Effects of Surface IR783 Density on the In Vivo Behavior and Imaging Performance of Liposomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
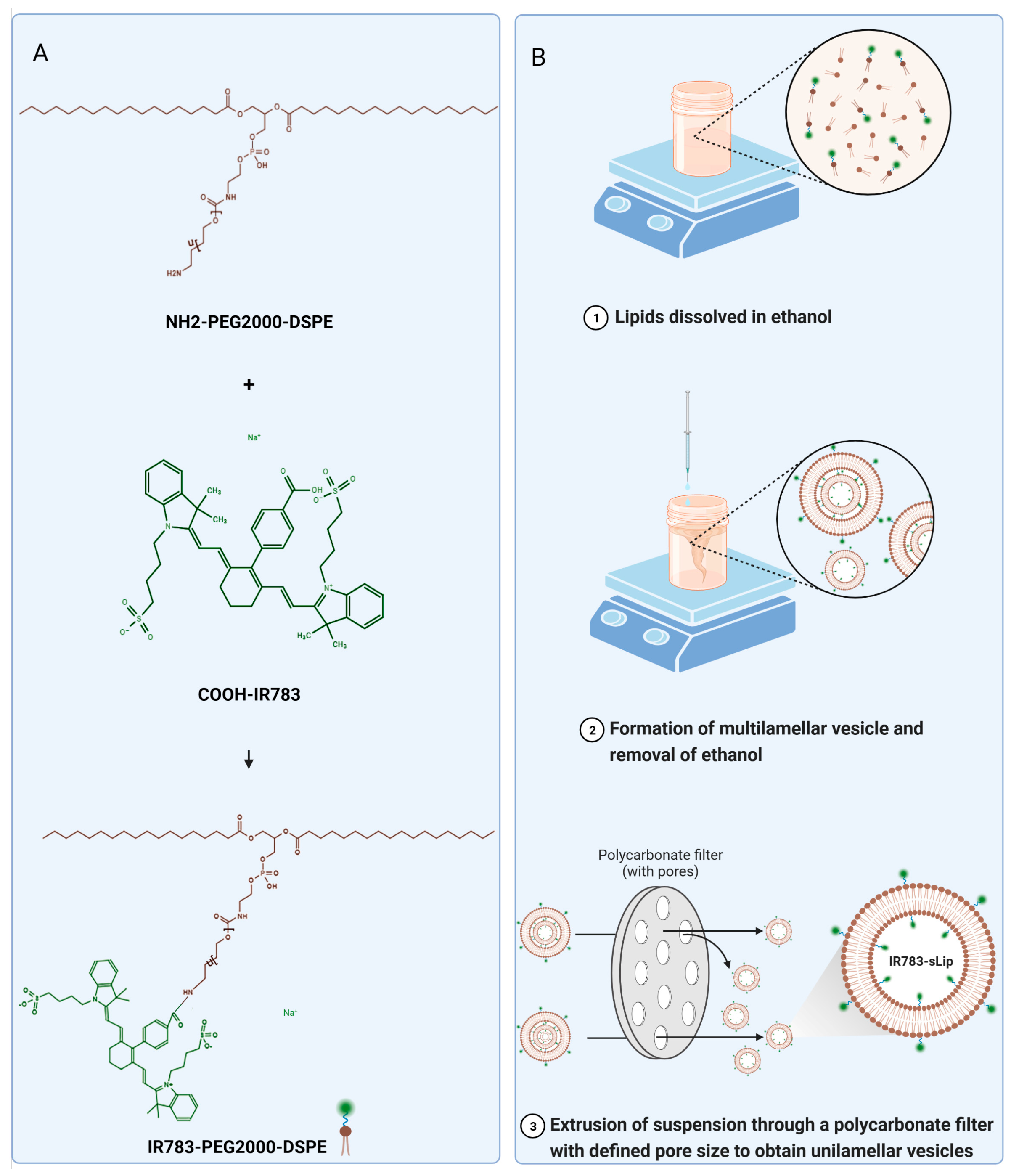
2.2. Synthesis of IR783-PEG2000-DSPE
2.3. Preparation and Characterization of Liposomes (sLip, sLip/DiI, IR783-sLip, sLip/FAM, and IR783-sLip/FAM)
2.4. Characterization of Protein Corona In Vitro and In Vivo
2.5. Western Blot Analysis
2.6. Cytotoxicity Analysis
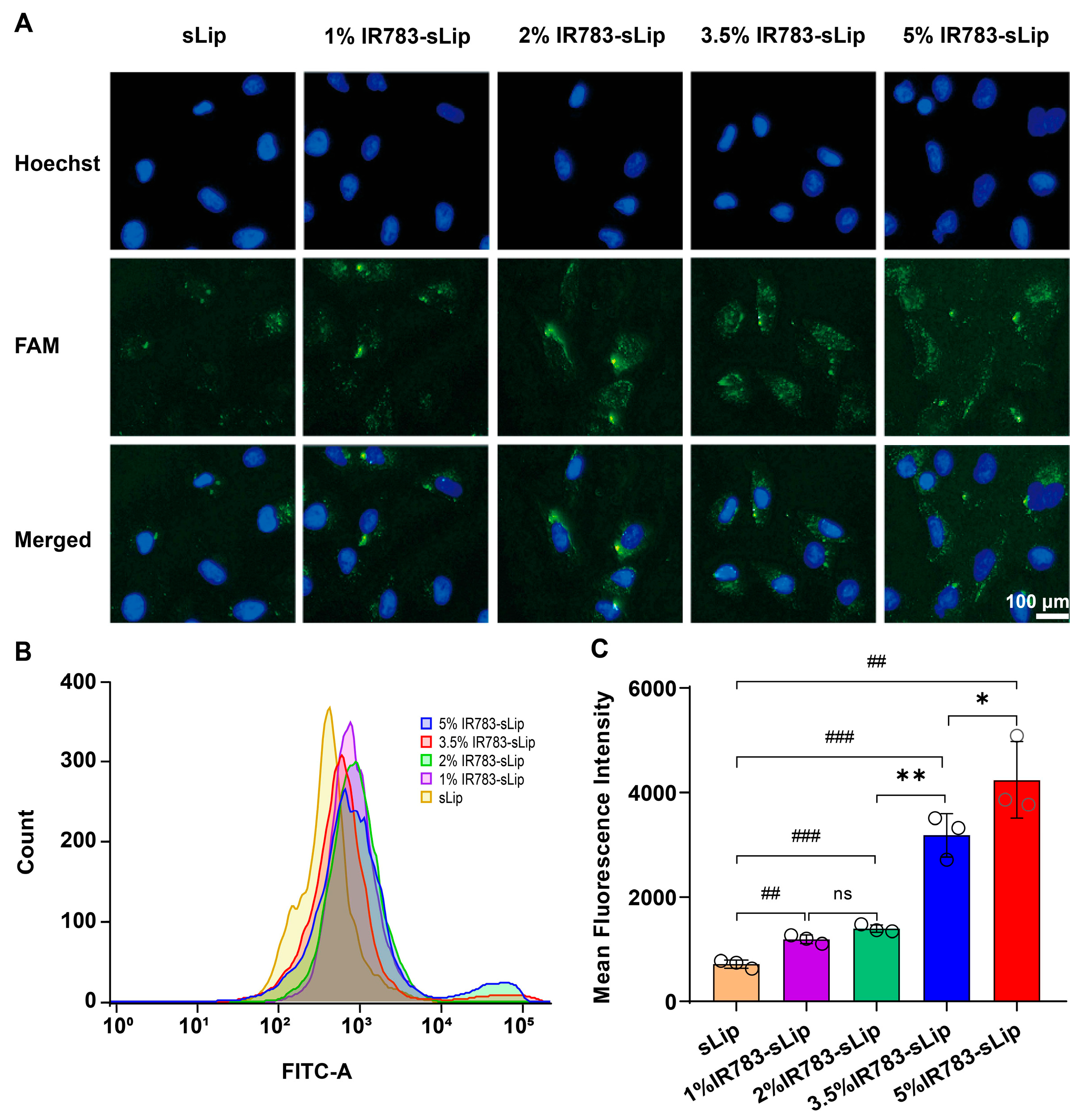
2.7. Cellular Uptake
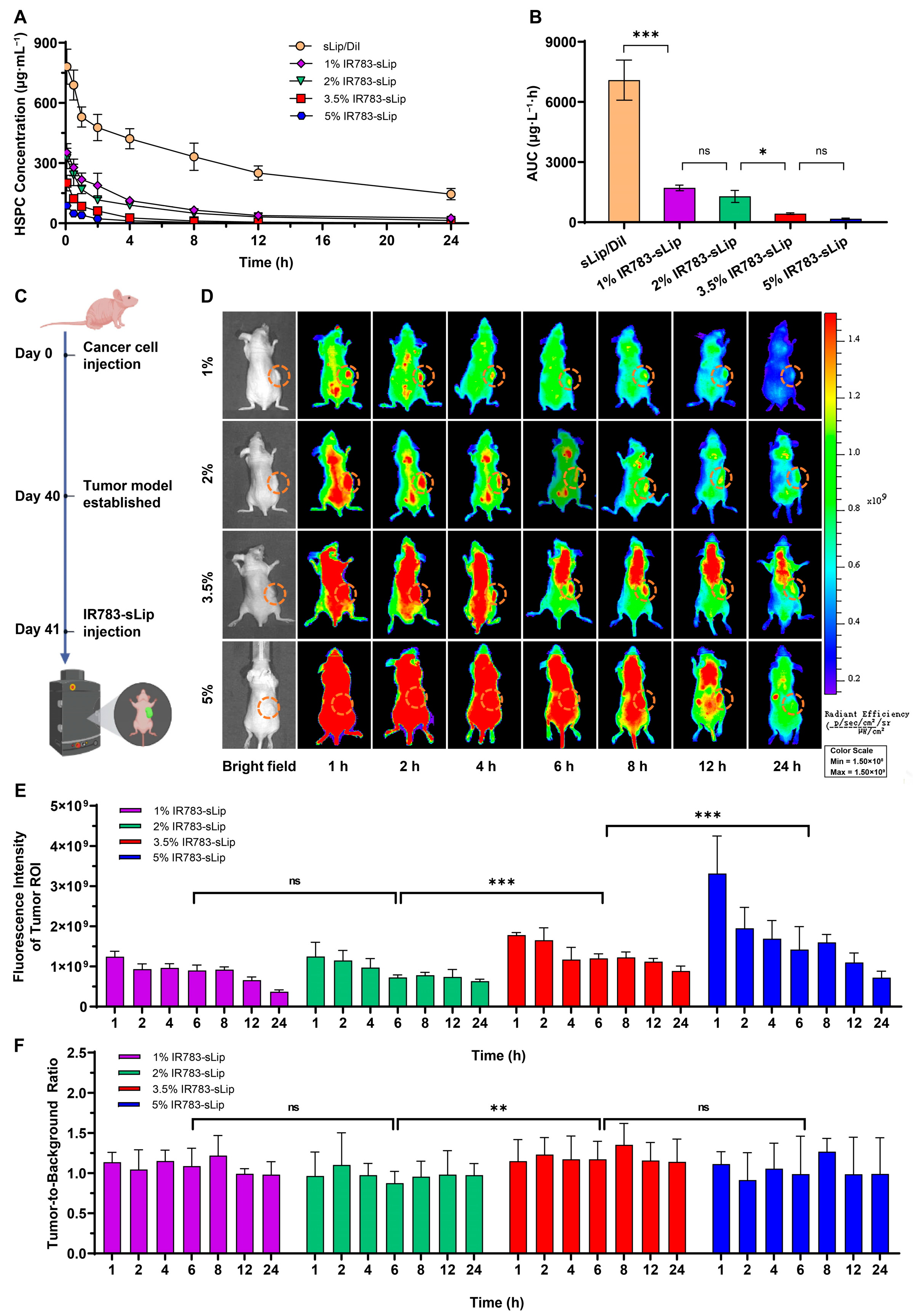
2.8. Pharmacokinetic Evaluation
2.9. Subcutaneous Tumor Model
2.10. In Vivo and Ex Vivo Fluorescence Imaging
2.11. In Vivo Safety Evaluation
2.12. Statistical Analysis
3. Results
3.1. Characterization of IR783-sLip
3.2. Dense IR783 Modification Enhances the Liposomal Cellular Uptake Efficiency
3.3. IR783 Modification Impacts the Liposomal Pharmacokinetic Profile
3.4. IR783 Modification Density Affects the Tumor-to-Background Ratio
3.5. IR783 Modification Density Affects the Distribution of Liposomes
3.6. IR783 Modification Density Correlates with Protein Corona Absorption on Liposomes
3.7. Safety Evaluation of IR783-sLip
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.; Barick, K.C.; Hassan, P.A. Recent advances in active targeting of nanomaterials for anticancer drug delivery. Adv. Colloid Interface Sci. 2021, 296, 102509. [Google Scholar] [CrossRef]
- Beh, C.Y.; Prajnamitra, R.P.; Chen, L.L.; Hsieh, P.C. Advances in biomimetic nanoparticles for targeted cancer therapy and diagnosis. Molecules 2021, 26, 5052. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xie, J.; Tong, Y.W.; Wang, C.H. Effect of PEG conformation and particle size on the cellular uptake efficiency of nanoparticles with the HepG2 cells. J. Control. Release 2007, 118, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Kim, H.S.; Palanikumar, L.; Go, E.M.; Jana, B.; Park, S.A.; Kim, H.Y.; Kim, K.; Seo, J.K.; Kwak, S.K.; et al. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat. Commun. 2018, 9, 4548. [Google Scholar] [CrossRef] [PubMed]
- Kue, C.S.; Kamkaew, A.; Burgess, K.; Kiew, L.V.; Chung, L.Y.; Lee, H.B. Small molecules for active targeting in cancer. Med. Res. Rev. 2016, 36, 494–575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, J. Surface engineering of nanomaterials with phospholipid-polyethylene glycol-derived functional conjugates for molecular imaging and targeted therapy. Biomaterials 2020, 230, 119646. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, G. Clinically approved liposomal nanomedicines: Lessons learned from the biomolecular corona. Nanoscale 2018, 10, 4167–4172. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wei, H.; Zhang, X.; Meng, C.; Kang, G.; Ma, W.; Ma, L.; Wang, B.; Yu, C. Synthesis of polymeric micelles with dual-functional sheddable PEG stealth for enhanced tumor-targeted drug delivery. Polym. Chem. 2020, 11, 4469–4476. [Google Scholar] [CrossRef]
- Zaichik, S.; Steinbring, C.; Jelkmann, M.; Bernkop-Schnürch, A. Zeta potential changing nanoemulsions: Impact of PEG-corona on phosphate cleavage. Int. J. Pharm. 2020, 581, 119299. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.; Chandra, S. Inorganic hybrid nanoparticles in cancer theranostics: Understanding their combinations for better clinical translation. Mater. Today Chem. 2020, 18, 100381. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Yue, X.; Dai, Z. Cyanine Conjugate-Based Biomedical Imaging Probes. Adv. Healthc. Mater. 2020, 9, e2001327. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of nanoparticles in biomedical imaging. Nanoscale 2019, 11, 799–819. [Google Scholar] [CrossRef] [PubMed]
- Hilderbrand, S.A.; Weissleder, R. Near-infrared fluorescence: Application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Pysz, M.A.; Gambhir, S.S.; Willmann, J.K. Molecular imaging: Current status and emerging strategies. Clin. Radiol. 2010, 65, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, W.; Su, X.; Lu, N.; Wu, G.; Ou-Yang, L.; Dang, M.; Tao, J.; Teng, Z.J. Facile preparation of near-infrared fluorescence and magnetic resonance dual-modality imaging probes based on mesoporous organosilica nanoparticles. Colloid Interface Sci. 2019, 539, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, P.; Gong, W.; Wang, Q.; Zhou, J.; Zhu, W.-H.; Cheng, Y. Dendron-Grafted Polylysine-Based Dual-Modal Nanoprobe for Ultra-Early Diagnosis of Pancreatic Precancerosis via Targeting a Urokinase-Type Plasminogen Activator Receptor. Adv. Healthc. Mater. 2018, 7, 1700912. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yue, X.; Wang, Y.; Qian, C.; Huang, P.; Lizak, M.; Niu, G.; Wang, F.; Rong, P.; Kiesewetter, D.O.; et al. A symmetrical fluorous dendron-cyanine dyeconjugated bimodal nanoprobe for quantitative 19F MRI and NIR fluorescence bioimaging. Adv. Healthc. Mater. 2014, 3, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Usama, S.M.; Lin, C.-M.; Burgess, K. On the mechanisms of uptake of tumor-seeking cyanine dyes. Bioconjugate Chem. 2018, 29, 3886–3895. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhu, A.; Tian, Y. Functional surface engineering of C-dots for fluorescent biosensing and in vivo bioimaging. Acc. Chem. Res. 2014, 47, 20–30. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, N.; Yao, Y.; Zhang, X.; Peng, X.; Zhao, L.; Wang, J.; Peng, L.; Wang, Z.; Mochizuki, K.; et al. Breaking the nanoparticle’s dispersible limit via rotatable surface ligands. Nat. Commun. 2022, 13, 3581. [Google Scholar] [CrossRef] [PubMed]
- Moyano, D.F.; Liu, Y.; Peer, D.; Rotello, V.M. Modulation of immune response using engineered nanoparticle surfaces. Small 2016, 12, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, R.; Liu, Q.; Lin, Z.; Shi, Y.; Li, J.; Wang, L.; Li, L.; Xiao, X.; Wu, Y.J. Engineering surface patterns on nanoparticles: New insights into nano-bio interactions. Mater. Chem. B 2022, 10, 2357–2383. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, H.; He, L.; Yao, Y.; Zhou, Y.; Wu, J.; Liu, J.; Ding, J. Aptamer density dependent cellular uptake of lipid-capped polymer nanoparticles for polyvalent targeted delivery of vinorelbine to cancer cells. RSC Adv. 2015, 5, 16931–16939. [Google Scholar] [CrossRef]
- Hak, S.; Helgesen, E.; Hektoen, H.H.; Huuse, E.M.; Jarzyna, P.A.; Mulder, W.J.M.; Haraldseth, O.; Davies, C.d.L. The Effect of Nanoparticle Polyethylene Glycol Surface Density on Ligand-Directed Tumor Targeting Studied In Vivo by Dual Modality Imaging. ACS Nano 2012, 6, 5648–5658. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Huang, F.Z.; Cheng, L.F.; Zhu, Y.Q.; Hu, Q.; Li, L.; Wei, L.; Chen, D.W. GE11-modified liposomes for non-small cell lung cancer targeting: Preparation, ex vitro and in vivo evaluation. Int. J. Nanomed. 2014, 9, 921–935. [Google Scholar] [CrossRef]
- Tang, H.; Chen, X.; Rui, M.; Sun, W.; Chen, J.; Peng, J.; Xu, Y. Effects of surface displayed targeting ligand GE11 on liposome distribution and extravasation in tumor. Mol. Pharm. 2014, 11, 3242–3250. [Google Scholar] [CrossRef] [PubMed]
- Shmeeda, H.; Tzemach, D.; Mak, L.; Gabizon, A.J. Her2-targeted pegylated liposomal doxorubicin: Retention of target-specific binding and cytotoxicity after in vivo passage. J. Control. Release 2009, 136, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Shahin, M.; Soudy, R.; Aliabadi, H.M.; Kneteman, N.; Kaur, K.; Lavasanifar, A. Engineered breast tumor targeting peptide ligand modified liposomal doxorubicin and the effect of peptide density on anticancer activity. Biomaterials 2013, 34, 4089–4097. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Zhang, L.; Teply, B.A.; Mann, N.; Wang, A.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc. Natl. Acad. Sci. USA 2008, 105, 2586–2591. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, W.; Larregieu, C.A.; Cheng, M.; Yan, B.; Chu, T.; Li, H.; Mao, S.-j. Development of synthetic peptide-modified liposomes with LDL receptor targeting capacity and improved anticancer activity. Mol. Pharm. 2014, 11, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Guan, J.; Wang, M.; Long, Q.; Liu, X.; Qian, J.; Wei, X.; Lu, W.; Zhan, C.J. Natural IgM dominates in vivo performance of liposomes. J. Control. Release 2020, 319, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Li, H.; Yang, M.; Li, X.; Gao, J.; Yuan, Z. IR783 Encapsulated in TR-Conjugated Liposomes for Enhancing NIR Imaging-Guided Photothermal and Photodynamic Therapy. ChemistrySelect 2022, 7, e202202560. [Google Scholar] [CrossRef]
- Maitani, Y.; Igarashi, S.; Sato, M.; Hattori, Y. Cationic liposome (DC-Chol/DOPE = 1:2) and a modified ethanol injection method to prepare liposomes, increased gene expression. Int. J. Pharm. 2007, 342, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guan, J.; Jiang, Z.; Yang, Y.; Liu, J.; Hua, W.; Mao, Y.; Li, C.; Lu, W.; Qian, J.; et al. Brain-targeted drug delivery by manipulating protein corona functions. Nat. Commun. 2019, 10, 3561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, W.; Wen, H.; Wu, E.; Ding, T.; Gu, J.; Lv, Z.; Zhan, C. Evaluation of CTB-sLip for targeting lung metastasis of colorectal cancer. Pharmaceutics 2022, 14, 868. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.J.; Anilkumar, T.S.; Chuang, C.C.; Chen, J.P. Liposomal IR-780 as a highly stable nanotheranostic agent for improved photothermal/photodynamic therapy of brain tumors by convection-enhanced delivery. Cancers 2021, 13, 3690. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Teh, C.; Ang, C.Y.; Tan, S.Y.; Luo, Z.; Qu, Q.; Zhang, Y.; Korzh, V.; Zhao, Y. Near-infrared light-absorptive stealth liposomes for localized photothermal ablation of tumors combined with chemotherapy. Adv. Funct. Mater. 2015, 25, 5602–5610. [Google Scholar] [CrossRef]
- Wang, H.; Ding, T.; Guan, J.; Liu, X.; Wang, J.; Jin, P.; Hou, S.; Lu, W.; Qian, J.; Wang, W.; et al. Interrogation of folic acid-functionalized nanomedicines: The regulatory roles of plasma proteins reexamined. ACS Nano 2020, 14, 14779–14789. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Q.; Zhao, X.; Gao, L.; Liu, M.; Pan, F.; Gao, X.; Zhan, C.; Chen, Y.; Wang, J.; Qian, J. Effects of Surface IR783 Density on the In Vivo Behavior and Imaging Performance of Liposomes. Pharmaceutics 2024, 16, 744. https://doi.org/10.3390/pharmaceutics16060744
Long Q, Zhao X, Gao L, Liu M, Pan F, Gao X, Zhan C, Chen Y, Wang J, Qian J. Effects of Surface IR783 Density on the In Vivo Behavior and Imaging Performance of Liposomes. Pharmaceutics. 2024; 16(6):744. https://doi.org/10.3390/pharmaceutics16060744
Chicago/Turabian StyleLong, Qianqian, Xinmin Zhao, Lili Gao, Mengyuan Liu, Feng Pan, Xihui Gao, Changyou Zhan, Yang Chen, Jialei Wang, and Jun Qian. 2024. "Effects of Surface IR783 Density on the In Vivo Behavior and Imaging Performance of Liposomes" Pharmaceutics 16, no. 6: 744. https://doi.org/10.3390/pharmaceutics16060744




