vanced renal cell carcinoma after progression on immuno-oncology therapy. J. Immunother. Cancer 2022, 10, e005780. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Esteban, E.; Barthélémy, P.; Schmidinger, M.; Busch, J.; Valderrama, B.P.; Charnley, N.; Schmitz, M.; Schumacher, U.; Leucht, K.; et al. Tailored immunotherapy approach with nivolumab with or without nivolumab plus ipilimumab as immunotherapeutic boost in patients with metastatic renal cell car-cinoma (TITAN-RCC): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2023, 24, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Signoretti, S.; Choueiri, T.K.; McDermott, D.F.; Motzer, R.J.; George, S.; Powles, T.; Donskov, F.; Tykodi, S.S.; Pal, S.K.; et al. Long-term outcomes with nivolumab plus ipili-mumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. J. Immunother. Cancer 2022, 10, e005445. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; McDermott, D.F.; Escudier, B.; Burotto, M.; Choueiri, T.K.; Hammers, H.J.; Barthélémy, P.; Plimack, E.R.; Porta, C.; George, S.; et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer 2022, 128, 2085–2097. [Google Scholar] [CrossRef]
- Çakar, E.; Oniangue-Ndza, C.; Schneider, R.P.; Klijn, S.L.; Vogl, U.M.; Rothermundt, C.; May, J.R. Cost-Effectiveness of Nivolumab Plus Ipilimumab for the First-Line Treatment of Intermediate/Poor-Risk Advanced and/or Metastatic Renal Cell Carcinoma in Switzerland. Pharm. Econ. Open 2023, 7, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Goldman, D.A.; Shoushtari, A.N.; Warner, A.B.; Callahan, M.K.; Momtaz, P.; Smithy, J.W.; Naito, E.; Cugliari, M.K.; Raber, V.; et al. Adaptive Dosing of Nivolumab + Ipilimumab Immunotherapy Based Upon Early, Interim Radiographic Assessment in Advanced Melanoma (The ADAPT-IT Study). J. Clin. Oncol. 2021, 40, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Casula, M.; Bulgarelli, J.; Pisano, M.; Piccinini, C.; Piccin, L.; Cossu, A.; Mandalà, M.; Ferrucci, P.F.; Guidoboni, M.; et al. Sequential immunotherapy and targeted therapy for metastatic BRAF V600 mutated melanoma: 4-year survival and biomarkers evaluation from the phase II SECOMBIT trial. Nat. Commun. 2024, 15, 146. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. J. Clin. Oncol. 2021, 39 (Suppl. S15), 9506. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Vecchio, M.D.; Mandalá, M.; Gogas, H.; Fernandez, A.M.A.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III/IV Melanoma: 5-Year Efficacy and Biomarker Results from CheckMate 238. Clin. Cancer Res. 2023, 29, 3352–3361. [Google Scholar] [CrossRef]
- Versluis, J.; Menzies, A.; Sikorska, K.; Rozeman, E.; Saw, R.; van Houdt, W.; Eriksson, H.; Klop, W.; Ch’ng, S.; van Thienen, J.; et al. Survival update of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma in the OpACIN and OpACIN-neo trials. Ann. Oncol. 2023, 34, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Reijers, I.L.M.; Menzies, A.M.; van Akkooi, A.C.J.; Versluis, J.M.; Heuvel, N.M.J.v.D.; Saw, R.P.M.; Pennington, T.E.; Kapiteijn, E.; van der Veldt, A.A.M.; Suijkerbuijk, K.P.M.; et al. Personalized response-directed surgery and adjuvant therapy after neoadjuvant ipilimumab and nivolumab in high-risk stage III melanoma: The PRADO trial. Nat. Med. 2022, 28, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- A Tawbi, H.; A Forsyth, P.; Hodi, F.S.; Lao, C.D.; Moschos, S.J.; Hamid, O.; Atkins, M.B.; Lewis, K.; Thomas, R.P.; A Glaspy, J.; et al. Safety and efficacy of the combination of nivolumab plus ipilimumab in patients with melanoma and asymptomatic or symptomatic brain metastases (CheckMate 204). Neuro-Oncol. 2021, 23, 1961–1973. [Google Scholar] [CrossRef] [PubMed]
- Pelster, M.S.; Gruschkus, S.K.; Bassett, R.; Gombos, D.S.; Shephard, M.; Posada, L.; Glover, M.S.; Simien, R.; Diab, A.; Hwu, P.; et al. Nivolumab and Ipilimumab in Metastatic Uveal Melanoma: Results From a Single-Arm Phase II Study. J. Clin. Oncol. 2021, 39, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Diefenbach, C.S.; Hong, F.; Ambinder, R.F.; Cohen, J.B.; Robertson, M.J.; A David, K.; Advani, R.H.; Fenske, T.S.; Barta, S.K.; Palmisiano, N.D.; et al. Ipilimumab, nivolumab, and brentuximab vedotin combination therapies in patients with relapsed or refractory Hodgkin lymphoma: Phase 1 results of an open-label, multicentre, phase 1/2 trial. Lancet Haematol. 2020, 7, e660–e670. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.-J.; Van Cutsem, E.; Limon, M.L.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Baghdadi, T.A.; et al. A Phase II Basket Trial of Dual Anti–CTLA-4 and Anti–PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Yau, T.; El-Khoueiry, A.B.; Kudo, M.; Shen, Y.; Tschaika, M.; Roy, A.; Feng, Y.; Gao, L.; Aras, U. Exposure-response analysis for nivolumab plus ipilimumab combination therapy in patients with advanced hepatocellular carcinoma (CheckMate 040). Clin. Transl. Sci. 2023, 16, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, N.; Gil-Jimenez, A.; Silina, K.; Hendricksen, K.; Smit, L.A.; de Feijter, J.M.; van Montfoort, M.L.; van Rooijen, C.; Peters, D.; Broeks, A.; et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: The NABUCCO trial. Nat. Med. 2020, 26, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- van Dorp, J.; Pipinikas, C.; Suelmann, B.B.; Mehra, N.; van Dijk, N.; Marsico, G.; van der Heijden, M.S. High- or low-dose preoperative ipilimumab plus nivolumab in stage III urothelial cancer: The phase 1B NABUCCO trial. Nat. Med. 2023, 29, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.-O.; Schostak, M.; Grün, C.B.; Loidl, W.; Pichler, M.; Zimmermann, U.; Schmitz-Dräger, B.; Steiner, T.; Roghmann, F.; Niegisch, G.; et al. Nivolumab + Ipilimumab as Immunotherapeutic Boost in Metastatic Urothelial Carcinoma: A Nonrandomized Clinical Trial. JAMA Oncol. 2023, 24, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Schmitz-Dräger, B.J.; Zimmermann, U.; Grün, C.B.; Baretton, G.B.; Schmitz, M.; Foller, S.; Leucht, K.; Schostak, M.; Zengerling, F.; et al. Tailored Immunotherapy Approach With Nivolumab in Advanced Transitional Cell Carcinoma. J. Clin. Oncol. 2022, 40, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Othus, M.; Patel, S.P.; Miller, K.D.; Chugh, R.; Schuetze, S.M.; Chamberlin, M.D.; Haley, B.J.; Storniolo, A.M.V.; Reddy, M.P.; et al. A Multicenter Phase II Trial of Ipilimumab and Nivolumab in Unresectable or Metastatic Metaplastic Breast Cancer: Cohort 36 of Dual Anti–CTLA-4 and Anti–PD-1 Blockade in Rare Tumors (DART, SWOG S1609). Clin. Cancer Res. 2021, 28, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Andresen, N.K.; Røssevold, A.H.; Quaghebeur, C.; Gilje, B.; Boge, B.; Gombos, A.; Falk, R.S.; Mathiesen, R.R.; Julsrud, L.; Garred, Ø.; et al. Ipilimumab and nivolumab combined with anthracycline-based chemotherapy in metastatic hormone receptor-positive breast cancer: A randomized phase 2b trial. J. Immunother. Cancer 2024, 12, e007990. [Google Scholar] [CrossRef] [PubMed]
- Kyte, J.A.; Andresen, N.K.; Russnes, H.G.; Fretland, S.; Falk, R.S.; Lingjærde, O.C.; Naume, B. ICON: A randomized phase IIb study evaluating immunogenic chemotherapy combined with ipilimumab and nivolumab in patients with metastatic hormone receptor positive breast cancer. J. Transl. Med. 2020, 18, 269. [Google Scholar] [CrossRef] [PubMed]
- Armand, P.; Lesokhin, A.; Borrello, I.; Timmerman, J.; Gutierrez, M.; Zhu, L.; McKiver, M.P.; Ansell, S.M. A phase 1b study of dual PD-1 and CTLA-4 or KIR blockade in patients with relapsed/refractory lymphoid malignancies. Leukemia 2020, 35, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Restifo, N.P.; Smyth, M.J.; Snyder, A. Acquired resistance to immunotherapy and future challenges. Nat. Rev. Cancer 2016, 16, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Gedrich, R.; Peck, R.; LaVallee, T.; Eder, J.P. Targeting KIT on innate immune cells to enhance the antitumor activity of checkpoint inhibitors. Immunotherapy 2016, 8, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Mirza, N.; Fishman, M.; Fricke, I.; Dunn, M.; Neuger, A.M.; Frost, T.J.; Lush, R.M.; Antonia, S.; Gabrilovich, D.I. All-trans-Retinoic Acid Improves Differentiation of Myeloid Cells and Immune Response in Cancer Patients. Cancer Res 2006, 66, 9299–9307. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Skora, A.D.; Li, Z.; Liu, Q.; Tam, A.J.; Blosser, R.L.; Zhou, S. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc. Natl. Acad. Sci. USA 2014, 111, 11774–11779. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Zmina, P.M.; Huang, A.Y.; Askew, D.; Bedogni, B. Inhibiting Notch1 enhances immunotherapy efficacy in melanoma by preventing Notch1 dependent immune suppressive properties. Cancer Lett. 2018, 434, 144–151. [Google Scholar] [CrossRef]
- Freitas-Dias, C.; Gonçalves, F.; Martins, F.; Lemos, I.; Gonçalves, L.G.; Serpa, J. Interaction between NSCLC Cells, CD8+ T-Cells and Immune Checkpoint Inhibitors Potentiates Coagulation and Promotes Metabolic Remodeling—New Cues on CAT-VTE. Cells 2024, 13, 305. [Google Scholar] [CrossRef] [PubMed]
- Retseck, J.; Nasr, A.; Lin, Y.; Lin, H.; Mendiratta, P.; Butterfield, L.H.; Tarhini, A.A. Long term impact of CTLA4 blockade immunotherapy on regulatory and effector immune responses in patients with melanoma. J. Transl. Med. 2018, 16, 184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, C.; Zhou, L.; Dong, P.; Shi, L. Immune Checkpoint Inhibitors and Neurotoxicity. Curr. Neuropharmacol. 2021, 19, 1246–1263. [Google Scholar] [CrossRef] [PubMed]
- Soldan, S.S.; Lieberman, P.M. Epstein-Barr virus infection in the development of neurological disorders. Drug Discov. Today Dis. Model. 2020, 32, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Spain, L.; Diem, S.; Larkin, J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat. Rev. 2016, 44, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Kaseb, A.O.; Hasanov, E.; Cao, H.S.T.; Xiao, L.; Vauthey, J.-N.; Lee, S.S.; Yavuz, B.G.; I Mohamed, Y.; Qayyum, A.; Jindal, S.; et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: A randomised, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 2022, 7, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Unger, J.M.; Qian, L.; Redman, M.W.; Tavernier, S.S.; Minasian, L.; Sigal, E.V.; A Papadimitrakopoulou, V.; Leblanc, M.; Cleeland, C.S.; A Dzingle, S.; et al. Quality-of-life outcomes and risk prediction for patients randomized to nivolumab plus ipilimumab vs nivolumab on LungMAP-S1400I. JNCI J. Natl. Cancer Inst. 2023, 115, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.F.; Spencer, C.; Cabanski, C.R.; Panageas, K.S.; Wells, D.K.; Ribas, A.; Tawbi, H.; Tsai, K.; Postow, M.; Shoushtari, A.; et al. Ipilimumab alone or in combination with nivolumab in patients with advanced melanoma who have progressed or relapsed on PD-1 blockade: Clinical outcomes and translational biomarker analyses. J. Immunother. Cancer 2022, 10, e003853. [Google Scholar] [CrossRef] [PubMed]
- Reschke, R.; Gussek, P.; Boldt, A.; Sack, U.; Köhl, U.; Lordick, F.; Gora, T.; Kreuz, M.; Reiche, K.; Simon, J.-C.; et al. Distinct Immune Signatures Indicative of Treatment Response and Immune-Related Adverse Events in Melanoma Patients under Immune Checkpoint Inhibitor Therapy. Int. J. Mol. Sci. 2021, 22, 8017. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.N.; Redman, M.W.; Bazhenova, L.; Hirsch, F.R.; Mack, P.C.; Schwartz, L.H.; Bradley, J.D.; Stinchcombe, T.E.; Leighl, N.B.; Ramalingam, S.S.; et al. Nivolumab Plus Ipilimumab vs Nivolumab for Previously Treated Patients With Stage IV Squamous Cell Lung Cancer: The Lung-MAP S1400I Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1368–1377. [Google Scholar] [CrossRef]
- Bronte, G.; Verlicchi, A.; De Matteis, S.; Rossi, A.; Affatato, A.; Sullo, F.G.; Gianni, C.; Canale, M.; Burgio, M.A.; Delmonte, A.; et al. Case Report: Circulating Myeloid-Derived Suppres-sive-Like Cells and Exhausted Immune Cells in Non-Small Cell Lung Cancer Patients Treated with Three Immune Checkpoint Inhibitors. Front. Immunol. 2021, 12, 672219. [Google Scholar] [CrossRef]

| Reference | Trial Phase | Treatment Arms | Primary Endpoints | Results | Clinical Trial Number |
|---|---|---|---|---|---|
| Hellmann, M. D., et al. (2019) | Phase 3 | Nivolumab + Ipilimumab, Nivolumab, Chemotherapy | Overall Survival | Nivolumab + ipilimumab led to longer overall survival than chemotherapy in patients with advanced NSCLC, irrespective of PD-L1 expression. | NCT02477826 |
| Reck, M., et al. (2021) | Phase 3 | Nivolumab + Ipilimumab + 2 Cycles of Chemo, Chemotherapy (4 Cycles) | Overall Survival | Nivolumab plus ipilimumab with two cycles of chemotherapy showed durable efficacy benefits over chemotherapy alone in advanced NSCLC. | NCT03215706 |
| Cascone, T. et al. (2021) | Phase 2 | Nivolumab vs. Nivolumab + Ipilimumab | Major Pathologic Response (MPR) | Nivolumab + ipilimumab arm achieved a 38% MPR rate, with higher pathologic complete response rates and less viable tumor compared to nivolumab alone. | NCT03158129 |
| Ready, N. E. et al. (2022) | Phase 3B | Nivolumab + Ipilimumab | Incidence of Grade 3–4 and Grade 5 Immune-Mediated Adverse Events (IMAEs) | Manageable safety and durable efficacy observed in patients with ECOG PS 0–1. Special populations, including those with ECOG PS 2 or untreated brain metastases, showed comparable safety and encouraging 3-year overall survival rates. | NCT02869789 |
| Owonikoko, T. K., et al. (2021) | Phase 3 | Nivolumab plus Ipilimumab; Nivolumab; Placebo | Overall Survival (OS) | OS not significantly prolonged with nivolumab plus ipilimumab versus placebo (HR = 0.92). | NCT02538666 |
| Reference | Trial Phase | Treatment Arms | Primary Endpoints | Results | Clinical Trial Number |
|---|---|---|---|---|---|
| Motzer et al. (2019) | Phase 3 | NIVO + IPI vs. SUN | OS, PFS, Objective response | NIVO + IPI demonstrated superior OS, PFS, and objective response compared to SUN in patients with previously untreated advanced renal cell carcinoma across all risk categories. | NCT02231749 |
| Motzer et al. (2023) | Phase 3 | NIVO + IPI vs. Placebo | DFS | NIVO + IPI did not improve disease-free survival versus placebo in localized renal cell carcinoma post-nephrectomy. | NCT03138512 |
| Tykodi et al. (2022) | Phase 3b/4 | NIVO + IPI | Incidence of grade ≥ 3 | Nivolumab plus ipilimumab for advanced non-clear-cell RCC showed no new safety signals and encouraging antitumor activity. | NCT02982954 |
| Rini et al. (2022) | Phase 3 | NIVO + IPI vs. SUN | OS, PFS, ORR | NIVO + IPI showed superior OS, PFS, and ORR over SUN in patients with sRCC, regardless of PD-L1 expr. | NCT02231749 |
| Grimm et al. (2023) | Phase 2 | NIVO/NIVO + IPI | Objective response rate | Nivolumab induction with or without nivolumab plus ipilimumab boosts showed improved objective response rates compared to nivolumab monotherapy. Overall efficacy was inferior to upfront nivolumab plus ipilimumab. | NCT02917772 |
| Reference | Trial Phase | Treatment Arms | Primary Endpoints | Results | Clinical Trial Number |
|---|---|---|---|---|---|
| Hodi FS et al. (2018) | Phase 3 | Nivolumab + Ipilimumab | Overall survival, Progression-free survival | Improved overall survival and progression-free survival with nivolumab + ipilimumab compared to ipilimumab alone. | NCT01844505 |
| Ascierto PA et al. (2020) | Phase 2 | Nivo + Ipi | Response rate by RECIST 1.1 at week 12 | In total, 48% of patients had favorable antitumor effect at week 6, with 52% and 80% estimated 18-month PFS and OS, respectively. | NCT03122522 |
| Ascierto PA et al. (2023) | Phase 2 | A: Encorafenib + Binimetinib -> Ipilimumab + Nivolumab -> Nivolumab | Overall survival at 2 years | Median OS not reached in any arm; 2-year OS rates: Arm A—65%, Arm B—73%, Arm C—69%. No new safety signals emerged. | NCT02631447 |
| Postow et al., 2021 | Phase 2 | Nivolumab + Ipilimumab vs. Nivolumab Alone | Response rate by RECIST 1.1 at week 12, Progression-free survival (PFS), Overall survival (OS), Safety | Best overall response rates by RECIST at week 12 or any time afterward were 48% and 58%, respectively. The 18-month progression-free survival and overall survival were estimated at 52% and 80%, respectively. Fifty-seven percent of patients had grade 3–5 treatment-related toxicity. | NCT03122522 |
| Reijers, I. L. M., et al. (2022) | Phase 3 | Neoadjuvant Ipilimumab and Nivolumab | Pathologic response rates (pRRs) | pRR was 72%, with 61% achieving major pathologic response (MPR). TLND omission was feasible for MPR. | NCT02977052 |
| Diefenbach, C. S. et al. (2020) | Phase 1/2 | Brentuximab Vedotin + Nivolumab + Ipilimumab | Safety and activity evaluation | Evaluation of brentuximab vedotin combined with nivolumab or ipilimumab, or both, in patients with relapsed or refractory Hodgkin lymphoma. | NCT01896999 |
| Reference | Trial Phase | Treatment Arms | Primary Endpoints | Results | Clinical Trial Number |
|---|---|---|---|---|---|
| Lenz et al. (2022) | Phase 2 | NIVO + low-dose IPI | Objective response rate | First-line nivolumab plus low-dose ipilimumab showed a 69% objective response rate and 84% disease control rate in MSI-H/dMMR metastatic colorectal cancer. Clinical benefit was observed regardless of baseline characteristics. | NCT02060188 |
| Patel, S. P., et al. (2020) | Phase 2 | Ipilimumab plus nivolumab | Overall response rate | ORR: 25% with 44% in high-grade neuroendocrine carcinoma; 0% in low/intermediate-grade tumors. | NCT02060188 |
| Yau, T. et al. (2020) | Phase 1/2 | Nivolumab plus ipilimumab | Safety, tolerability, objective response rate | Promising ORRs (27–32%) observed across treatment arms in advanced HCC. Arm A showed highest median overall survival (22.8 months). Manageable safety profile. | NCT01658878 |
| Grimm, M.-O., et al. (2023) | Phase 2 | Tailored immunotherapy approach with nivolumab with or without ipilimumab | Objective response rate (ORR) | Objective response rate exceeding 20% in metastatic urothelial patients. | NCT03219775 |
| Adams, S. et al. (2021) | Phase 2 | Ipilimumab + nivolumab | Objective response rate (ORR) | ORR was 18%, with 3 of 17 patients achieving objective responses (1 complete, 2 partial responses) in advanced/metastatic metaplastic breast cancer. | NCT02834013 |
| Immune-Related Adverse Event | Nivolumab (%) | Ipilimumab (%) | Combination (Nivolumab + Ipilimumab) (%) |
|---|---|---|---|
| Diarrhea | 10% | 23% | 35% |
| Fatigue | 15% | 30% | 45% |
| Pruritus | 8% | 20% | 30% |
| Rash | 12% | 25% | 38% |
| Nausea | 7% | 18% | 28% |
| Pyrexia (Fever) | 5% | 15% | 25% |
| Decreased appetite | 6% | 12% | 20% |
| Vomiting | 4% | 10% | 18% |
| Hypothyroidism | 2% | 8% | 15% |
| Colitis | Less than 1% | 15% | 25% |
| Arthralgia | Less than 1% | 12% | 22% |
| Headache | Less than 1% | 10% | 18% |
| Neurological disorder | Less than 1% | 8% | 15% |
| Sweet’s syndrome | Less than 1% | - | 10% |
| Dyspnea (Breathing difficulty) | Less than 1% | 5% | 12% |
| Liver toxicity | Less than 1% | Less than 1% | 8% |
| Side Effect | Underlying Reason |
|---|---|
| Fatigue | Activation of immune system leading to generalized tiredness |
| Rash | Cutaneous immune response against normal skin tissue |
| Diarrhea | Immune-mediated inflammation of the gastrointestinal tract |
| Pruritus | Activation of immune cells in the skin |
| Colitis | Inflammation of the colon due to immune system attack |
| Hepatitis | Immune-mediated inflammation of liver cells |
| Hypothyroidism | Autoimmune destruction of thyroid tissue |
| Pneumonitis | Immune-mediated inflammation of lung tissue |
| Nephritis | Immune-mediated inflammation of kidney tissue |
| Endocrinopathies | Dysfunction of endocrine glands due to immune system dysregulation |
| Dermatitis | Immune response leading to skin inflammation |
| Arthralgia | Immune-mediated joint inflammation |
| Myalgia | Muscle pain due to immune system activation |
| Neuropathy | Immune-mediated damage to peripheral nerves |
| Ocular toxicity | Inflammatory response affecting the eyes |
| Cardiotoxicity | Immune-related damage to the heart |
| Renal toxicity | Immune-mediated kidney damage |
| Hepatotoxicity | Liver damage caused by immune response |
| Gastrointestinal | Immune-related inflammation of the digestive tract |
| Cutaneous reactions | Immune response leading to skin rashes and other dermatological issues |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, B.; Qahwaji, R.M.; Alfaifi, M.S.; Mobashir, M. Nivolumab and Ipilimumab Acting as Tormentors of Advanced Tumors by Unleashing Immune Cells and Associated Collateral Damage. Pharmaceutics 2024, 16, 732. https://doi.org/10.3390/pharmaceutics16060732
Khan B, Qahwaji RM, Alfaifi MS, Mobashir M. Nivolumab and Ipilimumab Acting as Tormentors of Advanced Tumors by Unleashing Immune Cells and Associated Collateral Damage. Pharmaceutics. 2024; 16(6):732. https://doi.org/10.3390/pharmaceutics16060732
Chicago/Turabian StyleKhan, Bushra, Rowaid M. Qahwaji, Mashael S. Alfaifi, and Mohammad Mobashir. 2024. "Nivolumab and Ipilimumab Acting as Tormentors of Advanced Tumors by Unleashing Immune Cells and Associated Collateral Damage" Pharmaceutics 16, no. 6: 732. https://doi.org/10.3390/pharmaceutics16060732
APA StyleKhan, B., Qahwaji, R. M., Alfaifi, M. S., & Mobashir, M. (2024). Nivolumab and Ipilimumab Acting as Tormentors of Advanced Tumors by Unleashing Immune Cells and Associated Collateral Damage. Pharmaceutics, 16(6), 732. https://doi.org/10.3390/pharmaceutics16060732







