Process-Induced Crystal Surface Anisotropy and the Impact on the Powder Properties of Odanacatib
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Recrystallisation to Obtain Single Crystals
2.3. Specific Surface Area Determination Using BET
2.3.1. N2 Adsorption
2.3.2. IGC Octane Isotherm
2.3.3. Particle Size Distribution (PSD)
2.4. Surface Energy Heterogeneity Analysis
2.5. Contact Angle Measurements
2.6. X-ray Diffraction (PXRD)
2.6.1. Powder X-ray Diffraction (PXRD)
2.6.2. Single-Crystal X-ray Diffraction (SC-XRD)
2.7. X-ray Photoelectron Spectroscopy (XPS)
2.7.1. Powder Samples
2.7.2. Single Crystal
3. Results
3.1. Surface Area Using N2 Adsorption and IGC
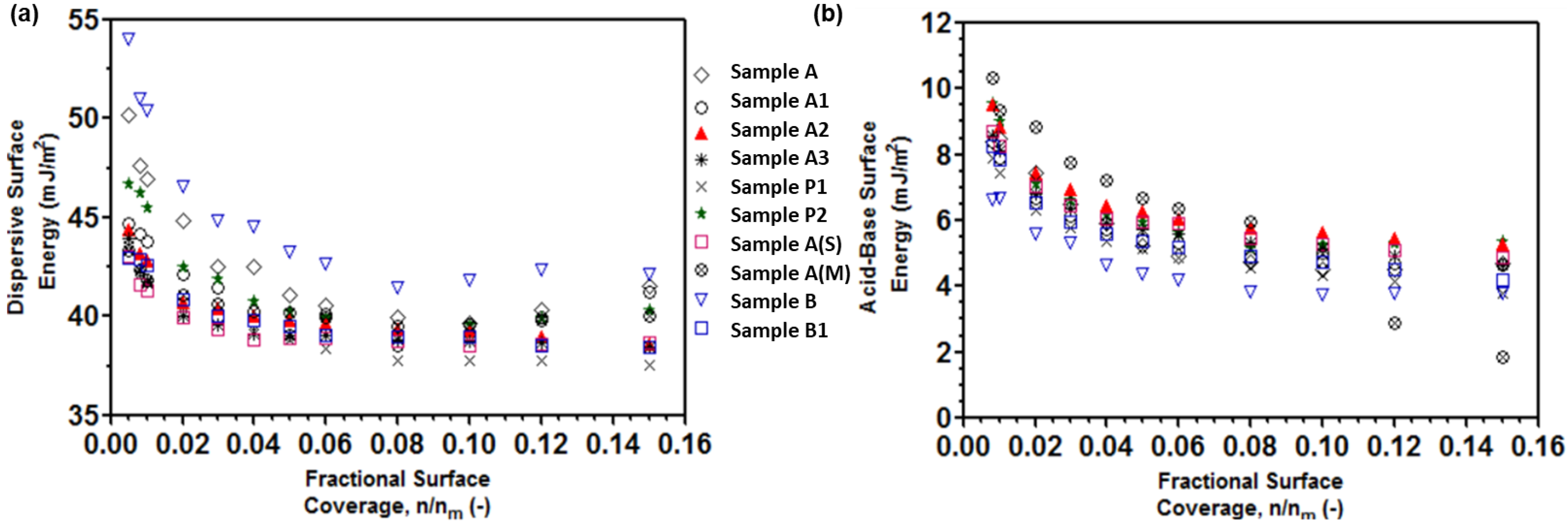
3.2. Surface Energy Heterogeneity of Processed APIs
3.3. Surface Wettability as a Function of Storage Time
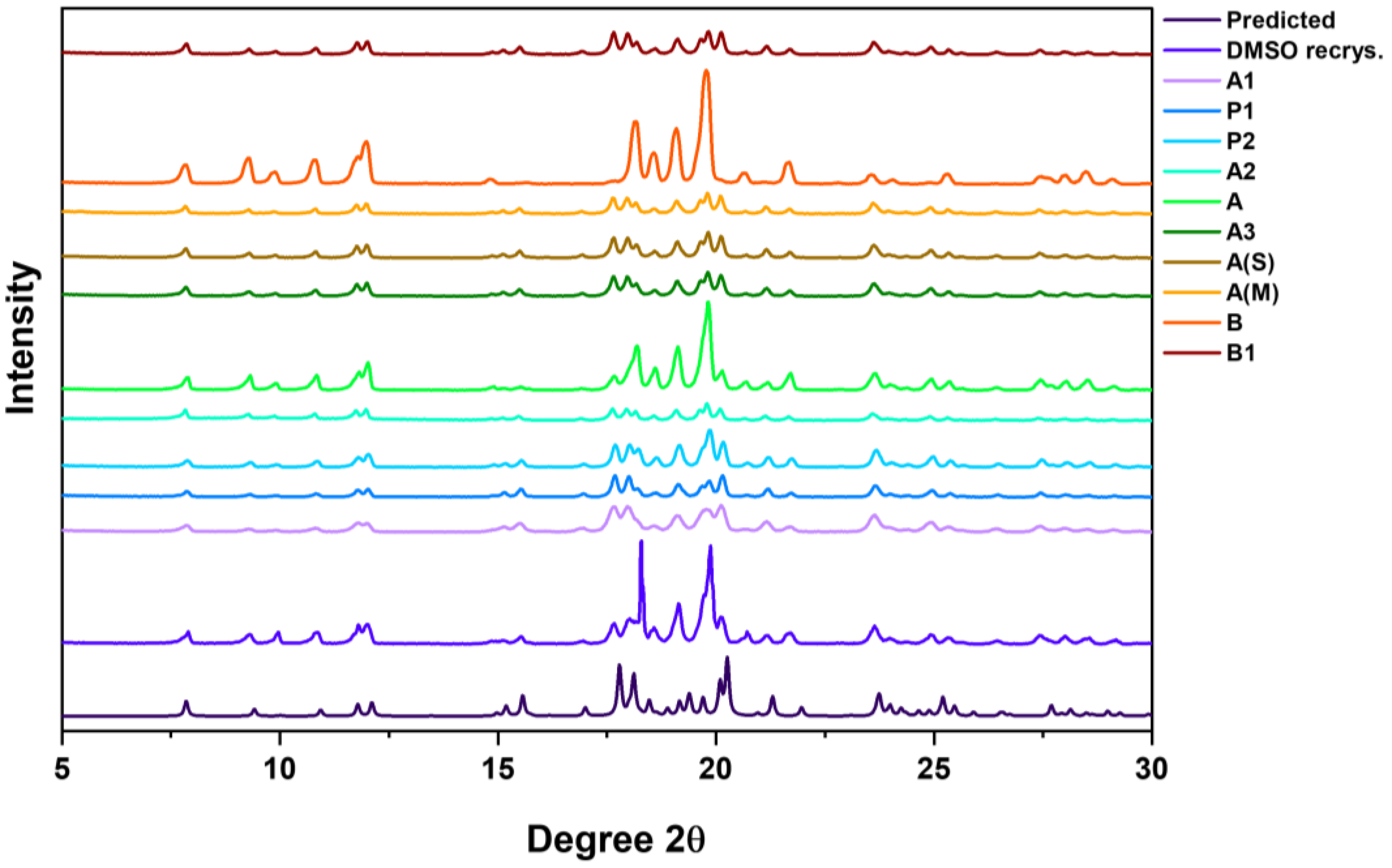
3.4. PXRD
3.5. XPS
3.5.1. Single-Crystal Facet Analysis and Indexing
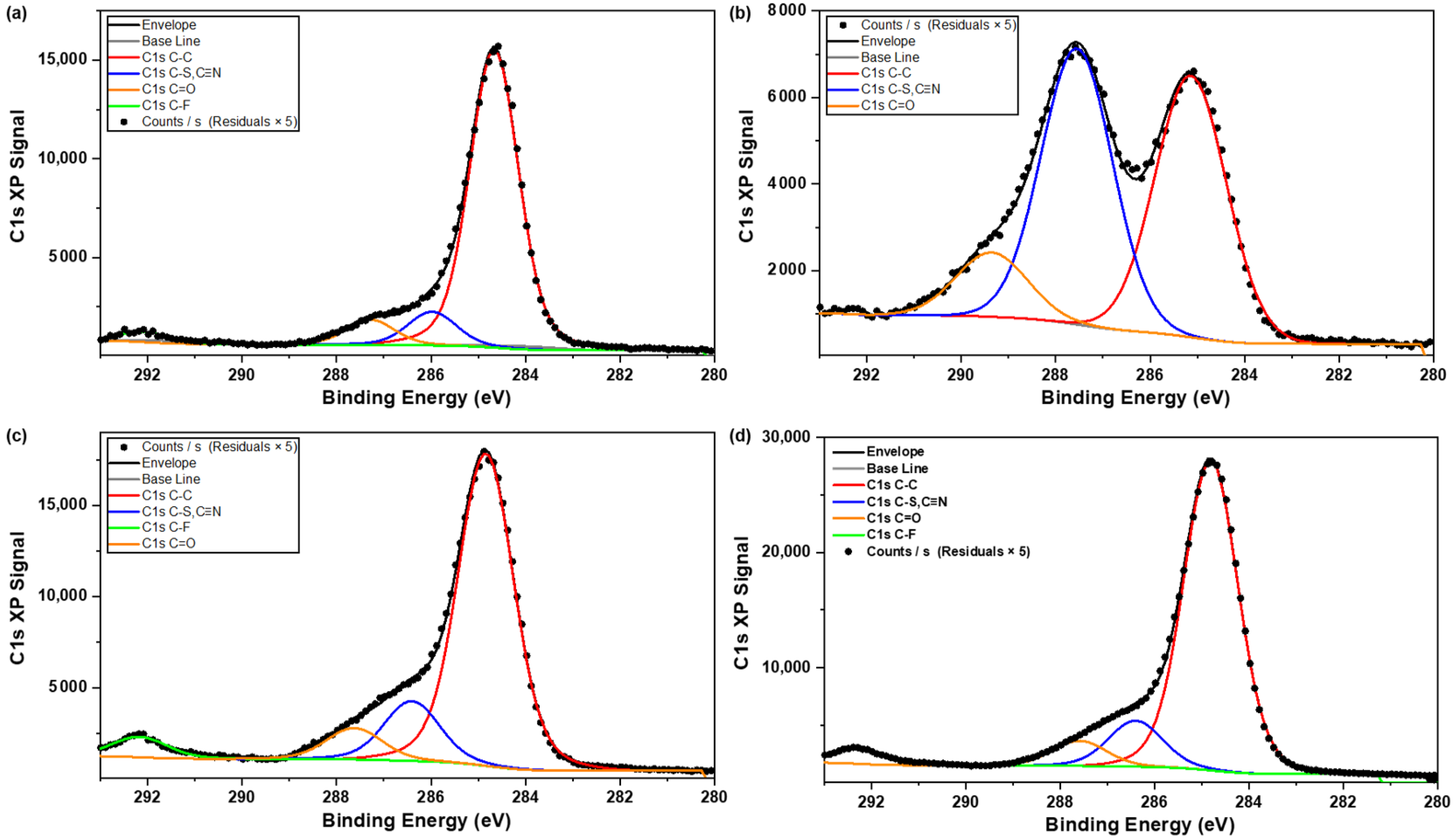
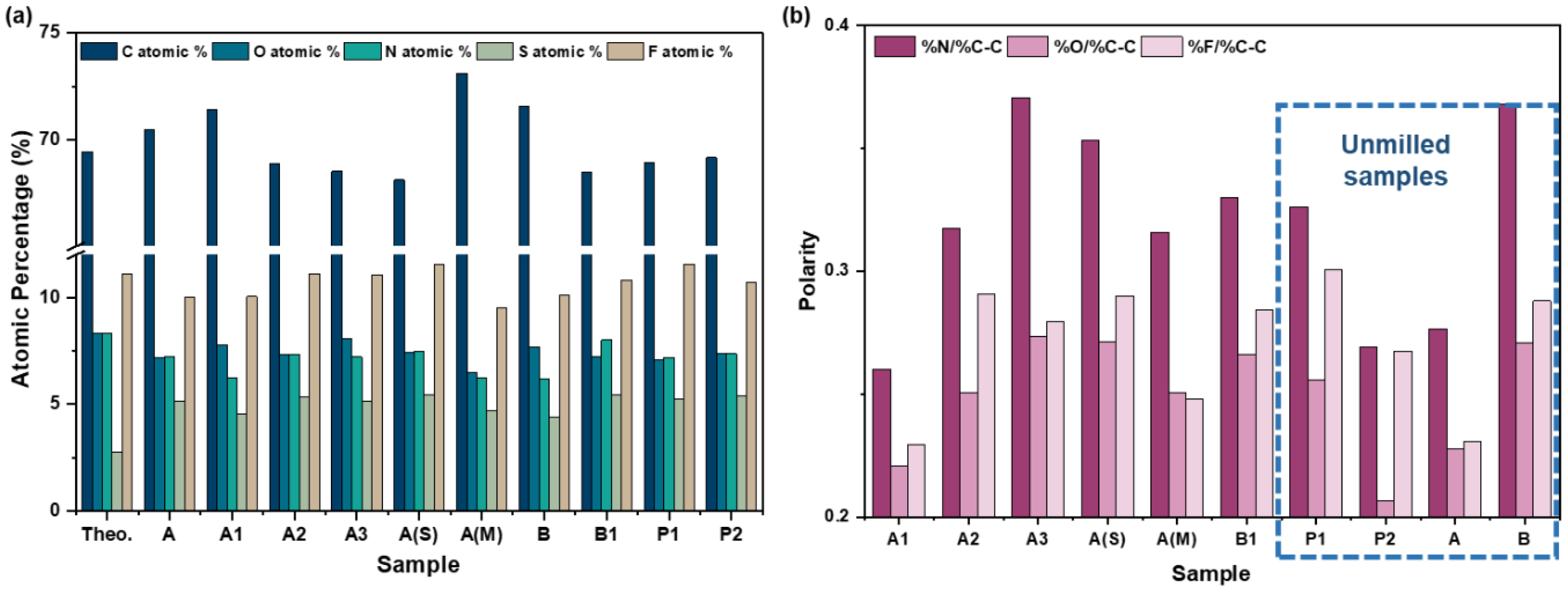
3.5.2. Powder Samples
4. Discussion
4.1. Correlations between Orthogonal Techniques
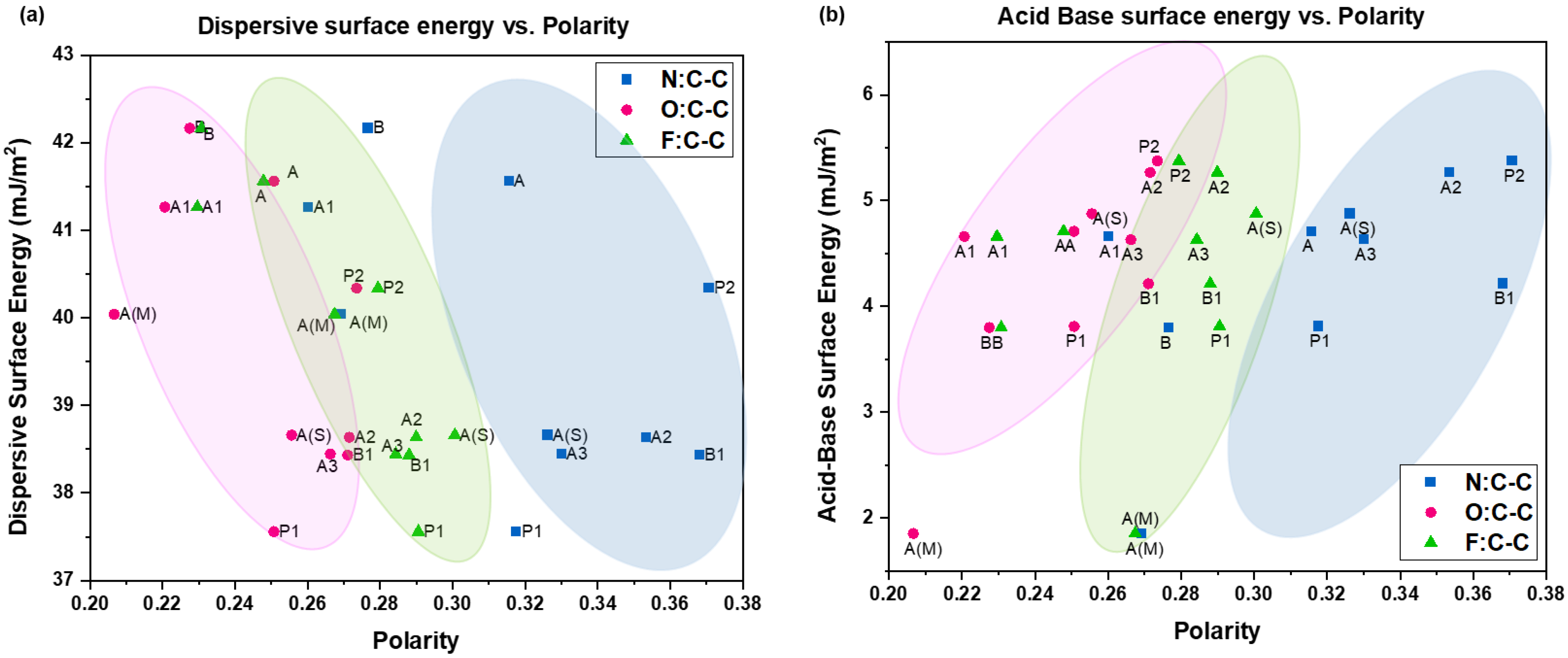
4.1.1. Surface Energy vs. Polarity
4.1.2. Processing History and Polarity
4.2. Correlations between Powder Properties and Performance Indicators
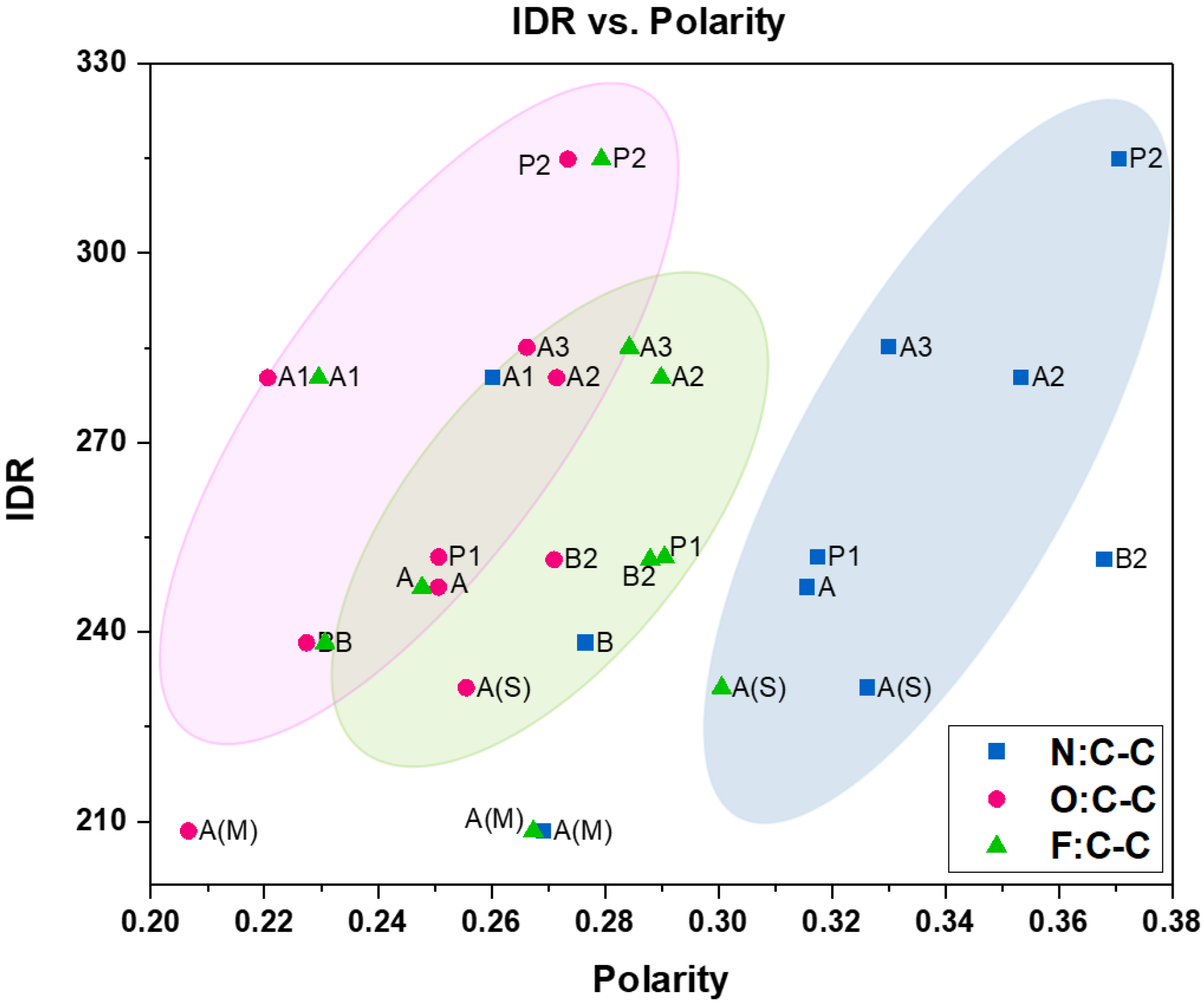
4.2.1. Polarity vs. Intrinsic Dissolution Rate (IDR)
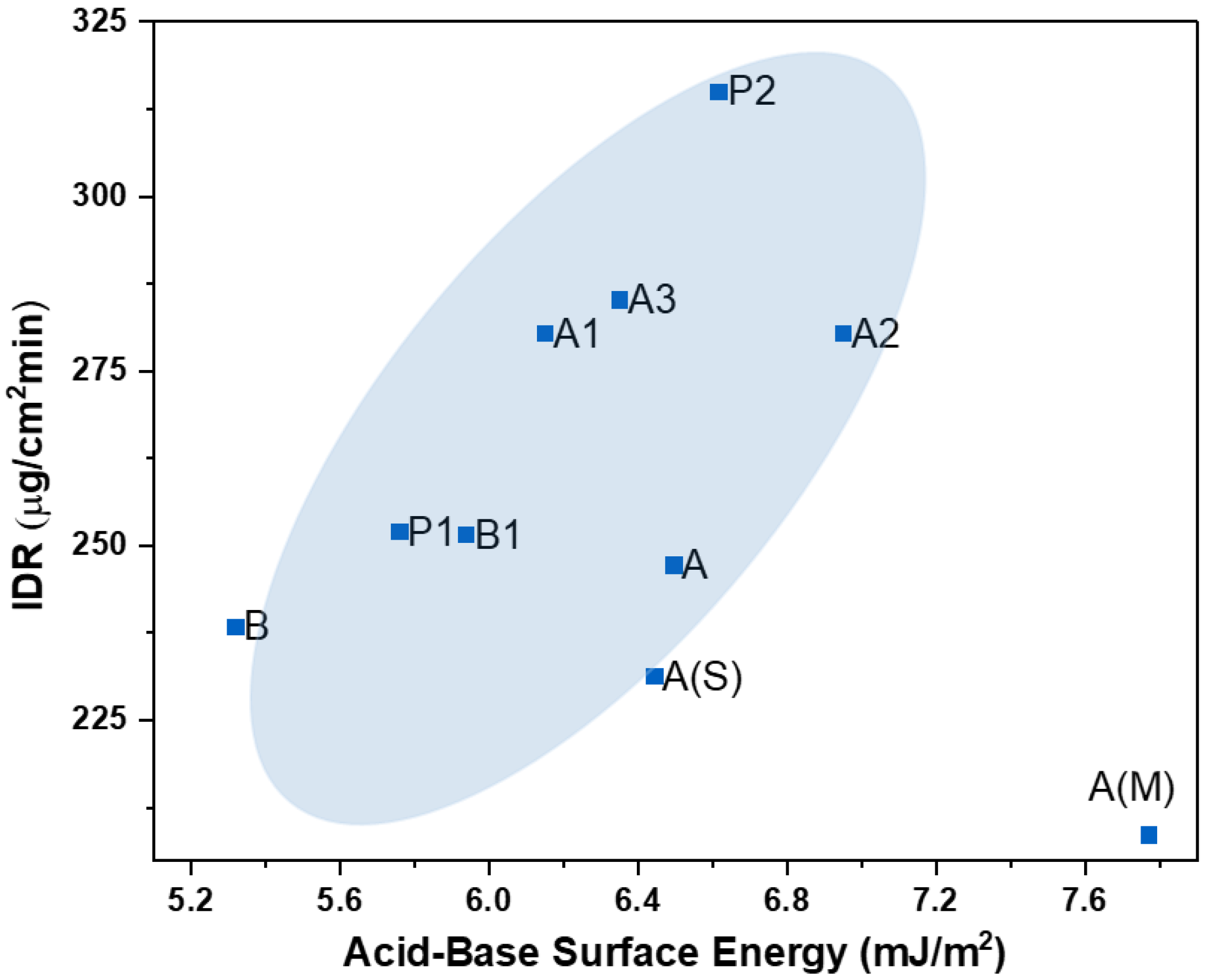
4.2.2. Intrinsic Dissolution Rate (IDR) vs. Acid–Base Surface Energy
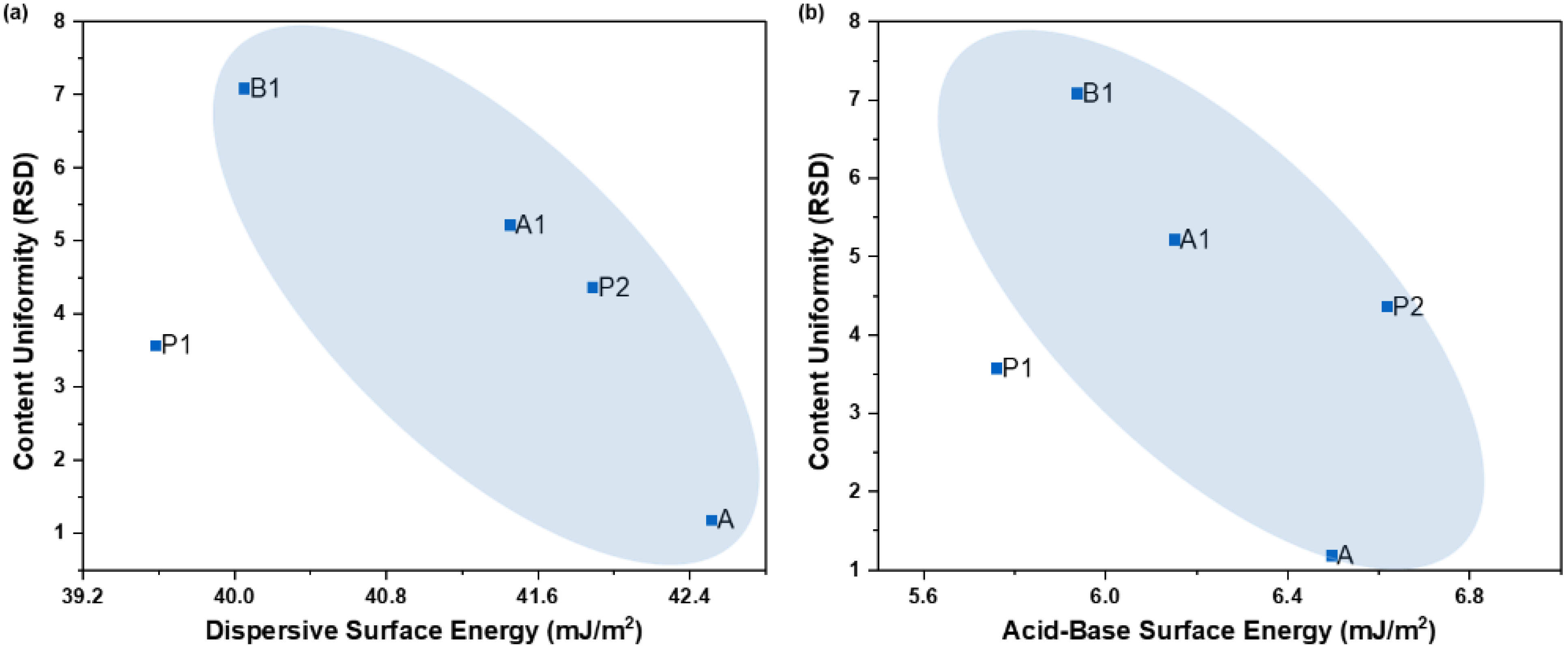
4.2.3. Content Uniformity vs. Surface Energy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karde, V.; Ghoroi, C. Influence of surface modification on wettability and surface energy characteristics of pharmaceutical excipient powders. Int. J. Pharm. 2014, 475, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Das, S.C.; Zhou, Q.; Morton, D.A.V.; Larson, I.; Stewart, P.J. Use of surface energy distributions by inverse gas chromatography to understand mechanofusion processing and functionality of lactose coated with magnesium stearate. Eur. J. Pharm. Sci. 2011, 43, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Jallo, L.J.; Ghoroi, C.; Gurumurthy, L.; Patel, U.; Davé, R.N. Improvement of flow and bulk density of pharmaceutical powders using surface modification. Int. J. Pharm. 2012, 423, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Castillo, C.; Sayedahmed, M.; Davé, R.N. Reduced Fine API Agglomeration After Dry Coating for Enhanced Blend Uniformity and Processability of Low Drug Loaded Blends. Pharm. Res. 2022, 39, 3155–3174. [Google Scholar] [CrossRef] [PubMed]
- Schenck, L.; Neri, C.; Jia, X.; Schafer, W.; Axnanda, S.; Canfield, N.; Li, F.; Shah, V. A Co-Processed API Approach for a Shear Sensitive Compound Affording Improved Chemical Stability and Streamlined Drug Product Processing. J. Pharm. Sci. 2021, 110, 3238–3245. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ghoroi, C.; To, D.; Chen, Y.; Davé, R. Simultaneous micronization and surface modification for improvement of flow and dissolution of drug particles. Int. J. Pharm. 2011, 415, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, H.; He, N.; Deng, Y. Effects of surface modifications on the physicochemical properties of iron oxide nanoparticles and their performance as anticancer drug carriers. Chin. Chem. Lett. 2018, 29, 1829–1833. [Google Scholar] [CrossRef]
- Swaminathan, S.; Ganapathy, B.; Wang, M.; Wang, F.; Wooding, J.; Frankel, J.; Chiruvolu, S.; Rengarajan, S.; Narwankar, P. Improving the processability of pharmaceutical powders using atomic layer coating. Powder Technol. 2023, 425, 118525. [Google Scholar] [CrossRef]
- Alvarez, A.J.; Myerson, A.S. Continuous plug flow crystallization of pharmaceutical compounds. Cryst. Growth Des. 2010, 10, 2219–2228. [Google Scholar] [CrossRef]
- Hadjittofis, E.; Isbell, M.A.; Karde, V.; Varghese, S.; Ghoroi, C.; Heng, J.Y.Y. Influences of Crystal Anisotropy in Pharmaceutical Process Development. Pharm. Res. 2018, 35, 100. [Google Scholar] [CrossRef]
- Heng, J.Y.Y.; Bismarck, A.; Williams, D.R. Anisotropic surface chemistry of crystalline pharmaceutical solids. AAPS PharmSciTech 2006, 7, E12–E20. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Worku, Z.A.; Gao, Y.; Kamaraju, V.K.; Glennon, B.; Babu, R.P.; Healy, A.M. Comparison of wet milling and dry milling routes for ibuprofen pharmaceutical crystals and their impact on pharmaceutical and biopharmaceutical properties. Powder Technol. 2018, 330, 228–238. [Google Scholar] [CrossRef]
- Shekunov, B.Y.; York, P. Crystallization processes in pharmaceutical technology and drug delivery design. J. Cryst. Growth 2000, 211, 122–136. [Google Scholar] [CrossRef]
- Kim, S.; Lotz, B.; Lindrud, M.; Girard, K.; Moore, T.; Nagarajan, K.; Alvarez, M.; Lee, T.; Nikfar, F.; Davidovich, M.; et al. Control of the particle properties of a drug substance by crystallization engineering and the effect on drug product formulation. Org. Process Res. Dev. 2005, 9, 894–901. [Google Scholar] [CrossRef]
- Ter Horst, J.H.; Geertman, R.M.; Van Rosmalen, G.M. The effect of solvent on crystal morphology. J. Cryst. Growth 2001, 230, 277–284. [Google Scholar] [CrossRef]
- Heng, J.Y.Y.; Thielmann, F.; Williams, D.R. The effects of milling on the surface properties of form I paracetamol crystals. Pharm. Res. 2006, 23, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.; Naderi, M.; Heng, J.Y.Y.; Williams, D.R.; Thielmann, F.; Bouza, P.; Keith, A.R.; Thiele, G.; Burnett, D.J. Effect of milling on particle shape and surface energy heterogeneity of needle-Shaped crystals. Pharm. Res. 2012, 29, 2806–2816. [Google Scholar] [CrossRef]
- Han, X.; Jallo, L.; To, D.; Ghoroi, C.; Davé, R. Passivation of high-surface-energy sites of milled ibuprofen crystals via dry coating for reduced cohesion and improved flowability. J. Pharm. Sci. 2013, 102, 2282–2296. [Google Scholar] [CrossRef] [PubMed]
- Shah, U.V.; Wang, Z.; Olusanmi, D.; Narang, A.S.; Hussain, M.A.; Tobyn, M.J.; Heng, J.Y.Y. Effect of milling temperatures on surface area, surface energy and cohesion of pharmaceutical powders. Int. J. Pharm. 2015, 495, 234–240. [Google Scholar] [CrossRef]
- Dujardin, N.; Willart, J.F.; Dudognon, E.; Hédoux, A.; Guinet, Y.; Paccou, L.; Chazallon, B.; Descamps, M. Solid state vitrification of crystalline α and β-D-glucose by mechanical milling. Solid State Commun. 2008, 148, 78–82. [Google Scholar] [CrossRef]
- Gil-Chávez, J.; Padhi, S.S.P.; Leopold, C.S.; Smirnova, I. Application of Aquasolv Lignin in ibuprofen-loaded pharmaceutical formulations obtained via direct compression and wet granulation. Int. J. Biol. Macromol. 2021, 174, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Shirazian, S.; Ranade, V.; Walker, G.M.; Kumar, A. Challenges and opportunities in modelling wet granulation in pharmaceutical industry—A critical review. Powder Technol. 2022, 403, 117380. [Google Scholar] [CrossRef]
- Sen, M.; Ramachandran, R. A multi-dimensional population balance model approach to continuous powder mixing processes. Adv. Powder Technol. 2013, 24, 51–59. [Google Scholar] [CrossRef]
- Fathollahi, S.; Faulhammer, E.; Glasser, B.J.; Khinast, J.G. Impact of powder composition on processing-relevant properties of pharmaceutical materials: An experimental study. Adv. Powder Technol. 2020, 31, 2991–3003. [Google Scholar] [CrossRef]
- Mortier, S.T.F.C.; De Beer, T.; Gernaey, K.V.; Remon, J.P.; Vervaet, C.; Nopens, I. Mechanistic modelling of fluidized bed drying processes of wet porous granules: A review. Eur. J. Pharm. Biopharm. 2011, 79, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Kenekar, V.V.; Ghugare, S.B.; Patil-Shinde, V. Multi-objective optimization of high-shear wet granulation process for better granule properties and fluidized bed drying characteristics. Powder Technol. 2023, 420, 118373. [Google Scholar] [CrossRef]
- Dai, S.; Xu, B.; Zhang, Z.; Yu, J.; Wang, F.; Shi, X.; Qiao, Y. A compression behavior classification system of pharmaceutical powders for accelerating direct compression tablet formulation design. Int. J. Pharm. 2019, 572, 118742. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Tanaka, C.; Yuasa, H.; Sakamoto, T. Utility of Microcrystalline Cellulose for Improving Drug Content Uniformity in Tablet Manufacturing Using Direct Powder Compression. AAPS PharmSciTech 2019, 20, 151. [Google Scholar] [CrossRef] [PubMed]
- Karde, V.; Khala, M.J.; Hare, C.; Heng, J.Y.Y. Use of shear sensitive coloured guest component to track powder mixing in adhesive binary mixtures. Powder Technol. 2023, 414, 118068. [Google Scholar] [CrossRef]
- Salehi, H.; Karde, V.; Hajmohammadi, H.; Dissanayake, S.; Larsson, S.H.; Heng, J.Y.Y.; Bradley, M. Understanding flow properties of mannitol powder at a range of temperature and humidity. Int. J. Pharm. 2021, 596, 120244. [Google Scholar] [CrossRef]
- Karde, V.; Jefferson, A.E.; Hebbink, G.A.; Heng, J.Y.Y. Investigating sizing induced surface alterations in crystalline powders using surface energy heterogeneity determination. Powder Technol. 2022, 395, 645–651. [Google Scholar] [CrossRef]
- Chen, W.; Karde, V.; Cheng, T.N.H.; Ramli, S.S.; Heng, J.Y.Y. Surface hydrophobicity: Effect of alkyl chain length and network homogeneity. Front. Chem. Sci. Eng. 2021, 15, 90–98. [Google Scholar] [CrossRef]
- Ho, R.; Hinder, S.J.; Watts, J.F.; Dilworth, S.E.; Williams, D.R.; Heng, J.Y.Y. Determination of surface heterogeneity of d-mannitol by sessile drop contact angle and finite concentration inverse gas chromatography. Int. J. Pharm. 2010, 387, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Krishna, D.N.G.; Philip, J. Review on surface-characterization applications of X-ray photoelectron spectroscopy (XPS): Recent developments and challenges. Appl. Surf. Sci. Adv. 2022, 12, 100332. [Google Scholar] [CrossRef]
- Heng, J.Y.Y.; Bismarck, A.; Lee, A.F.; Wilson, K.; Williams, D.R. Anisotropic surface energetics and wettability of macroscopic form I paracetamol crystals. Langmuir 2006, 22, 2760–2769. [Google Scholar] [CrossRef]
- Heng, J.Y.Y.; Williams, D.R. Wettability of paracetamol polymorphic forms I and II. Langmuir 2006, 22, 6905–6909. [Google Scholar] [CrossRef]
- Wang, Z.; Solomos, M.; Axnanda, S.; Chen, C.; Figus, M.; Schenck, L.; Sun, C.C. Varied Bulk Powder Properties of Micro-Sized API within Size Specifications as a Result of Particle Engineering Methods. Pharmaceutics 2022, 14, 1901. [Google Scholar] [CrossRef]
- Gauthier, J.Y.; Chauret, N.; Cromlish, W.; Desmarais, S.; Duong, L.T.; Falgueyret, J.P.; Kimmel, D.B.; Lamontagne, S.; Léger, S.; LeRiche, T.; et al. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg. Med. Chem. Lett. 2008, 18, 923–928. [Google Scholar] [CrossRef]
- Biran, I.; Rosenne, S.; Weissman, H.; Tsarfati, Y.; Houben, L.; Rybtchinski, B. Organic Crystal Growth: Hierarchical Self-Assembly Involving Nonclassical and Classical Steps. Cryst. Growth Des. 2022, 22, 6647–6655. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am.Chem.Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Ho, R.; Heng, J.Y.Y. A review of inverse gas chromatography and its development as a tool to characterize anisotropic surface properties of pharmaceutical solids. KONA Powder Part. J. 2012, 30, 164–180. [Google Scholar] [CrossRef]
- Schultz, J.; Lavielle, L.; Martin, C. The Role of the Interface in Carbon Fibre-Epoxy Composites. J. Adhes. 1987, 23, 45–60. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Niederquell, A.; Vraníková, B.; Kuentz, M. Study of Disordered Mesoporous Silica Regarding Intrinsic Compound Affinity to the Carrier and Drug-Accessible Surface Area. Mol. Pharm. 2023, 20, 6301–6310. [Google Scholar] [CrossRef] [PubMed]
- Maclean, N.; Khadra, I.; Mann, J.; Abbott, A.; Mead, H.; Markl, D. Formulation-dependent stability mechanisms affecting dissolution performance of directly compressed griseofulvin tablets. Int. J. Pharm. 2023, 631, 122473. [Google Scholar] [CrossRef]
- Bravais, A. Études Cristallographiques; Gauthier-Villars: Paris, France, 1913. [Google Scholar]
- Friedel, G. Leçons de Cristallographie; A. Hermann: Paris, France, 1911. [Google Scholar]
- Donnay, J.D.H.; Harker, D. A New Law of Crystal Morphology Extending the Law of Bravais. Am. Miner. 1937, 22, 446. [Google Scholar]
- Cypes, S.H.; Wenslow, R.M.; Thomas, S.M.; Chen, A.M.; Dorwart, J.G.; Corte, J.R.; Kaba, M. Drying an organic monohydrate: Crystal form instabilities and a factory-scale drying scheme to ensure monohydrate preservation. Org. Process Res. Dev. 2004, 8, 576–582. [Google Scholar] [CrossRef]
- Borsos, Á.; Lakatos, B.G. Investigation and simulation of crystallization of high aspect ratio crystals with fragmentation. Chem. Eng. Res. Des. 2014, 92, 1133–1141. [Google Scholar] [CrossRef]
- Perini, G.; Salvatori, F.; Ochsenbein, D.R.; Mazzotti, M.; Vetter, T. Filterability prediction of needle-like crystals based on particle size and shape distribution data. Sep. Purif. Technol. 2019, 211, 768–781. [Google Scholar] [CrossRef]




| Sample | d50 (µm) | Description |
|---|---|---|
| A | 21.8 | Heat cool in acetone:DIW parent lot for dry milling |
| A1 | 3.4 | Spiral milled Sample A, annealed |
| A2 | 3.2 | Spiral milled Sample A, annealed (2nd sample, different lot to assess PSD impact) |
| A3 | 4.9 | Spiral milled Sample A, held at −8 °C after milling |
| A(S) | 1.9 | Spiral milled Sample A, with water soluble processing aid (1% SSF) |
| A(M) | 2.6 | Spiral milled Sample A, with water insoluble processing aid (1% MgSt) |
| B | 15.8 | MTBE:acetone recrystallization—modified solvent system to alter morphology and explore impact on breakage planes during spiral milling |
| B1 | 1.6 | Spiral milled sample B |
| P1 | 2.7 | Direct precipitation from DMF:DIW |
| P2 | 4.9 | Direct precipitation from acetone:DIW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bade, I.; Karde, V.; Schenck, L.; Solomos, M.; Figus, M.; Chen, C.; Axnanda, S.; Heng, J.Y.Y. Process-Induced Crystal Surface Anisotropy and the Impact on the Powder Properties of Odanacatib. Pharmaceutics 2024, 16, 883. https://doi.org/10.3390/pharmaceutics16070883
Bade I, Karde V, Schenck L, Solomos M, Figus M, Chen C, Axnanda S, Heng JYY. Process-Induced Crystal Surface Anisotropy and the Impact on the Powder Properties of Odanacatib. Pharmaceutics. 2024; 16(7):883. https://doi.org/10.3390/pharmaceutics16070883
Chicago/Turabian StyleBade, Isha, Vikram Karde, Luke Schenck, Marina Solomos, Margaret Figus, Chienhung Chen, Stephanus Axnanda, and Jerry Y. Y. Heng. 2024. "Process-Induced Crystal Surface Anisotropy and the Impact on the Powder Properties of Odanacatib" Pharmaceutics 16, no. 7: 883. https://doi.org/10.3390/pharmaceutics16070883
APA StyleBade, I., Karde, V., Schenck, L., Solomos, M., Figus, M., Chen, C., Axnanda, S., & Heng, J. Y. Y. (2024). Process-Induced Crystal Surface Anisotropy and the Impact on the Powder Properties of Odanacatib. Pharmaceutics, 16(7), 883. https://doi.org/10.3390/pharmaceutics16070883






