Development and Quality Control of a Population Pharmacokinetic Model Library for Caspofungin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Development of Model Library
2.1.1. Search Strategy
2.1.2. Literature Evaluation
2.1.3. Data Extraction
2.1.4. Model Re-Implementation
2.2. Quality Control of Model Library
2.2.1. Comparison of Studies
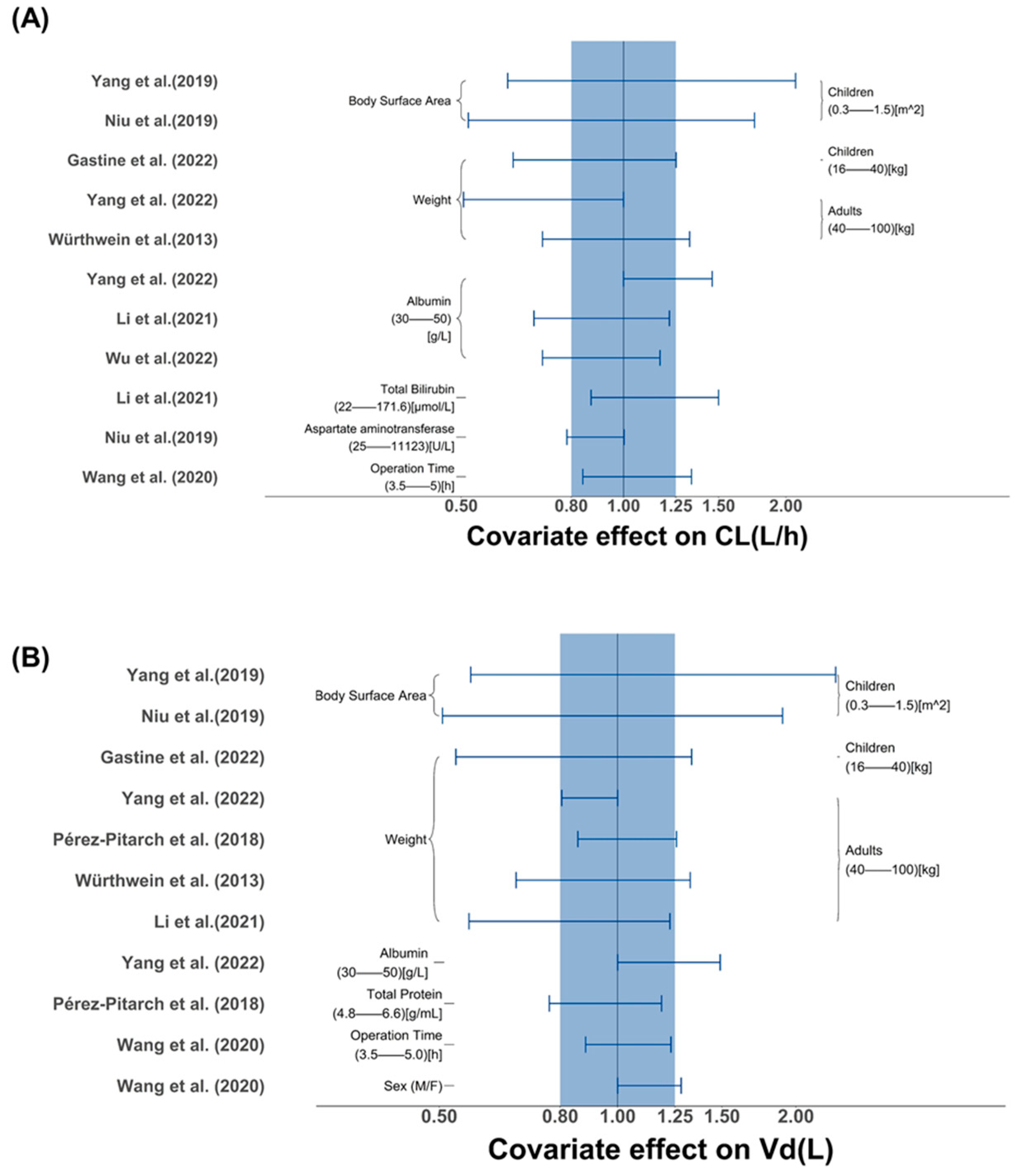
2.2.2. Assessment of Covariates Impact
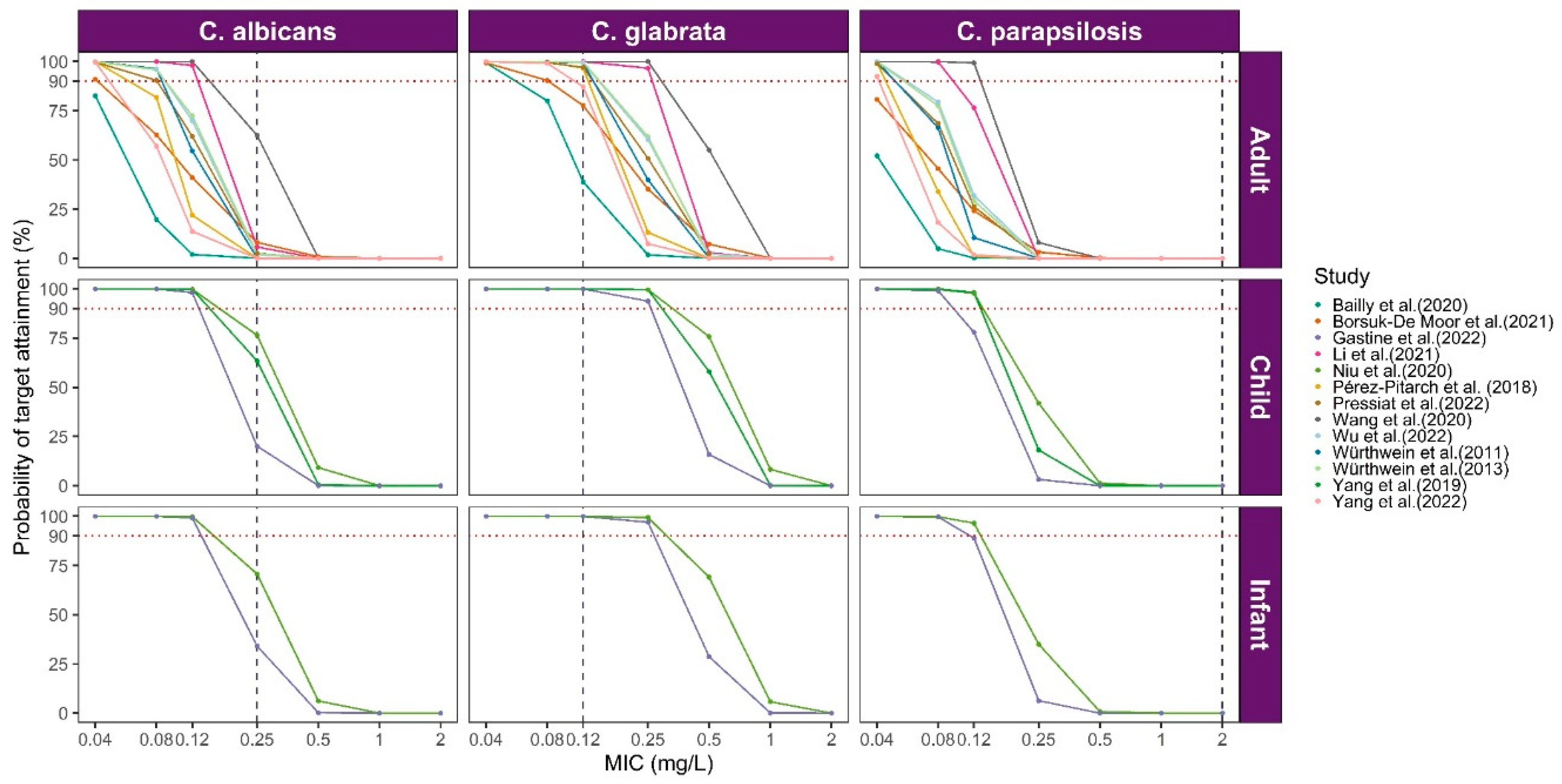
2.3. Application of PopPK Model Library
3. Results
3.1. Overview of Included PopPK Studies for Caspofungin
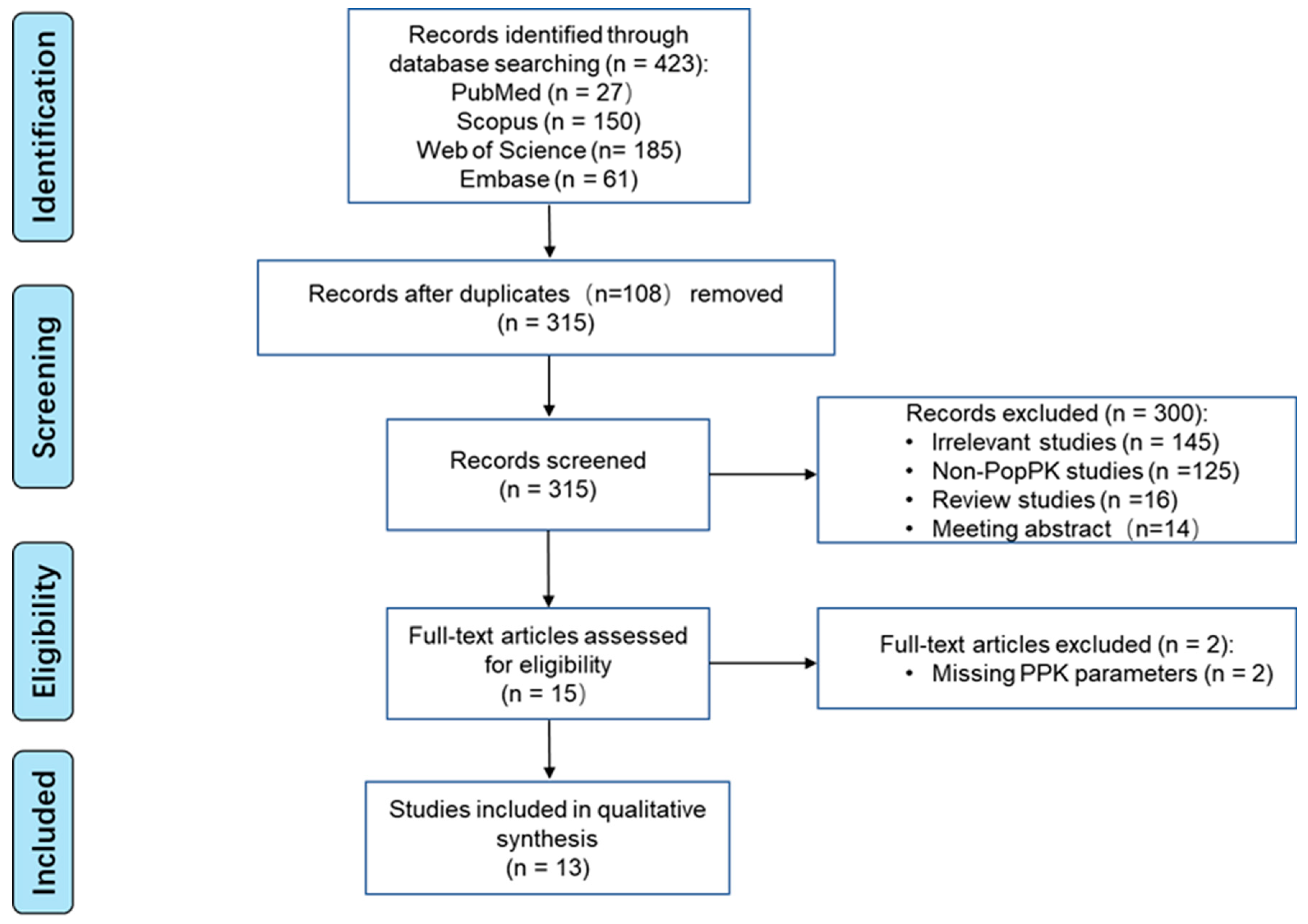
3.1.1. Identification of Included Studies
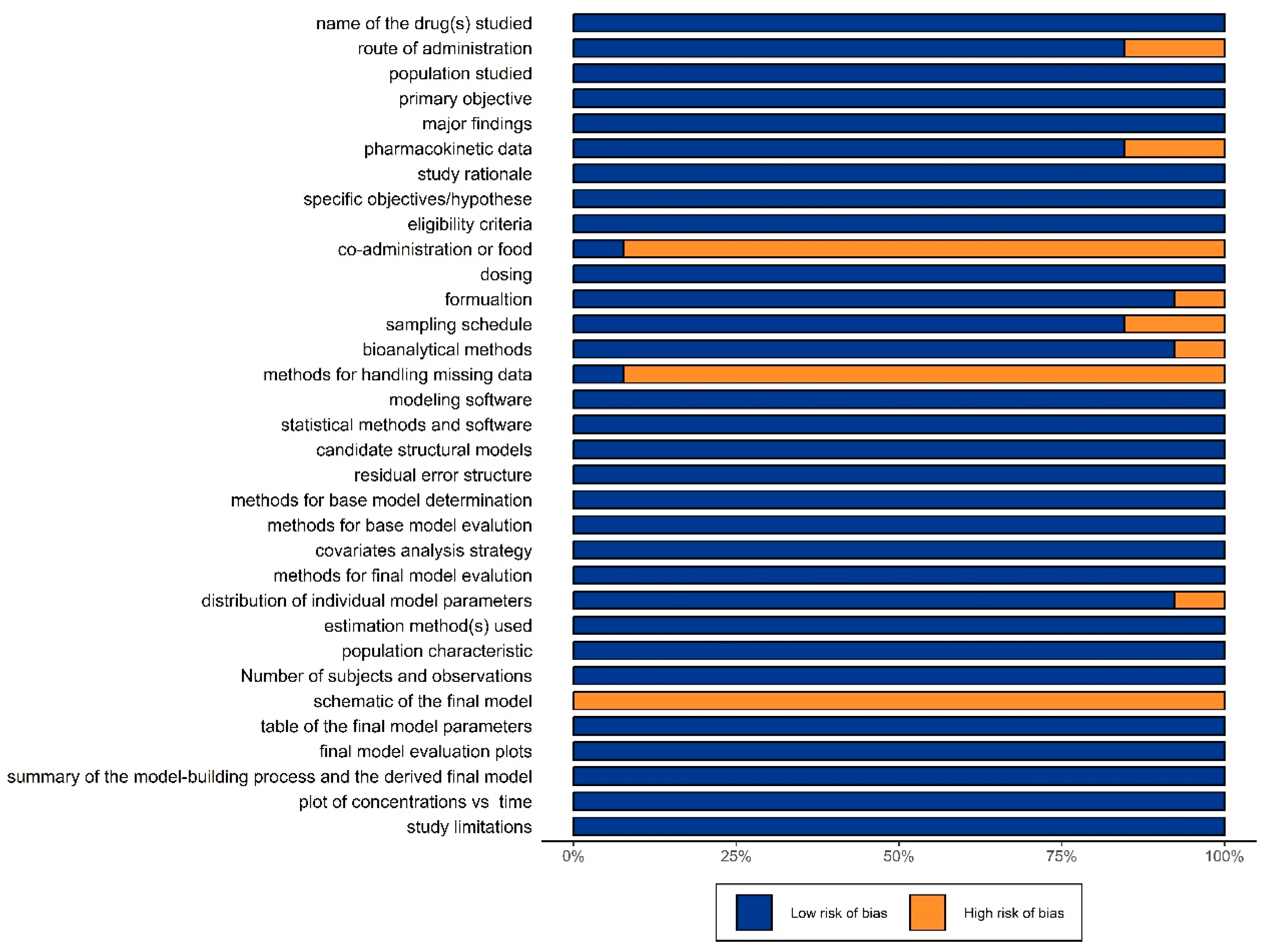
3.1.2. Evaluation of Literature
3.1.3. Study and PopPK Model Characteristics
3.2. Overview of PopPK Model Library
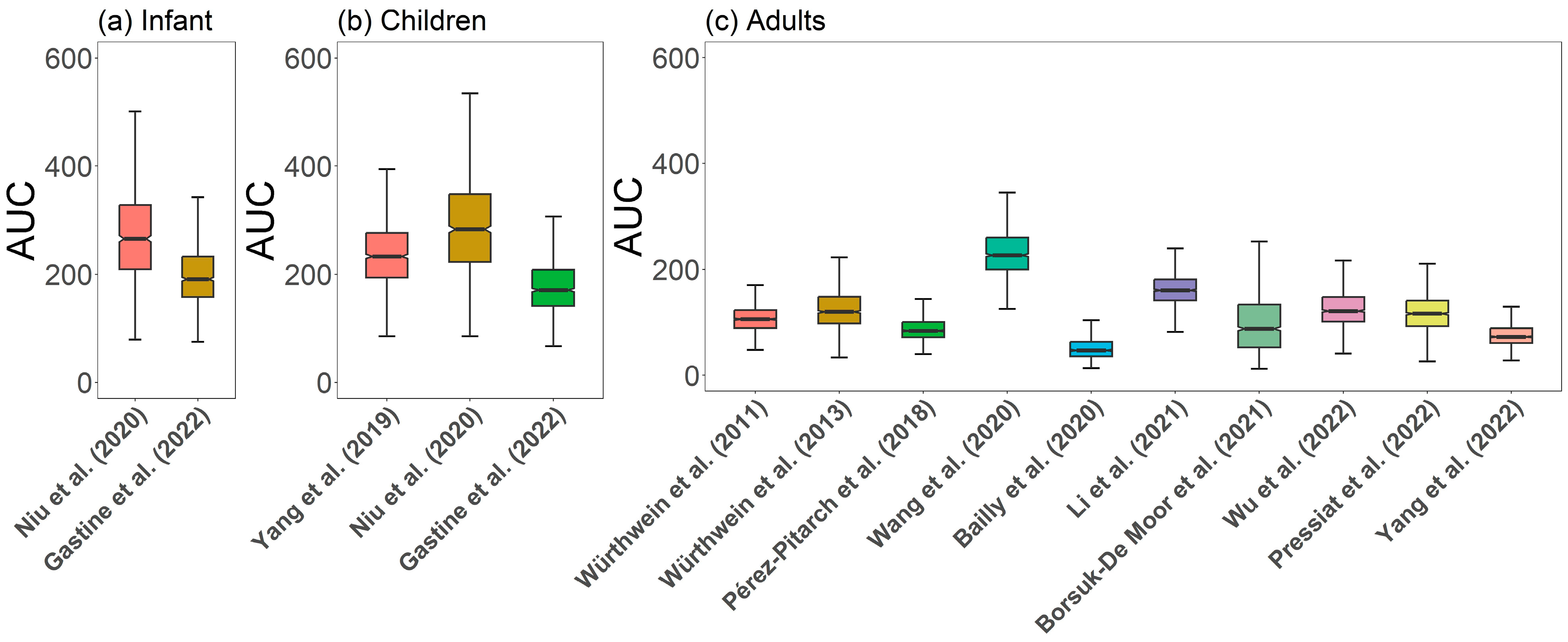
3.2.1. Comparison of Caspofungin PK Profiles
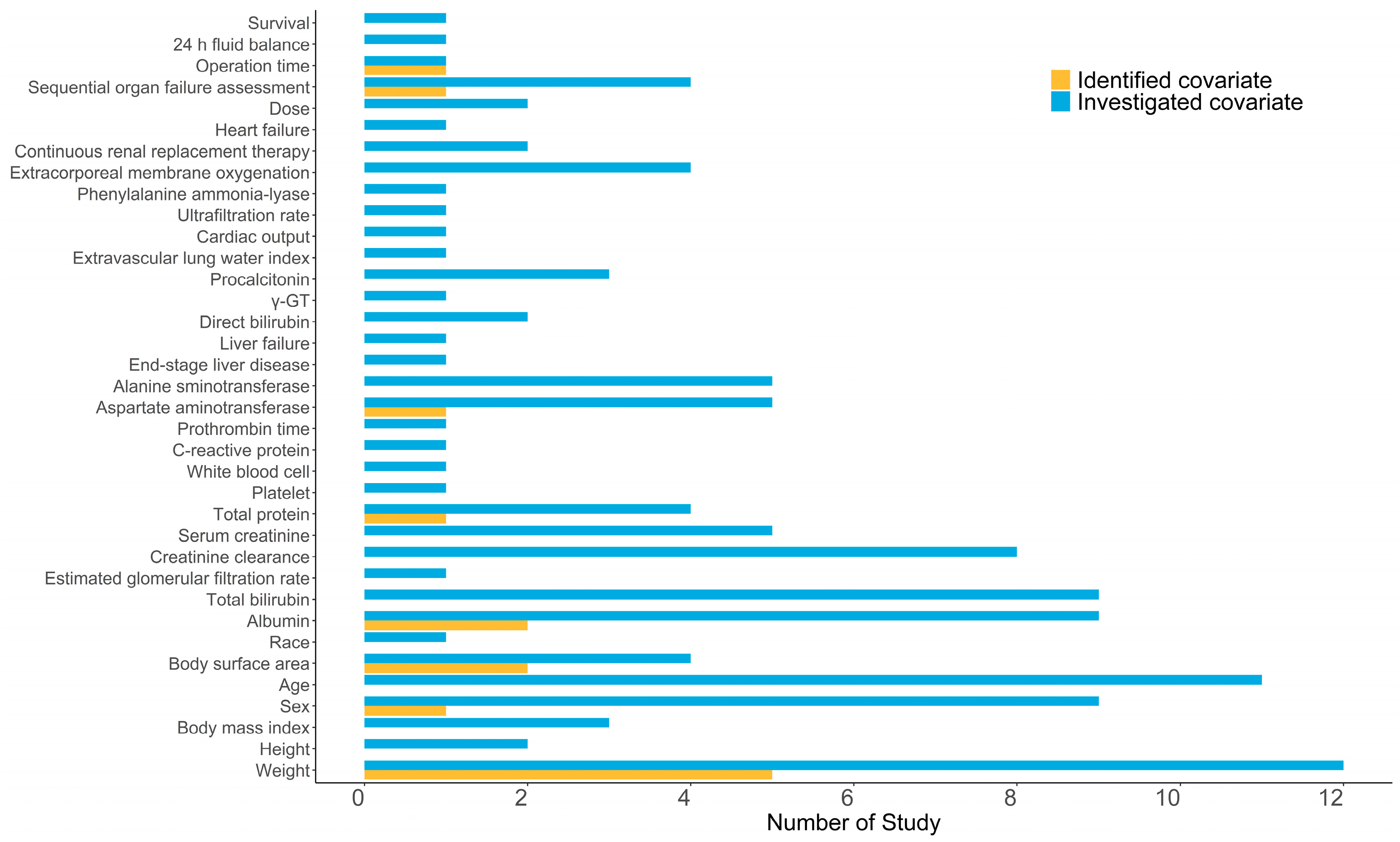
3.2.2. Covariate Screening and Influence Analysis
3.3. Model Library Applications
4. Discussion
4.1. Pediatric Patients
4.2. Adult Patients
4.3. Towards Precision Dosing
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Hope, W.W.; Castagnola, E.; Groll, A.H.; Roilides, E.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Cornely, O.A.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Prevention and management of invasive infections in neonates and children caused by Candida spp. Clin. Microbiol. Infect. 2012, 18 (Suppl. S7), 38–52. [Google Scholar] [CrossRef] [PubMed]
- HIGHLIGHTS OF PRESCRIBING INFORMATION for CANCIDAS-Merck. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/021227Orig1s040lbl.pdf (accessed on 17 September 2023).
- Sinnollareddy, M.G.; Roberts, J.A.; Lipman, J.; Akova, M.; Bassetti, M.; De Waele, J.J.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. Pharmacokinetic variability and exposures of fluconazole, anidulafungin, and caspofungin in intensive care unit patients: Data from multinational Defining Antibiotic Levels in Intensive care unit (DALI) patients Study. Crit. Care 2015, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- van der Elst, K.C.; Veringa, A.; Zijlstra, J.G.; Beishuizen, A.; Klont, R.; Brummelhuis-Visser, P.; Uges, D.R.; Touw, D.J.; Kosterink, J.G.; van der Werf, T.S.; et al. Low Caspofungin Exposure in Patients in Intensive Care Units. Antimicrob. Agents Chemother. 2017, 61, e01582-16. [Google Scholar] [CrossRef] [PubMed]
- van Vianen, W.; de Marie, S.; ten Kate, M.T.; Mathot, R.A.; Bakker-Woudenberg, I.A. Caspofungin: Antifungal activity in vitro, pharmacokinetics, and effects on fungal load and animal survival in neutropenic rats with invasive pulmonary aspergillosis. J. Antimicrob. Chemother. 2006, 57, 732–740. [Google Scholar] [CrossRef]
- Ambrose, P.G.; Bhavnani, S.M.; Rubino, C.M.; Louie, A.; Gumbo, T.; Forrest, A.; Drusano, G.L. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: It’s not just for mice anymore. Clin. Infect. Dis. 2007, 44, 79–86. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Kontoyiannis, D.P.; Chi, J.; Prince, R.A.; Tam, V.H.; Lewis, R.E. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: Evidence of concentration-dependent activity. J. Infect. Dis. 2004, 190, 1464–1471. [Google Scholar] [CrossRef]
- Louie, A.; Deziel, M.; Liu, W.; Drusano, M.F.; Gumbo, T.; Drusano, G.L. Pharmacodynamics of caspofungin in a murine model of systemic candidiasis: Importance of persistence of caspofungin in tissues to understanding drug activity. Antimicrob. Agents Chemother. 2005, 49, 5058–5068. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Diekema, D.J.; Pfaller, M.A.; Bohrmuller, J.; Marchillo, K.; Lepak, A. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob. Agents Chemother. 2010, 54, 2497–2506. [Google Scholar] [CrossRef]
- Borsuk-De Moor, A.; Sysiak-Sławecka, J.; Rypulak, E.; Borys, M.; Piwowarczyk, P.; Raszewski, G.; Onichimowski, D.; Czuczwar, M.; Wiczling, P. Nonstationary Pharmacokinetics of Caspofungin in ICU Patients. Antimicrob. Agents Chemother. 2020, 64, e00345-20. [Google Scholar] [CrossRef]
- Pérez-Pitarch, A.; Ferriols-Lisart, R.; Aguilar, G.; Ezquer-Garín, C.; Belda, F.J.; Guglieri-López, B. Dosing of caspofungin based on a pharmacokinetic/pharmacodynamic index for the treatment of invasive fungal infections in critically ill patients on continuous venovenous haemodiafiltration. Int. J. Antimicrob. Agents 2018, 51, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Sheiner, L.B.; Beal, S.; Rosenberg, B.; Marathe, V.V. Forecasting individual pharmacokinetics. Clin. Pharmacol. Ther. 1979, 26, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ju, G.; Yang, W.; Chen, L.; Xu, N.; He, Q.; Zhu, X.; Ouyang, D. Escitalopram Personalized Dosing: A Population Pharmacokinetics Repository Method. Drug Des. Devel. Ther. 2023, 17, 2955–2967. [Google Scholar] [CrossRef] [PubMed]
- Jamsen, K.M.; McLeay, S.C.; Barras, M.A.; Green, B. Reporting a population pharmacokinetic-pharmacodynamic study: A journal’s perspective. Clin. Pharmacokinet. 2014, 53, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Kanji, S.; Hayes, M.; Ling, A.; Shamseer, L.; Chant, C.; Edwards, D.J.; Edwards, S.; Ensom, M.H.; Foster, D.R.; Hardy, B.; et al. Reporting Guidelines for Clinical Pharmacokinetic Studies: The ClinPK Statement. Clin. Pharmacokinet. 2015, 54, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Fancher, K.M.; Sacco, A.J.; Gwin, R.C.; Gormley, L.K.; Mitchell, C.B. Comparison of two different formulas for body surface area in adults at extremes of height and weight. J. Oncol. Pharm. Pract. 2016, 22, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.R.; Wang, C.Y.; Zhu, X.; Jiao, Z. Population Pharmacokinetics of Levetiracetam: A Systematic Review. Clin. Pharmacokinet. 2021, 60, 305–318. [Google Scholar] [CrossRef]
- Lewis, R.E. Current concepts in antifungal pharmacology. Mayo Clin. Proc. 2011, 86, 805–817. [Google Scholar] [CrossRef]
- Ernst, E.J.; Klepser, M.E.; Ernst, M.E.; Messer, S.A.; Pfaller, M.A. In vitro pharmacodynamic properties of MK-0991 determined by time-kill methods. Diagn. Microbiol. Infect. Dis. 1999, 33, 75–80. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts; CLSI: Berwyn, PA, USA, 2022. [Google Scholar]
- Li, F.; Zhou, M.; Jiao, Z.; Zou, Z.; Yu, E.; He, Z. Caspofungin pharmacokinetics and probability of target attainment in ICU patients in China. J. Glob. Antimicrob. Resist. 2021, 25, 238–263. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Imaizumi, M.; Ishiwada, N.; Kaneko, T.; Goto, H.; Kato, K.; Hara, J.; Kosaka, Y.; Koike, K.; Kawamoto, H.; et al. Pharmacokinetics, efficacy, and safety of caspofungin in Japanese pediatric patients with invasive candidiasis and invasive aspergillosis. J. Infect. Chemother. 2015, 21, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.H.; Xu, H.; Gao, L.L.; Nie, Y.M.; Xing, L.P.; Yu, L.P.; Wu, S.L.; Wang, Y. Population Pharmacokinetics of Caspofungin and Dosing Optimization in Children with Allogeneic Hematopoietic Stem Cell Transplantation. Front. Pharmacol. 2020, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Pressiat, C.; Ait-Ammar, N.; Daniel, M.; Hulin, A.; Botterel, F.; Levesque, E. Pharmacokinetics/Pharmacodynamics of Caspofungin in Plasma and Peritoneal Fluid of Liver Transplant Recipients. Antimicrob. Agents Chemother. 2022, 66, e0118721. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.M.; Leroux, S.; Storme, T.; Zhang, D.L.; de Beaumais, T.A.; Shi, H.Y.; Yang, Y.L.; Wang, X.L.; Zhao, W.; Jacqz-Aigrain, E. Body Surface Area-Based Dosing Regimen of Caspofungin in Children: A Population Pharmacokinetics Confirmatory Study. Antimicrob. Agents Chemother. 2019, 63, e00248-19. [Google Scholar] [CrossRef] [PubMed]
- Würthwein, G.; Cornely, O.A.; Trame, M.N.; Vehreschild, J.J.; Vehreschild, M.J.; Farowski, F.; Müller, C.; Boos, J.; Hempel, G.; Hallek, M.; et al. Population pharmacokinetics of escalating doses of caspofungin in a phase II study of patients with invasive aspergillosis. Antimicrob. Agents Chemother. 2013, 57, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Lan, J.; Wang, X.; Wu, Y.; Yao, F.; Wang, Y.; Zhao, B.X.; Wang, Y.; Chen, J.; Chen, C. Population Pharmacokinetics of Caspofungin and Dose Simulations in Heart Transplant Recipients. Antimicrob. Agents Chemother. 2022, 66, e0224921. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Z.; Liu, D.; Chen, W.; Cui, G.; Li, P.; Zhang, X.; Li, M.; Zhan, Q.; Wang, C. Population Pharmacokinetics of Caspofungin among Extracorporeal Membrane Oxygenation Patients during the Postoperative Period of Lung Transplantation. Antimicrob. Agents Chemother. 2020, 64, e00687-20. [Google Scholar] [CrossRef]
- Bailly, S.; Gautier-Veyret, E.; Lê, M.P.; Bouadma, L.; Andremont, O.; Neuville, M.; Mourvillier, B.; Sonneville, R.; Magalhaes, E.; Lebut, J.; et al. Impact of Loading Dose of Caspofungin in Pharmacokinetic-Pharmacodynamic Target Attainment for Severe Candidiasis Infections in Patients in Intensive Care Units: The CASPOLOAD Study. Antimicrob. Agents Chemother. 2020, 64, e01545-20. [Google Scholar] [CrossRef]
- Würthwein, G.; Young, C.; Lanvers-Kaminsky, C.; Hempel, G.; Trame, M.N.; Schwerdtfeger, R.; Ostermann, H.; Heinz, W.J.; Cornely, O.A.; Kolve, H.; et al. Population pharmacokinetics of liposomal amphotericin B and caspofungin in allogeneic hematopoietic stem cell recipients. Antimicrob. Agents Chemother. 2012, 56, 536–543. [Google Scholar] [CrossRef]
- Gastine, S.; Hempel, G.; Neely, M.N.; Walsh, T.J.; Groll, A.H. Pharmacokinetic modelling of caspofungin to develop an extended dosing regimen in paediatric patients. J. Antimicrob. Chemother. 2022, 77, 2209–2216. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, T.; Zhang, Y.; Sun, D.; Zheng, X.; Du, Q.; Wang, X.; Cheng, X.; Xing, J.; Dong, Y. The recommended dosage regimen for caspofungin in patients with higher body weight or hypoalbuminaemia will result in low exposure: Five years of data based on a population pharmacokinetic model and Monte-Carlo simulations. Front. Pharmacol. 2022, 13, 993330. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, P.; Lee, W.; Xu, X.; Leake, B.F.; Yamazaki, M.; Stone, J.A.; Lin, J.H.; Pearson, P.G.; Kim, R.B. Hepatic uptake of the novel antifungal agent caspofungin. Drug Metab. Dispos. 2005, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Stader, F.; Wuerthwein, G.; Groll, A.H.; Vehreschild, J.J.; Cornely, O.A.; Hempel, G. Physiology-based pharmacokinetics of caspofungin for adults and paediatrics. Pharm. Res. 2015, 32, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Adamson, P.C.; Seibel, N.L.; Flynn, P.M.; Neely, M.N.; Schwartz, C.; Shad, A.; Kaplan, S.L.; Roden, M.M.; Stone, J.A.; et al. Pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrob. Agents Chemother. 2005, 49, 4536–4545. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Llorens, X.; Macias, M.; Maiya, P.; Pineros, J.; Jafri, H.S.; Chatterjee, A.; Ruiz, G.; Raghavan, J.; Bradshaw, S.K.; Kartsonis, N.A.; et al. Pharmacokinetics and safety of caspofungin in neonates and infants less than 3 months of age. Antimicrob. Agents Chemother. 2009, 53, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Sinha, J.; Al-Sallami, H.S.; Duffull, S.B. Choosing the Allometric Exponent in Covariate Model Building. Clin. Pharmacokinet. 2019, 58, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Fortmann, I.; Hartz, A.; Paul, P.; Pulzer, F.; Müller, A.; Böttger, R.; Proquitté, H.; Dawczynski, K.; Simon, A.; Rupp, J.; et al. Antifungal Treatment and Outcome in Very Low Birth Weight Infants: A Population-based Observational Study of the German Neonatal Network. Pediatr. Infect. Dis. J. 2018, 37, 1165–1171. [Google Scholar] [CrossRef]
- Roch, A.; Woloch, C.; Blayac, D.; Solas, C.; Quaranta, S.; Mardelle, V.; Castanier, M.; Papazian, L.; Sampol-Manos, E. Effect of fluid loading during hypovolaemic shock on caspofungin pharmacokinetic parameters in pig. Crit. Care 2011, 15, R219. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef]
- Gasperetti, T.; Welte, R.; Oberacher, H.; Marx, J.; Lorenz, I.; Schellongowski, P.; Staudinger, T.; Burgmann, K.; Eller, P.; Santner, T.; et al. Penetration of echinocandins into wound secretion of critically ill patients. Infection 2021, 49, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Betts, R.F.; Nucci, M.; Talwar, D.; Gareca, M.; Queiroz-Telles, F.; Bedimo, R.J.; Herbrecht, R.; Ruiz-Palacios, G.; Young, J.A.; Baddley, J.W.; et al. A Multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin. Infect. Dis. 2009, 48, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Kurland, S.; Furebring, M.; Löwdin, E.; Eliasson, E.; Nielsen, E.I.; Sjölin, J. Pharmacokinetics of Caspofungin in Critically Ill Patients in Relation to Liver Dysfunction: Differential Impact of Plasma Albumin and Bilirubin Levels. Antimicrob. Agents Chemother. 2019, 63, e02466-18. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ju, G.; Huang, X.; Yang, W.; Chen, L.; Li, C.; He, Q.; Xu, N.; Zhu, X.; Ouyang, D. Escitalopram population pharmacokinetics and remedial strategies based on CYP2C19 phenotype. J. Affect. Disord. 2024, 346, 64–74. [Google Scholar] [CrossRef]
- Kurland, S.; Löwdin, E.; Furebring, M.; Shams, A.; Chryssanthou, E.; Lagerbäck, P.; Tängden, T.; Breuer, O.; Sjölin, J. Human plasma protein levels alter the in vitro antifungal activity of caspofungin: An explanation to the effect in critically ill? Mycoses 2022, 65, 79–87. [Google Scholar] [CrossRef]
| Study (Publication Year) | Country (Type of Study) | Number of Subjects (Male/Female) | Basic Entry Standard | Number of Observations | Sampling Schedule | Age (Years) Mean ± SD Median [Range] | Weight (kg) Mean ± SD Median [Range] | Daily Dose Mean ± SD Median [Range] | Bioassay [LOQ] |
|---|---|---|---|---|---|---|---|---|---|
| Würthwein et al. (2011) [32] | Germany (clinical trial) | 19 (11/8) | Adults allo-HSCT recipients, immunocompromized | 239 | Day 1 and Day 4: immediately before administration; 0.5 to 1.5 h, 1.5 to 3 h, 3 to 5 h, 5 to 11 h, and 22 to 23 h after administration; Thereafter: random time points twice weekly until the end of treatment | 43.4 [20.1–57.6] | 71.2 [56–99.2] | 70 mg QD on day 1, followed by 50 mg QD, IV infusion over 60 min | HPLC [0.15 mg/L] |
| Würthwein et al. (2013) [28] | Germany (clinical trial) | 46 (21/25) | Adults with invasive Aspergillosis, immunocompro-mized | 468 | day 1 (immediately prior to dosing and 2 h [peak level], 3 h, 5 to 7 h, and 24 h [trough level] after the start of infusion); peak and trough time points on days 4, 7, 14, 28 h | 61 [18–74] | 76 [43–104] | 70 mg, QD; 100 mg, QD; 150 mg, QD; 200 mg, QD, IV infusion over 120 min | LC-MS/MS [0.084 mg/L] |
| Pérez-Pitarch et al. (2018) [12] | Spain (clinical trial) | 12 (6/6) | Critically ill adults on CVVHD | 105 | Day 3 and later: predose, 0.5, 1, 1.5, 2, 2.5, 3, 5, 7, 9, 24 h after the start of infusion | 73 [56–78] | 75 [60–88] | NR | HPLC [NR] |
| Yang et al. (2019) [27] | France (clinical trial) | 48 (28/20) | Children in ICU | 159 | NR | 6.07 ± 2.74 5.09 [2.05–11.77] | 22.78 ± 8.71 21 [11.8–47.5] | 70 mg/m2 (loading dose on day 1), 50 mg/m2 QD. | HPLC/MS [0.25 mg/L] |
| Wang et al. (2020) [30] | China (clinical trial) | ECMO group 12 (9/3) | Adults on ECMO after LT | 271 | predose, 0.5, 1, 2, 4, 8, 12, 24 h after the start of the infusion | 65 [60–67] | 64 [59–69.3] | 50 mg QD | UPLC-MS/MS [0.39 mg/L] |
| Control group 7 (5/2) | Adult patients never on ECMO after LT | 59 [56–62] | 65 [53–65] | ||||||
| Bailly et al. (2020) [31] | France (observational study) | 13 (10/3) | Adult Patients in ICU with proven or suspected invasive candidiasis | NR | 0, 2, 3, 5, 7, 24 h postinfusion | 53 [34–55] | 76.5 [60–85] | 50 mg QD with a 140 mg loading dose, IV infusion over 60 min | LC-MS/MS [0.5 mg/L] |
| Niu et al. (2020) [25] | China (clinical trial) | 48 (31/17) | Children with allo-HSCT | 139 | an opportunistic sampling strategy | 6.58 ± 3.7 [0.61–14] | 21.7 ± 10.3 [7.5–54] | loading dose of 70 mg/m2 followed by 50 mg/m2 | HPLC [0.6 mg/L] |
| Borsuk-De Moor et al. (2021) [11] | Poland (observational study) | 30 (16/14) | ICU patients | 180 | 0.5, 2, 4, 8, 12, 24 h | 53 [28–76] | 74 [40–150] | 70 mg intravenously on the first day and at 50 mg i.v on the consecutive days | HPLC [0.18 μg/mL] |
| Li et al. (2021) [23] | China (observational study) | 42 (31/11) | ICU patients with IFIs | 140 | 1,3,6, 24 h on Day 4 | 56.82 ± 16.39 [20–88] | 59.18 ± 11.40 [41–84.5] | a 70 mg loading dose and a 50 mg maintenance dose | LC-MS/MS [0.2 μg/mL] |
| Gastine et al. (2022) [33] | Germany (clinical trial) | 48 (26/22) | Children aged 3–17 | NR | Day 1, Day 4 and Day 9 | 6 [0–16] | 21.5 [9.4–79.5] | CAS I: 1 mg/kg | NR |
| CAS II: 50 mg/m2 | |||||||||
| CAS III: 70 mg/m2 | |||||||||
| CAS IV: 50 mg/m2 | |||||||||
| Wu et al. (2022) [29] | China (clinical trial) | HTx group 27 (22/5) | 27 HTx | 414 | predose, 1, 2, 6, 10, 16, 24 h | HTx group 50 [20–73] | HTx group 59.5 [43.5–76] | 1 h IV infusion at a dose of 50 mg QD after a loading dose of 70 mg | LC-MS/MS [0.4 mg/L] |
| non-HTx group 31 (21/10) | 31 non-HTx | Control group 58 [22–78] | Control group 62 [48–100] | ||||||
| Pressiat et al. (2022) [26] | France (clinical trial) | 20 (9/11) | Adult LT recipients admitted to the liver ICU | 395 plasma and 50 PF samples | predose, 1, 2, 4, 8, 12, 24 h D1, D3, D8 | 45 [40.7–50] | 72 [62–81] | A loading dose of 70 mg and then 50 mg per day (or 70 mg per day if the recipient > 80 kg), IV infusion over 1 h | HPLC [0.5 mg/L] |
| Yang et al. (2022) [34] | China (observational study) | 299 (207/92) | Patients who have been diagnosed with confirmed or probable candidiasis | 921 plasma samples | Cmin samples at interval windows of 22–24 h post-dose, other samples at interval windows of 0–12 h and 12–24 h post-dose | 44 [18–99] | 62.3 [30–100] | 1. standard dosage regimen of 70/50 mg; 2. Patients with hepatic insufficiency received 70/35 mg; 3. patients > 75 kg received 70/70 mg | LC-MS [NR] |
| Study (Publication Year) | Software/ Algorithm | Fixed Effect Parameters (RSE) | Between-Subject Variability (%) | Residual Unexplained Variability | Internal Validation | External Validation | Simulation Target | Modeling Application | |
|---|---|---|---|---|---|---|---|---|---|
| Würthwein et al. (2011) [32] | NONMEM/FOCE-I | CL (L/h) | =0.462 | 25 | prop.err = 21% | VPC GOF Bootstrap | NR | NR | Evaluate covariate effects |
| V1 (L) | =8.33 | 29 | |||||||
| Q (L/h) | =1.25 | / | |||||||
| V2 (L) | =3.59 | / | |||||||
| Würthwein et al. (2013) [28] | NONMEM/FOCE-I | CL (L/h) | =0.411 × [1 + 0.0102 × (BW-76)] | 28.5 | prop.err = 14.3% | pcVPC VPC GOF Bootstrap | 36/456 | NR | Evaluate covariate effects |
| V1 (L) | =5.85 × [1 + 0.0102 × (BW-76)] | 28.8 | |||||||
| Q (L/h) | =0.843 | / | |||||||
| V2 (L) | =6.53 | 66.8 | |||||||
| IOV(CL) | =0.16 | / | |||||||
| Cor-CL-V1 | =0.802 | / | |||||||
| Pérez-Pitarch et al. (2018) [12] | NONMEM/FOCE-I | Ke (h−1) | =0.0899 | 11.8 | add.error = 0.0941 mg/L | GOF Bootstrap VPC | NR | AUC/MIC: C. albicans 865; C. glabrata 450; C. parapsilosis 1185; Cmax/MEC: Aspergillus spp. 10–20 | Evaluate the efficacy of different dosages |
| V1 (L) | =6.46 × (BW/75) × [1–0.233 × (TP-5.6)] | 21.4 | |||||||
| K12 (h−1) | =0.494 | / | |||||||
| K21 (h−1) | =0.392 | / | |||||||
| Yang et al. (2019) [27] | NONMEM/FOCE-I | CL (L/h) | =0.165 × (BSA/0.79)1.3 | 24.2 | prop.err = 19.6% | GOF VPC NPDEs Bootstrap | NR | Cmin | Evaluate the efficacy of the dosing regimen; describe PK in a specific population |
| V1 (L) | =1.730 × (BSA/0.79)1.5 | / | |||||||
| Q (L/h) | =0.351 | 161.6 | |||||||
| V2 (L) | =0.943 | 76.6 | |||||||
| Wang et al. (2020) [30] | NONMEM/NR | CL (L/h) | =0.21 × (OPT/5)1.3 | 20 | Add.error = 0.73 mg/L | GOF VPC Bootstrap | NR | NR | Evaluate covariate effects; describe PK in a specific population |
| V1 (L) | =(2.21 + SEX × 0.62) × (OPT/5)0.93 | 10 | |||||||
| V2 (L) | =2.87 | 48.0 | |||||||
| Q (L/h) | =0.84 × (SOFA/7)1.98 | / | |||||||
| Bailly et al. (2020) [31] | Monolix/SEAM | CL (L/h) | =0.98 | 42.3 | prop.err = 12.2% | GOF VPC | NR | AUC/MIC: 50, 450, 865; Cmax/MIC 5, 10, 15, 20 | Evaluate different dosages |
| V1 (L) | =9.01 | 42.6 | |||||||
| Q (L/h) | =5.12 | 79.9 | |||||||
| V2 (L) | =11.9 | 77.2 | |||||||
| Niu et al. (2020) [25] | Phoenix NLIME | CL (L/h) | =0.1 × (BSA/0.79)0.89 × (lnAST/3.38)−0.23 | 33.3 | prop.err = 26.6% | GOF VPC NPDEs Bootstrap | NR | AUC24/MIC | Dosing optimization against Candida spp. |
| V1 (L) | =1.36 × (BSA/0.79) | 32.9 | |||||||
| Borsuk-De Moor et al. (2021) [11] | NONMEM/FOCE-I | CL (L/h)D1 | =0.563 × (BW/70)0.75 | 24.7 | prop.err = 19.9% | pcVPC GOF Bootstrap | NR | AUC24/MIC C.albicans 865; C. glabrata 450; C.parapsilosis 1185 | Evaluate covariate effects; describe PK in a specific population |
| CL (L/h)D2 | =0.737 * (BW/70)0.75 | 24.7 | |||||||
| CL (L/h)D3 | =1.01 × (BW/70)0.75 | 24.7 | |||||||
| V1 (L) D1 | =6.04 × (BW/70) | 28.6 | |||||||
| V1 (L) D2 | =7.32 × (BW/70) | 28.6 | |||||||
| V1 (L) D3 | =7.70 × (BW/70) | 28.6 | |||||||
| Q (L/h) | =1.31 | / | |||||||
| V2 (L) | =5.13 | 49.4 | |||||||
| Cor-CL-V1 | =0.868 | / | |||||||
| Li et al. (2021) [23] | NONMEM/FOCE-I | CL (L/h) | = 0.323 × 0.89 × (35/ALB)1.27 (TBIL ≤ 22 μmol/L) = 0.323 × (35/ALB)1.27 × (TBIL/22)0.265 (TBIL >22 μmol/L) | 22.4 | prop.err = 24% | pcVPC GOF NPDEs | NR | AUC24/MIC C. albicans 865; C. glabrata 450; C. parapsilosis 1185 | Evaluate covariate effects |
| V1 (L) | =6.77 × (WT/70)1.08 | / | |||||||
| Q (L/h) | =0.923 | / | |||||||
| V2 (L) | =4.58 | / | |||||||
| Gastine et al. (2022) [33] | NONMEM/FOCE-I | CL (L/h/70 kg) | =0.790 | 27.5 | prop.err = 19.4% | GOF VPC Bootstrap | NR | AUC24/MIC: C. albicans 865; C. glabrata 450; C. parapsilosis 1185; fAUC24/MIC: (10–20) | Assess extended twice-weekly dosage using caspofungin |
| V1 (L/70 kg) | =7.75 | 31.5 | |||||||
| Q (L/h/70 kg) | =1.20 | / | |||||||
| V2 (L/70 kg) | =5.29 | 15.1 | |||||||
| IOV(CL) | =17.2% | / | |||||||
| Wu et al. (2022) [29] | NONMEM/NR | CL (L/h) | =0.385 × (ALB/37.42)−1.01 | 33.5 | prop.err = 13.4% add.error = 0.213 mg/L | GOF Bootstrap VPC NPDE | NR | AUC24/MIC: C. albicans 865; C. glabrata 450; C. parapsilosis 1185 | Evaluate covariate effects |
| V1 (L) | =4.27 | 67.5 | |||||||
| Q (L/h) | =2.85 | 0.0 | |||||||
| V2 (L) | =6.01 | 47.7 | |||||||
| Pressiat et al. (2022) [26] | Monolix/ FOCE-I | CL (L/h) | =0.38 | 33.0 | prop.err = 36% | GOF pcVPC | NR | fAUC24/MIC: C. albicans 25.9; C. glabrata 13.5; C. parapsilosis 35.5 | Analyze the PK/PD of caspofungin in a specific population |
| V1 (L) | =6.24 | 59.0 | |||||||
| Q (L/h) | =2.58 | / | |||||||
| V2 (L) | =6.44 | 107.0 | |||||||
| Keff,13 | =0.08 | 54 | |||||||
| Keff,31 | =0.26 | / | |||||||
| Yang et al. (2022) [34] | NONMEM/FOCE-I | CL (L/h) | =0.32 × (1 + 0.46 × ALB*) × (1 + 0.98 × WT*) (ALB* = 1, ALB < 35 g/L; ALB* = 0, ALB ≥ 35 g/L; WT* = 1, WT ≥ 70 kg, WT* = 0, WT< 70 kg) | 29.2 | prop.err = 19.3% | Bootstrap GOF pcVPC | NR | fAUC24/MIC C. albicans 20; C. glabrata 7; C. parapsilosis 7 | Evaluate covariate effects; describe PK in a specific population |
| V1 (L) | =13.31 × (1 + 0.49 × ALB*) × (1 + 0.24 × WT*) (ALB* = 1, ALB < 35 g/L; ALB* = 0, ALB ≥ 35 g/L; WT* = 1, WT ≥ 70 kg, WT* = 0, WT< 70 kg) | 59.2 | |||||||
| Study (Publication Year) | Tested Covariates | Covariate Selection Criteria | Significant Covariates | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Demographic | Laboratory Tests | Co-Administration | Forward Inclusion | Backward Elimination | CL | V1 | Q | V2 | |
| Würthweinet al. (2011) [32] | Sex, Age, Weight, BSA | TBIL, CLCR | Liposomal amphotericin B | p < 0.001 | p < 0.001 | NR | NR | NR | NR |
| Würthwein et al. (2013) [28] | Dose, Sex, Age, Weight | TBIL, CLCR | NR | p < 0.05 | p < 0.01 | Weight | Weight | NR | NR |
| Pérez-Pitarch et al. (2018) [12] | Age, Sex, Weight | TP, SCR, TBIL, CLCR | NR | p < 0.05 | p < 0.01 | NR | Weight, TP | NR | NR |
| Yang et al. (2019) [27] | Age, Weight, BSA | SCR, ALB | NR | NR | p < 0.05 | BSA | BSA | NR | NR |
| Wang et al. (2020) [30] | Age, Sex, Weight, BMI, ECMO, OPT, 24 h fluid balance | SOFA, ALT, AST, ALB, TBIL, CLCR, PCT | NR | p < 0.05 | p < 0.01 | OPT | Sex | SOFA | NR |
| Bailly et al. (2020) [31] | Age, Weight, ECMO | ALB, PAL, TBIL, AST, ALT, SCR, SOFA | NR | NR | NR | NR | NR | NR | NR |
| Niu et al. (2020) [25] | BSA, Weight, | CR, eGFR, ALB, TBIL, DBIL, ALT, AST, γ-GT | NR | p < 0.05 | p < 0.01 | BSA, AST | BSA | NR | NR |
| Borsuk-De Moor et al. (2021) [11] | Age, Weight, Height, Sex, ECMO, CRRT, Survival, dose | SOFA, PCT, UF, ELWI, Cardiac Output, ALB, TP, Liver Failure | NR | NR | p < 0.01 | NR | NR | NR | NR |
| Li et al. (2021) [23] | Sex, Age, Weight | ALT, AST, TBIL, ALB, CLCR | NR | p < 0.05 | p < 0.01 | ALB, TBIL | Weight | NR | NR |
| Gastine et al. (2022) [33] | Weight, Age, BSA, Sex, Race | ALB, CLCR | Acyclovir, Vancomycin, Dexamethasone | p < 0.05 | p < 0.01 | Weight | Weight | Weight | Weight |
| Wu. et al. (2022) [29] | Age, Sex, Weight, Height, BMI, ECMO, CRRT | ALB, CLCR, AST, ALT, DBIL, TBIL, PCT, PLT, SCR, TP | NR | p < 0.05 | p < 0.001 | ALB | NR | NR | NR |
| Pressiat et al. (2022) [26] | Age, Sex, Weight, BMI | ALB, TP, TBIL, SCR, CLCR, SOFA, WBC, CRP, MELD, PT | NR | p < 0.05 | NR | NR | NR | NR | NR |
| Yang et al. (2022) [34] | Weight, CMT, SOP, SOT, ICU | ALB, MM, CYC, MET | NR | p < 0.05 | p < 0.001 | Weight, ALB | Weight, ALB | NR | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, N.; Shi, Y.; Wang, Y.; Mak, W.; Yang, W.; Ng, K.W.; Wu, Y.; Tang, Z.; He, Q.; Yan, G.; et al. Development and Quality Control of a Population Pharmacokinetic Model Library for Caspofungin. Pharmaceutics 2024, 16, 819. https://doi.org/10.3390/pharmaceutics16060819
Xu N, Shi Y, Wang Y, Mak W, Yang W, Ng KW, Wu Y, Tang Z, He Q, Yan G, et al. Development and Quality Control of a Population Pharmacokinetic Model Library for Caspofungin. Pharmaceutics. 2024; 16(6):819. https://doi.org/10.3390/pharmaceutics16060819
Chicago/Turabian StyleXu, Nuo, Yufei Shi, Yixue Wang, Wenyao Mak, Wenyu Yang, Kar Weng Ng, Yue Wu, Zhijia Tang, Qingfeng He, Gangfeng Yan, and et al. 2024. "Development and Quality Control of a Population Pharmacokinetic Model Library for Caspofungin" Pharmaceutics 16, no. 6: 819. https://doi.org/10.3390/pharmaceutics16060819
APA StyleXu, N., Shi, Y., Wang, Y., Mak, W., Yang, W., Ng, K. W., Wu, Y., Tang, Z., He, Q., Yan, G., Xiang, X., & Zhu, X. (2024). Development and Quality Control of a Population Pharmacokinetic Model Library for Caspofungin. Pharmaceutics, 16(6), 819. https://doi.org/10.3390/pharmaceutics16060819








