The Distinctive Role of Gluconic Acid in Retarding Percutaneous Drug Permeation: Formulation of Lidocaine-Loaded Chitosan Nanoparticles
Abstract
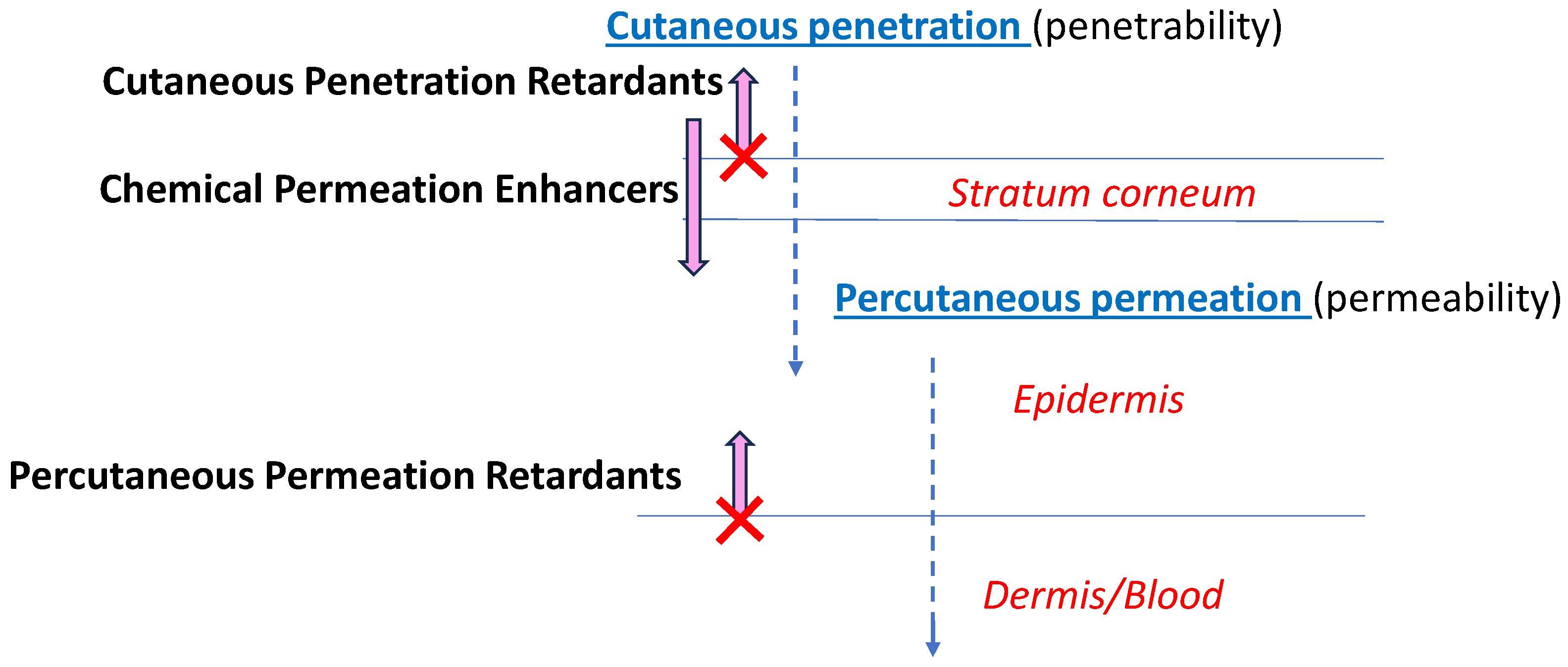
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanoparticles
2.3. Nanoparticle Tracking Analysis (NTA)
2.4. Zeta (ζ) Potential (ZP)
2.5. Skin Permeation Testing
2.5.1. The Ex-Vivo Study
2.5.2. Skin Integrity Testing
2.5.3. Skin Extraction
2.6. HPLC Analysis of Samples
2.7. Calculation of Lidocaine Permeation through the Skin
2.8. Statistical Analysis
3. Results
3.1. Nanoparticle Characterization
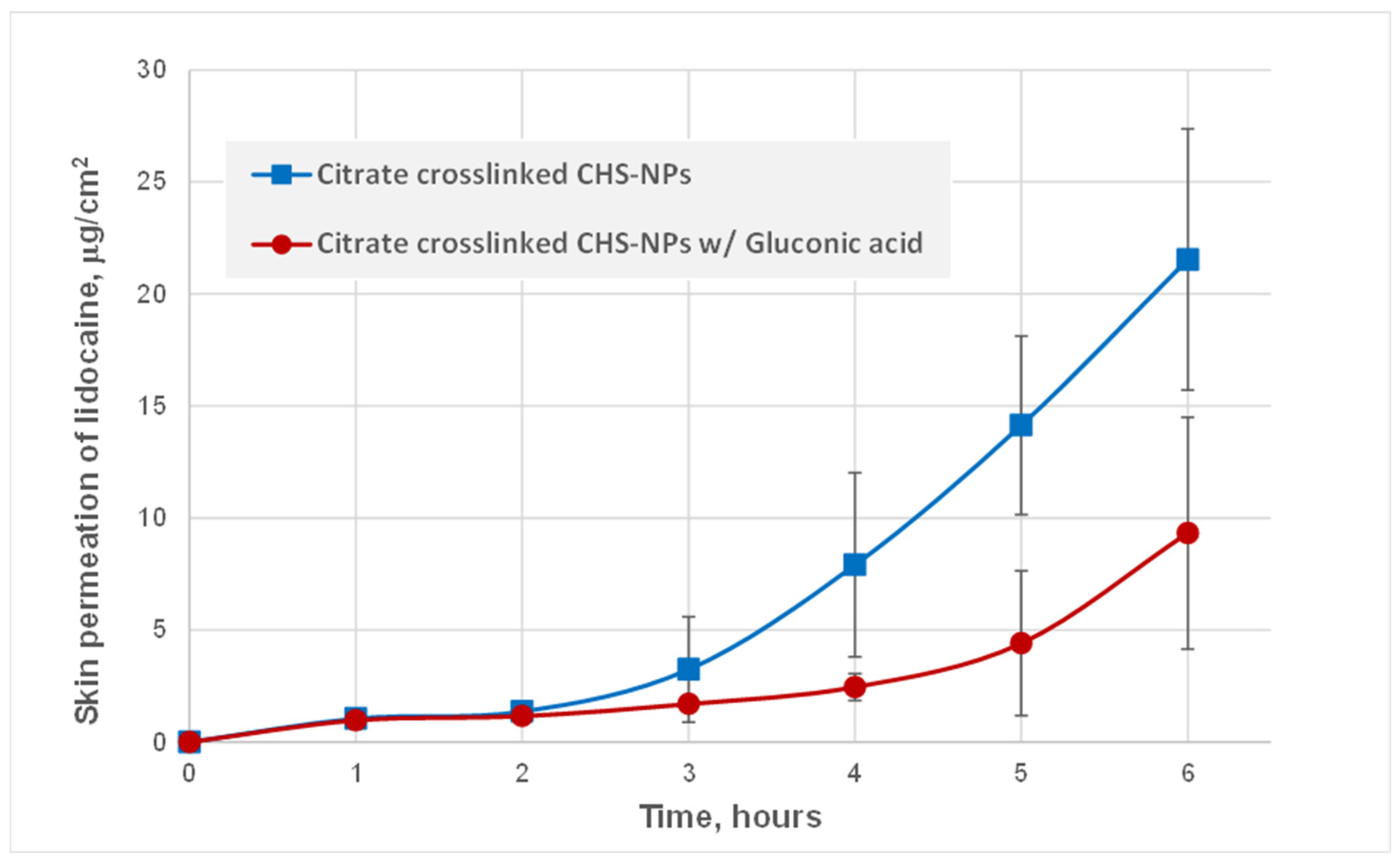
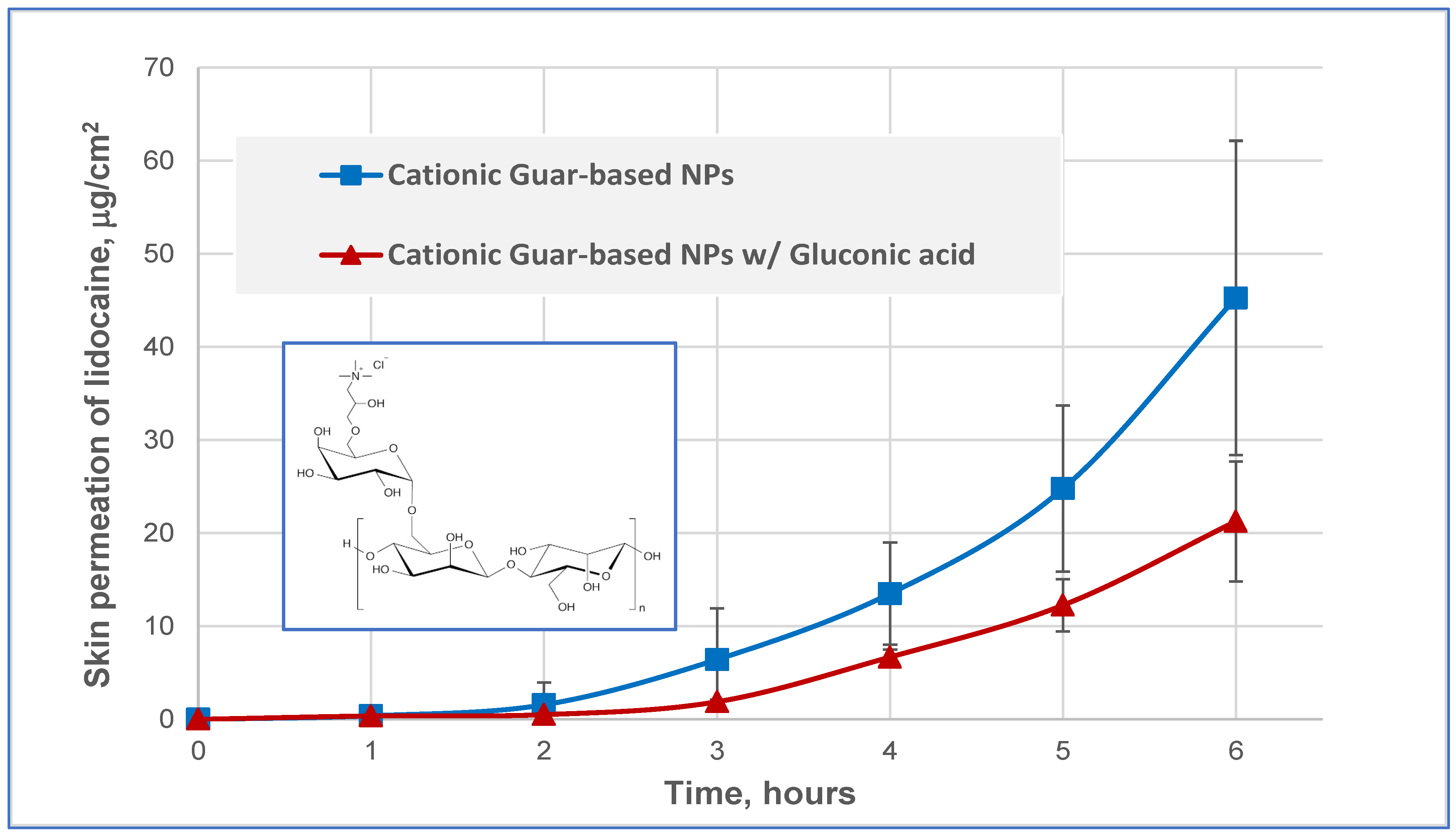
3.2. Skin Permeation of Lidocaine
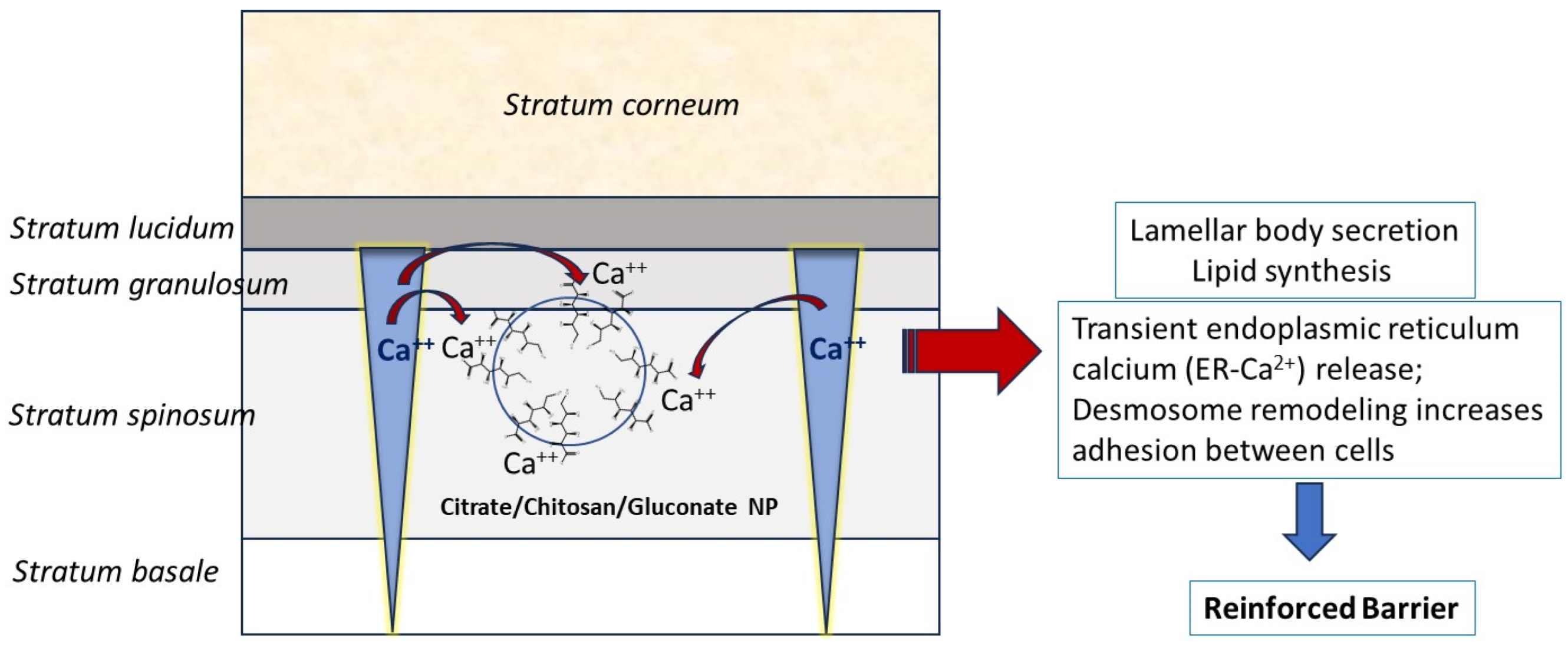
3.3. Mechanistic Explanation of the Skin Drug Retardation by Gluconate-Containing NPs
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velasco-Aguirre, C.; Sintov, A.C. Microemulsions and Nanoparticles as Carriers for Dermal and Transdermal Drug Delivery. J. Pharmacol. Pharm. Res. 2019, 2, 1–12. [Google Scholar] [CrossRef]
- Leong, M.Y.; Kong, Y.L.; Burgess, K.; Wong, W.F.; Sethi, G.; Looi, C.Y. Recent Development of Nanomaterials for Transdermal Drug Delivery. Biomedicines 2023, 11, 1124. [Google Scholar] [CrossRef]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Dermato-Endocrinol. 2009, 1, 197–206. [Google Scholar] [CrossRef]
- Sintov, A.C. Nanoparticulate System Based on Calcium-Crosslinked Carbomer Retards Percutaneous Drug Permeation: New Insight Into Skin Barrier Functions. Pharm. Res. 2022, 39, 3331–3343. [Google Scholar] [CrossRef]
- de Araujo, D.R.; da Silva, D.C.; Barbosa, R.M.; Franz-Montan, M.; Cereda, C.M.S.; Padula, C.; Santi, P.; de Paula, E. Strategies for delivering local anesthetics to the skin: Focus on liposomes, solid lipid nanoparticles, hydrogels and patches. Expert Opin. Drug Deliv. 2013, 10, 1551–1563. [Google Scholar] [CrossRef]
- Sakdiset, P.; Arce, F., Jr.; See, G.L.; Sawatdee, S.; Yoon, A.S. Preparation and characterization of lidocaine HCl-loaded proniosome gels with skin penetration enhancers. J. Drug Del. Sci. Technol. 2023, 86, 104639. [Google Scholar] [CrossRef]
- Marine, E.; Bozdaganyan, M.E.; Orekhov, P.S. Synergistic Effect of Chemical Penetration Enhancers on Lidocaine Permeability Revealed by Coarse-Grained Molecular Dynamics Simulations. Membranes 2021, 11, 410. [Google Scholar] [CrossRef]
- Yuan, J.S.; Yip, A.; Nguyen, N.; Chu, J.; Wen, X.-Y.; Acosta, E.J. Effect of surfactant concentration on transdermal lidocaine delivery with linker microemulsions. Int. J. Pharmaceut. 2010, 392, 274–284. [Google Scholar] [CrossRef]
- Sintov, A.C.; Shapiro, L. New microemulsion vehicle facilitates percutaneous penetration in vitro and cutaneous drug bioavailability in vivo. J. Control. Release 2004, 95, 173–183. [Google Scholar] [CrossRef]
- Zhang, D.; Ye, D.; Jing, P.; Tan, X.; Qiu, L.; Li, T.; Shen, L.; Sun, Y.; Hou, H.; Zhang, Y.; et al. Design, optimization, and evaluation of co-surfactant free microemulsion based hydrogel with low surfactant for enhanced transdermal delivery of lidocaine. Int. J. Pharmaceut. 2020, 586, 119415. [Google Scholar] [CrossRef]
- Menon, G.K.; Feingold, K.R.; Elias, P.M. Lamellar body secretory response to barrier disruption. J. Investig. Dermatol. 1992, 98, 279–289. [Google Scholar] [CrossRef]
- Feingold, K.R.; Man, M.Q.; Menon, G.K.; Cho, S.S.; Brown, B.E.; Elias, P.M. Cholesterol synthesis is required for cutaneous barrier function in mice. J. Clin. Investig. 1990, 86, 1738–1745. [Google Scholar] [CrossRef]
- Holleran, W.M.; Feingold, K.R.; Man, M.Q.; Gao, W.N.; Lee, J.M.; Elias, P.M. Regulation of epidermal sphingolipid synthesis by permeability barrier function. J. Lipid Res. 1991, 32, 1151–1158. [Google Scholar] [CrossRef]
- Celli, A.; Crumrine, D.; Meyer, J.M.; Mauro, T.M. Endoplasmic reticulum calcium regulates epidermal barrier response and desmosomal structure. J. Investig. Dermatol. 2016, 136, 1840–1847. [Google Scholar] [CrossRef]
- Hobbs, R.P.; Amargo, E.V.; Somasundaram, A.; Simpson, C.L.; Prakriya, M.; Denning, M.F.; Green, K.J. The calcium ATPase SERCA2 regulates desmoplakin dynamics and intercellular adhesive strength through modulation of PKC&alpha signaling. FASEB J. 2011, 25, 990–1001. [Google Scholar] [CrossRef]
- Sintov, A.C.; Enden, G. New doxorubicin nanoparticles engineered from calcium-crosslinked carbomer and a microemulsion precursor. Drug Dev. Ind. Pharm. 2017, 43, 830–838. [Google Scholar] [CrossRef]
- Mittal, A.; Sara, U.V.S.; Ali, A.; Aqil, M. Status of Fatty Acids as Skin Penetration Enhancers-A Review. Curr. Drug Del. 2009, 6, 274–279. [Google Scholar] [CrossRef]
- Menon, G.K.; Price, L.F.; Bommannan, B.; Elias, P.M.; Feingold, K.R. Selective obliteration of the epidermal calcium gradient leads to enhanced lamellar body secretion. J. Investig. Dermatol. 1994, 102, 789–795. [Google Scholar] [CrossRef]
- Choi, E.H.; Kim, M.J.; Yeh, B.I.; Ahn, S.K.; Lee, S.H. Iontophoresis and sonophoresis stimulate epidermal cytokine expression at energies that do not provoke a barrier abnormality: Lamellar body secretion and cytokine expression are linked to altered epidermal calcium levels. J. Investig. Dermatol. 2003, 121, 1138–1144. [Google Scholar] [CrossRef]

| Citrate-Crosslinked CHS-NPs | Citrate-Crosslinked CHS-NPs w/Gluconic Acid | Citrate-Crosslinked CHS-NPs w/Caproic Acid | |
|---|---|---|---|
| Mean particle size (nm) | 159.5 | 177.0 | 155.2 |
| PDI | 0.22 | 0.20 | 0.18 |
| D10 (nm) | 96.5 | 107.4 | 98.0 |
| D50 (nm) | 138.1 | 155.9 | 135.0 |
| D90 (nm) | 239.2 | 269.0 | 235.8 |
| NP concentration (NPs/mL) (±SD) | 2.76 × 1013 (±1.49 × 1012) | 2.26 × 1013 (±6.36 × 1011) | 3.12 × 1013 (±2.98 × 1012) |
| ζ potential (±SD) (mV) | 29.5 (±0.5) | 23.0 (±1.00) | 35.4 (±1.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sintov, A.C. The Distinctive Role of Gluconic Acid in Retarding Percutaneous Drug Permeation: Formulation of Lidocaine-Loaded Chitosan Nanoparticles. Pharmaceutics 2024, 16, 831. https://doi.org/10.3390/pharmaceutics16060831
Sintov AC. The Distinctive Role of Gluconic Acid in Retarding Percutaneous Drug Permeation: Formulation of Lidocaine-Loaded Chitosan Nanoparticles. Pharmaceutics. 2024; 16(6):831. https://doi.org/10.3390/pharmaceutics16060831
Chicago/Turabian StyleSintov, Amnon C. 2024. "The Distinctive Role of Gluconic Acid in Retarding Percutaneous Drug Permeation: Formulation of Lidocaine-Loaded Chitosan Nanoparticles" Pharmaceutics 16, no. 6: 831. https://doi.org/10.3390/pharmaceutics16060831
APA StyleSintov, A. C. (2024). The Distinctive Role of Gluconic Acid in Retarding Percutaneous Drug Permeation: Formulation of Lidocaine-Loaded Chitosan Nanoparticles. Pharmaceutics, 16(6), 831. https://doi.org/10.3390/pharmaceutics16060831






