Vaccinium myrtillus L. Leaf Waste as a Source of Biologically Potent Compounds: Optimization of Polyphenol Extractions, Chemical Profile, and Biological Properties of the Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Herbal Material, Reagents, and Standards
2.2. Extraction Procedures
2.2.1. Maceration
2.2.2. Heat-Assisted Extraction (HAE)
2.2.3. Ultrasound-Assisted Extraction (UAE)
2.2.4. Microwave-Assisted Extraction (MAE)
2.2.5. Freeze-Drying of the Samples
2.3. Chemical Characterization of the Extracts
2.3.1. Total Polyphenols
2.3.2. Total Flavonoids
2.3.3. Condensed Tannins
2.3.4. Total Proteins
2.3.5. FT-IR Spectroscopy
2.3.6. LC-MS Analysis
2.4. Antioxidant Activity Tests
2.4.1. ABTS Method
2.4.2. DPPH Method
2.4.3. FRAP Method
2.4.4. CUPRAC Method
2.5. Antimicrobial Analysis
2.6. Determination of the Cell Viability
2.7. Anti-Inflammatory Activity Analysis
2.7.1. Cell-Based ELISA
2.7.2. Red Blood Cell (RBC) Membrane Stabilization Assay
2.8. Physical Characterization
2.8.1. Extraction Yield
2.8.2. Measurement of the Conductivity
2.8.3. Determination of Density, Surface Tension, and Viscosity
2.9. Statistics
3. Results
3.1. Influence of Plant Material:Medium Ratio, Extraction Medium, and Period on Extracted Polyphenols
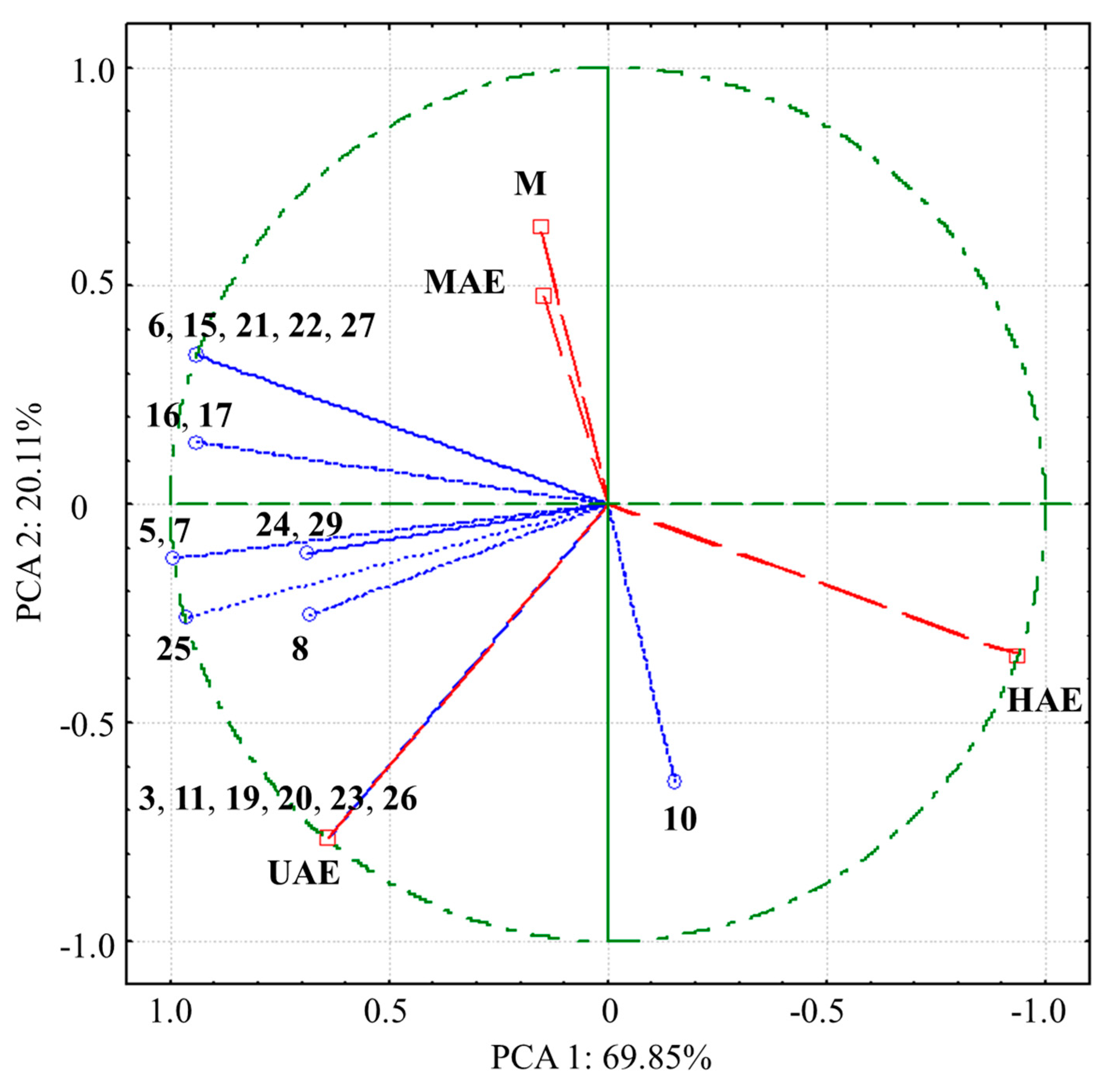
3.2. The Influence of Procedures on Extracted Polyphenol Compounds
3.3. Experimental Design (Full Factorial Design)
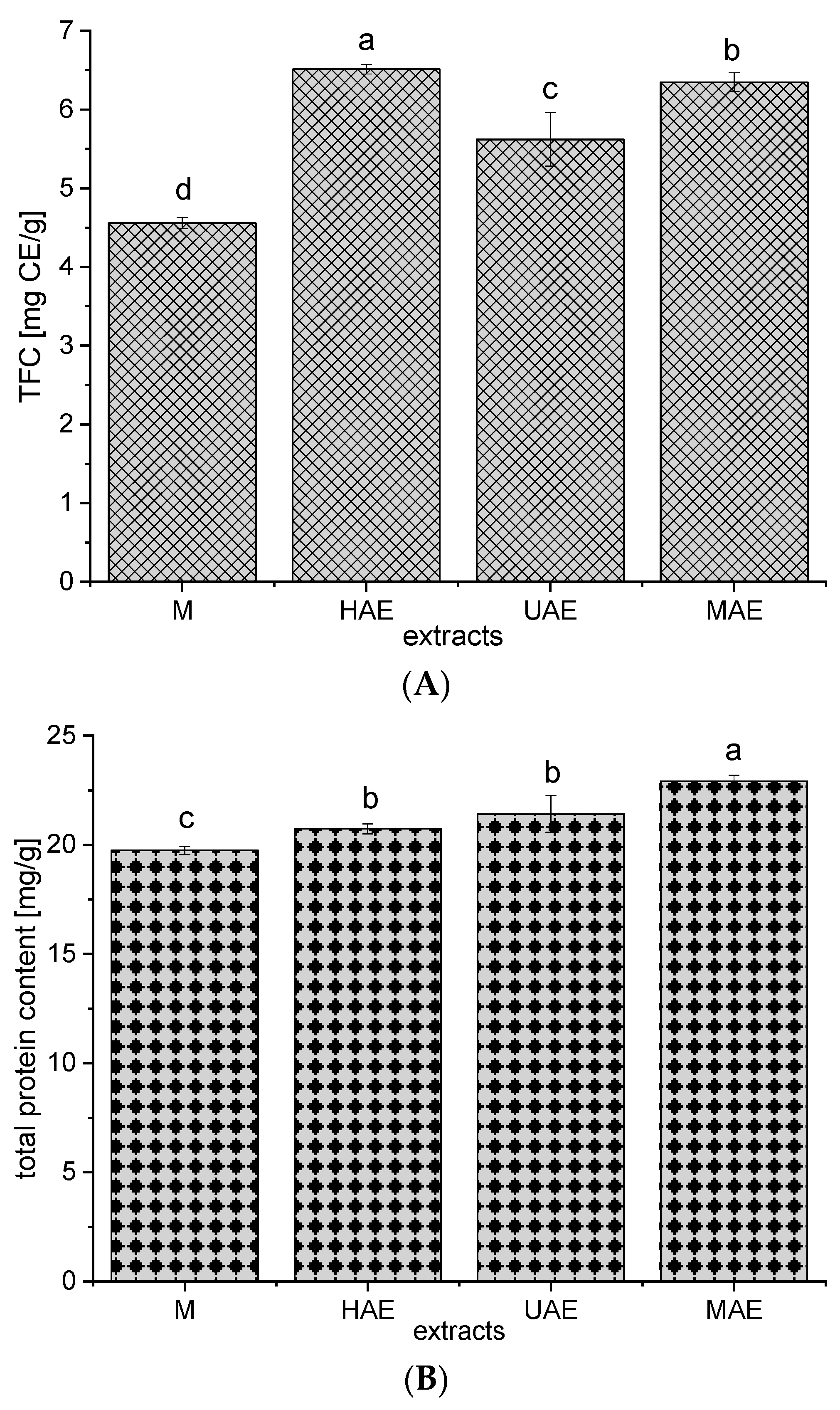
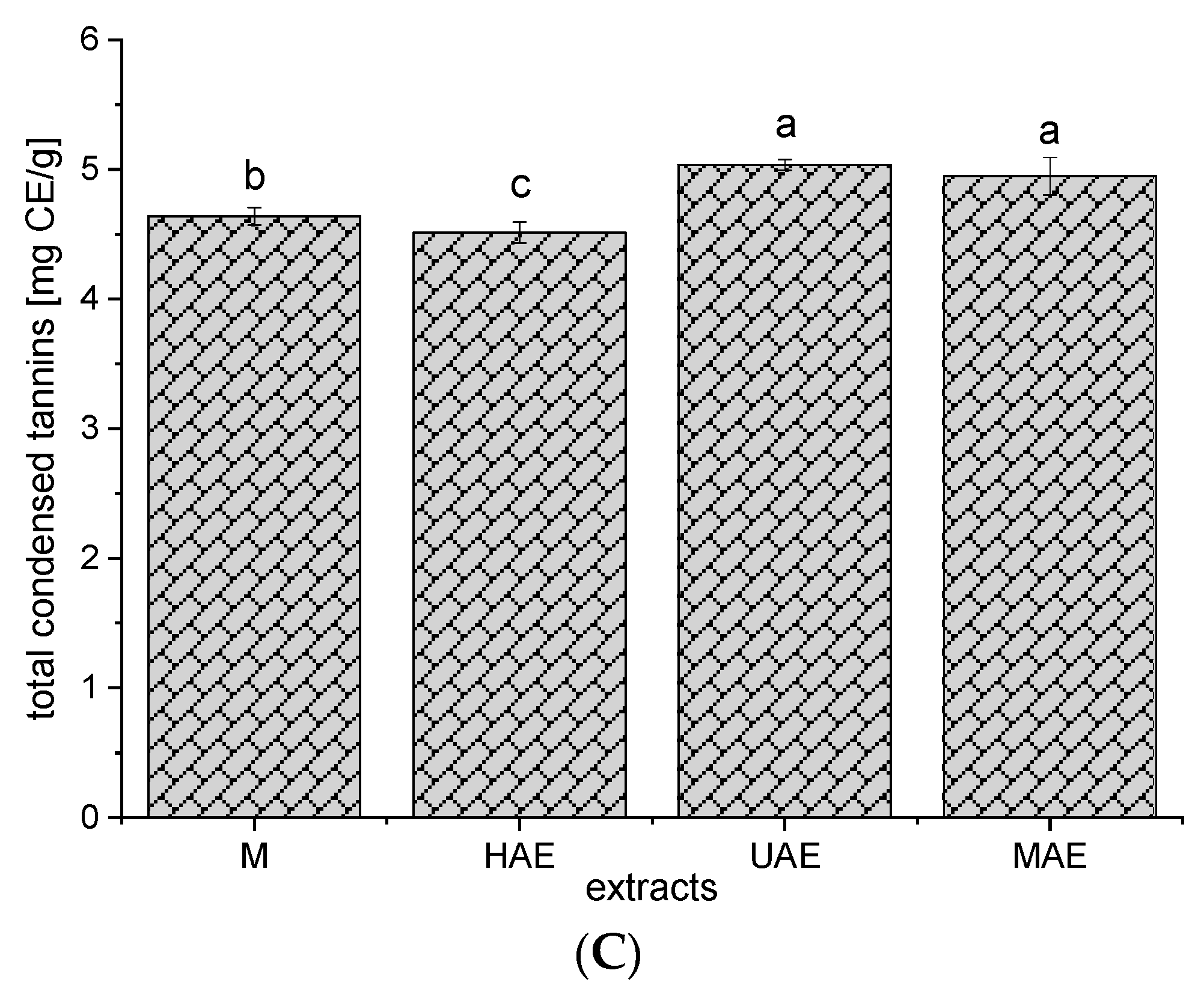
3.4. Total Flavonoids, Condensed Tannins, and Total Proteins of the Extracts
3.5. FT-IR Spectra of the Extracts
3.6. LC-MS Analysis of the Extracts
3.6.1. Qualitative LC-MS Analysis of the Extracts
| No * | Rt, min | λmax, nm | MS Data (250 V) | Compound | Ref./ Standard *** |
|---|---|---|---|---|---|
| 1 | 7.10 | 328 | 707 (100) ** [M-H]−, 513, 417, 343, 305, 191 | Caffeic acid derivative | [58] |
| 2 | 7.89 | 328 | 707 (100) [M-H]−, 513, 417, 343, 305, 191 | Caffeic acid derivative | [58] |
| 3 | 8.71 | 328 | 707 (100) [M-H]−, 643, 593, 441, 191 | Caffeic acid derivative | [58] |
| 4 | 9.55 | 328 | 707 (100) [M-H]−, 577, 513, 191 | Caffeic acid derivative | [58] |
| 5 | 5.71 | 328 | 353 [M-H]−, 191 (100), 161, 135 | Chlorogenic acid | St. |
| 6 | 6.69 | 328 | 353 [M-H]−, 191 (100), 179, 173, 161, 135 | Isochrologenic acid | [51] |
| 7 | 12.05 | 230, 278 | 577 (100) [M-H]−, 451, 425, 407, 381, 339, 289 | Procyanidin B2 | St. |
| 8 | 12.99 | 230, 278 | 865 (100) [M-H]−, 713, 691, 603, 575, 289, 245, 221, 137, 123 | Procyanidin B-type trimer | [58,59,60] |
| 9 | 14.70 | 230, 278 | 881 (100) [M-H]−, 755, 575, 289, 191 | (epi)Gallocatechin-(epi) catechin-(epi)catechin trimer | UV/MS |
| 10 | 16.59 | 230, 278 | 863 (100) [M-H]−, 711, 575, 451, 411, 289 | Procyanidin A-type trimer | [58,59,60] |
| 11 | 16.87 | 230, 278 | 865 (100) [M-H]−, 713, 577, 575, 425, 289 | Procyanidin C1 | St. |
| 12 | 20.02 | 230, 278 | 765 (100) [M-H]−, 739, 725, 588, 449, 289 | Catechin derivative | UV/MS |
| 13 | 20.55 | 256, 265sh, 301sh, 354 | 609 (100) [M-H]−, 503, 301, 271, 243 | Quercetin 3-O-rutinoside (rutin) | St. |
| 14 | 20.87 | 230, 278 | 535 (100) [M-H]−, 425, 371, 311, 289, 191, 163, 147, 119 | Catechin derivative | UV/MS |
| 15 | 21.41 | 256, 265sh, 301sh, 354 | 463 (100) [M-H]−, 425, 301, 301, 271, 255, 243, 151 | Quercetin 3-O-galactoside (hyperoside) | St. |
| 16 | 21.59 | 256, 265sh, 301sh, 354 | 463 (100) [M-H]−, 301, 285 | Quercetin 3-O-glucoside (isoquercitrin) | St. |
| 17 | 21.59 | 256, 265sh, 301sh, 354 | 477 (100) [M-H]−, 301, 271 | Quercetin 3-O-glucuronide (miquelianin) | St. |
| 18 | 19.90 | 230, 278 | 765 [M-H]−, 635, 551, 535 (100), 373, 311, 289, 191, 163 | Catechin derivative | UV/MS |
| 19 | 22.51 | 290, 306 | 573 [M-H]−, 411 (100), 163, 145, 117 | p-Coumaroyl malonyldihexoside | [54,58] |
| 20 | 23.01 | 290, 306 | 593 (100) [M-H]−, 409, 341, 163, 145, 117 | p-Coumaroyl diacetylhexoside derivative | [54,58] |
| 21 | 23.44 | 256, 265sh, 301sh, 354 | 505 [M-H]−, 463, 301 (100), 245, 271, 255, 243 | Quercetin acetylhexoside | UV/MS |
| 22 | 24.32 | 256, 265sh, 301sh, 354 | 579 (100) [M-H]−, 447, 301, 285, 271, 255, 243, 227 | Quercetin pentosyldeoxyhexoside | UV/MS |
| 23 | 24.45 | 256, 265sh, 301sh, 354 | 447 (100) [M-H]−, 445, 341, 301, 285, 271, 255, 243, 189 | Quercetin 3-O-rhamnoside (quercitrin) | St. |
| 24 | 24.79 | 286, 306 | 411 (100) [M-H]−, 341, 163, 145, 117 | p-Coumaroyl malonylhexoside | [54,58] |
| 25 | 25.15 | 286, 306 | 409 (100) [M-H]−, 357, 315, 307, 163, 145, 119 | p-Coumaroyl diacetylhexoside | [54,58] |
| 26 | 25.46 | 286, 306 | 411 (100) [M-H]−, 249, 231, 163, 146, 145, 119, 117 | p-Coumaroyl malonylhexoside | [54,58] |
| 27 | 25.90 | 286, 306 | 411 (100) [M-H]−, 249, 163, 145, 119, 117 | p-Coumaroyl malonylhexoside | [54,58] |
| 28 | 26.38 | 286, 306 | 823 (100) [M-H]−, 489, 441, 435, 411, 341 | p-Coumaroyl malonylhexoside dimer | [54,58] |
| 29 | 27.81 | 256, 265sh, 301sh, 354 | 591 (100) [M-H]−, 489, 447, 341, 301, 271, 243, 189 | Quercetin hydroxy- methylglutaryl deoxyhexoside | [65] |
3.6.2. Quantitative LC-MS Analysis of the Extracts
| Compounds | Extracts | |||
|---|---|---|---|---|
| M | HAE | UAE | MAE | |
| Quantity ** (%, g of compound/100 g of dried extract) | ||||
| 1 * | 0.003 ± 0.000** | 0.004 ± 0.000 | 0.002 ± 0.000 | 0.003 ± 0.000 |
| 2 *** | 0.022 ± 0.007 | 0.046 ± 0.007 | 0.027 ± 0.004 | 0.020 ± 0.002 |
| 3 | 0.023 ± 0.003 | 0.017 ± 0.001 | 0.019 ± 0.000 | 0.023 ± 0.000 |
| 4 | 0.020 ± 0.000 | 0.033 ± 0.003 | 0.015 ± 0.000 | 0.021 ± 0.001 |
| 5 | 6.963 ± 0.039 | 12.661 ± 0.108 | 4.967 ± 0.016 | 6.439 ± 0.017 |
| 6 | 0.152 ± 0.008 | 0.255 ± 0.009 | 0.106 ± 0.003 | 0.128 ± 0.006 |
| 7 | 0.040 ± 0.000 | 0.560 ± 0.050 | 0.030 ± 0.000 | 0.008 ± 0.000 |
| 8 | 0.003 ± 0.000 | 0.004 ± 0.000 | 0.001 ± 0.000 | 0.002 ± 0.000 |
| 9 | <LOQ | <LOQ | <LOQ | <LOQ |
| 10 | 0.023 ± 0.006 | 0.011 ± 0.000 | 0.013 ± 0.004 | 0.009 ± 0.000 |
| 11 | 0.012 ± 0.000 | 0.021 ± 0.000 | 0.008 ± 0.000 | 0.007 ± 0.000 |
| 12 | <LOQ | <LOQ | <LOQ | <LOQ |
| 13 | 0.617 ± 0.020 | 1.132 ± 0.023 | 0.459 ± 0.011 | 0.584 ± 0.020 |
| 14 | <LOQ | <LOQ | <LOQ | <LOQ |
| 15 | 1.057 ± 0.014 | 2.060 ± 0.124 | 0.774 ± 0.031 | 0.987 ± 0.044 |
| 16 | 1.989 ± 0.057 | 3.545 ± 0.116 | 1.417 ± 0.038 | 1.399 ± 0.021 |
| 17 | 2.458 ± 0.008 | 4.707 ± 0.053 | 1.787 ± 0.009 | 2.068 ± 0.020 |
| 18 | <LOQ | <LOQ | <LOQ | <LOQ |
| 19 | 0.082 ± 0.007 | 0.151 ± 0.007 | 0.063 ± 0.004 | 0.082 ± 0.006 |
| 20 | 0.124 ± 0.010 | 0.184 ± 0.012 | tr. | 0.136 ± 0.008 |
| 21 | 0.559 ± 0.018 | 1.073 ± 0.020 | 0.426 ± 0.005 | 0.523 ± 0.011 |
| 22 | 0.188 ± 0.006 | 0.276 ± 0.025 | 0.144 ± 0.003 | 0.191 ± 0.007 |
| 23 | 0.440 ± 0.001 | 0.753 ± 0.076 | 0.312 ± 0.029 | 0.422 ± 0.034 |
| 24 | 0.005 ± 0.000 | 0.023 ± 0.009 | 0.011 ± 0.003 | 0.018 ± 0.006 |
| 25 | 0.230 ± 0.007 | 0.447 ± 0.011 | 0.171 ± 0.003 | 0.228 ± 0.068 |
| 26 | 0.028 ± 0.006 | 0.062 ± 0.001 | 0.023 ± 0.003 | 0.031 ± 0.004 |
| 27 | 0.547 ± 0.011 | 1.046 ± 0.020 | 0.407 ± 0.003 | 0.526 ± 0.008 |
| 28 | tr. | tr. | tr. | tr. |
| 29 | 0.081 ± 0.006 | 0.170 ± 0.010 | 0.065 ± 0.004 | 0.088 ± 0.006 |
3.7. The Impact of the Parameters on the Antioxidant Capacity of Bilberry Extracts
3.8. Antimicrobial Activity of the Extracts
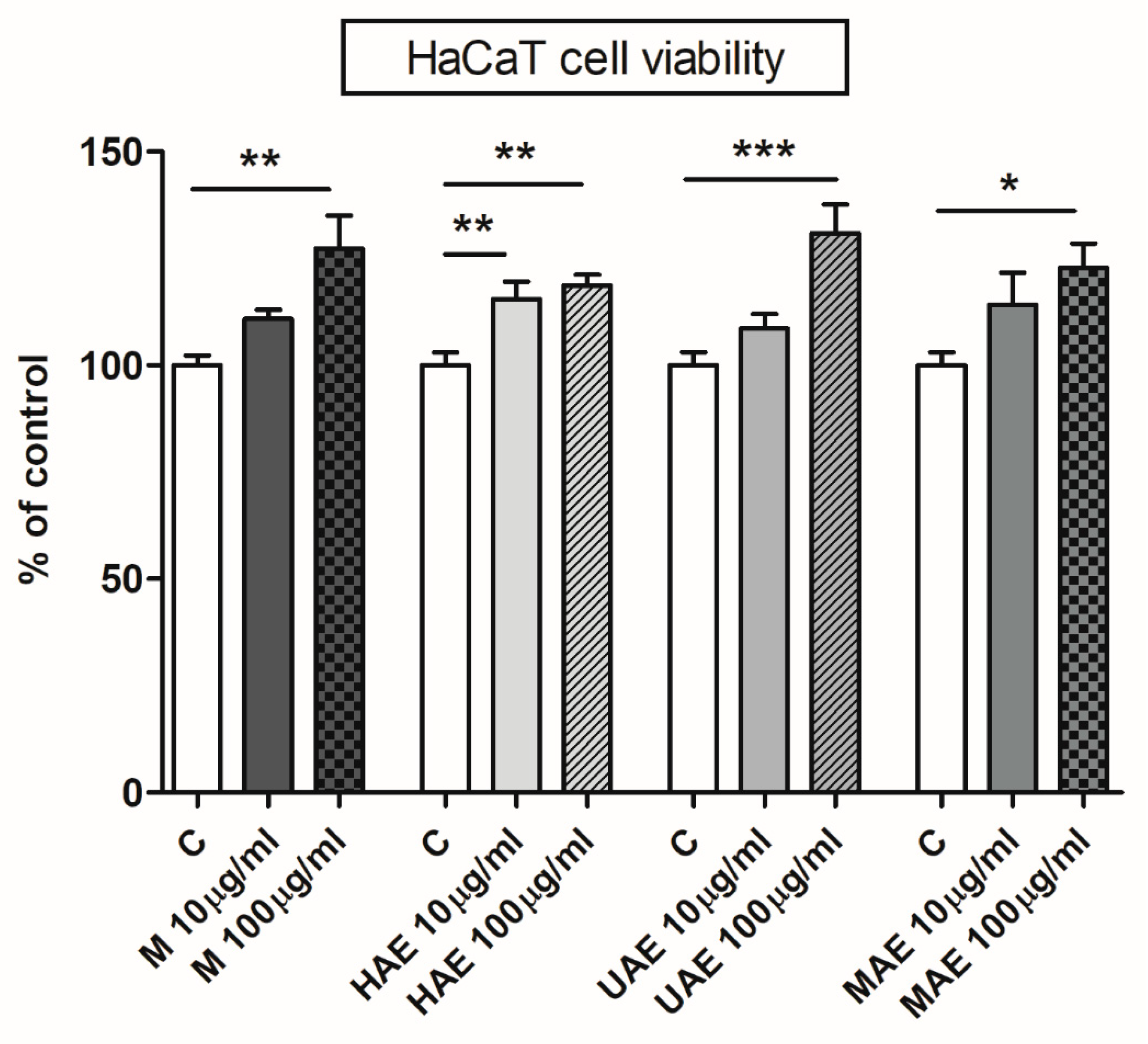
3.9. The Effect of Bilberry Extracts on the Viability of HaCaT Cells
3.10. In Vitro Anti-Inflammatory Effects of Bilberry Extracts
3.11. Physical Characteristics of Bilberry Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vrancheva, R.; Ivanov, I.; Dincheva, I.; Badjakov, I.; Pavlov, A. Triterpenoids and other non-polar compounds in leaves of wild and cultivated Vaccinium species. Plants 2021, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.D.; Krogfelt, K.A.; Cornett, C.; Hansen, S.H.; Christensen, S.B. Hydrophilic carboxylic acids and iridoid glycosides in the juice of American and European cranberries (Vaccinium macrocarpon and V. oxycoccos), lingonberries (V. vitis-idaea), and blueberries (V. myrtillus). J. Agric. Food Chem. 2002, 50, 6871–6874. [Google Scholar] [CrossRef] [PubMed]
- Riihinen, K.; Jaakola, L.; Karenlampi, S.; Hohtola, A. Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and ‘northblue’ blueberry (Vaccinium corymbosum x V. angustifolium). Food Chem. 2008, 110, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Ștefănescu, R.; Laczkó-Zöld, E.; Ősz, B.E.; Vari, C.E. An updated systematic review of Vaccinium myrtillus leaves: Phytochemistry and pharmacology. Pharmaceutics 2023, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Cignarella, A.; Nastasi, M.; Cavalli, E.; Puglisi, L. Novel lipid-lowering properties of Vaccinium myrtillus L. leaves, a traditional antidiabetic treatment, in several models of rat dyslipidaemia: A comparison with ciprofibrate. Thromb. Res. 1996, 84, 311–322. [Google Scholar] [CrossRef]
- Helmstädter, A.; Schuster, N. Vaccinium myrtillus as an antidiabetic medicinal plant–research through the ages. Pharmazie 2010, 65, 315–321. [Google Scholar] [CrossRef]
- Arora, R.; Sharma, J.; Selvamurthy, W.; Shivashankara, A.R.; Mathew, N.; Baliga, M.S. Asian medicinal remedies for alleviating aging effects. In Bioactive Food as Dietary Interventions for the Aging Population; Watson, R.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 305–320. [Google Scholar]
- Ieri, F.; Martini, S.; Innocenti, M.; Mulinacci, N. Phenolic distribution in liquid preparations of Vaccinium myrtillus L. and Vaccinium vitis idaea L. Phytochem. Anal. 2013, 24, 467–475. [Google Scholar] [CrossRef]
- Kenichi, I. Anti-aging effects of bilberry extract through up-regulation of GABA synthesizing enzyme (GAD) in dermal fibroblasts. Frag. J. 2006, 34, 48–53. [Google Scholar]
- Vuleta, G.; Milić, J.; Savić, S. Farmaceutska tehnologija. In Faculty of Pharmacy; University of Belgrade: Belgrade, Serbia, 2012. (In Serbian) [Google Scholar]
- Jovanović, A.; Petrović, P.; Djordjević, V.; Zdunić, G.; Šavikin, K.; Bugarski, B. Polyphenols extraction from plant sources. Lek. Sirovine 2017, 37, 45–49. [Google Scholar] [CrossRef]
- Nobre, B.P.; Gouveia, L.; Matos, P.G.S.; Cristino, A.F.; Palavra, A.F.; Mendes, R.L. Supercritical extraction of lycopene from tomato industrial wastes with ethane. Molecules 2012, 17, 8397–8407. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical fluid extraction of carotenoids from vegetable waste matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef]
- D’Alessandro, L.G.; Dimitrov, K.; Vauchel, P.; Nikov, I. Kinetics of ultrasound assisted extraction of anthocyanins from Aronia melanocarpa (black chokeberry) wastes. Chem. Eng. Res. Des. 2014, 92, 1818–1826. [Google Scholar] [CrossRef]
- Jovanović, A.; Vajić, J.; Mijin, D.; Zdunić, G.; Šavikin, K.; Branković, S.; Kitić, D.; Bugarski, B. Polyphenol extraction in microwave reactor using by-product of Thymus serpyllum L. and biological potential of the extract. Res. J. Med. Plant. 2022, 31, 100417. [Google Scholar] [CrossRef]
- Meullemiestre, A.; Petitcolas, E.; Maache-Rezzoug, Z.; Chemat, F.; Rezzoug, S.A. Impact of ultrasound on solid–liquid extraction of phenolic compounds from maritime pine sawdust waste. Kinetics, optimization and large scale experiments. Ultrason. Sonochem. 2016, 28, 230–239. [Google Scholar] [CrossRef]
- Rabelo, R.S.; MacHado, M.T.C.; Martínez, J.; Hubinger, M.D. Ultrasound assisted extraction and nanofiltration of phenolic compounds from artichoke solid wastes. J. Food Eng. 2016, 178, 170–180. [Google Scholar] [CrossRef]
- Prakash, M.J.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Microwave assisted extraction of pectin from waste Citrullus lanatus fruit rinds. Carbohydr. Polym. 2014, 101, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Seixas, F.L.; Fukuda, D.L.; Turbiani, F.R.B.; Garcia, P.S.; Petkowicz, C.L.D.O.; Jagadevan, S.; Gimenes, M.L. Extraction of pectin from passion fruit peel (Passiflora edulis F. Flavicarpa) by microwave-induced heating. Food Hydrocoll. 2014, 38, 186–192. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Dan, M.; Boran, S. The use of bilberry leaves (Vaccinium myrtillus L.) as an efficient adsorbent for cationic dye removal from aqueous solutions. Polymers 2022, 14, 978. [Google Scholar] [CrossRef]
- Regulations on the Quality of Tea, Herbal Tea, and Their Products of the Republic of Serbia, “Official Gazette of the Republic of Serbia”, No. 4 of January 20, 2012. Available online: https://pravno-informacioni-sistem.rs/eli/rep/sgrs/ministarstva/pravilnik/2012/4/2 (accessed on 30 April 2024). (in Serbian).
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels: I. Solvent extraction method. Sep. Purif. Technol. 2006, 48, 182–188. [Google Scholar] [CrossRef]
- Xi, J.; Yan, L. Optimization of pressure-enhanced solid-liquid extraction of flavonoids from Flos Sophorae and evaluation of their antioxidant activity. Sep. Purif. Technol. 2017, 175, 170–176. [Google Scholar] [CrossRef]
- Priyanthi, C.; Sivakanesan, R. The total antioxidant capacity and the total phenolic content of rice using water as a solvent. Int. J. Food Sci. 2021, 2021, 5268584. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 7, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Milutinović, V.; Niketić, M.; Ušjak, L.; Nikolić, D.; Krunić, A.; Zidorn, C.; Petrović, S. Methanol extracts of 28 Hieracium species from the Balkan Peninsula-comparative LC-MS analysis, chemosystematic evaluation of their flavonoid and phenolic acid profiles and antioxidant potentials. Phytochem. Anal. 2018, 29, 30–47. [Google Scholar] [CrossRef] [PubMed]
- International Conference on Harmonisation (ICH) 2005. ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2 (R1). ICH: Geneva, Switzerland. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 30 April 2024).
- Li, L.; Yang, Y.; Hou, X.; Gu, D.; Ba, H.; Abdulla, R.; Wu, G.; Xin, X.; Aisa, H.A. Bioassay-guided separation and purification of water-soluble antioxidants from Carthamus tinctorius L. by combination of chromatographic techniques. Sep. Purif. Technol. 2013, 104, 200–207. [Google Scholar] [CrossRef]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Petrović, P.; Ivanović, K.; Jovanović, A.; Simović, M.; Milutinović, V.; Kozarski, M.; Petković, M.; Cvetković, A.; Klaus, A.; Bugarski, B. The impact of puffball autolysis on selected chemical and biological properties: Puffball extracts as potential ingredients of skin-care products. Arch. Biol. Sci. 2019, 71, 721–733. [Google Scholar] [CrossRef]
- Borotová, P.; Galovičová, L.; Valková, V.; Ďúranová, H.; Vuković, N.; Vukić, M.; Babošová, M.; Kačániová, M. Biological activity of essential oil from Foeniculum vulgare. Acta Hortic. Regiotect. 2021, 24, 148–152. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar]
- Bojić-Trbojević, Ž.; Božić, M.; Vićovac, L.J. Steroid hormones modulate galectin-1 in the trophoblast HTR-8/SVneocell line. Arch. Biol. Sci. 2008, 60, 11–23. [Google Scholar] [CrossRef]
- Yesmin, S.; Paul, A.; Naz, T.; Rahman, A.B.M.A.; Akhter, S.F.; Wahed, M.I.I.; Emran, T.B.; Siddiqu, S.A. Membrane stabilization as a mechanism of the anti-inflammatory activity of ethanolic root extract of Choi (Piper chaba). Clin. Phytosci. 2020, 6, 59. [Google Scholar] [CrossRef]
- Anosike, C.A.; Obidoa, O.; Ezeanyika, L.U. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). Daru 2012, 20, 76. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Coelho, N.; Romano, A. Thymus lotocephalus wild plants and in vitro cultures produce different profiles of phenolic compounds with antioxidant activity. Food Chem. 2012, 135, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Pompeu, D.R.; Silva, E.M.; Rogez, H. Optimisation of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using response surface methodology. Bioresour. Technol. 2009, 100, 6076–6082. [Google Scholar] [CrossRef]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef]
- Luthria, D. Influence of experimental conditions on the extraction of phenolic compounds from parsley (Petroselinum crispum) flakes using a pressurized liquid extractor. Food Chem. 2008, 107, 745–752. [Google Scholar] [CrossRef]
- Paz, J.W.; Márquez, D.M.; Ávila, G.M.; Cerda, R.B.; Aguilar, C. Ultrasound-assisted extraction of polyphenols from native plants in the Mexican desert. Ultrason. Sonochem. 2015, 22, 474–481. [Google Scholar] [CrossRef]
- Torres-Rivero, K.; Bastos-Arrieta, J.; Fiol, N.; Florido, A. Metal and metal oxide nanoparticles: An integrated perspective of the green synthesis methods by natural products and waste valorization: Applications and challenges. Compr. Anal. Chem. 2021, 94, 433–469. [Google Scholar] [CrossRef]
- Šeremet, D.; Štefančić, M.; Petrović, P.; Kuzmić, S.; Doroci, S.; Mandura Jarić, A.; Vojvodić Cebin, A.; Pjanović, R.; Komes, D. Development, characterization and incorporation of alginate-plant protein covered liposomes containing ground ivy (Glechoma hederacea L.) extract into candies. Foods 2022, 11, 1816. [Google Scholar] [CrossRef]
- Verma, N.; Rakhra, M.; Wasim Bhatt, M.; Garg, U. Engineering technology characterization of source solution for ZnO and their data analytics effect with Aloe vera extract. Neurosci. Inform. 2022, 2, 100015. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, M.; Yadav, A.; Yadav, J.P. Impact of spatial and climatic conditions on phytochemical diversity and in vitro antioxidant activity of Indian Aloe vera (L.) Burm.f. S. Afr. J. Bot. 2017, 111, 50–59. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Lević, S.; Kalušević, A.; Špoljarić, I.; Djordjević, V.; Komes, D.; Mršić, G.; Nedović, V. Efficiency assessment of natural biopolymers as encapsulants of green tea (Camellia sinensis L.) bioactive compounds by spray drying. Food Bioprocess Technol. 2015, 8, 2444–2460. [Google Scholar]
- Jovanović, A.; Lević, S.; Pavlović, V.; Marković, S.; Pjanović, R.; Djordjević, V.; Nedović, V.; Bugarski, B. Freeze versus spray drying for dry wild thyme (Thymus serpyllum L.) extract formulations: The impact of gelatin as a coating material. Molecules 2021, 26, 3933. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Heneczkowski, M.; Kopacz, M.; Nowak, D.; Kuźniar, A. Infrared spectrum analysis of some flavonoids. Acta Pol. Pharm. 2001, 58, 415–420. [Google Scholar] [PubMed]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Brezoiu, A.M.; Deaconu, M.; Mitran, R.A.; Prelipcean, A.M.; Matei, C.; Berger, D. Optimisation of polyphenols extraction from wild bilberry leaves—Antimicrobial properties and stability studies. Molecules 2023, 28, 5795. [Google Scholar] [CrossRef]
- Gil-Martínez, L.; Aznar-Ramos, M.J.; del Carmen Razola-Diaz, M.; Mut-Salud, N.; Falcón-Piñeiro, A.; Baños, A.; Enrique Guillamón, E.; Gómez-Caravaca, A.M.; Verardo, V. Establishment of a sonotrode extraction method and evaluation of the antioxidant, antimicrobial and anticancer potential of an optimized Vaccinium myrtillus L. leaves extract as functional ingredient. Foods 2023, 12, 1688. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. A comparison of fruit quality parameters of wild bilberry (Vaccinium myrtillus L.) growing at different locations. J. Agric. Food. Chem. 2015, 95, 776–785. [Google Scholar] [CrossRef]
- Bljajić, K.; Petlevski, R.; Vujić, L.; Čačić, A.; Šoštarić, N.; Jablan, J.; Saraiva de Carvalho, I.; Zovko Končić, M. Chemical composition, antioxidant and α-glucosidase-inhibiting activities of the aqueous and hydroethanolic extracts of Vaccinium myrtillus leaves. Molecules 2017, 22, 703. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanović Nedeljković, S.; Radan, M.; Ćujić Nikolić, N.; Mutavski, Z.; Krgović, N.; Marković, S.; Stević, T.; Živković, J.; Šavikin, K. Microencapsulated bilberry and chokeberry leaf extracts with potential health benefits. Plants 2023, 12, 3979. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liimatainen, J.; Alanne, A.L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Bujor, O.C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal variations of the phenolic constituents in bilberry (Vaccinium myrtillus L.) leaves, stems and fruits, and their antioxidant activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Hellström, J.; Sinkkonen, J.; Karonen, M.; Mattila, P. Isolation and structure elucidation of procyanidin oligomers from saskatoon berries (Amelanchier alnifolia). J. Agric. Food Chem. 2007, 55, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Rue, E.A.; Rush, M.D.; van Breemen, R.B. Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, S.; Kumar, B. LC-MS identification of proanthocyanidins in bark and fruit of six Terminalia species. Nat. Prod. Commun. 2018, 13, 1934578X1801300511. [Google Scholar] [CrossRef]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B.; Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany, 1970. [Google Scholar]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Ek, S.; Kartimo, H.; Mattila, S.; Tolonen, A. Characterization of phenolic compounds from lingonberry (Vaccinium vitis-idaea). J. Agric. Food Chem. 2006, 54, 9834–9842. [Google Scholar] [CrossRef]
- Hokkanen, J.; Mattila, S.; Jaakola, L.; Pirttilä, A.M.; Tolonen, A. Identification of polyphenols from lingonberry (Vaccinium vitis-idaea L.), bilberry (Vaccinium myrtillus L.) and hybrid bilberry (Vaccinium x intermedium Ruthe L.) leaves. J. Agric. Food Chem. 2009, 57, 9437–9447. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.M.; Khoo, K.S.; Weng Sit, N. Interactions between plant extracts and cell viability indicators during cytotoxicity testing: Implications for ethnopharmacological studies. Trop. J. Pharm. Res. 2015, 14, 1991–1998. [Google Scholar] [CrossRef]
- Farooq, S.; Mir, S.A.; Shah, M.A.; Manickavasagan, A. Extraction techniques. In Plant Extracts: Applications in the Food Industry; Mir, S.A., Manickavasagan, A., Shah, M.A., Eds.; Academic Press: London, UK, 2022; pp. 23–37. [Google Scholar]
- Jurinjak Tušek, A.; Benković, M.; Valinger, D.; Jurina, T.; Belščak-Cvitanović, A.; Gajdoš Kljusurić, J. Optimizing bioactive compounds extraction from different medicinal plants and prediction through nonlinear and linear models. Ind. Crop. Prod. 2018, 126, 449–458. [Google Scholar] [CrossRef]
- Saad Suliman, R.; Asmani, F.; Saad Suliman, M.; Hussain, M.; Khan, J.; Kaleemullah, M.; Bin Othman, N.; Tofigh, A.; Yusuf, E. A new approach for predicting antioxidant property of herbal extracts. Int. J. Pharmacogn. Pharm. Res. 2015, 7, 166–174. [Google Scholar]
- Gao, M.; Huang, W.; Moytri, R.C.; Liu, C. Microwave assisted extraction of scutellarin from Erigeron breviscapus Hand-Mazz and its determination by high-performance liquid chromatography. Anal. Chim. Acta 2007, 591, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Dent, M.; Dragović-Uzelac, V.; Penić, M.; Brnčić, M.; Bosiljkov, T.; Levaj, B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol. Biotech. 2013, 51, 84–91. [Google Scholar]
- Fecka, I.; Turek, S. Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: Thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chem. 2008, 108, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.; Đorđević, V.; Zdunić, G.; Pljevljakušić, D.; Šavikin, K.; Gođevac, D.; Bugarski, B. Optimization of the extraction process of polyphenols from Thymus serpyllum L, herb using maceration, heat- and ultrasound-assisted extraction. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef]
- Michel, P.; Dobrowolska, A.; Kicel, A.; Owczarek, A.; Bazylko, A.; Granica, S.; Piwowarski, J.P.; Olszewska, M.A. Polyphenolic profile, antioxidant and anti-inflammatory activity of eastern teaberry (Gaultheria procumbens L.) leaf extracts. Molecules 2014, 19, 20498–20520. [Google Scholar] [CrossRef]
- Vajić, U.-J.; Grujić-Milanović, J.; Živković, J.; Šavikin, K.; Gođevac, D.; Miloradović, Z.; Bugarski, B.; Mihailović-Stanojević, N. Optimization of extraction of stinging nettle leaf phenolic compounds using response surface methodology. Ind. Crop. Prod. 2015, 74, 912–917. [Google Scholar] [CrossRef]
- Friedman, M.; Kim, S.-Y.; Lee, S.-J.; Han, G.-P.; Han, J.-S.; Lee, K.-R.; Kozuke, N. Distribution of catechins, theaflavins, caffeine, and theobromine in 77 teas consumed in the United States. J. Food Sci. 2005, 70, 550–559. [Google Scholar] [CrossRef]
- Vergara-Salinas, J.; Pérez-Jiménez, J.; Torres, J.L.; Agosin, E.; Pérez-Correa, J. Effects of temperature and time on polyphenolic content and antioxidant activity in the pressurized hot water extraction of deodorized thyme (Thymus vulgaris). J. Agri. Food Chem. 2012, 60, 10920–10929. [Google Scholar] [CrossRef] [PubMed]
- Simić, V.; Rajković, K.; Stojičević, S.; Veličković, D.; Nikolić, N.; Lazić, M.; Karabegović, I. Optimization of microwave-assisted extraction of total polyphenolic compounds from chokeberries by response surface methodology and artificial neural network. Sep. Purif. Technol. 2016, 160, 89–97. [Google Scholar] [CrossRef]
- Miron, T.L.; Plaza, M.; Bahrim, G.; Ibáñez, E.; Herrero, M. Chemical composition of bioactive pressurized extracts of Romanian aromatic plants. J. Chromatogr. A 2011, 1218, 4918–4927. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weller, C. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Horžić, D.; Režek Jambrak, A.; Belščak-Cvitanović, A.; Komes, D.; Lelas, V. Comparison of conventional and ultrasound assisted extraction techniques of yellow tea and bioactive composition of obtained extracts. Food Bioprocess Technol. 2012, 5, 2858–2870. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical composition and biological activities of the nord-west Romanian wild bilberry (Vaccinium myrtillus L.) and lingonberry (Vaccinium vitis-idaea L.) leaves. Antioxidants 2020, 9, 495. [Google Scholar] [CrossRef]
- Lisperguer, J.; Saravia, Y.; Vergara, E. Structure and thermal behavior of tannins from acacia dealbata bark and their reactivity toward formaldehyde. J. Chil. Chem. Soc. 2016, 61, 3188–3190. [Google Scholar] [CrossRef]
- Adamczyk, B.; Simon, J.; Kitunen, V.; Adamczyk, S.; Smolander, A. Tannins and their complex interaction with different organic nitrogen compounds and enzymes: Old paradigms versus recent advances. Chem. Open 2017, 6, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Daré, R.G.; Nakamura, C.V.; Ximenes, V.F.; Lautenschlager, S.O.S. Tannic acid, a promising anti-photoaging agent: Evidences of its antioxidant and anti-wrinkle potentials, and its ability to prevent photodamage and MMP−1 expression in L929 fibroblasts exposed to UVB. Free Radic. Biol. Med. 2020, 160, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Hu, T.; Xu, Z.; Jin, L.; McAllister, T.A.; Acharya, S.; Zeller, W.E.; Mueller-Harvey, I.; Wang, Y. Composition and protein precipitation capacity of condensed tannins in purple prairie clover (Dalea purpurea Vent.). Front. Plant Sci. 2021, 12, 715282. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Pavan. Determination of total protein content and soluble sugar in wheat crop. Int. J. Sci. Res. Sci. Technol. 2013, 3, 81–86. [Google Scholar]
- Lin, L.; Perets, A.; Harel, Y.; Varma, D.; Li, M.; Lazarovici, P.; Woerdeman, D.L.; Lelkes, P.I. Alimentary ‘green’ proteins as electrospun scaffolds for skin regenerative engineering. J. Tissue Eng. Regen. Med. 2013, 7, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Bremner, D.; Burgess, A.; Chand, R. The chemistry of ultrasonic degradation of organic compounds. Curr. Org. Chem. 2011, 15, 168–177. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Sözgen Başkan, K.; Erçağ, E.; Esin Çelik, S.; Baki, S.; Yıldız, L.; Karamanc, S.; Apak, R. A comprehensive review of CUPRAC methodology. Anal. Meth. 2011, 11, 2439–2453. [Google Scholar] [CrossRef]
- Ginova, A.; Mihalev, K.; Kondakova, V. Antioxidant capacity of petals and leaves from different rose (Rosa damascena mill.) plantations in Bulgaria. Int. J. Pure Appl. Biosci. 2013, 1, 38–43. [Google Scholar]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Wu, L. Effect of chlorogenic acid on antioxidant activity of Flos Lonicerae extracts. J. Zhejiang Univ. Sci. B 2007, 8, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Kwang, J.L.; You Chang, O.; Won Kyung, C.; Jin, Y.M. Antioxidant and anti-inflammatory activity determination of one hundred kinds of pure chemical compounds using offline and online screening HPLC assay. J. Evid. Based Complement. Altern. Med. 2015, 2015, 165457. [Google Scholar] [CrossRef] [PubMed]
- Jayameena, P.; Sivakumari, K.; Ashok, K.; Rajesh, S. In vitro antiinflammatory (membrane stabilization) and antioxidant potential of rutin. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2018, 4, 265–274. [Google Scholar] [CrossRef]
- Lang, G.P.; Han, Y.Y. Rutin ameliorates H2O2-induced oxidative stress injury in HaCaT cells via the Nrf2-regulated pathway. J. Evol. Biochem. Physiol. 2022, 58, 1389–1400. [Google Scholar] [CrossRef]
- Xiao, Z.; He, L.; Hou, X.; Wei, J.; Ma, X.; Gao, Z.; Yuan, Y.; Xiao, J.; Li, P.; Yue, T. Relationships between structure and antioxidant capacity and activity of glycosylated flavonols. Foods 2021, 10, 849. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Q.; Wang, T.; Liu, J.; Chen, D. Comparison of the antioxidant effects of quercitrin and isoquercitrin: Understanding the role of the 6″-OH group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef]
- Jayachandran, M.; Wu, Z.; Ganesan, K.; Khalid, S.; Chung, S.M.; Xu, B. Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2019, 303, 62–69. [Google Scholar] [CrossRef]
- Ha, A.T.; Rahmawati, L.; You, L.; Hossain, M.A.; Kim, J.H.; Cho, J.Y. Anti-inflammatory, antioxidant, moisturizing, and antimelanogenesis effects of quercetin 3-O-β-D-glucuronide in human keratinocytes and melanoma cells via activation of NF-κB and AP-1 pathways. Int. J. Mol. Sci. 2021, 23, 433. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Esin Karademir, S.; Erçağ, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef]
- Sadowska, B.; Paszkiewicz, M.; Podsędek, A.; Redzynia, M.; Różalska, B. Vaccinium myrtillus leaves and Frangula alnus bark derived extracts as potential antistaphylococcal agents. Acta Biochim. Pol. 2014, 61, 163–169. [Google Scholar] [CrossRef]
- Tian, Y.; Puganen, A.; Alakomi, H.-L.; Uusitupa, A.; Saarela, M.; Yang, B. Antioxidative and antibacterial activities of aqueous ethanol extracts of berries, leaves, and branches of berry plants. Food Res. Int. 2018, 106, 291–303. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Vučić, D.M.; Petković, M.R.; Rodić-Grabovac, B.B.; Stefanović, O.; Vasić, S.M.; Čomić, L.J. Antibacterial and antioxidant activities of bilberry (Vaccinium myrtillus L.) in vitro. Afr. J. Microbiol. Res. 2013, 7, 5130–5136. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr. 2022, 29, 943911. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Novović, K.; Malešević, M.; Dinić, M.; Stojković, D.; Jovčić, B.; Soković, M. Polyphenols as inhibitors of antibiotic resistant bacteria—Mechanisms underlying rutin interference with bacterial virulence. Pharmaceuticals 2022, 15, 385. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Q.; Zhang, H.; He, Y.; Wang, R.; Lu, X. Isolation, identification, and antibacterial activities of flavonoids from jujube (Zizipus jujuba Mill.) fruit. Int. J. Fruit Sci. 2023, 23, 51–61. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Al-Mana, F.A.; Al-Yafrsi, M.A. Antioxidant and biological activities of Acacia saligna and Lawsonia inermis natural populations. Plants 2020, 9, 908. [Google Scholar] [CrossRef]
- Nohynek, L.J.; Alakomi, H.-L.; Kähkönen, M.P.; Heinonen, M.; Helander, I.M.; Oksman-Caldentey, K.-M.; Puupponen-Pimiä, R.H. Berry phenolics: Antimicrobial properties and mechanisms of action against severe human pathogens. Nutr. Cancer 2006, 54, 18–32. [Google Scholar] [CrossRef]
- Deters, A.; Dauer, A.; Schnetz, E.; Fartasch, M.; Hensel, A. High molecular compounds (polysaccharides and proanthocyanidins) from Hamamelis virginiana bark: Influence on human skin keratinocyte proliferation and differentiation and influence on irritated skin. Phytochemistry 2001, 58, 949–958. [Google Scholar] [CrossRef]
- Werdin, F.; Tennenhaus, M.; Schaller, H.E.; Rennekampff, H.O. Evidence-based management strategies for treatment of chronic wounds. Eplasty 2009, 9, e19. [Google Scholar]
- Wang, R.; Lechtenberg, M.; Sendker, J.; Petereit, F.; Deters, A.; Hensel, A. Wound-healing plants from TCM: In vitro investigations on selected TCM plants and their influence on human dermal fibroblasts and keratinocytes. Fitoterapia 2013, 84, 308–317. [Google Scholar] [CrossRef]
- Gu, I.; Brownmiller, C.; Stebbins, N.B.; Mauromoustakos, A.; Howard, L.; Lee, S.O. Berry phenolic and volatile extracts inhibit pro-inflammatory cytokine secretion in LPS-stimulated RAW264.7 cells through suppression of NF-κB signaling pathway. Antioxidants 2020, 9, 871. [Google Scholar] [CrossRef]
- Felgus-Lavefve, L.; Howard, L.; Adams, S.H.; Baum, J.I. The effects of blueberry phytochemicals on cell models of inflammation and oxidative stress. Adv. Nutr. 2022, 13, 1279–1309. [Google Scholar] [CrossRef]
- Su, X.; Zhang, J.; Wang, H.; Xu, J.; He, J.; Liu, L.; Zhang, T.; Chen, R.; Kang, J. Phenolic acid profiling, antioxidant, and anti-inflammatory activities, and miRNA regulation in the polyphenols of 16 blueberry samples from China. Molecules 2017, 22, 312. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, L.; Liu, X.; Yin, Z. Effect of chlorogenic acid on LPS-induced COX-2 in vivo and vitro. J. Henan Normal Univ. Sci. Ed. 2017, 45, 49–52. [Google Scholar] [CrossRef]
- Kim, S.J.; Um, J.Y.; Lee, J.Y. Anti-inflammatory activity of hyperoside through the suppression of nuclear factor-κB activation in mouse peritoneal macrophages. Am. J. Chin. Med. 2011, 39, 171–181. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Yao, Y.; Ru, G.; Lan, C.; Li, L.; Huang, T. Protective effect of isoquercitrin on UVB-induced injury in HaCaT cells and mice skin through anti-inflammatory, antioxidant, and regulation of MAPK and JAK2-STAT3 pathways. Photochem. Photobiol. 2024, 1–12. [Google Scholar] [CrossRef]
- Won, Y.S.; Kim, J.H.; Lizardo, R.C.M.; Min, H.J.; Cho, H.D.; Hong, S.M.; Seo, K.I. The flavonol isoquercitrin promotes mitochondrial-dependent apoptosis in SK-Mel-2 melanoma cell via the PI3K/AKT/mTOR pathway. Nutrients 2020, 12, 3683. [Google Scholar] [CrossRef]
- Islam, M.; Al Shamsh Prottay, A.; Sultana, I.; Al Faruq, A.; Bappi, M.H.; Akbor, M.S.; Asha, A.I.; Hossen, M.M.; Machado, P.E.M.; Secundo Junior, I.J.; et al. Phytochemical screening and evaluation of antioxidant, anti-inflammatory, antimicrobial, and membrane-stabilizing activities of different fractional extracts of Grewia nervosa (Lour.) Panigrahi. Food Biosci. 2023, 54, 102933. [Google Scholar] [CrossRef]
- Zamora-Mendoza, L.; Vispo, S.N.; De Lima, L.; Mora, J.R.; Machado, A.; Alexis, F. Hydrogel for the controlled delivery of bioactive components from extracts of Eupatorium glutinosum Lam. leaves. Molecules 2023, 28, 1591. [Google Scholar] [CrossRef]
- Bonarska-Kujawa, D.; Cyboran-Mikołajczyk, S.; Kleszczyńska, H. Molecular mechanism of action of chlorogenic acid on erythrocyte and lipid membranes. Mol. Membr. Biol. 2015, 32, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Wang, G.; Wang, X.; Tang, J.; Li, C. Chlorogenic acid improves lipid membrane peroxidation and morphological changes in nitrite-induced erythrocyte model of methemoglobinemia. J. Food Biochem. 2020, 44, e13172. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Selvarajan, G. Phytochemical investigation and hypotonicity induced membrane stabilization studies of Prosopis chilensis. J. Adv. Sci. Res. 2021, 12, 180–183. [Google Scholar]
- Leal, F.P.; Maia, B.N.; Carmello, A.C.K.; Catharino, R.R.; Eberlin, M.N.; Meireles, M.A.A. Sweet basil (Ocimum basilicum) extracts obtained by supercritical fluid extraction (SFE): Global yields, chemical composition, antioxidant activity, and estimation of the cost of manufacturing. Food Bioprocess Technol. 2008, 1, 326–338. [Google Scholar]
- Teofilović, B. Biochemical and Chemical Characterization of Basil Extracts and Influence of Pharmaceutical Technological Formulations on the Glycemic, Lipid and Oxidative Status in Experimental Animals. Ph.D. Thesis, University of Novi Sad, Novi Sad, Serbia, 2016. [Google Scholar]
- Čutović, N.; Batinić, P.; Marković, T.; Radanović, D.; Marinković, A.; Bugarski, B.; Jovanović, A. The influence of extraction time and solid-to-solvent ratio on physicochemical properties of Satureja montana extracts. Chem. Ind. 2023, 77, 251–263. [Google Scholar] [CrossRef]
- Mladenović, J.; Đurić, M.; Šekularac, G.; Brković, D.; Stepanović, J.; Mašković, P.; Bošković Rakočević, L. Determination of the content of bioactive components in different extracts of Portulaca oleracea L. Acta Agric. Serb. 2018, 23, 223–231. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciu, S.; Amariei, S.; Gutt, G. Correlations between density, viscosity, surface tension and ultrasonic velocity of different mono- and di-saccharides. J. Mol. Liq. 2015, 207, 145–151. [Google Scholar] [CrossRef]
- Singh, M.P.; Singh, R.K. Correlation between ultrasonic velocity, surface tension, density and viscosity of ionic liquids. Fluid Ph. Equilib. 2011, 304, 1–6. [Google Scholar] [CrossRef]
- Boros, D.; Marquardt, R.R.; Slominski, B.A.; Guenter, W. Extract viscosity as an indirect assay for water-soluble pentosan content in rye. Cereal Chem. 1993, 70, 575–580. [Google Scholar]
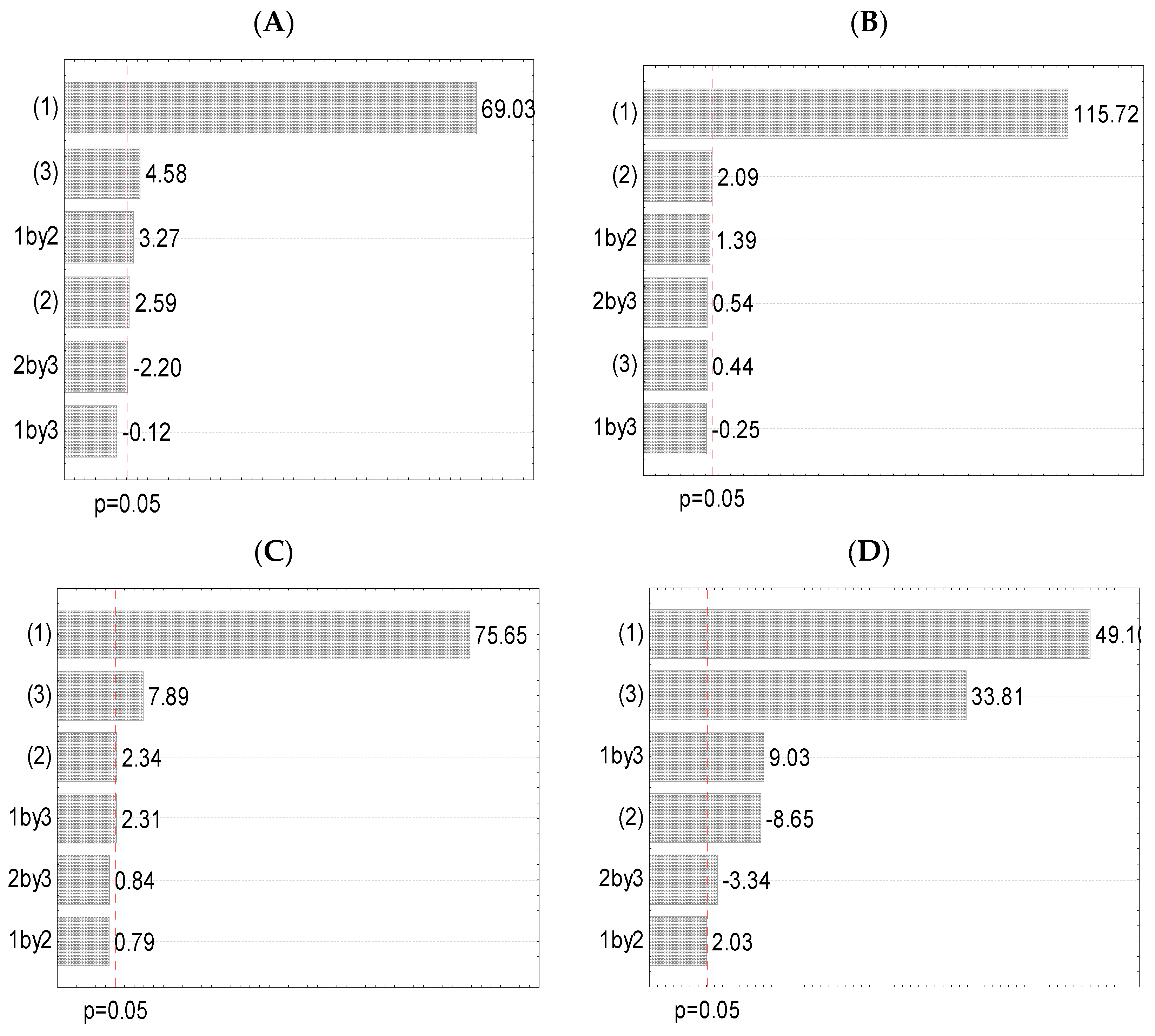


| Compound | Regression Equations | r2 | Linear Range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| Caffeic acid | y = 13,866.0695x − 2.1700 | 0.9999 | 0.07–1.75 | 0.022 | 0.066 |
| Chlorogenic acid | y = 7359.9706x + 21.8506 | 0.9999 | 0.05–4.00 | 0.015 | 0.044 |
| Procyanidin B2 | SIM: y = 43,695,889.4939x + 227,090.6258 | 0.9995 | 0.08–0.80 | 0.043 | 0.132 |
| Quercetin 3-O-galactoside | y = 2755.8034x + 22.2383 | 0.9999 | 0.02–4.00 | 0.010 | 0.060 |
| Quercetin 7-O-glucoside | y = 3450.5525x + 19.4631 | 0.9999 | 0.07–4.00 | 0.020 | 0.103 |
| Quercetin 3-O-glucoside | SIM: y = 11,180,730.8564x + 263,073.4506 | 0.9971 | 0.08–0.80 | 0.043 | 0.132 |
| Quercetin 3-O-glucuronide | SIM: y = 24,101,774.7157x + 872,524.4889 | 0.9944 | 0.08–0.80 | 0.043 | 0.132 |
| Quercetin 3-O-rhamnoside | y = 2941.0244x + 7.5674 | 0.9999 | 0.05–3.50 | 0.030 | 0.082 |
| p-Coumaric acid | y = 16,845.1037x + 47.4654 | 0.9998 | 0.08–1.75 | 0.026 | 0.078 |
| TPC (mg Gallic Acid Equivalent/g of Dried Herbal Matrix) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Level | Extraction Procedures | |||||||
| Maceration | HAE | UAE | MAE | ||||||
| Plant material:medium ratio (g/mL) | 1:10 | 19.96 ± 0.70 c* | 20.01 ± 0.41 c | 20.44 ± 0.52 c | 19.26 ± 0.60 c | ||||
| 1:20 | 38.32 ± 0.88 b | 37.92 ± 0.79 b | 38.27 ± 0.75 b | 38.57 ± 0.40 b | |||||
| 1:30 | 54.64 ± 1.10 a | 55.65 ± 0.23 a | 55.62 ± 1.70 a | 56.48 ± 1.68 a | |||||
| Extraction medium | 50% EtOH | 55.47 ± 1.28 a | 55.55 ± 0.35 a | 56.49 ± 0.40 a | 57.06 ± 1.90 a | ||||
| 70% EtOH | 55.71 ± 1.48 a | 56.10 ± 0.68 a | 56.04 ± 0.84 a | 56.26 ± 1.03 a | |||||
| Water | 47.43 ± 1.73 b | 48.20 ± 1.93 b | 47.54 ± 1.68 b | 49.95 ± 0.98 b | |||||
| Period ** (min) | 30 | 53.37 ± 0.34 b | 15 | 56.37 ± 1.23 a | 5 | 53.79 ± 1.06 b | 1 | 41.55 ± 0.42 c | |
| 45 | 55.38 ± 0.51 a | 30 | 55.43 ± 1.32 a | 15 | 56.82 ± 1.17 a | 2 | 56.48 ± 1.68 a | ||
| 60 | 55.16 ± 0.70 a | 45 | 55.47 ± 1.02 a | 30 | 56.73 ± 0.89 a | 3 | 49.95 ± 1.12 b | ||
| Ratio * | Extraction Medium | Period | Ratio (g/mL) | Extraction Medium | Period (min) | TPC (mg Gallic Acid Equivalents/g) | |
|---|---|---|---|---|---|---|---|
| Maceration | |||||||
| Design | Factor levels | Measured | Predicted | ||||
| −1 | −1 | −1 | 1:20 | 50% EtOH | 30 | 38.32 ± 0.62 | 38.09 |
| −1 | 1 | −1 | 1:20 | 70% EtOH | 30 | 38.23 ± 0.25 | 38.45 |
| 1 | −1 | 1 | 1:30 | 50% EtOH | 45 | 55.80 ± 0.23 | 56.02 |
| 1 | 1 | 1 | 1:30 | 70% EtOH | 45 | 55.38 ± 0.18 | 55.16 |
| −1 | −1 | 1 | 1:20 | 50% EtOH | 45 | 39.50 ± 0.50 | 39.72 |
| −1 | 1 | 1 | 1:20 | 70% EtOH | 45 | 39.27 ± 0.30 | 39.05 |
| 1 | −1 | −1 | 1:30 | 50% EtOH | 30 | 53.37 ± 0.24 | 53.59 |
| 1 | 1 | −1 | 1:30 | 70% EtOH | 30 | 54.71 ± 0.55 | 54.49 |
| Heat extraction | |||||||
| Measured | Predicted | ||||||
| −1 | −1 | −1 | 1:20 | 50% EtOH | 15 | 37.92 ± 0.56 | 37.95 |
| −1 | −1 | 1 | 1:20 | 70% EtOH | 15 | 38.00 ± 0.50 | 37.92 |
| −1 | 1 | −1 | 1:30 | 50% EtOH | 30 | 55.93 ± 0.11 | 56.07 |
| −1 | 1 | 1 | 1:30 | 70% EtOH | 30 | 55.43 ± 0.22 | 55.45 |
| 1 | −1 | −1 | 1:20 | 50% EtOH | 30 | 38.00 ± 0.20 | 37.97 |
| 1 | −1 | 1 | 1:20 | 70% EtOH | 30 | 38.13 ± 0.51 | 38.16 |
| 1 | 1 | −1 | 1:30 | 50% EtOH | 15 | 54.54 ± 0.16 | 54.51 |
| 1 | 1 | 1 | 1:30 | 70% EtOH | 15 | 55.10 ± 0.48 | 54.96 |
| Ultrasound extraction | |||||||
| Measured | Predicted | ||||||
| −1 | −1 | −1 | 1:20 | 50% EtOH | 5 | 36.87 ± 0.15 | 37.07 |
| −1 | 1 | −1 | 1:20 | 70% EtOH | 5 | 37.43 ± 0.21 | 37.23 |
| 1 | −1 | 1 | 1:30 | 50% EtOH | 15 | 55.62 ± 1.20 | 55.82 |
| 1 | 1 | 1 | 1:30 | 70% EtOH | 15 | 55.90 ± 0.30 | 55.73 |
| −1 | −1 | 1 | 1:20 | 50% EtOH | 15 | 38.36 ± 0.41 | 38.15 |
| −1 | 1 | 1 | 1:20 | 70% EtOH | 15 | 38.50 ± 0.62 | 38.70 |
| 1 | −1 | −1 | 1:30 | 50% EtOH | 5 | 53.88 ± 0.13 | 53.68 |
| 1 | 1 | −1 | 1:30 | 70% EtOH | 5 | 54.00 ± 0.10 | 54.20 |
| Microwave extraction | |||||||
| Measured | Predicted | ||||||
| −1 | −1 | −1 | 1:20 | 50% EtOH | 3 | 41.00 ± 1.1 | 40.53 |
| −1 | −1 | 1 | 1:20 | 70% EtOH | 3 | 37.83 ± 0.28 | 38.30 |
| −1 | 1 | −1 | 1:30 | 50% EtOH | 2 | 56.48 ± 0.15 | 56.10 |
| −1 | 1 | 1 | 1:30 | 70% EtOH | 2 | 52.52 ± 0.42 | 52.99 |
| 1 | −1 | −1 | 1:20 | 50% EtOH | 2 | 38.57 ± 0.30 | 39.04 |
| 1 | −1 | 1 | 1:20 | 70% EtOH | 2 | 35.27 ± 0.64 | 34.80 |
| 1 | 1 | −1 | 1:30 | 50% EtOH | 3 | 45.55 ± 0.32 | 45.02 |
| 1 | 1 | 1 | 1:30 | 70% EtOH | 3 | 44.50 ± 0.54 | 44.03 |
| Sample | Concentration (µg/mL) | Inhibition of Hemolysis at 54 °C (%, Mean ± SD) | Inhibition of Hemolysis in Hypotonic Solution (%, Mean ± SD) |
|---|---|---|---|
| Maceration | 50 | 55.7 ± 1.8 f,1* | 51.9 ± 1.1 h,2 |
| 100 | 72.7 ± 0.7 c,1 | 73.1 ± 1.7 ef,1 | |
| 250 | 81.5 ± 1.5 b,1 | 80.8 ± 1.2 c,1 | |
| HAE | 50 | 62.8 ± 1.0 e,1 | 57.4 ± 0.6 g,2 |
| 100 | 74.6 ± 1.5 c,2 | 78.5 ± 1.4 d,1 | |
| 250 | 83.9 ± 1.7 b,2 | 86.1 ± 1.0 b,1 | |
| UAE | 50 | 52.7 ± 1.1 g,1 | 52.9 ± 0.9 h,1 |
| 100 | 70.6 ± 0.8 d,2 | 75.9 ± 1.2 de,1 | |
| 250 | 82.5 ± 1.2 b,2 | 85.6 ± 1.4 b,1 | |
| MAE | 50 | 57.2 ± 1.0 f,1 | 49.8 ± 1.5 h,2 |
| 100 | 72.6 ± 1.1 c,1 | 71.3 ± 0.6 f,1 | |
| 250 | 82.9 ± 1.0 b,1 | 84.4 ± 1.8 b,1 | |
| Diclofenac (control) | 75 | 91.0 ± 0.5 a,2 | 93.9 ± 0.8 a,1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elferjane, M.R.; Milutinović, V.; Jovanović Krivokuća, M.; Taherzadeh, M.J.; Pietrzak, W.; Marinković, A.; Jovanović, A.A. Vaccinium myrtillus L. Leaf Waste as a Source of Biologically Potent Compounds: Optimization of Polyphenol Extractions, Chemical Profile, and Biological Properties of the Extracts. Pharmaceutics 2024, 16, 740. https://doi.org/10.3390/pharmaceutics16060740
Elferjane MR, Milutinović V, Jovanović Krivokuća M, Taherzadeh MJ, Pietrzak W, Marinković A, Jovanović AA. Vaccinium myrtillus L. Leaf Waste as a Source of Biologically Potent Compounds: Optimization of Polyphenol Extractions, Chemical Profile, and Biological Properties of the Extracts. Pharmaceutics. 2024; 16(6):740. https://doi.org/10.3390/pharmaceutics16060740
Chicago/Turabian StyleElferjane, Muna Rajab, Violeta Milutinović, Milica Jovanović Krivokuća, Mohammad J. Taherzadeh, Witold Pietrzak, Aleksandar Marinković, and Aleksandra A. Jovanović. 2024. "Vaccinium myrtillus L. Leaf Waste as a Source of Biologically Potent Compounds: Optimization of Polyphenol Extractions, Chemical Profile, and Biological Properties of the Extracts" Pharmaceutics 16, no. 6: 740. https://doi.org/10.3390/pharmaceutics16060740
APA StyleElferjane, M. R., Milutinović, V., Jovanović Krivokuća, M., Taherzadeh, M. J., Pietrzak, W., Marinković, A., & Jovanović, A. A. (2024). Vaccinium myrtillus L. Leaf Waste as a Source of Biologically Potent Compounds: Optimization of Polyphenol Extractions, Chemical Profile, and Biological Properties of the Extracts. Pharmaceutics, 16(6), 740. https://doi.org/10.3390/pharmaceutics16060740








