Therapeutic Applications of Nanoformulated Resveratrol and Quercetin Phytochemicals in Colorectal Cancer—An Updated Review
Abstract
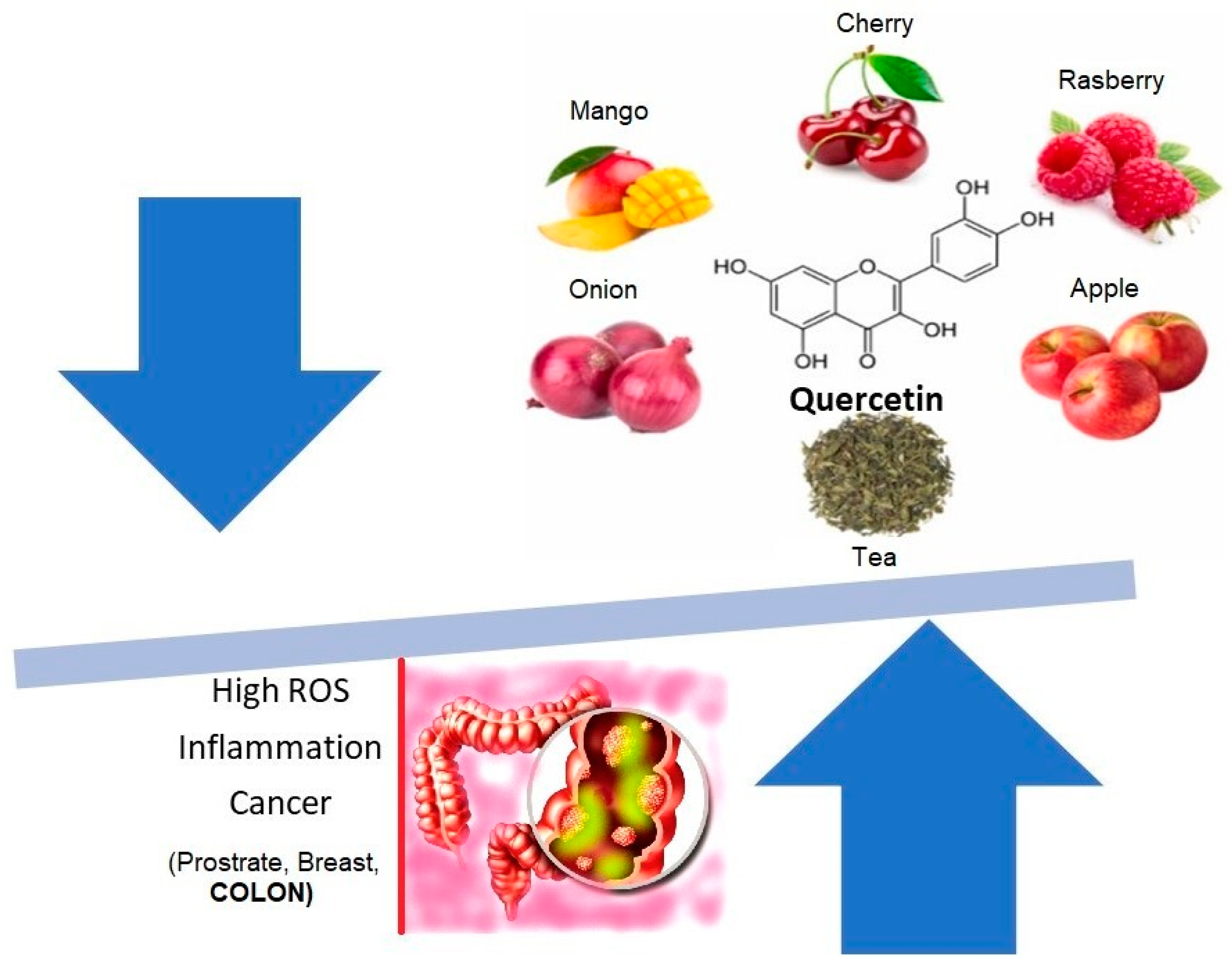
:1. Introduction
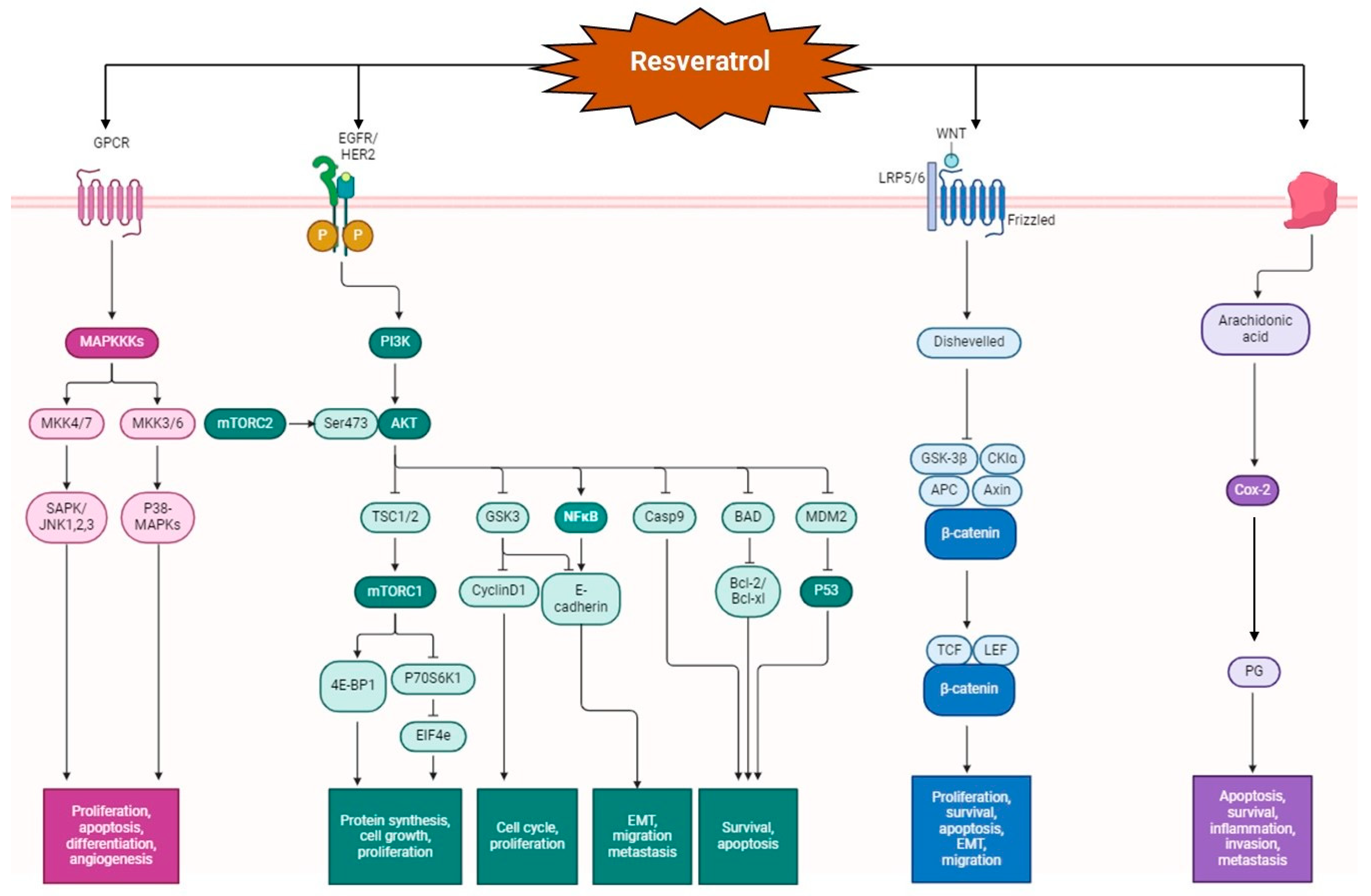
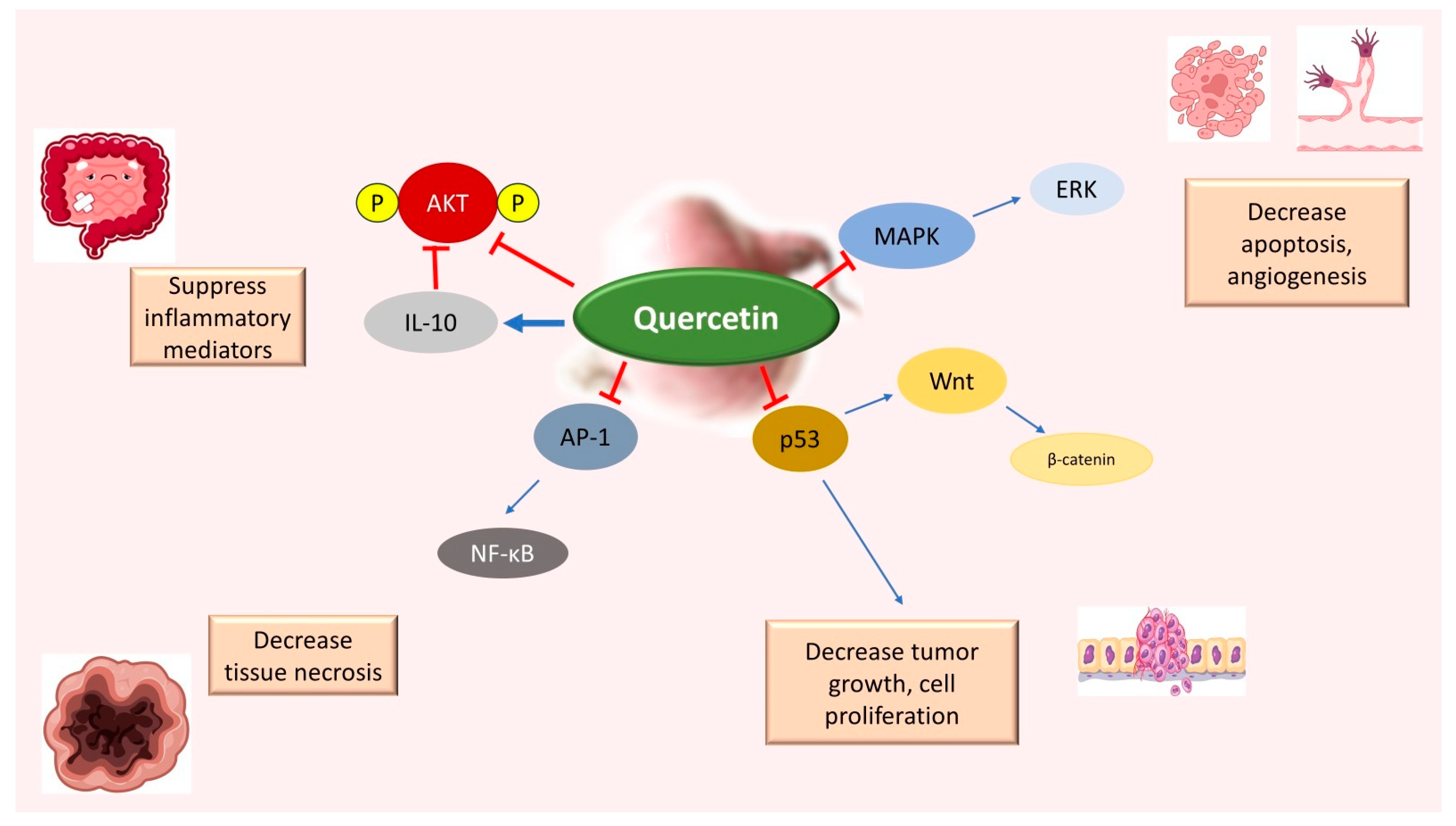
2. Resveratrol and Quercetin: Mechanism of Action in Colon Cancer
3. Challenges Regarding Oral Administration of Resveratrol and Quercetin
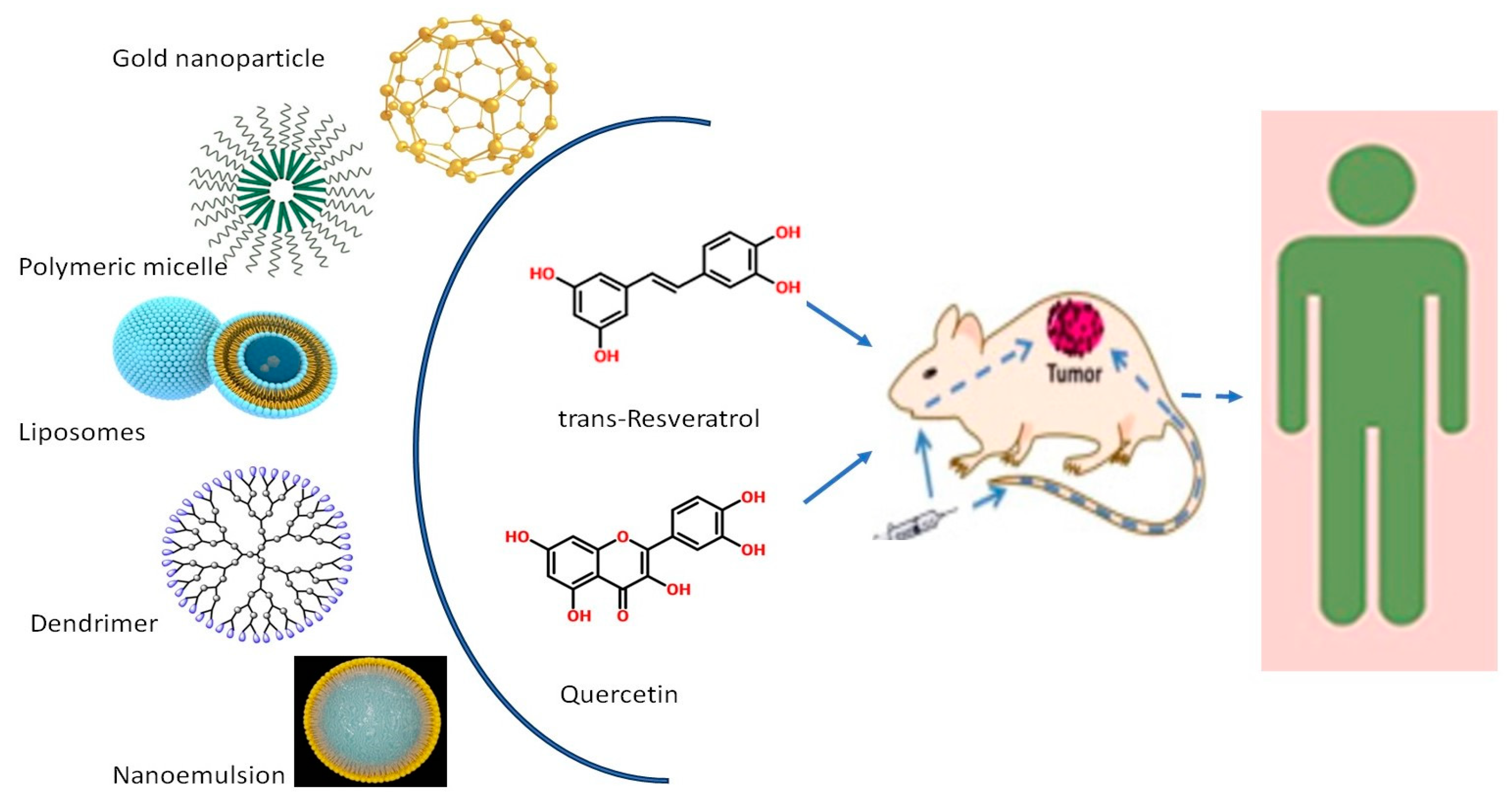
4. Chemotherapeutic Applications of Various Nanoformulations of Resveratrol and Quercetin in CRC Therapy
4.1. Liposomes
4.2. Polymeric Micelles
4.3. Gold Nanoparticles
4.4. Nanoemulsions
4.5. Dendrimers
5. Synergetic Effect of Co-Loading the Phytoconstituents in Pharmaceutical Formulations
6. Clinical Trials of Resveratrol and Quercetin in Colon Cancer
7. Toxicity and Production Feasibility
8. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global Burden of Colorectal Cancer in 2020 and 2040: Incidence and Mortality Estimates from GLOBOCAN. Gut 2022, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.M. Colorectal Cancer: An Overview. In Gastrointestinal Cancers; Exon Publications: Brisbane, Australia, 2017; pp. 1–12. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, M. Molecular Network of Colorectal Cancer and Current Therapeutic Options. Front. Oncol. 2022, 12, 852927. [Google Scholar] [CrossRef] [PubMed]
- García-Alfonso, P.; Muñoz, A.; Jiménez-Castro, J.; Jiménez-Fonseca, P.; Pericay, C.; Longo-Muñoz, F.; Reyna-Fortes, C.; Argilés-Martínez, G.; González-Astorga, B.; Gómez-Reina, M.J.; et al. Early Clinical Experience with Trifluridine/Tipiracil for Refractory Metastatic Colorectal Cancer: The ROS Study. Cancers 2021, 13, 4514. [Google Scholar] [CrossRef] [PubMed]
- Annaji, M.; Poudel, I.; Boddu, S.H.S.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. Resveratrol-Loaded Nanomedicines for Cancer Applications. Cancer Rep. 2021, 4, e1353. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, J.M. Resveratrol: Twenty Years of Growth, Development and Controversy. Biomol. Ther. 2019, 27, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ying, K.; Bai, B.; Gao, X.; Xu, Y.; Wang, H.; Xie, B. Orally Administrable Therapeutic Nanoparticles for the Treatment of Colorectal Cancer. Front. Bioeng. Biotechnol. 2021, 9, 670124. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, Dietary Sources and Bioavailability. Ann. Dell’istituto Super. Sanita 2007, 43, 348–361. [Google Scholar] [PubMed]
- Savic Gajic, I.; Savic, I.; Boskov, I.; Žerajić, S.; Markovic, I.; Gajic, D. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Black Locust (Robiniae pseudoacaciae) Flowers and Comparison with Conventional Methods. Antioxidants 2019, 8, 248. [Google Scholar] [CrossRef]
- N′ soukpoé-Kossi, C.N.; St-Louis, C.; Beauregard, M.; Subirade, M.; Carpentier, R.; Hotchandani, S.; Tajmir-Riahi, H.A. Resveratrol Binding to Human Serum Albumin. J. Biomol. Struct. Dyn. 2006, 24, 277–283. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I Randomized, Double-Blind Pilot Study of Micronized Resveratrol (SRT501) in Patients with Hepatic Metastases—Safety, Pharmacokinetics, and Pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
- Ren, X.; Wang, N.; Zhou, Y.; Song, A.; Jin, G.; Li, Z.; Luan, Y. An Injectable Hydrogel Using an Immunomodulating Gelator for Amplified Tumor Immunotherapy by Blocking the Arginase Pathway. Acta Biomater. 2021, 124, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, S.; Kilinc, O.K.; Selamoğlu, Z. Antioxidant Activity of Quercetin: A Mechanistic Review. Turk. J. Agric. Food Sci. Technol. 2016, 4, 1134–1138. [Google Scholar] [CrossRef]
- Parasuraman, S.; Anand David, A.; Arulmoli, R. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yin, O.Q.P.; Zuo, Z.; Chow, M.S.S. Pharmacokinetics and Modeling of Quercetin and Metabolites. Pharm. Res. 2005, 22, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative Stress: Role of Physical Exercise and Antioxidant Nutraceuticals in Adulthood and Aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [PubMed]
- Justino, G.C.; Santos, M.R.; Canário, S.; Borges, C.; Florêncio, M.H.; Mira, L. Plasma Quercetin Metabolites: Structure–Antioxidant Activity Relationships. Arch. Biochem. Biophys. 2004, 432, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Alrawaiq, N.; Abdullah, A. A Review of Flavonoid Quercetin: Metabolism, Bioactivity and Antioxidant Properties. Int. J. PharmTech Res. 2014, 6, 933–941. [Google Scholar]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.-K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and Bioavailability of Quercetin Glycosides in Humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef]
- Mirazimi, S.M.A.; Dashti, F.; Tobeiha, M.; Shahini, A.; Jafari, R.; Khoddami, M.; Sheida, A.H.; EsnaAshari, P.; Aflatoonian, A.H.; Elikaii, F.; et al. Application of Quercetin in the Treatment of Gastrointestinal Cancers. Front. Pharmacol. 2022, 13, 860209. [Google Scholar] [CrossRef]
- Paul, S.; Rimando, A.M.; Lee, H.J.; Ji, Y.; Reddy, B.S.; Suh, N. Anti-Inflammatory Action of Pterostilbene Is Mediated through the P38 Mitogen-Activated Protein Kinase Pathway in Colon Cancer Cells. Cancer Prev. Res. 2009, 2, 650–657. [Google Scholar] [CrossRef]
- Honari, M.; Shafabakhsh, R.; Reiter, R.J.; Mirzaei, H.; Asemi, Z. Resveratrol Is a Promising Agent for Colorectal Cancer Prevention and Treatment: Focus on Molecular Mechanisms. Cancer Cell Int. 2019, 19, 180. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Cesana, E.M.; Noonan, D. Cancer Stem Cells and the Tumor Microenvironment: Soloists or Choral Singers. Current Pharm. Biotechnol. 2011, 12, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Boghossian, S.; Hawash, A. Chemoprevention in Colorectal Cancer—Where We Stand and What We Have Learned from Twenty Year’s Experience. Surgeon 2012, 10, 43–52. [Google Scholar] [CrossRef]
- Cao, Y.; Fu, Z.D.; Wang, F.; Liu, H.Y.; Han, R. Anti-Angiogenic Activity of Resveratrol, a Natural Compound from Medicinal Plants. J. Asian Nat. Prod. Res. 2005, 7, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M. Epithelial–Mesenchymal Transition and Tumour Invasion. Int. J. Biochem. Cell Biol. 2007, 39, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Trapp, V.; Parmakhtiar, B.; Papazian, V.; Willmott, L.; Fruehauf, J.P. Anti-Angiogenic Effects of Resveratrol Mediated by Decreased VEGF and Increased TSP1 Expression in Melanoma-Endothelial Cell Co-Culture. Angiogenesis 2010, 13, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Panaro, M.A.; Carofiglio, V.; Acquafredda, A.; Cavallo, P.; Cianciulli, A. Anti-Inflammatory Effects of Resveratrol Occur via Inhibition of Lipopolysaccharide-Induced NF-κB Activation in Caco-2 and SW480 Human Colon Cancer Cells. Br. J. Nutr. 2012, 108, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Cao, N.; Li, Z.; Han, J.; Li, L. Resveratrol, an Activator of SIRT1, Induces Protective Autophagy in Non-Small-Cell Lung Cancer via Inhibiting Akt/MTOR and Activating P38-MAPK. OncoTargets Ther. 2018, 11, 7777–7786. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An Overview of Its Anti-Cancer Mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef]
- Colakoglu, T.; Yildirim, S.; Kayaselcuk, F.; Nursal, T.Z.; Ezer, A.; Noyan, T.; Karakayali, H.; Haberal, M. Clinicopathological Significance of PTEN Loss and the Phosphoinositide 3-Kinase/Akt Pathway in Sporadic Colorectal Neoplasms: Is PTEN Loss Predictor of Local Recurrence? Am. J. Surg. 2008, 195, 719–725. [Google Scholar] [CrossRef]
- Agarwal, E.; Chaudhuri, A.; Leiphrakpam, P.D.; Haferbier, K.L.; Brattain, M.G.; Chowdhury, S. Akt Inhibitor MK-2206 Promotes Anti-Tumor Activity and Cell Death by Modulation of AIF and Ezrin in Colorectal Cancer. BMC Cancer 2014, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, Z.; Liu, X.; Wang, D. New Development of Inhibitors Targeting the PI3K/AKT/MTOR Pathway in Personalized Treatment of Non-Small-Cell Lung Cancer. Anti-Cancer Drugs 2015, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Neradugomma, N.K.; Subramaniam, D.; Tawfik, O.W.; Goffin, V.; Kumar, T.; Jensen, R.A.; Anant, S. Prolactin Signaling Enhances Colon Cancer Stemness by Modulating Notch Signaling in a Jak2-STAT3/ERK Manner. Carcinogenesis 2013, 35, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, G.; Jin, G.; Yao, K.; Zhao, Z.; Bie, L.; Guo, Y.; Li, N.; Deng, W.; Chen, X.; et al. Resveratrol Suppresses Colon Cancer Growth by Targeting the AKT/STAT3 Signaling Pathway. Int. J. Mol. Med. 2019, 43, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Hsu, L.S.; Shia, Y.T.; Lin, M.W.; Lin, C.M. The β-Catenin/TCF Complex as a Novel Target of Resveratrol in the Wnt/β-Catenin Signaling Pathway. Biochem. Pharmacol. 2012, 84, 1143–1153. [Google Scholar] [CrossRef]
- Saunier, E.; Antonio, S.; Regazzetti, A.; Auzeil, N.; Laprévote, O.; Shay, J.W.; Coumoul, X.; Barouki, R.; Benelli, C.; Huc, L.; et al. Resveratrol Reverses the Warburg Effect by Targeting the Pyruvate Dehydrogenase Complex in Colon Cancer Cells. Sci. Rep. 2017, 7, 6945. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Mukazhanova, Z.; Knut, E.; Turgumbayeva, A.; Kipchakbayeva, A.; Seitimova, G.; Mahomoodally, M.F.; Lobine, D.; Koay, A.; et al. Resveratrol-Based Nanoformulations as an Emerging Therapeutic Strategy for Cancer. Front. Mol. Biosci. 2021, 8, 649395. [Google Scholar] [CrossRef]
- Lugli, E.; Ferraresi, R.; Roat, E.; Troiano, L.; Pinti, M.; Nasi, M.; Nemes, E.; Bertoncelli, L.; Gibellini, L.; Salomoni, P.; et al. Quercetin Inhibits Lymphocyte Activation and Proliferation without Inducing Apoptosis in Peripheral Mononuclear Cells. Leuk. Res. 2009, 33, 140–150. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA Damage-Induced Cell Death by Apoptosis. Trends Mol. Med. 2006, 12, 440–450. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.-H.; Wang, Z.-J. Flavones and Flavonols Exert Cytotoxic Effects on a Human Oesophageal Adenocarcinoma Cell Line (OE33) by Causing G2/M Arrest and Inducing Apoptosis. Food Chem. Toxicol. 2008, 46, 2042–2053. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.-H.; Wang, Z.-J. Cytotoxicity of Flavones and Flavonols to a Human Esophageal Squamous Cell Carcinoma Cell Line (KYSE-510) by Induction of G2/M Arrest and Apoptosis. Toxicol. In Vitro 2009, 23, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wang, B.; Zhu, L. Regulation of Survivin and Bcl-2 in HepG2 Cell Apoptosis Induced by Quercetin. Chem. Biodivers. 2009, 6, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K.; Xia, Y.; Zweier, J.L.; Kinzler, K.W.; Vogelstein, B. A Model for P53-Induced Apoptosis. Nature 1997, 389, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Amstad, P.; He, P.; Robles, A.; Lupold, S.; Kaneko, I.; Ichimiya, M.; Sengupta, S.; Mechanic, L.; Okamura, S.; et al. P53-Induced Up-Regulation of MnSOD and GPx but Not Catalase Increases Oxidative Stress and Apoptosis. Cancer Res. 2004, 64, 2350–2356. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Nakamura, Y.; Arakawa, H. Identification of ALDH4 as a P53-Inducible Gene and Its Protective Role in Cellular Stresses. J. Hum. Genet. 2004, 49, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, S.; Fujii, M.; Hou, D.X. Stabilization of P53 Is Involved in Quercetin-Induced Cell Cycle Arrest and Apoptosis in HepG2 Cells. Biosci. Biotechnol. Biochem. 2008, 72, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cooper, E.L.; Cossarizza, A. Quercetin and Cancer Chemoprevention. Evid.-Based Complement. Altern. Med. 2011, 2011, 591356. [Google Scholar] [CrossRef]
- Metodiewa, D.; Jaiswal, A.K.; Cenas, N.; Dickancaité, E.; Segura-Aguilar, J. Quercetin May Act as a Cytotoxic Prooxidant after Its Metabolic Activation to Semiquinone and Quinoidal Product. Free Radic. Biol. Med. 1999, 26, 107–116. [Google Scholar] [CrossRef]
- Awad, H.M.; Boersma, M.G.; Vervoort, J.; Rietjens, I.M.C.M. Peroxidase-Catalyzed Formation of Quercetin Quinone Methide–Glutathione Adducts. Arch. Biochem. Biophys. 2000, 378, 224–233. [Google Scholar] [CrossRef]
- Prossomariti, A.; Piazzi, G.; Alquati, C.; Ricciardiello, L. Are Wnt/β-Catenin and PI3K/AKT/MTORC1 Distinct Pathways in Colorectal Cancer? Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 491–506. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential Mechanisms of Quercetin in Cancer Prevention: Focus on Cellular and Molecular Targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Wang, M.; Qian, Y.; Dong, X.; Gu, H.; Wang, H.; Guo, S.; Hisamitsu, T. Quercetin-Induced Apoptosis of HT-29 Colon Cancer Cells via Inhibition of the Akt-CSN6-Myc Signaling Axis. Mol. Med. Rep. 2016, 14, 4559–4566. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.B.; Rajendiran, V.; Kasinathan, N.K.; Amrithalakshmi, P.; Venkatabalasubramanian, S.; Murali, M.R.; Devaraj, H.; Devaraj, S.N. Differential Cytotoxic Activity of Quercetin on Colonic Cancer Cells Depends on ROS Generation through COX-2 Expression. Food Chem. Toxicol. 2017, 106, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Holz, M.K.; Blenis, J. Identification of S6 Kinase 1 as a Novel Mammalian Target of Rapamycin (MTOR)-Phosphorylating Kinase. J. Biol. Chem. 2005, 280, 26089–26093. [Google Scholar] [CrossRef] [PubMed]
- Refolo, M.G.; D’Alessandro, R.; Malerba, N.; Laezza, C.; Bifulco, M.; Messa, C.; Caruso, M.G.; Notarnicola, M.; Tutino, V. Anti Proliferative and pro Apoptotic Effects of Flavonoid Quercetin Are Mediated by CB1 Receptor in Human Colon Cancer Cell Lines. J. Cell. Physiol. 2015, 230, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Mock, C.; Liang, D. Pre-Formulation Studies of Resveratrol. Drug Dev. Ind. Pharm. 2014, 41, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Aguda, R.; Chen, C.C. Solubility of Nutraceutical Compounds in Generally Recognized as Safe Solvents at 298 K. Int. J. Chem. Eng. Appl. 2016, 7, 289–294. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Patel, K.R.; Brown, V.A.; Jones, D.J.L.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical Pharmacology of Resveratrol and Its Metabolites in Colorectal Cancer Patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.L.; Wang, L.; Ong, P.S.; et al. Resveratrol for Cancer Therapy: Challenges and Future Perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Loncaric, A.; Lamas, J.; Guerra, E.; Lores, M. Increasing water solubility of Quercetin by increasing the temperature. In Proceedings of the 15th Instrumental Analysis Conference—Expoquimia 2017, Barcelona, Spain, 3–5 October 2017. [Google Scholar]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, M.; Fu, J.; Ao, H.; Wang, W.; Wang, X. Enhancement of Oral Bioavailability of Quercetin by Metabolic Inhibitory Nanosuspensions Compared to Conventional Nanosuspensions. Drug Deliv. 2021, 28, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; ElHack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Scalarone, D.; Brunella, V.; Ugazio, E.; Sapino, S.; Berlier, G. Thermoresponsive Copolymer-Grafted SBA-15 Porous Silica Particles for Temperature-Triggered Topical Delivery Systems. Express Polym. Lett. 2017, 11, 96–105. [Google Scholar] [CrossRef]
- Maurer, N.; Fenske, D.B.; Cullis, P.R. Developments in Liposomal Drug Delivery Systems. Expert Opin. Biol. Ther. 2001, 1, 923–947. [Google Scholar] [CrossRef] [PubMed]
- Bonechi, C.; Martini, S.; Ciani, L.; Lamponi, S.; Rebmann, H.; Rossi, C.; Ristori, S. Using Liposomes as Carriers for Polyphenolic Compounds: The Case of Trans-Resveratrol. PLoS ONE 2012, 7, e41438. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Q.; Bai, Q.; Jiang, W. Advances of Smart Nano-Drug Delivery Systems in Osteosarcoma Treatment. J. Mater. Chem. B 2021, 9, 5439–5450. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cao, Y.; Sun, J.; Liang, Z.; Wu, Q.; Cui, S.; Zhi, D.; Guo, S.; Zhen, Y.; Zhang, S. Anti-Breast Cancer Activity of Resveratrol Encapsulated in Liposomes. J. Mater. Chem. B 2020, 8, 27–37. [Google Scholar] [CrossRef]
- Toopkanloo, S.P.; Tan, T.B.; Abas, F.; Alharthi, F.A.; Nehdi, I.A.; Tan, C.P. Impact of Quercetin Encapsulation with Added Phytosterols on Bilayer Membrane and Photothermal-Alteration of Novel Mixed Soy Lecithin-Based Liposome. Nanomaterials 2020, 10, 2432. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Gao, Y.; Liu, S.; Li, K.; Wang, S.; Gao, L.; Shi, M.; Liu, Z.; Han, Z.; et al. Effect of a Drug Delivery System Made of Quercetin Formulated into PEGylation Liposomes on Cervical Carcinoma in Vitro and in Vivo. J. Nanomater. 2021, 2021, 9389934. [Google Scholar] [CrossRef]
- Dana, P.; Thumrongsiri, N.; Tanyapanyachon, P.; Chonniyom, W.; Punnakitikashem, P.; Saengkrit, N. Resveratrol Loaded Liposomes Disrupt Cancer Associated Fibroblast Communications within the Tumor Microenvironment to Inhibit Colorectal Cancer Aggressiveness. Nanomaterials 2023, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, F.; Dorfaki, M.; Bardania, H.; Khosravani, F.; Nazari, P.; Ghalamfarsa, G. Quercetin-Loaded Liposomes Effectively Induced Apoptosis and Decreased the Epidermal Growth Factor Receptor Expression in Colorectal Cancer Cells: An in Vitro Study. Iran. J. Med. Sci. 2023, 48, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Melchior, S.; Codrich, M.; Gorassini, A.; Mehn, D.; Ponti, J.; Verardo, G.; Tell, G.; Calzolai, L.; Calligaris, S. Design and Advanced Characterization of Quercetin-Loaded Nano-Liposomes Prepared by High-Pressure Homogenization. Food Chem. 2023, 428, 136680. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, Y.; Gregoriou, G.; Yilmaz, V.; Kapnisis, K.; Prokopi, M.; Anayiotos, A.; Strati, K.; Dietis, N.; Constantinou, A.I.; Andreou, C. Resveratrol Loaded Polymeric Micelles for Theranostic Targeting of Breast Cancer Cells. Nanotheranostics 2021, 5, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Sudha, T.; El-Far, A.H.; Mousa, D.S.; Mousa, S.A. Resveratrol and Its Nanoformulation Attenuate Growth and the Angiogenesis of Xenograft and Orthotopic Colon Cancer Models. Molecules 2020, 25, 1412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ji, Y.; Hu, N.; Yu, Q.; Zhang, X.; Li, J.; Wu, F.; Xu, H.; Tang, Q.; Li, X. Ferroptosis-Induced Anticancer Effect of Resveratrol with a Biomimetic Nano-Delivery System in Colorectal Cancer Treatment. Asian J. Pharm. Sci. 2022, 17, 751–766. [Google Scholar] [CrossRef]
- Chang, M.; Wu, M.; Li, H. Antitumor Activities of Novel Glycyrrhetinic Acid-Modified Curcumin-Loaded Cationic Liposomes in Vitro and in H22 Tumor-Bearing Mice. Drug Deliv. 2018, 25, 1984–1995. [Google Scholar] [CrossRef] [PubMed]
- Sunoqrot, S.; Abujamous, L. pH-Sensitive Polymeric Nanoparticles of Quercetin as a Potential Colon Cancer-Targeted Nanomedicine. J. Drug Deliv. Sci. Technol. 2019, 52, 670–676. [Google Scholar] [CrossRef]
- Colpan, R.D.; Erdemir, A. Co-Delivery of Quercetin and Caffeic-Acid Phenethyl Ester by Polymeric Nanoparticles for Improved Antitumor Efficacy in Colon Cancer Cells. J. Microencapsul. 2021, 38, 381–393. [Google Scholar] [CrossRef]
- Shahidi, S.; Rostamizadeh, K.; Fathi, M.; Nedaei, K.; Ramazani, A. Combination of Quercetin Or/and SiRNA-Loaded DDAB-MPEG-PCL Hybrid Nanoparticles Reverse Resistance to Regorafenib in Colon Cancer Cells. BMC Complement. Med. Ther. 2022, 22, 340. [Google Scholar] [CrossRef]
- Das, A.; Adhikari, S.; Deka, D.; Baildya, N.; Sahare, P.; Banerjee, A.; Paul, S.; Bisgin, A.; Pathak, S. An Updated Review on the Role of Nanoformulated Phytochemicals in Colorectal Cancer. Medicina 2023, 59, 685. [Google Scholar] [CrossRef] [PubMed]
- Rashedi, J.; Haghjo, G.A.; Abbasi, M.M.; Tabrizi, D.A.; Yaqoubi, S.; Sanajou, D.; Jigheh, A.Z.; Namvaran, A.; Mohammadi, A.; Mohammadi Khoshraj, J.; et al. Anti-Tumor Effect of Quercetin Loaded Chitosan Nanoparticles on Induced Colon Cancer in Wistar Rats. Adv. Pharm. Bull. 2019, 9, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.; Chadha, V.D.; Dhawan, D.K. Physiological Uptake and Retention of Radiolabeled Resveratrol Loaded Gold Nanoparticles (99mTc-Res-AuNP) in Colon Cancer Tissue. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Karanastasis, A.; Chatziathanasiadou, M.V.; Oguz, M.; Kougioumtzi, A.; Clemente, N.; Kellici, T.F.; Zafeiropoulos, N.E.; Avgeropoulos, A.; Mavromoustakos, T.; et al. Inclusion of Quercetin in Gold Nanoparticles Decorated with Supramolecular Hosts Amplifies Its Tumor Targeting Properties. ACS Appl. Bio Mater. 2019, 2, 2715–2725. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F.; Gavini, E.; Rassu, G.; Giunchedi, P. Nanoemulsions for “Nose-To-Brain” Drug Delivery. Pharmaceutics 2019, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Choradiya, B.R.; Patil, S.B. A Comprehensive Review on Nanoemulsion as an Ophthalmic Drug Delivery System. J. Mol. Liq. 2021, 339, 116751. [Google Scholar] [CrossRef]
- Kotta, S. Formulation of Resveratrol Nanoemulsion by Phase Inversion Technique and Evaluation of Anti-Cancer Activity on Human Colon Cancer Cell Lines. Indian J. Pharm. Educ. Res. 2021, 55, S623–S629. [Google Scholar] [CrossRef]
- Serini, S.; Cassano, R.; Corsetto, P.; Rizzo, A.; Calviello, G.; Trombino, S. Omega-3 PUFA Loaded in Resveratrol-Based Solid Lipid Nanoparticles: Physicochemical Properties and Antineoplastic Activities in Human Colorectal Cancer Cells In Vitro. Int. J. Mol. Sci. 2018, 19, 586. [Google Scholar] [CrossRef]
- Feng, M.; Zhong, L.X.; Zhan, Z.Y.; Huang, Z.-H.; Xiong, J.P. Enhanced Antitumor Efficacy of Resveratrol-Loaded Nanocapsules in Colon Cancer Cells: Physicochemical and Biological Characterization. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 375–382. [Google Scholar] [PubMed]
- Lotfi, M.; Kazemi, S.; Ebrahimpour, A.; Shirafkan, F.; Pirzadeh, M.; Hosseini, M.; Moghadamnia, A.A. Protective Effect of Quercetin Nanoemulsion on 5-Fluorouracil-Induced Oral Mucositis in Mice. J. Oncol. 2021, 2021, 5598230. [Google Scholar] [CrossRef]
- Enin, H.A.A.; Alquthami, A.F.; Alwagdani, A.M.; Yousef, L.M.; Albuqami, M.S.; Alharthi, M.A.; Alsaab, H.O. Utilizing TPGS for Optimizing Quercetin Nanoemulsion for Colon Cancer Cells Inhibition. Colloids Interfaces 2022, 6, 49. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. Dendritic Macromolecules: Synthesis of Starburst Dendrimers. Macromolecules 1986, 19, 2466–2468. [Google Scholar] [CrossRef]
- Tomalia, D.A. The Dendritic State. Mater. Today 2005, 8, 34–46. [Google Scholar] [CrossRef]
- Newkome, G.R.; Yao, Z.; Baker, G.R.; Gupta, V.K. Micelles. Part 1. Cascade Molecules: A New Approach to Micelles. A [27]-Arborol. J. Org. Chem. 1985, 50, 2003–2004. [Google Scholar] [CrossRef]
- de Brabander-van den Berg, E.M.M.; Meijer, E.W. Poly(propylene imine) Dendrimers: Large-Scale Synthesis by Hetereogeneously Catalyzed Hydrogenations. Angew. Chem. Int. Ed. 1993, 32, 1308–1311. [Google Scholar] [CrossRef]
- Wörner, C.; Mülhaupt, R. Polynitrile- and Polyamine-Functional Poly(trimethylene imine) Dendrimers. Angew. Chem. Int. Ed. 1993, 32, 1306–1308. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, F.; Lu, K.; Hui, Q.; Miao, M. Characterizations and Bioavailability of Dendrimer-like Glucan Nanoparticulate System Containing Resveratrol. J. Agric. Food Chem. 2020, 68, 6420–6429. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zichri, S.; Meltzer, M.; Lacham-Hartman, S.; Kolusheva, S.; Hadad, U.; Papo, N.; Jelinek, R. Synergistic Activity of Anticancer Polyphenols Embedded in Amphiphilic Dendrimer Nanoparticles. ACS Appl. Polym. Mater. 2022, 4, 8913–8925. [Google Scholar] [CrossRef]
- Mouria, M.; Gukovskaya, A.S.; Jung, Y.; Buechler, P.; Hines, O.J.; Reber, H.A.; Pandol, S.J. Food-Derived Polyphenols Inhibit Pancreatic Cancer Growth through Mitochondrial Cytochrome c Release and Apoptosis. Int. J. Cancer 2002, 98, 761–769. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Percival, S.S. Ellagic Acid and Quercetin Interact Synergistically with Resveratrol in the Induction of Apoptosis and Cause Transient Cell Cycle Arrest in Human Leukemia Cells. Cancer Lett. 2005, 218, 141–151. [Google Scholar] [CrossRef]
- Zamin, L.L.; Filippi-Chiela, E.C.; Dillenburg-Pilla, P.; Horn, F.; Salbego, C.; Lenz, G. Resveratrol and Quercetin Cooperate to Induce Senescence-like Growth Arrest in C6 Rat Glioma Cells. Cancer Sci. 2009, 100, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Nácher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernàndez-Busquets, X.; Carbone, C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of Quercetin and Resveratrol Co-Incorporated in Liposomes against Inflammatory/Oxidative Response Associated with Skin Cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Cadena, P.G.; Pereira, M.A.; Cordeiro, R.B.; Cavalcanti, I.M.; Neto, B.B.; Pimentel, M.D.C.C.; Filho, J.L.L.; Silva, V.L.; Santos-Magalhães, N.S. Nanoencapsulation of Quercetin and Resveratrol into Elastic Liposomes. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Vaz, G.R.; Clementino, A.R.; Bidone, J.; Villetti, M.A.; Falkembach, M.; Batista, M.; Barros, P.M.; Sonvico, F. Curcumin and Quercetin-Loaded Nanoemulsions: Physicochemical Compatibility Study and Validation of a Simultaneous Quantification Method. Nanomaterials 2020, 10, 1650. [Google Scholar] [CrossRef] [PubMed]
- Elnawasany, S.; Haggag, Y.A.; Shalaby, S.M.; Soliman, N.A.; EL Saadany, A.A.; Ibrahim, M.A.A.; Badria, F. Anti-Cancer Effect of Nano-Encapsulated Boswellic Acids, Curcumin and Naringenin against HepG-2 Cell Line. BMC Complement. Med. Ther. 2023, 23, 270. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.Y.; Chiu, G.N.C. Simultaneous Liposomal Delivery of Quercetin and Vincristine for Enhanced Estrogen-Receptor-Negative Breast Cancer Treatment. Anti-Cancer Drugs 2010, 21, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jia, R.; Li, J.; Tian, X.; Qian, Y. Curcumin- and Resveratrol-Co-Loaded Nanoparticles in Synergistic Treatment of Hepatocellular Carcinoma. J. Nanobiotechnol. 2022, 20, 339. [Google Scholar] [CrossRef]
- Soltantabar, P.; Calubaquib, E.L.; Mostafavi, E.; Biewer, M.C.; Stefan, M.C. Enhancement of Loading Efficiency by Coloading of Doxorubicin and Quercetin in Thermoresponsive Polymeric Micelles. Biomacromolecules 2020, 21, 1427–1436. [Google Scholar] [CrossRef]
- Hauqe, M.; Anamika; Jha, B.N.; Kumari, P.; Jana, R.; Shukla, S.; Kumar, A.; Bhadauria, S.S.; Yadav, P.; Ashique, S.; et al. Development and Characterization of Drug loaded Novel Carrier for the Treatment of Arthritis. Eur. Chem. Bull. 2023, 12, 6950–6965. [Google Scholar] [CrossRef]
- Pinisetti, D.; Aditi Balvanbhai, P.; Kakadiya, J. Role of Quercetin as an Effective Bioenhancer in Curcumin Absorption, In Vitro Study. Res. J. Pharm. Technol. 2022, 15, 4867–4870. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C.; Holcombe, R.F. Results of a Phase I Pilot Clinical Trial Examining the Effect of Plant-Derived Resveratrol and Grape Powder on Wnt Pathway Target Gene Expression in Colonic Mucosa and Colon Cancer. Cancer Manag. Res. 2009, 1, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef] [PubMed]
- Sulindac and Plant Compounds in Preventing Colon Cancer. Clin.Larvol.com. Available online: https://clin.larvol.com/trial-detail/NCT00003365 (accessed on 13 May 2024).
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Wu, M.; Feng, Z.; Deng, Y.; Zhong, C.; Zhao, X. Folate-Conjugated Human Serum Albumin-Encapsulated Resveratrol Nanoparticles: Preparation, Characterization, Bioavailability and Targeting of Liver Tumors. Artif. Cells Nanomed. Biotechnol. 2019, 47, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Samir, B.; El-Kamel, A.; Zahran, N.; Heikal, L. Resveratrol-Loaded Invasome Gel: A Promising Nanoformulation for Treatment of Skin Cancer. Drug Deliv. Transl. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, E.; El-Derany, M.O.; Biondo, F.; Tiboni, M.; Casettari, L.; Soliman, M.E. Quercetin Loaded Monolaurate Sugar Esters-Based Niosomes: Sustained Release and Mutual Antioxidant—Hepatoprotective Interplay. Pharmaceutics 2020, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Tripathi, A.K.; Parveen, A.; Parveen, S.; Banerjee, M. PLGA-Quercetin Nano-Formulation Inhibits Cancer Progression via Mitochondrial Dependent Caspase-3,7 and Independent FoxO1 Activation with Concomitant PI3K/AKT Suppression. Pharmaceutics 2022, 14, 1326. [Google Scholar] [CrossRef]
- Marcantonio, D.H.; Matteson, A.; Presler, M.; Burke, J.M.; Hagen, D.R.; Hua, F.; Apgar, J.F. Early Feasibility Assessment: A Method for Accurately Predicting Biotherapeutic Dosing to Inform Early Drug Discovery Decisions. Front. Pharmacol. 2022, 8, 864768. [Google Scholar] [CrossRef]
- Patel, M.; Bueters, T. Can Quantitative Pharmacology Improve Productivity in Pharmaceutical Research and Development? Expert Opin. Drug Discov. 2020, 15, 1111–1114. [Google Scholar] [CrossRef]


| Description | Resveratrol | Quercetin | ||
| Chemical Properties [57,58] | ||||
| Solubility | PEG-400 (373 mg/mL), Ethanol (88 mg/mL), Labrasol (14 mg/mL), Tween 80 (7 mg/mL), Cremophore EL (6.7 mg/mL), Polysorbate 80 (5 mg/mL), water (0.05 mg/mL) | DMSO (67 mg/mL), Ethanol (21 mg/mL), Acetone (80 mmol/L), t-amyl alcohol (67 mmol/L), Acetonitrile (5.4 mmol/L), water (0.4 mg/L) | ||
| Stability | Unstable at high temperatures and at alkaline pH | Unstable at higher temperatures and at alkaline pH | ||
| Pharmacological Targets in CRC [23,24,25,26,27,28,29,30,31,32,33,34,35,51,52,53,54,55,56] | ||||
| Pathways/Molecules | Upregulated | Downregulated | Upregulated | Downregulated |
| Anti-cancer | SIRT1, p38-MAPK, PDH, | MMP-9, VEGF, EMT, HIF-1alpha, TSP1, NO, MMP-2, iNOS, AKT/mTOR, Wnt, | P53, BAX, caspase-3, and caspase-9, | Wnt/β-catenin, PI3K/AKT, p-STAT3, NF-κB, |
| Description | Liposomes | Nanoemulsions | Dendrimers | Polymeric Micelles |
|---|---|---|---|---|
| Advantages |
|
|
|
|
| Limitations |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unnikrishnan Meenakshi, D.; Narde, G.K.; Ahuja, A.; Al Balushi, K.; Francis, A.P.; Khan, S.A. Therapeutic Applications of Nanoformulated Resveratrol and Quercetin Phytochemicals in Colorectal Cancer—An Updated Review. Pharmaceutics 2024, 16, 761. https://doi.org/10.3390/pharmaceutics16060761
Unnikrishnan Meenakshi D, Narde GK, Ahuja A, Al Balushi K, Francis AP, Khan SA. Therapeutic Applications of Nanoformulated Resveratrol and Quercetin Phytochemicals in Colorectal Cancer—An Updated Review. Pharmaceutics. 2024; 16(6):761. https://doi.org/10.3390/pharmaceutics16060761
Chicago/Turabian StyleUnnikrishnan Meenakshi, Dhanalekshmi, Gurpreet Kaur Narde, Alka Ahuja, Khalid Al Balushi, Arul Prakash Francis, and Shah Alam Khan. 2024. "Therapeutic Applications of Nanoformulated Resveratrol and Quercetin Phytochemicals in Colorectal Cancer—An Updated Review" Pharmaceutics 16, no. 6: 761. https://doi.org/10.3390/pharmaceutics16060761
APA StyleUnnikrishnan Meenakshi, D., Narde, G. K., Ahuja, A., Al Balushi, K., Francis, A. P., & Khan, S. A. (2024). Therapeutic Applications of Nanoformulated Resveratrol and Quercetin Phytochemicals in Colorectal Cancer—An Updated Review. Pharmaceutics, 16(6), 761. https://doi.org/10.3390/pharmaceutics16060761







