Transformation of ABT-199 Nanocrystal Suspensions into a Redispersible Drug Product—Impact of Vacuum Drum Drying, Spray Drying and Tableting on Re-Nanodispersibility
Abstract
1. Introduction
- (a)
- To produce a stable nanosuspension using techniques like wet ball milling, where ionic and/or steric stabilizers are employed.
- (b)
- To solidify the nanosuspension through a drying process using drying protectants/bulking agents (such as sugars or sugar alcohols), which act as spacers to prevent crystal-to-crystal contact.
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Manufacture of ABT-199 Nanosuspension
2.2.2. Nanosuspension Characterization—Particle Size Distribution
Dynamic Light Scattering
Laser Diffraction
2.2.3. Solidification/Drying of Nanosuspensions
Vacuum Drum Drying
Spray Drying
2.2.4. Comminution/Deagglomeration
2.2.5. Redispersibility of Intermediates and Tablets Using Laser Diffraction
2.2.6. Intermediate Characterization
Particle Size Distribution (PSD) of Dry Powder Using Laser Diffraction
Powder Bulk/Tapped Density and Particle Density
Flowability (Ringshear Tester)
Loss on Drying
2.2.7. Compression Analysis and Tableting
2.2.8. Disintegration
3. Results
3.1. Particle Size Analysis of the ABT-199 Nanosuspensions
3.2. Effect of Drying Technique on Solid Yield and on Particle Size upon Redispersion in Water
3.3. Characterization of VDD and SD Intermediates
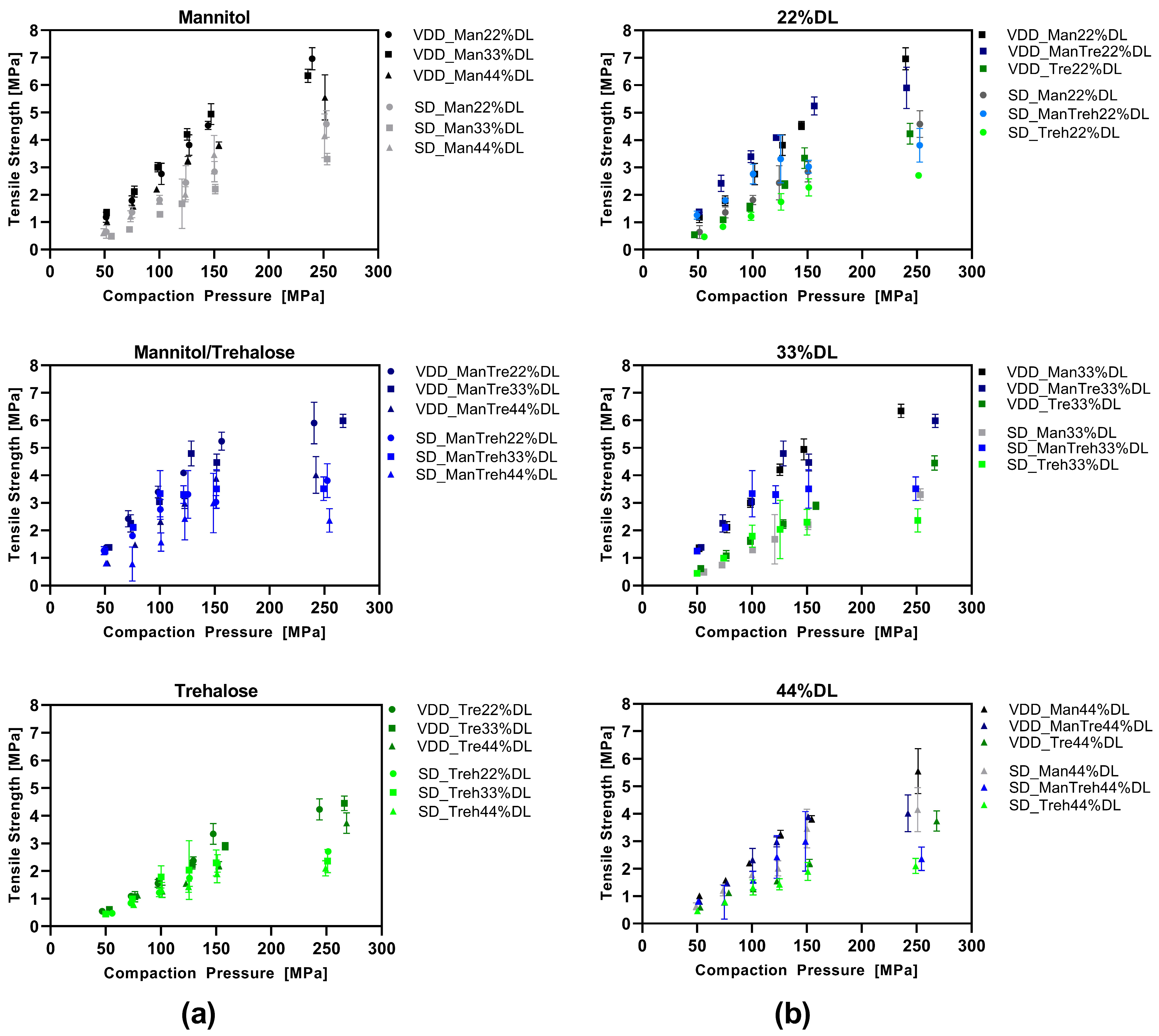
3.4. Compression Analysis of VDD and SD Intermediate Powders—Tabletability
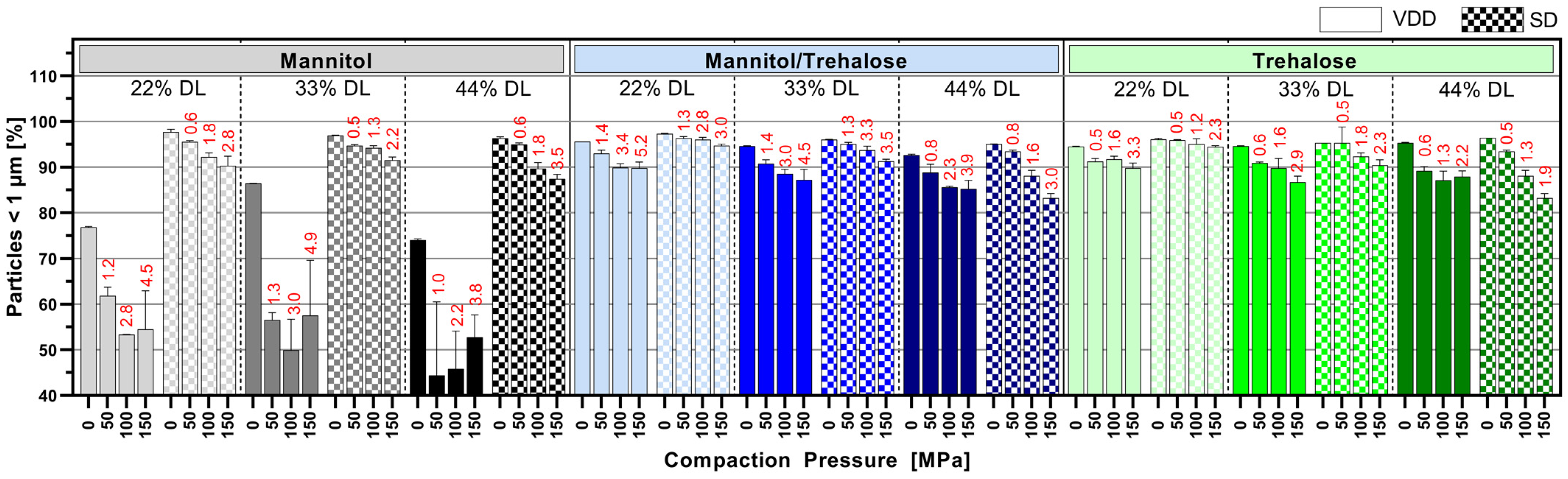
3.5. Impact of Tableting on Nanoparticle Size upon Redispersion in Water
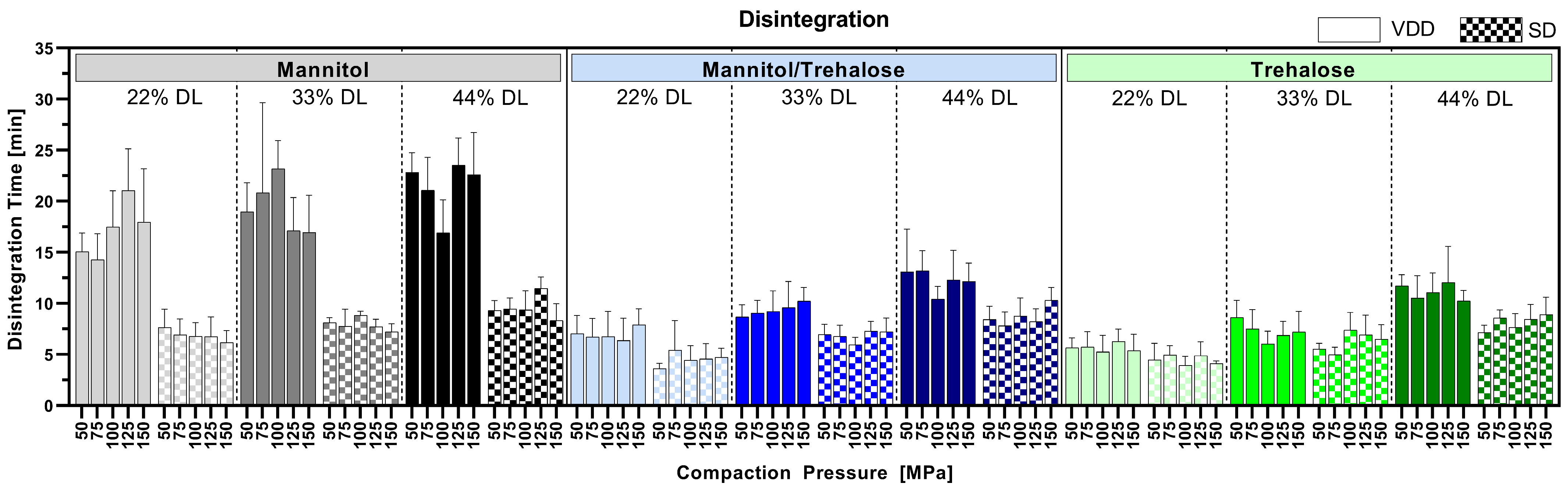
3.6. Disintegration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Khan, K.U.; Minhas, M.U.; Badshah, S.F.; Suhail, M.; Ahmad, A.; Ijaz, S. Overview of nanoparticulate strategies for solubility enhancement of poorly soluble drugs. Life Sci. 2022, 291, 120301. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Otto, D.; De Villiers, M. Physicochemical Principles of Nanosized Drug Delivery Systems; Springer: New York, NY, USA, 2009; Volume 10, pp. 3–33. [Google Scholar]
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Jermain, S.V.; Brough, C.; Williams, R.O. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery—An update. Int. J. Pharm. 2018, 535, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, L. Developing early formulations: Practice and perspective. Int. J. Pharm. 2007, 341, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Rong, X.; Laru, J.; van Veen, B.; Kiesvaara, J.; Hirvonen, J.; Laaksonen, T.; Peltonen, L. Nanosuspensions of poorly soluble drugs: Preparation and development by wet milling. Int. J. Pharm. 2011, 411, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Yoo, J.Y.; Kwak, H.-S.; Uk Nam, B.; Lee, J. Role of polymeric stabilizers for drug nanocrystal dispersions. Curr. Appl. Phys. 2005, 5, 472–474. [Google Scholar] [CrossRef]
- Lestari, M.L.A.D.; Müller, R.H.; Möschwitzer, J.P. Systematic Screening of Different Surface Modifiers for the Production of Physically Stable Nanosuspensions. J. Pharm. Sci. 2015, 104, 1128–1140. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Liang, X.; Xu, J.; Lee, C.; Liang, Q.; Tao, P.; Deng, T. Dispersion stability of thermal nanofluids. Prog. Nat. Sci. Mater. Int. 2017, 27, 531–542. [Google Scholar] [CrossRef]
- Ahmadi Tehrani, A.; Omranpoor, M.M.; Vatanara, A.; Seyedabadi, M.; Ramezani, V. Formation of nanosuspensions in bottom-up approach: Theories and optimization. DARU J. Pharm. Sci. 2019, 27, 451–473. [Google Scholar] [CrossRef] [PubMed]
- Möschwitzer, J.P. Drug nanocrystals in the commercial pharmaceutical development process. Int. J. Pharm. 2013, 453, 142–156. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Zhang, H.; Gao, J.; Zheng, A. Progress in the development of stabilization strategies for nanocrystal preparations. Drug Deliv. 2021, 28, 19–36. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 3. [Google Scholar] [CrossRef]
- Malamatari, M.; Somavarapu, S.; Taylor, K.M.G.; Buckton, G. Solidification of nanosuspensions for the production of solid oral dosage forms and inhalable dry powders. Expert Opin. Drug Deliv. 2016, 13, 435–450. [Google Scholar] [CrossRef]
- Patel, D.; Zode, S.S.; Bansal, A.K. Formulation aspects of intravenous nanosuspensions. Int. J. Pharm. 2020, 586, 119555. [Google Scholar] [CrossRef] [PubMed]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef]
- Chogale, M.M.; Ghodake, V.N.; Patravale, V.B. Performance Parameters and Characterizations of Nanocrystals: A Brief Review. Pharmaceutics 2016, 8, 26. [Google Scholar] [CrossRef]
- Czyz, S.; Wewers, M.; Finke, J.H.; Kwade, A.; van Eerdenbrugh, B.; Juhnke, M.; Bunjes, H. Spray drying of API nanosuspensions: Importance of drying temperature, type and content of matrix former and particle size for successful formulation and process development. Eur. J. Pharm. Biopharm. 2020, 152, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wewers, M.; Finke, J.H.; Czyz, S.; Eerdenbrugh, B.; John, E.; Büch, G.; Juhnke, M.; Bunjes, H.; Kwade, A. Evaluation of the Formulation Parameter-Dependent Redispersibility of API Nanoparticles from Fluid Bed Granules. Pharmaceutics 2022, 14, 1688. [Google Scholar] [CrossRef] [PubMed]
- Jakubowska, E.; Bielejewski, M.; Milanowski, B.; Lulek, J. Freeze-drying of drug nanosuspension– study of formulation and processing factors for the optimization and characterization of redispersible cilostazol nanocrystals. J. Drug Deliv. Sci. Technol. 2022, 74, 103528. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, J.; Jia, W.; Liu, Y.; Zhang, L.; Yang, Q.; Guo, J.; Zhao, J.; Yan, B.; Wang, Y. A Comparison of Spray-Drying and Freeze-Drying for the Production of Stable Silybin Nanosuspensions. J. Nanosci. Nanotechnol. 2020, 20, 3598–3603. [Google Scholar] [CrossRef] [PubMed]
- Sahnen, F.; Kamps, J.P.; Langer, K. Conversion of indomethacin nanosuspensions into solid dosage forms via fluid bed granulation and compaction. Eur. J. Pharm. Biopharm. 2020, 154, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, B.; Westedt, U.; Wagner, K.G. Vacuum drum drying—A novel solvent-evaporation based technology to manufacture amorphous solid dispersions in comparison to spray drying and hot melt extrusion. Int. J. Pharm. 2021, 596, 120233. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, B.V.; Westedt, U.; Keller, B.L.; Wagner, K.G. Transformation of Ritonavir Nanocrystal Suspensions into a Redispersible Drug Product via Vacuum Drum Drying. AAPS PharmSciTech 2022, 23, 137. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.H.; Parmentier, J.; Low, A.; Möschwitzer, J.P. Downstream drug product processing of itraconazole nanosuspension: Factors influencing tablet material properties and dissolution of compacted nanosuspension-layered sugar beads. Int. J. Pharm. 2017, 532, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Chaubal, M.V.; Popescu, C. Conversion of Nanosuspensions into Dry Powders by Spray Drying: A Case Study. Pharm. Res. 2008, 25, 2302–2308. [Google Scholar] [CrossRef]
- FDA. Venclexta (Venetoclax) Clinical Pharmacology and Biopharmaceutics Review(s) Application Number: 208573Orig1s000. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208573Orig1s000ClinPharmR.pdf (accessed on 21 May 2024).
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Cang, S.; Iragavarapu, C.; Savooji, J.; Song, Y.; Liu, D. ABT-199 (venetoclax) and BCL-2 inhibitors in clinical development. J. Hematol. Oncol. 2015, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Itchaki, G.; Brown, J.R. The potential of venetoclax (ABT-199) in chronic lymphocytic leukemia. Ther. Adv. Hematol. 2016, 7, 270–287. [Google Scholar] [CrossRef] [PubMed]
- B-290 Data Sheet 11594126 H en 1907, version G; BÜCHI Labortechnik AG: Flawil, Switzerland.
- Zuo, B.; Sun, Y.; Li, H.; Liu, X.; Zhai, Y.; Sun, J.; He, Z. Preparation and in vitro/in vivo evaluation of fenofibrate nanocrystals. Int. J. Pharm. 2013, 455, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Ćurić, A.; Keller, B.-L.; Reul, R.; Möschwitzer, J.; Fricker, G. Development and lyophilization of itraconazole loaded poly(butylcyanoacrylate) nanospheres as a drug delivery system. Eur. J. Pharm. Sci. 2015, 78, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Malamatari, M.; Somavarapu, S.; Kachrimanis, K.; Buckton, G.; Taylor, K.M.G. Preparation of respirable nanoparticle agglomerates of the low melting and ductile drug ibuprofen: Impact of formulation parameters. Powder Technol. 2017, 308, 123–134. [Google Scholar] [CrossRef]
- Sun, W.; Ni, R.; Zhang, X.; Li, L.C.; Mao, S. Spray drying of a poorly water-soluble drug nanosuspension for tablet preparation: Formulation and process optimization with bioavailability evaluation. Drug Dev. Ind. Pharm. 2015, 41, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Y.; Aisha, M.; Wu, J.; Wang, H.; Huang, F.; Sun, M. Preparation of loratadine nanocrystal tablets to improve the solubility and dissolution for enhanced oral bioavailability. J. Pharm. Pharmacol. 2021, 73, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Malamatari, M.; Charisi, A.; Malamataris, S.; Kachrimanis, K.; Nikolakakis, I. Spray Drying for the Preparation of Nanoparticle-Based Drug Formulations as Dry Powders for Inhalation. Processes 2020, 8, 788. [Google Scholar] [CrossRef]
- Torge, A.; Grützmacher, P.; Mücklich, F.; Schneider, M. The influence of mannitol on morphology and disintegration of spray-dried nano-embedded microparticles. Eur. J. Pharm. Sci. 2017, 104, 171–179. [Google Scholar] [CrossRef]
- Sun, C.C. Mechanism of moisture induced variations in true density and compaction properties of microcrystalline cellulose. Int. J. Pharm. 2008, 346, 93–101. [Google Scholar] [CrossRef]
- Osei-Yeboah, F.; Sun, C.C. Effect of drug loading and relative humidity on the mechanical properties and tableting performance of Celecoxib–PVP/VA 64 amorphous solid dispersions. Int. J. Pharm. 2023, 644, 123337. [Google Scholar] [CrossRef] [PubMed]
- Ohrem, H.L.; Schornick, E.; Kalivoda, A.; Ognibene, R. Why is mannitol becoming more and more popular as a pharmaceutical excipient in solid dosage forms? Pharm. Dev. Technol. 2014, 19, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.S.; Patel, C.; Sevak, V.; Chan, M. Effect of Kollidon VA®64 particle size and morphology as directly compressible excipient on tablet compression properties. Drug Dev. Ind. Pharm. 2018, 44, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Kolter, K.; Flick, D. Structure and Dry Binding Activity of Different Polymers, Including Kollidon® VA 64. Drug Dev. Ind. Pharm. 2000, 26, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.C.; Lang, S. Pharmazeutische Hilfsstoffe: Eigenschaften, Anwendung und Handelsprodukte; Govi-Verlag: Eschborn, Germany, 2013. [Google Scholar]
- Kosugi, A.; Leong, K.H.; Tsuji, H.; Hayashi, Y.; Kumada, S.; Okada, K.; Onuki, Y. Characterization of Powder- and Tablet Properties of Different Direct Compaction Grades of Mannitol Using a Kohonen Self-organizing Map and a Lasso Regression Model. J. Pharm. Sci. 2020, 109, 2585–2593. [Google Scholar] [CrossRef] [PubMed]
- Skelbæk-Pedersen, A.L.; Al-Sharabi, M.; Vilhelmsen, T.K.; Rantanen, J.; Zeitler, J.A. Effect of particle size and deformation behaviour on water ingress into tablets. Int. J. Pharm. 2020, 587, 119645. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, S.; Johno, I.; Ito, Y.; Teramura, S.; Okado, J. Effects of hardness on the disintegration time and the dissolution rate of uncoated caffeine tablets. J. Pharm. Pharmacol. 1975, 27, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, J.; Hu, A.; Nie, T.; Cheng, Z.; Liu, W. A Critical Review on Engineering of d-Mannitol Crystals: Properties, Applications, and Polymorphic Control. Crystals 2022, 12, 1080. [Google Scholar] [CrossRef]
- Peltonen, L.; Strachan, C.J. Degrees of order: A comparison of nanocrystal and amorphous solids for poorly soluble drugs. Int. J. Pharm. 2020, 586, 119492. [Google Scholar] [CrossRef]
- Quodbach, J.; Kleinebudde, P. A critical review on tablet disintegration. Pharm. Dev. Technol. 2016, 21, 763–774. [Google Scholar] [CrossRef]
- Sun, C.C. Microstructure of Tablet—Pharmaceutical Significance, Assessment, and Engineering. Pharm. Res. 2017, 34, 918–928. [Google Scholar] [CrossRef] [PubMed]
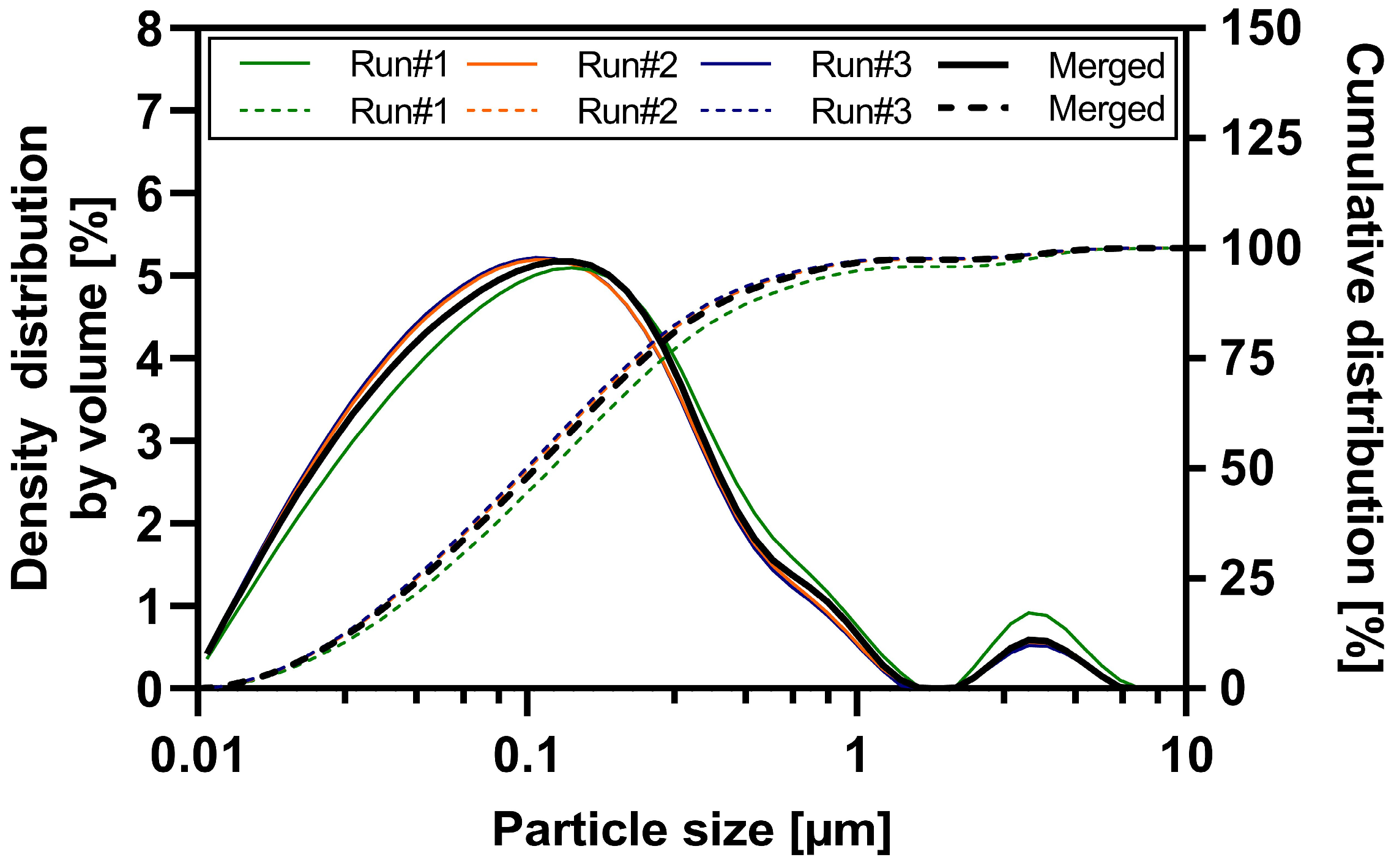
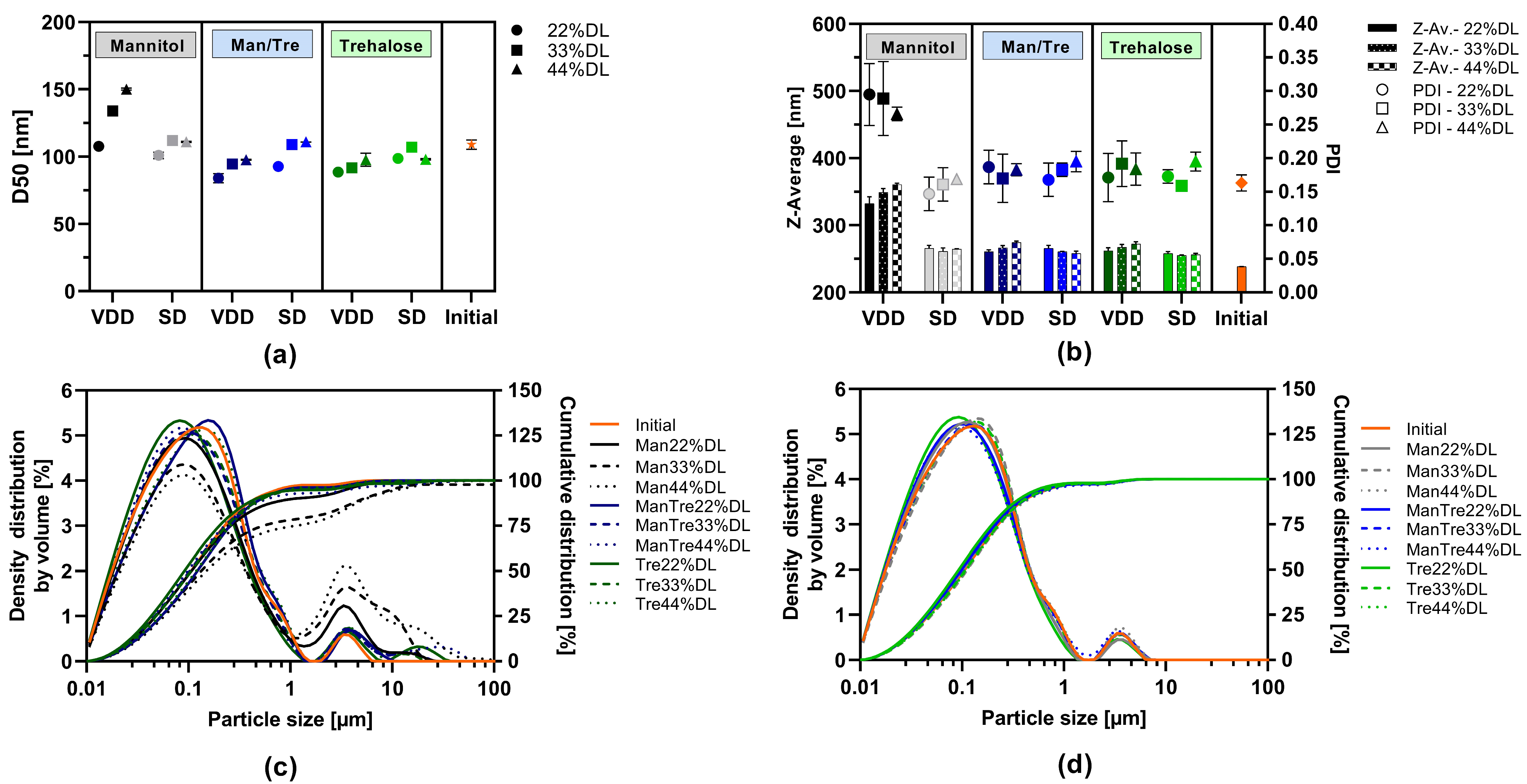
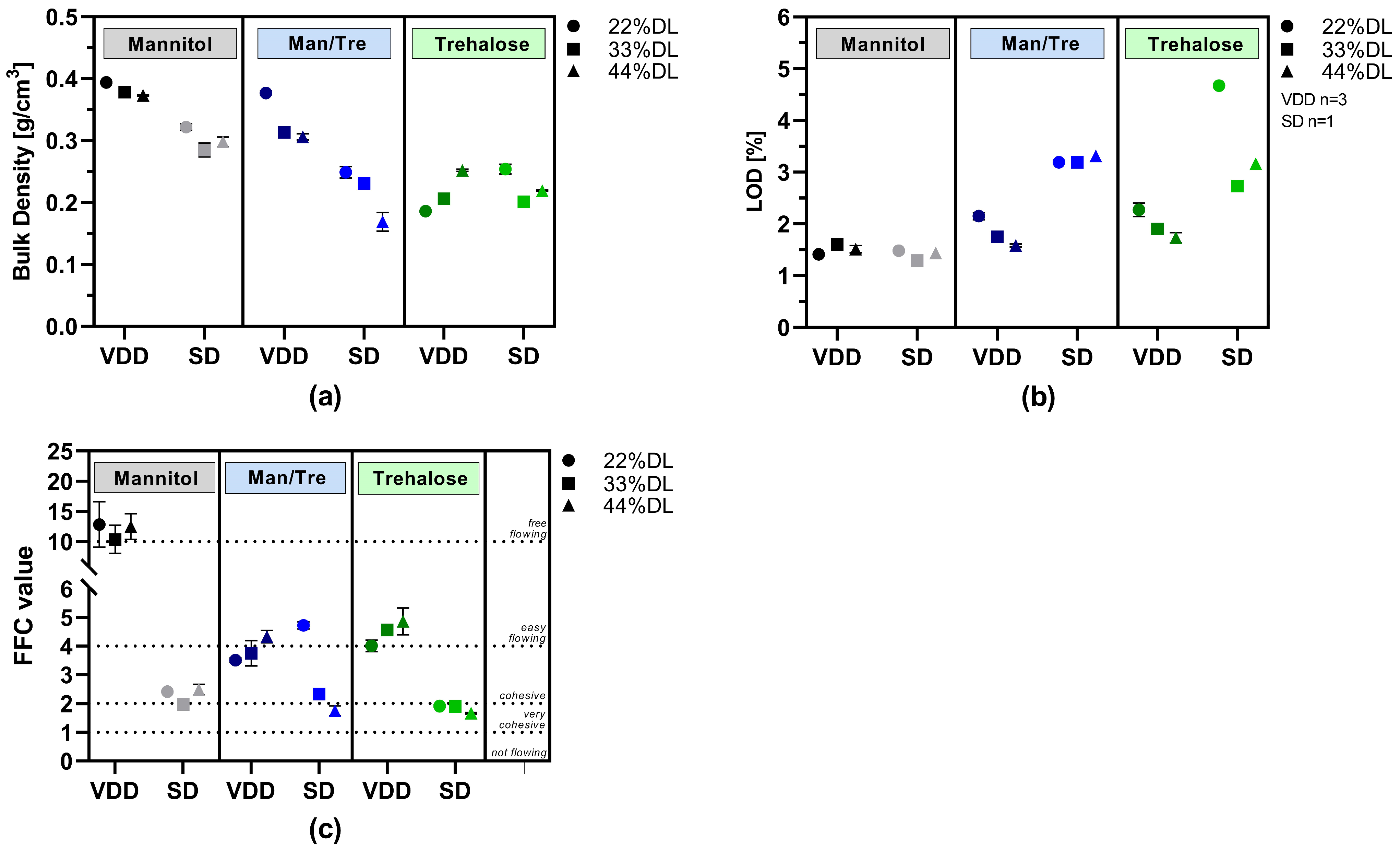
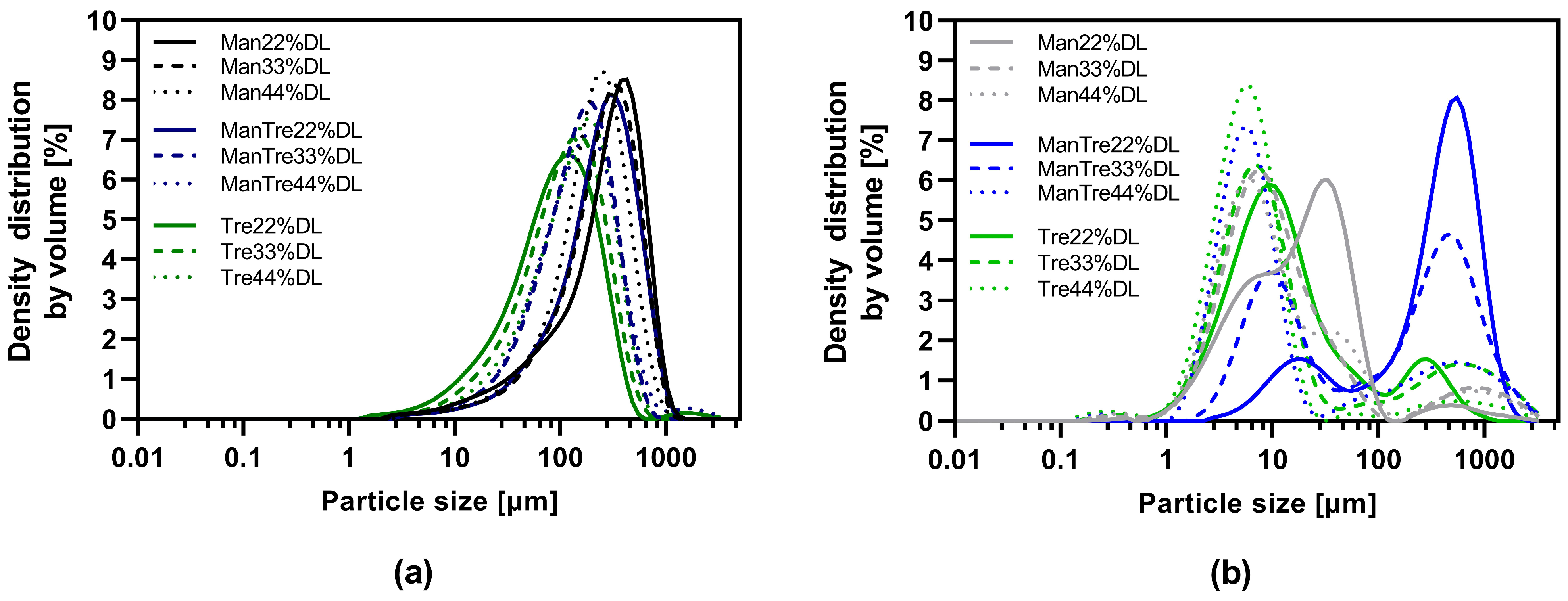
| Material | Function | Amount [% w/w] |
|---|---|---|
| ABT-199 | Active pharmaceutical ingredient | 15.00 |
| Sodium deoxycholate | Electrostatic stabilizer | 0.85 |
| Copovidone | Steric stabilizer | 2.55 |
| Demineralized water | Dispersion media | 81.60 |
| Mannitol | Mannitol/Trehalose | Trehalose | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 22% DL | 33% DL | 44% DL | 22% DL | 33% DL | 44% DL | 22% DL | 33% DL | 44% DL | |
| ABT-199 | 22.00 | 33.00 | 44.00 | 22.00 | 33.00 | 44.00 | 22.00 | 33.00 | 44.00 |
| Sodium deoxycholate | 1.25 | 1.87 | 2.49 | 1.25 | 1.87 | 2.49 | 1.25 | 1.87 | 2.49 |
| Copovidone | 12.53 | 16.79 | 21.06 | 12.53 | 16.79 | 21.06 | 12.53 | 16.79 | 21.06 |
| Mannitol | 64.23 | 48.33 | 32.45 | 32.12 | 24.17 | 16.23 | - | - | - |
| Trehalose | - | - | - | 32.12 | 24.17 | 16.23 | 64.23 | 48.33 | 32.45 |
| Z-Average [nm] | PDI | |
|---|---|---|
| 1 | 239.4 | 0.15 |
| 2 | 237.4 | 0.18 |
| 3 | 238.3 | 0.16 |
| 4 | 242.2 | 0.13 |
| 5 | 238.4 | 0.18 |
| 6 | 235.7 | 0.16 |
| Mean | 238.6 | 0.16 |
| SD | 2.1 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schönfeld, B.; Sundermann, J.; Keller, B.-L.; Westedt, U.; Heinzerling, O. Transformation of ABT-199 Nanocrystal Suspensions into a Redispersible Drug Product—Impact of Vacuum Drum Drying, Spray Drying and Tableting on Re-Nanodispersibility. Pharmaceutics 2024, 16, 782. https://doi.org/10.3390/pharmaceutics16060782
Schönfeld B, Sundermann J, Keller B-L, Westedt U, Heinzerling O. Transformation of ABT-199 Nanocrystal Suspensions into a Redispersible Drug Product—Impact of Vacuum Drum Drying, Spray Drying and Tableting on Re-Nanodispersibility. Pharmaceutics. 2024; 16(6):782. https://doi.org/10.3390/pharmaceutics16060782
Chicago/Turabian StyleSchönfeld, Barbara, Julius Sundermann, Benjamin-Luca Keller, Ulrich Westedt, and Oliver Heinzerling. 2024. "Transformation of ABT-199 Nanocrystal Suspensions into a Redispersible Drug Product—Impact of Vacuum Drum Drying, Spray Drying and Tableting on Re-Nanodispersibility" Pharmaceutics 16, no. 6: 782. https://doi.org/10.3390/pharmaceutics16060782
APA StyleSchönfeld, B., Sundermann, J., Keller, B.-L., Westedt, U., & Heinzerling, O. (2024). Transformation of ABT-199 Nanocrystal Suspensions into a Redispersible Drug Product—Impact of Vacuum Drum Drying, Spray Drying and Tableting on Re-Nanodispersibility. Pharmaceutics, 16(6), 782. https://doi.org/10.3390/pharmaceutics16060782







